Abstract
Background
Diabetes mellitus (DM) is a leading contributor to morbidity and mortality in the United States (US). Prior DM prevalence estimates in Asian Americans are predominantly from Asians aggregated into a single group, but the Asian American population is heterogenous.
Objective
To evaluate self-reported DM prevalence in disaggregated Asian American subgroups to inform targeted management and prevention.
Design
Serial cross-sectional analysis.
Participants
Respondents to the US Behavioral Risk Factor Surveillance System surveys who self-identify as non-Hispanic Asian American (NHA, N=57,001), comprising Asian Indian (N=11,089), Chinese (N=9458), Filipino (N=9339), Japanese (N=10,387), and Korean Americans (N=2843), compared to non-Hispanic White (NHW, N=2,143,729) and non-Hispanic Black (NHB, N=215,957) Americans.
Main Measures
Prevalence of self-reported DM. Univariate Satterthwaite-adjusted chi-square tests compared the differences in weighted DM prevalence by sociodemographic and health status.
Key Results
Self-reported fully adjusted DM prevalence was 8.7% (95% confidence interval 8.2–9.3) in NHA, compared to 14.3% (14.0–14.6) in NHB and 10.0% (10.0–10.1) in NHW (p<0.01 for difference). In NHA subgroups overall, DM prevalence was 14.4% (12.6–16.3) in Filipino, 13.4% (10.9–16.2) in Japanese, 10.7% (9.6–11.8) in Asian Indian, 5.1% (4.2–6.2) in Chinese, and 4.7% (3.4–6.3) in Korean Americans (p<0.01). Among those aged ≥65 years, DM prevalence was highest in Filipino (35.0% (29.4–41.2)) and Asian Indian (31.5% (25.9–37.8)) Americans. Adjusted for sex, education, and race/ethnicity-specific obesity category, NHA overall had a 21% higher DM prevalence compared to NHW (prevalence ratio 1.21 [1.14–1.27]), while prevalence ratios were 1.42 (1.24–1.63) in Filipinos and 1.29 (1.14–1.46) in Asian Indians.
Conclusions
Adjusted self-reported DM prevalence is higher in NHA compared with NHW. Disaggregating NHA reveals heterogeneity in self-reported DM prevalence, highest in Filipino and Asian Indian Americans.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-06909-z.
KEY WORDS: diabetes mellitus, cardiovascular disease, Asian American, surveillance
INTRODUCTION
Diabetes mellitus (DM) is a leading cause of morbidity and mortality in the United States (US). The current estimates indicate that approximately 13% of Americans have DM.1 However, there is considerable variation in diabetes prevalence by race/ethnicity. A recent report using a nationally representative sample showed that total (diagnosed and undiagnosed) DM prevalence ranged from 12% in non-Hispanic White (NHW) populations to approximately 19% in non-Hispanic Asians (NHA) and 20% in non-Hispanic Black (NHB) populations. Among Asian Americans disaggregated by region of ancestry, DM prevalence ranged from 14% among East Asians, to 22% in Southeast Asians, and 23% in South Asians.2
Asian Americans are among the fastest-growing minority groups in the US.3 However, the population group is not homogeneous and health data in Asian Americans could be disaggregated into Asian ancestry subgroups to unmask important heterogeneity that can identify subgroups with elevated burden. Disaggregated subgroup statistics can inform targeted clinical and public health prevention.4,5 Disaggregated Asian American cardiovascular health data may assist in the appropriate targeting of preventive strategies. For example, NHA in the US have a lower prevalence of hypertension (21%) compared to the NHW population in the US (34%). However, disaggregated data reveals that hypertension prevalence is as low as 17% in Asian Indians in the US, but as high as 41% in Japanese Americans, a disproportionately high prevalence that was masked due to aggregation and may in part be due to differences in age distributions of NHA subgroups.6
Given the considerable cultural, health behavioral, socioeconomic, and demographic differences between the largest Asian subgroups in the US, their DM prevalence may vary widely.5 To help create targeted clinical and public health interventions and public health prevention measures tailored to health-related behaviors unique to NHA subgroups, we quantified nationally representative contemporary self-reported DM prevalence as a measure of diagnosed diabetes in the five largest Asian American subgroups (Chinese, Asian Indian, Filipino, Japanese, and Korean Americans) using the Behavioral Risk Factor Surveillance System (BRFSS) data from 2013 to 2019.
METHODS
Data Source
BRFSS is an annual, state-based telephone survey of over 400,000 civilian non-institutionalized population aged ≥18 years in the US. Annual overall BRFSS median response rates ranged between 45.1 (in 2017) and 49.9% (in 2018). BRFSS data from 2013 to 2019 were evaluated to obtain contemporary prevalence estimates of DM in the US. Participants were contacted via random digit dialing of both mobile and landline telephone in all 50 states and the District of Columbia.
Variable Definitions
DM was identified by self-report. Estimated DM prevalence was calculated for the participants who responded “yes” to whether they had ever been told they had DM by a physician, nurse, or other healthcare professional. Female respondents who reported they had been told they had DM were subsequently asked if this report of DM was only when they were pregnant. Women who reported exclusively gestational diabetes were excluded from the analysis. Self-reported race and ethnicity data were categorized as NHW, NHB, and NHA. For NHA participants who selected Asian race, they were further asked to select a subgroup identification. Asian American participants had the option to self-identify as an Asian subgroup beginning in the 2013 survey cycle. The five largest NHA groups were identified and analyzed: Asian Indian, Chinese, Filipino, Japanese, and Korean. An “other Asian” category comprised subgroups with smaller sample sizes. In BRFSS, these groups are “Vietnamese” and “other Asian,” which were combined because small sample sizes of both groups limited reliability of statistical comparison.
Demographic and socioeconomic characteristics were based on self-report during the BRFSS survey (see Supplemental Table 1 for specific questions asked of participants). Primary characteristics included age (categorized as 18–64 and ≥65 years), sex (women and men), educational attainment (up to high school, and more than high school), health insurance status (had insurance or no insurance at the time of the survey), annual family income (less than $25,000 per year, $25,000–$50,000 per year, and more than $50,000 per year). Co-morbid health behaviors and health factors were also based on self-report. History of cardiovascular disease (CVD) was defined as a report of myocardial infarction, angina, coronary artery disease, or stroke. Obesity was calculated based on body mass index (BMI) derived from self-reported height and weight and defined by standard categorization for obesity (BMI ≥30 kg/m2) in NHW and NHB participants and Asian-specific categorization (BMI ≥27.5 kg/m2) in NHA participants.7 Smoking was categorized as current, former, or never smoker. Physical activity was categorized as physically active versus sedentary based on the report of participation in leisure time physical activity (“yes” or “no” answer to a question asking if participants engage in non-employment-related physical activity). Participants missing data for sociodemographic (except for income), health behavior, or health factor variables were excluded from analysis. Hypertension and hyperlipidemia were not included as a covariate since self-reported high blood pressure and high cholesterol were only asked in odd years in BRFSS.
Statistical Analysis
We estimated univariate Satterthwaite-adjusted chi-square tests to compare the differences in weighted percentage for sociodemographic characteristics. Using logistic regression analyses, we estimated the DM prevalence for the three racial/ethnic groups and disaggregated Asian subgroups adjusted for age, sex, education level, obesity, history of CVD, insurance, annual household income, physical activity, and smoking status. As a covariate, obesity was categorized using two approaches. In the first obesity definition, all participants were categorized as having obesity using standard categorization for obesity (BMI ≥30 kg/m2). In the second obesity definition, to account for differences in BMI categorization of obesity in Asian individuals, Asian participants were categorized as having obesity if BMI ≥27.5 kg/m2, and non-Hispanic White and Black participants were categorized as having obesity if BMI ≥30 kg/m2. Multinomial logistic regression models were used to calculate prevalence ratios of DM, adjusted for age group, sex, education level, and either standard obesity categorization or race/ethnicity-specific obesity categorization with NHW as the referent. In a secondary analysis, prevalence ratios were additionally adjusted for access to care variables (presence of a primary provider, inability to see a doctor because of cost, and time since last routine checkup). Two-sided p values for Wald F statistics <0.05 indicated statistical significance.
This study was exempt from human subjects review by the Centers for Disease Control and Prevention. This study used both publicly available BRFSS data and restricted data on Asian subgroups (under a data use agreement). Analyses were conducted using SAS-enabled SUDAAN version 11.0.3 (RTI International, Research Triangle Park, NC, USA) and accounted for the BRFSS complex sample design and respondent sampling weights.
Role of the Funding Source
Research reported in this study was supported in part by the National Heart, Lung, and Blood Institute, grant F32HL149187 to NSS. The funding agency was not involved in study design, conduct, or reporting.
RESULTS
Participant Characteristics
Between 2013 and 2019, there were 57,001 NHA, 215,957 NHB, and 2,143,729 NHW BRFSS participants. Among the NHA participants, 11,089 were Asian Indian, 9458 were Chinese, 9339 were Filipino, 10,387 were Japanese, 2843 were Korean, and 7382 were other Asian Americans. Age, sex, education, insurance status, annual family income, history of CVD, physical activity, smoking, and BMI distributions by race are displayed in Table 1. NHA participants were younger and more frequently had higher education, higher income, no reported history of CVD, and participated in physical activity. NHA participants were also more likely to be non-smokers and not classified as having obesity, compared to NHB and NHW participants (p<0.01 for comparison of the distribution of characteristics between race categories).
Table 1.
Distribution of Participants by Characteristics in the USA, 2013–2019
| Non-Hispanic White N=2,143,729 | Non-Hispanic Black N=215,957 | Non-Hispanic Asian N=57,001 | p* | ||||
|---|---|---|---|---|---|---|---|
| N | Weighted % (95% CI) | N | Weighted % (95% CI) | N | Weighted % (95% CI) | ||
| Age (years) | |||||||
| 18–64 | 1324747 | 75.8 (75.7–75.9) | 154705 | 84.2 (83.9–84.4) | 45797 | 88.5 (87.9–89.1) | <0.01 |
| ≥65 | 818982 | 24.2 (24.1–24.3) | 61252 | 15.8 (15.6–16.1) | 11204 | 11.5 (10.9–12.1) | |
| Sex | |||||||
| Men | 954762 | 49.7 (49.5–49.8) | 82761 | 46.6 (46.2–47.0) | 29192 | 50.3 (49.4–51.1) | <0.01 |
| Women | 1188967 | 50.3 (50.2–50.5) | 133196 | 53.4 (53.0–53.8) | 27809 | 49.7 (48.9–50.6) | |
| Education | |||||||
| Up to high school | 687808 | 36.3 (36.2–36.4) | 91478 | 45.1 (45.0–45.8) | 11125 | 21.7 (20.9–22.5) | <0.01 |
| >High school | 1455921 | 63.7 (63.6–63.9) | 124479 | 54.9 (54.2–55.0) | 45876 | 78.3 (77.6–79.1) | |
| Health insurance | |||||||
| Yes | 2011314 | 91.6 (91.5–91.7) | 191988 | 85.1 (84.8–85.4) | 52770 | 91.2 (90.8–91.7) | <0.01 |
| No | 132415 | 8.4 (8.3–8.5) | 23969 | 14.9 (14.6–15.2) | 4231 | 8.8 (8.3–9.3) | |
| Annual family income | |||||||
| <25k/year | 412480 | 18.0 (17.9–18.1) | 76594 | 33.8 (33.4–34.1) | 9540 | 16.2 (15.6–16.8) | <0.01 |
| 25–50k/year | 466773 | 20.2 (20.1–20.3) | 48118 | 22.4 (22.1–22.7) | 10468 | 16.1 (15.4–16.7) | |
| >50k/year | 982706 | 49.1 (49.0–49.3) | 61625 | 30.3 (30.0–30.7) | 29594 | 52.9 (52.0–53.7) | |
| Missing data | 281770 | 12.7 (12.6–12.8) | 29620 | 13.5 (13.3–13.8) | 7399 | 14.9 (14.2–15.5) | |
| History of CVD | |||||||
| Yes | 257323 | 9.4 (9.3–9.4) | 26511 | 8.9 (8.7–9.1) | 2958 | 3.8 (3.5–4.1) | <0.01 |
| No | 1886406 | 90.7 (90.6–90.7) | 189446 | 91.1 (90.9–91.3) | 54043 | 96.2 (95.9–96.5) | |
| Physical activity | |||||||
| Yes | 1622196 | 77.0 (76.9–77.1) | 148083 | 70.8 (70.4–71.1) | 45338 | 80.7 (80.0–81.4) | <0.01 |
| No | 521533 | 23.0 (22.9–23.1) | 67874 | 29.3 (28.9–29.6) | 11663 | 19.3 (18.6–20.0) | |
| Smoking | |||||||
| Current | 312927 | 17.4 (17.3–17.5) | 37947 | 18.7 (18.4–19.0) | 5045 | 8.2 (7.8–8.6) | <0.01 |
| Former | 663824 | 28.5 (28.4–28.6) | 45162 | 16.9 (16.6–17.2) | 9944 | 13.4 (12.9–14.0) | |
| Never | 1166978 | 54.1 (54.0–54.2) | 132848 | 64.4 (64.0–64.7) | 42012 | 78.4 (77.7–79.1) | |
| Obesity** | |||||||
| Yes | 629530 | 28.9 (28.8–29.0) | 88623 | 38.4 (38.0–38.8) | 12110 | 20.2 (19.4–20.9) | <0.01 |
| No | 1510682 | 71.1 (71.0–71.2) | 126870 | 61.6 (61.2–62.0) | 40927 | 79.8 (79.1–80.5) | |
CVD cardiovascular disease (comprised of self-reported myocardial infarction, angina, coronary artery disease, or stroke). Weighted percent represents estimated national population distribution of characteristics. *p value, univariate Satterthwaite-adjusted χ2 test for comparison of distribution between race categories. **Obesity defined as body mass index ≥30 kg/m2 in non-Hispanic White and Black and ≥27.5 kg/m2 in non-Hispanic Asian
Comparison of characteristics between NHA subgroups is shown in Table 2. Asian Indian Americans had the highest frequency of more than high school education and annual family income >$50,000 per year. Japanese Americans had the highest frequency of being insured and the highest frequency of reporting a history of CVD. Compared to other Asian subgroups, Chinese Americans reported the highest frequency of participating in physical activity, Korean Americans reported the highest frequency of current smoking, and Filipino and Japanese Americans reported the greatest frequency of obesity. Comparisons of distributions of all characteristics between NHA subgroups were statistically significant (p<0.01).
Table 2.
Distribution of Non-Hispanic Asian Participants by Selected Characteristics and Asian Subgroups in the USA, 2013–2019
| Asian Indian N=11,089 | Chinese N=9458 | Filipino N=9339 | Japanese N=10,387 | Korean N=2843 | Other Asian N=7382 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | |
| Age (years)* | ||||||||||||
| 18–64 | 9781 | 91.3 (90.3–92.2) | 7829 | 89.1 (87.6–90.5) | 7334 | 82.8 (80.8–84.6) | 6072 | 67.3 (64.0–70.5) | 2508 | 93.6 (91.5–95.2) | 6661 | 93.1 (91.8–94.1) |
| ≥65 | 1308 | 8.7 (7.8–9.7) | 1629 | 10.9 (9.5–12.4) | 2005 | 17.2 (15.4–19.2) | 4315 | 32.7 (29.5–36.0) | 335 | 6.4 (4.9–8.5) | 721 | 7.0 (5.9–8.2) |
| Sex* | ||||||||||||
| Men | 6768 | 56.9 (55.3–58.6) | 4776 | 48.6 (46.6–50.5) | 4016 | 40.8 (38.5–43.1) | 4744 | 43.6 (40.5–46.8) | 1381 | 48.2 (44.7–51.7) | 4075 | 51.7 (49.6–53.8) |
| Women | 4321 | 43.1 (41.4–44.7) | 4682 | 51.4 (49.5–53.4) | 5323 | 59.2 (56.9–61.5) | 5643 | 56.4 (53.2–59.5) | 1462 | 51.8 (48.3–55.3) | 3307 | 48.3 (46.2–50.4) |
| Education* | ||||||||||||
| Up to high school | 1207 | 16.7 (15.4–18.2) | 1292 | 17.1 (15.5–18.9) | 2745 | 25.5 (23.3–27.2) | 2125 | 22.4 (19.6–25.4) | 571 | 20.0 (17.4–22.9) | 1943 | 29.6 (27.6–31.6) |
| >High school | 9882 | 83.3 (81.8–84.6) | 8166 | 82.9 (81.1–84.5) | 6594 | 74.5 (72.3–76.7) | 8262 | 77.6 (74.6–80.4) | 2272 | 80.0 (77.1–82.6) | 5439 | 70.5 (68.4–72.4) |
| Health insurance* | ||||||||||||
| Yes | 10384 | 92.5 (91.6–93.4) | 8884 | 93.9 (93.0–94.7) | 8578 | 92.0 (90.6–93.2) | 10020 | 95.0 (93.6–96.1) | 2561 | 88.6 (86.3–90.5) | 6467 | 86.6 (85.1–87.9) |
| No | 705 | 7.5 (6.6–8.4) | 574 | 6.2 (5.4–7.1) | 761 | 8.0 (6.8–9.4) | 367 | 5.0 (3.9–6.4) | 282 | 11.4 (9.5–13.7) | 915 | 13.4 (12.1–14.9) |
| Annual family income* | ||||||||||||
| <25k/year | 1370 | 13.2 (12.0–14.4) | 1424 | 15.9 (14.5–17.4) | 2164 | 19.2 (17.3–21.1) | 1438 | 11.4 (9.8–13.2) | 471 | 13.3 (11.4–15.4) | 1569 | 20.5 (19.0–22.1) |
| 25–50k/year | 1293 | 12.2 (11.1–13.4) | 1517 | 14.0 (12.7–15.5) | 2259 | 19.2 (17.4–21.1) | 2171 | 18.2 (15.7–20.9) | 496 | 17.4 (14.9–20.3) | 1592 | 19.6 (18.0–21.3) |
| >50k/year | 7003 | 61.4 (59.8–63.0) | 5222 | 54.9 (53.0–56.9) | 3835 | 47.8 (45.4–50.2) | 5671 | 57.4 (54.2–60.5) | 1493 | 53.7 (50.2–57.2) | 3098 | 43.1 (41.1–45.3) |
| History of CVD* | ||||||||||||
| Yes | 482 | 3.8 (3.2–4.4) | 370 | 2.8 (2.2–3.6) | 555 | 5.7 (4.6–7.1) | 840 | 6.7 (5.2–8.4) | 105 | 2.2 (1.5–3.3) | 294 | 3.0 (2.3–3.8) |
| No | 10607 | 96.3 (95.6–96.8) | 9088 | 97.2 (96.4–97.8) | 8784 | 94.3 (92.9–95.4) | 9547 | 93.3 (91.6–94.8) | 2738 | 97.8 (96.7–98.5) | 7088 | 97.1 (96.2–97.7) |
| Physical activity* | ||||||||||||
| Yes | 8967 | 80.3 (79.0–81.6) | 7822 | 83.1 (81.6–84.6) | 7037 | 79.5 (77.5–81.3) | 8248 | 80.9 (78.2–83.3) | 2333 | 82.4 (79.7–84.8) | 5710 | 78.8 (77.1–80.4) |
| No | 2122 | 19.7 (18.4–21.0) | 1636 | 16.9 (15.4–18.4) | 2302 | 20.5 (18.7–22.5) | 2139 | 19.1 (16.7–21.8) | 510 | 17.6 (15.2–20.3) | 1672 | 21.2 (19.7–22.9) |
| Smoking* | ||||||||||||
| Current | 780 | 6.2 (5.5–7.0) | 555 | 6.0 (5.2–7.0) | 929 | 9.1 (7.9–10.5) | 933 | 9.7 (7.9–12.0) | 424 | 14.0 (12.0–16.3) | 801 | 9.7 (8.6–10.9) |
| Former | 1423 | 10.8 (9.9–11.7) | 1189 | 10.1 (9.0–11.3) | 1737 | 17.1 (15.4–18.9) | 2972 | 26.5 (23.7–29.6) | 646 | 18.6 (16.2–21.2) | 1005 | 11.8 (10.6–13.1) |
| Never | 8886 | 83.0 (81.8–84.1) | 7714 | 83.9 (82.4–85.2) | 6673 | 73.8 (71.7–75.9) | 6482 | 63.8 (60.6–66.9) | 1773 | 67.4 (64.3–70.4) | 5576 | 78.5 (76.9–80.1) |
| Obesity* | ||||||||||||
| BMI ≥27.5 | 2165 | 21.7 (20.4–23.2) | 1295 | 12.1 (10.8–13.5) | 2601 | 27.4 (25.3–29.7) | 2733 | 27.2 (24.2–30.4) | 525 | 16.2 (13.9–18.8) | 1545 | 21.3 (19.6–23.1) |
| BMI <27.5 | 7917 | 78.3 (76.9–79.6) | 7565 | 87.9 (86.6–89.2) | 6191 | 72.6 (70.3–74.7) | 7276 | 72.8 (69.6–75.9) | 2131 | 83.8 (81.2–86.1) | 5259 | 78.7 (76.7–80.4) |
CVD cardiovascular disease (comprised of self-reported myocardial infarction, angina, coronary artery disease, or stroke), BMI body mass index (kg/m2). Percent column represents weighted percent estimating national population distribution of characteristics. Participants missing data for income not shown in table. *p<0.01, univariate Satterthwaite-adjusted χ2 test for comparison of distribution between Asian subgroup categories
Diabetes Prevalence
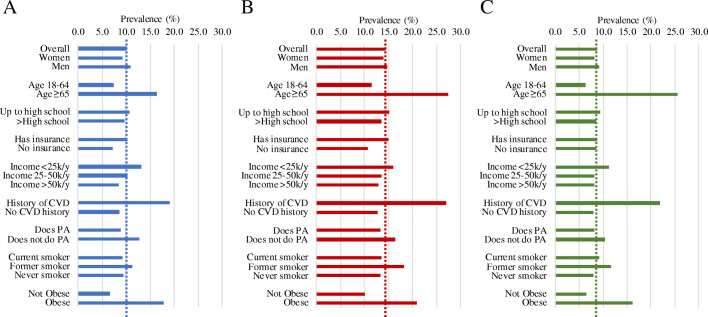
Adjusted self-reported DM prevalence by demographic and cardiovascular health characteristics is shown in race groups in Figure 1 and Supplemental Table 2. Overall adjusted self-reported DM prevalence in NHA was 8.7% (95% confidence interval 8.2–9.3), compared to 14.3% (14.0–14.6) in NHB and 10.0% (10.0–10.1) in NHW. Among participants age ≥65 years, DM prevalence was 25.5% (22.8–28.4) in NHA, compared to 27.4% (26.6–28.2) in NHB, and 16.3% (16.2–16.5) in NHW. Among women, DM prevalence was 8.2% (7.5–9.0) in NHA, 14.0% (13.6–14.3) in NHB, and 9.2% (9.1–9.3) in NHW. Among men, DM prevalence was 9.1% (8.5–9.8) in NHA, 14.7% (14.3–15.1) in NHB, and 10.9% (10.8–11.0) in NHW. Among participants who reported a history of CVD, 21.8% (18.4–25.6) of NHA reported DM, compared to 26.9% (25.9–28.0) in NHB and 19.0% (18.8–19.3) in NHW. The distribution of DM prevalence between race/ethnicity groups across all demographic and clinical characteristics was statistically significant (p<0.01).
Figure 1.
Adjusted self-reported diabetes prevalence in the United States among race/ethnic groups, 2013–2019. CVD cardiovascular disease, BMI body mass index, NHA non-Hispanic Asian, NHB non-Hispanic Black, NHW non-Hispanic white, PA physical activity. p<0.01 for differences across race subgroups in adjusted diabetes prevalence by characteristic for all comparisons. 95% confidence intervals of weighted diabetes prevalence are shown in Supplemental Table 3. Obesity defined as body mass index ≥30 kg/m2 in non-Hispanic White and Black and ≥27.5 kg/m2 in non-Hispanic Asian. Prevalence is adjusted for age, sex, education level, obesity, history of CVD, insurance, annual household income, physical activity, and smoking status. Where prevalence is stratified by one factor, estimates are adjusted for all other factors. Dotted line represents overall prevalence of each group.
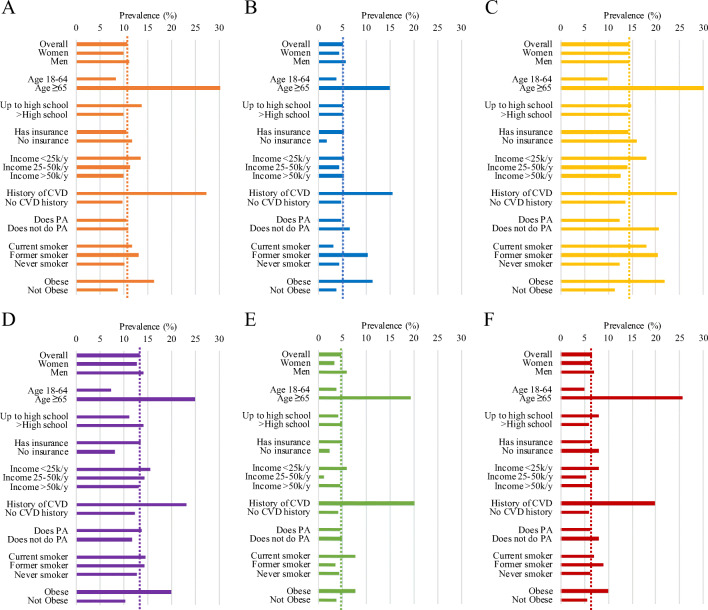
Adjusted self-reported DM prevalence by demographic and cardiovascular health characteristics in NHA subgroups is shown in Figure 2 and Supplemental Table 3. Overall prevalence was 14.4% (12.6–16.3) in Filipino American, 13.4% (10.9–16.2) in Japanese, 10.7% (9.6–11.8) in Asian Indians, 5.1% (4.2–6.2) in Chinese, and 4.7% (3.4–6.3)% in Korean Americans. Among older participants age ≥65 years, DM prevalence was the highest in Filipino Americans (35.0% [29.4–41.2]) and Asian Indian Americans (31.5% [25.9–37.8]). The distribution of DM prevalence across all demographic and clinical characteristics between NHA subgroups was statistically significant overall, and by age, sex, income, history of CVD, physical activity, smoking status, and BMI (p<0.01). By gender, DM prevalence was highest in Filipina American (14.4% [12.2–17.0]) and Japanese American women (12.8% [9.9–16.5]), and Filipino American (14.3% [12.0–16.9]) and Japanese American men (14.1% [10.7–18.4]).
Figure 2.
Adjusted self-reported diabetes prevalence in the United States among Asian subgroups, 2013–2019. CVD cardiovascular disease, BMI body mass index, PA physical activity. p<0.01 for differences across Asian subgroups in adjusted diabetes prevalence overall and by age, income, history of CVD, participation in physical activity, smoking status, and obesity. 95% confidence intervals of weighted diabetes prevalence are shown in Table 2. Prevalence is adjusted for age, sex, education level, obesity, history of CVD, insurance, annual household income, physical activity, and smoking status. Where prevalence is stratified by one factor, estimates are adjusted for all other factors. Dotted line represents overall prevalence of each group.
Finally, crude and adjusted prevalence ratios of DM in race groups are listed in Table 3 and Table 4. After adjustment for age, sex, education, and race/ethnicity-specific obesity categorization, the prevalence ratio of DM in NHA was 1.21 (95% confidence interval (1.14–1.27) compared to NHW and 0.85 (0.80–0.90) compared to NHB. In Asian subgroups, the highest adjusted DM prevalence ratios relative to NHW were in Filipino Americans (1.42 [1.24–1.63]) and Asian Indian Americans (1.29 [1.14–1.46]). Conversely, adjusted DM prevalence ratios were lower relative to NHW in Chinese Americans (0.70 [0.57–0.85]) and Korean Americans (0.74 [0.56–0.97]). In a secondary analysis further adjusting for access to care variables, findings were similar (Supplemental Table 4).
Table 3.
Adjusted Prevalence Ratios of Diabetes in the USA among Race/Ethnic Groups, 2013–2019
| Non-Hispanic White N=2,143,729 | Non-Hispanic Black N=215,957 | Non-Hispanic Asian N=57,001 | p* | |
|---|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | ||
| Relative to non-Hispanic White | ||||
| Crude | 1 (reference) | 1.43 (1.40–1.46) | 0.86 (0.81–0.92) | <0.01 |
| Model 1 | 1 (reference) | 1.56 (1.53–1.59) | 1.10 (1.04–1.16) | <0.01 |
| Model 2 | 1 (reference) | 1.42 (1.40–1.45) | 1.37 (1.30–1.44) | <0.01 |
| Model 3 | 1 (reference) | 1.42 (1.40–1.45) | 1.21 (1.14–1.27) | <0.01 |
| Relative to non-Hispanic Black | ||||
| Crude | 0.70 (0.69–0.71) | 1 (reference) | 0.61 (0.57–0.65) | <0.01 |
| Model 1 | 0.64 (0.63–0.65) | 1 (reference) | 0.71 (0.67–0.75) | <0.01 |
| Model 2 | 0.70 (0.69–0.72) | 1 (reference) | 0.96 (0.91–1.01) | <0.01 |
| Model 3 | 0.70 (0.69–0.72) | 1 (reference) | 0.85 (0.80–0.90) | <0.01 |
PR prevalence ratio, CI confidence interval. Model 1 (adjusted for age, sex, education), model 2 (model 1 + adjusted for obesity defined by standard BMI categories), model 3 (model 1 + race/ethnicity-specific obesity categorization, defined by standard BMI for non-Hispanic White and Black and Asian-specific BMI for non-Hispanic Asian). *p value, Wald F statistic for comparison of prevalence between racial/ethnic groups
Table 4.
Adjusted Prevalence Ratio of Diabetes in the USA among Asian Subgroups, 2013–2019
| Non-Hispanic White N=2,143,729 | Asian Indian N=11,089 | Chinese N=9458 | Filipino N=9339 | Japanese N=10,387 | Korean N=2843 | Other Asian N=7382 | P | |
|---|---|---|---|---|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | |||
| Crude | 1 (ref.) | 0.93 (0.82–1.05) | 0.46 (0.37–0.56) | 1.26 (1.09–1.45) | 1.16 (0.95–1.43) | 0.45 (0.33–0.59) | 0.59 (0.49–0.72) | <0.01 |
| Model 1 | 1 (ref.) | 1.18 (1.04–1.33) | 0.56 (0.46–0.69) | 1.36 (1.18–1.57) | 0.94 (0.76–1.16) | 0.62 (0.47–0.82) | 0.78 (0.65–0.94) | <0.01 |
| Model 2 | 1 (ref.) | 1.47 (1.30–1.68) | 0.76 (0.62–0.93) | 1.62 (1.41–1.87) | 1.13 (0.92–1.39) | 0.83 (0.63–1.09) | 0.98 (0.81–1.18) | <0.01 |
| Model 3 | 1 (ref.) | 1.29 (1.14–1.46) | 0.70 (0.57–0.85) | 1.42 (1.24–1.63) | 0.97 (0.79–1.19) | 0.74 (0.56–0.97) | 0.86 (0.71–1.03) | <0.01 |
PR prevalence ratio, CI confidence interval. Model 1 (adjusted for age, sex, education), model 2 (model 1 + adjusted for obesity defined by standard BMI categories), model 3 (model 1 + adjusted for race/ethnicity-specific obesity categorization, defined by standard BMI for non-Hispanic White and Black and Asian-specific BMI for non-Hispanic Asian). *p value Wald F statistics for comparison of prevalence between Asian subgroups
DISCUSSION
In this contemporary nationally representative analysis, self-reported DM prevalence in an aggregate NHA category masked important heterogeneity in the burden of DM among Asian Americans. NHA in aggregate reported a lower DM prevalence compared to NHB and NHW; however, after adjusting for sex, education, and BMI, NHA had an approximately 20% higher prevalence of DM compared to NHW. Filipino Americans had the highest prevalence of DM compared to all other NHA subgroups and compared to NHB and NHW. Asian Indians and Japanese Americans also reported a higher prevalence of DM compared to NHW, but Chinese and Korean Americans reported a lower prevalence. DM prevalence in NHA was generally higher in older individuals, men, individuals with a history of CVD, current or former smokers, those who were not physically active, and those categorized as having obesity.
These data provide a more detailed understanding of self-reported DM prevalence at the self-identified NHA subgroup level and identify important differences in known (i.e., diagnosed) DM between NHA subgroups in the USA indicating that health status in the NHA population is likely heterogenous. Prevalence estimates from the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2016 showed a similar relative prevalence of DM, higher in South and Southeast Asian Americans compared to that in East Asian Americans. These NHANES data notably identified a 27% overall adjusted prevalence (self-reported plus undiagnosed DM) and 17% adjusted prevalence of self-reported DM in NHA adults (adjusted for age, sex, and BMI).2 Our findings of self-reported DM prevalence in BRFSS of 8.9 percentage points lower than found in NHANES may represent a sampled population with a higher rate of undiagnosed DM. Identifying and addressing undiagnosed DM is an important public health strategy to prevent DM-related morbidity and mortality, particularly for NHA subgroups that are disproportionately affected in the context of projected increase of diabetes prevalence by 54% to approximately 55 million in the US by 2030.8 Prior analysis in NHANES suggests that over 50% of DM in NHA (in aggregate) is undiagnosed, indicating substantial opportunity for improvement.9
The findings in BRFSS confirm important differences in DM prevalence between NHA subgroups that may be related to differences in sociocultural factors and social determinants of health, dietary patterns, physical activity norms, metabolic physiology, and demographics including age distribution of individual subgroups (e.g., Japanese Americans). For example, prior research has shown Asian Indians may have lower beta cell function and lower ability to compensate for higher glucose levels compared to individuals in other race/ethnic groups, leading to relatively higher DM prevalence.10,11 High rates of DM have also previously been described in the Filipino American population in non-nationally representative samples,12,13 and behavioral factors were hypothesized as a primary contributing factor.14 There are also differences in diabetes prevalence in NHA subgroups in the US and their respective counterpart populations in Asia. In 2019, estimated overall age-adjusted diabetes prevalence was 10.4% in India, 9.2% in China, 7.1% in the Philippines, 5.6% in Japan, and 6.9% in South Korea.15 Whether genetic differences in susceptibility to DM exist in individuals of Asian ancestry and between Asian ancestry subgroups remains an area of active study, although currently identified type 2 DM genetic variants do not appear to explain disproportionate susceptibility to type 2 DM compared to populations of European ancestry.16 Overall, however, a paucity of data exist on contributors to variations in DM among Asian American subgroups, and additional research is needed to appreciate how multi-level factors—including environment, social determinants, behavioral factors such as nutrition and physical activity, and physiologic differences—may account for these differences between subgroups, and differences between NHA subgroups and their counterpart populations in Asia.
DM is a strong risk factor for CVD,17 which is responsible for significant premature mortality in NHA.18,19 Differences in DM may contribute to the disproportionate burden of CVD in specific NHA subgroups, such as in Asian Indians.20 We demonstrate that in NHA in aggregate, adjusted DM prevalence is similar to that of the NHW population in the US. However, disaggregation reveals wide differences in the prevalence of diagnosed DM that are masked in aggregated data, with particularly high prevalence in Asian Indian and Filipino Americans. Undiagnosed DM may show similar variability, as certain Asian American subgroups may experience barriers to health care utilization related to health literacy, English language fluency, and cultural differences in attitudes and acceptance of health care services, which may lead to lower awareness of, and less frequent identification and a lower likelihood of, screening for DM.21,22 These findings have important implications for targeting early prevention of type 2 DM, timely diagnosis, and adequate DM management strategies particularly to populations experiencing both high prevalence of DM and excess CVD in order to reduce disparities. Our findings may therefore inform efforts to reduce the burden of DM in the US at the individual, health system, community, and broader population levels.
The main strength of these data is that prevalence estimates are disaggregated into the five largest NHA subgroups in the US, which is necessary for accurate representation of the NHA population in health research.23 There are, however, several limitations. First, our results are based on self-report information, which may be subject to recall and reporting bias. Second, our estimates reflect the prevalence of diagnosed DM, as we are not able to assess undiagnosed DM in this sample since laboratory measures are not collected in BRFSS. The state-level BRFSS data were not weighted to be representative of NHA subcategories, so our findings provide an estimate of the relative burden of self-reported DM. Additionally, because of the limitations of the data collected in BRFSS and lack of survey administration in Asian-specific languages, we are unable to study in detail the roles of healthcare utilization, health literacy, English language fluency, environmental factors, immigration-related factors including time since immigration or immigrant generation, and social and cultural contributors in the observed prevalence distributions. Furthermore, these data are unable to identify differences in rates of undiagnosed diabetes that may exist between NHA subgroups, since only self-reported data are available. Given relatively small sample sizes of NHA subgroups, we also could not reliably estimate DM prevalence in smaller age groups, and we acknowledge that age-related DM prevalence differences may exist within our identified age groups.
This report provides a valuable description of the burden of DM among Asian American subgroups in the US. Overall, from 2013 to 2019, DM prevalence in NHA in aggregate (8.7%) was approximately similar to that of NHW populations (10.0%). However, disaggregated data showed significant variability in DM prevalence in NHA subgroups, with Chinese and Korean Americans having the lowest DM prevalence and Filipino, Japanese, and Asian Indian Americans having the highest. These data can inform public health practitioners and clinicians to more directly tailor programs and interventions to specific populations.
Supplementary Information
(DOCX 46 kb)
Funding
Funding support includes National Heart, Lung, and Blood Institute grant F32HL149187 to NSS.
Conflict of Interest
The authors report no conflicts of interest related to this work.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020.
- 2.Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, et al. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011-2016. JAMA. 2019;322(24):2389–98. doi: 10.1001/jama.2019.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pew Research Center. “Asian Americans are the fastest-growing racial or ethnic group in the U.S.” 2021. Accessed at https://www.pewresearch.org/fact-tank/2021/04/09/asian-americans-are-the-fastest-growing-racial-or-ethnic-group-in-the-u-s/ on 12 April 2021.
- 4.Holland AT, Palaniappan LP. Problems with the collection and interpretation of Asian-American health data: omission, aggregation, and extrapolation. Ann Epidemiol. 2012;22(6):397–405. doi: 10.1016/j.annepidem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palaniappan LP, Araneta MR, Assimes TL, Barrett-Connor EL, Carnethon MR, Criqui MH, et al. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122(12):1242–52. doi: 10.1161/CIR.0b013e3181f22af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang J, Luncheon C, Patel A, Ayala C, Gillespie C, Greenlund KJ, et al. Self-Reported Prevalence of Hypertension and Antihypertensive Medication Use Among Asian Americans: Behavioral Risk Factor Surveillance System 2013, 2015 and 2017. J Immigr Minor Health. 2020. [DOI] [PMC free article] [PubMed]
- 7.Expert Consultation WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 8.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag. 2017;20(1):6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015;314(10):1021–9. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 10.Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ, et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621–8. doi: 10.2337/dc13-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanaya AM, Wassel CL, Mathur D, Stewart A, Herrington D, Budoff MJ, et al. Prevalence and correlates of diabetes in South asian indians in the United States: findings from the metabolic syndrome and atherosclerosis in South asians living in america study and the multi-ethnic study of atherosclerosis. Metab Syndr Relat Disord. 2010;8(2):157–64. doi: 10.1089/met.2009.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karter AJ, Schillinger D, Adams AS, Moffet HH, Liu J, Adler NE, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE) Diabetes Care. 2013;36(3):574–9. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller-Thomson E, Roy A, Tsz-Kit Chan K, Kobayashi KM. Diabetes among non-obese Filipino Americans: Findings from a large population-based study. Can J Public Health. 2017;108(1):e36–e42. doi: 10.17269/CJPH.108.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas P. Dietary Intake and Obesity among Filipino Americans in New Jersey. J Environ Public Health. 2018;2018:6719861. doi: 10.1155/2018/6719861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Diabetes Federation. “Diabetes Atlas.” 2019. Accessed at https://diabetesatlas.org on April 5 2021. [PubMed]
- 16.Qi Q, Wang X, Strizich G, Wang T. Genetic Determinants of Type 2 Diabetes in Asians. Int J Diabetol Vasc Dis Res. 2015;2015(Suppl 1). [DOI] [PMC free article] [PubMed]
- 17.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–58. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jose PO, Frank AT, Kapphahn KI, Goldstein BA, Eggleston K, Hastings KG, et al. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol. 2014;64(23):2486–94. doi: 10.1016/j.jacc.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer DG, Shah NS, Hastings KG, Hu J, Rodriguez F, Boothroyd DB, et al. Years of Potential Life Lost Because of Cardiovascular Disease in Asian-American Subgroups, 2003-2012. J Am Heart Assoc. 2019;8(7):e010744. doi: 10.1161/JAHA.118.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volgman AS, Palaniappan LS, Aggarwal NT, Gupta M, Khandelwal A, Krishnan AV, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: Epidemiology, risk factors, and treatments: A scientific statement from the American Heart Association. Circulation. 2018;138(1):e1–e34. doi: 10.1161/CIR.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 21.Tung EL, Baig AA, Huang ES, Laiteerapong N, Chua KP. Racial and Ethnic Disparities in Diabetes Screening Between Asian Americans and Other Adults: BRFSS 2012-2014. J Gen Intern Med. 2017;32(4):423–9. doi: 10.1007/s11606-016-3913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Martinez G, Ma GX, Hsu CE, Robinson ES, Bawa J, et al. Barriers to health care access in 13 Asian American communities. Am J Health Behav. 2010;34(1):21–30. doi: 10.5993/AJHB.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah NS, Kandula NR. Addressing Asian American Misrepresentation and Underrepresentation in Research. Ethn Dis. 2020;30(3):513–6. doi: 10.18865/ed.30.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 46 kb)