Abstract
The significance of bacterial biofilm formation in chronic bacterial lung infections has long been recognized [1]. Likewise, chronic biofilm formation on medical devices is well accepted as a nidus for recurrent bacteremia [2,3]. Even though the prevailing paradigm relies on the dominance of planktonic bacteria in acute endobronchial infections, our understanding of the bacterial organization during acute infection is, so far, limited - virtually absent. However, by comparing similar clinical samples, we have recently demonstrated massive bacterial biofilm formation during acute lung infections resembling the immense bacterial biofilm formation during chronic lung infections. These findings pose major challenges to the basic paradigm of chronic infections being dominated by biofilm forming bacteria while acute infections are dominated by planktonic bacteria. As opposed to the similar high amount of bacterial biofilm found in chronic and acute lung infections, we found that the fast bacterial growth in acute lung infections differed from the slow bacterial growth in chronic lung infections. By highlighting these new findings, we review modes of improved treatment of biofilm infections and the relevance of bacterial growth rates for other bacterial biofilm infections than human lung infections.
1. Introduction
During the past 40 years microbiologists have proposed that bacteria exist in two growth states: as single independent cells (planktonic) or as extracellular matrix-embedded aggregates termed biofilms [4]. In 1982, the biofilm pioneer Professor Bill Costerton and colleagues demonstrated for the first time a difference between acute and chronic infection where planktonic bacteria in blood were readily treatable with antibiotics, whereas bacteria in biofilm covering a pacemaker were tolerant to the same treatment [[2], [3]]. Amalgamating this early observation of biofilm-related high antibiotic tolerance with the dominance of Pseudomonas aeruginosa microcolonies persisting massive antibiotic treatment in the lungs of patients with cystic fibrosis with chronic lung infection, spawned the concept of chronic infections being caused by biofilm growing bacteria, while acute infections are caused by planktonic bacteria [1,5,6]. This current paradigm was soon exemplified by considering bacteria in a shaken, liquid culture growing as planktonic single cells (chains or clusters) while bacteria aggregated in clumps or associated with surfaces are considered to grow as biofilms. However, our understanding of biofilm biology has recently been modernized to focus directly on bacteria in the in vivo situation [5,6]. In chronic lung infections, the biofilms induce local continuous pro-inflammatory host responses characterized by persistent and progressing pathology [7] and biofilm-associated bacteria in the lung are typically slow-growing [8,9]. In addition, persisting local inflammation and slow growth of the microorganisms has been proposed to be common to various biofilm infections [4,7]. If an infection causes clinical symptoms such as inflammation or fever it may be eradicated spontaneously by the host response or by antibiotics. Such infections are known as acute infections. If the infection persists, despite the immune response and antibiotic therapy it is known as a chronic infection [10].
2. Similar bacterial organization in acute and chronic lungs infections
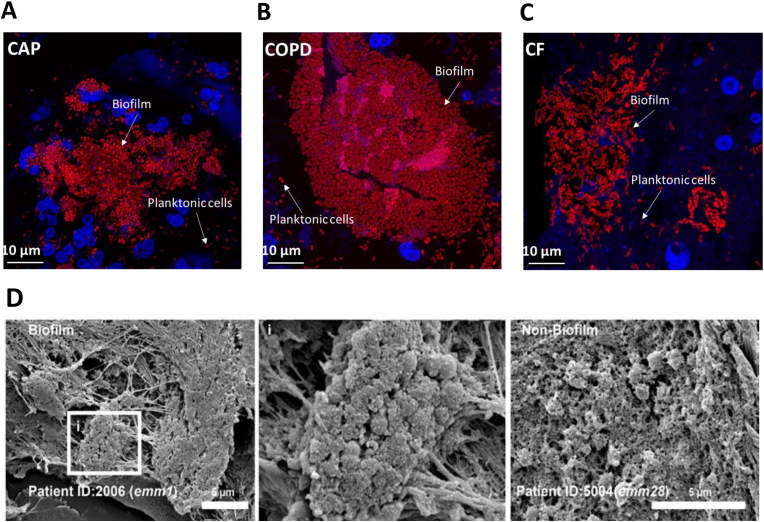
While the predominance of the biofilm lifestyle in chronic infections was confirmed, the prevalence of the biofilm lifestyle in acute infections has recently been challenged in a comparison between chronic and acute lung infections [11] (Fig. 1). This study demonstrates that a majority of bacteria are in biofilms in both chronic and acute infections. Interestingly, organization of bacteria in biofilm has also been observed in other acute infections in patients with e.g. necrotizing fasciitis [12,13] (Fig. 1) and acute otitis media [14] as well as in experimental acute wounds and in experimental blister wounds [15,16]. These observations suggest that we need a new bacterial infection paradigm. Since the bacterial growth rate was found to be different in chronic and acute infections [11], we propose the new paradigm to emphasize on characterization of the infections according to the bacterial metabolism rather than bacterial aggregation. In chronic lung infections the growth of biofilm forming bacteria is linked to the activity of the inflammatory cells [8,9]. Surprisingly, the bacteria grew faster in sputum from acute infections even though similar amounts of inflammatory cells accumulated around the biofilms in all types of infections. This unexpected fast bacterial growth may rely on incomplete maturation of the acquired immune response, which, when matured, may accelerate the inflammatory response as seen in chronic lung infections [17]. Furthermore, the expression of virulence factors, such as quorum sensing-mediated inactivation of the inflammatory response, may be lost during chronic biofilm infections [18,19].
Fig. 1.
Biofilmsareobservedacrossinfectiontypes.(A-C) Representative projections of confocal images of sputum samples of CAP with no detected pathogen (A), COPD with Moraxella sp. (B) and CF with Pseudomonasaeruginosa (C). Specimens were stained with Tamra-5 (red) using PNA -FISH probes specific to bacterial 16S rRNA and DAPI m (blue). Scale bar is 10 μm. [11]. (D) Representative scanning electronmicrographs of GAS biofilm and non biofilm single-cocci are as in patient biopsies. The boxed area (i) is shown in larger magnification (× 10,000) to visualize the biofilm community [38].
3. Significance of biofilm formation in acute infections
While the detrimental contribution of biofilm has long been recognized in chronic infections [20], the demonstrated high prevalence of biofilm in acute infections calls for investigations of the significance of biofilm in acute infections. Considering the high mortality rate of necrotizing fasciitis [21], which contain massive amounts of biofilm [12], we need to consider whether it is the biofilm i.e. the aggregation of bacteria which is important or also the physiology of bacteria and the infectious microenvironment [22]. Bacteria seem to inherently aggregate in close proximity and we know that within confined space the biofilm formation is typically by clonal expansion. However, the presence of aggregated bacteria within a matrix, the hallmark of a biofilm, in acute fast progressing infections like acute pneumonia and even more in necrotizing fasciitis clearly calls for a revision of the biofilm paradigm, especially categorizing acute and chronic infections. Extracellular polysaccharide (EPS) is likely a part of matrix components of infectious biofilm, but if EPS is differentially expressed in the bacterial aggregates in acute and chronic pneumonia is unclear. However, the role of EPS changes temporally over the course of an infection as EPS loses its impact on virulence which is highest in acute infections [23]. However, this has not been demonstrated in acute and chronic pneumonia where EPS was detected in both infection types [11].
4. Optimizing antibiotic treatment according to the bacterial growth rate
The distinct growth rates of bacteria in acute and chronic lung infections may provide important clues for advancing antibiotic treatment. The knowledge that the effect of antibiotic treatment of bacterial biofilm is enhanced when the growth rate of the bacteria is accelerated by supplemental oxygen [[24], [25], [26], [27], [28]] could serve a strategy for improving treatment of chronic lung infections, where clinical cure is complicated and rare. In addition, supplemental nutrients or metal traces may induce oxidative stress-mediated enhancement of the activity of antibiotics against biofilm [[29], [30], [31], [32], [33], [34]]. In acute lung infections clinical cure is frequent and approximates 80%, but a high number of patients still experience clinical failure [35]. This could indicate failure of the empirical treatment regimens even though the bacteria display sensitive growth physiology [11] and antimicrobial resistance is reported in less than 5% of the isolates [36]. The “one size fits all” mindset of empirical antibiotic treatment must be re-evaluated so that individualized antibiotic treatment [37] can be offered to patients with acute pneumonia. This is especially important in those situations where there has not been a sufficient effect of the empirical treatment, or where a deterioration in the patient's condition is seen. Thus, our new findings call for reevaluating bacterial behavior in infections, and a changed approach towards categorizing infections. The acute/chronic – planktonic/biofilm paradigm needs to be revised with an emphasis on the metabolism of the infecting bacteria and possibly the local inflammatory response as well as an appreciation of the infectious microenvironment. Science is a dynamic process and all the developments within the field of bacterial biofilms contribute pieces to a greater understanding. We as scientists need to adapt to the increasing knowledge, just as infecting bacteria are adapting to specific microenvironments.
CRediT authorship contribution statement
Mette Kolpen: Writing – review & editing. Peter Østrup Jensen: Writing – review & editing. Daniel Faurholt-Jepsen: Writing – review & editing. Thomas Bjarnsholt: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hoiby N. Pseudomonas aeruginosa infection in cystic fibrosis. Diagnostic and prognostic significance of pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. A survey. Acta Pathol Microbiol Scand Suppl. 1977;(262):1–96. [PubMed] [Google Scholar]
- 2.Marrie T.J., Nelligan J., Costerton J.W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- 3.Marrie T.J., Costerton J.W. Morphology of bacterial attachment to cardiac pacemaker leads and power packs. J Clin Microbiol. 1984;19(6):911–914. doi: 10.1128/jcm.19.6.911-914.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013;(136):1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 5.Coenye T., Kjellerup B., Stoodley P., Bjarnsholt T., Biofilm Bash P. The future of biofilm research - report on the '2019 Biofilm Bash. Biofilms. 2020;2 doi: 10.1016/j.bioflm.2019.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burmolle M., Thomsen T.R., Fazli M., Dige I., Christensen L., Homoe P., et al. Biofilms in chronic infections - a matter of opportunity - monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol. 2010;59(3):324–336. doi: 10.1111/j.1574-695X.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoiby N., Bjarnsholt T., Moser C., Bassi G.L., Coenye T., Donelli G., et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(1):S1–S25. doi: 10.1016/j.cmi.2014.10.024. the official publication of the European Society of Clinical Microbiology and Infectious Diseases. [DOI] [PubMed] [Google Scholar]
- 8.Kolpen M., Hansen C.R., Bjarnsholt T., Moser C., Christensen L.D., van Gennip M., et al. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax. 2010;65(1):57–62. doi: 10.1136/thx.2009.114512. [DOI] [PubMed] [Google Scholar]
- 9.Kragh K.N., Alhede M., Jensen P.O., Moser C., Scheike T., Jacobsen C.S., et al. Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect Immun. 2014;82(11):4477–4486. doi: 10.1128/IAI.01969-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Høiby N., Bjarnsholt T., Moser C., Bassi G.L., Coenye T., Donelli G., et al. ESCMID ∗ guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21:S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Kolpen M., Kragh K.N., Enciso J.B., Faurholt-Jepsen D., Lindegaard B., Egelund G.B., et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax. 2022 doi: 10.1136/thoraxjnl-2021-217576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siemens N., Chakrakodi B., Shambat S.M., Morgan M., Bergsten H., Hyldegaard O., et al. Biofilm in group A streptococcal necrotizing soft tissue infections. JCI Insight. 2016;1(10) doi: 10.1172/jci.insight.87882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afzal M., Saccenti E., Madsen M.B., Hansen M.B., Hyldegaard O., Skrede S., et al. Integrated univariate, multivariate, and correlation-based network analyses reveal metabolite-specific effects on bacterial growth and biofilm formation in necrotizing soft tissue infections. J Proteome Res. 2020;19(2):688–698. doi: 10.1021/acs.jproteome.9b00565. [DOI] [PubMed] [Google Scholar]
- 14.Thornton R.B., Wiertsema S.P., Kirkham L.A., Rigby P.J., Vijayasekaran S., Coates H.L., et al. Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media--a potential treatment target. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0053837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bay L., Kragh K.N., Eickhardt S.R., Poulsen S.S., Gjerdrum L.M.R., Ghathian K., et al. Bacterial aggregates establish at the edges of acute epidermal wounds. Adv Wound Care. 2018;7(4):105–113. doi: 10.1089/wound.2017.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaber J.A., Triffo W.J., Suh S.J., Oliver J.W., Hastert M.C., Griswold J.A., et al. Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect Immun. 2007;75(8):3715–3721. doi: 10.1128/IAI.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen P.O., Givskov M., Bjarnsholt T., Moser C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol. 2010;59(3):292–305. doi: 10.1111/j.1574-695X.2010.00706.x. [DOI] [PubMed] [Google Scholar]
- 18.Bjarnsholt T., Jensen P.O., Jakobsen T.H., Phipps R., Nielsen A.K., Rybtke M.T., et al. Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marvig R.L., Sommer L.M., Molin S., Johansen H.K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet. 2015;47(1):57–64. doi: 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 20.Bjarnsholt T., Alhede M., Alhede M., Eickhardt-Sorensen S.R., Moser C., Kuhl M., et al. The in vivo biofilm. Trends Microbiol. 2013;21(9):466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Anaya D.A., McMahon K., Nathens A.B., Sullivan S.R., Foy H., Bulger E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg. 2005;140(2):151–157. doi: 10.1001/archsurg.140.2.151. ; discussion 8. [DOI] [PubMed] [Google Scholar]
- 22.Bjarnsholt T., Whiteley M., Rumbaugh K.P., Stewart P.S., Jensen P.O., Frimodt-Moller N. The importance of understanding the infectious microenvironment. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming D., Niese B., Redman W., Vanderpool E., Gordon V., Rumbaugh K.P. Contribution of Pseudomonas aeruginosa exopolysaccharides Pel and Psl to wound infections. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.835754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolpen M., Mousavi N., Sams T., Bjarnsholt T., Ciofu O., Moser C., et al. Reinforcement of the bactericidal effect of ciprofloxacin on Pseudomonas aeruginosa biofilm by hyperbaric oxygen treatment. Int J Antimicrob Agents. 2016;47(2):163–167. doi: 10.1016/j.ijantimicag.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Kolpen M., Lerche C.J., Kragh K.N., Sams T., Koren K., Jensen A.S., et al. Hyperbaric oxygen sensitizes anoxic Pseudomonas aeruginosa biofilm to ciprofloxacin. Antimicrob. Agents Chemother. 2017;61(11) doi: 10.1128/AAC.01024-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moller S.A., Jensen P.O., Hoiby N., Ciofu O., Kragh K.N., Bjarnsholt T., et al. Hyperbaric oxygen treatment increases killing of aggregating Pseudomonas aeruginosa isolates from cystic fibrosis patients. J Cyst Fibros : Off J Eur Cystic Fibrosis Soc. 2019 doi: 10.1016/j.jcf.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Jensen P.O., Moller S.A., Lerche C.J., Moser C., Bjarnsholt T., Ciofu O., et al. Improving antibiotic treatment of bacterial biofilm by hyperbaric oxygen therapy: not just hot air. Biofilms. 2019;1 doi: 10.1016/j.bioflm.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerche C.J., Christophersen L.J., Kolpen M., Nielsen P.R., Trostrup H., Thomsen K., et al. Hyperbaric oxygen therapy augments tobramycin efficacy in experimental Staphylococcus aureus endocarditis. Int J Antimicrob Agents. 2017 doi: 10.1016/j.ijantimicag.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Bao X., Bove M., Coenye T. Organic acids and their salts potentiate the activity of selected antibiotics against Pseudomonas aeruginosa biofilms grown in a synthetic cystic fibrosis sputum medium. Antimicrob. Agents Chemother. 2022;66(1) doi: 10.1128/AAC.01875-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morones-Ramirez J.R., Winkler J.A., Spina C.S., Collins J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med. 2013;5(190) doi: 10.1126/scitranslmed.3006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabbe A., Jensen P.O., Bjarnsholt T., Coenye T. Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. 2019 doi: 10.1016/j.tim.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Su Y.B., Peng B., Han Y., Li H., Peng X.X. Fructose restores susceptibility of multidrug-resistant Edwardsiella tarda to kanamycin. J Proteome Res. 2015;14(3):1612–1620. doi: 10.1021/pr501285f. [DOI] [PubMed] [Google Scholar]
- 33.Singh K.S., Sharma R., Keshari D., Singh N., Singh S.K. Down-regulation of malate synthase in Mycobacterium tuberculosis H37Ra leads to reduced stress tolerance, persistence and survival in macrophages. Tuberculosis. 2017;106:73–81. doi: 10.1016/j.tube.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Slachmuylders L., Van Acker H., Brackman G., Sass A., Van Nieuwerburgh F., Coenye T. Elucidation of the mechanism behind the potentiating activity of baicalin against Burkholderia cenocepacia biofilms. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oosterheert J.J., Bonten M.J., Schneider M.M., Buskens E., Lammers J.W., Hustinx W.M., et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. doi: 10.1136/bmj.38993.560984.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collaborators A.R. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022 doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser C., Lerche C.J., Thomsen K., Hartvig T., Schierbeck J., Jensen P.O., et al. Antibiotic therapy as personalized medicine - general considerations and complicating factors. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2019;127(5):361–371. doi: 10.1111/apm.12951. [DOI] [PubMed] [Google Scholar]
- 38.Siemens Nikolai, Chakrakodi Bhavya, Srikanth Mairpady Shamba, Marina Morgan, Helena Bergsten, Ole Hyldegaard, et al. Biofilm in group A streptococcal necrotizing soft tissue infections. JCI Insight. 2016;1(10):e87882. doi: 10.1172/jci.insight.87882. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]