Abstract
Pregnancy and lactation are highly metabolically demanding states. Maternal glucose is a key fuel source for the growth and development of the fetus, as well as for the production of milk during lactation. Hence, the maternal body undergoes major adaptations in the systems regulating glucose homeostasis to cope with the increased demand for glucose. As part of these changes, insulin levels are elevated during pregnancy and lower in lactation. The increased insulin secretion during pregnancy plays a vital role in the periphery; however, the potential effects of increased insulin action in the brain have not been widely investigated. In this review, we consider the impact of pregnancy on brain access and brain levels of insulin. Moreover, we explore the hypothesis that pregnancy is associated with site-specific central insulin resistance that is adaptive, allowing for the increases in peripheral insulin secretion without the consequences of increased central and peripheral insulin functions, such as to stimulate glucose uptake into maternal tissues or to inhibit food intake. Conversely, the loss of central insulin actions may impair other functions, such as insulin control of the autonomic nervous system. The potential role of low insulin in facilitating adaptive responses to lactation, such as hyperphagia and suppression of reproductive function, are also discussed. We end the review with a list of key research questions requiring resolution.
Keywords: insulin, lactation, pregnancy
1 ∣. PREGNANCY
1.1 ∣. Glucose homeostasis undergoes significant changes during pregnancy
Glucose is the main energy source used by the fetus. Therefore, the mother must provide a constant supply across the placenta for the successful growth and development of offspring. Glucose transport from mother to fetus occurs by passive diffusion, relying on the maintenance of a higher glucose concentration on the maternal side compared to the fetal side. As pregnancy progresses and the fetus grows, this glucose gradient becomes harder to maintain and requires adaptations in maternal glucose regulation.
Insulin is the key hormone regulating blood glucose levels. When glucose levels rise, insulin is secreted to increase glucose uptake into peripheral insulin-sensitive tissues. As part of the normal response to pregnancy, maternal tissues such as muscle and fat become relatively insulin resistant1-5 thereby impeding glucose uptake (Figure 1) and favouring increased delivery of glucose to the fetus. These maternal tissues instead increase use of other fuel sources such as fatty acids and ketone bodies.6 However, excessive exposure to glucose can also be detrimental to optimal fetal growth. Therefore, part of the changes in glucose regulation act to prevent excessive glucose exposure. For example, normal pregnancy reduces the threshold for glucose-stimulated insulin secretion from the insulin-secreting beta-cells of the pancreas,7 so that, in response to increases in maternal blood glucose, there is a greater insulin response to attempt to rapidly reduce the high blood glucose level despite the relative insulin resistance of many maternal tissues (Figure 1). Alongside this lowered threshold for glucose-stimulated insulin secretion, beta-cells undergo expansion during pregnancy, and this expansion begins prior to the development of peripheral insulin resistance in other tissues.7 The pregnancy-induced beta-cell expansion represents an “anticipatory” adaptation, a physiological change that meets the future needs of increased insulin during pregnancy.8 Activation of the prolactin receptor, either through the hormone prolactin or its homologue, placental lactogen, plays a key role in driving beta-cell expansion during pregnancy.7-9
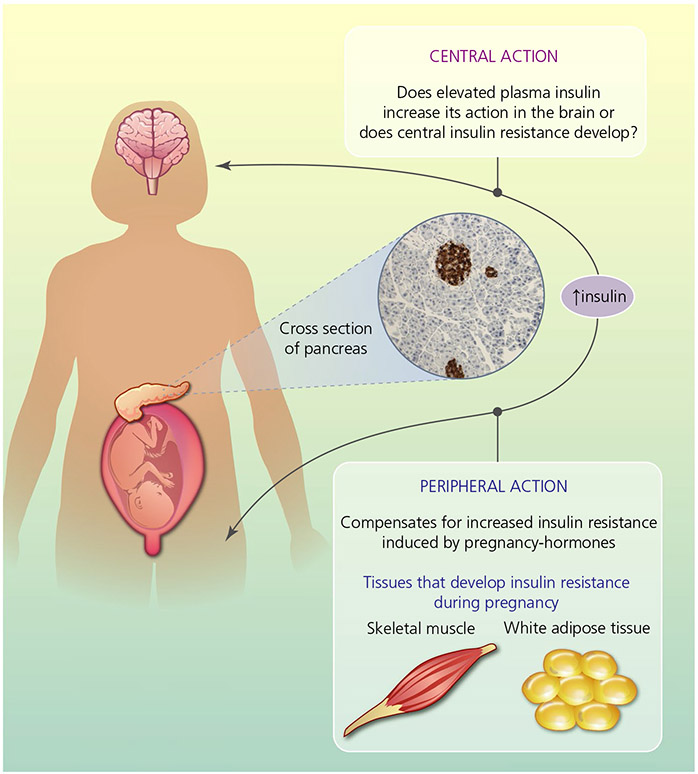
FIGURE 1.
The key question of central insulin action in the brain during pregnancy: does elevated insulin level increase central insulin function or does central insulin resistance develop? Insulin is secreted from beta-cells in the pancreas (as indicated by the brown immunoreactive staining in the cross-section of pancreas) and maternal adaptation in glucose regulation leads to increased insulin levels to compensate for increased peripheral insulin resistance during pregnancy. The effect of increased insulin levels during pregnancy on central insulin functions are yet to be thoroughly explored
The fine balancing act to maintain optimal levels of glucose transfer to the fetus and to protect against excessive exposure tends to increase circulating insulin levels during pregnancy. Glucose-stimulated insulin secretion is elevated7,8 and, in humans, fasting insulin levels increase.10-12 Whether similar rises in circulating insulin levels occur in pregnant animal models depends on the species, satiety status and experimental protocols. In the often-studied rat, under fasting conditions, pregnant insulin levels are similar to virgin levels.13-15 Pentobarbital anaesthetised pregnant rats also have similar insulin levels as virgins.16,17 Similar to many anaesthetics, pentobarbital can alter the regulation of glucose18-20 and thus caution should be taken in the interpretation of insulin levels measured under anaesthetic. However, unanaesthetised rats, mice and rabbits all show elevated basal insulin levels in the non-fasting state.15,21-24 Our data in rats and mice indicate that this elevation is detectable from mid-pregnancy,21,23 although others have not seen significant increases until late pregnancy (day 18 in rats).25 Some studies have only investigated a single time point in late pregnancy, showing increased insulin levels compared to virgins following 6 hours of food deprivation (although the timing of this fast was not stated).26
In the periphery, insulin plays the key role with respect to promoting uptake of glucose by insulin-sensitive tissues, such as liver, muscle and fat. Insulin receptors are also found in the brain 27,28; however, insulin does not drive the uptake of glucose by the brain as it does for peripheral tissues. Nevertheless, insulin can act in the brain to regulate a wide range of other functions, including food intake and energy homeostasis, peripheral glucose levels, reproduction, the sympathetic nervous system and cognition.29 The increase in circulating insulin levels during pregnancy therefore would suggest the potential for increased insulin action in the brain during this state. However, the actions of insulin in the brain also depend on the rate of uptake of insulin into the brain and insulin receptor responsiveness. Indeed, although increases in peripheral insulin are required for optimal offspring development, increases in some of the central functions of insulin, in particular inhibition of food intake and suppression of hepatic glucose production, would be counterproductive. Therefore, the aim of this review is to discuss the impact of pregnancy on brain access and responsiveness to insulin (Figure 1), as well as the resulting changes in select central insulin actions.
1.2 ∣. Insulin access to the brain during pregnancy
Insulin is produced in the periphery and to have effects in the brain, it needs to gain access across the blood-brain barrier (BBB).30,31 Insulin is too large to simply diffuse into the brain but, instead, moves across the BBB via an active, saturable trans-endothelial transport mechanism. In rodent models of obesity causing insulin resistance and hyperinsulinaemia, impaired access of insulin into the brain is considered to play a major role in the attenuated central insulin actions.32,33 During pregnancy, insulin transport into the brain is mostly unaffected, although transport of insulin across the choroid plexus, into cerebrospinal fluid (CSF), is increased.17 Thus, although other insulin-resistant states such as obesity and ageing decrease insulin transport into brain,32,34,35 this does not appear to be the case during pregnancy.
The level of insulin in the brain is affected not only by plasma insulin levels and transport into brain, but also by degradation. Interestingly, parenchymal degradation of insulin in the brain is elevated during pregnancy, which may explain why pregnancy does not alter brain insulin levels, despite elevated plasma levels.11 In vitro studies have shown that insulin itself can increase levels of insulin-degrading enzyme (IDE) in neurones36 and others have shown that oestrogen can increase IDE in primary cultures of rat hippocampal neurones.37 Whether elevated degradation of insulin in the brain is a result of a pregnancy-induced increase in IDE level or activity, as well as what role hormonal changes associated with pregnancy play in driving any potential change in degradation, remains to be investigated. Another outstanding question is what is the specific location of this increased degradation?
The impact of pregnancy on CSF levels of insulin has also been investigated, with varying results. Insulin levels were decreased in both late pregnancy in rats38 and rabbits,16 but, in another study, they were unchanged in late pregnant rats.17 The variability may be a result of methodological differences or, more simply, the action of pregnancy to increase plasma insulin levels and transport into CSF on the one hand but increase degradation on the other. Regardless, insulin transport via the CSF is not considered to be a major access point of insulin to the brain, and so it is unclear what these CSF values reflect in terms of functionality.17,30 Overall, the evidence suggests that pregnancy does not significantly alter levels of insulin in brain interstitial fluid.
1.3 ∣. Insulin-induced intracellular signalling in the brain during pregnancy
During pregnancy, in tissues such as skeletal muscle and adipose tissue, insulin resistance has been associated with attenuated insulin-induced activation of intracellular signalling pathways. Insulin predominantly acts through a phosphoinositide 3-kinase (PI3K) pathway,39 and pregnancy-induced insulin resistance in skeletal muscle down-regulates various steps in this signalling cascade, including reduced phosphorylation of the insulin receptor (IR) and reduced expression of insulin receptor substrate 1 (IRS-1).40 Emerging evidence also suggests the appearance of central insulin resistance (Figure 2). For example, early in the dark phase, which is a time of increased food consumption in rats, plasma insulin concentrations are greater in pregnant rats (day 14 of gestation) than in virgins. Despite this, pregnant rats show markedly lower phosphorylated Akt (pAkt) levels in the arcuate nucleus, a key site of insulin action in the brain, compared to virgin rats.15 Even in other areas of the hypothalamus, pAkt levels were similar between pregnant and virgin rats despite the difference in plasma insulin.15 Of course, Akt is not solely phosphorylated because of interactions between insulin and its receptor, and so these data are only suggestive of impaired sensitivity of neurones to endogenous insulin. When insulin-induced pAkt was directly assessed following acute, central administration of exogenous insulin, insulin sensitivity in the arcuate and ventromedial nuclei of the hypothalamus (VMH) was substantially attenuated during pregnancy. Yet other hypothalamic areas, the paraventricular nucleus (PVN) and dorsomedial nucleus (DMH), remained responsive in terms of insulin-induced pAkt.15 These data indicate that, within the hypothalamus, there are site-specific reductions in insulin sensitivity during pregnancy (Figure 2).
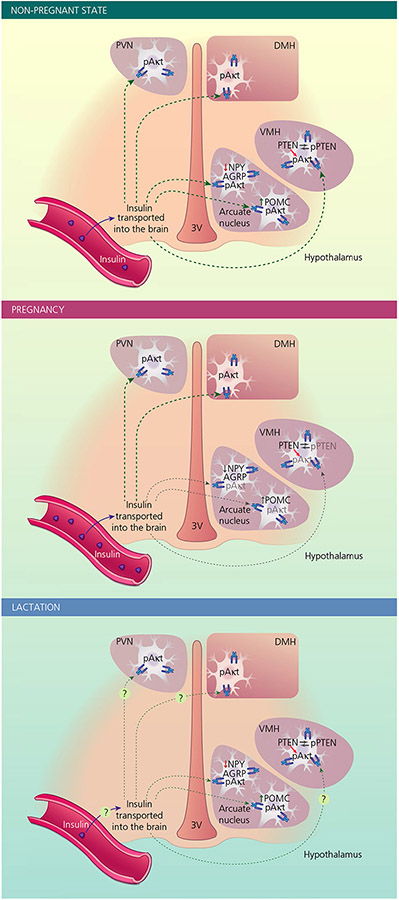
FIGURE 2.
Changes in cellular insulin signalling in various hypothalamic nuclei in the non-pregnant and pregnant state. Insulin is transported from the blood into the brain, where it acts in various regions, including hypothalamic areas such as the arcuate nucleus, paraventricular nucleus (PVN), dorsomedial nucleus (DMH) and ventromedial nucleus (VMH). Interaction of insulin with its receptor leads to phosphorylation of Akt (pAkt) as part of insulin-induced intracellular signalling cascade. In the arcuate nucleus, insulin suppresses (solid red arrow) mRNA expression of orexigenic neuropeptide Y (NPY) and agouti-related peptide (AGRP) and stimulates (solid green arrow) mRNA expression of anorectic pro-opiomelanocortin (POMC). Phosphatase and tensin homolog (PTEN) is a negative regulator of Akt and phosphorylation leads to deactivation of this function of PTEN. During pregnancy (middle) insulin transport into the brain is not reduced, whereas insulin-induced pAkt in the arcuate nucleus and VMH is attenuated. NPY, AgRP and POMC mRNA expression in the arcuate nucleus is dissociated from peripheral insulin levels. Reduced phosphorylated PTEN (pPTEN) in the VMH suggests that a reduction in the deactivation of this inhibitory signalling molecule might contribute to attenuated insulin-induced pAkt during pregnancy. During lactation (bottom), the low plasma insulin concentrations are likely to limit the effects of insulin in the brain. Exogenously administrated insulin can regulate NPY, AgRP and POMC mRNA levels, indicating that there is a restoration of insulin sensitivity in these neurones compared to pregnancy, yet the low endogenous insulin levels likely contribute to high NPY and AgRP mRNAs and low POMC mRNA observed during lactation. Whether there are changes in insulin transport into the brain or insulin responses in other neurone populations during lactation has yet to be determined. Green arrows indicate stimulatory effects of insulin, whereas red arrows indicate inhibitory effects. Grey arrows/text indicates the attenuated actions of insulin during pregnancy. 3V, third ventricle
Within the arcuate nucleus, insulin has been shown to act on key neuronal populations involved in the regulation of energy homeostasis: the anorectic pro-opiomelanocortin (POMC) neurones and orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurones. In alignment with its anorectic function, insulin can increase the expression of POMC mRNA41 and inhibit the expression of NPY mRNA and AgRP mRNA in the arcuate nucleus42-44 (Figure 2). During pregnancy, POMC mRNA is relatively constant or reduced,45-49 and NPY mRNA and AgRP mRNA are either similarly constant or increased.46,48-50 Although other factors can influence expression of these neuropeptides, these changes are opposite to those predicted based on the increases in insulin levels, hinting further that insulin sensitivity is reduced in the arcuate nucleus in particular at this time.
As yet, the mechanisms underlying the suppressed insulin-induced pAkt in the arcuate nucleus and VMH during pregnancy are unknown. IR protein levels in these two nuclei do not change during pregnancy (Figure 2), ruling out a reduction in IR expression as a cause of the attenuated insulin sensitivity.15 In animal models of obesity, arcuate IR expression is also unchanged,51,52 and central insulin resistance is instead associated with impaired insulin signalling as a result of increases in tyrosine phosphatases that dephosphorylate the IR and IRS.53 Further investigations are required to determine if similar mechanisms are involved during pregnancy. In the VMH, reduced levels of phosphorylated phosphatase and tensin homolog (PTEN) during pregnancy have been reported, potentially leading to increased activity of this negative regulator of PI3K,15 which might contribute to reduced insulin signalling (Figure 2). Interestingly, pPTEN levels were unaffected during pregnancy in the arcuate nucleus, suggesting that different mechanisms underlie changes in insulin sensitivity in these two regions that show this pregnancy-associated reduction in insulin-induced pAkt.15
1.4 ∣. Impact of pregnancy on the central functions of insulin
As introduced earlier, IR is expressed in many brain regions,27,28 particularly the hypothalamus, and insulin influences a wide range of brain functions. Region-specific “insulin resistance” in the brain of pregnant animals, as described in the previous section (Figure 2), would suggest that some of these actions may be unaffected, whereas others might be impaired. Insulin resistance in the arcuate nucleus and VMH may be adaptive to prevent insulin actions that may hinder optimal pregnancy outcomes, at the same time as allowing insulin actions that remain responsive to help meet the demands of pregnancy. Although many of the roles of insulin in the brain have not been well characterised during pregnancy, perhaps the best studied are the involvement of insulin in the central regulation of energy balance and in the autonomic control of cardiovascular function.
1.4.1 ∣. Increases in food intake during pregnancy
There is extensive evidence indicating that insulin can act in the brain, specifically the hypothalamus, to influence food intake and energy homeostasis. IR is found in key hypothalamic areas involved in energy regulation, such as the arcuate nucleus, VMH and PVN. Early investigations in baboons demonstrated that central administration of insulin can suppress food intake54 and this has subsequently been repeated in many other species, including rats55-57 and mice.58 Central infusions of insulin antibodies or IR antisense oligonucleotides to block central insulin action drive an increase in food intake and bodyweight.57,59,60 There is not complete uniformity in results with respect to investigating the anorectic effects of central insulin as some studies report no satiety effect of insulin with only small changes in protocols.61,62
Pregnancy is associated with increased food intake despite elevations in insulin levels, which is highly suggestive that the brain becomes insensitive to its anorectic actions. This has yet to be confirmed with a functional assessment of food intake following insulin administration during pregnancy. Given that the arcuate nucleus is a key site for insulin regulation of food intake and that insulin-induced intracellular signalling is impaired in this area during pregnancy,15 it would certainly be predicted that there is a lack of central insulin-induced satiety during pregnancy. One of the mechanisms by which central insulin action is thought to suppress food intake is through its ability to increase sensitivity to acute satiety factors such as the meal terminating hormone cholecystokinin (CCK).63 Pregnancy in rodents increases meal duration64 and impairs the acute satiety actions of CCK.65 It is possible that any attenuation of central insulin action may contribute to this reduction in CCK sensitivity during pregnancy, although this has yet to be investigated directly. Similar to central pregnancy-induced leptin insensitivity,66,67 it also remains unknown whether insulin insensitivity in brain circuits regulating food intake drives hyperphagia during pregnancy, or merely facilitates it by removing potential inhibition via this hormone.
1.4.2 ∣. Reductions in physical activity during pregnancy
Insulin action in the brain has also been suggested to influence levels of physical activity, although this effect can be variable depending on the physiological situation. Central insulin administration can increase locomotor activity in lean mice but diet-induced obese mice are insensitive to this effect of insulin.68 Other studies have shown that a high dose of insulin given centrally can suppress locomotor activity69 and that insulin activation of melanin-concentrating hormone (MCH) neurones in the lateral hypothalamus leads to a suppression of locomotion.70 The insulin-induced decrease in locomotion through MCH neurones is specifically activated when exogenous insulin is elevated, such as in diet-induced obesity, and may represent a neuronal population that does not develop insulin insensitivity in obesity.70 Mice with a deletion of insulin receptors from either NPY, AgRP or POMC neurones show no physical activity phenotype, suggesting these key insulin-responsive neuronal populations are unlikely to mediate any effect of insulin on locomotion.71,72 Pregnancy is associated with reductions in physical activity64,73-75 and whether the elevated insulin levels contribute to these lower levels of ambulation through activation of MCH neurones70 or through insensitivity of other neuronal populations that mediate the insulin-induced increases in physical activity68 is yet to be determined, although it poses an interesting unresolved question.
1.4.3 ∣. Pregnancy-induced changes in cardiovascular regulation
The maternal cardiovascular system undergoes major adaptations to support the developing fetus. These include a primary decrease in systemic vascular resistance, which drives, at least in part, substantial increases in heart rate (HR), cardiac output and blood volume, all of which assure adequate delivery of oxygen and nutrients to the uteroplacental unit. Pregnancy in humans also progressively increases muscle sympathetic nerve activity (SNA); in animal models (mostly rodent) increases in basal SNA to several organs have been reported.76 This sympathoexcitation helps to counteract widespread vasodilation, thereby limiting the fall in arterial pressure, and also promotes fluid retention. However, the mechanisms have yet to be fully explained. Conversely, the sensitivity of the baroreceptor reflex progressively decreases during pregnancy.76 Baroreflex control of both HR and lumbar or muscle sympathetic activity are impaired, which underlies the increased incidence of orthostatic hypotension and a reduced ability to maintain blood pressure during haemorrhage.77,78
Similar to pregnancy, insulin increases SNA to several organs, although lumbar SNA (in rats) and muscle SNA (in humans) are particularly stimulated. Insulin binds to receptors in only one brain site, the arcuate nucleus, to increase SNA79-81 through inhibition of sympathoinhibitory NPY neurones and activation of sympathoexcitatory POMC neurones that project to the hypothalamic paraventricular nucleus (PVN).82,83 Glutamatergic neurones in the PVN are stimulated, which drive vasomotor neurones in the rostral ventrolateral medulla.84 Also, in both rodents and humans, acute infusion of insulin, either central administration or i.v. administration, respectively, enhances baroreflex sensitivity.85,86
The pregnancy-induced increase in basal SNA is driven in part by the arcuate nucleus, specifically by decreasing NPY and increasing POMC inputs to the PVN, just like insulin action87; therefore, insulin was considered a plausible mediator. However, in late pregnant rats, the sympathoexcitatory response to i.c.v. insulin (or leptin) was completely abolished.17 More importantly, bilateral local blockade of arcuate IR, through nano-injections of the IR antagonist S691, failed to lower SNA,17 unlike its action in insulin-resistant obese male rats.88 Therefore, insulin does not contribute to the basal sympathoexcitation of pregnancy.17
As noted above, during pregnancy, baroreflex-induced increases in SNA in response to hypotension are impaired. This change may be partially a result of the marked elevations in basal SNA, closer to the highest SNA levels reached when blood pressure is low, and also because of greater inhibition of vasomotor neurones in the rostral ventrolateral medulla.76 Because insulin does not contribute to basal sympathoexcitation, as a result of the development of stark central insulin resistance, insulin also cannot explain the increase in basal SNA closer to the baroreflex maximum. The cardiac or HR baroreflex is also impaired; HR baroreflex sensitivity or gain is reduced and, similar to SNA, maximal HR levels in response to hypotension are decreased during pregnancy. These changes are a result of suppressed responsiveness of both the sympathetic and parasympathetic control of HR.75
It was hypothesised that whole-body insulin resistance may contribute to the mechanisms underlying arterial cardiac baroreflex dysfunction during pregnancy.89 In support of this concept, other conditions show similar links between whole-body insulin resistance and baroreflex dysfunction.90 In pregnant rabbits, reductions in whole-body insulin sensitivity were correlated with reductions in baroreflex gain, demonstrating a similar time course of progression.16 Similarly, in rats, both baroreflex sensitivity and whole-body insulin sensitivity are reduced at a similar time in mid- and late pregnancy, with a transient resumption of near-normal baroreflex and insulin sensitivities between these time points.25,89 Although it was initially hypothesised that peripheral insulin resistance might reduce access of insulin to the brain,16 as outlined above, recent work showing that pregnancy does not impair insulin transport into the brain or reduce brain insulin levels17 does not support this initial hypothesis. Nevertheless, i.c.v. insulin infusion in conscious38 and urethane-anaesthetised17 rats did improve cardiac baroreflex gain or sensitivity without normalising the decrease in maximal HR achieved with hypotension. Thus, it appears that this depressed feature of baroreflex function (gain) retains some sensitivity to insulin, whereas the blunted maximal reflex tachycardia could be secondary to the central insulin resistance that develops. In support, i.c.v. infusion of the artificial CSF vehicle decreased the HR baroreflex maximum in conscious virgin but not pregnant rats, suggesting a factor in brain that normally supports baroreflex function in virgin (but not pregnant) rats was diluted by the infusion. As previously discussed,38 this factor could be insulin (levels or responsiveness). Alternatively, a factor that contributes to peripheral insulin resistance could in parallel decrease hypothalamic insulin sensitivity, thereby causing the marked decreases in HR baroreflex function that occur during pregnancy. Thus, the links between the reduced (peripheral and central) insulin sensitivity and the substantial impairment of cardiac baroreflex function during pregnancy remain unresolved and require further study.
1.4.4 ∣. Hepatic glucose production during pregnancy
Hepatic glucose production plays an important role in maintenance of blood glucose levels (particularly when food is scarce) and is readily responsive to changes in energy balance. Insulin directly suppresses hepatic glucose production. Central insulin action can also regulate blood glucose levels,91 particularly through the control of hepatic glucose production.91-94 Arcuate nucleus AgRP neurones are a key site of action, since mice with a specific deletion of insulin receptors from arcuate AgRP neurones show increased hepatic glucose production.42 Intranasal insulin administration in humans can also suppress glucose production independently of changes of peripheral insulin levels, indicating that preclinical animal studies provide a valuable model for central insulin's ability to inhibit hepatic glucose production.95
Hepatic glucose production is increased in later stages of pregnancy in humans 96-98 and rats.14,99 As described above, the central actions of insulin on hepatic glucose output appear to be mediated by AgRP neurones. Because insulin-induced pAkt is reduced in the arcuate nucleus during pregnancy,15 reduced insulin sensitivity of AgRP neurones may underlie the increase in glucose production. However, this has yet to be directly examined. Hepatic glucose production is complex and there are multiple redundancies in the control of this process94 that could allow for other mechanisms to play a more critical role in driving increases in hepatic glucose production during pregnancy, such as decreased liver sensitivity to insulin.14 Nevertheless, attenuated central insulin control of hepatic glucose production during pregnancy is an intriguing hypothesis that warrants investigation.
1.4.5 ∣. Cognition
Cognition, particularly memory and learning, can be affected by pregnancy. The anecdotal concept of “pregnancy brain” or “mommy brain” is certainly widely accepted and a recent meta-analysis of studies focusing on objective measures of cognition found a significant decline in cognitive function in pregnant women compared to non-pregnant women, particularly in the third trimester.100 Memory recall was the main function that was negatively impacted.100-102 In self-reported studies, a wider range of cognitive issues were impaired during pregnancy, including an inability to concentrate or maintain attention, increased confusion and a lack of coordination.103-105
Insulin influences cognition, with acute increases in insulin proving to be beneficial in both human and animal studies, in particular, by improving memory formation.106 This action likely is initiated in the hippocampus, where insulin influences signalling cascades that underlie hippocampal plasticity, learning and memory.107-109 Furthermore, long-term studies using intranasal insulin administration in humans have shown improved memory without any negative effects on glycaemia.110
Insulin resistance in the brain has been linked as a contributing factor to cognitive decline in Alzheimer's disease, ageing, obesity and type 2 diabetes.111 Given that a decline in cognitive abilities can be associated with states of systemic insulin resistance when insulin levels are typically elevated, it is suggested that central insulin resistance may also develop. Insulin resistance in the hippocampus could lead to reductions in insulin's effects on cognition despite elevated insulin levels.111 Directly increasing brain insulin levels can overcome this reduction in central insulin sensitivity because models of impaired cognition and insulin resistance can show improvements in cognition with chronic intranasal administration of insulin.112,113 This suggests that the mechanism underlying this central insulin insensitivity in these cases is more likely to involve access and the availability of insulin in the brain rather than impaired intracellular activation. Given that insulin transport into the brain is unaffected by pregnancy,17 whether a mechanism similar to this develops in pregnancy appears unlikely, although future work will be needed to confirm this. It is tempting to speculate that decreased insulin sensitivity in the hippocampus may contribute to any transient deterioration in cognitive function experienced during pregnancy. This is an unexplored area and perhaps initial work should focus on whether there is reduced insulin sensitivity in the hippocampus similar to the arcuate nucleus and VMH in the hypothalamus during pregnancy.
Of note, the results from animal studies are not indicative of declined cognitive function during pregnancy,114 raising the question of whether pregnancy may represent more of a shift in focus of aspects of cognition with respect to optimising tasks important for nurturing offspring that are not adequately tested for in current measures of cognitive function.115 Such an adaptive view of “mommy brain” as opposed to the maladaptive perception that is commonly considered in the popular media, is more consistent with the evolutionary perspective that hormone-induced adaptations in the maternal brain are predominantly beneficial to ensure a healthy pregnancy.116
1.4.6 ∣. Other actions of insulin in brain
Central insulin action has been implicated in many other functions, including increases in body temperature via actions in the preoptic area of the hypothalamus117,118 and changes in olfaction via action in the olfactory bulb.119,120 However, as with most of the specific actions of central insulin discussed above, there is a lack of studies directly assessing these effects in pregnancy. Thus, although the growing list of central actions of insulin currently provides many opportunities for speculation about how this might be impacted by pregnancy, it also means that there are many opportunities for future investigation.
2 ∣. LACTATION
2.1 ∣. Glucose homeostasis during lactation
Lactation is an enormous metabolic investment for the mother, and in species with multiple young, such as rodents, the energy demands of milk production are extreme. Lactating rats and mice consume two to three times their virgin level of food121,122 to meet these energy demands. Glucose is a key requirement for milk production, especially for the production of lactose in milk. As a result, blood glucose levels are on the lower side of normal as a result of avid sequestration by the mammary gland and, in parallel, insulin levels also decrease. Lower blood glucose levels presented to the pancreas, rather than changes in beta-cell function, are thought to mediate reduced insulin secretion during lactation.123,124 Despite the relative hypoinsulinaemia,125,126 selective resistance is maintained in some maternal tissues, such as fat and muscle, to direct glucose to the mammary gland.127,128 Over the years, the hypoinsulinaemia of lactation has been hypothesised to contribute to a number of functional changes in brain, in particular hyperphagia and infertility.
2.2 ∣. Impact of lactation on the central functions of insulin
2.2.1 ∣. Hyperphagia during lactation
Although the energetic drain of lactation in terms of milk production might appear to be a sufficient driving factor for hyperphagia, the suckling stimulus and associated hormonal changes alone can drive higher levels of food intake compared to virgin levels.122 Along with increased food intake, there is increased NPY mRNA and AgRP mRNA in the arcuate nucleus during lactation.129-131 Furthermore, during lactation, NPY mRNA expression greatly increases in the DMH, contributing to the hyperphagia of this state.132,133 It is possible that the low levels of insulin during lactation facilitate these increases in orexigenic neuropeptide mRNA and thus contribute to the hyperphagia of lactation by removing insulin inhibition on these neurones. However, chronic infusion of insulin during lactation in rats to increase insulin levels to control virgin levels has no effect on food intake,126 suggesting a level of insulin resistance. Interestingly, these infusions did reduce the high NPY mRNA levels seen in lactation down to virgin levels, and slightly but significantly reduced the lactation-induced increase in AgRP mRNA.126 The low levels of POMC mRNA during lactation were also elevated after this insulin treatment.126 First, these data suggest that the arcuate nucleus remains somewhat sensitive to changes in insulin during lactation, as reflected by the ability of insulin to regulate NPY, AgRP and POMC mRNAs. Second, the data suggest that, at this stage of lactation, these arcuate nucleus orexigenic peptides are not the major factors inducing the hyperphagia of lactation.
The ability of insulin to regulate NPY, AgRP and POMC mRNAs126 would suggest that insulin signalling, or at least insulin-induced pAkt, in the arcuate nucleus is restored at some point between mid-pregnancy and mid-lactation, although this has yet to be specifically investigated. Furthermore, whether insulin sensitivity is restored during lactation in the VMH, an area where attenuated insulin-induced pAkt signalling is associated with pregnancy,15 remains unknown. Further investigations are required to determine when this transition in central insulin sensitivity between pregnancy and lactation takes place, and whether there are differences as to if or when this process occurs in various different insulin-responsive regions.
2.2.2 ∣. Lactational infertility
Lactation is a state of suppressed reproductive function, characterised by a suppression of pulsatile luteinising hormone (LH) secretion, which is a key part of the reproductive axis driving follicle development and ovulation. There is a long history of work linking metabolic fuel availability with reproductive ability134 and hence one hypothesis for the suppression of LH pulses during lactational infertility is that this state of negative energy balance, and the hormonal milieu that goes along with this, contribute to this suppression. Increases in insulin have been associated with resumption of LH pulses following food restriction,135 and increasing insulin levels can lead to increased LH secretion.136 Furthermore, female mice lacking IR in the brain demonstrate lower LH levels,137 although this should be interpreted with caution because one-off LH measurements are not a reliable substitution for serial measurements of pulsatile LH secretion. In terms of lactation, however, the evidence would suggest it is unlikely that low insulin levels act as a metabolic signal to the brain that suppresses fertility at this time. Studies utilising continuous infusion of insulin during lactation to increase insulin to virgin levels have not led to restoration of LH secretion,126,138 indicating that low central actions of insulin alone do not drive suppression of LH as part of lactational infertility.
2.2.3 ∣. Functional changes in magnocellular neurones during lactation
Magnocellular neurones (MCNs) in the supraoptic nucleus of the hypothalamus have been proposed to act as metabolic sensors by utilising insulin-dependent mechanisms for glucose uptake, similar to VMH glucose-sensing neurones. More specifically, glucose depolarises the membrane through glucose kinase (GK)-mediated inaction of ATP-sensitive potassium channels, which in turn stimulates oxytocin (OT) and vasopressin (VP) secretion from the posterior pituitary.139 During lactation, blocking GK activity in these neurones does not prevent the insulin and glucose-stimulated release of OT and VP as it does in males (nonlactating females were not included as a control), indicating perhaps a change in intracellular signal transduction by which these neurones respond to insulin and glucose with release of OT and VP.140 The exact mechanism has not been defined, although it may be related to a potential change in how these neurones take up and use glucose during lactation, switching from low-affinity GK, which is used as a metabolic sensor, to higher affinity glycolytic hexokinases to provide increased levels of fuel for cellular activity.140 Thus, during lactation, MCN neurones maximise their glucose uptake and metabolism to meet the demands of increased OT production required for milk letdown, at the expense of their ability to act as a glucose monitor. In parallel, astrocytes withdraw support for MCN, allowing for more synaptic connections between MCNs141,142 and more efficient and synchronised activity in OT release.143 Thus, it is possible that, during lactation, MCNs move from lactate for cellular energy to other fuel sources such as insulin-mediated glucose uptake.140 Although much of the details of this proposed change in metabolic process of MCN during lactation require additional work for confirmation, this does provide an example of how one specific neuronal population may undergo a change in insulin-mediated function during lactation.
3 ∣. BEYOND PREGNANCY AND LACTATION
The process of pregnancy and lactation represent a major biological challenge to the female body and, as such, is associated with a wide range of physiological adaptations to meet this challenge. In particular, becoming a mother leads to many alterations in the brain that can have both short- and long-term impacts.144 Whether the decreases in central insulin sensitivity that develop during pregnancy,145 despite some restoration in lactation,126 have any lasting impact on insulin function in the maternal brain has yet to be investigated; however, there are some intriguing possibilities.
Recent work has highlighted that undergoing pregnancy and lactation (termed ‘reproductive experience’) leads to long-term biological changes in body weight regulation, in particular a reduction in locomotion activity.145,146 Could a long-term change in insulin sensitivity in the lateral hypothalamus contribute to this lower locomotion activity? As described above, the exact role of insulin with respect to promoting or suppressing locomotion in different physiological states is not completely understood, although it begs the question of what this effect may be in reproductively experienced female.
Evidence for long-term cognitive changes as a result of parity in humans is somewhat equivocal; however, in animal models, it has been demonstrated that reproductive experience leads to enhanced cognitive function, particularly with memory and learning (reviewed in 144). Alongside this, reproductive experience in animal models induces positive neuroplasticity, such as increases in synaptic proteins,147,148 a higher level of hippocampal neurogenesis149-151 and a general slowing down of age-dependent decline in neurogenesis.144 Insulin has been shown to promote neuroplasticity both in development and in the adult, contributing to a healthy brain.152 Future investigations examining long-term changes in insulin sensitivity in the hippocampus after pregnancy and lactation may reveal a contributing role for insulin in these changes in neuroplasticity and cognition after reproductive experience.
4 ∣. PERSPECTIVES/CONCLUSIONS
Pregnancy and lactation are times of major adaptation in the regulation of glucose homeostasis as the maternal body adjusts to meet the metabolically demanding challenge of fetal growth and development, as well as subsequent milk production for offspring following birth. Peripheral insulin resistance develops during pregnancy, and recent studies indicate that central insulin resistance is also present, leading to reduced insulin actions in the brain. This central insulin resistance is site-specific and may reflect adaptive changes in the brain that decrease some of insulin's central effects at the same time as maintaining other functions that support a healthy pregnancy.
Yet, our understanding of these homeostatic mechanisms is limited. Much more information is required, especially given the importance of glucose and insulin levels and their actions on fetal development, as well as maternal health. We offer the following as key unanswered questions that deserve particular attention. First, is pregnancy-induced central insulin resistance widespread and what are the mechanisms? Here, we might gain clues to the cellular mechanisms based on what is known about peripheral insulin resistance and/or central insulin resistance associated with obesity, although it is also possible these cellular mechanisms may be unique to pregnancy. Furthermore, the factors driving these changes in central insulin sensitivity needs investigation. We know that prolactin and placental lactogens play a key role in changes in insulin secretion during pregnancy 8 and hence may also be involved in preventing excessive insulin function in the brain at this time. Indeed, how the hormonal milieu of pregnancy influences central insulin sensitivity will be a key area of investigation in the future. Second, does lactation produce central insulin resistance, and if so, which brain sites and functions are affected? Does lactation alter transport of insulin into the brain or brain insulin levels? Of particular interest will be the investigation of how the transition from pregnancy to lactation impacts on central insulin sensitivity and/or other aspects of central insulin function that may differ between these two states. Finally, we ask, does the same pattern of site-specific central insulin resistance develop in gestational diabetes as in unaffected pregnancies, or perhaps pregnancy-induced central insulin resistance is intensified with this condition as it is in the periphery. Addressing these key questions in the future will expand our knowledge of how the maternal body adapts to these unique reproductive states and allow us to better understand how pregnancy complications may impact on the health and well-being of both mother and baby.
ACKNOWLEDGEMENTS
We thank scientific illustrator Alison Schroeer for her contribution with respect to generating the illustrations used in the figures.
REFERENCES
- 1.Catalano P The diabetogenic state of maternal metabolism in pregnancy. NeoReviews. 2002;3:e165. [Google Scholar]
- 2.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. [DOI] [PubMed] [Google Scholar]
- 3.Nolan CJ, Proietto J. The feto-placental glucose steal phenomenon is a major cause of maternal metabolic adaptation during late pregnancy in the rat. Diabetologia. 1994;37:976–984. [DOI] [PubMed] [Google Scholar]
- 4.Ramos MP, Crespo-Solans MD, del Campo S, Cacho J, Herrera E. Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am J Physiol Endocrinol Metab. 2003;285:E318–E328. [DOI] [PubMed] [Google Scholar]
- 5.Hornnes PJ. On the decrease of glucose tolerance in pregnancy. A review. Diabete Metab 1985;11:310–315. [PubMed] [Google Scholar]
- 6.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 1256S;71(5 Suppl):1256S–S1261. [DOI] [PubMed] [Google Scholar]
- 7.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29:301–307. [DOI] [PubMed] [Google Scholar]
- 8.Baeyens L, Hindi S, Sorenson RL, German MS. beta-Cell adaptation in pregnancy. Diabetes Obes Metab. 2016;18(Suppl 1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee RR, Cyphert HA, Walker EM, et al. Gestational Diabetes Mellitus From Inactivation of Prolactin Receptor and MafB in Islet beta-Cells. Diabetes. 2016;65:2331–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano PM, Drago NM, Amini SB. Longitudinal changes in pancreatic beta-cell function and metabolic clearance rate of insulin in pregnant women with normal and abnormal glucose tolerance. Diabetes Care. 1998;21:403–408. [DOI] [PubMed] [Google Scholar]
- 11.Perichart-Perera O, Munoz-Manrique C, Reyes-Lopez A,Tolentino-Dolores M, Espino YSS, Ramirez-Gonzalez MC. Metabolic markers during pregnancy and their association with maternal and newborn weight status. PLoS One. 2017;12:e0180874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonagra AD, Biradar SM, Dattareya K, Murthy DSJ. Normal pregnancy- a state of insulin resistance. J Clin Diagn Res. 2014;8:CC01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladyman SR, Grattan DR. Central effects of leptin on glucose homeostasis are modified during pregnancy in the rat. J Neuroendocrinol. 2016;28:e12431. [DOI] [PubMed] [Google Scholar]
- 14.Leturque A, Burnol AF, Ferre P, Girard J. Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am J Physiol. 1984;246:E25–31. [DOI] [PubMed] [Google Scholar]
- 15.Ladyman SR, Grattan DR. Region-Specific Suppression of Hypothalamic Responses to Insulin To Adapt to Elevated Maternal Insulin Secretion During Pregnancy. Endocrinology. 2017;158:4257–4269. [DOI] [PubMed] [Google Scholar]
- 16.Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2188–R2195. [DOI] [PubMed] [Google Scholar]
- 17.Shi Z, Hansen KM, Bullock KM, Morofuji Y, Banks WA, Brooks VL. Resistance to the sympathoexcitatory effects of insulin and leptin in late pregnant rats. J Physiol. 2019;597:4087–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen O, Vaaler S, Jorde R, Reikeras O. Increased plasma glucose levels after Hypnorm anaesthesia, but not after Pentobarbital anaesthesia in rats. Lab Anim. 1994;28:244–248. [DOI] [PubMed] [Google Scholar]
- 19.Windelov JA, Pedersen J, Holst JJ. Use of anesthesia dramatically alters the oral glucose tolerance and insulin secretion in C57Bl/6 mice. Physiol Rep. 2016;4:e12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuurbier CJ, Keijzers PJ, Koeman A, Van Wezel HB, Hollmann MW. Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesth Analg. 2008:106:135–142. [DOI] [PubMed] [Google Scholar]
- 21.Khant Aung Z, Grattan DR, Ladyman SR. Pregnancy-induced adaptation of central sensitivity to leptin and insulin. Mol Cell Endocrinol. 2020;516:110933. [DOI] [PubMed] [Google Scholar]
- 22.Menchetti L, Brecchia G, Canali C, et al. Food restriction during pregnancy in rabbits: effects on hormones and metabolites involved in energy homeostasis and metabolic programming. Res Vet Sci. 2015;98:7–12. [DOI] [PubMed] [Google Scholar]
- 23.Phillipps HR, Ladyman SR, Grattan DR. Maintained expression of genes associated with metabolism in the ventromedial hypothalamic nucleus despite development of leptin resistance during pregnancy in the rat. Physiol Rep. 2013;1:e00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiyama C, Yamamoto M, Kotani T, Kikkawa F, Murata Y, Hayashi Y. Fertility and pregnancy-associated ss-cell proliferation in mice deficient in proglucagon-derived peptides. PLoS One. 2012;7:e43745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz C, Lopez-Luna P, Herrera E. Glucose and insulin tolerance tests in the rat on different days of gestation. Biol Neonate. 1995;68:282–291. [DOI] [PubMed] [Google Scholar]
- 26.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. [DOI] [PubMed] [Google Scholar]
- 27.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. [DOI] [PubMed] [Google Scholar]
- 28.Marks JL, Porte D Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. [DOI] [PubMed] [Google Scholar]
- 29.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray SM, Barrett EJ. Insulin transport into the brain. Am J Physiol Cell Physiol. 2018;315:C125–C136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhea EM, Banks WA. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front Neurosci. 2019;13:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. [DOI] [PubMed] [Google Scholar]
- 33.Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. [DOI] [PubMed] [Google Scholar]
- 35.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Teter B, Morihara T, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci. 2004;24:11120–11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: relevance to Alzheimer's prevention. Neurobiol Aging. 2011;32:1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azar AS, Brooks VL. Impaired baroreflex gain during pregnancy in conscious rats: role of brain insulin. Hypertension. 2011;57:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48:1807–1814. [DOI] [PubMed] [Google Scholar]
- 41.Benoit SC, Air EL, Coolen LM, et al. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konner AC, Janoschek R, Plum L, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz MW, Sipols AJ, Marks JL, et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. [DOI] [PubMed] [Google Scholar]
- 44.Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44:147–151. [DOI] [PubMed] [Google Scholar]
- 45.Douglas AJ, Bicknell RJ, Leng G, Russell JA, Meddle SL. Beta-endorphin cells in the arcuate nucleus: projections to the supraoptic nucleus and changes in expression during pregnancy and parturition. J Neuroendocrinol. 2002;14:768–777. [DOI] [PubMed] [Google Scholar]
- 46.Ladyman SR, Tups A, Augustine RA, Swahn-Azavedo A, Kokay IC, Grattan DR Loss of hypothalamic response to leptin during pregnancy associated with development of melanocortin resistance. J Neuroendocrinol. 2009;21:449–456. [DOI] [PubMed] [Google Scholar]
- 47.Mann PE, Rubin BS, Bridges RS. Differential proopiomelanocortin gene expression in the medial basal hypothalamus of rats during pregnancy and lactation. Brain Res Mol Brain Res. 1997;46:9–16. [DOI] [PubMed] [Google Scholar]
- 48.Pazos P, Lima L, Casanueva FF, Dieguez C, Garcia MC. Interleukin 6 deficiency modulates the hypothalamic expression of energy balance regulating peptides during pregnancy in mice. PLoS One. 2013;8:e72339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocha M, Bing C, Williams G, Puerta M. Pregnancy-induced hyperphagia is associated with increased gene expression of hypothalamic agouti-related peptide in rats. Regul Pept. 2003;114:159–165. [DOI] [PubMed] [Google Scholar]
- 50.Garcia MC, Lopez M, Gualillo O, Seoane LM, Dieguez C, Senaris RM. Hypothalamic levels of NPY, MCH, and prepro-orexin mRNA during pregnancy and lactation in the rat: role of prolactin. FASEB J. 2003;17:1392–1400. [DOI] [PubMed] [Google Scholar]
- 51.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288:R981–R986. [DOI] [PubMed] [Google Scholar]
- 52.Shi Z, Cassaglia PA, Pelletier NE, Brooks VL. Sex differences in the sympathoexcitatory response to insulin in obese rats: role of neuropeptide Y. J Physiol. 2019;597:1757–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ono H Molecular mechanisms of hypothalamic insulin resistance. Int J Mol Sci. 2019;20:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. [DOI] [PubMed] [Google Scholar]
- 55.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav. 2002;72:423–429. [DOI] [PubMed] [Google Scholar]
- 56.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. [DOI] [PubMed] [Google Scholar]
- 57.McGowan MK, Andrews KM, Grossman SP. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol Behav. 1992;51:753–766. [DOI] [PubMed] [Google Scholar]
- 58.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. [DOI] [PubMed] [Google Scholar]
- 59.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. [DOI] [PubMed] [Google Scholar]
- 60.Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav. 1977;19:309–313. [DOI] [PubMed] [Google Scholar]
- 61.Jessen L, Clegg DJ, Bouman SD. Evaluation of the lack of anorectic effect of intracerebroventricular insulin in rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R43–50. [DOI] [PubMed] [Google Scholar]
- 62.Me Allister E, Pacheco-Lopez G, Woods SC, Langhans W. Inconsistencies in the hypophagic action of intracerebroventricular insulin in mice. Physiol Behav. 2015;151:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiol Behav. 1995;58:755–760. [DOI] [PubMed] [Google Scholar]
- 64.Ladyman SR, Carter KM, Grattan DR. Energy homeostasis and running wheel activity during pregnancy in the mouse. Physiol Behav. 2018;194:83–94. [DOI] [PubMed] [Google Scholar]
- 65.Ladyman SR, Sapsford TJ, Grattan DR. Loss of acute satiety response to cholecystokinin in pregnant rats. J Neuroendocrinol. 2011;23:1091–1098. [DOI] [PubMed] [Google Scholar]
- 66.Ladyman SR, Fieldwick DM, Grattan DR. Suppression of leptin-induced hypothalamic JAK/STAT signalling and feeding response during pregnancy in the mouse. Reproduction. 2012;144:83–90. [DOI] [PubMed] [Google Scholar]
- 67.Ladyman SR, Grattan DR. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology. 2004;145:3704–3711. [DOI] [PubMed] [Google Scholar]
- 68.Hennige AM, Sartorius T, Lutz SZ, et al. Insulin-mediated cortical activity in the slow frequency range is diminished in obese mice and promotes physical inactivity. Diabetologia. 2009;52:2416–2424. [DOI] [PubMed] [Google Scholar]
- 69.Sartorius T, Hennige AM, Fritsche A, Haring HU. Sustained Treatment with Insulin Detemir in Mice Alters Brain Activity and Locomotion. PLoS One. 2016;11:e0162124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hausen AC, Ruud J, Jiang H, et al. Insulin-dependent activation of MCH neurons impairs locomotor activity and insulin sensitivity in obesity. Cell Rep. 2016;17:2512–2521. [DOI] [PubMed] [Google Scholar]
- 71.Loh K, Zhang L, Brandon A, et al. Insulin controls food intake and energy balance via NPY neurons. Mol Metab. 2017;6:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin AC, Filatova N, Lindtner C, et al. Insulin Receptor Signaling in POMC, but Not AgRP, Neurons Controls Adipose Tissue Insulin Action. Diabetes. 2017;66:1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amezcua-Prieto C, Olmedo-Requena R, Jimenez-Mejias E, Mozas-Moreno J, Lardelli-Claret P, Jimenez-Moleon JJ. Factors associated with changes in leisure time physical activity during early pregnancy. Int J Gynaecol Obstet. 2013;121:127–131. [DOI] [PubMed] [Google Scholar]
- 74.Gaston A, Vamos CA. Leisure-time physical activity patterns and correlates among pregnant women in Ontario, Canada. Matern Child Health J. 2013;17:477–484. [DOI] [PubMed] [Google Scholar]
- 75.Slonaker JR. The effect of copulation, pregnancy, pseudopregnancy and lactation on the voluntary activity and food consumption of the albino rat. Am J Physiol. 1925;71:362–394. [Google Scholar]
- 76.Brooks VL, Fu Q, Shi Z, Heesch CM. Adaptations in autonomic nervous system regulation in normal and hypertensive pregnancy. Handb Clin Neurol. 2020;171:57–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clow KA, Giraud GD, Ogden BE, Brooks VL. Pregnancy alters hemodynamic responses to hemorrhage in conscious rabbits. Am J Physiol Heart Circ Physiol. 2003;284:H1110–H1118. [DOI] [PubMed] [Google Scholar]
- 78.Easterling TR, Schmucker BC, Benedetti TJ. The hemodynamic effects of orthostatic stress during pregnancy. Obstet Gynecol. 1988;72:550–552. [PubMed] [Google Scholar]
- 79.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol. 2013;304:H1538–H1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muntzel MS, Morgan DA, Mark AL, Johnson AK. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol. 1994;267:R1350–R1355. [DOI] [PubMed] [Google Scholar]
- 82.Cassaglia PA, Shi Z, Brooks VL. Insulin increases sympathetic nerve activity in part by suppression of tonic inhibitory neuropeptide Y inputs into the paraventricular nucleus in female rats. Am J Physiol Regul Integr Comp Physiol. 2016;311:R97–R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010;55:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. [DOI] [PubMed] [Google Scholar]
- 86.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol. 2010;588:3593–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi Z, Cassaglia PA, Gotthardt LC, Brooks VL. Hypothalamic Paraventricular and Arcuate Nuclei Contribute to Elevated Sympathetic Nerve Activity in Pregnant Rats: Roles of Neuropeptide Y and alpha-Melanocyte-Stimulating Hormone. Hypertension. 2015;66:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Z, Zhao D, Cassaglia PA, Brooks VL. Sites and sources of sympathoexcitation in obese male rats: role of brain insulin. Am J Physiol Regul Integr Comp Physiol. 2020;318:R634–R648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2010;299:R439–R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grassi G, Seravalle G, Colombo M, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–2042. [DOI] [PubMed] [Google Scholar]
- 91.Tups A, Benzler J, Sergi D, Ladyman SR, Williams LM. Central Regulation of Glucose Homeostasis. Compr Physiol. 2017;7:741–764. [DOI] [PubMed] [Google Scholar]
- 92.Inoue H Central insulin-mediated regulation of hepatic glucose production [Review]. Endocr J. 2016;63:1–7. [DOI] [PubMed] [Google Scholar]
- 93.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. [DOI] [PubMed] [Google Scholar]
- 94.Rojas JM, Schwartz MW. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab. 2014;16(Suppl 1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes. 2015;64:766–774. [DOI] [PubMed] [Google Scholar]
- 96.Assel B, Rossi K, Kalhan S. Glucose metabolism during fasting through human pregnancy: comparison of tracer method with respiratory calorimetry. Am J Physiol. 1993;265:E351–E356. [DOI] [PubMed] [Google Scholar]
- 97.Catalano PM, Tyzbir ED, Wolfe RR, Roman NM, Amini SB, Sims EA. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am J Obstet Gynecol. 1992;167:913–919. [DOI] [PubMed] [Google Scholar]
- 98.Kalhan SC, D'Angelo LJ, Savin SM, Adam PA. Glucose production in pregnant women at term gestation. Sources of glucose for human fetus. J Clin Invest. 1979;63:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baumann G, Puavilai G, Freinkel N, Domont LA, Metzger BE, Levene HB. Hepatic insulin and glucagon receptors in pregnancy: their role in the enhanced catabolism during fasting. Endocrinology. 1981;108:1979–1986. [DOI] [PubMed] [Google Scholar]
- 100.Davies SJ, Lum JA, Skouteris H, Byrne LK, Hayden MJ. Cognitive impairment during pregnancy: a meta-analysis.Med J Aust. 2018;208:35–40. [DOI] [PubMed] [Google Scholar]
- 101.Glynn LM. Giving birth to a new brain: hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010;35:1148–1155. [DOI] [PubMed] [Google Scholar]
- 102.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol. 2007;29:793–803. [DOI] [PubMed] [Google Scholar]
- 103.Brett M, Baxendale S. Motherhood and memory: a review. Psychoneuroendocrinology. 2001;26:339–362. [DOI] [PubMed] [Google Scholar]
- 104.Casey P, Huntsdale C, Angus G, Janes C. Memory in pregnancy. II: Implicit, incidental, explicit, semantic, short-term, working and prospective memory in primigravid, multigravid and postpartum women. J Psychosom Obstet Gynaecol. 1999;20:158–164. [DOI] [PubMed] [Google Scholar]
- 105.Parsons C, Redman S. Self-reported cognitive change during pregnancy. Aust J Adv Nurs. 1991;9:20–29. [PubMed] [Google Scholar]
- 106.Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. 2012;14:214–221. [DOI] [PubMed] [Google Scholar]
- 107.Mainardi M, Fusco S, Grassi C. Modulation of hippocampal neural plasticity by glucose-related signaling. Neural Plast. 2015;2015:657928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spinelli M, Fusco S, Grassi C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front Neurosci. 2019;13:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spinelli M, Fusco S, Mainardi M, et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat Commun. 2017;8:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. [DOI] [PubMed] [Google Scholar]
- 111.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev. 2016;96:1169–1209. [DOI] [PubMed] [Google Scholar]
- 112.Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19:758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lv H, Tang L, Guo C, et al. Intranasal insulin administration may be highly effective in improving cognitive function in mice with cognitive dysfunction by reversing brain insulin resistance. Cogn Neurodyn. 2020;14:323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Macbeth AH, Gautreaux C, Luine VN. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain Res. 2008;1241:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–467. [DOI] [PubMed] [Google Scholar]
- 116.Grattan DR, Ladyman SR. Neurophysiological and cognitive changes in pregnancy. Handb Clin Neurol. 2020;171:25–55. [DOI] [PubMed] [Google Scholar]
- 117.Benedict C, Brede S, Schioth HB, et al. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes. 2011;60:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanchez-Alavez M, Osborn O, Tabarean IV, et al. Insulin-like growth factor 1-mediated hyperthermia involves anterior hypothalamic insulin receptors. J Biol Chem. 2011;286:14983–14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aime P, Hegoburu C, Jaillard T, et al. A physiological increase of insulin in the olfactory bulb decreases detection of a learned aversive odor and abolishes food odor-induced sniffing behavior in rats. PLoS One. 2012;7:e51227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brunner YF, Benedict C, Freiherr J. Intranasal insulin reduces olfactory sensitivity in normosmic humans. J Clin Endocrinol Metab. 2013;98:E1626–E1630. [DOI] [PubMed] [Google Scholar]
- 121.Speakman JR. The physiological costs of reproduction in small mammals. Philos Trans R Soc Lond B Biol Sci. 2008;363:375–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woodside B Prolactin and the hyperphagia of lactation. Physiol Behav. 2007;91:375–382. [DOI] [PubMed] [Google Scholar]
- 123.Jones RG, Ilic V, Williamson DH. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochem J. 1984;220:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Madon RJ, Ensor DM, Flint DJ. Hypoinsulinaemia in the lactating rat is caused by a decreased glycaemic stimulus to the pancreas. J Endocrinol. 1990;125:81–88. [DOI] [PubMed] [Google Scholar]
- 125.Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69:299–307. [DOI] [PubMed] [Google Scholar]
- 126.Xu J, Kirigiti MA, Grove KL, Smith MS. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology. 2009;150:4231–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vernon RG. Lipid metabolism during lactation: a review of adipose tissue-liver interactions and the development of fatty liver. J Dairy Res. 2005;72:460–469. [DOI] [PubMed] [Google Scholar]
- 128.Vernon RG, Pond CM. Adaptations of maternal adipose tissue to lactation. J Mammary Gland Biol Neoplasia. 1997;2:231–241. [DOI] [PubMed] [Google Scholar]
- 129.Chen P, Li C, Haskell-Luevano C, Cone RD, Smith MS. Altered expression of agouti-related protein and its colocalization with neuropeptide Y in the arcuate nucleus of the hypothalamus during lactation. Endocrinology. 1999;140:2645–2650. [DOI] [PubMed] [Google Scholar]
- 130.Pickavance L, Dryden S, Hopkins D, et al. Relationships between hypothalamic neuropeptide Y and food intake in the lactating rat. Biochem Soc Trans. 1996;24:236S. [DOI] [PubMed] [Google Scholar]
- 131.Smith MS. Lactation alters neuropeptide-Y and proopiomelanocortin gene expression in the arcuate nucleus of the rat. Endocrinology. 1993;133:1258–1265. [DOI] [PubMed] [Google Scholar]
- 132.Chen P, Smith MS. Regulation of hypothalamic neuropeptide Y messenger ribonucleic acid expression during lactation: role of prolactin. Endocrinology. 2004;145:823–829. [DOI] [PubMed] [Google Scholar]
- 133.Li C, Chen P, Smith MS. Neuropeptide Y (NPY) neurons in the arcuate nucleus (ARH) and dorsomedial nucleus (DMH), areas activated during lactation, project to the paraventricular nucleus of the hypothalamus (PVH). Regul Pept. 1998;75–76:93–100. [DOI] [PubMed] [Google Scholar]
- 134.Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16:235–272. [DOI] [PubMed] [Google Scholar]
- 135.Szymanski LA, Schneider JE, Friedman MI, et al. Changes in insulin, glucose and ketone bodies, but not leptin or body fat content precede restoration of luteinising hormone secretion in ewes. J Neuroendocrinol. 2007;19:449–460. [DOI] [PubMed] [Google Scholar]
- 136.Burcelin R, Thorens B, Glauser M, Gaillard RC, Pralong FP. Gonadotropin-releasing hormone secretion from hypothalamic neurons: stimulation by insulin and potentiation by leptin. Endocrinology. 2003;144:4484–4491. [DOI] [PubMed] [Google Scholar]
- 137.Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. [DOI] [PubMed] [Google Scholar]
- 138.Butler ST, Pelton SH, Butler WR. Insulin increases 17 beta-estradiol production by the dominant follicle of the first postpartum follicle wave in dairy cows. Reproduction. 2004;127:537–545. [DOI] [PubMed] [Google Scholar]
- 139.Song Z, Levin BE, Stevens W, Sladek CD. Supraoptic oxytocin and vasopressin neurons function as glucose and metabolic sensors. Am J Physiol Regul Integr Comp Physiol. 2014;306:R447–R456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sladek CD, Stevens W, Song Z, Johnson GC, MacLean PS. The "metabolic sensor" function of rat supraoptic oxytocin and vasopressin neurons is attenuated during lactation but not in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310:R337–R345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Theodosis DT, Poulain DA. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984;11:183–193. [DOI] [PubMed] [Google Scholar]
- 142.Theodosis DT, Poulain DA. Evidence that oxytocin-secreting neurones are involved in the ultrastructural reorganisation of the rat supraoptic nucleus apparent at lactation. Cell Tissue Res. 1984;235:217–219. [DOI] [PubMed] [Google Scholar]
- 143.GI Hatton, Wang YF. Neural mechanisms underlying the milk ejection burst and reflex. Prog Brain Res. 2008;170:155–166. [DOI] [PubMed] [Google Scholar]
- 144.Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. [DOI] [PubMed] [Google Scholar]
- 145.Ladyman SR, Khant Aung Z, Grattan DR. Impact of Pregnancy and Lactation on the Long-Term Regulation of Energy Balance in Female Mice. Endocrinology. 2018;159:2324–2336. [DOI] [PubMed] [Google Scholar]
- 146.Teixeira PDS, Ramos-Lobo AM, Furigo IC, Donato J Brain STAT5 Modulates Long-Term Metabolic and Epigenetic Changes Induced by Pregnancy and Lactation in Female Mice. Endocrinology. 2019;160:2903–2917. [DOI] [PubMed] [Google Scholar]
- 147.Cui J, Jothishankar B, He P, Staufenbiel M, Shen Y, Li R. Amyloid precursor protein mutation disrupts reproductive experience-enhanced normal cognitive development in a mouse model of Alzheimer's disease. Mol Neurobiol. 2014;49:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rossetti MF, Varayoud J, Lazzarino GP, Luque EH, Ramos JG. Pregnancy and lactation differentially modify the transcriptional regulation of steroidogenic enzymes through DNA methylation mechanisms in the hippocampus of aged rats. Mol Cell Endocrinol. 2016;429:73–83. [DOI] [PubMed] [Google Scholar]
- 149.Barha CK, Lieblich SE, Chow C, Galea LA. Multiparity-induced enhancement of hippocampal neurogenesis and spatial memory depends on ovarian hormone status in middle age. Neurobiol Aging. 2015;36:2391–2405. [DOI] [PubMed] [Google Scholar]
- 150.Eid RS, Chaiton JA, Lieblich SE, Bodnar TS, Weinberg J, Galea LAM. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1–17. [DOI] [PubMed] [Google Scholar]
- 151.Galea LAM, Roes MM, Dimech CJ, et al. Premarin has opposing effects on spatial learning, neural activation, and serum cytokine levels in middle-aged female rats depending on reproductive history. Neurobiol Aging. 2018;70:291–307. [DOI] [PubMed] [Google Scholar]
- 152.Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: Anatomical, functional and temporal contexts. Neuropharmacology. 2018;136:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]