Abstract
Intraductal papillary mucinous neoplasms (IPMN) are mucin producing tumors which arise from epithelial cells of the main pancreatic duct, pancreatic branch ducts, or both. They are characterized by mucin-producing columnar cells, papillary ductal proliferation, cyst formation, and varying degrees of dysplasia. IPMNs are classified as main duct or branch duct based upon the pancreatic duct anatomy which the IPMN is arising from. Additionally, they can be classified based on their histologic subtypes, which carry varying associations with dysplasia and/or malignancy. Many patients have incidentally identified IPMNs, which are asymptomatic. However, patients may also present with pancreatitis, elevation of liver enzymes, dilation of the pancreatic duct or bile duct as well as distention of the ampullary pancreatic orifice(s), due to impaction and obstruction with mucus. This is known as an endoscopically visualized “fish eye” sign. Patients may also develop exocrine and endocrine pancreatic insufficiency and maldigestion. Some studies also suggest that patients with IPMNs may also be at increased risk for gastric, colorectal, biliary, renal cell, and thyroid malignancies. Rarely, IPMNs can be complicated by fistulation between the main pancreatic duct and neighboring organs. Herein, we present an unusual case of simultaneous fistulation to both the gastric body and the duodenum.
Keywords: Pancreatic fistula, IPMN, Upper gastrointestinal bleed, Mucinous tumor, Papillary neoplasm
Introduction
Intraductal papillary mucinous neoplasms (IPMN) of the pancreas are a spectrum of benign to malignant neoplasms, characterized by papillary proliferations of mucin-producing epithelial cells, ductal dilation and cyst formation [1], [2], [3]. IPMNs have different subtypes, however, all originate from epithelial cells of the pancreatic ducts [4]. The currently identified phenotypes include intestinal, pancreaticobiliary, oncocytic and gastric [4]. Additionally, IPMNs are classified as main duct or branch duct based upon the pancreatic duct anatomy which the IPMN is arising from. Main duct IPMNs (MD-IPMN) involve the main pancreatic duct and are more likely to harbor a malignancy then a branch duct IPMN [5,6]. Some publications suggest that the risk of carcinoma in situ or invasive carcinoma in main duct IPMNs is up to 70%t [7,8]. Whereas branch duct IPMNs (BD-IPMN) are at a significantly lower risk for developing malignancy approaching approximately 20% at 10 years [1,2].
The risk factors and etiology of IPMNs are not clear [4]. However, recent studies have shown an association with diabetic patients on insulin, smokers, those with chronic pancreatitis and a family history of pancreatic ductal adenocarcinoma [4]. Nearly 5% of all cystic pancreatic lesions are IPMNs and the majority are diagnosed in older male patients [1].The incidence of IPMNs are gradually increasing due to the advancements in diagnostic technologies [1,4].
Many patients are asymptomatic with IPMNs being identified incidentally [2,3]. The clinical approach to management is contingent on clinical risk factors, symptoms, complications and potential for progression to invasive carcinoma. Current guidelines suggest surveillance at 2-3 years for cysts less than 1 cm, annual follow-up for a total of 2 years in those with cysts sized 1-2 cm, and closer surveillance every 3-6 months for patients with cysts larger than 2 centimeters [2].
Case
A 76-year-old female with a past medical history of coronary artery disease, hypertension, chronic kidney disease, aortic stenosis, and sick sinus syndrome with automatic implanted cardiac defibrillator, presented to the Emergency Department (ED) with acute epigastric abdominal pain and unintentional 10-pound weight loss over the course of 6 months. Examination revealed an alert, afebrile African American female in no acute distress with epigastric tenderness to superficial and deep palpation. Bowel sounds were normoactive, abdomen was soft and non-distended. Physical examination otherwise was unremarkable. Labs revealed leukocytosis without left shift or bandemia (white blood cells 20.2 × 10^3/mm3; reference range 4.5-11.0), microcytic anemia (hemoglobin 7.4 g/dL; reference range 12-16, hematocrit 21.9 %; reference range 36-46, mean corpuscular volume 79.9 fL; reference range 80-100), abnormal renal function due to underlying chronic kidney disease (blood urea nitrogen 50 mg/dL; reference range 7-20, Creatinine 2.62 mg/dL; reference range 0.6-1.3), and hyperglycemia (glucose 141 mg/dL; reference range <100). Liver function enzymes, lipase and amylase were within normal limits.
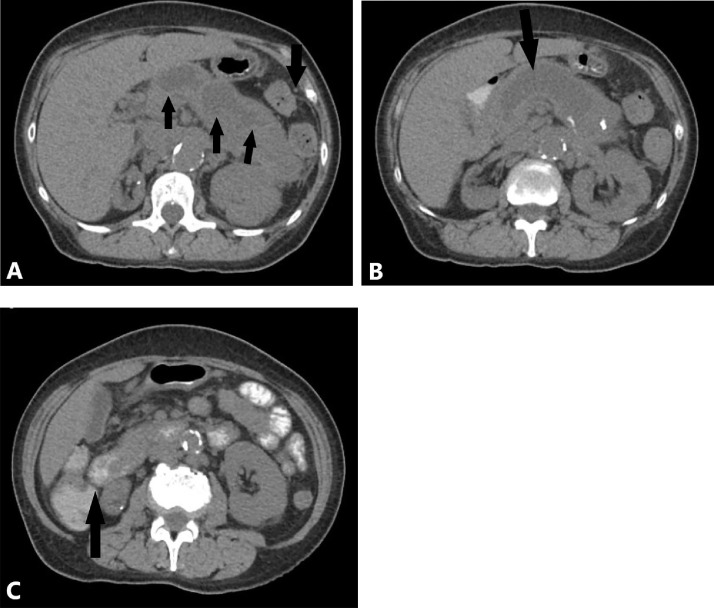
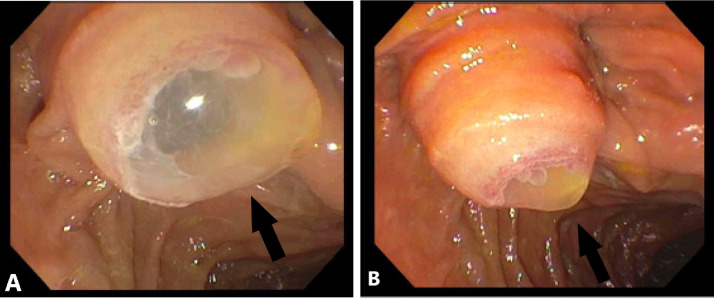
A computerized-tomography (CT) scan of the abdomen was obtained and revealed marked dilatation of the pancreatic duct, measuring 3.8 cm, with distention of the ampulla causing intraluminal protrusion into the duodenum. Numerous cysts were visualized in the tail of the pancreas measuring up to 5 centimeters in maximal cross sectional diameter. The spleen was displaced laterally and superiorly due to enlargement of the pancreatic tail (Fig. 1). A diagnostic endoscopic ultrasound and endoscopic retrograde cholangiopancreatography (ERCP) were performed. A main duct IPMN was identified with a bulging ampulla and classical “fish-eye” appearance of inspissated mucin visualized on ERCP (Fig. 2). No focal source of malignancy was noted. Pathology confirmed the presence of an IPMN and she was referred to surgery for a distal pancreatectomy.
Fig. 1.
(A-C) Enlarged and cystic pancreas on computerized tomography (CT). CT without contrast, axial view demonstrating (A) laterally and superiorly displaced spleen (arrow) due to diffusely enlarged pancreatic tail with numerous large cysts (arrows), measuring up to 5 centimeters with focal inflammation, (B) dilated pancreatic duct measuring up to 3.8 centimeters in diameter and (C) distended ampulla (arrow) protruding into the duodenum.
Fig. 2.
(A-B) Endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) demonstrating “fish-eye” appearance. Arrows indicate fish mouth appearance of major papilla extruding mucin. Copious amounts of thick mucinous material were found in the pancreatic duct.
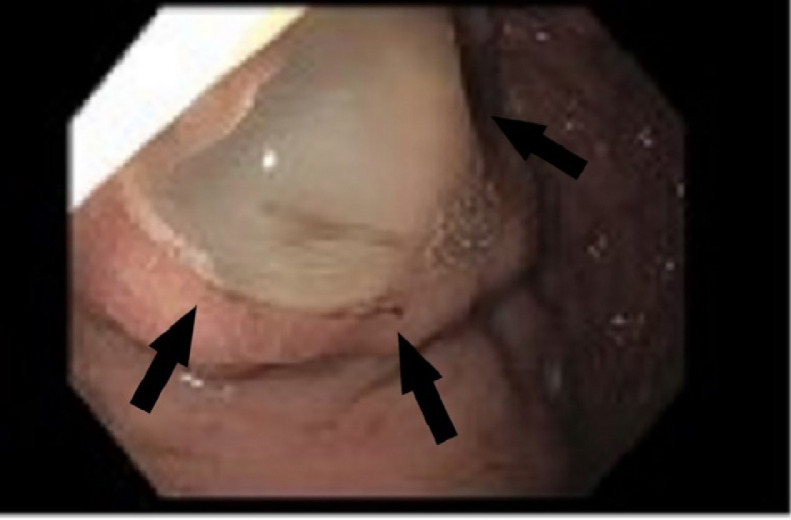
Due to intractable abdominal pain, she was re-admitted to the hospital and treated for transverse-descending colitis with intravenous piperacillin-tazobactam. A repeat CT of the abdomen was performed demonstrating interval progression of the dilatation involving the main pancreatic duct with extension of the ampulla into the lateral wall of the duodenum and questionable extension into the spleen and gastric fundus concerning for fistulous communication between the spleen and gastric fundus. Esophagogastroduodenoscopy was performed, revealing mucin protruding from a large ulcerated and bleeding gastropancreatic fistula along the greater curvature of the proximal gastric body (Fig. 3). The patient immediately underwent a diagnostic laparoscopy, exploratory laparotomy, takedown and repair of a 2-cm gastropancreatic fistula from the superior portion of the pancreatic body to the gastric body and a repair of a 0.5-cm colopancreatic fistula from the inferior portion of the pancreatic body to the splenic flexure. The surgeons also found diffusely thick, large pancreas with mucin extravasation with dense adhesions and another pancreatic fistula within the greater omentum and gastric buttresses requiring partial omentectomy. Due to recurrent bleeding, the patient ultimately underwent total pancreatectomy, gastric wedge resection and splenectomy. Biopsy results demonstrated invasive mucinous adenocarcinoma arising from the IPMN with abundant fibrosis and fat necrosis replacing the entire parenchyma of the pancreatic tail. The final staging was T3N1M0. Postoperatively, she developed an enterocutaneous fistula and was referred to Oncology for chemotherapy with FOLFIRINOX C1D2.
Fig. 3.
Esophagogastroduodenoscopy (EGD) demonstrating mucin producing tumor. Arrow indicates mucin protruding from a large gastro-pancreatic fistula along the greater curvature of the proximal gastric body.
Discussion
In this case, we observed an unusual presentation of an IPMN forming simultaneous fistulous tracts to the gastric, splenic, duodenal, colonic lumens as well as the omentum. Although the IPMN was pathologically benign at time of fistulation to the gastric body, the final pathology results were positive for invasive adenocarcinoma at the time of fistulation to other organs.
IPMNs are generally complicated by biliary/pancreatic ductal dilation, pancreatitis, hemorrhage, acute pancreatitis, abscess, or perforation; however, fistulation is not common [3]. The first known case of fistulation to adjacent organs was reported in 1980 as a pancreaticobiliary fistula, however other studies have shown fistula formation in the duodenum, common bile duct or stomach [1,4]. Fistulous tracts predominate along main duct-IPMNs and are incidentally found in multiples, usually within the same organ tissue [9]. Main duct-IPMN's however, are not found in multiple organs simultaneously [9]. Fistula formation into an adjacent structure may occur from direct invasion, elevated intraductal pressures, or secondary to inflammation [3].
Pancreatic IPMN's progress from benign neoplasms to invasive neoplasms in an average span of 5-6 years [4,10]. Noninvasive neoplasms can be classified into three types of cytoarchitectural atypia, stratifying noninvasive IPMNs into low, intermediate or high grade dysplasia [10]. When they become invasive carcinoma, they are classified by 2 types: tubular, which arises from the pancreaticobiliary structures, and colloid carcinoma, which is of the intestinal mucinous glands. Colloid carcinoma has an overall better prognosis 4,10]. Advances in genetics have allowed clinicians to better understand the pathogenesis behind this malignant transformation [11]. Additionally, advances in technology over the last two decades have allowed clinicians to diagnose and treat IPMN's with the use of MRCP, ERCP, endoscopic ultrasound, but also allows for surveillance for malignant transformation [5,7].
It is important to closely monitor patients who have developed fistulae, as not only do they progress to malignancy but also have varying complications depending on the organs invaded [9,12]. Once IPMN progress to invasive carcinoma, tumors can cause symptoms such as pain, weight loss, pancreatitis, or pancreatic insufficiency [12]. IPMN fistula can be treated endoscopically [2].
Conclusion
Many IPMNs are found incidentally and can rarely be complicated with fistulae. When treating IPMNs that have become complicated by the presence of fistula/ae, they must be removed to avoid possible malignant dissemination and/or be more frequently surveillance outpatient for growth.
Authorship statement
Tagliaferri, A., M.D. and Estifan E., M.D. performed the literature review and wrote the manuscript, Farohkian, A., M.D. and Melki, G., M.D. assisted in the collection of the patient's clinical data and final editing. All work was performed at St. Joseph's University Medical Center, at the following address:
Patient consent statement
As this is a case report, consent was obtained for the purpose of this paper.
Footnotes
Competing Interests: The authors report no conflict of interest. Ethical review is not necessary, because this is a case report. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments: We would like to acknowledge the patient and her family for allowing us to share this case with our colleagues. We would also like to thank Dr. Cavanagh and Dr. Grossman for their support in writing and submitting this article.
References
- 1.KY Vusal Aliyev, Badawy Amr. Pancreatico-gastric fistula: a rare complication of intraductal papillary mucinous neoplasm. JOP: J Pancreas. 2017;18(4):362–364. [Google Scholar]
- 2.Patel A, Allen A, Kuwahara J, Wadsworth T, Loeffler DM, Xie KL. Intraductal papillary mucinous neoplasm complicated by a gastropancreatic fistula. Radiol Case Rep. 2018;14:320–323. doi: 10.1016/j.radcr.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estifan E, Farokhian A, Melki G, Cavanagh Y, Grossman M. S1487 Simultaneous pancreatico-enteric fistulae: a complication of pancreatic intraductal papillary mucinous neoplasm. Am J Gastroenterol. 2020;115:S757. doi: 10.14309/01.ajg.0000707996.90276.b0. [DOI] [Google Scholar]
- 4.Puckett Y, Sharma B, Kasi A. StatPearls; Treasure IslandFL: 2020. Intraductal papillary mucinous cancer of the pancreas. [PubMed] [Google Scholar]
- 5.Serikawa M, Sasaki T, Fujimoto Y, Kuwahara K, Chayama K. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol. 2006;40:856–862. doi: 10.1097/01.mcg.0000225609.63975.6f. [DOI] [PubMed] [Google Scholar]
- 6.Lévy P, Jouannaud V, O'Toole D, Couvelard A, Vullierme MP, Palazzo L, et al. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460–468. doi: 10.1016/j.cgh.2006.01.018. 10.1016/j.cgh.2006.01.018 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 8.Crippa S, Aleotti F, Longo E, Belfiori G, Partelli S, Tamburrino D, et al. Main duct thresholds for malignancy are different in intraductal papillary mucinous neoplasms of the pancreatic head and body-tail. Clin Gastroenterol Hepatol. 2022;20:390–399. doi: 10.1016/j.cgh.2020.12.028. e397. [DOI] [PubMed] [Google Scholar]
- 9.Ravaud S, Laurent V, Jausset F, Cannard L, Mandry D, Oliver A, et al. CT and MR imaging features of fistulas from intraductal papillary mucinous neoplasms of the pancreas to adjacent organs: a retrospective study of 423 patients. Eur J Radiol. 2015;84:2080–2088. doi: 10.1016/j.ejrad.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012;43:1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Wei T, Zhang J, Liang T. Intraductal papillary mucinous neoplasms of the pancreas: a review of their genetic characteristics and mouse models. Cancers (Basel) 2021;13 doi: 10.3390/cancers13215296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortegoso Valdivia P, Bruno M, Gaia S, Saracco GM, De Angelis C. A rare case of gastric fistulization of a main-duct intraductal papillary mucinous neoplasm. Minerva Gastroenterol Dietol. 2018;64:383–385. doi: 10.23736/s1121-421x.18.02486-8. [DOI] [PubMed] [Google Scholar]