Abstract
Introduction
Atropine sulfate is an FDA-approved medical countermeasure (MCM) for the treatment of organophosphorus nerve agent and organophosphate pesticide toxicity. Sufficient MCM supplies must be available in an incident involving a mass human exposure either from an accidental chemical release or a terrorist attack.
Methods
We performed a randomized, 3-sequence, 3-period phase I crossover study to assess the bioavailability and pharmacokinetics (PK) of a single dose (0.5 mg and 1.0 mg) of 1% ophthalmic atropine sulfate solution administered sublingually to 15 healthy adult volunteers. The primary endpoint was evaluation of the bioavailability of each of the two sublingual doses against a 1.0 mg reference intravenous (IV) atropine dose. Secondary endpoints included the safety and tolerability (xerostomia scale) of atropine sulfate administered sublingually.
Results
Sublingual atropine was safe (no severe AEs or SAEs were reported with either dose) and well tolerated, with a single subject reaching maximum xerostomia on a single dosing day. The geometric mean AUC∞ was 286.40, 493.81, and 816.47 min*ng/mL for the 0.5 mg and 1.0 mg sublingual doses, and the 1.0 mg IV dose, respectively. Compared to IV administration, the 1.0 mg sublingual dose produced 0.60 (90% CI: 0.55–0.66) of the overall concentration of atropine over time (AUC∞).
Conclusion
Sublingual atropine sulfate 1% ophthalmic solution may be an alternative formulation and route of administration combination which expands the capacity and dosing options of atropine as a nerve agent MCM.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13181-021-00873-0.
Keywords: Atropine, Sublingual, Pharmacokinetics, Clinical trial
Introduction
The Biomedical Advanced Research and Development Authority (BARDA), as part of the US Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response, is tasked with supporting the development and procurement of strategic medical countermeasures (MCMs) to make MCMs available for the USA that address the public health and medical consequences of chemical, biological, radiological, and nuclear (CBRN) threats, pandemic influenza, and emerging infectious diseases. Typically, this effort involves supporting novel drug development through the US Food and Drug Administration (FDA) approval and procuring the products for national preparedness. However, after a large nerve agent poisoning, pre-deployed MCMs are likely to be inadequate due to limited availability of community caches (CHEMPACK), logistical challenges in bringing the resource to the site of treatment, depletion of the resource before all patients needing treatment receive it, expiration or unavailability of approved MCMs, or unavailability of preferred delivery platforms (autoinjectors). In the largest and most well-researched nerve agent mass exposure, 528 mildly (defined as only ocular symptoms) poisoned patients were observed at St. Luke’s Hospital following the March 1995 Tokyo subway sarin attack [1]. The following description of nerve agent MCM requirements, utilization, and resupply required to treat the moderately and severely poisoned patients from the same incident is taken directly from the original article describing the hospital response [1]. The description suggests that the moderately and severely poisoned patients’ MCM requirements may exceed the supply of parenteral atropine formulations available:
Initially, we stored 100 ampules of 2-pyridine aldoxime methiodide (2-PAM) (1 ampule contains 500 mg of 2-PAM) and 1,030 ampules of atropine sulfate (1 ampule contains 0.5 mg of atropine sulfate). While this supply permitted initial treatment of the moderate to severely ill patients, our pharmaceutical department made an additional order to wholesale dealers at an early stage of the disaster…We used 700 ampules of 2-PAM and 2,800 ampules of atropine sulfate [emphasis added]
Immediately accessing adequate supplies of atropine for an incident of similar size could pose a challenge for all but the very best-resourced metropolitan areas in the USA. Additionally, while atropine autoinjectors are easier and faster to use, they are often unavailable in adequate quantities in under-resourced countries at highest risk for nerve agent attacks as was tragically demonstrated in the Syrian civil war [2, 3].
Several approaches to capacity limitations on atropine availability in the event of a nerve agent attack have been proposed. In preparation for the 1996 Atlanta Olympic Games, Geller et al. described augmenting hospital supplies of parenteral atropine formulations with bulk atropine powder and published their plan for rapid reconstitution by healthcare facility-based pharmacists in the event of a nerve agent attack [4]. Contingency antimuscarinic countermeasures, alternative pharmaceuticals, and routes of administration have recently been reviewed [5] and are the subject of a position statement by the American College of Medical Toxicology [6]. In a 2017 article, Calvano et al. proposed sublingual ophthalmic atropine solution as an alternative to limited atropine autoinjector supplies [7]. Additional community sources of ophthalmic atropine may include shelf stocks in consumer pharmacies and in optometry and ophthalmology practices. Bryant et al. estimated the emergency department of their community hospital had only 150 mg of parenteral atropine available in drug-dispensing cabinets and advanced cardiac life support carts, but the hospital’s pharmacies, departments, and clinics combined, had the equivalent of over 40,000 mg of antimuscarinics, including atropine, cyclopentolate, tropicamide, and homatropine in the form of high concentration eye drops [8].
The initial approval of atropine sulfate by the US FDA occurred in 1960 and included the indication [as] “a muscarinic antagonist indicated for temporary blockade of severe or life-threatening muscarinic effects.” [9] Atropine is used clinically to treat organophosphorus (OP) chemical warfare nerve agent and pesticide poisonings; symptomatic bradycardia; in the context of several Advanced Cardiac Life Support algorithms; and to dilate pupils for posterior chamber eye examination or for symptomatic ciliary spasm. Atropine is administered by intramuscular injection, intravenous injection, and as ocular drops. The ophthalmic drop formulation also has been administered sublingually in a number of off-label clinical settings. A literature review by the authors — to collect safety data on the ophthalmic formulation and sublingual route of administration — identified several publications detailing 1% atropine sulfate’s use in mitigating drug-induced sialorrhea [10], excessive drooling in children [11] and adults [12], and easing the final breaths taken by hospice patients [13].
In this phase I study, the US government conducted a clinical trial evaluating the bioavailability and pharmacokinetics of sublingually-administered atropine sulfate ophthalmic solution 1% USP at 0.5 mg (50 µL) and 1.0 mg (100 µL) doses compared to the IV criterion standard route of administration (ROA) of atropine sulfate at a 1.0 mg dose (0.4 mg/mL).
Methods
Patient Population
Healthy male and non-pregnant females 18 to 55 years of age were enrolled at a single center, High Point Clinical Trials Center (High Point, NC). Inclusion and exclusion criteria are listed on clinicaltrials.gov identifier: NCT04290039. Written informed consent was obtained before each subject underwent any study-related procedures. The protocol, informed consent, and other subject-facing documents, including any amendments, were reviewed and approved by IntegReview Institutional Review Board (Austin, TX) prior to implementation. Rho Federal Systems Division, Inc. (Durham, NC) served as the full-service contract research organization (CRO).
The trial site selected held a US Federal Wide Assurance issued by the Office for Human Research Protections at the US Department of Health and Human Services. The clinical study was conducted in accordance with current International Council for Harmonisation (ICH), Good Clinical Practice (GCP), the Declaration of Helsinki, US Code of Federal Regulations (CFR) Chapter 21 Part 50 (Protection of Human Subjects) and Part 56 (Institutional Review Boards) guidelines.
Study Design
This was a randomized, 3-sequence, 3-period crossover study to assess the bioavailability and PK of a single dose of atropine administered sublingually in healthy adult volunteers. Fifteen healthy male and female volunteers were enrolled to obtain approximately 12 fully evaluable subjects. Eligible subjects meeting all of the inclusion criteria and none of the exclusion criteria were randomized at a 1:1:1 ratio to receive 1 of 3 dosing sequences (A, B, or C) as depicted in Table 1.
Table 1.
Study design scheme by dosing sequence.
| Dosing sequence | Expected number of evaluable subjects (N) | Period 1 (visit 1; day 1) |
Period 2 (visit 2; day 8) |
Period 3 (visit 3; day 15) |
|---|---|---|---|---|
| A | 4 | Low-dose sublingual | High-dose sublingual | IV |
| B | 4 | High-dose sublingual | IV | Low-dose sublingual |
| C | 4 | IV | Low-dose sublingual | High-dose sublingual |
IV, intravenous atropine sulfate 1.0 mg (0.4 mg/mL) administered via IV route
Low-dose sublingual = 0.5 mg (50 µL) of atropine sulfate 1% ophthalmic solution, administered via sublingual route
High-dose sublingual = 1.0 mg (100 µL) of atropine sulfate 1% ophthalmic solution, administered via sublingual route
Once randomized, each subject was to receive 3 doses of atropine according to their assigned dosing sequence. Each dose was separated by a washout period of 6 ± 1 days. Blood samples to measure atropine plasma concentrations and determine PK parameters were collected via an indwelling venous catheter during each dosing visit at the following time points: time 0 (predose); post-dose at 2, 4, 6, 10, 15, 20, 30, 45, and 60 min; and post-dose at 2, 4, 6, and 8 h. As this was an open-label study, both study site staff and subjects could have ascertained the identity of treatments given during the study. Site staff recorded subjects’ reports of their subjective xerostomia predose and every 10 min up to the first hour after dosing as described below. Subjects were discharged from the clinic after the 8 h blood sample collection.
Administration of Atropine
Atropine sulfate ophthalmic solution, USP 1% (Akorn Laboratories, Inc.; Lake Forest, IL; NDC 17478–215-15) was administered sublingually using a calibrated Pipetman (Gilson, Middleton, WI). For the low-dose (0.5 mg) sublingual cohort, 50 µL of atropine sulfate ophthalmic, USP 1% was administered via the sublingual route. For high-dose (1.0 mg) sublingual treatment, 100 µL of atropine sulfate ophthalmic, USP 1% was administered via the sublingual route. Before administration of sublingual atropine, subjects were asked to swallow. Following administration, subjects were instructed to try not to swallow for 30 s and thereafter swallow as they normally would. For IV administration (1.0 mg), 2.5 mL of atropine sulfate injection, USP (0.4 mg/mL; Fresenius Kabi USA, LLC; Lake Zurich, IL; NDC 63323–580-20) was administered (1/4 dose every 15 s) via an indwelling catheter in the opposite arm from that catheterized for blood collection.
Pharmacokinetics Assessment and Bioavailability
PK sample collection time points were as noted under study design. Actual PK sample collection times were used in analyses. If a collection time was incomplete or missing, the nominal time was used for analyses. Subject time points without evaluable results were excluded from analyses. Plasma samples for pharmacokinetic assessment were analyzed by National Medical Services Labs (Willow Grove, PA), using a validated method for measurement of atropine in human plasma by high performance liquid chromatography/tandem mass spectrometry (LC/MS–MS). The commercial, validated method had a limit of detection (LOD) of 0.004 ng/ml and a lower limit of quantitation (LLOQ) of 0.2 ng/ml and a linear range of 0.2 to 50 ng/ml. BARDA site auditors performed a site visit and qualified NMS Labs prior to study commencement.
Pharmacokinetics parameters were estimated for each subject and route of administration using a non-compartmental model based on the linear log trapezoidal method and uniform weighting for plasma. The terminal elimination rate constant (λz) was calculated using the best fit method. Parameters AUC∞, t½, CL/F, and Vd/F were considered non-evaluable when there were fewer than 3 data points beyond Tmax in the time-concentration curve. Parameter estimates were obtained from Phoenix WinNonlin (Certara, Princeton, NJ, USA). The primary endpoint of bioavailability of sublingual dosing was defined as the ratios of the following PK parameters: AUC∞, AUCt, and Cmax.
Xerostomia Assessment
Subjects were assessed for xerostomia using a subset of questions from a validated scoring system developed for patients presenting with salivary dysfunction due to rheumatological condition as described in Pai et al. [14]. Subjects were asked to rate the degrees of tongue dryness, lip dryness, and difficulty swallowing due to mouth dryness on a scale of 1 (not dry at all/not difficult at all) to 10 (very dry/very difficult). Scoring assessments were performed predose, and every 10 min until one of the following conditions had been met: 1 h had elapsed, the subject scored a maximum on one or more of the xerostomia assessment questions, or qualitatively reported intolerable xerostomia or asked for a drink. When any of these conditions were met, the assessment was terminated, and the subject was provided water ad libitum. Time to assessment termination was also recorded. Summary statistics for xerostomia scores and time to assessment termination were performed.
Safety Assessments
Safety was evaluated using physical examination, vital signs, electrocardiogram (ECG), and clinical chemistry. Xerostomia, a physiological endpoint in this study, was not considered as an AE for the purposes of the safety assessment. A physical examination was performed at the screening visit to assess and confirm eligibility. The examination included a general assessment of all major organ systems.
Vital sign measurements, including oral temperature, heart rate, respiratory rate, and diastolic and systolic blood pressure (after the subject was seated for at least 5 min), were collected at the initial screening visit, and pre-dose prior to any blood draws for each atropine dose (days 1, 8, and 15). Post-dose automated blood pressure and heart rate measurements were recorded using the arm opposite from the blood collection arm every 10 min for the first hour, every 20 min for the second hour, every 30 min for the third and fourth hours, and thereafter as deemed clinically necessary by the investigator until the end of each visit. Respiratory rate, if regular, was assessed over 30 s and doubled, but in no case was it assessed over a period of less than 30 s. If the respiratory rate was irregular, it was assessed over 60 s.
A standard 12-lead ECG was recorded and assessed at the initial screening visit. An ECG was also performed within 5 min after completing IV atropine administration and could be repeated as needed at the investigator’s discretion during any time thereafter. ECGs were reviewed by a medically qualified individual to verify whether any abnormalities were clinically significant. In general, clinically significant abnormal ECGs were expected to be reported in the subject’s medical history (when detected at screening).
Clinical Chemistry
Venous blood was collected for routine clinical laboratory safety evaluations, including standard chemistry and hematology, at screening, and samples were analyzed by Laboratory Corporation of America, Burlington, NC. Urine was collected for a urine drug screen at the screening visit (analyzed by LabCorp) and prior to each dosing (analyzed on site) — per the clinical trial site’s standard operating procedures to ensure participant safety. Female subjects were screened at initial intake with a serum human chorionic gonadotropin (HCG) quantitative assay, and at each dosing visit with a urine HCG qualitative test.
Statistical Methods
A statistical analysis plan, which pre-specified the analyses that were to be conducted, was developed before any study-related activities commenced. Summary statistics were calculated for baseline demographic characteristics, xerostomia assessments, and atropine blood levels. Pharmacokinetic parameters were summarized using descriptive statistics, including geometric mean and coefficient of variation of the geometric mean for AUC parameters and Cmax. The PK analysis population was defined as all subjects who received at least one study drug dose, and had samples collected for the applicable period. Subjects in the PK analysis population were considered evaluable for bioavailability if they received at least two study drug doses and had evaluable time-concentration profiles for the applicable periods. The safety population was defined as any subject who received at least one dose of study drug. For the primary analyses of bioavailability of sublingual dosing, linear mixed models were fit to the log-transformed PK parameters, including terms for dosing sequence, dose, and period as fixed effects, and subject nested within sequence as a random effect in the model. Ratios of least squares means of key plasma PK parameters and associated 90% confidence intervals from the mixed model were anti-log transformed to obtain estimates on the original scale. For ratios of low dose versus IV, unadjusted ratios were multiplied by 2 to obtain the dose-corrected ratio. Descriptive statistics and analyses were performed using SAS version 9.4 (Cary, NC).
Results
Patient Population
This study enrolled 15 healthy volunteer subjects, including 8 (53.3%) male subjects and 7 (46.7%) female subjects. Median age at screening was 36.0 years (range from 23 to 45 years). These volunteer subjects were Black or African American (8 subjects; 53.3%), White (5 subjects; 33.3%), Hispanic or Latino (4 subjects; 16.4%). Median BMI in the study population was 29.55 (range 21.1 to 39.0 kg/m2). The study population is summarized in Table 2.
Table 2.
Demographic and baseline physical characteristics by dosing sequence population: safety.
| Dosing sequence A N = 5 |
Dosing sequence B N = 5 |
Dosing Sequence C N = 5 |
Overall total N = 15 |
|
|---|---|---|---|---|
| Characteristics | ||||
| Sex, n (%) | ||||
| Male | 5 (100.0%) | 1 (20.0%) | 2 (40.0%) | 8 (53.3%) |
| Female | 0 | 4 (80.0%) | 3 (60.0%) | 7 (46.7%) |
| Age (years) | ||||
| Mean | 37.2 | 29.2 | 35.0 | 33.8 |
| SD | 7.5 | 5.8 | 3.9 | 6.5 |
| Race, n (%) | ||||
| American Indian or Alaska Native | 0 | 0 | 0 | 0 |
| Black or African American | 2 (40.0%) | 3 (60.0%) | 3 (60.0%) | 8 (53.3%) |
| Asian | 0 | 0 | 0 | 0 |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 0 |
| White | 1 (20.0%) | 2 (40.0%) | 2 (40.0%) | 5 (33.3%) |
| More than one race | 1 (20.0%) | 0 | 0 | 1 ( 6.7%) |
| Missing | 1 (20.0%) | 0 | 0 | 1 ( 6.7%) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 1 (20.0%) | 0 | 1 (20.0%) | 2 (13.3%) |
| Not Hispanic or Latino | 4 (80.0%) | 5(100.0%) | 4 (80.0%) | 13 (86.7%) |
| Not reported | 0 | 0 | 0 | 0 |
| Baseline Weight (kg) | ||||
| Mean | 72.8 | 77.7 | 89.1 | 79.8 |
| SD | 13.7 | 13.9 | 14.7 | 14.8 |
| Baseline Height (cm) | ||||
| Mean | 169.5 | 169.1 | 161.9 | 166.9 |
| SD | 5.7 | 5.7 | 10.1 | 7.8 |
| BMI (kg/m2) | ||||
| Mean | 25.3 | 27.4 | 34.0 | 28.9 |
| SD | 4.2 | 6.3 | 4.6 | 6.1 |
| Screening xerostomia assessment—difficulty swallowing (0–10) | ||||
| Mean | 0.0 | 0.0 | 0.0 | 0.0 |
| SD | 0.0 | 0.0 | 0.0 | 0.0 |
| Screening xerostomia assessment—dryness of lips(0–10) | ||||
| Mean | 0.4 | 1.6 | 0.2 | 0.7 |
| SD | 0.5 | 2.1 | 0.4 | 1.3 |
| Screening xerostomia assessment—dryness of tongue (0–10) | ||||
| Mean | 0.0 | 0.0 | 0.0 | 0.0 |
| SD | 0.0 | 0.0 | 0.0 | 0.0 |
BMI, body mass index; SD, standard deviation
Note: Dosing sequence A: Period 1, low-dose sublingual; Period 2, high-dose sublingual; Period 3, intravenous. Dosing sequence B: Period 1, high-dose sublingual; Period 2, intravenous; Period 3, low-dose sublingual. Dosing sequence C: Period 1, intravenous; Period 2, low-dose sublingual; Period 3, high-dose sublingual
Note: Percentages were based on the number of subjects (N) in the dosing sequence. Body mass index was calculated as weight (kg)/[height (m)2]
Note: Xerostomia scores were subject reported and based on a scale of 0 to 10, with 0 being not difficult/dry at all and 10 being very difficult/dry
A total of 14 subjects completed the study; 1 subject withdrew from the study early due to experiencing an adverse event that was deemed not related to study drug. The PK analysis population consisted of all 15 subjects; 13 subjects met the requirement of at least 2 evaluable PK profiles to be included in the analysis of bioavailability. Table 3 shows the number of evaluable PK profiles by dosage level for each pharmacokinetic parameter.
Table 3.
Bioavailability and pharmacokinetic summary by dose received population: PK analysis.
| Parameter Statistic | Low Dose sublingual | High Dose sublingual | Intravenous Observed | ||
|---|---|---|---|---|---|
| Observed | Ratio to IV [1] | Observed | Ratio to IV [1] | ||
| AUC∞ (min*ng/mL) | |||||
| n | 11 | 11 | 14 | 13 | 14 |
| Geometric mean | 286.4 | 0.347 | 493.8 | 0.603 | 816.5 |
| Geometric coefficient of variation (%) | 26.6 | 18.7 | 27.3 | 22.4 | 22.0 |
| AUCt (min*ng/mL) | |||||
| n | 14 | 14 | 15 | 14 | 14 |
| Geometric mean | 218.2 | 0.304 | 408.1 | 0.559 | 717.7 |
| Geometric coefficient of variation (%) | 20.2 | 22.5 | 23.9 | 23.0 | 23.2 |
| Cmax (ng/mL) | |||||
| n | 14 | 14 | 15 | 14 | 14 |
| Geometric mean | 0.883 | 0.048 | 1.64 | 0.089 | 18.2 |
| Geometric coefficient of variation (%) | 27.2 | 81.1 | 30.5 | 72.6 | 66.9 |
| tmax (min) | |||||
| n | 14 | NA | 15 | NA | NA |
| Mean | 125.4 | 107.1 | |||
| SD | 69.8 | 47.8 | |||
| t1/2 (min) | |||||
| n | 11 | 11 | 14 | 13 | 14 |
| Mean | 176.2 | 1.01 | 171.3 | 1.04 | 179.3 |
| SD | 75.1 | 0.42 | 50.0 | 0.38 | 60.4 |
| CL/F [2] (mL/min) | |||||
| n | 11 | 14 | |||
| Mean | 1800.8 | 2098.8 | |||
| SD | 473.4 | 632.8 | |||
| Vd/F [2] (L) | |||||
| n | 11 | 14 | |||
| Mean | 423.7 | 496.7 | |||
| SD | 119.8 | 138.8 | |||
| AUC45 (min*ng/mL) | |||||
| n | 14 | 14 | 15 | 14 | 14 |
| Geometric mean | 7.31 | 0.030 | 11.6 | 0.049 | 240.3 |
| Geometric coefficient of variation (%) | 73.7 | 108.7 | 94.7 | 99.3 | 40.3 |
| AUC60 (min*ng/mL) | |||||
| n | 14 | 14 | 15 | 14 | 14 |
| Geometric mean | 12.4 | 0.044 | 22.6 | 0.081 | 279.7 |
| Geometric coefficient of variation (%) | 82.4 | 107.5 | 106.9 | 112.7 | 35.4 |
| AUC120 (min*ng/mL) | |||||
| n | 14 | 14 | 15 | 14 | 14 |
| Geometric mean | 48.5 | 0.119 | 99.4 | 0.243 | 406.6 |
| Geometric coefficient of variation (%) | 56.4 | 66.5 | 51.5 | 52.3 | 27.0 |
| AUC240 (min*ng/mL) | |||||
| n | 14 | 14 | 15 | 14 | 14 |
| Geometric mean | 130.8 | 0.231 | 253.1 | 0.440 | 566.9 |
| Geometric coefficient of variation (%) | 29.1 | 35.8 | 27.0 | 27.0 | 24.1 |
AUC∞, area under curve to infinity; AUCt, AUC to last quantifiable data point; AUC45, AUC to 45-min timepoint; AUC60, AUC to 60-min timepoint; AUC120, AUC to 120-min timepoint; AUC240, AUC to 240-min timepoint; CL/F, apparent total body clearance after extravascular administration; Cmax, maximum concentration; IV, intravenous; n, count of subjects with non-missing data; NA, not applicable; PK, pharmacokinetic; tmax, time of Cmax; t1/2, apparent terminal half-life; Vd/F, apparent volume of distribution after extravascular administration
Note: tmax was not estimable in intravenous administration
Subject-specific ratios were summarized
CL/F and Vd/F are not applicable to intravenous administration
Safety
A total of 6 subjects (40.0%) experienced at least 1 AE; the proportion of subjects experiencing AEs was higher during the IV dosing period (4 subjects; 28.6%) compared with the low-dose sublingual dosing period (1 subject; 7.1%) and high-dose sublingual dosing period (1 subject; 6.7%). There were 7 AEs recorded during this study and a table of all AEs is provided in Supplementary Appendix A.
No subjects experienced SAEs or severe AEs. Five subjects (33.3%) experienced an AE that was considered related to study drug: 4 subjects during their IV dosing period and 1 subject during their low-dose sublingual dosing period. No subjects experienced an AE leading to early termination of study drug or an AE leading to death. In addition, one female subject who received high-dose sublingual atropine during the first dosing period experienced a non-study drug–related AE (an upper respiratory infection acquired during the intervening week) leading to her early study withdrawal. All six AEs in this study occurred from dosing day 1 through 7 days after the last study drug administration.
Analysis of Pharmacokinetics
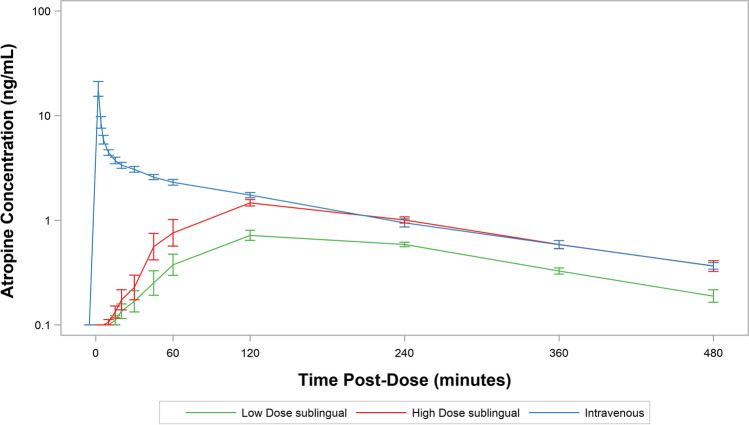
Figure 1 shows the composite subject-level averages of atropine plasma concentration–time curves for each dose on a semi-log scale. All PK parameters are summarized in Table 3. For IV administration, the peak plasma levels were observed immediately post-dose (2 min), and the geometric mean Cmax was 18.25 ng/mL. Plasma peaks for low-dose (0.5 mg) and high-dose (1 mg) SL atropine were 0.88 and 1.64 ng/mL, respectively, with mean Tmax values of 125.4 min for the low-dose sublingual route and 107.1 min for the high-dose sublingual route (Table 3). Cmax values for low-dose and high-dose SL atropine exhibit good dose proportionality. The calculated absorption rates for low-dose and high-dose SL atropine were 0.010 and 0.018/min, respectively, with dose-adjusted rates of 0.020 and 0.018/min, respectively.
Fig. 1.
Atropine plasma concentration–time curves for all subjects.
The AUC∞ geometric means were 286.40, 493.81, and 816.47 min*ng/mL for the low-dose sublingual, high-dose sublingual, and IV routes, respectively (Table 3). The estimated geometric mean ratio of AUC∞ between the high-dose sublingual route and IV route was 0.60 (90% CI: 0.55 to 0.66), indicating 60% bioavailability for atropine administered sublingually (Table 4). The estimated geometric mean ratio of AUC∞ between the low-dose sublingual route and IV route indicated 0.35 (90% CI: 0.32 to 0.38; Table 4) bioavailability. The estimated dose-corrected geometric mean ratio of AUC∞ between low-dose sublingual and high-dose sublingual routes was 1.16 (90% CI: 1.05 to 1.27), demonstrating good dose proportionality with respect to AUC∞.
Table 4.
Bioavailability and pharmacokinetic comparisons between doses received population: PK analysis.
| Low-dose sublingual/high-dose sublingual | Low-dose sublingual/intravenous | High dose sublingual/intravenous | |
|---|---|---|---|
| Parameter statistic | |||
| AUC∞ (min*ng/mL) | |||
| Ratio | |||
| Geometric mean | 0.579 | 0.349 | 0.603 |
| 90% CI | (0.526, 0.636) | (0.317, 0.384) | (0.552, 0.658) |
| Dose corrected ratio | |||
| Geometric Mean | 1.157 | 0.698 | NA |
| 90% CI | (1.053, 1.272) | (0.634, 0.768) | |
| AUCt (min*ng/mL) | |||
| Ratio | |||
| Geometric mean | 0.546 | 0.306 | 0.561 |
| 90% CI | (0.497, 0.600) | (0.279, 0.337) | (0.510, 0.616) |
| Dose corrected ratio | |||
| Geometric mean | 1.093 | 0.613 | NA |
| 90% CI | (0.994, 1.201) | (0.558, 0.673) | |
| Cmax (ng/mL) | |||
| Ratio | |||
| Geometric mean | 0.549 | 0.049 | 0.090 |
| 90% CI | (0.424, 0.712) | (0.038, 0.064) | (0.069, 0.117) |
| Dose corrected ratio | |||
| Geometric mean | 1.098 | 0.099 | NA |
| 90% CI | (0.847, 1.424) | (0.076, 0.128) | |
| AUC45 (min*ng/mL) | |||
| Ratio | |||
| Geometric mean | 0.635 | 0.031 | 0.049 |
| 90% CI | (0.432, 0.931) | (0.021, 0.045) | (0.033, 0.072) |
| Dose corrected ratio | |||
| Geometric mean | 1.269 | 0.062 | NA |
| 90% CI | (0.865, 1.862) | (0.042, 0.091) | |
| AUC60 (min*ng/mL) | |||
| Ratio | |||
| Geometric mean | 0.558 | 0.045 | 0.081 |
| 90% CI | (0.380, 0.821) | (0.031, 0.066) | (0.055, 0.119) |
| Dose corrected ratio | |||
| Geometric Mean | 1.117 | 0.090 | NA |
| 90% CI | (0.759, 1.643) | (0.061, 0.132) | |
| AUC120 (min*ng/mL) | |||
| Ratio | |||
| Geometric mean | 0.499 | 0.121 | 0.242 |
| 90% CI | (0.393, 0.634) | (0.095, 0.153) | (0.190, 0.307) |
| Dose corrected ratio | |||
| Geometric mean | 0.998 | 0.241 | NA |
| 90% CI | (0.786, 1.267) | (0.190, 0.307) | |
| AUC240 (min*ng/mL) | |||
| Ratio | |||
| Geometric mean | 0.529 | 0.233 | 0.440 |
| 90% CI | (0.462, 0.605) | (0.203, 0.266) | (0.385, 0.504) |
| Dose corrected ratio | |||
| Geometric mean | 1.058 | 0.466 | NA |
| 90% CI | (0.924, 1.210) | (0.407, 0.533) |
AUC∞, area under curve to infinity; AUCt, AUC to last quantifiable data point; AUC45, AUC to 45-min timepoint; AUC60, AUC to 60-min timepoint; AUC120, AUC to 120-min timepoint; AUC240, AUC to 240-min timepoint; CI, confidence interval; Cmax, maximum concentration; NA, not applicable; PK, pharmacokinetic
Note: Only subjects who had at least 2 periods where study drug was received with an evaluable PK profile were included in the comparisons. All statistics are back-transformed and based on a random effects linear model with log10 numeric response variable, adjusting for study dosage received, dosing sequence, and period, with a random effect for subject nested within dosing sequence
Note: Dose corrected ratios do not apply to the ratio of high-dose sublingual to intravenous due to the doses being equivalent
The AUCt geometric means were 218.20, 408.06, and 717.67 min*ng/mL for the low-dose sublingual, high-dose sublingual, and IV routes, respectively (Table 3). The estimated geometric mean ratio of AUCt between the high-dose sublingual route and IV route was 0.56 (90% CI: 0.51 to 0.62), again demonstrating a sublingual atropine bioavailability of approximately 60%. The estimated geometric mean ratio of AUCt between the low-dose sublingual route and IV route was 0.31 (90% CI: 0.28 to 0.34). The estimated dose-corrected geometric mean ratio of AUCt between low-dose sublingual and high-dose sublingual routes was 1.09 (90% CI: 0.99 to 1.20 [Table 4]).
Elimination of atropine showed first-order kinetics. The t½ means were 176.19 (SD: 75.09), 171.26 (SD: 49.98), and 179.33 (SD: 60.41) min for the low-dose sublingual, high-dose sublingual, and IV routes, respectively (Table 3). The mean CL/F was 1800.80 (SD: 473.36) mL/min for the low-dose sublingual route and 2098.81 (SD: 632.82) mL/min for the high-dose sublingual route, and the mean Vd/F was 423.71 (SD: 119.77) L for the low-dose sublingual route and 496.70 (SD: 138.81) L for the high-dose sublingual route (Table 3). Additional AUC values for select time points of operational clinical interest (possible time of first contact with prehospital providers, e.g.) are also presented in Table 3.
Discussion
A phase I bioavailability (BA) study was completed to determine the pharmacokinetics of 1% ophthalmic atropine sulfate solution administered via the sublingual route, as well as the safety and tolerability of the ROA. Ophthalmic atropine (1%) is a potential high concentration, high-capacity contingency formulation of atropine. Ophthalmic atropine contains 10 mg/ml atropine sulfate and 8.3 mg/ml of free atropine, and commercially available sizes include 2-ml (20 mg atropine sulfate) up to 15-ml (150 mg atropine sulfate) plastic bottles that may be readily available in healthcare facilities’ emergency departments, automated drug-dispensing cabinets, pharmacies, and outpatient ophthalmology clinics. The formulation itself allows ease of sublingual administration directly from the bottle. Our study was not meant to prescribe a specific methodology for sublingual administration. (We utilized pipettes to insure a precise sublingual dose for pharmacokinetic comparison with an equally precise IV dose.) A 50 ul drop of 1% ophthalmic atropine solution contains 0.5 mg of atropine sulfate; based on the AUC-derived bioavailability from this study, this volume is equivalent to a 0.25 mg IV dose of atropine. However, the use of a precision pipette to administer exactly 50 ul of study drug was never meant to be proscriptive in terms of a clinical dosing method. Any mechanism for administration — including administering drops directly from the bottle and use of a needleless tuberculin or other small volume syringe — would be appropriate if those resources were available.
Targeted atropinization necessary to stabilize one severely poisoned OP patient often requires in excess of 50–100 mg of atropine in the first 24 h [15]. Previous authors have examined the pharmacokinetics of sublingually injected atropine sulfate, however not by direct application of drops to the sublingual mucosa. Rajpal et al. used a needle and syringe to inject 2 mg atropine sulfate in 0.1 ml volume (2%) sublingually in 6 healthy volunteers and showed a higher serum concentration at t = 10 min (14 ng/mL) than the therapeutic peak of 6 to 8 ng/mL obtained with a 2 mg intramuscular dose which occurs at 30 ± 31 min post IM injection [16]. This study is not directly comparable to the present study due to the use of an intraoral injection. However, both studies demonstrate the SL space as a well-recognized site of pharmaceutical dosing (sublingual nitroglycerin sprays and tablets, e.g.).
The sublingual ROA appeared safe and well-tolerated based on a small number of subjects in this phase 1 study; however, the number of subjects (14 who completed the study) and number of person-doses administered is a limitation for any phase I clinical trial. De Simone et al. administered sublingual 1% atropine sulfate (0.5 mg; 2 drops [gtt]) q6h × 48 h to 22 patients with esophageal or gastric cancer to mitigate drooling without adverse safety events [12]. Norderyd et al. administered 1% atropine sulfate solution 1 gtt/day × 4 weeks followed by 2 gtt per day for a second four weeks to 26 children aged 5–18 years old with disabilities and a history of excessive drooling; 19 children (mean age 11.6 ± 4.7 years) completed the study, and the only AEs reported were mild [11]. Unstimulated salivary secretion rate (USSR), measured with cotton balls weighed dry and after placement in the mouth during a study visit, decreased significantly (p = 0.032). Parental reporting on a 100-point Visual Analog Scale (VAS) of decreased drooling also was statistically significant (p = 0.004) [11]. Heisler et al. studied the administration of 1 mg of 1% ophthalmic atropine sulfate solution to 160 terminally ill adults in the final moments of life to decrease “death rattle” due to retained airway secretions; no significant changes in HR were noted though no other AEs were reported [13]. Hyson et al. administered 1 gtt of 1% atropine sulfate ophthalmic solution bid × 7d to seven patients (62–82 years old) with Parkinsonism and drooling [17]. One SAE was reported (delirium secondary to UTI) that was unrelated to study drug. Finally, Matos-Santana administered 1% atropine sulfate ophthalmic solution (1–2 gtt nightly for 7 days) to three male schizophrenic patients with clozapine-induced sialorrhea; in their study no AEs were reported [10].
The Cmax of the SL high-dose administration was 1.64 ng/ml, and the Tmax occurred at 107.1 min. The extent to which these parameters would change with a higher sublingual dose is limited by safety concerns in dosing a healthy volunteer population with 2 mg or more of atropine. For comparison, the following parameters are derived from the 2.1 mg atropine fraction of a DuoDote (Meridian Medical Technologies, Inc, Columbia, MD) Autoinjector System: Cmax = 13 ± 3 ng/ml; Tmax = 31 ± 30 min [18]. In our study, atropine ophthalmic solution administered sublingually had a Tmax of 125.4 min for the low-dose sublingual route and 107.1 min for the high-dose sublingual route. Clearly the sublingual route of administration is not suitable as a “rescue” medication; however, the slower and flatter PK profile for sublingual atropine appears to be well-suited for treatment of mild exposure to nerve agent or as a maintenance regimen to preserve the stores of more rapid acting IM and IV formulations of atropine stock for those more severely affected. For mildly poisoned patients with lacrimation, rhinorrhea, and/or sialorrhea, the ability to administer SL 1% ophthalmic atropine drops to achieve symptom resolution has several advantages. Mildly poisoned patients could be treated with SL atropine which could preserve scarce IM (autoinjector, e.g.) and parenteral (IV) formulations of atropine for moderately and severely poisoned patients. SL atropine has a longer Tmax than other ROA, so there is risk of iatrogenic atropine toxicity if clinicians do not recognize there may be a delay in symptom resolution in mildly poisoned patients dosed sublingually. The SL route also allows for redosing of mildly poisoned patients who initially achieved symptom resolution, but have return of lacrimation, rhinorrhea, and/or sialorhea (during a period of observation, for example).
Repurposing widely stocked, commercially available, and FDA-approved pharmaceuticals (and formulations allowing alternative ROA dosing) as contingency chemical medical countermeasures has several advantages. Immediate access to MCMs in the event of a large chemical incident is paramount; organophosphorus nerve agent exposure may lead to irreversible inactivation of the acetylcholinesterase enzyme (“aging”) within minutes if MCMs are not administered rapidly. Contingency treatments and preparations may be available for their FDA-approved indications in greater quantities and in more community healthcare-related venues than specific chemical MCMs stockpiled in one or a few locations for an exceedingly rare occurrence. Commonly used drugs have the added advantage of physician familiarity due to providers’ routine clinical experience with their use. For non-parenteral ROA such as sublingual administration, more prehospital first responders of varying levels of training and certification may be able to expand the response capacity to treat larger numbers of exposed patients.
Studies such as the one described here are useful to evaluate the proposed alternative ROA for BA/BE equivalence, as well as to identify the relevant PK parameters that may inform dosing recommendations or guidelines from experts in toxicology, pharmacology, or emergency management if and when a commonly used medication is called to action as a contingency MCM. There are several limitations to this study, including small sample size and the potential confounding effect actual OP nerve agent induced sialorrhea might have on the absorption of atropine solution from the sublingual space. The study data was obtained under ideal conditions and administering SL atropine in patients with actual OP nerve–induced sialorrhea may result in marked differences in absorption and the resulting parameters. Additionally, study subjects were asked to avoid swallowing immediately after administration to maximize dwell time of the dose in the SL space; however, the possibility of some GI absorption cannot be ruled-out.
Conclusion
A phase I clinical trial demonstrated that 1% atropine sulfate ophthalmic solution administered sublingually was well tolerated with an acceptable safety profile. The primary endpoint of the study was demonstration of the bioavailability of sublingual 1% ophthalmic atropine; the trial determined that bioavailability based on the AUC∞ was 60%. The Cmax of a 1 mg SL dose achieved a plasma concentration less than that achieved by an IV dose of 1 mg atropine; a direct comparison of SL Cmax to IM Cmax was not a component of this study which did not include an IM or autoinjector dosing arm. The Tmax was 107.1 min for 1 mg atropine SL; this is later than the Tmax achieved with parenteral administration. In patients suffering mild OP intoxication symptoms, SL ophthalmic atropine may be a readily available and high capacity alternative to currently stockpiled atropine autoinjectors and multidose vials, preserving these more expedient MCM formulations for the subset of the exposed population experiencing severe or life-threatening poisoning.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was performed by the US Government.
No additional funding supported this work.
Declarations
Ethics Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Conflicts of Interest
None.
Disclaimer
For Schwartz, Raulli, Laney, Coley, Walker, O’Rourke, Raine, Horwith, Gao, Eisnor, Lu, Wolling: “The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the US Department of Health and Human Services or its components. The information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the US Department of Health and Human Services. It does not represent and should not be construed to represent any agency determination or policy.”
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael D. Schwartz, Email: Michael.schwartz@hhs.gov
Brenda Wolling, Email: Bwolling@yahoo.com.
References
- 1.Okumura T, Suzuki K, Fukuda A, Kohama A, Takasu N, Ishimatsu S, Hinohara S. The Tokyo subway sarin attack: disaster management, part 2: hospital response. Acad Emerg Med. 1998;5(6):618–624. doi: 10.1111/j.1553-2712.1998.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 2.U.N. Mission to investigate allegations of the use of chemical weapons in the Syrian Arab Republic: report on the alleged use of chemical weapons in the Ghouta Area of Damascus on 21 August 2013. United Nations. 13 September 2013. https://reliefweb.int/sites/reliefweb.int/files/resources/Secretary_General_Report_of_CW_Investigation.pdf . Accessed 22 May 2021.
- 3.Doctors Without Borders. Thousands suffering neurotoxic symptoms treated in hospitals supported by MSF. https://www.msf.org/syria-thousands-suffering-neurotoxic-symptoms-treated-hospitals-supported-msf . Accessed 22 May 2021.
- 4.Geller RJ, Lopez GP, Cutler S, Lin D, Bachman GF, Gorman SE. Atropine availability as an antidote for nerve agent casualties: validated rapid reformulation of high-concentration atropine from bulk powder. Ann Emerg Med. 2003;41(4):453–456. doi: 10.1067/mem.2003.103. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz MD, Sutter ME, Eisnor D, Kirk MA. Contingency medical countermeasures for mass nerve-agent exposure: use of pharmaceutical alternatives to community stockpiled antidotes. Disaster Med Public Health Prep. 2018;1–8. 10.1017/dmp.2018.99. [DOI] [PubMed]
- 6.Stolbach A, Bebarta V, Beuhler M, Carstairs S, Nelson L, Wahl M, Wax PM, McKay C. ACMT Position statement: alternative or contingency countermeasures for acetylcholinesterase inhibiting agents. J Med Toxicol. 2018;14(3):261–263. doi: 10.1007/s13181-018-0658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvano CJ, Enzenauer RW, Eisnor DE, Mazzoli RA. Atropine eye drops: a proposed field expedient substitute in the absence of atropine autoinjectors. J Spec Oper Med. 2017;17(3):81–83. doi: 10.55460/DQ96-STYU. [DOI] [PubMed] [Google Scholar]
- 8.Bryant SM, Rhee JW, Thompson TM, Aks SE. Pretreating rats with parenteral ophthalmic antimuscarinic agents decreases mortality from lethal organophosphate poisoning. Acad Emerg Med. 2007;14:370–2. doi: 10.1197/j.aem.2006.10.099. [DOI] [PubMed] [Google Scholar]
- 9.United States Food and Drug Administration. Atropine sulfate. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021146s015lbl.pdf . Accessed 7 Jul 2021.
- 10.Matos Santana TE, Capurso N, Ranganathan M, Yoon G. Sublingual atropine in the treatment of clozapine-induced sialorrhea. Schizophr Res. 2017;182:144–145. doi: 10.1016/j.schres.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Norderyd J, Graf J, Marcusson A. Sublingual administration of atropine eyedrops in children with excessive drooling - a pilot study. Int J Paediatr Dent. 2017;27(1):22–29. doi: 10.1111/ipd.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Simone GG, Ersenchlas JH, Junin M, Pereyra F, Brizuela R. Atropine drops for drooling: a randomized controlled trial. Palliat Med. 2006;20(7):665–671. doi: 10.1177/0269216306071702. [DOI] [PubMed] [Google Scholar]
- 13.Heisler M, Hamilton G, Abbott A, Chengalaram A, Koceja T, Gerkin R. Randomized double-blind trial of sublingual atropine vs. placebo for the management of death rattle. J Pain Symptom Manage. 2013;45(1):14–22. doi: 10.1016/j.jpainsymman.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Pai S, Ghezzi EM, Ship JA. Development of a visual analogue scale questionnaire for subjective assessment of salivary gland dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol. 2001;91(3):311–316. doi: 10.1067/moe.2001.111551. [DOI] [PubMed] [Google Scholar]
- 15.Eddleston M, Buckley NA, Checketts H, Senarathna L, Mohamed F, Sheriff MH, Dawson A. Speed of initial atropinisation in significant organophosphate pesticide poisoning. Clin Toxicol. 2004;42(6):865–75. doi: 10.1081/clt-200035223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajpal S, Mittal G, Sachdeva R, Chhillar M, Ali R, Agrawal SS, Kashyap R, Bhatnagar A. Development of atropine sulphate nasal drops and its pharmacokinetic safety evaluation in healthy human volunteers. Environ Toxicol Pharmacol. 2009;27:206–211. doi: 10.1016/j.etap.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Hyson HC, Johnson AM, Jog MS. Sublingual atropine for sialorrhea secondary to parkinsonism: a pilot study. Mov Disord. 2002;17(6):1318–1320. doi: 10.1002/mds.10276. [DOI] [PubMed] [Google Scholar]
- 18.Meridian Medical Technologies, Inc., a Pfizer company. DUODOTE (atropine and pralidoxime chloride injection). https://www.meridianmeds.com/sites/default/files/duodote_uspi_2017.pdf . Accessed 5 Aug 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.