Abstract
Female genital schistosomiasis (FGS) is the gynaecological presentation of Schistosoma haematobium infection, resulting from egg deposition in the female genital tract. Despite the fact that this condition has been reported in the early days of the discovery of S. haematobium in Egypt, its existence has been grossly neglected, causing many women in schistosomiasis-endemic areas to go through a preventable, debilitating, and stigmatizing presentation of FGS. To prevent this, increasing awareness of FGS is necessary for all, especially healthcare providers, to improve the diagnosis, management, and treatment. As proposed by the FAST package project, several healthcare professionals with different specializations are expected to be involved in the management of FGS. It is therefore important that basic updated knowledge on the parasitology of the disease be acquired by healthcare professionals. This review provides basic information necessary to improve the knowledge of FGS among healthcare professionals in areas endemic to schistosomiasis. Armed with these basic details, healthcare professionals can improve their confidence in the management and treatment of FGS, contributing significantly to the control and prevention of FGS in endemic areas.
Keywords: Schistosoma haematobium, Urogenital schistosomiasis, Female genital schistosomiasis, Genital lesions, Praziquantel
Graphical abstract
Highlights
-
•
A review of female genital schistosomiasis.
-
•
Data on the life-cycle of Schistosoma haematobium, and pathogenesis and clinical features of female genital schistosomiasis.
-
•
Summary of diagnostic methods and treatment, control and prevention of female genital schistosomiasis.
-
•
Increasing awareness among healthcare workers is key to the management, control, and prevention of the disease.
1. Introduction
Female genital schistosomiasis (FGS) is the gynaecological manifestation of Schistosoma haematobium infection characterized by the deposition of parasite eggs in the genital tract of young girls and women (Kjetland et al., 1996, 2012; Nour, 2010). Schistosoma haematobium is the only species of the genus that causes disease in the urinary tract aptly called urinary schistosomiasis due to migration and deposition of the eggs in the bladder and urethra, often resulting in gross haematuria among those infected (Gundersen et al., 1996; Barsoum, 2013; Ghieth & Lotfy, 2017; Nelwan, 2019). However, it is observed that the migration of S. haematobium eggs is not only confined to the urinary tract, as there are reports of eggs migrating to other sites such as the brain (Imai et al., 2011), spinal cord (Freitas & Angerami, 2013), eyes (Guirou et al., 2021), skin and genital tract (Nour, 2010; Kjetland et al., 2012; Costain et al., 2018). Migration and deposition of the eggs in the genital tract is the commonly reported site outside the urinary tract probably because the genital tract shares vascular interconnections with the urinary tract (Poggensee et al., 2001a; Nation et al., 2020).
FGS was reported for the first time in 1899 in Egypt close to the discovery of S. haematobium (Wu & Halim, 2000). There were also report from scholars in the 1940s, 1950s, 1980s, and early 2000 which suggest that FGS might be the rule rather than the exception (Poggensee et al., 2000, 2001a). Despite these reports heavily suggesting that FGS might be a common occurrence and probably existing in most women with symptoms of urinary schistosomiasis (Poggensee et al., 2000, 2001a), it is rarely considered among females with urinary schistosomiasis in endemic areas (Gyapong &Theobald, 2015). Gyapong and Theobald (2015) estimated 20–120 million cases of FGS and indicated that this estimate may not reflect the true burden of FGS in areas of endemic schistosomiasis in the urinary tract, as the condition is grossly under-reported probably due to poor diagnosis as a result of inadequate knowledge or lack of awareness among health workers in endemic regions.
The World Health Organization (WHO, 2009) recommended that urinary schistosomiasis caused by S. haematobium is officially referred to as urogenital schistosomiasis in a bid to emphasize and spark up more awareness of the genital aspect of the disease. The WHO considered it important that this emphasis be made, owing to reports that deposition of S. haematobium eggs in the genital tract induce lesions which cause severe complications in females such as increased risk of HIV infection (Feldmeier et al., 1994; Downs et al., 2011; O’Brien et al., 2019; Patel et al., 2021), gynaecological complications such as infertility, ectopic pregnancy, abortions, and other varied painful and stigmatizing gynaecological symptoms (WHO, 2009; Nour, 2010; Gyapong & Theobald, 2015). Unfortunately, despite the WHO efforts, awareness of FGS in many endemic countries has not improved, as people in endemic areas, especially health workers, are still grappling with poor knowledge of FGS (Kukula et al., 2019; Mazigo et al., 2021).
Adequate knowledge and awareness among healthcare workers is a veritable tool in the control and prevention of neglected tropical diseases (Emeto & Salawu, 2021). Controlling and preventing FGS and its debilitating complications, through improved diagnosis and treatment of women and girls, can be achieved by improving awareness and knowledge of FGS among healthcare workers (SCI Foundation, 2021). Training of healthcare workers is one of the important multifaceted components of the FGS accelerated scale together (FAST) package project that focuses on reducing the morbidity associated with FGS among women and girls (SCI Foundation, 2021). The FAST package, among other things, targets healthcare professionals in schistosomiasis-endemic areas from varied fields and specialties such as gynaecologists, primary care physicians, nurses and midwives, community health workers, and even peer educators (SCI Foundation, 2021). These healthcare professionals have different levels of knowledge of schistosomiasis and FGS, and it is necessary that their training improves their knowledge by providing basic information on the parasite that causes FGS, its transmission dynamics, life-cycle, and pathogenesis, as well as symptoms and signs, diagnosis, and prevention of acquisition of infections. This review intends to provide these basic details necessary to improve the knowledge of FGS among healthcare professionals, particularly in schistosomiasis-endemic areas.
2. Brief history, distribution, and disease burden of S. haematobium
There is evidence suggesting that S. haematobium has been with humans for several thousands of years (Di Bella et al., 2021). Scientific studies conducted on fossilized remains of human pelvic bones, found in the Middle East, identified eggs of S. haematobium (Di Bella et al., 2021). Haematuria was reported to be rampant among people living in Egypt by physicians like Prospero Alpini in 1591 and Renault in 1798. Finally, in 1851, Theodor Maximilian Bilharz, a German physician working in Cairo, Egypt, isolated the parasite during an autopsy (Di Bella et al., 2021) and named it Distomum haematobium Bilharz, 1852. Two generic names, Bilharzia Meckel von Hemsbach, 1856 and Schistosoma Weinland, 1858, have been proposed for D. haematobium. To avoid confusion in medical literature, the International Commission of Zoological Nomenclature suppressed the name Bilharzia and validated the name Schistosoma (Hemming, 1954).
Schistosoma haematobium is the main parasite responsible for FGS (Kjetland et al., 1996, 2012). It is one of the seven species of the genus that cause human infections in many parts of the world (CDC, 2019; WHO, 2022). Schistosoma haematobium and Schistosoma mansoni are the main species that cause human infections in Africa, though human infections are also seen in the Middle East and South America (CDC, 2019; WHO, 2022). Other less common species seen in Africa include Schistosoma intercalatum in the Democratic Republic of the Congo and Schistosoma guineensis, in some parts of West Africa (CDC, 2019). Schistosoma japonicum causes human infections in China, Indonesia, and the Philippines (CDC, 2019; WHO, 2022), while Schistosoma mekongi is found in Cambodia and Laos (CDC, 2019; WHO, 2022). Schistosoma malayensis have been reported to cause zoonotic infections in certain parts of Malaysia (Sagin et al., 2001).
All species of the genus Schistosoma are dioecious and have the same life-cycle that involves humans and freshwater snails in streams and rivers in warm tropical climates of Africa, South America, the Middle East, China and Southeast Asia (CDC, 2019; WHO, 2022). The diseases caused by S. haematobium (urinary and genital schistosomiasis) are endemic in 54 African and Mediterranean countries and contribute significantly to the 235 million cases of schistosomiasis annually reported worldwide (Ahmed, 2020; WHO, 2022). Infections are recorded in both adults and children of both sexes and complications such as bladder cancer and renal failure are observed in chronic infections involving the urinary tract (Barsoum, 2013; Antwi et al., 2014), while complications like infertility, ectopic pregnancy, miscarriages, and increased risk of HIV infections are observed in chronic infections involving the genital tract especially among females (Feldmeier et al., 1994; WHO, 2009; Nour, 2010; Downs et al., 2011; Gyapong & Theobald, 2015).
3. Transmission
Women and girls with FGS acquire S. haematobium infection through contact with infested water bodies such as streams, rivers, and lakes (Nour, 2010; Aula et al., 2021). These water bodies are usually contaminated when the urine of infected persons carries the eggs of S. haematobium into the water. Upon contact with these water bodies, the free-swimming larval forms of S. haematobium known as cercariae penetrate the skin and enter the human body (Grimes et al., 2015; Braun et al., 2018). Activities that increase human contact with streams, rivers and lakes are replete in endemic areas, especially in rural communities of Africa (Grimes et al., 2015; Braun et al., 2018). Many of these water contact activities are driven by the lack of potable water for drinking and domestic use (Grimes et al., 2015). However, some are behavioral, such as recreational swimming, while others are inevitable as a means of livelihood, farming and fishing, and children crossing shallow streams on their way to school (Grimes et al., 2015). Women and girls are particularly involved in many of these water contact activities like washing, cleaning, and fetching of water for domestic use, making them susceptible to infections (Nour, 2010; Aula et al., 2021). Water fetched from infested streams, lakes, or rivers for domestic use also poses a risk of infection as cercariae skin penetration can still occurs especially when the fetched water is used within 48 h (Grimes et al., 2015).
4. Life-cycle and migration of schistosomula, adult worms and eggs of S. haematobium
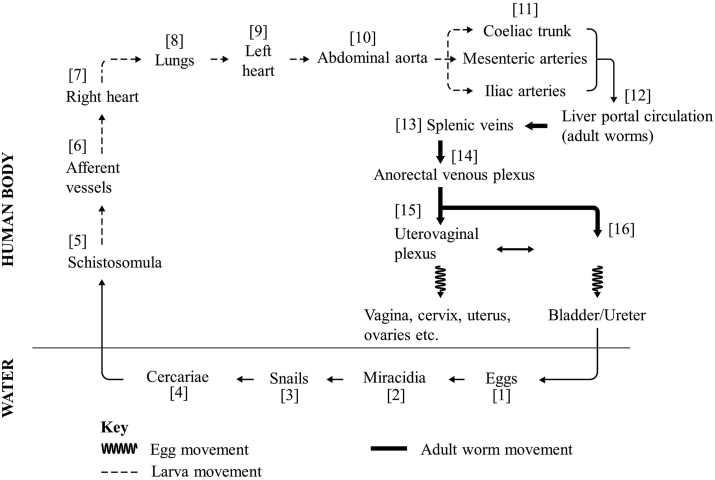
The life-cycle of S. haematobium involves two hosts. Female adult worms in the definitive hosts (humans) produce large numbers of eggs which are released into the water bodies through urine (Fig. 1). Free-swimming miracidia [2] hatch from eggs immediately after release (Nelwan, 2019), seek out and infect the snail intermediate hosts (Bulinus spp.) where asexual reproduction occurs giving rise to the second free-swimming stage (cercaria). Miracidia have a very short life span but snail infection with a single miracidium results in release into the water of about 200 cercariae daily (Grimes et al., 2015; Nelwan, 2019), thus a water snail produces several thousand cercariae in its lifetime (Rozendaal et al., 1997; Grimes et al., 2015). Cercariae [4] seek out the definitive human hosts who come into contact with the water bodies, penetrate the skin, and transform into schistosomula [5]. Schistosomula traverse the epidermis and enter the blood vessels in the dermis and from there gain entry into afferent vessels [6] where they are carried to the right side of the heart [7] (Nelwan, 2019; Nation et al., 2020). From the right side of the heart and through the pulmonary artery they get into the lungs [8]. At 7 days post-cercariae penetration of the skin schistosomula of S. haematobium reach to the lungs and transit the lungs for almost 25 days (Nation et al., 2020). Schistosomula migrate from the lungs to the left side of the heart [9] through the pulmonary veins and from there enter the abdominal aorta [10]. From the abdominal aorta, schistosomula can either pass through the coeliac trunk, the inferior and superior mesenteric arteries or the iliac arteries [11] to reach their final destination, the portal veins of the liver [12] (Nation et al., 2020). In the portal veins of the liver, schistosomula lose their migratory ability and begin to grow and develop into either adult male or female worms (Nation et al., 2020). From the portal veins the male-female pairs travel against the blood flow in the venous circulation (Poggensee et al., 2001a; Nelwan, 2019; Nation et al., 2020).
Fig. 1.
Life-cycle and migration of schistosomula, adult worms, and eggs of Schistosoma haematobium.
The adult male-female pairs of S. haematobium exhibit a complex movement through the venous circulation but a more direct movement is down the splenic veins [13] and through the mesenteric veins into the anorectal venous plexus [14] and further down into the complex interconnection of the uterovaginal venous plexus [15] and the vesical venous plexus [16] (Poggensee et al., 2001a; Nation et al., 2020). These plexuses are complex with valve-less veins allowing back and forth movement of the adult worms between the plexuses (Poggensee et al., 2001a; Nation et al., 2020). The adult worms that settle in the uterovaginal plexuses produce eggs that migrate into the genital tract, i.e. vagina, cervix, uterus, fallopian tubes, and other parts of the genital tract (Poggensee et al., 2001a; Nation et al., 2020). Those that reside in the vesical venous plexus produce eggs that migrate to the bladder, ureter, and other parts of the urinary tract (Poggensee et al., 2001a; Nelwan, 2019; Nation et al., 2020).
These eggs permeate the wall of blood vessels, as well as the walls of the bladder or genital organs to gain entry into the bladder and the genital tract (Nation et al., 2020). Eggs that enter the genital tract are trapped and do not enter into the environment and therefore do not contribute to the transmission of S. haematobium (Poggensee et al., 1998; Costain et al., 2018). However, eggs that enter into the urinary tract exit the bladder through urine and enter water bodies where hatched miracidia continue the transmission cycle (Nelwan, 2019). It is important at this point to note that while S. haematobium is the most implicated species in FGS, there are also reports of FGS being caused by S. mansoni (Kjetland et al., 2012; Christinet et al., 2016; Leandro et al., 2021) and possibly from mixed infections and interactions of S. haematobium and S. mansoni (Cunin et al., 2003; Gouvras et al., 2013). Eggs migrating to the male genital tract resulting in male genital schistosomiasis (MGS) have also been reported, initially in 1911 in Egypt and more recently in other parts of Africa, affecting boys and men and causing various debilitating urogenital symptoms such as pelvic, coital and ejaculatory pain, haemospermia and others (Vilana et al., 1997; Leutscher et al., 2008; Kayuni et al., 2019a, 2019b, 2021).
5. Pathogenesis and clinical features of FGS
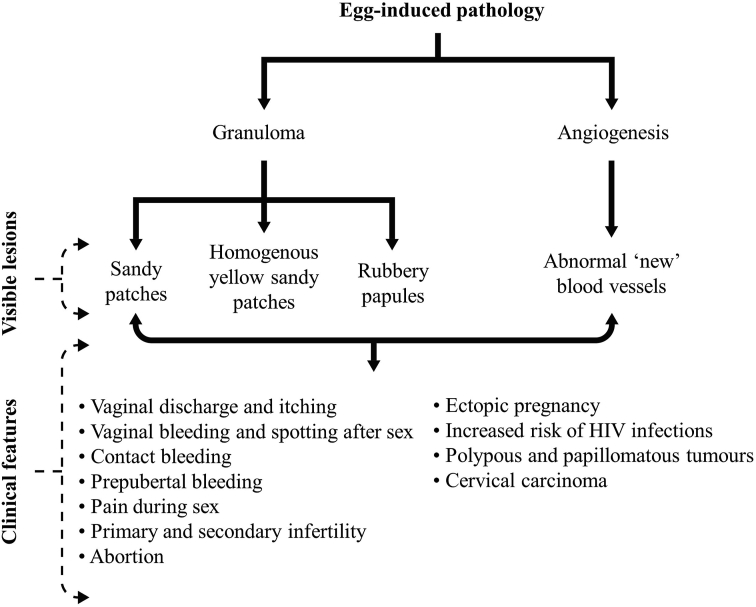
The pathogenesis of FGS is initiated by the presence of eggs in the female genital tract (Fig. 2) (Kjetland et al., 2012; Costain et al., 2018). The immune system mounts a response against eggs that are deposited in the genital tract whether live, dead or calcified (Kjetland et al., 2012). This immune response causes an influx of immune cells such as macrophages, neutrophils, eosinophils, lymphocytes, plasma cells, Langerhans giant cells, multinucleated histiocytes, and fibroblasts (Costain et al., 2018). These immune cells initiate an inflammatory response that results in the formation of granulomas surrounding the eggs (Kjetland et al., 2012; Costain et al., 2018). These granulomas formed in the genital tract in response to the deposited eggs result in various pathological mucosal changes in the genital tract, such as sandy patches (which present as grainy sandy patches and homogenous yellow sandy patches) and rubbery papules (Kjetland et al., 2012; Randrianasolo et al., 2015; Costain et al., 2018). In addition to the eggs invoking the immune-inflammatory response, they also induce angiogenesis around the site of deposition (Kjetland et al., 2005; Jourdan et al., 2011b; Costain et al., 2018) (Fig. 2).
Fig. 2.
Pathogenesis and clinical features of female genital schistosomiasis.
These abnormal pathological mucosal changes are responsible for several clinical manifestations and complications of FGS (Crump et al., 2000; Ekpo et al., 2017) (Fig. 2). These lesions, i.e. grainy sandy patches, homogenous yellow sandy patches, rubbery papules, and abnormal blood vessels, are mainly visible lesions that can be visualized in the vaginal wall and cervix during colposcopy (Renaud et al., 1989; Poggensee et al., 2001b; Kjetland et al., 2005; Randrianasolo et al., 2015; Costain et al., 2018). In some cases, the lesions can be seen on the vulva, and can be diagnostic, especially in prepubertal virgin girls where colposcopy is not encouraged (Savioli et al., 1990; Blum et al., 1998; Kjetland et al., 2012). In addition to being visible during colposcopy, these lesions result in a variety of clinical features in patients with FGS (Crump et al., 2000; Kjetland et al., 2005; Ekpo et al., 2017). The sandy patches cause irritation and discharge, which present as vaginal discharge and itching (Yirenya-Tawiah et al., 2011; Randrianasolo et al., 2015; Ekpo et al., 2017). They also make the epithelium easily friable and breakable resulting in bleeding as seen in pre-pubertal vaginal bleeding and spotting, contact bleeding (fresh bleeding during speculum or colposcopic examination) vaginal bleeding after sex, and pain during sex (Kjetland et al., 2012; Randrianasolo et al., 2015; Pillay et al., 2016).
Other clinical features that are often complications of FGS include primary and secondary infertility, ectopic pregnancy, abortions, and an increased risk of acquisition of HIV infection (Crump et al., 2000; Poggensee et al., 2000; Kjetland et al., 2006a; Swai et al., 2006; Jourdan et al., 2011a, 2011b; Randrianasolo et al., 2015; Costain et al., 2018) (Fig. 2). Pelvic inflammatory adhesions caused by these lesions, especially in the cervix, fallopian tubes, and uterus, could be responsible for infertility, ectopic pregnancy, and abortions experienced by women with FGS (El-Mahgoub, 1982; Vass & Lucey, 1982; Feldmeier et al., 1994; Crump et al., 2000; Schneider & Steyn, 2000; Hoffmann & Bauerfeind, 2003; Kjetland et al., 2010). The lesions in the vagina and cervix cause loss of mucosal integrity and coupled with the abnormal new blood vessels formation around the lesions increase the risk of acquisition of HIV and HPV infections among women with FGS (Poggensee et al., 2000; Downs et al., 2011; Randrianasolo et al., 2015; Costain et al., 2018). Benign and malignant lesions such as, polypous and papillomatous tumours, cervical carcinoma, and others, can also complicate FGS (El-Mahgoub, 1982; Schwartz, 1984; Helling-Giese et al., 1996; Pillay et al., 2016).
6. Diagnosis
The diagnosis of FGS begins with a high index of suspicion in female patients living in Schistosoma-endemic areas who present with vaginal symptoms with or without haematuria (Gundersen et al., 1996; WHO, 2015). Symptoms alone may not be definitive of FGS as many of the symptoms may be caused by several other debilitating disorders of the genital tract such as sexually transmitted diseases (Kjetland et al., 2008b; Shukla et al., 2019). Therefore, it is important that prompt and accurate diagnosis is reached for women with these worrisome symptoms. This will help reduce the anxiety and stigma of FGS by increasing awareness and support together with prompt treatment which will in turn improve the mental and social health of these women (Talaat et al., 2004; Yirenya-Tawiah et al., 2011; Hotez et al., 2020; Masong et al., 2021). Women living in endemic areas with these presentations should be further examined and investigated with a high index of suspicion to make a definitive diagnosis of FGS (WHO, 2015).
6.1. Colposcopy examination
The ability to visualize and recognize the egg-induced lesions (grainy sandy patches, homogeneous yellow sandy patches, rubbery tubercles and abnormal blood vessels) during pelvic examination using a colposcopy, is strongly diagnostic for FGS in women with clinical features (Kjetland et al., 2012; Randrianasolo et al., 2015; WHO, 2015; Ekpo et al., 2017). The presence of sandy patches (grainy sandy patches and homogenous yellow sandy patches) might signify a more chronic infection probably starting from childhood, while rubbery papules are seen more in younger women or girls and might signify a more recent infection (Randrianasolo et al., 2015). Colposcopy is limited to observing only vaginal lesions on the wall of the vagina and on the cervix (Randrianasolo et al., 2015). Many healthcare professionals are not knowledgeable in identifying these genital lesions and to overcome this, the WHO designed a pocket atlas to serve as a visual aid for healthcare professionals in endemic areas (WHO, 2015; Ekpo et al., 2017). However, the absence of lesions in colposcopy may not rule out FGS as the lesions might be in the uterus, fallopian tubes, or ovaries (WHO, 2015).
This diagnostic examination has cultural and ethical challenges because it can only be done in women who are sexually active and not virgin girls (Kjetland et al., 2012; Randrianasolo et al., 2015; FAST, 2021). Even sexually active women might be unwilling to undergo pelvic examination due to stigmatization (FAST, 2021).
6.2. Other diagnostic procedures
At the moment, there are no standardized laboratory diagnostic procedures yet for the diagnosis of FGS, but the use of some methods such as histological biopsy, PCR analysis on cervical lavage samples, vaginal swabs, and biopsies has been reported (Randrianasolo et al., 2015). Biopsies are not really encouraged because the procedure can further increase the risk of HIV transmission among sexually active women (Kjetland et al., 2012; O’Brien et al., 2019). In many S. haematobium-endemic areas, these laboratory diagnoses might not be feasible as the technology and logistics to carry them out are usually not available (Randrianasolo et al., 2015). However, there are ongoing studies to harness molecular techniques to overcome the challenges with colposcopy in resource-limited endemic areas such as PCR molecular analysis on a self-sampled vaginal swab (PCR self-swab), or in vaginal lavage, especially among virgin girls (Kjetland et al., 2009; Sturt et al., 2020; FAST, 2021).
7. Treatment
Praziquantel is the drug of choice for the treatment of S. haematobium infection (Richter, 2003; WHO, 2015; FAST, 2021). Although praziquantel has no effect on the deposited eggs, the rationale for use in the treatment of FGS is its ability to kill the adult worms and thus preventing egg production and subsequent deposition in the genital tract (Kjetland et al., 2006b). In S. haematobium-endemic areas, WHO recommends that praziquantel is prescribed to school-attending children including young and adolescent girls (WHO, 2009). This early treatment among young girls is considered effective in preventing FGS by killing the egg-producing adult worms thereby preventing egg deposition in the genital tract (Kjetland et al., 2006b, 2008a; WHO, 2009). It is believed that even a single dose of praziquantel in childhood can prevent half of the cases of FGS in endemic areas (WHO, 2009).
Women with suspected symptoms or complications as well as those with established FGS can still be treated with praziquantel to prevent further deposition of egg (Richter et al., 1996; Kjetland et al., 2012; WHO, 2015). Preventing further deposition of eggs in the genital tract can still be effective in resolving symptoms and complications of FGS despite praziquantel not having a direct impact on the deposited eggs (Richter et al., 1996; Kjetland et al., 2006b, 2008a; WHO, 2015). This beneficial effect was reported among women with infertility who were pregnant after almost two years of praziquantel treatment (Kjetland et al., 2012). Preemptive treatment is not only encouraged among women and girls living in endemic areas, but also among women with a history of contact with water bodies after a brief visit to endemic areas, because they also have substantial risk of developing FGS (Crump et al., 2000; FAST, 2021). Praziquantel is administered at 40 mg/kg body weight at least twice a year. In areas where reinfection is very likely, it can be used more often such as at a 6–8-week interval (which is the time taken for the adult worm to mature after infection) (WHO, 2015; FAST, 2021).
8. Control and prevention of FGS
With increasing awareness of FGS, several control and prevention measures have been proposed. They include intensifying existing prevention measures against urinary schistosomiasis, preventing FGS in women and young girls in endemic areas, through mass chemotherapy and preemptive treatment with praziquantel, and finally treatment of FGS with praziquantel after a proper and prompt diagnosis (Grimes et al., 2015; Tian-Bi et al., 2018; FAST, 2021). These interventions can be summarized as primary and secondary prevention of FGS.
8.1. Primary prevention of FGS
The main focus of this intervention is to prevent the entry of S. haematobium into the human body through the reduction or prevention of human exposure to cercariae in water bodies. To achieve this, various activities already being practiced in Schistosoma-endemic areas in the prevention of urinary schistosomiasis can be employed (Grimes et al., 2015; Braun et al., 2018). The provision of potable water for drinking and household chores as well as building bridges across rivers, lakes, and streams can reduce human exposure considerably (Grimes et al., 2015). Removal of water snails from water bodies through the removal of vegetation or the use of chemicals (molluscicides) has also been shown to be beneficial in reducing cercariae in the water bodies (Braun et al., 2018; Tian-Bi et al., 2018). Preventing open defecation through health education with an emphasis on the risk of not only urinary schistosomiasis but also FGS can help reduce exposure to infection by preventing the release of eggs into water bodies (Grimes et al., 2015; Saleem et al., 2019). Health education focused on increasing awareness of FGS in the community can also be a useful primary prevention strategy (Engels et al., 2020; FAST, 2021).
8.2. Secondary prevention of FGS
These are activities that aim to prevent full-blown disease among people living in endemic areas that are likely to be infected or diagnosed with infection (FAST, 2021). These activities are both effective against urinary schistosomiasis as well as FGS (Kjetland et al., 2012). Periodic praziquantel mass chemotherapy in endemic areas targeting urinary schistosomiasis is a potent secondary prevention strategy against FGS (Kjetland et al., 2012). Preemptive treatment of FGS is also a secondary prevention strategy where women who live in endemic areas or those with a history of exposure to water bodies in endemic areas presenting with symptoms are given praziquantel without a definitive diagnosis of FGS (FAST, 2021). Increasing awareness of FGS among healthcare providers and training them to recognize symptoms and signs will help improve easy identification and treatment (FAST, 2020). The FAST package project is proposing among other measures, integration of the diagnosis and treatment of FGS into existing reproductive and sexual health clinical services like STI/HIV, family planning, mother and child health, and cervical cancer screening services (FAST, 2020). Prompt treatment of women or young girls with symptoms and signs of FGS can ultimately prevent overarching debilitating complications that lead to death or reduced quality of life of these women (Kjetland et al., 2012; FAST, 2020, 2021).
8.3. Challenges of control and prevention
There are several challenges facing the control and prevention of FGS in endemic areas. First and foremost, preventing human contact with water bodies in endemic areas is a daunting task, as the provision of clean and potable water in some countries is almost impossible due to lack of political will, civil conflict, and wars (Evan Secor, 2014; Grimes et al., 2015). Sometimes the provision of potable water does not prevent people from having contact with water bodies (Evan Secor, 2014; Grimes et al., 2015). Many women and young girls are gainfully employed in places such as rice plantations, fishing, and local cloth cleaning outfits, where they may be constantly exposed to water bodies infested with Schistosoma spp. (Grimes et al., 2015; FAST, 2015). Provision of adequate toilet facilities in endemic countries could be another challenge, as many homes lack basic toilet facilities, thus encouraging open defecation (Saleem et al., 2019).
Unsustainable dependence on the donation of praziquantel in the treatment of FGS is another major challenge (Tchuem Tchuenté et al., 2017; FAST, 2021). Many governments in endemic areas are yet to throw their weight on financing the provision of praziquantel, probably because they are oblivious of the overarching consequences of Schistosoma spp. Infections in endemic areas (King & Dangerfield-Cha, 2008; Oyeyemi et al., 2020). While the available praziquantel in most endemic communities is donor-supplied for mass chemotherapy exercise targeting school children, there is no praziquantel for adults with FGS (Tchuem Tchuenté et al., 2017; FAST, 2021). This poses a strong challenge for the treatment of FGS in endemic areas.
9. Conclusions
With all the ongoing efforts of the relevant stakeholders to increase awareness among health workers and members of the community, it is very likely that FGS will soon be better appreciated in endemic areas. With the proposed involvement of healthcare professionals with varied specialty and qualifications in the diagnosis, management and treatment of FGS, the acquisition of basic knowledge of this parasitic disease is very crucial. Increased awareness of FGS among healthcare professionals will improve diagnosis and ensure prompt treatment of condition. A better understanding of FGS can throw more light on the true burden and implications of schistosomiasis in endemic areas, contributing greatly to its control and prevention.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT author statement
Verner N. Orish: conceived the idea for this review and wrote the first draft of the manuscript. Emmanuel Komla Senanu Morhe, Wisdom Azanu, Robert K. Alhassan and Margaret Gyapong: writing: reviewed & editing. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed S.H. Schistosomiasis (Bilharzia) 2020. https://emedicine.medscape.com/article/228392-overview#a6
- Antwi S., Aboah K.E.K., Saprong C.K.G. The unacknowledged impact of urinary schistosomiasis in children: 5 cases from Kumasi, Ghana. Ghana Med. J. 2014;48:228–233. doi: 10.4314/gmj.v48i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aula O.P., McManus D.P., Jones M.K., Gordon C.A. Schistosomiasis with a focus on Africa. Trop. Med. Infect. Dis. 2021;6 doi: 10.3390/tropicalmed6030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum R.S. Urinary schistosomiasis. J. Adv. Res. 2013;4:453–459. doi: 10.1016/j.jare.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J., Beck B., Strnad I., Hatz C. Vulvar lesion in urogenital schistosomiasis (S. haematobium) Z. Geburtshilfe Neonatol. 1998;202:255–257. [PubMed] [Google Scholar]
- Braun L., Grimes J.E., Templeton M.R. The effectiveness of water treatment processes against schistosome cercariae: a systematic review. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2019. Schistosoma species.https://www.cdc.gov/dpdx/schistosomiasis/index.html#:∼:text=Geographic%20Distribution,pockets%20of%20the%20Middle%20East [Google Scholar]
- Christinet V., Lazdins-Helds J.K., Stothard J.R., Reinhard-Rupp J. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int. J. Parasitol. 2016;46:395–404. doi: 10.1016/j.ijpara.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Costain A.H., MacDonald A.S., Smits H.H. Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump J.A., Murdoch D.R., Chambers S.T., Aickin D.R., Hunter L.A. Female genital schistosomiasis. J. Travel Med. 2000;7:30–32. doi: 10.2310/7060.2000.00008. [DOI] [PubMed] [Google Scholar]
- Cunin P., Tchuem Tchuenté L.A., Poste B., Djibrilla K., Martin P.M.V. Interactions between Schistosoma haematobium and Schistosoma mansoni in humans in north Cameroon. Trop. Med. Int. Health. 2003;8:110–117. doi: 10.1046/j.1360-2276.2003.01139.x. [DOI] [PubMed] [Google Scholar]
- Di Bella S., Riccardi N., Giacobbe D.R., Luzzati R. History of schistosomiasis (bilharziasis) in humans: from Egyptian medical papyri to molecular biology on mummies. Pathog. Glob. Health. 2021;112:268–273. doi: 10.1080/20477724.2018.1495357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J.A., Mguta C., Kaatano G.M., Mitchell K.B., Bang H., Simplice H., et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am. J. Trop. Med. Hyg. 2011;84:364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpo U.F., Odeyemi O.M., Sam-Wobo S.O., Onunkwor O.B., Mogaji H.O., Oluwole A.S., et al. Female genital schistosomiasis (FGS) in Ogun State, Nigeria: a pilot survey on genital symptoms and clinical findings. Parasitol. Open. 2017;3 doi: 10.1017/pao.2017.11. [DOI] [Google Scholar]
- El-Mahgoub S. Pelvic schistosomiasis and infertility. Int. J. Gynaecol. Obstet. 1982;20:201–206. doi: 10.1016/0020-7292(82)90072-8. [DOI] [PubMed] [Google Scholar]
- Emeto D.C., Salawu A.T. Recognition and reporting of neglected tropical diseases by primary health care workers in Ibadan, Nigeria. Pan Afr. Med. J. 2021;38:224. doi: 10.11604/pamj.2021.38.224.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels D., Hotez P.J., Ducker C., Gyapong M., Bustinduy A.L., Secor W.E., et al. Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull. World Health Organ. 2020;98:615–624. doi: 10.2471/BLT.20.252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan Secor W. Water-based interventions for schistosomiasis control. Pathog. Glob. Health. 2014;108:246–254. doi: 10.1179/2047773214Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAST FAST Package Project Launch Meeting. 2020. https://www.eliminateschisto.org/sites/gsa/files/content/attachments/2020-11-30/FAST%20Package_introduction%20slides_FINAL%2023SeptLaunch.pdf
- FAST FAST Package Workshop Female Genital Schistosomiasis, 2021. 2021. https://www.eliminateschisto.org/sites/gsa/files/content/attachments/2021-07-01/FAQ%20FGS%20training%20EN.pdf
- Feldmeier H., Krantz I., Poggensee G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. AIDS. 1994;5:368–372. doi: 10.1177/095646249400500517. [DOI] [PubMed] [Google Scholar]
- Freitas A.R.R., Angerami R.N. In: Parasitic diseases: schistosomiasis. El Ridi R., editor. IntechOpen; London: 2013. Spinal cord schistosomiasis. [DOI] [Google Scholar]
- Ghieth M.A., Lotfy A.M. Schistosomiasis haematobium prevalence among haematuric patients: parasitological and immuno-assay. Beni-Sueff Univ. J. Basic Appl. Sci. 2017;6:83–86. [Google Scholar]
- Gouvras A.N., Kariuki C., Koukounari A., Norton A.J., Lange C.N., Ireri E., et al. The impact of single versus mixed Schistosoma haematobium and S. mansoni infections on morbidity profiles amongst school-children in Taveta, Kenya. Acta Trop. 2013;128:309–317. doi: 10.1016/j.actatropica.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Grimes J.E., Croll D., Harrison W.E., Utzinger J., Freeman M.C., Templeton M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit. Vectors. 2015;8:156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirou N., Resnikoff S., Yakoura A.K.H., Gouda M., Bakayoko S., Napo A., et al. Orbital migration of schistosome eggs: a case report. BMC Ophthalmol. 2021;21:189. doi: 10.1186/s12886-021-01956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen S.G., Kjetland E.F., Poggensee G., Helling-Giese G., Richter J., Chitsulo L. Urine reagent strips for diagnosis of schistosomiasis haematobium in women of fertile age. Acta Trop. 1996;62:281–287. doi: 10.1016/s0001-706x(96)00029-0. [DOI] [PubMed] [Google Scholar]
- Gyapong M., Theobald S. The sexual and reproductive health issue you’ve probably never heard of. 2015. https://www.opendemocracy.net/en/5050/sexual-and-reproductive-health-issue-youve-probably-never-hear/
- Helling-Giese G., Sjaastad A., Poggensee G., Kjetland E.F., Richter J., Chitsulo L., et al. Female genital schistosomiasis (FGS): relationship between gynecological and histopathological findings. Acta Trop. 1996;62:257–267. doi: 10.1016/s0001-706x(96)00027-7. [DOI] [PubMed] [Google Scholar]
- Hemming F., editor. International Trust for Zoological Nomenclature; London, UK: 1954. pp. 201–208. (Opinions and declarations rendered by the International Commission on Zoological Nomenclature, Volume 4, Part 16). [Google Scholar]
- Hoffmann H., Bauerfeind I. High tissue egg burden mechanically impairing the tubal motility in genital schistosomiasis of the female. Acta Obstet. Gynecol. Scand. 2003;82:970–971. doi: 10.1034/j.1600-0412.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Harrison W., Fenwick A., Bustinduy A.L., Ducker C., Mbabazi P.S., et al. Correction to female genital schistosomiasis and HIV/AIDS: Reversing the neglect of girls and women. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Koibuchi T., Kumagai T., Maeda T., Osada Y., Ohta N., et al. Cerebral schistosomiasis due to Schistosoma haematobium confirmed by PCR analysis of brain specimen. J. Clin. Microbiol. 2011;49:3703–3706. doi: 10.1128/JCM.01073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan P.M., Holmen S.D., Gundersen S.G., Roald B., Kjetland E.F. HIV target cells in Schistosoma haematobium-infected female genital mucosa. Am. J. Trop. Med. Hyg. 2011;85:1060–1064. doi: 10.4269/ajtmh.2011.11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan P.M., Roald B., Poggensee G., Gundersen S.G., Kjetland E.F. Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl. Trop. Dis. 2011;5:e1170. doi: 10.1371/journal.pntd.0001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayuni S., Lampiao F., Makaula P., Juziwelo L., Lacourse E.J., Reinhard-Rupp J., et al. A systematic review with epidemiological update of male genital schistosomiasis (MGS): a call for integrated case management across the health system in sub-Saharan Africa. Parasite Epidemiol. Control. 2019;4:e00077. doi: 10.1016/j.parepi.2018.e00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayuni S.A., Alharbi M.H., Makaula P., Lampiao F., Juziwelo L., LaCourse E.J., et al. Male genital schistosomiasis along the shoreline of Lake Malawi: baseline prevalence and associated knowledge, attitudes and practices among local fishermen in Mangochi District, Malawi. Front. Public Health. 2021;9:615. doi: 10.3389/fpubh.2021.590695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayuni S.A., LaCourse E.J., Makaula P., Lampiao F., Juziwelo L., Fawcett J., et al. Case report: Highlighting male genital schistosomiasis (MGS) in fishermen from the southwestern shoreline of Lake Malawi, Mangochi District. Am. J. Trop. Med. Hyg. 2019;101:1331. doi: 10.4269/ajtmh.19-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.H., Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chron Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- Kjetland E.F., Ndhlovu P.D., Kurewa E.N., Midzi N., Gomo E., Mduluza T., et al. Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. Am. J. Trop. Med. Hyg. 2008;79:79–83. [PubMed] [Google Scholar]
- Kjetland E.F., Hove R.J.T., Gomo E., Midzi N., Gwanzura L., Mason P., et al. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am. J. Trop. Med. Hyg. 2009;81:1050–1055. doi: 10.4269/ajtmh.2009.09-0081. [DOI] [PubMed] [Google Scholar]
- Kjetland E.F., Kurewa E.N., Mduluza T., Midzi N., Gomo E., Friis H., et al. The first community-based report on the effect of genital Schistosoma haematobium infection on female fertility. Fertil. Steril. 2010;94:1551–1553. doi: 10.1016/j.fertnstert.2009.12.050. [DOI] [PubMed] [Google Scholar]
- Kjetland E.F., Kurewa E.N., Ndhlovu P.D., Midzi N., Gwanzura L., Mason P.R., et al. Female genital schistosomiasis - a differential diagnosis to sexually transmitted disease: genital itch and vaginal discharge as indicators of genital Schistosoma haematobium morbidity in a cross-sectional study in endemic rural Zimbabwe. Trop. Med. Int. Health. 2008;13:1509–1517. doi: 10.1111/j.1365-3156.2008.02161.x. [DOI] [PubMed] [Google Scholar]
- Kjetland E.F., Leutscher P.D., Ndhlovu P.D. A review of female genital schistosomiasis. Trends Parasitol. 2012;28:58–65. doi: 10.1016/j.pt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Kjetland E.F., Mduluza T., Ndhlovu P.D., Gomo E., Gwanzura L., Midzi N., et al. Genital schistosomiasis in women: a clinical 12-month in vivo study following treatment with praziquantel. Trans. R. Soc. Trop. Med. Hyg. 2006;100:740–752. doi: 10.1016/j.trstmh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Kjetland E.F., Ndhlovu P.D., Gomo E., Mduluza T., Midzi N., Gwanzura L., Gundersen S.G. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- Kjetland E.F., Ndhlovu P.D., Mduluza T., Gomo E., Gwanzura L., Mason P.R., et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am. J. Trop. Med. Hyg. 2005;72:311–319. [PubMed] [Google Scholar]
- Kjetland E.F., Poggensee G., Helling-Giese G., Richter J., Sjaastad A., Chitsulo L., Kumwenda N., et al. Female genital schistosomiasis due to Schistosoma haematobium. Clinical and parasitological findings in women in rural Malawi. Acta Trop. 1996;62:239–255. doi: 10.1016/s0001-706x(96)00026-5. [DOI] [PubMed] [Google Scholar]
- Kukula V.A., MacPherson E.E., Tsey I.H., Stothard J.R., Theobald S., Gyapong M. A major hurdle in the elimination of urogenital schistosomiasis revealed: identifying key gaps in knowledge and understanding of female genital schistosomiasis within communities and local health workers. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandro D.M., Reis F.P., Silva J.R.S., Soares A.F., de Oliveira A.D.S., Santos R.P.V., et al. Clinical and histopathological profile of female genital schistosomiasis. Res. Soc. Dev. 2021;10 e47410716652. [Google Scholar]
- Leutscher P.D.C., Ramarokoto C.E., Hoffmann S., Jensen J.S., Ramaniraka V., Randrianasolo B., et al. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin. Infect. Dis. 2008;47:775–782. doi: 10.1086/591127. [DOI] [PubMed] [Google Scholar]
- Masong M.C., Wepnje G.B., Marlene N.T., Gamba V., Mengue M.T., Kouokam E., et al. Female genital schistosomiasis (FGS) in Cameroon: a formative epidemiological and socioeconomic investigation in eleven rural fishing communities. PLOS Glob. Public Health. 2021;1:e0000007. doi: 10.1371/journal.pgph.0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazigo H.D., Samson A., Lambert V.J., Kosia A.L., Ngoma D.D., Murphy R., et al. We know about schistosomiasis but we know nothing about FGS”: a qualitative assessment of knowledge gaps about female genital schistosomiasis among communities living in Schistosoma haematobium endemic districts of Zanzibar and Northwestern Tanzania. PLoS Negl. Trop. Dis. 2021;15:e0009789. doi: 10.1371/journal.pntd.0009789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation C.S., Da’dara A.A., Marchant J.K., Skelly P.J. Schistosome migration in the definitive host. PLoS Negl. Trop. Dis. 2020;14:e0007951. doi: 10.1371/journal.pntd.0007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelwan M.L. Schistosomiasis: life cycle, diagnosis, and control. Curr. Ther. Res. 2019;91:5–9. doi: 10.1016/j.curtheres.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour N.M. Schistosomiasis: health effects on women. Rev. Obstet. Gynecol. 2010;3:28–32. [PMC free article] [PubMed] [Google Scholar]
- O’Brien D.P., Ford N., Djirmay A.G., Calmy A., Vitoria M., Jensen T.O., et al. Female genital schistosomiasis and HIV: research urgently needed to improve understanding of the health impacts of this important coinfection. J. Acquir. Immune Defic. Syndr. 2019;80:489–493. doi: 10.1097/QAI.0000000000001957. [DOI] [PubMed] [Google Scholar]
- Oyeyemi O.T., de Jesus Jeremias W., Grenfell R.F.Q. Schistosomiasis in Nigeria: gleaning from the past to improve current efforts towards control. One Health. 2020;11:100183. doi: 10.1016/j.onehlt.2020.100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Rose C.E., Kjetland E.F., Downs J.A., Mbabazi P.S., Sabin K., et al. Association of schistosomiasis and HIV infections: a systematic review and meta-analysis. Int. J. Infect. Dis. 2021;102:544–553. doi: 10.1016/j.ijid.2020.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay P., Van Lieshout L., Taylor M., Sebitloane M., Zulu S.G., Kleppa E., et al. Cervical cytology as a diagnostic tool for female genital schistosomiasis: correlation to cervical atypia and Schistosoma polymerase chain reaction. CytoJournal. 2016;13:10. doi: 10.4103/1742-6413.180784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggensee G., Feldmeier H. Female genital schistosomiasis: facts and hypotheses. Acta Trop. 2001;79:193–210. doi: 10.1016/s0001-706x(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Poggensee G., Kiwelu I., Saria M., Richter J., Krantz I., Feldmeier H. Schistosomiasis of the lower reproductive tract without egg excretion in urine. Am. J. Trop. Med. Hyg. 1998;59:782–783. doi: 10.4269/ajtmh.1998.59.782. [DOI] [PubMed] [Google Scholar]
- Poggensee G., Kiwelu I., Weger V., Göppner D., Diedrich T., Krantz I., et al. Female genital schistosomiasis of the lower genital tract: prevalence and disease-associated morbidity in northern Tanzania. J. Infect. Dis. 2000;181:1210–1213. doi: 10.1086/315345. [DOI] [PubMed] [Google Scholar]
- Poggensee G., Sahebali S., Van Marck E., Swai B., Krantz I., Feldmeier H. Diagnosis of genital cervical schistosomiasis: comparison of cytological, histopathological and parasitological examination. Am. J. Trop. Med. Hyg. 2001;65:233–236. doi: 10.4269/ajtmh.2001.65.233. [DOI] [PubMed] [Google Scholar]
- Randrianasolo B.S., Jourdan P.M., Ravoniarimbinina P., Ramarokoto C.E., Rakotomanana F., Ravaoalimalala V.E., et al. Gynecological manifestations, histopathological findings, and Schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: a cross-sectional study in Madagascar. J. Infect. Dis. 2015;212:275–284. doi: 10.1093/infdis/jiv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud G., Devidas A., Develoux M., Iamothe F., Blanchi G. Prevalence of vaginal schistosomiasis caused by Schistosoma haematobium in an endemic village in Niger. Trans. R. Soc. Trop. Med. Hyg. 1989;83:797. doi: 10.1016/0035-9203(89)90333-7. [DOI] [PubMed] [Google Scholar]
- Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Trop. 2003;86:161–183. doi: 10.1016/s0001-706x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Richter J., Poggensee G., Kjetland E.F., Helling-Giese G., Chitsulo L., Kumwenda N., et al. Reversibility of lower reproductive tract abnormalities in women with Schistosoma haematobium infection after treatment with praziquantel - an interim report. Acta Trop. 1996;62:289–301. doi: 10.1016/s0001-706x(96)00030-7. [DOI] [PubMed] [Google Scholar]
- Rozendaal J.A., Arie J., World Health Organization . Vector control: methods for use by individuals and communities. World Health Organization; Geneva: 1997. Freshwater snails.https://www.who.int/water_sanitation_health/resources/vector337to356.pdf [Google Scholar]
- Sagin D.D., Ismail G., Fui J.N., Jok J.J. Schistosomiasis malayensis-like infection among the penan and other interior tribes (orang ulu) in upper rejang river basin, sarawak, Malaysia. Southeast Asian J. Trop. Med. Publ. Health. 2001;32:27–32. [PubMed] [Google Scholar]
- Saleem M., Burdett T., Heaslip V. Health and social impacts of open defecation on women: a systematic review. BMC Publ. Health. 2019;19:158. doi: 10.1186/s12889-019-6423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli L., Gabrielli A., Neve H. Vulvar Schistosoma haematobium lesion treated with praziquantel. Trop Doct. 1990;20:45–46. doi: 10.1177/004947559002000119. [DOI] [PubMed] [Google Scholar]
- Schneider D., Steyn D.W. Genital schistosomiasis presenting as suspected ectopic pregnancy in the Western Cape. S. Afr. Med. J. 2000;90:609. [PubMed] [Google Scholar]
- Schwartz D.A. Carcinoma of the uterine cervix and schistosomiasis in West Africa. Gynecol. Oncol. 1984;19:365–370. doi: 10.1016/0090-8258(84)90205-1. [DOI] [PubMed] [Google Scholar]
- SCI Foundation FGS Acelerated Scale together (FAST) Package Project 2021. 2021. https://schistosomiasiscontrolinitiative.org/fast-research-project
- Shukla J.D., Kleppa E., Holmen S., Ndhlovu P.D., Mtshali A., Sebitloane M.H., et al. Female genital schistosomiasis and reproductive tract infections. A cross-sectional study in rural adolescents in South Africa. medRxiv. 2019:19009233. doi: 10.1097/LGT.0000000000000756. https://www.medrxiv.org/content/10.1101/19009233v1.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturt A.S., Webb E.L., Phiri C.R., Mweene T., Chola N., van Dam G.J., et al. Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in Zambian women: the BILHIV study. PLoS Negl. Trop. Dis. 2020;14:e0008337. doi: 10.1371/journal.pntd.0008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai B., Poggensee G., Mtweve S., Krantz I. Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health: a retrospective histopathological study from Tanzania. BMC Infect. Dis. 2006;6:134. doi: 10.1186/1471-2334-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat M., Watts S., Mekheimar S., Ali H.F., Hamed H. The social context of reproductive health in an Egyptian hamlet: a pilot study to identify female genital schistosomiasis. Soc. Sci. Med. 2004;58:515–524. doi: 10.1016/j.socscimed.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Tchuem Tchuenté L.A., Rollinson D., Stothard J.R., Molyneux D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect. Dis. Poverty. 2017;6:42. doi: 10.1186/s40249-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian-Bi Y.N.T., Ouattara M., Knopp S., Coulibaly J.T., Hürlimann E., Webster B., et al. Interrupting seasonal transmission of Schistosoma haematobium and control of soil-transmitted helminthiasis in northern and central Côte d’Ivoire: a SCORE study protocol. BMC Publ. Health. 2018;18:186. doi: 10.1186/s12889-018-5044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass A.C.R., Lucey J.J. Bilharzial granuloma of the fallopian tube: case report. Br. J. Obstet. Gynaecol. 1982;89:867–869. doi: 10.1111/j.1471-0528.1982.tb05044.x. [DOI] [PubMed] [Google Scholar]
- Vilana R., Corachan M., Gascon J., Valls E., Bru C. Schistosomiasis of the male genital tract: transrectal sonographic findings. J. Urol. 1997;158:1491–1493. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2009. Statement: WHO working group on urogenital schistosomiasis and HIV transmission, 1–2 October 2009.https://www.who.int/news/item/30-10-2009-statement-who-working-group-on-urogenital-schistosomiasis-and-hiv-transmission-1-2-october-2009 [Google Scholar]
- WHO . World Health Organization; Geneva: 2015. Female genital schistosomiasis: a pocket atlas for clinical health-care professionals (No. WHO/HTM/NTD/2015.4)https://apps.who.int/iris/handle/10665/180863 [Google Scholar]
- WHO . World Health Organization; Geneva: 2022. Schistosomiasis WHO facts.https://www.who.int/news-room/fact-sheets/detail/schistosomiasis [Google Scholar]
- Wu G.Y., Halim M.H. Schistosomiasis: progress and problems. World J. Gastroenterol. 2000;6:12. doi: 10.3748/wjg.v6.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirenya-Tawiah D., Amoah C., Apea-Kubi K.A., Dade M., Ackumey M., Annang T., et al. A survey of female genital schistosomiasis of the lower reproductive tract in the Volta Basin of Ghana. Ghana Med. J. 2011;45:16–21. doi: 10.4314/gmj.v45i1.68917. [DOI] [PMC free article] [PubMed] [Google Scholar]