Abstract
Neutral red (NR) was utilized as an electron mediator in microbial fuel cells consuming glucose to study both its efficiency during electricity generation and its role in altering anaerobic growth and metabolism of Escherichia coli and Actinobacillus succinogenes. A study of chemical fuel cells in which NADH, NR, and ferricyanide were the electron donor, the electronophore, and the electron acceptor, respectively, showed that electrical current produced from NADH was proportional to the concentration of NADH. Fourfold more current was produced from NADH in chemical fuel cells when NR was the electron mediator than when thionin was the electron mediator. In microbial fuel cells in which E. coli resting cells were used the amount of current produced from glucose when NR was the electron mediator (3.5 mA) was 10-fold more than the amount produced when thionin was the electron mediator (0.4 mA). The amount of electrical energy generated (expressed in joules per mole of substrate) and the amount of current produced from glucose (expressed in milliamperes) in NR-mediated microbial fuel cells containing either E. coli or A. succinogenes were about 10- and 2-fold greater, respectively, when resting cells were used than when growing cells were used. Cell growth was inhibited substantially when these microbial fuel cells were making current, and more oxidized end products were formed under these conditions. When sewage sludge (i.e., a mixed culture of anaerobic bacteria) was used in the fuel cell, stable (for 120 h) and equivalent levels of current were obtained with glucose, as observed in the pure-culture experiments. These results suggest that NR is better than other electron mediators used in microbial fuel cells and that sludge production can be decreased while electricity is produced in fuel cells. Our results are discussed in relation to factors that may improve the relatively low electrical efficiencies (1.2 kJ/mol) obtained with microbial fuel cells.
Electricity can be produced in different types of power plant systems, batteries (9, 12), or fuel cells (3). A biofuel cell is a device that directly converts microbial metabolic or enzyme catalytic energy into electricity by using conventional electrochemical technology (2, 16). Chemical energy can be converted to electric energy by coupling the biocatalytic oxidation of organic or inorganic compounds to the chemical reduction of an oxidant at the interface between the anode and cathode (22). It has been shown that direct electron transfer from microbial cells to electrodes occurs only at very low efficiency (1). In microbial fuel cells, two redox couples are required, one for coupling reduction of an electron mediator to bacterial oxidative metabolism and the other for coupling oxidation of the electron mediator to the reduction of the electron acceptor on the cathode surface (where the electron acceptor is regenerated with atmospheric oxygen) (4, 7).
The amount of free energy produced either by normal microbial metabolism or by microbial fuel cell systems is determined mainly by the potential difference (ΔE) between the electron donor and the acceptor according to the following equation: −ΔG = nFΔE, where ΔG is the variation in free energy, n is the number of electron moles, and F is the Faraday constant (96,487 J/V) (7). The coupling of metabolic oxidation of the primary electron donor (NADH) to reduction of the final electron acceptor (such as oxygen or fumarate in bacterial respiration systems) is very similar to the coupling of the electrochemical half-reaction of the reductant (electron donor) to the half-reaction of the oxidant (electron acceptor) in a fuel cell or battery system (6). Biological reducing power sources with low redox potentials, such as NADH (E0′ = −0.32 V), reduced ferredoxin (FdH2) (E0′ = −0.42 V), or reduced flavin adenine dinucleotide (E0′ = −0.19 V), can act as reductants for fuel cells, but they are not easily converted to electricity because the cytoplasmic membrane has to be nonconductive to maintain the membrane potential absolutely required for free energy (i.e., ATP) production (19).
For electron transfer from a microbial electron carrier to an electrode to occur, an electron mediator is required (8). Previous investigators (2, 5, 7, 16, 20) have reported that metabolic reducing power produced by Proteus vulgaris or Escherichia coli can be converted to electricity by using electron mediators, such as thionin or 2-hydroxy-1,4-naphthoquinone (HNQ) Tanaka et al. (17, 18) reported that light energy can be converted to electricity by Anabaena variabilis when HNQ is used as the electron mediator. Park et al. (13) confirmed that viologen dyes (10, 11) cross-linked with carbon polymers and absorbed on Desulfovibro desulfuricans cytoplasmic membranes can mediate electron transfer from bacterial cells to electrodes or from electrodes to bacterial cells. The electron transfer efficiencies in microbial fuel cells could be improved if more suitable electron mediators were used.
An ideal electron mediator for converting metabolic reducing power into electricity should form a reversible redox couple at the electrode, and it should link to NADH and have a high negative E0′ value in order to maximize electrical energy generation. It should also be stable in both the oxidized form and the reduced form and should not decompose during long-term redox cycling. The mediator polarity should be such that the mediator is soluble in aqueous systems (near pH 7.0) and can pass through or be absorbed by the microbial cytoplasmic membrane. We have shown previously (14, 15) that neutral red (NR) has all of these general properties and that electrically reduced NR chemically reduces NAD. In these studies we also demonstrated that NR functions as an electronophore (electron shuttle) for electron transfer across the cytoplasmic membrane (14), which allows a microbe to use electrical reducing power for both growth and metabolite production.
The purpose of this study was fourfold: (i) to determine the electrochemical redox properties of NR and thionin in relation to NADH oxidation; (ii) to demonstrate that NR is a better electron mediator than thionin for enhancing electricity production from glucose in a novel biofuel cell system in which either E. coli or Actinobacillus succinogenes is used; (iii) to study the physiological relationships in this fuel cell system between growing and resting cells and production of electricity; and (iv) to describe the first biofuel cell experiments performed with a mixed microbial culture (i.e., sewage sludge) and to show that electricity can be produced during waste treatment.
MATERIALS AND METHODS
Bacterial growth, cell preparation, and metabolite measurement.
A. succinogenes 130Z and E. coli K-12 were grown anaerobically for 16 and 20 h, respectively, in medium A (10 g of glucose per liter, 5 g of yeast extract per liter, 8.5 g of NaH2PO4 per liter, 10 g of NaHCO3 per liter) under an anaerobic N2-CO2 (80:20) atmosphere at 37°C in 150-ml serum vials or under a 100% N2 atmosphere in fuel cell system with a pH controller (21). The inoculum size was 3% (vol/vol) for both the vial and fuel cell experiments. Glucose was aseptically added to the medium after autoclaving. Resting cell suspensions were prepared by harvesting stationary-phase cultures at 4°C by centrifugation at 5,000 × g. The cells were washed twice with 50 mM phosphate buffer (pH 7.0) under a 100% N2 atmosphere. The washed cells were resuspended in 50 mM phosphate buffer (pH 7.0), and then the dissolved O2 was removed by gassing the preparations with N2 for 30 min. The cell density was adjusted to an optical density at 660 nm of 3.0.
Glucose, lactate, acetate, succinate, and ethanol were analyzed quantitatively by using a Waters high-performance liquid chromatograph equipped with a refractive index (RI) detector as described previously (15). Cell mass was calculated by using bacterial cell protein, which was extracted by boiling in NaOH; also, protein contents were measured by using the Bradford reagent as described elsewhere (15). The data reported below are means based on values that were obtained in triplicate experiments and were within 1 standard deviation of each other.
Fuel cell system.
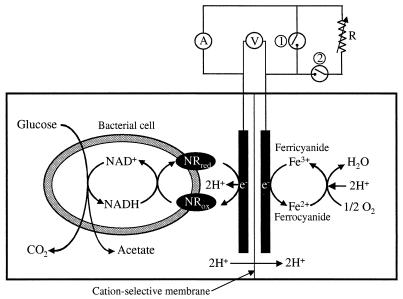
A two-compartment (anode and cathode) electrochemical cell was used as a fuel cell system for microbial electricity production (Fig. 1). NR at a concentration of 100 μM or thionin at a concentration of 300 μM was used as the electron mediator. The total volume and the working volume of each compartment were 1,600 and 1,300 ml, respectively. The electrodes, each of which was made of 12 g of fine woven graphite felt (0.47 m2/g; Electrosynthesis, Lancaster, N.Y.), were connected to a precision multimeter (model 45; Fluke, Everett, Wash.) with a platinum wire (diameter, 0.5 mm; resistance, <1.0 Ω cm−2; Sigma Chemical Co., St. Louis, Mo.). The platinum wire was connected to the graphite felt with graphite epoxy (resistance, <1.0 Ω cm−2; Electrosynthesis). The anode and cathode compartments were separated by a cation-selective membrane septum (diameter, 70 mm; Nafion; Electrosynthesis). The self-electric resistance of the fuel cell system between the anode and cathode was approximately 1,000 Ω; it was adjusted by using variable resistance for controlling current production, but it was not adjusted for measuring maximum potential or current production. The current and voltage between the anode and the cathode were measured with a precision multimeter (model 45; Fluke). The electrochemical half-reduction of ferric ion (as potassium ferricyanide; E0′ = 0.36 V), which was reoxidized by O2 (E0′ = 0.82 V), was coupled to NR or thionin half-oxidation, which in turn was reductively coupled to bacterial oxidative metabolism. In the fuel cell system in which resting cells were used, a bacterial cell suspension (optical density at 660 nm, 3.0) in 50 mM phosphate buffer (pH 7.2) containing 100 μM NR or 300 μM thionin and 100 mM phosphate buffer (pH 7.0) containing 50 mM ferricyanide were used as the anolyte and the catholyte, respectively. In the fuel cell system in which growing cells were used, medium A containing a fresh bacterial inoculum was the anolyte; the catholyte was the same as the catholyte described above. During experiments, completely anoxic conditions were maintained in the anode compartment by gassing the compartment with 100% N2 for 30 min before operation at N2 flow rates of 0.8 ml/min. The traces of oxygen contained in the N2 gas was removed in a furnace filled with pure copper fillings at 370°C. The cathode compartment was oxygenated by constant bubbling with air and stirring. The pH of the anode compartment was maintained at 7.0 by using an automatic pH controller (model pH-40; New Brunswick Scientific Co., Edison, N.J.).
FIG. 1.
Schematic diagram of the microbial fuel cell in which NR was used as an electronophore (i.e., electron mediator). Switches 1 and 2 were off when the circuit was open, switch 1 was on and switch 2 was off when the circuit was closed, and switch 1 was off and switch 2 was on when a circuit with external variable resistance was used.
Electrical parameters and measurements.
A joule is a unit of energy which is calculated by using the following equation: amperes × volts × time (in seconds). A coulomb is equal to amperes × seconds, and coulombs × volts is equal to joules. Thus, a joule is the amount of electrons (amperes) with a driving force (volts) in a closed circuit system per unit of time. To calculate the joule value, the current, potential, and time were all measured in the fuel cells which we used.
Current was measured with an ohmmeter connected to the line between the anode and the cathode in the closed circuit configuration. Current is inversely proportional to resistance and is directly proportional to potential (in volts). Potential was measured with a voltammeter. An open circuit was used to measure potential, while current was measured in the closed circuit configuration. The data reported below are means based on values that were obtained in triplicate experiments and were within 1 standard deviation of each other. The current and potential were nearly identical in replicate experiments.
Current production by chemical dye chemical oxidation coupled to NADH oxidation.
A small chemical fuel cell system (total volume, 50 ml; working volume, 30 ml) consisting of anode and cathode compartments equipped with 0.3-g fine woven graphite felt electrodes and a cation-selective membrane septum (diameter, 20 mm; Nafion; Electrosynthesis) was used. A 100 μM NR solution in 50 mM phosphate buffer (pH 7.0) and 100 mM phosphate buffer (pH 7.0) containing 50 mM ferricyanide were used as the anolyte and the catholyte, respectively. Oxygen was completely removed from the anode compartment by gassing the compartment with N2 for 30 min before NADH was added. The concentrated NADH solution in 50 mM phosphate buffer (pH 7.0) was gassed previously with N2 to remove O2.
Cyclic voltametry.
A 3-mm-diameter glassy carbon working electrode, a platinum wire counterelectrode, and an Ag-AgCl reference electrode (all obtained from BAS, West Lafayette, Ind.) were used in an electrochemical cell with a working volume of 3 ml. Cyclic voltametry was performed by using a cyclic voltametric potentiostat (model CV50W; BAS) linked to an IBM personal computer data acquisition system. Prior to use, the working electrode was polished with an aluminum-water slurry on cotton wool, and the electrochemical cell was thoroughly washed. Oxygen was purged from the reactant by bubbling it with oxygen-free N2 for 10 min before electrochemical measurements were obtained. The scanning rate used was 25 mV/s over the range from −0.3 to −0.8 V. Phosphate buffer (50 mM) containing 5 mM NaCl was used as the electrolyte. NR at a concentration of 100 μM and NAD at a concentration of 100 μM were used as the electron mediator and acceptor, respectively.
Anaerobic sludge.
Anaerobic sludge was obtained from the East Lansing, Mich., sewage treatment plant. The fresh anaerobic sludge was allowed to settle under an N2 atmosphere for 1 day to remove solid particles. The supernatant (1,200 ml) was used as a biocatalyst and anolyte for the fuel cell system, to which 3 g of glucose per ml was added as an energy source. The catholyte was 100 mM phosphate buffer (pH 7.0) containing 50 mM ferricyanide.
RESULTS AND DISCUSSION
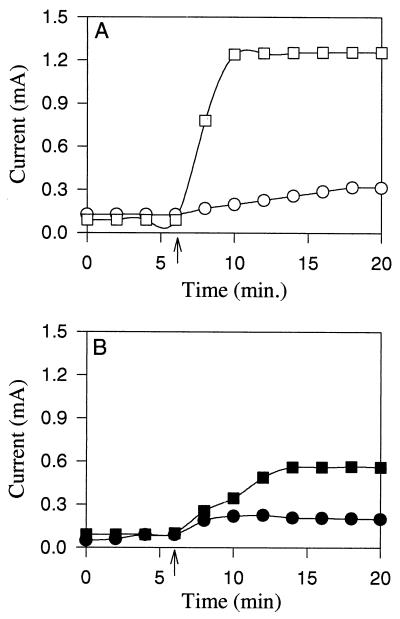
NR has not been used previously as an electron mediator in fuel cells. One reason that NR is a more suitable electron mediator than thionin for fuel cells is that the E0′ value of NR (−0.325 V) is more negative and provides a higher driving force for electron transfer than the E0 value of thionin (0.064 V). We performed experiments in which we compared NR and thionin as electron mediators for oxidation of NADH in chemical fuel cells producing electricity. Figure 2 shows that more current was generated from chemical oxidation of NADH when NR was the electron mediator than when thionin was the electron mediator. The amount of current produced also depended on the NADH concentration used. At low NADH concentrations the amount of current was quite small. High NADH concentrations were required to drive NR reduction because the E0′ value of NAD (−0.32 V) is nearly the same as the E0′ value of NR.
FIG. 2.
Production of current from NADH oxidation in a chemical fuel cell when 100 μM NR (A) or 300 μM thionin (B) was the electron mediator. The arrows indicate when 1 mM NADH (○ and ●) or 3.5 mM NADH (□ and ■) was added.
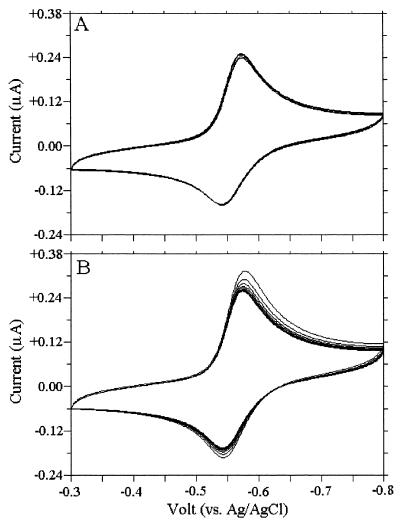
We performed cyclic voltametry experiments to electrochemically measure chemical oxidation-reduction coupling between NR and NAD. Figure 3 shows cyclic voltammograms of a NR solution with and without NAD+. The NR oxidation (upper) and reduction (lower) peaks did not shift during 20 scanning cycles with controls in the absence of NAD+ (Fig. 3A). Both peaks were higher when NAD+ was added (Fig. 3B). NAD+ allowed more electrons to pass unidirectionally from the electrode to NR to NAD and from NADH to the electrode via NR. These experiments established that transfer of electrons between oxidized and reduced forms of NR and NAD was reversible.
FIG. 3.
Cyclic voltammogram obtained with a glassy carbon electrode for successive cycles following introduction of the electrode into a 100 μM NAD+ solution. The current was fixed for 20 cycles with NR (A). NR oxidation and reduction peaks increased when the reaction was coupled to NAD oxidoreduction (B).
Figure 4 shows the amounts of current and potential generated in a fuel cell from glucose by E. coli resting cells when either NR or thionin was the electron mediator. Under the anaerobic conditions used, higher levels of current and potential were produced with NR than with thionin. In control experiments under aerobic conditions, significant levels of current or potential were not detected because NR and thionin could not oxidize NADH through the electron transport system since O2 was a much better electron acceptor (i.e., it had a much more positive E0′ value) than the two electron mediators.
FIG. 4.
Current and potential obtained in a glucose (10 g/liter) fuel cell when E. coli K-12 resting cells were used as the catalyst and 100 μM NR or 300 μM thionin was used as the electron mediator in closed circuit (current) (A) and open circuit (potential) (B) configurations. Symbols: ○ and ●, NR; □ and ■, thionin. The arrows indicate when the electron mediator was added (arrow 1) and when the circuit was converted to an open circuit (arrow 2).
Previous investigations (2, 20) have shown that in microbial fuel cells in which thionin is the electron mediator, the levels of both current and potential decrease when the resting cells are depleted of glucose (i.e., fuel). We performed fuel cell experiments with NR to determine the maximal electrical productivities and stabilities that could be generated by E. coli resting cells in the presence of different glucose concentrations. Table 1 shows the effect of glucose concentration on the maximal electrical productivities and stabilities in an open circuit and a closed circuit with and without 120 Ω of external resistance. The maximal levels of current, potential, and electrical energy produced by the fuel cell were proportional to the glucose (i.e., fuel) concentration. The maximum levels of current and coulombic yields obtained from glucose when NR was the electronophore far exceeded the levels and yields obtained with thionin in other investigations (Table 2).
TABLE 1.
Effect of initial glucose concentration on electrical productivity and stability of a microbial fuel cell in which E. coli resting cells were used and NR was the electron mediator
| Glucose concn (mM) | Open circuit
|
Closed circuit
|
Closed circuit with 120 Ω of resistance
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Potential (V) | Current (mA) | Potential (V) | Current (mA) | Potential (V) | Current (mA) | Electrical charge (C) | Electrical energy (J) | Electrical stability (h) | |
| 11.1 | 0.58 | 0.0 | 0.02 | 1.2 | 0.46 | 0.5 | 58 | 27 | 32 |
| 55.5 | 0.65 | 0.0 | 0.04 | 5.6 | 0.57 | 3.6 | 1,050 | 598 | 81 |
| 111 | 0.85 | 0.0 | 0.05 | 17.7 | 0.62 | 4.8 | 2,039 | 1,264 | 118 |
TABLE 2.
Production of electricity from glucose in microbial fuel cells when different electron mediators were used
| Authors | Reference | Microorganism | Electron mediator | Current (mA)a | Potential (V) | Electrical charge (C)b | Energy (J) | Energy rate (J/h) |
|---|---|---|---|---|---|---|---|---|
| Thurston et al.c | 20 | P. vulgaris | Thionine | 1.25 | 0.3d | 18 | 5.4 | 1.35 |
| Allen and Bennettoc | 2 | P. vulgaris | HNQ | 0.5 | 0.5 | 7.2 | 3.6 | 0.9 |
| Park and Zeikuse | This study | E. coli | NR | 4.5 | 0.68 | 64.8 | 44.1 | 11 |
Mean values.
Values for 4 h.
Resistance and potential were measured, and current was calculated.
Maximum value.
Resistance, potential, and current were measured.
In previous studies (2, 5, 7, 16, 20) of microbial fuel cells the workers did not examine the impact of NADH oxidation via generation of electricity on cellular physiology (i.e., the impact on growth and end product formation). In resting cell studies it was assumed that generation of electricity as a consequence of NADH oxidation would result in lower levels of reduced metabolic products and higher levels of oxidized products. We examined the impact of electrical generation in fuel cells on these physiological properties by using growing and resting cells of E. coli. Table 3 shows that the rate of glucose consumption was higher but the amounts of cell mass and electrical energy generated were lower in growing cells than in resting cells. Table 4 shows that both lower cell yields and lower total end product concentrations were obtained with growing E. coli cells that produced electricity than with growing control cells that were not coupled to the electrical generation system. These results were expected since NADH, ATP, and acetyl coenzyme A are required for cell synthesis. Also, more oxidized end products were formed by growing cells that were producing electricity than by growing control cells that were not coupled to generation of electricity.
TABLE 3.
Substrate consumption and production of electricity by growing and resting E. coli cells in a fuel cell when NR was used as the electron mediatora
| Cells | Glucose consumption (mM) | Glucose consumption rate (mM/h) | Cell mass (g/liter) | Rate of cell mass increase (g/liter/h) | Electric energy (J/mol of substrate) |
|---|---|---|---|---|---|
| Growingb | 45.1 | 7.52 | 1.74 | 0.29 | 100.8 |
| Restingc | 15.5 | 2.59 | 0.214 | 0.035 | 1,207.7 |
For this experiment we used medium A containing 100 μM NR and the standard fuel cell system.
Data obtained 4 to 6 h after inoculation.
Data obtained 18 h after inoculation.
TABLE 4.
Anaerobic metabolism of E. coli cells growing in the presence and in the absence of generation of electricitya
| Growth conditions | Glucose consumption (mM) | Cell mass (g/liter) | Ysub (g of cells/mol of substrate) | Sum of concn of end products (mM)b | End product oxidation/ reduction balanceb |
|---|---|---|---|---|---|
| Without generation of electricity | 60.6 | 3.1 | 50 | 11.9 | 2.3 |
| With generation of electricity | 66.3 | 1.4 | 22 | 8.9 | 5.0 |
Data were obtained after 6 h of growth in medium A containing 100 μM NR in a standard fuel cell.
The concentrations of end products without and with generation of electricity were as follows: lactate, 3.3 and 2.2 mM, respectively; succinate, 1.2 and 1.4 mM, respectively; formate, 1.6 and 1.3 mM, respectively; acetate, 4.4 and 2.2 mM, respectively; and ethanol, 1.5 and 1.8 mM, respectively.
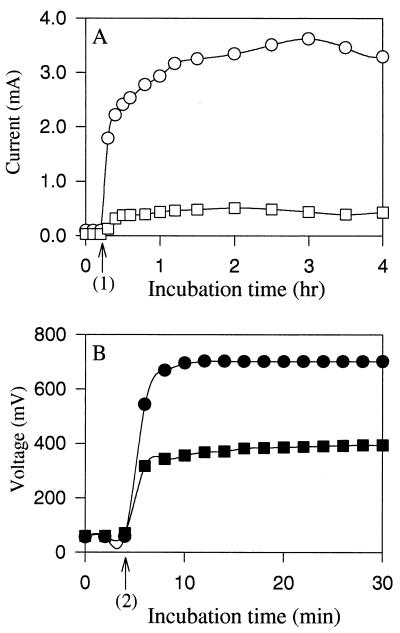
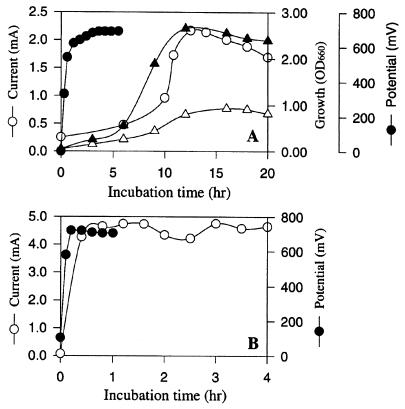
We wanted to test our fuel cell system containing NR by using a different microorganism to see if similar levels of current could be obtained from glucose and if growth was inhibited by generation of electricity. We reported previously that adding NR to culture medium alone did not alter the growth of A. succinogenes (14, 15). Figure 5 shows the levels of electrical current and potential obtained when A. succinogenes growing cells (Fig. 5A) and resting cells (Fig. 5B) were used. Control experiments (Fig. 5A) revealed that the growth yield and growth rate were much higher in the absence of generation of electricity than in the presence of generation of electricity. The potential rapidly increased to the maximum theoretical value (0.685 V), and the amount of electric current generated increased with cell growth (Fig. 5A). The potentials generated by growing and resting cells were similar, whereas the amount of current produced by resting cells was significantly greater (about twofold greater) than the amount of current produced by growing cells. The specific current produced per milligram of cell protein per hour was calculated at 10 h for growing cells (1.235 mA/mg of protein/h) and at 2 h for resting cells (2.595 mA/mg of protein/h) when the glucose levels were high. A total of 68 C was produced by growing cells at 20 h (after glucose was depleted), whereas the resting cells produced 90 C at 4 h.
FIG. 5.
Comparison of the electrical current and potential levels obtained when A. succinogenes growing cells (A) and resting cells (B) were used in a glucose (10 g/liter) fuel cell along with 100 μM NR as the electron mediator under anaerobic conditions. Symbols: ▵, growth; ○, current; ●, potential in open circuit configuration; ▴, normal growth under control conditions (electricity was not being produced with NR as an electron mediator). OD660, optical density at 660 nm.
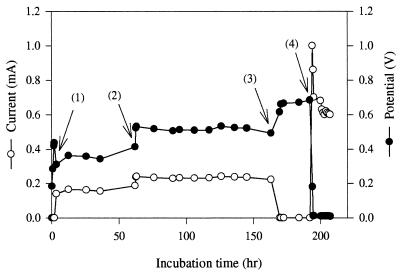
We performed experiments with anaerobic sludge in order to determine its potential as a catalyst for generation of electricity in a fuel cell containing NR as the electronophore. Figure 6 shows the effect of adding glucose on the amounts of current and potential generated in the presence of the sewage sludge, as well as the maximum amount of current produced in a closed circuit configuration and the maximum potential produced in an open circuit configuration. The electrical productivity of the glucose fuel cell when sewage sludge was used as the catalyst was calculated to be 370.8 C (ΔG = 162.82 J).
FIG. 6.
Current and potential produced in a glucose (3 g/liter) fuel cell when anaerobic sewage sludge was used as the catalyst and NR (100 μM) was used as the electronophore. The arrows indicate when the circuit was converted from an open circuit to a closed circuit with 2.2 kΩ of resistance (arrow 1), when 3 g of glucose per liter was added (arrow 2), when the circuit was converted from a closed circuit to an open circuit (arrow 3), and when the circuit was converted from an open circuit to a closed circuit without external resistance (arrow 4).
Table 2 shows the production of electricity from glucose when thionin, HNQ, and NR were used as electron mediators in different microbial fuel cell systems. It is clear that NR was the best electron mediator because it increased both the rate of electron transfer (current) and the yield of electrons transferred (coulombic yield). The efficiency of generation of electricity in a microbial fuel cell is much lower than the efficiency of generation of electricity in a chemical fuel cell for many reasons. The rate of microbial reducing power generation coupled to NR is lower than chemical reaction rates, such generation occurs in aqueous systems, and the metabolic reactions are spatially separated. However, it is quite possible that in the future microbial fuel cells can be improved by changing the electrode surface area, the bacterial cell mass, and the electron mediator type and concentration and by identifying better microbial strains. The microbial fuel cell improvements that could be made immediately include enhancing the current by increasing the electrode surface area, by immobilizing the cells on the electrode, and by covalently bonding the electron mediator on the electrode surface.
Nonetheless, the low levels of current (4 to 17 mA) obtained in microbial fuel cells when NR is used may still be useful. There may be potential applications for low-power direct-current microbial fuel cells in which waste materials are used as fuel, such as maintaining telecommunications in remote areas, including outer space. Further studies are necessary to assess whether generation of electricity during anaerobic sewage treatment can be utilized to reduce the amount of sludge that must be disposed of in waste treatment systems.
ACKNOWLEDGMENT
This research was supported by U.S. Department of Energy grant DE-F602-93ER20108.
REFERENCES
- 1.Allen M J. Cellular electrophysiology. In: Norris J R, Ribbons D W, editors. Methods in microbiology. New York, N.Y: Academic Press; 1972. pp. 247–283. [Google Scholar]
- 2.Allen, R. M., and H. P. Bennetto. 1993. Microbial fuel cells: electricity production from carbohydrates. Appl. Biochem. Biotechnol. 39–40:27–40.
- 3.Appleby A J, Foukes F R. Fuel cell handbook. Reinhol, New York: Van Nostrand; 1989. , N.Y. [Google Scholar]
- 4.Ardeleanu I, Margineaunu D-G, Vais H. Electrochemical conversion in biofuel cells using Clostridium butyricum or Staphylococcus aureus oxford. Bioelectrochem Bioenerg. 1983;11:273–277. [Google Scholar]
- 5.Bennetto H P, Delaney G M, Mason J R, Roller S K, Stirling J L, Thurston C F. The sucrose fuel cell: efficient biomass conversion using a microbial catalyst. Biotechnol Lett. 1985;7:699–705. [Google Scholar]
- 6.Chang R. Physical chemistry with application to biological systems. 2nd ed. New York, N.Y: Macmillan Publishing; 1981. [Google Scholar]
- 7.Dealney G M, Bennetto H P, Mason J R, Roller S B, Stirling J L, Thurston C F. Electron-transfer coupling in microbial fuel cells. 2. Performance of fuel cells containing selected microorganism-mediator-substrate combinations. Chem Tech Biotechnol. 1984;34B:13–27. [Google Scholar]
- 8.Fultz M L, Durst R A. Mediator compounds for the electrochemical study of biological redox systems: a compilation. Anal Chim Acta. 1982;140:1–18. [Google Scholar]
- 9.Higgins I J, Hill H A O. Bioelectrochemistry. Essays Biochem. 1985;21:119–145. [PubMed] [Google Scholar]
- 10.Kim C H, Kristjansseon J K, White M M, Hollocher T C. Benzyl viologen cation radical: first example of a perfectly selective anion ionophore of the carrier type. Biochem Biophys Res Commun. 1982;108:1126–1130. doi: 10.1016/0006-291x(82)92117-9. [DOI] [PubMed] [Google Scholar]
- 11.Morimyo M. Isolation and characterization of methyl viologen-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1988;170:2136–2142. doi: 10.1128/jb.170.5.2136-2142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldham H B, Myland J C. Fundamentals of electrochemical science. New York, N.Y: Academic Press; 1994. [Google Scholar]
- 13.Park D H, Kim B H, Moore B, Hill H A O, Song M K, Rhee H W. Electrode reaction of Desulfovibrio desulfuricans modified with organic conductive compounds. Biotech Technol. 1997;11:145–148. [Google Scholar]
- 14.Park D H, Laivenieks M, Guettler M V, Jain M K, Zeikus J G. Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl Environ Microbiol. 1999;65:2912–2917. doi: 10.1128/aem.65.7.2912-2917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park D H, Zeikus J G. Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J Bacteriol. 1999;181:2403–2410. doi: 10.1128/jb.181.8.2403-2410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roller S B, Bennetto H P, Delancy G M, Mason J R, Stirling J L, Thurston C F. Electron-transfer coupling in microbial fuel cells. 1. Comparison of redox-mediator reduction rates and respiratory rates of bacteria. J Chem Tech Biotechnol. 1984;34B:3–12. [Google Scholar]
- 17.Tanaka K, Kashiwagi N, Ogawa T. Effects of light on the electrical output of bioelectrochemical fuel-cells containing Anabaena variabilis M-2: mechanisms of the post-illumination burst. Chem Tech Biotechnol. 1988;42:235–240. [Google Scholar]
- 18.Tanaka K, Tamamuchi R, Ogawa T. Bioelectrochemical fuel-cells operated by the cyanobacterium, Anabaena variabilis. Chem Tech Biotechnol. 1985;35B:191–197. [Google Scholar]
- 19.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston C F, Bennetto H P, Delaney G M, Mason J R, Rooer S E, Stirling J L. Glucose metabolism in a microbial fuel cell. Stoichiometry of product formation in a thionine-mediated Proteus vulgaris fuel cell and its relation to coulombic yields. J Gen Microbiol. 1985;131:1393–1401. [Google Scholar]
- 21.van der Werf M J, Guettler M V, Jain M K, Zeikus J G. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol. 1997;167:332–342. doi: 10.1007/s002030050452. [DOI] [PubMed] [Google Scholar]
- 22.Willner I, Arad G, Katz E. A biofuel cell based on pyrroloquinoline quinone and microperoxidase-11 monolayer-functionalized electrodes. Bioelectrochem Bioenerg. 1998;44:209–214. [Google Scholar]