Abstract
Background:
Abnormal reward processing, typically anhedonia, is a hallmark of human depression and is accompanied by altered functional connectivity in reward circuits. Negative allosteric modulators of GABAA receptors (GABA-NAMs) have rapid antidepressant-like properties in rodents and exert few adverse effects, but molecular targets underlying their behavioral and synaptic effects remain undetermined. We hypothesized that GABA-NAMs act at the benzodiazepine site of GABAARs containing α5 subunits to increase gamma oscillatory activity, strengthen synapses in reward circuits, and reverse anhedonia.
Methods:
Anhedonia was induced by chronic stress in male mice and assayed by preferences for sucrose and female urine (n=5-7 mice/group). Hippocampal slices were then prepared for electrophysiological recording (n=4-6/mouse). EEG power was quantified in response to GABA-NAM and ketamine administration (n=7-9 mice/group).
Results:
Chronic stress reduced sucrose and female urine preferences, and hippocampal temporoammonic-CA1 synaptic strength. A peripheral injection of the GABA-NAM MRK-016 restored hedonic behavior and AMPA:NMDA ratios in wildtype mice. These actions were prevented by pretreatment with the benzodiazepine site antagonist flumazenil. MRK-016 administration increased gamma power over the prefrontal cortex in wildtype mice, but not α5 KO mice, whereas ketamine promoted gamma power in both genotypes. Hedonic behavior and AMPA:NMDA ratios were only restored by MRK-016 in stressed wildtype mice, but not α5 KO mice.
Conclusions:
Alpha5 selective GABA-NAMs exert rapid anti-anhedonic actions and restore the strength of synapses in reward regions by acting at the benzodiazepine site of α5-containing GABAARs. These results encourage human studies using GABA-NAMs to treat depression by providing readily translatable measures of target engagement.
Keywords: GABA, antidepressant, ketamine, depression, gamma, hippocampus
Major depressive disorder (MDD, depression) afflicts an estimated 264 million people worldwide. The cardinal symptoms of MDD are chronically depressed mood and the inability to experience pleasure from previously rewarding stimuli, or anhedonia. Feelings of anhedonia are strongly correlated to suicidal ideation1,2 and 66 - 90% of all completed suicides are carried out by those with mood disorders3.
Selective serotonin reuptake inhibitors (SSRIs) and other monoamine-modulating drugs are the standard treatment for MDD, but SSRIs typically require 4 to 8 weeks to elicit symptomatic relief, produce remission in only half of patients4, and exert significant side effects5. (S)-Ketamine, an NMDA antagonist, is the first FDA-approved fast-acting antidepressant, with relief of symptoms occurring within hours and persisting for days-to-weeks6,7. Ketamine, however, has significant risks for abuse and produces a dissociative alteration of consciousness. Because ketamine antagonizes NMDARs throughout the entire brain, it is difficult to minimize these undesirable effects while retaining its antidepressant actions.
Ketamine may exert its beneficial effects, at least in part, by acutely decreasing the excitation of inhibitory interneurons, thereby promoting synchronous neuronal discharges in pyramidal cells8-11 and activating several activity-dependent synaptic strengthening processes12-16. Negative allosteric modulators of GABAA receptors (GABA-NAMs) that contain α5 (alpha5) subunits represent a promising alternative to ketamine because they produce disinhibition directly and thereby promote increased excitatory glutamatergic transmission17-20. Drugs that target α5 subunit-containing GABAARs are uniquely attractive because expression of this class of receptor is greatly enriched in the hippocampus and prefrontal cortex21-23, two stress-sensitive brain regions implicated in regulating emotion and motivation.
Two common α5-preferring GABA-NAMs, MRK-016 and L655,708, have been shown to exert rapid and persistent beneficial behavioral effects in rodent models of stress-induced anhedonia24,25 and in tests of potential antidepressant efficacy, such as the forced swim test25-29. GABA-NAMs bind GABAARs with high affinity at the interface of an α and γ subunit, a site that is shared with benzodiazepine positive allosteric modulators30. GABA-NAMs bind to recombinant human GABAARs containing different α subunits with a range of affinities, selectivities, and potencies30-32. L655,708, for example, has 50-100-fold selectivity for binding to GABAARs containing α5 subunits, but only inhibits GABA responses by ca. 25%33. MRK-016, in contrast, binds with comparable nM-affinity to GABAARs containing α1, α2, α3, and α5 subunits, but potently inhibits responses only at α5-containing GABAARs (>50% inhibition)31. To advance α5-selective GABA-NAMs to clinical trials for human MDD, it is essential to determine whether they exert their antidepressant-like actions through engaging their predicted target.
We therefore used the potent GABA-NAM, MRK-016, to test the hypothesis that α5-preferring GABA-NAMs exert their antidepressant-like behavioral and synaptic actions by acting at the benzodiazepine binding site of α5-containing GABAARs. If true, then we predicted that both the non-subunit specific antagonist of the benzodiazepine site, flumazenil, and the genetic deletion of the α5 subunit, would be sufficient to prevent the rapid restoration of stress-induced deficits in hedonic behavior and synaptic strength.
Materials and Methods
Additional methodological details are provided in Supplemental Materials.
Animals and housing:
Male mice were group-housed with ad libitum access to water and standard rodent chow. Mice were 8 weeks-old at the start of chronic multimodal stress (CMMS) and were transferred to clean caging, where they remained singly housed through the duration of the experiment.
GABAAR α5 knockout (KO) mice:
Initial breeding pairs of global GABAAR α5 KO mice on a C57BL/6J background34 were transferred from UR’s colony at McLean Hospital to the University of Maryland.
Drug Treatment:
MRK-016 (Tocris Bioscience, R&D Systems, Minneapolis, MN) was prepared in 100% DMSO and injected at 3mg/kg intraperitoneally (i.p.), a dose shown to produce 85% receptor occupancy in rats31 (injection volume = 40-50 μL). Flumazenil (Tocris Bioscience) was prepared in 100% DMSO and injected at 20mg/kg. This dose was calculated to be sufficient to antagonize the benzodiazepine site based on half-life in the rodent brain and its ability to restore the benzodiazepine-induced loss of righting-reflex in mice35 for the duration of MRK-016’s rodent elimination half-life31. (R,S) ketamine was purchased from Sigma-Aldrich, prepared in 0.9% saline and injected at 10mg/kg i.p. (100-150 μL).
Behavioral Protocols
Chronic multi-modal stress (CMMS):
Mice were singly housed in a fresh cage at the onset of the stress protocol. Starting at 10 AM, each animal was immobilized in a translucent plastic restraint tube and subjected to white noise and strobe lighting for 4 hours a day for 10 concurrent days. All mice were returned to their home cages and singly housed for the duration of the experiment.
Sucrose preference test (SPT):
Bottles containing either 1% sucrose solution or tap water were placed in the home cage overnight. Sucrose preference was calculated by dividing the weight of 1% sucrose solution consumed by the total weight consumed from both bottles. This was repeated the following night with the two nightly sucrose preference values averaged. Mice displaying a sucrose preference of >65% at baseline which decreased by at least 10% to a value of <65% following stress were considered stress-susceptible and included in further sucrose preference tests.
Female urine sniff test (FUST):
Two cotton swabs individually soaked in male urine and urine from females in estrus were placed at opposite ends of the cage. Time spent sniffing each swab during a 3 min trial was quantified by a trained observer blinded to treatment and position of each swab. Female urine preference was calculated as the total time a mouse spent sniffing the female urine swab over the total time sniffing both swabs. Mice displaying a female urine preference of greater than 50% at baseline that decreased by at least 10% following stress were considered stress-susceptible and used in the FUST arm of the study.
Electrophysiology
Extracellular recording:
Mice were anesthetized with isoflurane and decapitated. The hippocampus was rapidly excised, mounted into a 3% agar block, and sectioned into 400μm transverse slices while submerged in ice-cold ACSF comprised of 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.5 mM MgSO4, 2.5 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose, and bubbled with 95% O2/5% CO2. All slices were kept in a humidified recovery chamber for at least one hour prior to recording. Hippocampal region CA3 was removed with a razor and slices were transferred to an immersion recording chamber. Glass recording electrodes were placed in stratum lacunosum-moleculare (SLM), with concentric bipolar tungsten stimulating electrodes placed approximately >500 μm away in the temporo-ammonic (TA) pathway.
Field EPSPs (fEPSPs) were recorded extracellularly in room temperature (20-22°C) Mg2+-free ACSF perfused at a rate of 1mL/min. Picrotoxin (100 μM) and CGP54626 (2μM) (Tocris Bioscience) were added to the bath to block GABAARs and GABABRs, respectively. Sequential 15-minute wash-ins of DNQX (50μM) and D,L - APV (80μM) (Tocris Bioscience) were used to isolate the components of the fEPSP mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartic acid (NMDA) receptors, respectively36. The initial slope (1.5ms after fEPSP onset prior to DNQX wash-in) was used to quantify the AMPAR-mediated component of the fEPSP and the slope 4.0ms after fEPSP onset, following DNQX wash-in, was used to quantify the NMDAR-mediated component. The slopes were normalized to their fiber volley amplitude. Traces at each stimulation intensity were averaged, and pairs of responses recorded at the same intensities before and after DNQX wash-ins with an NMDA component closest to 0.1mV/ms were divided to calculate an AMPA:NMDA ratio. Analysis was performed blind to animal genotype or treatment. Individual data points represent the average of all AMPA:NMDA ratios collected from slices from one individual animal (n=1-6 slices per animal), where responses in all individual slices are shown in the AMPA slope and NMDA slope graphs.
EEG recording:
12-week-old male GABAAR α5 KO mice and their wildtype littermate controls were anesthetized with 3% isoflurane, with anesthesia maintained using 1.5% isoflurane. An F20-EET radiotelemetric transmitter (Data Sciences International, Minneapolis, MN, USA) was embedded subcutaneously between the scapulae. Transmitter leads were implanted unilaterally above the prefrontal cortex. Mice were allowed to recover seven days prior to experiments.
EEG signals were recorded with the Ponemah software suite (Data Sciences International, Minneapolis, MN, USA). Baseline activity was recorded for 3 hours during the light cycle. Mice were then treated with a volume-equivalent dose of 100% DMSO vehicle (i.p., 40-50 μL) and the EEG was recorded for 90 minutes. The mice were then given 3 mg/kg MRK-016 (i.p.) and EEG recordings continued for another 90 minutes. Subsequent injections of saline and 10 mg/kg (R,S) ketamine were given 24 hours later using the same protocol. Power analysis was conducted in Neuroscore (DSI, Harvard Bioscience) and spectrograms were generated in MathWorks MATLAB with oscillations at delta frequencies defined as 1-3Hz, theta at 4-7Hz, alpha at 8-12Hz, beta at 13-29Hz and gamma at 30-80Hz. Post-treatment powers were normalized to their average power during the last 30 minutes of the baseline recording.
Statistical analysis:
All one-, two- and three-way ANOVA, Student’s t-tests, linear regressions and correlations were performed using GraphPad Prism 9 software. Three-way ANOVAs were calculated as uncorrected values and Šidák corrections were performed manually in Microsoft Excel. One and two-way ANOVAs employed Holm-Šidák corrections for multiple comparisons. Figures represent group average ± SEM.
Results
Pretreatment with the benzodiazepine site antagonist flumazenil prevents the effects of MRK-016 on reward behavior and synaptic strength
We first asked whether MRK-016 mediates its anti-anhedonic actions by acting at the benzodiazepine binding site of GABAARs. Flumazenil is a functionally neutral, competitive antagonist of benzodiazepine agonists37 and MRK-01631, and has minimal impact on GABAAR-mediated currents at physiological concentrations of GABA38. We therefore tested whether flumazenil would block the behavioral and synaptic actions of MRK-016 in chronically stressed mice.
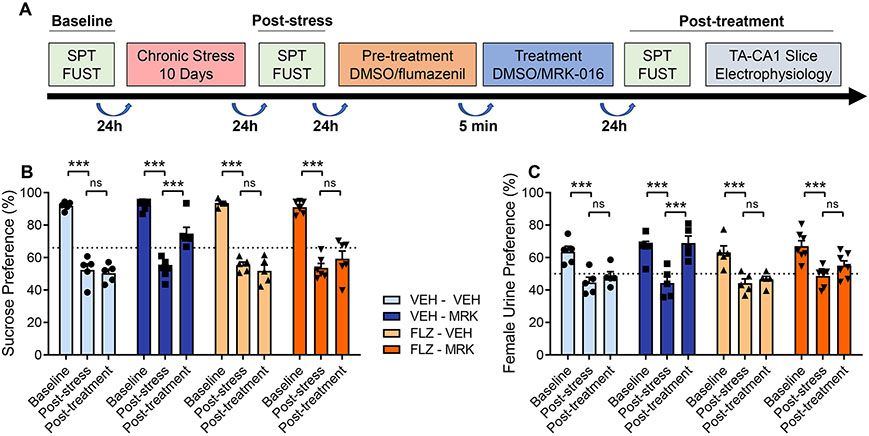
After measuring baseline behaviors, 8-week-old male C57BL/6J mice were exposed to a 10-day period of chronic multimodal stress (CMMS) (Fig 1A). Mice subjected to CMMS displayed significant decreases in their responses in two different hedonic behavioral assays: the sucrose preference test (SPT), measuring consumption of 1% sucrose solution compared to consumption of tap water, and the female urine sniffing test (FUST), measuring time spent interacting with cotton swabs soaked in urine from either female mice in estrous or male mice (Fig. 1B,C). Mice susceptible to CMMS displayed significant reductions in preference for both sucrose and female urine. A single 3mg/kg i.p. injection of MRK-016 significantly improved preferences in both SPT and FUST assays 24-48 hours later, compared to post-stress values. No significant change in reward behaviors was observed in mice treated with an equivalent volume of vehicle. In contrast, mice pretreated with 20mg/kg flumazenil, followed 5 minutes later by administration of either 3mg/kg of MRK-016 or an equivalent volume of vehicle showed no significant improvement in preferences in either the SPT and FUST assays, compared to post-stress values. No significant changes in reward behaviors were observed in mice treated with flumazenil alone.
Figure 1.
Flumazenil prevents restoration of hedonic behavior by MRK-016 in stressed mice. (A) Timeline of hedonic behavior assessment in relation to chronic stress and drug treatment. (B) 10 days of CMMS significantly decreased sucrose preference compared to baseline in all groups (vehicle-vehicle, n=5, p=0.0012; vehicle-MRK-016, n=6, p=0.0012; flumazenil-vehicle, n=5, 0.0012, MRK-016, n=5, p=0.0012). Administration of 3mg/kg MRK-016 significantly increased sucrose preference over post-stress values (p=0.0012) in vehicle pretreated mice. Pretreatment with 20mg/kg flumazenil prevented significant restoration of sucrose preference from their post-stress values (p=0.1256). Neither vehicle (p=0.6120) or flumazenil alone (p=0.6130) improved sucrose preferences following stress. Three-way ANOVA revealed a significant effect of stress (F 2,36 = 242.3, p<0.0001), MRK-016 (F 1,36 = 242.3, p<0.0001) and stress × MRK-016 (F 2,36 = 12.98, p<0.0001), but not stress × flumazenil × MRK (F 2,36 = 2.35, p=0.1098). (C) CMMS decreased female urine preference in each group (vehicle-vehicle, n=5, p=0.0012; vehicle-MRK-016, n=5, p=0.0012; flumazenil-vehicle, n=5, p=0.0012, MRK-016, n=7, p=0.0012). MRK-016 significantly increased female urine preferences following stress (p=0.0012), but this was prevented by flumazenil pretreatment (p=0.1869). Female urine preference was not significantly different following administration of vehicle (p=0.6678) or flumazenil (p=0.5281) alone. Three-way ANOVA revealed a significant effect of stress (F 2,36 = 59.44, p<0.0001), MRK-016 (F 1,18 = 6.661, p=0.0188) and stress × MRK-016 (F 2,36 = 7.272, p=0.0022), but not stress × flumazenil × MRK (F 2,36 = 3.038, p=0.0604). p<0.005 ***, ns: not significant.
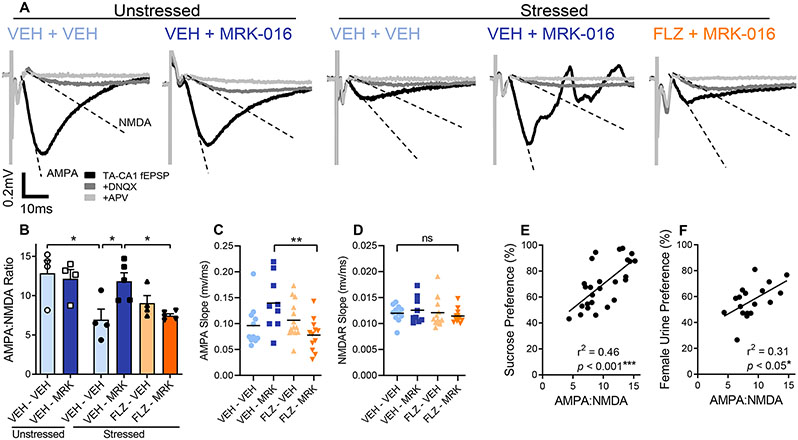
Following behavioral testing, these same mice were euthanized, and hippocampal slices were prepared in order to determine whether flumazenil administration had any effect on the ability of MRK-016 to restore the strength of TA-CA1 hippocampal synapses (Fig 2A,B). Concomitant with restoration of reward behavior, significantly higher TA-CA1 AMPA:NMDA ratios were observed in hippocampal slices taken from mice given 3 mg/kg MRK-016, compared to stressed mice treated with vehicle alone. AMPA:NMDA ratios in slices from stressed mice that received MRK-016 were not different to those from unstressed mice, with or without MRK-016 administration. Mice that were pretreated with flumazenil prior to MRK-016 administration displayed significantly smaller AMPA:NMDA ratios than those pretreated with vehicle prior to MRK-016. The increase in TA-CA1 AMPA:NMDA ratio was mediated by a selective increase in the slope of the AMPA-mediated component of the fEPSP (Fig 2C,D). There was a significant positive correlation between behavioral responses in the SPT and FUST of individual mice with their calculated AMPA:NMDA ratio (Fig 2E,F).
Figure 2.
Flumazenil prevents restoration of synaptic strength by MRK-016 in stressed mice. (A) Representative traces comparing AMPA:NMDA ratios in stressed animals given MRK-016 with or without flumazenil pretreatment. (B) CMMS decreases TA-CA1 synaptic strength. Stressed DMSO vehicle treated mice had a significantly lower TA-CA1 AMPA:NMDA ratio than unstressed mice (n=4 animals, p=0.0298). Among stressed mice, those administered 3mg/kg MRK-016 (n=5) have significantly higher AMPA:NMDA ratios than those receiving vehicle alone (n=4, p=0.0167) or MRK-016 in conjunction with flumazenil pretreatment (n=4, p=0.0199). Two-way ANOVA of treated stress-susceptible animals indicated significant pretreatment × treatment interaction (F 1,14 = 11.50, P=0.0044). (C) MRK-016 increases the AMPA-mediated component of the TA-CA1 fEPSP in stress-sensitive animals, which is prevented by flumazenil (n=individual slices from Fig. 2B, p=0.0079). One-way ANOVA indicated significant effect of treatment on AMPAR-mediated slope (F 3,42 = 4.47, P=0.0084). (D) MRK-016 administration does not change the NMDAR-mediated component of the TA-CA1 fEPSP. One-way ANOVA indicated no effect of treatment on NMDAR-mediated slope (F 3,42 = 0.4757, P=0.7009). (E) TA-CA1 synaptic strength correlates with an animal’s sucrose preference (n=26, r=0.6763, p=0.0001. Line of fit: Y = 0.1220*X + 1.465). (F) TA-CA1 synaptic strength correlates with an animal’s female urine sniffing preference (n=18, r=0.5561, p=0.0164. Line of fit: Y = 0.1167*X + 2.272). p<0.05 *, p<0.01 **, p<0.005 ***, ns: not significant
These behavioral and electrophysiological actions of MRK-016 are consistent with previous observations following chronic stress and administration of GABA-NAMs in rats24. We conclude that the ability of MRK-016 to produce an anti-anhedonic behavioral response and to strengthen stress-sensitive synapses requires its binding to the benzodiazepine site of GABAARs.
The behavioral and electrophysiological effects of MRK-016 require the GABAAR α5 subunit
Benzodiazepine binding sites can be formed by most GABAAR α subunits and MRK-016 has comparable affinity for several α subunits. We therefore asked whether an action at α5 subunits was required for the behavioral and electrophysiological effects of MRK-016 using mice with global knockout (KO) of the α5 subunit gene34.
Acute increases in electroencephalogram (EEG) power in the high frequency gamma band (30-80Hz) are a hallmark of a number of rapid acting antidepressant interventions in mice and humans15,39,40 and are believed essential to their persistent actions. We have previously demonstrated that MRK-016 administration also promotes increases in EEG gamma power quantified over the PFC27. We therefore first tested whether MRK-016 would increase EEG gamma power in α5 KO mice.
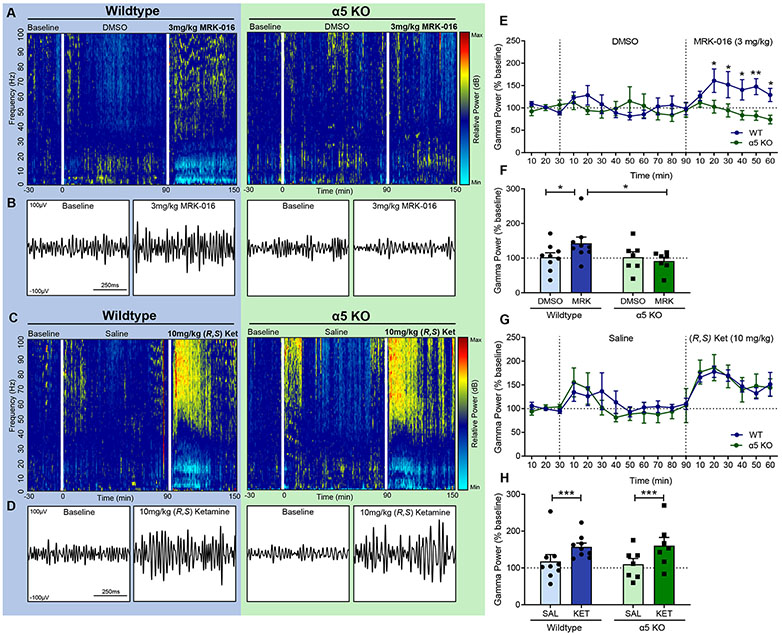
Cohorts of 12-week-old male α5 KO mice and wildtype (WT) littermate control mice were given DMSO vehicle injections (i.p.) followed 90 minutes later by 3mg/kg of MRK-016. Wildtype mice demonstrated significantly higher levels of gamma oscillatory activity 60 minutes following MRK-016 administration compared to α5 KO mice (Fig 3A,B,E,F). As a positive control for the ability of the α5 KO mice to generate gamma activity, these same mice were treated 24 hours later with saline vehicle followed by 10mg/kg (R,S) ketamine 90 minutes later. Both WT and α5 KO mice displayed significantly higher EEG gamma power 60 minutes after ketamine administration compared to their saline values (Fig 3C,D,G,H). We conclude that MRK-016 requires GABAARs containing α5 subunits to generate increases in EEG gamma power.
Figure 3.
Induction of EEG gamma activity by MRK-016 is absent in mice lacking GABAAR α5 subunits. (A, C) Representative EEG spectrograms showing oscillatory activity normalized to baseline for wildtype and α5 KO mice respectively. More relative activity at a given frequency range is indicated by “warmer” colors, and less by “cooler” colors. (B, D) Representative EEG gamma activity (750ms) at baseline and 30 minutes after treatment, filtered to 30-80Hz. (E) EEG gamma powers (30-80hz) were normalized to the last 30 minutes of baseline gamma activity following DMSO vehicle and MRK-016 administration. MRK-016 produces significantly higher cortical gamma power in wildtypes vs α5 KO animals beginning 20 minutes after administration. 2-way ANOVA identifies significant effect between time × genotype (F 17,238 = 2.678, p=0.0005). (F) There is no significant difference in gamma powers at any time point between wildtype or α5 KO animals in response to ketamine. Time × genotype (F 17,238 = 0.5589, p=0.9193). (G) MRK-016 promotes significant increases in gamma power over a 60-minute period after treatment compared to DMSO vehicle in wildtype (n=9, p=0.0462) but not α5 KO animals (n=7, p=0.9962). Wildtype animals generate significantly more gamma power compared to α5 KOs following MRK-016 administration (p=0.0390). 2-way ANOVA identifies significant effect between treatment × genotype (F 1,14 = 5.052, p=0.0412). (H) Normalized EEG gamma power quantified for 60 minutes after 10mg/kg (R,S) ketamine (i.p.) administration was significantly greater than after volume-equivalent saline vehicle for both wildtype (n=9, p=0.0033) and α5 KO cohorts (n=7, p=0.0027). 2-way ANOVA identifies significant treatment effect (F 1,14 = 28.35, p=0.0001), but no significant interaction between treatment × genotype (F 1,14 = 0.4164, p=0.5292). p<0.05 *, p<0.005 ***.
Concomitant with the increase in gamma power, MRK-016 produced a significant decrease in EEG power lasting >30 min within the theta (4-7 Hz), alpha (8-12 Hz), and beta (13-29 Hz) bands in wild type mice, but not in α5 KO mice (Fig S1). Decreases in low frequency EEG power were also observed in response to ketamine, however these decreases were more transient than those elicited by MRK-016 and were equivalent in both wild type and α5 KO mice (Fig S2).
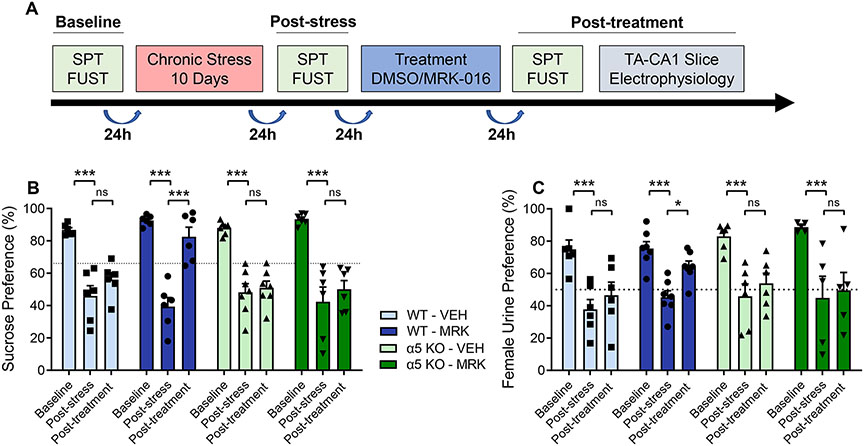
We next tested whether MRK-016 could reverse deficits in hedonic behavior and synaptic strength in stressed α5 KO mice. A cohort of 8-week-old male α5 KO mice and WT littermate controls were subjected to 10 days of CMMS. Stress-susceptible mice were treated with a single i.p. injection of 3mg/kg MRK-016 or an equivalent volume of vehicle (Fig 4A). Significant restoration of both SPT and FUST preferences compared to post-stress values was observed only in wildtype mice treated with MRK-016 (Fig 4B,C). No significant difference between post-stress and post-treatment values was observed in α5 KO mice given MRK-016 or in mice treated with vehicle alone.
Figure 4.
Restoration of hedonic behavior by MRK-016 is absent in mice lacking GABAAR α5 subunits. (A) Timeline outlining the experimental protocol of assessing hedonic behavior and chronic multimodal stress. (B) 10 days of CMMS significantly decreased sucrose preference compared to baseline among all groups and genotypes (wildtype-vehicle, n=6, p=0.0012; wildtype-MRK-016, n=6, p=0.0012; α5 KO-vehicle, n=7, 0.0012, α5 KO-MRK-016, n=6, p=0.0012). Wildtype animals given 3mg/kg MRK-016 demonstrated significantly increased sucrose preferences compared to post-stress values (p=0.0012), but this effect was absent in the α5 KO cohort (p=0.5079). Administration of DMSO vehicle did not significantly alter sucrose preference values after stress in either the wildtype (p=0.5472) or α5 KO groups (p=0.6923). Three-way ANOVA revealed a significant effect of stress (F 2,42 = 83.35, p<0.0001), stress × treatment (F 2,42 = 3.493, p=0.0395), but not stress × treatment × α5 KO (F 2,42 = 2.360, p=0.1069). (C) CMMS significantly lowered female urine preferences in all groups compared to baseline values (wildtype-vehicle, n=6, p=0.0012; wildtype-MRK-016, n=7, p=0.0012; a5 KO-vehicle, n=6, 0.0012, α5 KO-MRK-016, n=5, p=0.0012). MRK-016 significantly increases female urine preference following stress in wildtype (p=0.0296) but not α5 KO (p=0.5505) groups. Vehicle did not significantly improve female urine preferences following stress in either wildtype (p=0.5345) or α5 KO groups (p=0.4576). Three-way ANVOA showed a significant effect of stress (F 2,40 = 58.46, p<0.0001). Stress × treatment (F 2,40 = 2.019, p=0.1462). Stress × treatment × a5 KO (F 2,40 = 1.839, p=0.1722). p<0.05 *, p<0.005 ***, ns: not significant.
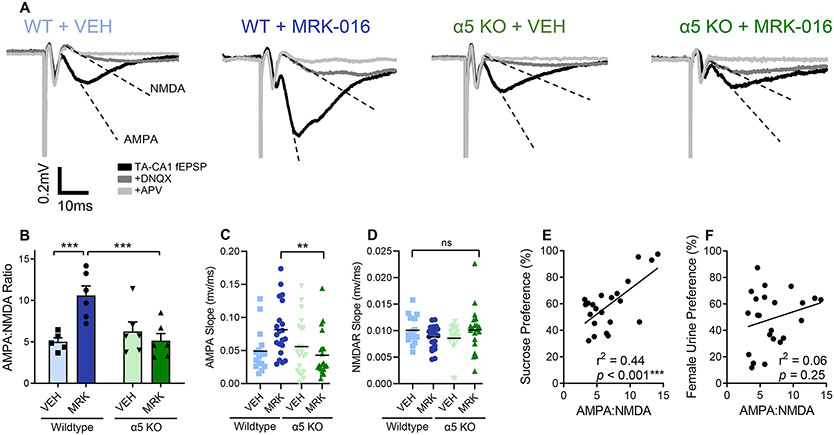
Following behavioral testing, hippocampal slices were prepared for electrophysiological recording of TA-CA1 synaptic strength. Significantly greater AMPA:NMDA ratios were observed in stressed WT mice given MRK-016, compared to stressed WT mice receiving vehicle (Fig 5A,B). In contrast, there was no significant difference between the AMPA:NMDA ratios of stressed α5 KO mice given either MRK-016 or vehicle. The increase in TA-CA1 AMPA:NMDA ratio was mediated by a selective increase in the slope of the AMPA-mediated component of the fEPSP(Fig 5C,D). An individual animal’s TA-CA1 synaptic strength was positively correlated with hedonic behavior in these experiments (Fig 5E,F).
Figure 5.
Restoration of synaptic strength by MRK-016 is absent in mice lacking GABAAR α5 subunits. (A) Representative traces showing larger AMPA:NMDA ratios in wildtype animals compared to α5 KOs. (B) CMMS decreases TA-CA1 synaptic strength. Among stress-sensitive wildtype mice, 3mg/kg MRK-016 (n=6 animals) significantly increased AMPA:NMDA ratios compared to vehicle (n=5) treated animals (p=0.0045). AMPA:NMDA ratios in stress-sensitive α5 KO mice were not significantly different between vehicle (n=6) or MRK-016 (n=6) groups (p=0.7749). Wildtype mice displayed significantly higher AMPA:NMDA ratios than α5 KO mice after MRK-016 administration (p=0.0044). Two-way ANOVA indicated significant interaction of genotype × MRK-016 (F 1,19 = 11.62, P=0.0029). (C) MRK-016 increases the AMPA-mediated component of the TA-CA1 fEPSP in stress-sensitive wildtype but not α5 KO mice (n=individual slices from Fig. 5B, p=0.0079). One-way ANOVA indicated significant effect of treatment on AMPAR-mediated slope (F 3,75 = 4.211, P=0.0083). (D) MRK-016 administration does not change the NMDAR-mediated component of the TA-CA1 fEPSP. One-way ANOVA indicated no effect of treatment on NMDAR-mediated slope (F 3,75 = 1.476, P=0.2279). (E) TA-CA1 synaptic strength correlates with an animal’s sucrose preference (n=23, r=0.6625, p=0.0006. Line of fit: Y = 3.791*X + 33.20). (F) TA-CA1 synaptic strength positively trends with an animal’s female urine sniffing preference (n=23, r=0.2481, p=0.2536. Line of fit: Y = 1.637*X + 37.66). p<0.01 **p<0.005 ***, ns: not significant.
To confirm the hedonic behavior of α5 KO mice is not innately refractory to rapid-acting antidepressant compounds, a separate cohort of α5 KO mice and WT littermate controls were chronically stressed for 10 days and given three treatments: an MRK-016 dose-equivalent volume of vehicle, 3mg/kg MRK-016, and 10mg/kg (R,S) ketamine, each spaced 72 hours apart (Fig S3). When given MRK-016, only the wildtype cohort showed significant restoration of hedonic behavior. Both SPT and FUST preferences in the α5 KO mice were significantly improved compared to post-stress values after administration of ketamine.
MRK-016 does not alter hedonic behavior or synaptic strength in unstressed mice
A separate cohort of unstressed, 10-week-old, male α5 KO mice and WT littermates were given 3mg/kg of MRK-016 or an equivalent volume of vehicle (Fig S4). Sucrose preferences remained unchanged following treatment with MRK-016 or vehicle, regardless of genotype. Hippocampal slices were prepared from these mice and TA-CA1 AMPA:NMDA ratios were found to be the same across genotypes and treatments.
Discussion
Negative allosteric modulators of α5 subunit-containing GABAARs are a promising class of compounds for the treatment of major depressive disorder. In preclinical models, GABA-NAMs produce a rapid and persistent restoration of reward behaviors in rats with stress-induced anhedonia24, a finding we replicate here in mice, while exerting fewer potential side effects than ketamine26,27, the only fast-acting antidepressant currently approved by the FDA. A critical next step in advancing GABA-NAMs from preclinical studies to FDA approval is to ensure that they exert their actions by engaging their expected target. This is especially important for putative α5-selective GABA-NAMs because many of them have comparable affinity at multiple GABAA receptor α subunits31. Here we tested the hypothesis that their behavioral and electrophysiological actions are mediated at the benzodiazepine binding site of GABAARs formed by α5 subunits, as predicted by in vitro binding assays31. We found that both the benzodiazepine site antagonist flumazenil and deletion of the α5 gene led to a failure of the GABA-NAM MRK-016 to exert an anti-anhedonic response in two assays of reward behavior or restore the strength of stress-weakened excitatory synapses in the hippocampus. We conclude the behavioral and electrophysiological actions of α5-selective GABA-NAMs require binding to the benzodiazepine site on α5 subunit-containing GABAARs.
Utility of stress-induced anhedonia as a readout of efficacy
Depression is a complex, uniquely human psychological disorder with a range of symptoms, including the two cardinal symptoms of persistently depressed mood and anhedonia. Preclinical studies have better predictive outcomes when they are based on models that are both based on shared putative causes and result in behavioral changes that are analogous to human symptoms. Chronic stress is known to be positively correlated with the likelihood of human depression. For example, depressed patients report more stressful life events than non-depressed subjects, including physical illness, troubled family relationships, and financial difficulty41. In the human brain, depression is associated with altered brain structure and functional connectivity across a range of regions, including reward processing regions42-44. In rodents, stress also has a number of deleterious effects on excitatory synaptic function, including dendritic atrophy45, decreases in dendritic spine density46,47, decreases in glutamate receptor expression36,48, and reduced expression of the synaptic growth-promoting factors, such as BDNF49. Our finding that the binding of GABA-NAMs to their appropriate target is required for their anti-anhedonic and synaptic actions in these models thus offers preclinical endpoints with the best available predictive reliability for future human trials.
We note that α5-selective positive allosteric modulators (GABA-PAMs) have been suggested as potentially useful antidepressant compounds50, which would be inconsistent with our proposed model. This suggestion was based on prior immunocytochemical and Western blotting studies of postmortem tissue samples from depressed subjects51,52 and preclinical stress models53, suggestive of GABAergic interneuron hypofunction that could be reversed by GABA-PAM administration. Effects of an α5-selective GABA-PAM after chronic stress were not seen in male mice, and were observed only for an anxiety behavior in stressed female mice50, suggestive of anxiolytic rather than anti-anhedonic actions.
Mechanisms underlying GABA-NAM action
Negative allosteric modulators of GABAARs decrease the ability of GABA to open the ion channel, thereby promoting cell and network excitability by effectively diminishing the “brake” on the depolarization of excitatory pyramidal cells54-56. While diminishing the efficacy of GABA action nonspecifically promotes seizures57, α5 subunit-selective GABA-NAMs do not produce or promote seizures in mice or human subjects31. This is likely because α5-containing GABAARs represent a small subset of all GABAARs, even in the hippocampus and PFC, where they are expressed at the highest levels21-23. Alpha5-selective GABA-NAMs do not produce significant off-target effects in preclinical models27,58 or in limited human studies31, perhaps because the ratio of α5-containing to α5-lacking GABAARs is much lower outside of the hippocampus and prefrontal cortex.
Alpha5-containing GABAARs contribute to both tonic and phasic inhibition18,19,59 of pyramidal cells in the hippocampus and PFC, and are also expressed at synapses formed by dendrites targeting inhibitory interneurons20,55,56. By reducing both forms of inhibition, α5 subunit-selective GABA-NAM can be predicted to promote pyramidal cell discharge, dendritic NMDAR activation, and plasticity of excitatory synapses55,60. Therefore, α5-selective GABA-NAMs provide the means to selectively and safely alter the balance of excitation and inhibition primarily within the hippocampus and PFC in a transient manner that leads to a persistent improvement in circuit function. Alpha5-containing GABAARs are also expressed at synapses between inhibitory interneurons61. We have not tested whether the critical α5-containing GABAARs contributing to anti-anhedonic action are expressed by pyramidal cells18,19,59 or interneurons34.
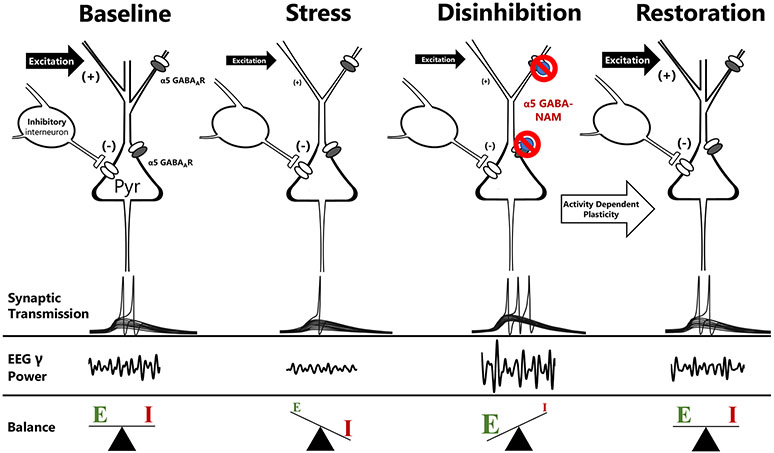
Our results suggest that α5-selective GABA-NAMs exert their anti-anhedonic actions through the following mechanism (Fig 6). GABA-NAM – induced increases in the excitability of pyramidal cells favor mutual excitation locally and promote excitatory drive in efferent projections to subcortical targets. Indeed, MRK-016 promotes an increase in EEG power in the gamma frequency band and decreases in power in the lower frequency bands over the PFC in wildtype mice, but not in mice lacking α5 subunits. Because ketamine increases gamma power in α5 KO mice, we can conclude that promotion of gamma oscillations by GABA-NAMs is specifically triggered at the benzodiazepine site formed by α5 GABAAR subunits, rather than a defect in the function of critical circuits in the absence of α5-containing GABAARs. Alpha5-containing GABAARs have been implicated in maintaining cortical functional connectivity through the generation of high frequency EEG gamma power17, however we observed no difference in EEG gamma power generated in vivo between wildtype and global α5 KO mice following ketamine administration. There is considerable evidence in preclinical models and humans for structural and functional deficits in synaptic connectivity in depression13,14,42,43,62. Such deficits in reward circuits could explain the symptom of anhedonia. GABA-NAM – induced increases in synchronous high frequency discharge is likely to be a powerful activator of a range of activity-dependent synaptic strengthening processes, including increased BDNF-trkB signaling, increased expression of AMPARs, or mTORC1 activation, much like ketamine12,14,16,29. It is widely believed that these mechanisms can then act to restore the function of stress-weakened synapses, both within the PFC and hippocampus, as well as in downstream targets receiving their synchronized output, such as the NAc63. Improved function in reward circuits could explain the anti-anhedonic actions of GABA-NAMs. These mechanisms can also account for the persistence of the therapeutic actions long after the compounds have been eliminated13,15,64. Indeed, increases in EEG gamma power are a hallmark of putative rapid-acting antidepressants, such as ketamine and its metabolites (e.g. hydroxynorketamines) in rodents65 and humans40,66.
Figure 6.
Mechanisms underlying the antidepressant actions of α5 GABA-NAMs. Excitability and function in reward circuits is determined by the balance of excitatory and inhibitory transmission. Chronic stress has numerous deleterious effects on excitatory synaptic function, including reductions in net excitability, spine density, and dendritic branching. This shifts the balance of excitation and inhibition and contributes to the genesis of the anhedonic state. Negative allosteric modulators of α5-containing GABAARs bind to benzodiazepine allosteric sites on α5-containing GABAARs, including perisomatic extrasynaptic receptors and synaptic receptors on distal dendrites, to reduce GABAergic inhibition acutely, thereby promoting glutamatergic transmission and favoring high-frequency gamma frequency oscillations. These oscillations promote endogenous activity dependent plasticity mechanisms, thereby strengthening stress-weakened synapses, restoring excitation/inhibition balance, and rescuing hedonic behavior.
Clinical potential of α5-selective GABA-NAMs
Originally developed for their nootropic potential, α5-selective GABA-NAMs represent promising tools for the treatment of MDD, as well as other disorders with significant comorbid mood dysregulation. They may also exert potential therapeutic actions in a variety of other conditions in which there is a pathological imbalance between synaptic inhibition and excitation30. For example, there is strong evidence that α5-selective GABA-NAMs can improve cognitive function by dampening excess inhibition in preclinical models of Downs syndrome20,67, although initial clinical trials have failed to detect benefits (ClinicalTrials.gov ID NCT02024789). They may also improve postsurgical cognitive outcomes following the use of common general anesthetics68,69.
We conclude that the rapid-acting antidepressant-like effects of MRK-016 are mediated via negative allosteric modulation of the GABAA receptor at an α5-containing benzodiazepine site, thereby promoting transient generation of EEG gamma power and increased hippocampal synaptic strength. Our findings directly link MRK-016’s target engagement with its downstream synaptic effects and the ultimate restoration of reward-seeking behaviors to a physiological normal. They also suggest two powerful approaches to ensure target engagement in future human trials, which will aid in their clinical development. First, displacement of radiolabeled GABAAR ligands can be readily performed in human subjects61 and EEG or MEG recording of changes in gamma power66 can be used to determine appropriate dosing and ensuring target engagement. These preclinical mechanistic results may provide a model for developing additional rapid-acting antidepressant-like compounds, as well as accelerating the use of GABA-NAMs in human clinical trials for mood disorders.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Chemical Compound or Drug | MRK-016 | Tocris Bioscience | Cat. No. 3817 | |
| Chemical Compound or Drug | flumazenil | Tocris Bioscience | Cat. No. 1328 | |
| Chemical Compound or Drug | picrotoxin | Tocris Bioscience | Cat. No. 1128/1G | |
| Chemical Compound or Drug | CGP54626 | Tocris Bioscience | Cat. No. 1088/10 | |
| Chemical Compound or Drug | DNQX | Sigma-Aldrich | Cat. No. D0540 | |
| Chemical Compound or Drug | APV | Tocris Bioscience | Cat. No. 0105/10 | |
| Chemical Compound or Drug | ketamine | Sigma-Aldrich | Cat. No. K2753 | |
| Organism/Strain | alpha5 knock-out mice (males) | PMCID: PMC4571505 |
Acknowledgements and disclosures
We thank Mackenzie Nelson for training in EEG transmitter implantation, Brent Stewart for assistance with EEG signal analysis and spectrogram generation, and Maxwell Madden for facilitating 3D printing of restraint tubes. Supported by grants from the NIH to SMT (R01 MH086828), TDG (R01-MH107615) and UR (R01MH095905-01A1 and R01GM128183-01A1); The Kahlert Foundation (SMT); U.S. Department of Veterans Affairs Merit Awards 101BX004062 and 101BX003631 (TDG); and a NARSAD Distinguished Investigator grant (# 25623) and a P&S Fund Investigator (UR).
The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
TAT and SMT conceived and designed the project and wrote the manuscript. TAT, PZ and PG performed all behavioral experiments. TAT performed all electrophysiological experiments. PZ, PG and TDG assisted with design and performance of behavioral and EEG experiments. UR provided α5 KO mice and assisted with genotyping. All authors contributed to the editing of the manuscript. Data from this manuscript was previously shown in posters at 2020 meetings of The Society for Neuroscience and American College of Neuropsychopharmacology under the title of “The GABAAR α5 subunit is required for the fast antidepressant-like actions of MRK-016 on stress-induced anhedonia and weakened synaptic function.”
The University of Maryland Baltimore has a patent pending (USPTO application number 15/300,984), on which SMT is listed as an inventor, covering the use of α5-selective GABA-NAMs to treat psychiatric disease. T.D.G. is listed as a co-author of patent applications related to the pharmacology and use of (2R,6R)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorder. TDG has received research funding from Allergan and Roche Pharmaceuticals and served as a consultant for FSV7 LLC during the preceding 3 years. TT, PZ, PG, and UR declare that they have no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Winer ES, Drapeau CW, Veilleux JC, Nadorff MR (2016): The association between anhedonia, suicidal ideation, and suicide attempts in a large student sample. 20:265–272. [DOI] [PubMed] [Google Scholar]
- 2.Bonanni L, Gualtieri F, Lester D, Falcone G, Nardella A, Fiorillo A, Pompili M (2019): Can anhedonia be considered a suicide risk factor? A review of the literature. Medicina (Lithuania) 55:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isometsä E (2014): Suicidal behaviour in mood disorders—Who, when, and why? Canadian J Psychiatry 59:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M., Rush AJ (2009): What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatric Service 60:1439–1445. [DOI] [PubMed] [Google Scholar]
- 5.Kelly K, Posternak M, Jonathan EA (2008): Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci 10:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000): Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- 7.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. (2006): A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Yoshida T, Katz DB, Lisman JE (2012): NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J Neurophysiol 107:3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivolta D, Heidegger T, Scheller B, Sauer A, Schaum M, Birkner K, et al. (2015): Ketamine dysregulates the amplitude and connectivity of high-frequency oscillations in cortical–subcortical networks in humans: Evidence from resting-state magnetoencephalography-recordings. Schizophrenia Bull 41:1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widman AJ, McMahon LL (2018): Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Nat Acad Sci USA, 115:E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, Kwan AC (2020): Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nature Communications 11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. (2011): Glutamate NMDA receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X (2015): An excitatory synapse hypothesis of depression. Trends Neurosci 38:279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016): Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould TD, Zarate CA, Thompson SM (2019): Molecular pharmacology and neurobiology of rapid-acting antidepressants. Ann Rev Pharmacol Toxicol 59:213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JW, Autry AE, Na ES, Adachi M, Björkholm C, Kavalali ET, Monteggia LM (2021): Sustained effects of rapidly acting antidepressants require BDNF-dependent MeCP2 phosphorylation. Nat Neurosci. doi: 10.1038/s41593-021-00868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, et al. (2004): α5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol 559:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glykys J, Mody I (2006): Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit–deficient mice. J Neurophysiol 95:2796–2807. [DOI] [PubMed] [Google Scholar]
- 19.Prenosil GA, Gasser EMS, Rudolph U, Keist R, Fritschy JM, Vogt KE (2006): Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol 96:846–857. [DOI] [PubMed] [Google Scholar]
- 20.Zorrilla de San Martin J, Donato C, Peixoto J, Aguirre A, Choudhary V, De Stasi AM, Lourenço J, Potier MC, Bacci A (2020): Alterations of specific cortical GABAergic circuits underlie abnormal network activity in a mouse model of Down syndrome. Elife. 9:e58731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritschy JM, Mohler H (1995): GABAA-receptor heterogeneity in the adult rat brain: Differential regional and cellular distribution of seven major subunits. J Comp Neurol 359:154–194. [DOI] [PubMed] [Google Scholar]
- 22.Malherbe P, Sigel E, Baur R, Persohn E, Richards JG, Möhler H. (1990): Functional expression and sites of gene transcription of a novel α subunit of the GABAA receptor in rat brain. FEBS Letters 260:261–265. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright A, Sirinathsinghji DJS, Oliver KR (2000): Expression of GABAA receptor α5 subunit-like immunoreactivity in human hippocampus. Mol Brain Res 80:228–232. [DOI] [PubMed] [Google Scholar]
- 24.Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM (2015): Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharmacol 40:2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong Z, Zhang K, Ishima T, Ren Q, Chang L, Chen J, Hashimoto K (2018): Comparison of rapid and long-lasting antidepressant effects of negative modulators of α5-containing GABAA receptors and (R)-ketamine in a chronic social defeat stress model. Pharmacol Biochem Behav 175:139–145. [DOI] [PubMed] [Google Scholar]
- 26.Carreno FR, Collins GT, Frazer A, Lodge DJ (2017): Selective pharmacological augmentation of hippocampal activity produces a sustained antidepressant-like response without abuse-related or psychotomimetic effects. Int J Neuropsychopharmacol 20:504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, Thompson SM (2017): A negative allosteric modulator for α5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. ENeuro 4:285–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu NZ, Ernst M, Treven M, Cerne R, Wakulchik M, Li X, et al. (2018): Negative allosteric modulation of alpha 5-containing GABA A receptors engenders antidepressant-like effects and selectively prevents age-associated hyperactivity in tau-depositing mice. Psychopharmacol 235: 1151–1161. [DOI] [PubMed] [Google Scholar]
- 29.Bugay V, McCoy AM, Lodge DJ, Brenner R, Frazer A, Carreno FR (2020): Mechanisms associated with the antidepressant-like effects of L-655,708. Neuropsychopharmacol 45:2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maramai S, Benchekroun M, Ward SE, Atack JR (2019): Subtype selective γ-aminobutyric acid type A receptor (GABAAR) modulators acting at the benzodiazepine binding site: An update. J Medicinal Chem 63:3425–3446. [DOI] [PubMed] [Google Scholar]
- 31.Atack JR, Maubach KA, Wafford KA, O’Connor D, Rodrigues AD, Evans DC, et al. (2009): In vitro and in vivo properties of 3-tert-Butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylmethoxy)-pyrazolo[1,5-d]-[1,2,4]triazine (MRK-016), a GABAA receptor α5 subtype-selective inverse agonist. J Pharmacol Exp Therapeutics 331:470–484. [DOI] [PubMed] [Google Scholar]
- 32.Sieghart W, Savić MM. International Union of Basic and Clinical Pharmacology. CVI (2018): GABAA Receptor subtype- and function-selective ligands: Key issues in translation to humans. Pharmacol Rev 70:836–878. [DOI] [PubMed] [Google Scholar]
- 33.Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR (2006): L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology 51:1023–1029. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers FC, Zarnowska ED, Laha KT, Engin E, Zeller A, Keist R, et al. (2015): Etomidate impairs long-term potentiation in vitro by targeting α5-subunit containing GABAA receptors on nonpyramidal cells. J Neurosci 35:9707–9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoz. (2013). Flumazenil Injection. Retrieved from https://www.sandoz.ca/sites/www.sandoz.ca/files/Flumazenil_Inj_PMe_20130612.doc.pdf
- 36.Kallarackal AJ, Kvarta MD, Cammarata E, Jaberi L, Cai X, Bailey AM, Thompson SM (2013): Chronic stress induces a selective decrease in AMPA receptor-mediated excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci 33:15669–15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Möhler H, Richards JG (1981): Agonist and antagonist benzodiazepine receptor interaction in vitro. Nature 294:763–765. [DOI] [PubMed] [Google Scholar]
- 38.Safavynia SA, Keating G, Spiegel I, Fidler JA, Kreuzer M, Rye DB, et al. (2016): The effects of γ-aminobutyric acid type A receptor modulation by flumazenil on emergence from general anesthesia. Anesthesiol 125:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farmer CA, Gilbert JR, Moaddel R, George J, Adeojo L, Lovett J, et al. (2020): Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacol 45:1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanos P, Thompson SM, Duman RS, Zarate CA Jr, Gould TD (2018): Convergent Mechanisms Underlying Rapid Antidepressant Action. CNS Drugs 32:197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billings AG, Cronkite RC, Moos RH (1983): Social-environmental factors in unipolar depression: Comparisons of depressed patients and nondepressed controls. J Abnormal Psychol 92:119–133. [DOI] [PubMed] [Google Scholar]
- 42.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996): Hippocampal atrophy in recurrent major depression. Proc Nat Acad Sci USA 93:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. (2017): Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang F, Peng W, Sweeney JA, Jia Z, Gong Q (2018): Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci Therapeutics 24:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magariños AM, McEwen BS (1995): Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neurosci 69:83–88. [DOI] [PubMed] [Google Scholar]
- 46.Silva-Gómez AB, Rojas D, Juárez I, Flores G (2003): Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res 983:128–136. [DOI] [PubMed] [Google Scholar]
- 47.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. (2019): Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science, 364:eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012): Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73:962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duman RS, Monteggia LM (2006): A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- 50.Piantadosi SC, French BJ, Poe MM, Timic T, Markovic BD, Pabba M, Seney ML, et al. (2016): Sex-dependent anti-stress effect of an a5 subunit containing GABAA receptor positive allosteric modulator. Front Pharmacol 7: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripp A, Oh H, Guilloux J-P, Martinowich K, Lewis DA, Sibille E (2012): Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 169: 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sibille E, Morris H, Kota R, Lewis DA (2011): GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. J Neuropsychopharmacol 14: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banasr M, Lepack A, Fee C, Duric V, Maldonado-Aviles J, DiLeone R, et al. (2017): Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Stress 1: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shu Y, Hasenstaub A, McCormick DA (2003): Turning on and off recurrent balanced cortical activity. Nature 423:288–293. [DOI] [PubMed] [Google Scholar]
- 55.Schulz JM, Knoflach F, Hernandez MC, Bischofberger J (2018): Dendrite-targeting interneurons control synaptic NMDA-receptor activation via nonlinear α5-GABA A receptors. Nature Comm 9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali AB, Thomson AM (2008): Synaptic α5 subunit–containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cerebral Cortex 18:1260–1271. [DOI] [PubMed] [Google Scholar]
- 57.Miczek KA, Weerts EM (1987): Seizures in drug-treated animals. Science 235:1127a. [DOI] [PubMed] [Google Scholar]
- 58.Hipp JF, Knoflach F, Comley R, Ballard TM, Honer M, Trube G, et al. (2021): Basmisanil, a highly selective GABAA-α5 negative allosteric modulator: preclinical pharmacology and demonstration of functional target engagement in man. Sci Rep 11:7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manzo MA, Wang DS, Li WW, Pinguelo A, Popa MO, Khodaei S, Atack JR, et al. (2021): Inhibition of a tonic inhibitory conductance in mouse hippocampal neurones by negative allosteric modulators of α5 subunit-containing γ-aminobutyric acid type A receptors: implications for treating cognitive deficits. Br J Anaesth 126:674–683. [DOI] [PubMed] [Google Scholar]
- 60.Davenport CM, Rajappa R, Katchan L, Taylor CR, Tsai MC, Smith CM, et al. (2021): Relocation of an extrasynaptic GABAA receptor to inhibitory synapses freezes excitatory synaptic strength and preserves memory. Neuron 109:123–134.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magnin E, Francavilla R, Amalyan S, Gervais E, David LS, Luo X, Topolnik L (2019): Input-specific synaptic location and function of the α5 GABA a receptor subunit in the mouse CA1 hippocampal neurons. J Neurosci 39:788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sapolsky RM (2000): The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry 48:755–765. [DOI] [PubMed] [Google Scholar]
- 63.LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, Thompson SM (2018): Reward behaviour is regulated by the strength of hippocampus-nucleus accumbens synapses. Nature 564:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duchon A, Gruart A, Albac C, Delatour B, Zorrilla de San Martin J, Delgado-García JM, et al. (2019): Long-lasting correction of in vivo LTP and cognitive deficits of mice modelling Down syndrome with an α5-selective GABAA inverse agonist. Br J Pharmacol 177: 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. (2016): NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA (2019): Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry 24:1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braudeau J, Delatour B, Duchon A, Pereira PL, Dauphinot L, de Chaumont F, et al. (2011): Specific targeting of the GABA-A receptor {alpha}5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. J Psychopharmacol 25: 1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eckenhoff RG, Maze M, Xie Z, Culley DJ, Goodlin SJ, Zuo Z, et al. (2020): Perioperative neurocognitive disorder: State of the preclinical science. Anesthesiol 132:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zurek AA, Yu J, Wang DS, Haffey SC, Bridgwater EM, Penna A, Lecker I, Lei G, Chang T, Salter EW, Orser BA (2014): Sustained increase in α5 GABAA receptor function impairs memory after anesthesia. J Clin Invest. 124:5437–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.