Abstract
The two-process model of sleep regulation posits two main processes regulating sleep: the circadian process controlled by the circadian clock and the homeostatic process that depends on the history of sleep and wakefulness. The model has provided a dominant conceptual framework for sleep research since its publication ∼40 years ago. The time of day and prior wake time are the primary factors affecting the circadian and homeostatic processes, respectively. However, it is critical to consider other factors influencing sleep. Since sleep is incompatible with other behaviors, it is affected by the need for essential behaviors such as eating, foraging, mating, caring for offspring, and avoiding predators. Sleep is also affected by sensory inputs, sickness, increased need for memory consolidation after learning, and other factors. Here, we review multiple factors influencing sleep and discuss recent insights into the mechanisms balancing competing needs.
Keywords: sleep, two-process model, circadian clock, sleep homeostasis, motivation
Introduction
The widely accepted two-process model of sleep regulation postulates that a process controlled by the circadian clock (Process C) interacts with a homeostatic process (Process S) to govern the timing and intensity of sleep [1,2]. Process S, reflecting sleep debt, increases during wakefulness and dissipates during sleep, and a transition to sleep or wake is triggered when Process S reaches the upper or lower threshold, respectively. Process C regulates the daily cycling of the thresholds according to the time of day [3]. EEG slow wave activity (SWA) and rebound sleep are the primary indicators of Process S [4], while body temperature and melatonin rhythms serve as major indicators of Process C [1,2]. The model successfully accounts for the timing and intensity of sleep in a variety of experimental conditions. It has been highly influential and has provided a dominant framework for sleep research for decades [2,5–7].
The molecular and neural basis of circadian rhythms has been studied extensively, and the highly conserved machinery of the molecular oscillators has been elucidated in detail [8]. In mammals, the circadian oscillators in the suprachiasmatic nuclei (SCN) of the hypothalamus synchronize the peripheral clocks [9]. The SCN influences sleep-wake timing in part through downstream subparaventricular zone-dorsomedial hypothalamic pathways acting on the ventrolateral preoptic area (VLPO) and the lateral hypothalamus (LH), as well as the paraventricular nuclei of the hypothalamus acting on the LH [10]. In Drosophila, ∼150 neurons in the central brain that contain the molecular clock machinery serve as the neural substrates for the circadian clock [11]. Among these, several clusters have been shown to affect sleep, including the ventral lateral neurons (LNvs), dorsal lateral neurons, and dorsal neuron group 1 (DN1), likely by acting on downstream targets such as the ellipsoid body and the pars intercerebralis [12].
In contrast to the circadian mechanisms, the molecular and neural mechanisms of sleep homeostasis remain elusive despite recent advances. In mammals, homeostatic control of sleep involves molecules such as adenosine, which increases during wake, promotes sleep, and acts on multiple brain regions [13]. Numerous brain regions in mammals involved in sleep regulation have been identified, facilitated by optogenetic and chemogenetic approaches in recent years [14,15]. However, the brain regions identified thus far are generally active when animals are either awake or asleep and may be involved in switching between wake and sleep states rather than sleep homeostasis [16]. On the other hand, several studies point to subsets of central complex neurons as a putative sleep homeostat in Drosophila [14]. Whether there are functionally analogous neuronal groups in mammals is unknown. Interestingly, recent mammalian and Drosophila studies show that calcium signaling in astrocytes reflects changes in sleep need after deprivation [17,18], suggesting a conversed mechanism of sleep homeostasis.
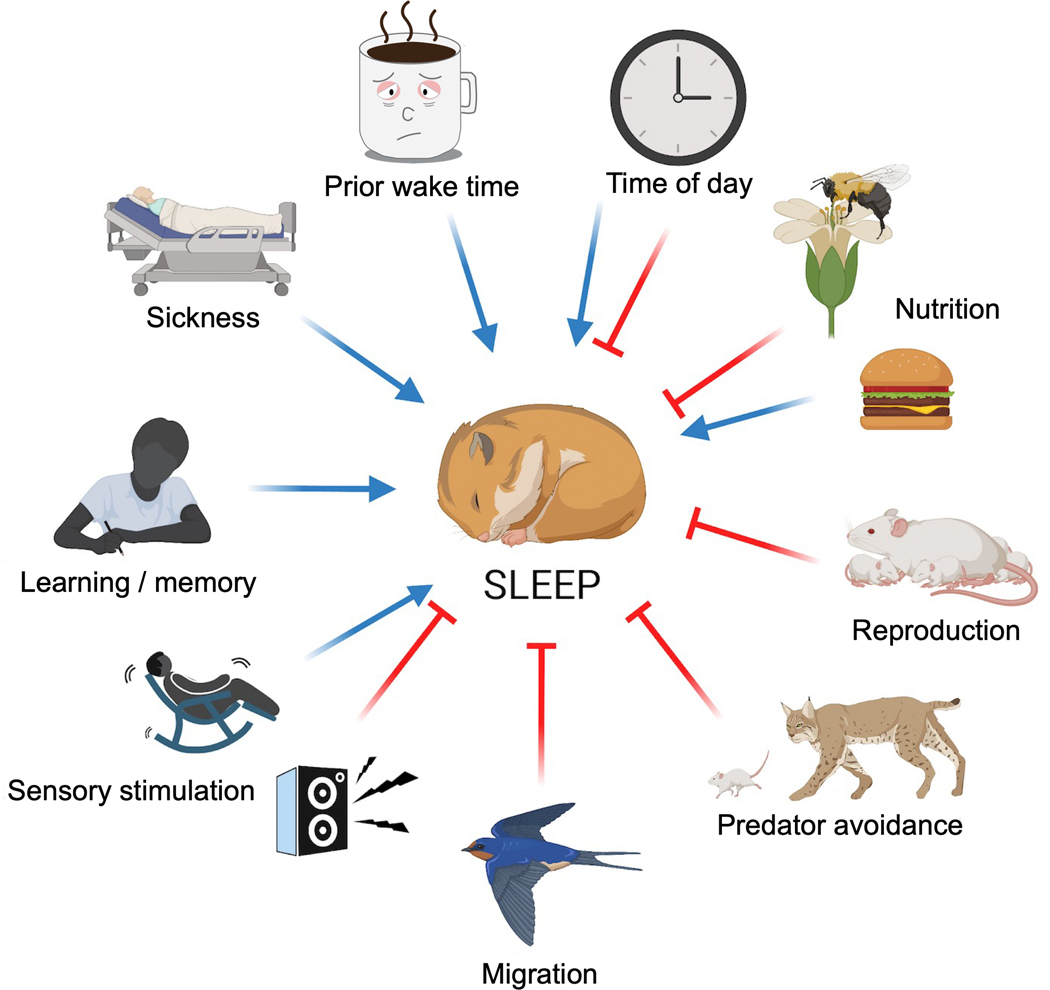
Although the circadian and homeostatic processes are central to sleep regulation, and the time of day and prior wake time are the primary factors affecting the two processes, it is also critical to consider other factors influencing sleep (Figure 1) [19–21]. Sleeping organisms cannot engage in essential behaviors such as eating, foraging, mating, caring for offspring, avoiding predators, and locomotion. Moreover, sensory inputs can interrupt or promote sleep, and certain conditions such as illness, fatigue, or increased need for memory consolidation after learning, may induce sleep. Thus, it is likely that organisms continually monitor various internal drives and environmental conditions to determine whether to sleep or engage in other behaviors. The preceding list of factors impacting sleep, while incomplete, underscores the need to go beyond the time of day and prior wake time for understanding sleep regulation.
Figure 1. Multiple factors influencing sleep.
In addition to the time of day and prior wake time, sleep is strongly influenced by other factors, a subset of which is depicted. Blue arrows indicate sleep promotion, and red lines indicate sleep suppression. The separate arrows and lines do not represent independent processes. The various factors may influence sleep in part through their effects on the circadian and homeostatic processes.
The various sleep-regulatory factors may influence sleep in part through their effects on the circadian clock and sleep homeostat. Circadian rhythms can be modulated by temperature, nutrition, immune challenges, social interactions, and mechanosensory stimulation, among other factors [22–26]. Similarly, the sleep homeostat and its response to sleep deprivation can be affected by stress, social cues, and infections [27–29]. Process C and Process S are separable, but there is evidence of interaction between them [6]. For example, sleep deprivation or increased sleep pressure can affect the magnitude of circadian changes elicited by light [30–32] and SCN neuronal activity [33,34]. Conversely, clock genes may impact the homeostatic control of sleep pressure and the response to sleep deprivation [35,36]. Several recent reviews have covered the circadian and homeostatic mechanisms of sleep regulation [10,12,14]. Here, we focus on some of the other factors influencing sleep as well as recent advances in understanding the genetic and neural mechanisms balancing various drives.
Nutrition
Proper nutrient intake is vital for the survival of an individual. Not surprisingly, food scarcity has a marked influence on sleep in several species. For example, food deprivation leads to reduced sleep in rodents and flies, presumably to allow extra time for foraging [37–40]. The striking reduction in sleep amount in cave-dwelling fish populations of A. mexicanus compared to their surface counterparts is also likely due to food scarcity and the increased need for foraging in caves [41]. The effects of starvation on sleep, however, may depend on the duration of food deprivation. In C. elegans, short-term starvation leads to heightened arousal and foraging behavior, but long-term food deprivation leads to increased sleep [42]. Human studies also suggest that acute and long-term starvation have different effects on sleep [43–45].
Consistent with sleep suppression by starvation, there is an increased propensity for sleep after meals. While human studies yielded variable results, perhaps due to differences across individuals and the amount and type of food consumed [46–50], a recent Drosophila study has documented acute sleep-promoting effects of food consumption in more controlled settings [51]. Rodent studies have also found that a high caloric diet increases rapid eye movement (REM) sleep and alters sleep architecture [52–54]. This section reviews the molecular and neural mechanisms of sleep regulation by the metabolic state, focusing on recent advances from rodent and fly studies.
Sleep suppression by starvation
Ghrelin, a “hunger hormone” that promotes food intake, is upregulated during fasting [55]. Notably, Ghrelin administration is sufficient to stimulate wakefulness in rodents [56,57], suggesting that it is involved in sleep suppression by food deprivation. Neuropeptide Y (NPY)- and Agouti-Related Peptide (AgRP)-expressing neurons mediate Ghrelin signaling, and those in the hypothalamic arcuate nucleus induce appetite-related behavior [55]. Activation of AgRP neurons increases wakefulness, while their inhibition suppresses sleep changes caused by food deprivation [58]. Some rodent studies have also shown that NPY promotes wakefulness and feeding behavior [59–62], although other studies have shown that it induces sleep states [63,64]. Drosophila studies provide supporting evidence that Neuropeptide F (NPF), the ortholog to mammalian NPY, contributes to sleep regulation by food deprivation [65,66]. NPF signaling can also increase wake behavior, and starvation-induced sleep loss requires NPF [66]. Subsets of NPF-expressing neurons in the circadian network and the fan-shaped body, a known sleep center, suppress sleep when activated [66], making them good candidates for sleep regulation by food deprivation. Overall, these studies point to a significant role for the Ghrelin/AgRP/NPY pathway in sleep suppression by starvation.
Orexin (Hypocretin) peptides were first discovered as regulators of feeding behavior in rodents [67]. The subsequent discovery of an Orexin Receptor mutation in narcoleptic dogs and narcolepsy in Orexin knockout mice indicated their role in maintaining wakefulness [68,69]. Orexins are expressed in the LH, a brain region regulating feeding, sleep, and motivated behaviors. Therefore, Orexin neurons are well suited for mediating the effects of food deprivation on sleep. Indeed, ablation of Orexin-expressing neurons abolishes fasting-induced increases in arousal [70]. Further evidence for the role of Orexin in the interaction between hunger and sleep comes from the Mexican cavefish, in which Orexin neurons mediate sleep suppression in the cave populations relative to surface populations [71].
Recent experiments in mice have demonstrated the role of Calretinin (CR), a calcium--binding protein, and CR-expressing neurons in the Paraventricular thalamus (PVTCR+) in starvation-induced wakefulness [72]. PVTCR+ neurons become activated in response to starvation, and their optogenetic activation promotes wakefulness. Significantly, chemogenetic inhibition of PVTCR neurons, specifically those projecting to the bed nucleus of the stria terminalis (BNST), attenuates starvation-induced arousal. These findings show that PVTCR+ neuronal activity reflects the arousal state under starvation and that the PVT-BNST pathway is critical for sleep suppression by starvation.
The first study documenting dramatically decreased sleep in starved flies found that two clock genes, Clk and Cyc, limit starvation-induced sleep loss [40]. Subsequent studies in Drosophila have identified several molecules and neurons involved in the process, including the Adipokinetic Hormone (AKH) pathway, which is analogous to the Glucagon pathway in mammals [73]. AKH Receptor-expressing neurons integrate information from AKH-expressing neurons and Insulin-producing cells (IPCs) for starvation-induced hyperactivity [74], and one of the Drosophila Insulin-like proteins, Dilp2, is necessary for starvation-dependent changes in arousal threshold [75]. Forkhead Box-O (FOXO), which regulates synaptic plasticity, functions downstream of AKH in the starvation-induced remodeling of dorsal projections of the small-LNv (s-LNv) clock neurons [73]. It would be interesting to determine if the role of circadian genes, Clk and Cyc, in limiting the starvation-induced sleep suppression is linked to the AKH/FOXO pathway’s role in remodeling the s-LNv dorsal projections.
The conserved RNA/DNA binding protein Translin is upregulated in starved flies and functions in Leukokinin (Lk)-expressing neurons for starvation-induced sleep suppression [76]. Strikingly, a single pair of Lk-expressing neurons is required to integrate sleep and metabolic state, likely through the action of Lk neuropeptide on IPCs in the central brain [77]. Also, increased serine during starvation acts as a wake-promoting factor through cholinergic neurotransmission [78], suggesting that serine metabolism in the brain regulates starvation-induced sleep suppression.
Metabolic control of sleep may also involve the circadian process. Work from the beginning of the 20th century showed that rats kept under constant light conditions with a regularly scheduled access to food showed anticipatory increases of locomotor activity before mealtime [84]. This phenomenon is controlled by food entrainable oscillators (FEOs), although their exact locations are unknown [85,86]. Whereas the SCN controls most behavioral outputs that occur in a circadian fashion, the FEOs can be entrained independently of the SCN [87–89].
How the nutritional regulation of sleep is integrated with the circadian and homeostatic processes requires further investigation.
Nutrient-specific sleep regulation
In addition to starvation, specific dietary components can affect sleep. Human studies assessing the effect of diet on sleep have found that a diet high in plant-derived foods, whole grains, legumes, and seafood and low in processed food is associated with improved sleep quality [79,80]. In Drosophila, dietary protein (provided mainly by yeast in the laboratory food) modulates sleep amount in a sex-dependent manner [81], and protein consumption leads to postprandial sleep through Lk Receptor neurons [51]. Furthermore, the absence of dietary protein increases sleep depth, a phenomenon dependent on the Dilp2 signaling pathway [75]. In addition to dietary protein, sugar content influences sleep amount and architecture through gustatory receptors [82], and dietary fatty acids promote sleep independently of taste perception [83].
Nutrition also modulates the balance between sleep and other behaviors, as discussed in detail below. In both rodents and fruit flies, metabolic control of sleep involves multiple molecular and neuronal pathways. How the various pathways work together to meet metabolic and sleep needs remains to be investigated.
Reproduction
Reproduction is essential for species survival; therefore, it acts as a strong motivational drive capable of robust sleep modulation. Several vertebrate and invertebrate examples illustrate the animals’ adaptive sleep reduction to enhance successful mating and offspring survival.
Mating
A striking example of sleep loss by sex drive comes from a study of arctic sandpipers [90]. Male sandpipers markedly reduce their sleep to compete for females during 3 weeks of female fertility. Notably, males that sleep the least produce the most offspring, highlighting the benefits of sacrificing sleep to satisfy reproductive needs and challenging the view that sleep loss necessarily leads to decreased performance.
Like sandpipers, flies also reduce sleep in a sexual context. Male flies paired with female flies forgo a lot of nighttime sleep to engage in courtship [28,91]. However, courtship does not always outcompete sleep. Sleep-deprived males (with high sleep drive) and sexually satiated males (with decreased sex drive) favor sleep over mating [91]. Thus, the decision between sleep and courtship depends on the relative strength of sleep and sex drive. Intriguingly, male sleep loss due to a sexual partner does not lead to homeostatic sleep rebound when the partner is removed [28]. Further, female pheromones can suppress rebound sleep that typically follows mechanical sleep deprivation, implying that sexual arousal can override homeostatic sleep drive. The fact that sexually aroused flies and sandpipers maintain reduced sleep for several days or weeks suggests plastic sleep needs depending on the context. Animals that lose sleep voluntarily to meet other pressing needs may sleep more efficiently, obviating the need for rebound sleep.
Recent studies in mice and flies have begun to identify the neural mechanisms by which sex drive competes with sleep drive. A study in mice showed that dopaminergic ventral tegmental area (VTA) neurons mediate wakefulness in the presence of salient stimuli, including a sexual partner [92]. Activation of VTA neurons promotes wakefulness and suppresses sleep, while their inhibition promotes sleep-related nesting behavior. VTA neurons are critical for arousal in response to not only sexual partners but also other ethologically relevant salient stimuli related to food and predators. These findings reveal a central role for the VTA dopaminergic circuit in integrating multiple arousal signals to decide between sleep and wakefulness.
Drosophila studies have also identified several neuronal populations, including dopaminergic neurons, involved in sexually motivated male sleep reduction [28,91,93–96]. At the center are male-specific Fruitless (Fru)-expressing P1 neurons, known primarily for integrating external sensory cues and internal sex drive to control courtship and mating behaviors [97,98]. However, P1 neuron activation also leads to sleep suppression [28,91,96], demonstrating the close link between sleep and courtship regulation. P1 neurons act downstream of a subset of octopaminergic neurons and upstream of a pair of dopaminergic neurons [91,93]. The circadian DN1 neurons function upstream and downstream of P1 neurons [96] and may provide the circadian input to the courtship and sleep balance.
Post-mating offspring care
Species survival relies on proper care of the offspring resulting from successful mating. Post-mating behavioral changes, including altered sleep patterns, have been observed in several species. Sleep changes can occur before and after the birth of the offspring.
Various sleep-related disorders have been documented in pregnant women, including restless legs syndrome, frequent awakenings, daytime sleepiness, and insomnia [99–102]. These problems may be attributable to the hormonal changes as well as physical discomfort. Pregnancy-related sleep changes have also been reported in rodents, although the exact nature of the sleep alterations is unclear. Studies have found a reduction in REM and non-REM (NREM) sleep during the light phase [103,104]. However, two studies showed that pregnancy generally increase NREM and REM sleep [104,105], whereas a third study found decreased sleep [103]. The mechanisms underlying these changes are not yet known but may involve hormones [106–108]. Notably, mating with vasectomized males results in nighttime sleep changes in female rats for up to 12 days post mating, similar to the duration of altered sleep observed in pregnant females [109]. This result implies that post-mating sleep changes in rodents are, at least in part, independent of fertilization.
Females of diverse Drosophila species exhibit post-mating sleep suppression [110–114]. This sleep suppression occurs even when the males lack sperm [110,111], suggesting that post-mating sleep changes in insects, as in rodents, are independent of fertilization. Sex Peptide and its receptor in the female’s SPSN-SAG neuronal circuit convey information about the mating status from the periphery to the central brain to elicit several behavioral changes, including increased egg laying, reduced sexual receptivity, and decreased sleep [111,115]. The reduced sleep may be due to egg laying or foraging for proper food substrates for egg laying.
In addition to prenatal care, postnatal offspring care can also lead to sleep reduction. Postpartum sleep reduction in humans can last more than 12 weeks [116,117], with maternal-infant co-sleeping generally leading to poorer sleep quality in the mothers than sleeping separately [118–120]. Whether postpartum sleep reduction is due only to the baby waking up the adult or also to an internal regulation of the adult’s sleep pattern, is unknown. In dolphins and killer whales, both mothers and offspring suppress rest behavior for several months after birth [121,122]. The early postpartum period in rodents is also associated with increased wakefulness and decreased NREM sleep [103,105], in part due to the external stimuli stemming from the pups [103]. Interestingly, maternal nursing in rabbits acts as a synchronizing factor on their offspring through brain regions that are not part of the central circadian oscillator, resembling the findings of the FEOs and suggesting that reproductive demands recruit novel neuronal substrates to regulate rest-activity patterns [123–125]. An invertebrate example of sleep regulation due to offspring care comes from worker bumble bees, which reduce their sleep amount in the presence of larvae and pupae [126]. Interestingly, bees show no apparent sleep rebound when the pupae are removed, suggesting, that this type of sleep loss does not lead to homeostatic recovery, as seen with sleep loss by sexual encounters in male flies.
Predator avoidance
Animals are vulnerable to predation during sleep, and thus they restrict sleep to certain places and times of the day to mitigate this danger. The circadian organization of rest-activity cycles is plastic, since the appearance of a novel predator or the disappearance of the threat can lead to changes in the diurnality/nocturnality of the prey [127,128]. In addition, sleep undergoes plastic changes depending on recent encounters with predators. For example, rats show increased wake time and decreased NREM and REM sleep after being chased by a simulated predator [129]. Some species regulate other aspects of sleep to facilitate predator avoidance. Mallard ducks located on the edges of a group exhibit increase unihemispheric NREM sleep and orient their opened eye away from the group’s center [130,131]. This ability allows the ducks in the periphery to better detect predators during sleep.
To our knowledge, the potential effects of predator avoidance on invertebrate sleep have not been investigated. However, given the recent demonstration of an internal state resembling fear [132], Drosophila could serve as an attractive model to uncover the molecular and neural mechanisms of sleep regulation by fear.
Migration/locomotion.
Migration in search of food, reproductive niches, or warmer weather can take several days, and it is sometimes associated with alterations in the amount or characteristics of sleep. For example, sleep in migratory birds is dramatically reduced during the migratory season [133–135], and episodes of unihemispheric sleep can occur in flight [135]. Performance on a cognitive task is preserved in white-crowned sparrows with reduced sleep during the migratory season, while sleep restriction during the non-migratory season impairs performance on the same task [134], suggesting that birds show resistance to the effects of sleep loss during migration.
The daily organization of rest-activity cycles is also affected by migration in songbirds, with the appearance of nocturnal activity during migration in otherwise diurnal animals [136]. This phenomenon appears to be controlled by a circadian oscillator that is independent of the one controlling diurnal activity [137]. Moreover, the circadian clock is responsive to seasonal changes in photoperiod, which controls migratory behaviors [138,139], suggesting that the circadian process mediates behavioral changes needed to accomplish the migration process, including nighttime sleep suppression.
Locomotion and physical activity also impact sleep in animals that do not migrate. In rodents, spontaneous activity affects the circadian organization of rest-activity rhythms [reviewed in 140], and voluntary wheel-running activity can consolidate sleep-wake cycles by increasing wakefulness during the active phase of the animal [141–143]. The study of how voluntary activity affects sleep is of particular interest since there is evidence that exercise also improves sleep quality in humans [144]. Finally, a certain level of constant locomotion is needed to assure optimal respiration in some species of sharks and cetacean mammals. Although sleep has not been described in cartilaginous fish that constantly swim [145], unihemispheric sleep in cetacean mammals allows them to fulfill their respiratory and sleep needs [146], pointing to the diverse regulatory effects that physical activity and locomotion can have on sleep.
Sensory stimulation
Multiple sensory inputs such as light, temperature, and food influence sleep, in part by entraining the circadian clock [147–149]. Besides its role in entraining the circadian clock [150], light directly affects sleep, which is referred to as masking [151]. In nocturnal rodents, light can promote sleep when applied during the dark, active phase, while dark pulses applied in the light phase can have the opposite effect [151]. In mammals, the direct effects of light on sleep rely on intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing the photopigment melanopsin [152–154]. These cells connect with brain structures, including the SCN, the intergeniculate leaflet (IGL), as well as with sleep regulatory neurons within the preoptic area (POA) [155,156]. Light induces the activation of both POA and IGL (among other regions downstream iPRGC), and GABAergic IGL and POA neurons have an important role in light-induced sleep promotion in rodents [156,157]. Moreover, light may also affect the homeostatic process since melanopsin-deficient mice show reduced SWA both at baseline and after sleep deprivation [154].
Sensory inputs can influence sleep both positively and negatively. Sensory gating filters out many sensory stimuli during sleep, but intense or salient stimuli such as a loud noise from an alarm clock or food-associated odor can interrupt sleep. On the other hand, certain stimuli such as rocking and soothing sounds can help us fall asleep. In this section, we focus on the latter, i.e., sleep promotion by sensory stimulation.
Anecdotal observations suggest that babies like to be rocked to sleep, and humans of all ages tend to fall asleep during long car rides. Experimental studies have confirmed that rocking promotes sleep in infants, adults, and mice [158–162]. Studies in adult humans found that lying on a bed rocking at 0.25 Hz reduced sleep latency and increased the duration of deep sleep [161,162]. Rocking increased the EEG power of slow wave activity (SWA), believed to reflect sleep need [163], and spindle activity, thought to be involved in sensory gating and memory consolidation [164–166]. Consistent with the well-known effects of sleep on memory consolidation, rocking also improved declarative memory in humans [161].
The first study showing that rocking promotes sleep in a non-human species involved the mouse [159]. Rocking mice at 1 Hz accelerates sleep onset, increased NREM sleep, and decreases wake time. The rocking effects on sleep depends on the maximum linear acceleration instead of the rocking frequency. Mutant mice that cannot encode linear acceleration due to missing otoliths, small particles in the inner ear, do not exhibit rocking-induced sleep changes, suggesting that the vestibular otolithic organs mediate the rocking effects [159].
Gentle vibration also promotes sleep in Drosophila [167]. Flies exhibit reduced sleep after vibration (‘negative rebound’), suggesting vibration-induced sleep contributes to sleep homeostasis. Additionally, flies show reduced arousability during vibration-induced sleep relative to baseline sleep, indicating that sleep during vibration is deeper than baseline sleep. Elevated arousal through the circadian clock, increased dopamine signaling, and reduced GABA signaling attenuate vibration-induced sleep [167], pointing to the suppression of arousal as a potential mechanism for sleep induction by vibration.
Repetitive acoustic stimuli or white noise can also enhance sleep SWA in humans [158,168–171]. Whether stimulation in other sensory modalities such as vision and olfaction can promote sleep has received relatively little attention. While some studies found that olfactory stimulation can promote sleep in humans and rats [172], another study found little effects of olfactory stimuli on human sleep [168]. Moreover, deprivation of olfactory stimulation reduces sleep in flies, consistent with sleep-promoting effects of olfactory inputs, whereas activation of olfactory receptor neurons or presentation of several odorants fails to elicit sleep increase in flies [173]. Differences in the odorants and delivery methods may account for the variable results. Intriguingly, disrupting flight in flies by cutting wings increases sleep through wing chemosensory neurons [174], suggesting that chemosensory stimulation associated with flight promotes sleep. Further investigation of the effects of stimuli in multiple modalities may provide insights into the mechanisms of sleep induction by sensory stimulation and determine whether different modalities with distinct peripheral sensory organs converge on the same central sleep circuits.
Two mechanisms of how repetitive sensory stimulation promotes sleep have been proposed: synchronization and habituation. The synchronization model suggests that sensory stimulation synchronizes neural activity and enhances sleep SWA [161,175]. The model is consistent with the finding that synchronizing brain activity through transcranial direct current stimulation and magnetic stimulation of the cortex can enhance sleep SWA [176–178]. Also consistent with the model are the sleep-promoting effects of rocking and brief tones delivered at low frequencies (≤ 1.5 Hz) in humans and mice [159,161,162,168]. Notably, a human rocking study found that slow oscillations and spindles tend to occur at specific time points relative to the rocking cycles, supporting the idea that rhythmic stimulation entrains brain oscillations. However, these studies did not test sensory stimuli outside the frequency range of SWA, leaving open the possibility that mechanisms other than the synchronization of brain activity to low-frequency stimuli may play a role in sleep induction.
The habituation model proposes that habituation, a form of simple non-associative learning, mediates sleep induction by repetitive stimuli [158,179,180]. The model suggests that habituation leads to a reduction in arousal and an increase in sleep through a common inhibitory mechanism. The finding that vibration-induced sleep in Drosophila requires GABA signaling, the primary inhibitory neurotransmission system, is consistent with the model [167]. More direct support for the model comes from the finding that flies fall asleep faster and sleep longer over multiple blocks of continuous vibration, and the improvement during continuous vibration does not generalize to intermittent vibration [167]. This finding demonstrates stimulus specificity, ruling out sensory adaptation and motor fatigue, and supports the view that habituation is involved in sleep induction by vibration. Furthermore, vibrations ranging from 3–200 Hz can induce sleep in flies [167], suggesting that synchronizing the brain activity to stimuli of a specific frequency range is not required for sleep induction. However, the habituation and synchronization models are not mutually exclusive, and determining their relative contributions under different stimulus conditions may be a promising future direction.
Learning/Memory
One of the essential functions of sleep is to enhance learning and memory. Numerous studies investigated the link between sleep and learning/memory and have shown an evolutionarily conserved role for sleep in facilitating learning and memory consolidation [181]. Almost all studies focused on how sleep influences learning and memory, as discussed in recent review articles [181,182]. A smaller number of studies showed that learning and related processes also influence sleep, demonstrating that the relationship is bi-directional. Here we focus on the effects of learning on sleep.
Sleep or SWA increases after learning tasks in several species [182]. Humans performing a rotation adaptation task, a learning paradigm in which people adapt to a systematic rotation imposed on a visuomotor task, show increased sleep SWA after rotation compared to no-rotation control tasks [183]. Notably, the increase in SWA is localized to the cortical regions relevant for the rotation adaptation task [184], suggesting local regulation of sleep based on synaptic changes associated with learning. Visual perceptual learning that depends on a restricted population of orientation-selective neurons in the occipital cortex increases the number of slow waves initiated in the area [185], further supporting local control of post-learning sleep. Sleep SWA in rats also increases locally after learning a task involving the motor cortex[186] and after locally infusing BDNF to induce synaptic potentiation [187]. In addition, sleep SWA in humans is elevated in cortical areas that are active during wakefulness [183,188,189], suggesting that sleep is locally regulated in a neural activity-dependent manner.
Related studies in Drosophila found that sleep is significantly increased following courtship conditioning, a learning and memory paradigm in which male flies learn to suppress their courtship behaviors following unsuccessful mating attempts toward a mated female [190–192]. Additionally, flies raised in a socially enriched environment sleep significantly more than those kept in isolation [193–196]. Socially enriched flies also show increased pre- and post-synaptic protein levels, suggesting that increased synaptic strength mediates the sleep increase following social enrichment [197]. Importantly, learning and memory mutants with impaired cAMP and ERK signaling do not exhibit sleep increase following social enrichment [193,197,198], suggesting that common molecular mechanisms underlie social enrichment-induced sleep and learning and memory processes.
The effects of cognitive tasks and social interaction on sleep may be mediated in part by their ability to entrain the circadian clock [199,200]. In nocturnal rats, the daily performance of a cognitive task requiring sustained attention during the light phase yields a diurnal activity pattern that persists for days in the absence of cognitive training [200]. Similarly, daily administration of foot shocks to rats alters their feeding and activity from the dark to the light phase, which is maintained in a free-running condition [201]. Interestingly, daily cognitive training can entrain SCN-lesioned animals, suggesting cognition entrainable oscillators outside the SCN [200], reminiscent of the FEOs.
Together, these studies demonstrate that learning, neural activity, and social behavior promote sleep, likely to meet the increased need for memory consolidation. However, as discussed below, a recent study reported an exciting discovery of sleep-independent memory under starvation conditions [202].
Sickness
Sickness caused by various conditions such as infection, excessive heat or cold, and injury results in various behavioral changes, including decreased activity, altered sleep, anorexia, reduced social interaction, cognitive impairments, among others [203–206]. While these responses generally have adaptative advantages such as reducing energy expenditure and promoting the recovery process, prolonged or exacerbated sickness responses can be detrimental [204,207]. This section reviews mechanisms of several types of sickness-induced sleep, focusing on insights from invertebrate studies [208].
Infection and immune response
The effects of inflammation on sleep in mammals have been reviewed elsewhere [209,210]. They include increased NREM sleep, decreased REM sleep, and sleep fragmentation. These changes are mediated by humoral factors such as Interleukins, Interferon and Tumor Necrosis Factor and activation of certain neural circuits such as the vagal circuit [209,210]. The effects of infection and immune response on sleep are observed in invertebrates as well. In Drosophila, infection with gram-negative bacteria increases baseline sleep, mediated by the Nuclear Factor-κβ-like transcription factor relish and the clock gene period [211,212]. Interestingly, a recently identified sleep gene, nemuri, encodes an antimicrobial peptide and has a role in infection-induced sleep increase in flies [213]. Further, intra-thoracic injections of human tumor necrosis factor and astrocytic expression of its Drosophila homolog Eiger promote sleep suggesting a conserved role for the molecular actors in sleep changes upon immune activation [214].
Viral infections with the pathogenic Drosophila C Virus also lead to increased sleep [215]. However, infections with S. pneumoniae and non-pathogenic immune activation via upregulation of the pattern recognition protein PGRP-Lca lead to reduced sleep [216,217]. Whether the divergent results are due to different experimental approaches or varying sleep responses elicited by activating distinct immune pathways remains to be investigated. Notably, proinflammatory markers are associated with the progression of several neurodegenerative diseases [218], which in turn are linked to disrupted sleep regulation [219].
Injury
Traumatic brain injury (TBI) is associated with a variety of sleep disturbances, including excessive daytime sleepiness, increased sleep need, insomnia, and sleep fragmentation in humans [220,221], mice [222], and flies [223]. A peripheral injury can also influence sleep. The removal of antennae or wings in flies increases daytime sleep for several hours [174,224]. Removing other sensory organs or silencing olfactory receptor neurons does not cause increased sleep, suggesting that post-injury sleep is not due to sensory deprivation or inflammatory response. Notably, pre-synaptic active zones of the severed neurons are removed within hours after injury, unlike the Wallerian degeneration of severed axons that requires several days [224]. Mutations that protect neurons from Wallerian degeneration attenuate post-injury sleep, and depriving flies of post-injury sleep impairs the removal of both active zones and axons, suggesting a bidirectional relationship between neural injury and sleep [224].
Cellular stress
Features of sleep such as behavioral quiescence and reduced response to sensory stimuli suggest that sleep may provide an opportunity for restorative processes. Consistent with the view, cellular stress leads to increased sleep in several species to facilitate the recovery from cellular damage. In C. elegans, several manipulations that lead to cellular stress, including noxious heat, cold, hypertonicity, tissue damage and UV light, increase sleep [225,226]. Stress-induced sleep is independent of the sensation of the stressor and is a response to the cellular damage [227], and likely restores protein homeostasis and promotes cellular recovery [225]. The current model of stress-induced sleep in C. elegans is that cellular stress promotes the release of LIN-3/EGF, which activates the ALA neuron, leading to increased sleep via the NLP-8, FLP-24, and FLP-13 neuropeptides [225,228,229]. FLP-13 acts through the GPCR MLSR-1, which inhibits wake-promoting neurons [230]. Interestingly, FLP-13 peptides are related to Drosophila FMRFa peptides, and flies mutant for FMRFa or its receptor show decreased sleep increase upon heat stress [231], suggesting a conserved mechanism for stress-induced sleep.
Endoplasmic reticulum (ER) stress, a form of cellular stress, also influences sleep [232,233]. Overexpression of immunoglobulin binding protein (BiP), an indicator of ER stress, elicits exaggerated recovery sleep after sleep deprivation in Drosophila, while reduced BiP activity has the opposite effect [234]. In addition, inducing ER stress leads to fragmented sleep, whereas relieving ER stress ameliorates sleep fragmentation in aged animals [235]. The role of ER stress regulators in sleep is likely conserved since activating Eukaryotic Initiation Factor 2α, an ER response related pathway, promotes sleep in rats [236,237], and inhibiting the ER kinase PERK reduces sleep in flies and zebrafish [238].
Reactive oxygen species
A proposed function of sleep is removing excess reactive oxygen species (ROS) that accumulate during wakefulness [239]. Studies involving acute sleep restriction have found inconsistent effects on antioxidant response and ROS accumulation in the brains of mice and rats [224,240–246]. However, recent studies show that chronic sleep loss is consistently associated with increased ROS. First, diverse short-sleeping fly mutants exhibit reduced survival after oxidative challenge, while inducing sleep in wild-type flies by genetic and pharmacological means increases resistance to oxidative stress [246]. Second, sleep loss elevates mitochondrial ROS in sleep-promoting neurons in Drosophila [247]. Third, sleep loss causes lethality through ROS accumulation in the gut of flies and mice [248]. These findings support the view that ROS accumulates during wakefulness and decreases during sleep.
Interestingly, overexpression of antioxidant genes in neurons reduces sleep in flies [246], suggesting that ROS plays a role in regulating sleep. A recently proposed mechanism states that accumulated ROS oxidates a cofactor for Hyperkinetic, a subunit of the Shaker potassium channel, and slows the inactivation of the A-type current to enhance the excitability of sleep-promoting neurons, thereby promoting sleep [247].
Nutritional modulation of the balance between competing needs
As we presented in previous sections, sleep is suppressed by various competing needs, such as reproduction, foraging, predator avoidance, and migration. However, the decision to sleep or not is unlikely to be a simple choice between two alternative behaviors. Several recent studies have revealed the complexity of sleep regulatory mechanisms by considering multiple factors simultaneously. We provide four examples of how an organism’s nutritional state can modulate the trade-off between sleep and other demands.
Sleep vs. courtship.
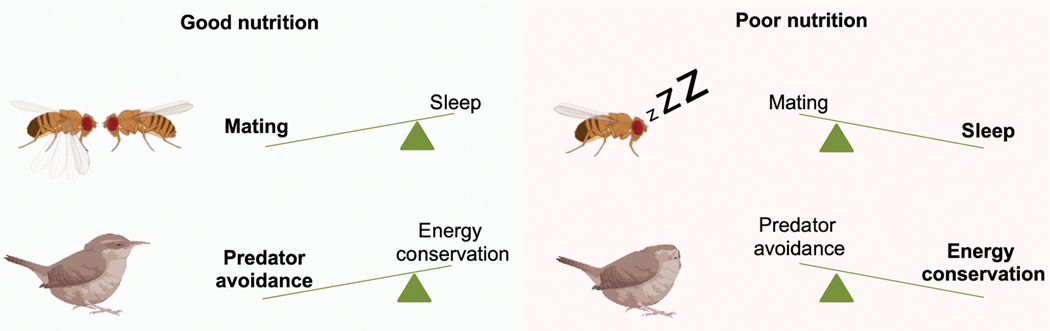
Whereas well-fed male flies are willing to forgo most of their nighttime sleep in favor of courtship when paired with females [28,91], protein-deprived males are less inclined to give up sleep to pursue sexual partners [93]. Although protein-deprived males are less likely to sacrifice sleep at night to engage in courtship, they are willing and able to court and mate during the day (Figure 2). These data suggest that flies engage in a sophisticated cost-benefit analysis to decide when to sleep or court. Since protein is necessary for potential offspring to survive, malnourished males may reason that even a successful mating is not likely to produce live offspring and choose sleep over courtship at night. But if they are already awake during the day, they may decide it is worth the effort to court in case protein becomes available later.
Figure 2. Nutrition modulates the balance between competing needs.
The nutritional status shifts the balance between sleep and other needs. Top: Under good nutritional conditions, male flies forgo their nighttime sleep to engage in reproductive behaviors. Lack of dietary protein, needed for offspring survival, causes males to reduce their nighttime courtship in favor of sleep [93]. Bottom: Garden warblers in good nutritional conditions choose to sleep in an untucked position, which allows for higher alertness and predatory avoidance. Poorly nourished warblers sleep with their heads tucked under the feathers, which favors energy conservancy over predator avoidance [249].
Predator avoidance vs. energy conservation.
Warblers, small migratory songbirds, cannot make their long-distance migration in one leg and make stopovers to rest and forage to restore energy reserves. The birds must reconcile competing needs for sleep, energy restoration/conservation, and predator avoidance during the stopovers. Ferretti et al. investigated sleep and metabolic patterns in garden warblers at a Mediterranean stopover site [249]. They found that songbirds in poor metabolic conditions sleep with the head tucked in their feathers, whereas those in good metabolic conditions sleep with a normal posture. They determined that birds that sleep with the head tucked in reduce energy consumption but respond slower to the noise of a potential predator than those that sleep with a normal posture. Thus, birds in poor metabolic conditions are willing to adopt an unsafe sleep posture to conserve energy, suggesting a trade-off between energy consumption and predator avoidance (Figure 2). Warblers adjust their sleeping posture, which impacts both processes, depending on their metabolic conditions.
Memory consolidation vs. foraging.
The need to sleep for memory consolidation is also affected by food availability [202]. Whereas well-fed flies increase their sleep after olfactory conditioning, starved flies do not. Moreover, post-training sleep deprivation in fed flies leads to poor memory consolidation, but memory is unaffected by sleep deprivation in starved flies. Distinct neural circuits mediate memory in fed vs. starved conditions. This plasticity in memory circuits may allow flies in a food-scarce environment to remember food-associated cues without giving up foraging [202].
Recovery from cellular stress vs. foraging.
Whereas food deprivation reduces sleep to allow time for foraging [38–41], cellular stress by noxious stimuli such as UV light and heat increases sleep to promote recovery from damage [225,228,230]. A study in C. elegans investigated the combined effects of starvation and cellular stress on sleep and found that food deprivation severely suppresses stress-induced sleep [250]. Interestingly, whereas mutant worms with defective stress-induced sleep exhibit shortened lifespan under a well-fed condition, food deprivation protected the mutant worms against the lethal effects of cellular damage [225]. This finding suggests that food deprivation inhibits stress-induced sleep, in part by reducing the need for sleep. Analogous to sleep-independent memory consolidation discussed above [202], recovery from cellular stress may involve distinct cellular pathways in fed and starved conditions.
These examples of nutritional modulation of the tradeoff between competing needs suggest that sophisticated cost-benefit analyses underlie the decision to sleep or not to sleep.
Concluding remarks
As our survey of the literature shows, sleep is strongly influenced by several factors beyond the time of day and prior wake time. Sleep is also influenced by many other factors not covered in this review, such as, exercise, aging, and neurodegeneration, anxiety and other emotions, and human-specific requirements such as jobs and deadlines [129,130,134,144,251–254]. To fully understand how sleep is regulated in health and diseases, it will be advantageous to investigate sleep in various contexts while simultaneously manipulating multiple variables.
Manipulation of specific groups of cells has identified numerous sleep-regulatory neuronal groups in mice, flies, and worms [12,255,256], and uncovered the involvement of glia in sleep regulation [257]. The diversity of sleep-regulatory loci may reflect the many factors that control sleep and explain why many neurodegenerative and neurodevelopmental diseases are linked to sleep disruptions [258]. While most of these loci were identified because their manipulation influences baseline sleep, others influence sleep only in specific circumstances [58,77,91]. Which neurons and glia are activated under various conditions and how they integrate diverse sleep-relevant information are important topics for future research.
A recurring theme in this review is that sleep loss does not necessarily lead to homeostatic rebound sleep or negative consequences. Starvation-induced sleep loss does not lead to rebound sleep [75] or poor memory consolidation [202] and can provide resistance to stress-induced lethality [250]. Similarly, sleep loss due to sexual activities does not result in rebound sleep or poor mating performance [28,90]. These results demonstrate plasticity in sleep regulation in that the physiological need for homeostatic rebound sleep depends on the context of sleep loss. Interestingly, sleep-dependent and sleep-independent memory consolidation employ distinct neural circuits [202]. Switching to alternative neural circuits or molecular pathways may also provide resistance to sleep loss in other contexts, which would be an important topic for future investigation.
Acknowledgements
We thank Laura Reynolds and Irene Cooper for commenting on the manuscript. This work was supported in part by a Pew Latin American Fellowship (to J.M.D.) and grants from the National Institute of Neurological Disorders and Stroke (R01NS086887 and R01NS109151 to K.K).
Abbreviations:
- AgRP
Agouti-Related Peptide
- AKH
Adipokinetic Hormone
- BDNF
Brain-derived neurotrophic factor
- BiP
Immunoglobulin binding protein
- BNST
Bed nucleus of the stria terminalis
- cAMP
Cyclic Adenosine mono-phosphate
- CR
Calretinin
- DN1
dorsal neuron group 1
- EGF
Epidermal growth factor
- ER
Endoplasmic reticulum
- ERK
Extracellular signal-regulated kinase
- FEO
Food entrainable oscillator
- FOXO
Forkhead Box-O
- GPCR
G protein-coupled receptors
- IGL
Intergeniculate leaflet
- IPCs
Insulin-producing cells
- ipRGCs
Intrinsically photosensitive retinal ganglion cells
- LK
Leukokinin
- NPF
Neuropeptide F
- NPY
Neuropeptide Y
- NREM
Non-rapid eye movement
- PERK
Phosporilated-extracellular signal-regulated kinase
- PGRP-Lca
Peptidoglycan-recognition protein LCa
- POA
Preoptic area
- PVT
Paraventricular thalamus
- REM
Rapid eye movement
- s-LNv
Small ventral lateral neurons
- SWA
Slow wave activity
- VLPO
Ventrolateral preoptic area
- VTA
Ventral tegmental area
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Borbély AA (1982) A two process model of sleep regulation. Hum Neurobiol 1, 195–204. [PubMed] [Google Scholar]
- 2.Borbély AA, Daan S, Wirz-Justice A & Deboer T (2016) The two-process model of sleep regulation: a reappraisal. J Sleep Res 25, 131–143. [DOI] [PubMed] [Google Scholar]
- 3.Daan S, Beersma DGM & Borbely AA (1984) Timing of human sleep: Recovery process gated by a circadian pacemaker. Am J Physiol - Regul Integr Comp Physiol 15. [DOI] [PubMed] [Google Scholar]
- 4.Borbély A & Achermann P (1999) Sleep Homeostasis and Models of Sleep Regulation. J Biol Rhythms 14, 559–570. [DOI] [PubMed] [Google Scholar]
- 5.Skeldon AC, Derks G & Dijk D-J (2016) Modelling changes in sleep timing and duration across the lifespan: Changes in circadian rhythmicity or sleep homeostasis? Sleep Med Rev 28, 96–107. [DOI] [PubMed] [Google Scholar]
- 6.Deboer T (2018) Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other’s functioning? Neurobiol Sleep Circadian Rhythm 5, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achermann P (2004) The two-process model of sleep regulation revisited. Aviat Space Environ Med 75, A37–43. [PubMed] [Google Scholar]
- 8.Zheng X & Sehgal A (2012) Speed control: cogs and gears that drive the circadian clock. Trends Neurosci 35, 574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JA (2016) Collective timekeeping among cells of the master circadian clock. J Endocrinol 230, R27–R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez REA, Kalume F & de la Iglesia HO (2021) Sleep timing and the circadian clock in mammals: Past, present and the road ahead. Semin Cell Dev Biol. [DOI] [PMC free article] [PubMed]
- 11.Hermann-Luibl C & Helfrich-Förster C (2015) Clock network in Drosophila. Curr Opin Insect Sci 7, 65–70. [DOI] [PubMed] [Google Scholar]
- 12.Shafer OT & Keene AC (2021) The Regulation of Drosophila Sleep. Curr Biol 31, R38–R49. [DOI] [PubMed] [Google Scholar]
- 13.Scammell TE, Arrigoni E & Lipton JO (2017) Neural Circuitry of Wakefulness and Sleep. Neuron 93, 747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donlea JM, Alam MN & Szymusiak R (2017) Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr Opin Neurobiol 44, 228–235. [DOI] [PubMed] [Google Scholar]
- 15.Weber F & Dan Y (2016) Circuit-based interrogation of sleep control. Nature 538, 51–59. [DOI] [PubMed] [Google Scholar]
- 16.Frank MG (2021) Challenging sleep homeostasis. Neurobiol sleep circadian Rhythm 10, 100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum ID, Keleş MF, Baz E-S, Han E, Park K, Luu S, Issa H, Brown M, Ho MCW, Tabuchi M, Liu S & Wu MN (2021) Astroglial Calcium Signaling Encodes Sleep Need in Drosophila. Curr Biol 31, 150–162.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingiosi AM, Hayworth CR, Harvey DO, Singletary KG, Rempe MJ, Wisor JP & Frank MG (2020) A Role for Astroglial Calcium in Mammalian Sleep and Sleep Regulation. Curr Biol 30, 4373–4383.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckwith EJ & French AS (2019) Sleep in Drosophila and Its Context. Front Physiol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eban-Rothschild A, Giardino WJ & de Lecea L (2017) To sleep or not to sleep: neuronal and ecological insights. Curr Opin Neurobiol 44, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eban-Rothschild A, Appelbaum L & de Lecea L (2018) Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology 43, 937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW & Bass J (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6, 414–21. [DOI] [PubMed] [Google Scholar]
- 23.Simoni A, Wolfgang W, Topping MP, Kavlie RG, Stanewsky R & Albert JT (2014) A mechanosensory pathway to the Drosophila circadian clock. Science 343, 525–8. [DOI] [PubMed] [Google Scholar]
- 24.O’Callaghan EK, Anderson ST, Moynagh PN & Coogan AN (2012) Long-lasting effects of sepsis on circadian rhythms in the mouse. PLoS One 7, e47087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lone SR & Sharma VK (2011) Circadian Consequence of Socio-Sexual Interactions in Fruit Flies Drosophila melanogaster. PLoS One 6, e28336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V & Hut RA (2014) Cold and hunger induce diurnality in a nocturnal mammal. Proc Natl Acad Sci U S A 111, 15256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rijo-Ferreira F, Bjorness TE, Cox KH, Sonneborn A, Greene RW & Takahashi JS (2020) Sleeping Sickness Disrupts the Sleep-Regulating Adenosine System. J Neurosci 40, 9306–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckwith EJ, Geissmann Q, French AS & Gilestro GF (2017) Regulation of sleep homeostasis by sexual arousal. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olini N, Rothfuchs I, Azzinnari D, Pryce CR, Kurth S & Huber R (2017) Chronic social stress leads to altered sleep homeostasis in mice. Behav Brain Res 327, 167–173. [DOI] [PubMed] [Google Scholar]
- 30.Mistlberger RE, Landry GJ & Marchant EG (1997) Sleep deprivation can attenuate light-induced phase shifts of circadian rhythms in hamsters. Neurosci Lett 238, 5–8. [DOI] [PubMed] [Google Scholar]
- 31.Challet E, Turek FW, Laute M & Van Reeth O (2001) Sleep deprivation decreases phase-shift responses of circadian rhythms to light in the mouse: role of serotonergic and metabolic signals. Brain Res 909, 81–91. [DOI] [PubMed] [Google Scholar]
- 32.Burgess HJ (2010) Partial sleep deprivation reduces phase advances to light in humans. J Biol Rhythms 25, 460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deboer T, Détári L & Meijer JH (2007) Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep 30, 257–62. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A, Gais S, Schabus M, Desseilles M, Dang-Vu TT, Salmon E, Balteau E, Degueldre C, Luxen A, Maquet P, Cajochen C & Peigneux P (2009) Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science 324, 516–9. [DOI] [PubMed] [Google Scholar]
- 35.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C & Turek F (2005) Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 28, 395–409. [DOI] [PubMed] [Google Scholar]
- 36.Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM & Franken P (2002) A role for cryptochromes in sleep regulation. BMC Neurosci 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danguir J & Nicolaidis S (1979) Dependence of sleep on nutrients’ availability. Physiol Behav 22, 735–740. [DOI] [PubMed] [Google Scholar]
- 38.Borbély AA (1977) Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res 124, 457–471. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs BL & McGinty DJ (1971) Effects of food deprivation on sleep and wakefulness in the rat. Exp Neurol 30, 212–222. [DOI] [PubMed] [Google Scholar]
- 40.Keene AC, Duboué ER, McDonald DM, Dus M, Suh GSB, Waddell S & Blau J (2010) Clock and cycle limit starvation-induced sleep loss in drosophila. Curr Biol 20, 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaggard J, Robinson BG, Stahl BA, Oh I, Masek P, Yoshizawa M & Keene AC (2017) The lateral line confers evolutionarily derived sleep loss in the Mexican cavefish. J Exp Biol 220, 284–293. [DOI] [PubMed] [Google Scholar]
- 42.Skora S, Mende F & Zimmer M (2018) Energy Scarcity Promotes a Brain-wide Sleep State Modulated by Insulin Signaling in C. elegans. Cell Rep 22, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacFayden UM, Oswald I & Lewis SA (1973) Starvation and human slow-wave sleep. J Appl Physiol 35, 391–394. [DOI] [PubMed] [Google Scholar]
- 44.Karacan I, Rosenbloom AL, Londono JH, Salis PJ, Thornby JI & Williams RL (1973) The Effect of Acute Fasting on Sleep and the Sleep-Growth Hormone Response. Psychosomatics 14, 33–37. [DOI] [PubMed] [Google Scholar]
- 45.Crisp AH, Stonehill E & Fenton GW (1971) The relationship between sleep, nutrition and mood: a study of patients with anorexia nervosa. Postgrad Med J 47, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells AS, Read NW, Idzikowski C & Jones J (1998) Effects of meals on objective and subjective measures of daytime sleepiness. J Appl Physiol 84, 507–515. [DOI] [PubMed] [Google Scholar]
- 47.Orr WC, Shadid G, Harnish MJ & Elsenbruch S (1997) Meal Composition and Its Effect on Postprandial Sleepiness. Physiol Behav 62, 709–712. [DOI] [PubMed] [Google Scholar]
- 48.Stahl ML, Orr WC & Bollinger C (1983) Postprandial Sleepiness: Objective Documentation via Polysomnography. Sleep 6, 29–35. [DOI] [PubMed] [Google Scholar]
- 49.Zammit GK, Kolevzon A, Fauci M, Shindledecker R & Ackerman S (1995) Postprandial sleep in healthy men. Sleep 18, 229–31. [PubMed] [Google Scholar]
- 50.Zammit GK, Ackerman SH, Shindledecker R, Fauci M & Smith GP (1992) Postprandial sleep and thermogenesis in normal men. Physiol Behav 52, 251–259. [DOI] [PubMed] [Google Scholar]
- 51.Murphy KR, Deshpande SA, Yurgel ME, Quinn JP, Weissbach JL, Keene AC, DawsonScully K, Huber R, Tomchik SM & Ja WW (2016) Postprandial sleep mechanics in Drosophila. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perron IJ, Pack BSAI & Veasey S (2015) Diet/Energy Balance Affect Sleep and Wakefulness Independent of Body Weight. Sleep 38, 1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panagiotou M, Meijer JH & Deboer T (2018) Chronic high-caloric diet modifies sleep homeostasis in mice. Eur J Neurosci 47, 1339–1352. [DOI] [PubMed] [Google Scholar]
- 54.Luppi M, Cerri M, Martelli D, Tupone D, Del Vecchio F, Di Cristoforo A, Perez E, Zamboni G & Amici R (2014) Waking and sleeping in the rat made obese through a high-fat hypercaloric diet. Behav Brain Res 258, 145–152. [DOI] [PubMed] [Google Scholar]
- 55.Sovetkina A, Nadir R, Fung JNM, Nadjarpour A & Beddoe B (2020) The Physiological Role of Ghrelin in the Regulation of Energy and Glucose Homeostasis. Cureus 12, e7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinton CM, Fitch TE & Gershenfeld HK (1999) The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res 8, 197–203. [DOI] [PubMed] [Google Scholar]
- 57.Szentirmai E, Hajdu I, Obal F & Krueger JM (2006) Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res 1088, 131–140. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein N, Levine BJ, Loy KA, Duke WL, Meyerson OS, Jamnik AA & Carter ME (2018) Hypothalamic Neurons that Regulate Feeding Can Influence Sleep/Wake States Based on Homeostatic Need. Curr Biol 28, 3736–3747.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szentirmai E & Krueger JM (2006) Central administration of neuropeptide Y induces wakefulness in rats. Am J Physiol Integr Comp Physiol 291, R473–R480. [DOI] [PubMed] [Google Scholar]
- 60.Stanley BG & Leibowitz SF (1984) Neuroreptide Y: Stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci 35, 2635–2642. [DOI] [PubMed] [Google Scholar]
- 61.Ushimura A, Tsuji T, Tanaka S, Kogo M & Yamamoto T (2015) Neuropeptide-Y modulates eating patterns and masticatory muscle activity in rats. Behav Brain Res 278, 520–526. [DOI] [PubMed] [Google Scholar]
- 62.Tóth A, Hajnik T, Záborszky L & Détári L (2007) Effect of basal forebrain neuropeptide Y administration on sleep and spontaneous behavior in freely moving rats. Brain Res Bull 72, 293–301. [DOI] [PubMed] [Google Scholar]
- 63.Akanmu MA, Ukponmwan OE, Katayama Y & Honda K (2006) Neuropeptide-Y Y2-receptor agonist, PYY3–36 promotes non-rapid eye movement sleep in rat. Neurosci Res 54, 165–170. [DOI] [PubMed] [Google Scholar]
- 64.Fuxe K, Agnati LF, Harfstrand A, Zini I, Tatemoto K, Pich EM, Hokfelt T, Mutt V & Terenius L (1983) Central administration of neuropeptide., 189–192. [DOI] [PubMed]
- 65.Lin S, Senapati B & Tsao C-H (2019) Neural basis of hunger-driven behaviour in Drosophila. Open Biol 9, 180259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung BY, Ro J, Hutter SA, Miller KM, Guduguntla LS, Kondo S & Pletcher SD (2017) Drosophila Neuropeptide F Signaling Independently Regulates Feeding and Sleep-Wake Behavior. Cell Rep 19, 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ & Yanagisawa M (1998) Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- 68.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Clay Williams S, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB & Yanagisawa M (1999) Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- 69.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, De Jong PJ, Nishino S & Mignot E (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376. [DOI] [PubMed] [Google Scholar]
- 70.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami KI, Sugiyama F, Goto K, Yanagisawa M & Sakurai T (2003) Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38, 701–713. [DOI] [PubMed] [Google Scholar]
- 71.Jaggard JB, Stahl BA, Lloyd E, Prober DA, Duboue ER & Keene AC (2018) Hypocretin underlies the evolution of sleep loss in the Mexican cavefish. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hua R, Wang X, Chen X, Wang X, Huang P, Li P, Mei W & Li H (2018) Calretinin Neurons in the Midline Thalamus Modulate Starvation-Induced Arousal. Curr Biol 28, 3948–3959.e4. [DOI] [PubMed] [Google Scholar]
- 73.He Q, Du J, Wei L & Zhao Z (2020) AKH-FOXO pathway regulates starvation-induced sleep loss through remodeling of the small ventral lateral neuron dorsal projections. PLOS Genet 16, e1009181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Y, Huang R, Ye J, Zhang V, Wu C, Cheng G, Jia J & Wang L (2016) Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila. Elife 5, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown EB, Shah KD, Faville R, Kottler B & Keene AC (2020) Drosophila insulin-like peptide 2 mediates dietary regulation of sleep intensity. PLoS Genet 16, e1008270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murakami K, Yurgel ME, Stahl BA, Masek P, Mehta A, Heidker R, Bollinger W, Gingras RM, Kim YJ, Ja WW, Suter B, Diangelo JR & Keene AC (2016) Translin Is Required for Metabolic Regulation of Sleep. Curr Biol 26, 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yurgel ME, Kakad P, Zandawala M, Nässel DR, Godenschwege TA & Keene AC (2019) A single pair of leucokinin neurons are modulated by feeding state and regulate sleep–metabolism interactions. PLOS Biol 17, e2006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sonn JY, Lee J, Sung MK, Ri H, Choi JK, Lim C & Choe J (2018) Serine metabolism in the brain regulates starvation-induced sleep suppression in Drosophila melanogaster. Proc Natl Acad Sci U S A 115, 7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Godos J, Grosso G, Castellano S, Galvano F, Caraci F & Ferri R (2021) Association between diet and sleep quality: A systematic review. Sleep Med Rev 57, 101430. [DOI] [PubMed] [Google Scholar]
- 80.St-Onge MP, Mikic A & Pietrolungo CE (2016) Effects of diet on sleep quality. Adv Nutr 7, 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catterson JH, Knowles-Barley S, James K, Heck MMS, Harmar AJ & Hartley PS (2010) Dietary modulation of Drosophila sleep-wake behaviour. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Linford NJ, Chan TP & Pletcher SD (2012) Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pamboro ELS, Brown EB & Keene AC (2020) Dietary fatty acids promote sleep through a taste‐independent mechanism. Genes, Brain Behav 19. [DOI] [PubMed] [Google Scholar]
- 84.Richter CP (1922) A behavioristic study of the activity of the rat. Comp Psychol Monogr.
- 85.Pendergast JS & Yamazaki S (2018) The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J Biol Rhythms 33, 458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mistlberger RE (2020) Food as circadian time cue for appetitive behavior. F1000Research 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stephan FK, Swann JM & Sisk CL (1979) Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol 25, 545–554. [DOI] [PubMed] [Google Scholar]
- 88.Stephan FK, Swann JM & Sisk CL (1979) Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol 25, 346–363. [DOI] [PubMed] [Google Scholar]
- 89.Boulos Z, Rosenwasser AM & Terman M (1980) Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res 1, 39–65. [DOI] [PubMed] [Google Scholar]
- 90.Lesku JA, Rattenborg NC, Valcu M, Vyssotski AL, Kuhn S, Kuemmeth F, Heidrich W & Kempenaers B (2012) Adaptive sleep loss in polygynous pectoral sandpipers. Science 337, 1654–8. [DOI] [PubMed] [Google Scholar]
- 91.Machado DR, Afonso DJ, Kenny AR, Öztürk-Çolak A, Moscato EH, Mainwaring B, Kayser M & Koh K (2017) Identification of octopaminergic neurons that modulate sleep suppression by male sex drive. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR & De Lecea L (2016) VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duhart JM, Baccini V, Zhang Y, Machado DR & Koh K (2020) Modulation of sleepcourtship balance by nutritional status in drosophila. Elife 9, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang W, Guo C, Chen D, Peng Q & Pan Y (2018) Hierarchical Control of Drosophila Sleep, Courtship, and Feeding Behaviors by Male-Specific P1 Neurons. Neurosci Bull 34, 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu S, Guo C, Zhao H, Sun M, Chen J, Han C, Peng Q, Qiao H, Peng P, Liu Y, Luo SD & Pan Y (2019) Drosulfakinin signaling in fruitless circuitry antagonizes P1 neurons to regulate sexual arousal in Drosophila. Nat Commun 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen D, Sitaraman D, Chen N, Jin X, Han C, Chen J, Sun M, Baker BS, Nitabach MN & Pan Y (2017) Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat Commun 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang SX, Rogulja D & Crickmore MA (2016) Dopaminergic Circuitry Underlying Mating Drive. Neuron 91, 168–181. [DOI] [PubMed] [Google Scholar]
- 98.Zhang SX, Rogulja D & Crickmore MA (2019) Recurrent Circuitry Sustains Drosophila Courtship Drive While Priming Itself for Satiety. Curr Biol 29, 3216–3228.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mindell JA, Cook RA & Nikolovski J (2015) Sleep patterns and sleep disturbances across pregnancy. Sleep Med 16, 483–488. [DOI] [PubMed] [Google Scholar]
- 100.Sedov ID, Cameron EE, Madigan S & Tomfohr-Madsen LM (2018) Sleep quality during pregnancy: A meta-analysis. Sleep Med Rev 38, 168–176. [DOI] [PubMed] [Google Scholar]
- 101.Sedov ID, Anderson NJ, Dhillon AK & Tomfohr-Madsen LM (2020) Insomnia symptoms during pregnancy: A meta-analysis. J Sleep Res, 1–10. [DOI] [PubMed]
- 102.Chen SJ, Shi L, Bao YP, Sun YK, Lin X, Que JY, Vitiello MV., Zhou YX, Wang YQ & Lu L (2018) Prevalence of restless legs syndrome during pregnancy: A systematic review and meta-analysis. Sleep Med Rev 40, 43–54. [DOI] [PubMed] [Google Scholar]
- 103.Tóth A, Pethő M, Keserű D, Simon D, Hajnik T, Détári L & Dobolyi Á (2020) Complete sleep and local field potential analysis regarding estrus cycle, pregnancy, postpartum and post-weaning periods and homeostatic sleep regulation in female rats. Sci Rep 10, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kimura M, Zhang SQ & Inoué S (1996) Pregnancy-associated sleep changes in the rat. Am J Physiol - Regul Integr Comp Physiol 271. [DOI] [PubMed] [Google Scholar]
- 105.Sivadas N, Radhakrishnan A, Aswathy BS, Kumar VM & Gulia KK (2017) Dynamic changes in sleep pattern during post-partum in normal pregnancy in rat model. Behav Brain Res 320, 264–274. [DOI] [PubMed] [Google Scholar]
- 106.Lord C, Sekerovic Z & Carrier J (2014) Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol Biol 62, 302–310. [DOI] [PubMed] [Google Scholar]
- 107.Santiago JR, Nolledo MS, Kinzler W & Santiago TV. (2001) Sleep and Sleep Disorders in Pregnancy. Ann Intern Med 134, 396. [DOI] [PubMed] [Google Scholar]
- 108.Parry B, Orff H, Meliska C & Martinez F (2014) The influence of sex and gonadal hormones on sleep disorders. ChronoPhysiology Ther, 15. [Google Scholar]
- 109.Kimura M, Zhang SQ & Inoué S (1998) An animal model for pregnancy-associated sleep disorder. Psychiatry Clin Neurosci 52, 209–211. [DOI] [PubMed] [Google Scholar]
- 110.Isaac RE, Li C, Leedale AE & Shirras AD (2010) Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc R Soc B Biol Sci 277, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garbe DS, Vigderman AS, Moscato E, Dove AE, Vecsey CG, Kayser MS & Sehgal A (2016) Changes in Female Drosophila Sleep following Mating Are Mediated by SPSN-SAG Neurons. J Biol Rhythms 31, 551–567. [DOI] [PubMed] [Google Scholar]
- 112.Ferguson CTJ, O’Neill TL, Audsley N & Isaac RE (2015) The sexually dimorphic behaviour of adult Drosophila suzukii: Elevated female locomotor activity and loss of siesta is a post-mating response. J Exp Biol 218, 3855–3861. [DOI] [PubMed] [Google Scholar]
- 113.Geissmann Q, Beckwith EJ & Gilestro GF (2019) Most sleep does not serve a vital function: Evidence from Drosophila melanogaster. Sci Adv 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garbe DS, Bollinger WL, Vigderman A, Masek P, Gertowski J, Sehgal A & Keene AC (2015) Context-specific comparison of sleep acquisition systems in Drosophila. Biol Open 4, 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng K, Palfreyman MT, Häsemeyer M, Talsma A & Dickson BJ (2014) Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron 83, 135–148. [DOI] [PubMed] [Google Scholar]
- 116.Nishihara K & Horiuchi S (1998) Changes in sleep patterns of young women from late pregnancy to postpartum: Relationships to their infants’ movements. Percept Mot Skills 87, 1043–1056. [DOI] [PubMed] [Google Scholar]
- 117.Shinkoda H, Matsumoto K & Park YM (1999) Changes in sleep-wake cycle during the period from late pregnancy to puerperium identified through the wrist actigraph and sleep logs. Psychiatry Clin Neurosci 53, 133–135. [DOI] [PubMed] [Google Scholar]
- 118.Teti DM, Shimizu M, Crosby B & Kim B-R (2016) Sleep arrangements, parent-infant sleep during the first year, and family functioning. Dev Psychol 52, 1169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Volkovich E, Ben-Zion H, Karny D, Meiri G & Tikotzky L (2015) Sleep patterns of co-sleeping and solitary sleeping infants and mothers: A longitudinal study. Sleep Med 16, 1305–1312. [DOI] [PubMed] [Google Scholar]
- 120.Volkovich E, Bar-Kalifa E, Meiri G & Tikotzky L (2018) Mother-infant sleep patterns and parental functioning of room-sharing and solitary-sleeping families: A longitudinal study from 3 to 18 months. Sleep 41, 1–14. [DOI] [PubMed] [Google Scholar]
- 121.Lyamin O, Pryaslova J, Lance V & Siegel J (2005) Continuous activity in cetaceans after birth. Nature 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lyamin O, Pryaslova J, Kosenko P & Siegel J (2007) Behavioral aspects of sleep in bottlenose dolphin mothers and their calves. Physiol Behav 92, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meza E, Waliszewski SM & Caba M (2011) Circadian nursing induces PER1 protein in neuroendocrine tyrosine hydroxylase neurones in the rabbit doe. J Neuroendocrinol 23, 472–480. [DOI] [PubMed] [Google Scholar]
- 124.Meza E, Juárez C, Morgado E, Zavaleta Y & Caba M (2008) Brief daily suckling shifts locomotor behavior and induces PER1 protein in paraventricular and supraoptic nuclei, but not in the suprachiasmatic nucleus, of rabbit does. Eur J Neurosci 28, 1394–1403. [DOI] [PubMed] [Google Scholar]
- 125.Caba M, González-Mariscal G & Meza E (2018) Circadian rhythms and clock genes in reproduction: insights from behavior and the female rabbit’s brain. Front Endocrinol (Lausanne) 9, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagari M, Gera A, Jonsson S & Bloch G (2019) Bumble Bee Workers Give Up Sleep to Care for Offspring that Are Not Their Own. Curr Biol 29, 3488–3493.e4. [DOI] [PubMed] [Google Scholar]
- 127.Fenn MGP & Macdonald DW (1995) Use of Middens by Red Foxes: Risk Reverses Rhythms of Rats. J Mammal 76, 130–136. [Google Scholar]
- 128.Swarts HM, Crooks KR, Willits N & Woodroffe R (2009) Possible contemporary evolution in an endangered species, the Santa Cruz Island fox. Anim Conserv 12, 120–127. [Google Scholar]
- 129.Lesku JA, Bark RJ, Martinez-Gonzalez D, Rattenborg NC, Amlaner CJ & Lima SL (2008) Predator-induced plasticity in sleep architecture in wild-caught Norway rats (Rattus norvegicus). Behav Brain Res 189, 298–305. [DOI] [PubMed] [Google Scholar]
- 130.Rattenborg NC, Lima SL & Amlaner CJ (1999) Half-awake to the risk of predation. Nature 397, 397–398. [DOI] [PubMed] [Google Scholar]
- 131.Rattenborg NC, Lima SL & Amlaner CJ (1999) Facultative control of avian unihemispheric sleep under the risk of predation. Behav Brain Res 105, 163–172. [DOI] [PubMed] [Google Scholar]
- 132.Gibson WT, Gonzalez CR, Fernandez C, Ramasamy L, Tabachnik T, Du RR, Felsen PD, Maire MR, Perona P & Anderson DJ (2015) Behavioral Responses to a Repetitive Visual Threat Stimulus Express a Persistent State of Defensive Arousal in Drosophila. Curr Biol 25, 1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fuchs T, Haney A, Jechura TJ, Moore FR & Bingman VP (2006) Daytime naps in night-migrating birds: behavioural adaptation to seasonal sleep deprivation in the Swainson’s thrush, Catharus ustulatus. Anim Behav 72, 951–958. [Google Scholar]
- 134.Rattenborg NC, Mandt BH, Obermeyer WH, Winsauer PJ, Huber R, Wikelski M & Benca RM (2004) Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol 2, E212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rattenborg NC, Voirin B, Cruz SM, Tisdale R, Dell’Omo G, Lipp H-P, Wikelski M & Vyssotski AL (2016) Evidence that birds sleep in mid-flight. Nat Commun 7, 12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumar V, Wingfield JC, Dawson A, Ramenofsky M, Rani S & Bartell P (2010) Biological clocks and regulation of seasonal reproduction and migration in birds. Physiol Biochem Zool 83, 827–835. [DOI] [PubMed] [Google Scholar]
- 137.Bartell PA & Gwinner E (2005) A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J Biol Rhythms 20, 538–49. [DOI] [PubMed] [Google Scholar]
- 138.Denlinger DL, Hahn DA, Merlin C, Holzapfel CM & Bradshaw WE (2017) Keeping time without a spine: What can the insect clock teach us about seasonal adaptation? Philos Trans R Soc B Biol Sci 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stevenson TJ & Kumar V (2017) Neural control of daily and seasonal timing of songbird migration. J Comp Physiol A Neuroethol Sensory, Neural, Behav Physiol 203, 399–409. [DOI] [PubMed] [Google Scholar]
- 140.Webb IC, Antle MC & Mistlberger RE (2014) Regulation of circadian rhythms in mammals by behavioral arousal. Behav Neurosci 128, 304–25. [DOI] [PubMed] [Google Scholar]
- 141.Welsh DK, Richardson GS & Dement WC (1988) Effect of running wheel availability on circadian patterns of sleep and wakefulness in mice. Physiol Behav 43, 771–777. [DOI] [PubMed] [Google Scholar]
- 142.Edgar DM, Kilduff TS, Martin CE & Dement WC (1991) Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav 50, 373–378. [DOI] [PubMed] [Google Scholar]
- 143.Lancel M, Droste SK, Sommer S & Reul JMHM(2003) Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. Eur J Neurosci 17, 2171–2179. [DOI] [PubMed] [Google Scholar]
- 144.Kelley GA & Kelley KS (2017) Exercise and sleep: a systematic review of previous metaanalyses. J Evid Based Med 10, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kelly ML, Collin SP, Hemmi JM & Lesku JA (2019) Evidence for Sleep in Sharks and Rays: Behavioural, Physiological, and Evolutionary Considerations. Brain Behav Evol 94, 37–50. [DOI] [PubMed] [Google Scholar]
- 146.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM & Siegel JM (2008) Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev 32, 1451–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wams EJ, Woelders T, Marring I, van Rosmalen L, Beersma DGM, Gordijn MCM & Hut RA (2017) Linking Light Exposure and Subsequent Sleep: A Field Polysomnography Study in Humans. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harding EC, Franks NP & Wisden W (2019) The temperature dependence of sleep. Front Neurosci 13, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.French AS, Geissmann Q, Beckwith EJ & Gilestro GF (2021) Sensory processing during sleep in Drosophila melanogaster. Nature 598, 479–482. [DOI] [PubMed] [Google Scholar]
- 150.Golombek DA & Rosenstein RE (2010) Physiology of Circadian Entrainment. Physiol Rev, 1063–1102. [DOI] [PubMed]
- 151.Hubbard J, Ruppert E, Gropp CM & Bourgin P (2013) Non-circadian direct effects of light on sleep and alertness: Lessons from transgenic mouse models. Sleep Med Rev 17, 445–452. [DOI] [PubMed] [Google Scholar]
- 152.Altimus CM, Guler AD, Villa KL, McNeill DS, LeGates TA & Hattar S (2008) Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci 105, 19998–20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lupi D, Oster H, Thompson S & Foster RG (2008) The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci 11, 1068–73. [DOI] [PubMed] [Google Scholar]
- 154.Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P & Bourgin P (2009) Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biol 7, e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau K-W & Berson DM (2006) Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 497, 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Z, Beier C, Weil T & Hattar S (2021) The retinal ipRGC-preoptic circuit mediates the acute effect of light on sleep. Nat Commun 12, 5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shi HY, Xu W, Guo H, Dong H, Qu WM & Huang ZL (2020) Lesion of intergeniculate leaflet GABAergic neurons attenuates sleep in mice exposed to light. Sleep 43, 1–12. [DOI] [PubMed] [Google Scholar]
- 158.Gunilla bohlin (1971) MONOTONOUS STIMULATION REACTION SLEEP ONSET AND HABITUATION. Electroencephalogr Clin Neurophysiol 31, 593–601. [DOI] [PubMed] [Google Scholar]
- 159.Kompotis K, Hubbard J, Emmenegger Y, Perrault A, Mühlethaler M, Schwartz S, Bayer L & Franken P (2019) Rocking Promotes Sleep in Mice through Rhythmic Stimulation of the Vestibular System. Curr Biol 29, 392–401. [DOI] [PubMed] [Google Scholar]
- 160.Korner AF, Guilleminault C, Van Den Hoed J & Baldwin RB (1978) Reduction of sleep apnea and bradycardia in preterm infants on oscillating water beds: a controlled polygraphic study. Pediatrics 61, 528–533. [PubMed] [Google Scholar]
- 161.Perrault AA, Khani A, Quairiaux C, Kompotis K, Franken P, Muhlethaler M, Schwartz S & Bayer L (2019) Whole-Night Continuous Rocking Entrains Spontaneous Neural Oscillations with Benefits for Sleep and Memory. Curr Biol 29, 402–411.e3. [DOI] [PubMed] [Google Scholar]
- 162.Bayer L, Constantinescu I, Perrig S, Vienne J, Vidal PP, Mühlethaler M & Schwartz S (2011) Rocking synchronizes brain waves during a short nap. Curr Biol 21, R461–R462. [DOI] [PubMed] [Google Scholar]
- 163.Greene RW & Frank MG (2010) Slow Wave Activity During Sleep: Functional and Therapeutic Implications. Neurosci 16, 618–633. [DOI] [PubMed] [Google Scholar]
- 164.Fernandez LMJ & Lüthi A (2020) Sleep spindles: Mechanisms and functions. Physiol Rev 100, 805–868. [DOI] [PubMed] [Google Scholar]
- 165.Wimmer RD, Astori S, Bond CT, Rovó Z, Chatton JY, Adelman JP, Franken P & Lüthi A (2012) Sustaining sleep spindles through enhanced SK2-channel activity consolidates sleep and elevates arousal threshold. J Neurosci 32, 13917–13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schabus M, Dang-Vu TT, Heib DPJ, Boly M, Desseilles M, Vandewalle G, Schmidt C, Albouy G, Darsaud A, Gais S, Degueldre C, Balteau E, Phillips C, Luxen A & Maquet P (2012) The fate of incoming stimuli during NREM sleep is determined by spindles and the phase of the slow oscillation. Front Neurol APR, 1–11. [DOI] [PMC free article] [PubMed]
- 167.Öztürk-Çolak A, Inami S, Buchler JR, McClanahan PD, Cruz A, Fang-Yen C & Koh K (2020) Sleep Induction by Mechanosensory Stimulation in Drosophila. Cell Rep 33, 108462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tononi G, Riedner BA, Hulse BK, Ferrarelli F & Sarasso S (2010) Enhancing sleep slow waves with natural stimuli. Medicamundi 54, 82–88. [Google Scholar]
- 169.Gluck H R V (1959) Defensive conditioning of electrographic arousal with delayed and differentiated auditory stimuli. Electroencephalogr Clin Neurophysiol 11, 485–96. [DOI] [PubMed] [Google Scholar]
- 170.Ngo HV V, Miedema A, Faude I, Martinetz T, Mölle M & Born J (2015) Driving sleep slow oscillations by auditory closed-loop stimulation—A self-limiting process. J Neurosci 35, 6630–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Spencer JAD, Moran DJ, Lee A & Talbert D (1990) White noise and sleep induction. Arch Dis Child 65, 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perl O, Arzi A, Sela L, Secundo L, Holtzman Y, Samnon P, Oksenberg A, Sobel N & Hairston IS (2016) Odors enhance slow-wave activity in non-rapid eye movement sleep. J Neurophysiol 115, 2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hsu CT, Choi JTY & Sehgal A (2021) Manipulations of the olfactory circuit highlight the role of sensory stimulation in regulating sleep amount. Sleep 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Melnattur K, Zhang B & Shaw PJ (2020) Disrupting flight increases sleep and identifies a novel sleep-promoting pathway in Drosophila. Sci Adv 6, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bellesi M, Riedner BA, Garcia-Molina GN, Cirelli C & Tononi G (2014) Enhancement of sleep slow waves: Underlying mechanisms and practical consequences. Front Syst Neurosci 8, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fehér KD, Wunderlin M, Maier JG, Hertenstein E, Schneider CL, Mikutta C, Züst MA, Klöppel S & Nissen C (2021) Shaping the slow waves of sleep: A systematic and integrative review of sleep slow wave modulation in humans using non-invasive brain stimulation. Sleep Med Rev 58, 101438. [DOI] [PubMed] [Google Scholar]
- 177.Marshall L, Helgadóttir H, Mölle M & Born J (2006) Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613. [DOI] [PubMed] [Google Scholar]
- 178.Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ & Tononi G (2007) Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U S A 104, 8496–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pavlov IP (1927) Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford Univ. Press, Oxford, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sokolov E (1963) Perception and the conditioned reflex (Oxford: Pergamon press; ). . [Google Scholar]
- 181.Tononi G & Cirelli C (2014) Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 81, 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vorster AP & Born J (2015) Sleep and memory in mammals, birds and invertebrates. Neurosci Biobehav Rev 50, 103–119. [DOI] [PubMed] [Google Scholar]
- 183.Huber R, Ghilardi MF, Massimini M & Tononi G (2004) Local sleep and learning. Nature 430, 78–81. [DOI] [PubMed] [Google Scholar]
- 184.Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A & Eidelberg D (2000) Patterns of regional brain activation associated with different forms of motor learning. Brain Res 871, 127–145. [DOI] [PubMed] [Google Scholar]
- 185.Mascetti L, Muto V, Matarazzo L, Foret A, Ziegler E, Albouy G, Sterpenich V, Schmidt C, Degueldre C, Leclercq Y, Phillips C, Luxen A, Vandewalle G, Vogels R, Maquet P & Balteau E (2013) The impact of visual perceptual learning on sleep and local slow-wave initiation. J Neurosci 33, 3323–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hanlon EC, Faraguna U, Vyazovskiy VV., Tononi G & Cirelli C (2009) Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep 32, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Faraguna U, Vyazovskiy VV., Nelson AB, Tononi G & Cirelli C (2008) A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci 28, 4088–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ & Tononi G (2006) Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci 9, 1169–1176. [DOI] [PubMed] [Google Scholar]
- 189.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ & Tononi G (2007) TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One 2, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Siegel RW & Hall JC (1979) Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A 76, 3430–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sakai T, Tamura T, Kitamoto T & Kidokoro Y (2004) A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc Natl Acad Sci U S A 101, 16058–16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Inami S, Sato S, Kondo S, Tanimoto H, Kitamoto T & Sakai T (2020) Environmental light is required for maintenance of long-term memory in drosophila. J Neurosci 40, 1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ganguly-Fitzgerald I, Donlea J & Shaw PJ (2006) Waking experience affects sleep need in Drosophila. Science (80- ) 313, 1775–1781. [DOI] [PubMed] [Google Scholar]
- 194.Brown MK, Strus E & Naidoo N (2017) Reduced sleep during social isolation leads to cellular stress and induction of the unfolded protein response. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu C, Haynes PR, Donelson NC, Aharon S & Griffith LC (2015) Sleep in Populations of Drosophila Melanogaster. eNeuro 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zimmerman JE, Chan MT, Jackson N, Maislin G & Pack AI (2012) Genetic background has a major impact on differences in sleep resulting from environmental influences in Drosophila. Sleep 35, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Donlea JM, Ramanan N & Shaw PJ (2009) Use-dependent plasticity in clock neurons regulates sleep need in drosophila. Science (80- ) 324, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vanderheyden WM, Gerstner JR, Tanenhaus A, Yin JC & Shaw PJ (2013) ERK phosphorylation regulates sleep and plasticity in Drosophila. PLoS One 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mistlberger RE & Skene DJ (2004) Social influences on mammalian circadian rhythms: Animal and human studies. Biol Rev Camb Philos Soc 79, 533–556. [DOI] [PubMed] [Google Scholar]
- 200.Gritton HJ, Stasiak AM, Sarter M & Lee TM (2013) Cognitive Performance as a Zeitgeber: Cognitive Oscillators and Cholinergic Modulation of the SCN Entrain Circadian Rhythms. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pellman BA, Kim E, Reilly M, Kashima J, Motch O, de la Iglesia HO & Kim JJ (2015) Time-Specific Fear Acts as a Non-Photic Entraining Stimulus of Circadian Rhythms in Rats. Sci Rep 5, 14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chouhan NS, Griffith LC, Haynes P & Sehgal A (2020) Availability of food determines the need for sleep in memory consolidation. Nature, 1–4. [DOI] [PMC free article] [PubMed]
- 203.Kelley KW & Kent S (2020) The Legacy of Sickness Behaviors. Front Psychiatry 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dantzer R, O’Connor JC, Freund GG, Johnson RW & Kelley KW (2008) From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Obradovich N, Migliorini R, Mednick SC & Fowler JH (2017) Nighttime temperature and human sleep loss in a changing climate. Sci Adv 3, e1601555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sandsmark DK, Elliott JE & Lim MM (2017) Sleep-Wake Disturbances After Traumatic Brain Injury: Synthesis of Human and Animal Studies. Sleep 40, zsx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sylvia KE & Demas GE (2017) A return to wisdom: Using sickness behaviors to integrate ecological and translational research. Integr Comp Biol 57, 1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Davis KC & Raizen DM (2017) A mechanism for sickness sleep: lessons from invertebrates. J Physiol 595, 5415–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Imeri L & Opp MR (2009) How (and why) the immune system makes us sleep. Nat Rev Neurosci 10, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Irwin MR (2019) Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol 19, 702–715. [DOI] [PubMed] [Google Scholar]
- 211.Kuo TH, Pike DH, Beizaeipour Z & Williams JA (2010) Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFκB Relish. BMC Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Williams JA, Sathyanarayanan S, Hendricks JC & Sehgal A (2007) Interaction between sleep and the immune response in Drosophila: A role for the NFκB relish. Sleep 30, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Toda H, Williams JA, Gulledge M & Sehgal A (2019) A sleep-inducing gene, nemuri, links sleep and immune function in Drosophila. Science (80- ) 363, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vanderheyden WM, Goodman AG, Taylor RH, Frank MG, Van Dongen HPA & Gerstner JR (2018) Astrocyte expression of the Drosophila TNF-alpha homologue, Eiger, regulates sleep in flies. PLoS Genet 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vale PF & Jardine MD (2015) Sex-specific behavioural symptoms of viral gut infection and Wolbachia in Drosophila melanogaster. J Insect Physiol 82, 28–32. [DOI] [PubMed] [Google Scholar]
- 216.Shirasu-Hiza MM, Dionne MS, Pham LN, Ayres JS & Schneider DS (2007) Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr Biol 17, 353–355. [DOI] [PubMed] [Google Scholar]
- 217.Mallon EB, Alghamdi A, Holdbrook RTK & Rosato E (2014) Immune stimulation reduces sleep and memory ability in drosophila melanogaster. PeerJ 2014, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stephenson J, Nutma E, van der Valk P & Amor S (2018) Inflammation in CNS neurodegenerative diseases. Immunology 154, 204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ahmed RM, Ke YD, Vucic S, Ittner LM, Seeley W, Hodges JR, Piguet O, Halliday G & Kiernan MC (2018) Physiological changes in neurodegeneration-mechanistic insights and clinical utility. Nat Rev Neurol 14, 259–271. [DOI] [PubMed] [Google Scholar]
- 220.Baumann CR (2012) Traumatic brain injury and disturbed sleep and wakefulness. NeuroMolecular Med 14, 205–212. [DOI] [PubMed] [Google Scholar]
- 221.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE & Kuna ST (2007) Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med 3, 349–356. [PMC free article] [PubMed] [Google Scholar]
- 222.Rowe RK, Striz M, Bachstetter AD, Van Eldik LJ, Donohue KD, O’Hara BF & Lifshitz J (2014) Diffuse brain injury induces acute post-traumatic sleep. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Barekat A, Gonzalez A, Mauntz RE, Kotzebue RW, Molina B, El-Mecharrafie N, Conner CJ, Garza S, Melkani GC, Joiner WJ, Lipinski MM, Finley KD & Ratliff EP (2016) Using Drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci Rep 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Singh P & Donlea JM (2020) Bidirectional Regulation of Sleep and Synapse Pruning after Neural Injury. Curr Biol 30, 1063–1076.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hill AJ, Mansfield R, Lopez JMNG, Raizen DM & Van Buskirk C (2014) Cellular stress induces a protective sleep-like state in C. elegans. Curr Biol 24, 2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.DeBardeleben HK, Lopes LE, Nessel MP & Raizen DM (2017) Stress-Induced Sleep After Exposure to Ultraviolet Light Is Promoted by p53 in Caenorhabditis elegans. Genetics 207, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Goetting DL, Mansfield R, Soto R & Van Buskirk C (2020) Cellular damage, including wounding, drives C. elegans stress-induced sleep. J Neurogenet 34, 430–439. [DOI] [PubMed] [Google Scholar]
- 228.Nelson MD, Lee KH, Churgin MA, Hill AJ, Van Buskirk C, Fang-Yen C & Raizen DM (2014) FMRFamide-like FLP-13 Neuropeptides Promote Quiescence following Heat Stress in Caenorhabditis elegans. Curr Biol 24, 2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nath RD, Chow ES, Wang H, Schwarz EM & Sternberg PW (2016) C. elegans Stress-Induced Sleep Emerges from the Collective Action of Multiple Neuropeptides. Curr Biol 26, 2446–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Iannacone MJ, Beets I, Lopes LE, Churgin MA, Fang-Yen C, Nelson MD, Schoofs L & Raizen DM (2017) The RFamide receptor DMSR-1 regulates stress-induced sleep in C. elegans. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lenz O, Xiong J, Nelson MD, Raizen DM & Williams JA (2015) FMRFamide signaling promotes stress-induced sleep in Drosophila. Brain Behav Immun 47, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Naidoo N (2009) Cellular stress/the unfolded protein response: Relevance to sleep and sleep disorders. Sleep Med Rev 13, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Williams JA & Naidoo N (2020) Sleep and cellular stress. Curr Opin Physiol 15, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Naidoo N, Casiano V, Cater J, Zimmerman J & Pack AI (2007) A Role for the Molecular Chaperone Protein BiP/GRP78 in Drosophila Sleep Homeostasis. Sleep 30, 557–565. [DOI] [PubMed] [Google Scholar]
- 235.Brown MK, Chan MT, Zimmerman JE, Pack AI, Jackson NE & Naidoo N (2014) Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol Aging 35, 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Methippara MM, Bashir T, Kumar S, Alam N, Szymusiak R & McGinty D (2009) Salubrinal, an inhibitor of protein synthesis, promotes deep slow wave sleep. Am J Physiol Integr Comp Physiol 296, R178–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Methippara M, Mitrani B, Schrader FX, Szymusiak R & McGinty D (2012) Salubrinal, an endoplasmic reticulum stress blocker, modulates sleep homeostasis and activation of sleep- and wake-regulatory neurons. Neuroscience 209, 108–118. [DOI] [PubMed] [Google Scholar]
- 238.Ly S, Lee DA, Strus E, Prober DA & Naidoo N (2020) Evolutionarily Conserved Regulation of Sleep by the Protein Translational Regulator PERK. Curr Biol 30, 1639–1648.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Reimund E (1994) The free radical flux theory of sleep. Med Hypotheses 43, 231–233. [DOI] [PubMed] [Google Scholar]
- 240.Almeida DM & Kessler RC (1998) Everyday stressors and gender differences in daily distress. J Pers Soc Psychol 75, 670–680. [DOI] [PubMed] [Google Scholar]
- 241.Kanazawa LKS, Vecchia DD, Wendler EM, Hocayen P de AS, dos Reis Lívero FA, Stipp MC, Barcaro IMR, Acco A & Andreatini R (2016) Quercetin reduces manic-like behavior and brain oxidative stress induced by paradoxical sleep deprivation in mice. Free Radic Biol Med 99, 79–86. [DOI] [PubMed] [Google Scholar]
- 242.Ramanathan L, Gulyani S, Nienhuis R & Siegel JM (2002) Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport 13, 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Süer C, Dolu N, Artis AS, Sahin L, Yilmaz A & Cetin A (2011) The effects of long-term sleep deprivation on the long-term potentiation in the dentate gyrus and brain oxidation status in rats. Neurosci Res 70, 71–77. [DOI] [PubMed] [Google Scholar]
- 244.Silva RH, Abílio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, Medrano WA, Calzavara MB, Registro S, Andersen ML, Machado RB, Carvalho RC, Ribeiro R d. A, Tufik S & Frussa-Filho R (2004) Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 46, 895–903. [DOI] [PubMed] [Google Scholar]
- 245.Villafuerte G, Miguel-Puga A, Murillo Rodríguez E, Machado S, Manjarrez E & AriasCarrión O (2015) Sleep deprivation and oxidative stress in animal models: A systematic review. Oxid Med Cell Longev 2015. [DOI] [PMC free article] [PubMed]
- 246.Hill VM, O’Connor RM, Sissoko GB, Irobunda IS, Leong S, Canman JC, Stavropoulos N & Shirasu-Hiza M (2018) A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol 16, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kempf A, Song SM, Talbot CB & Miesenböck G (2019) A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature 568, 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vaccaro A, Kaplan Dor Y, Nambara K, Pollina EA, Lin C, Greenberg ME & Rogulja D (2020) Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell, 1–22. [DOI] [PubMed]
- 249.Ferretti A, Rattenborg NC, Ruf T, McWilliams SR, Cardinale M & Fusani L (2019) Sleeping Unsafely Tucked in to Conserve Energy in a Nocturnal Migratory Songbird. Curr Biol 29, 2766–2772.e4. [DOI] [PubMed] [Google Scholar]
- 250.Goetting DL, Soto R & Van Buskirk C (2018) Food-Dependent Plasticity in Caenorhabditis elegans Stress-Induced Sleep Is Mediated by TOR-FOXA and TGF-β Signaling. Genetics 209, 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Crain TL, Brossoit RM & Fisher GG (2018) Work, Nonwork, and Sleep (WNS): a Review and Conceptual Framework. J Bus Psychol 33, 675–697. [Google Scholar]
- 252.Konjarski M, Murray G, Lee VV & Jackson ML (2018) Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies. Sleep Med Rev 42, 47–58. [DOI] [PubMed] [Google Scholar]
- 253.Pillai JA & Leverenz JB (2017) Sleep and Neurodegeneration. Chest 151, 1375–1386. [DOI] [PubMed] [Google Scholar]
- 254.Gulia KK & Kumar VM (2018) Sleep disorders in the elderly: a growing challenge. Psychogeriatrics 18, 155–165. [DOI] [PubMed] [Google Scholar]
- 255.Saper CB & Fuller PM (2017) Wake–sleep circuitry: an overview. Curr Opin Neurobiol 44, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Trojanowski NF & Raizen DM (2016) Call it Worm Sleep. Trends Neurosci 39, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Artiushin G & Sehgal A (2020) The Glial Perspective on Sleep and Circadian Rhythms. Annu Rev Neurosci 43, 119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Anderson K (2011) Sleep disturbance and neurological disease. Clin Med (Northfield Il) 11, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]