Summary
Hyaluronic acid-based filler is the most popular injectable augmentation preparation due to its efficacy and safety compared to other injection fillers. The complication of infected filler is known, but it is unknown exactly how long filler persists prior to reabsorption. A case was presented of filler-exacerbated facial cellulitis that occurred 2.5 years after hyaluronic acid-based filler administration. The presence of residual filler was confirmed with magnetic resonance imaging, suggesting that hyaluronic acid-based fillers may persist longer than previously thought and act as a reservoir for regional bacterial infections refractory to antibiotics.
Keywords: Hyaluronic acid, Hyaluronidase, Soft-tissue filler, Cellulitis, Complications of cosmetic procedures
Introduction
Hyaluronic acid-based (HA) filler has become the most popular injectable augmentation preparation1 due to its biocompatibility, moderate length of efficacy (3–12 months), and reversibility of many complications using hyaluronidase.2 However, its use is not without complications. Complications from fillers are divided into two subtypes, immediate/early and delayed. Immediate/early complications occur within the first few weeks after augmentation. In the case of HA fillers, immediate/early complications primarily include infection, migration, scarring, and very rarely vascular occlusion. HA fillers are much less immunogenic than its predecessors, and theoretically do not carry a risk of allergy or foreign-body reaction.3 However, there are multiple reports of presumed granulomatous inflammatory nodules in response to HA fillers.4
Delayed complications include inflammation or infection that can occur months to years after HA filler injection.1 Delayed infections are thought to be the result of biofilms forming on the filler deposit, potentially associated with multiple needle passes or poor aseptic technique.5,6 These biofilms may remain dormant for weeks to years before being “activated” by trauma, hematogenous spread of an existing infection, or a compromised immune system, resulting in local infections like cellulitis or abscesses.5
The expected permanence of HA fillers has been cited as up to 12 months,4, 7 with potential longer-lasting or permanent effects from stimulation of local collagen production.7 However, there have been cases described of patients with delayed infections 2 years after augmentation with HA fillers,1 suggesting that components of the HA filler may persist longer than previously thought. Here, we describe a case of refractory facial cellulitis 2.5 years delayed after augmentation, confirmed to be associated with residual filler on MR imaging.
Case
The patient was a 46 year-old male with a history of hypertension for which he takes lisinopril. He was neither diabetic or immunocompromised. Two weeks prior to presentation, he developed a fever to 39 °C, a sore throat, and rhinorrhea. An outpatient COVID test was negative. Five days prior to presentation, he developed a pruritic rash over his left scalp and forehead, which slowly subsided. Starting the day prior to presentation, he developed rapidly progressive swelling of the left midface (Figure 1a). Upon presentation, he was also noted to have a medialized left tonsil. He denied any subjective dyspnea, though oxygen was delivered through a nasal canula prophylactically by the emergency medicine team. His Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score at this time was 6 (Table 1) .8 Sepsis protocol was initiated, blood cultures were drawn, and a computed tomography (CT) scan of his face was performed.
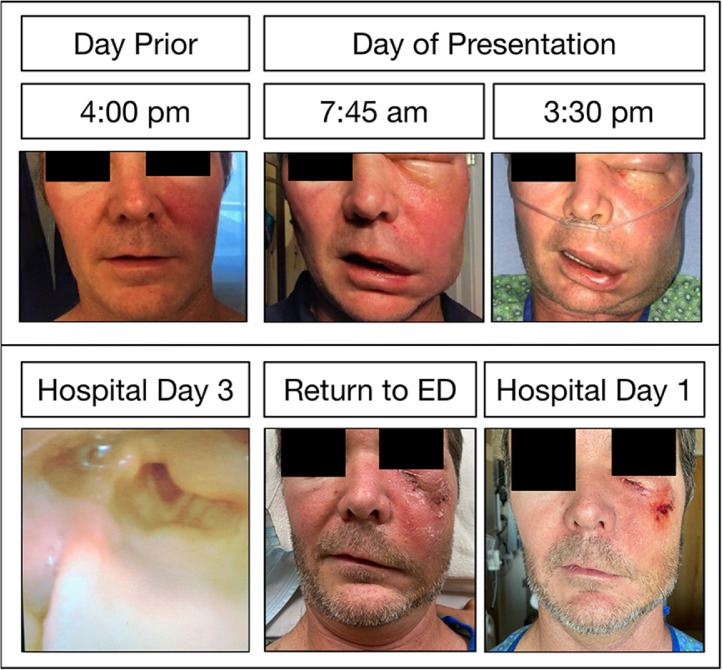
Figure 1.
Clinical photos of facial cellulitis demonstrating rapid progression the day prior to and the day of presentation (A) and supraglottic edema on hospital day 3 (B). After returning to the Emergency Department (C, left panel), he was given hyaluronidase and demonstrated marked improvement the following morning (C, right panel).
Table 1.
The patient's LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score at the time of presentation. Presenting LRINEC Score.
| Variable (Units) | Patient Value | Score | |
|---|---|---|---|
| C-Reactive Protein (mg/L) | 189.6 | (>150) | 4 |
| Total white cell count (per mm3) | 27 | (>25) | 2 |
| Hemoglobin (g/dL) | 14.6 | (>13.5) | 0 |
| Sodium (mmol/L) | 137 | (>135) | 0 |
| Creatinine (mg/dL) | 1.08 | (<1.6) | 0 |
| Glucose (mg/dL) | 115 | (<180) | 0 |
| Total Score | 6 | ||
The CT imaging demonstrated cellulitis without a drainable fluid collection, though there was evidence of mild ipsilateral sinusitis. He was admitted to the hospital under the internal medicine service for close monitoring and administration of broad-spectrum IV antibiotics, which were recommended by the infectious disease team to include ceftriaxone, metronidazole, and linezolid. Clindamycin was added for its antitoxin effect. Because angioedema was included in the differential diagnosis, his lisinopril was held, and histamine blockade was initiated.
Over the first three hospital days, his physical examination remained stable and his blood cultures did not grow any organisms, though his CRP continued to rise. On hospital day three, his CRP peaked at 296, and a repeat CT face was performed. Again, there were no drainable fluid collections, though there was new evidence of ipsilateral laryngeal edema. Bedside flexible laryngoscopy was performed, demonstrating mild edema of the left arytenoid and aryepiglottic fold (Figure 1b). He continued to be asymptomatic of any dyspnea or signs of impending airway compromise.
Over the subsequent 3 days, the patient began clinically improving with diminishing facial edema and pain. Clindamycin and linezolid were discontinued, and he remained on ceftriaxone and metronidazole. A sinus regimen was initiated with a saline rinse followed by a nasal steroid. He was discharged on hospital day six on oral cefpodoxime and metronidazole.
He returned to the Emergency Department the following day with a mild increase in facial edema and new nausea and vomiting. His inflammatory markers were reassuring, but due to the complicated clinical course, slow improvement, and new symptoms, a magnetic resonance image (MRI) of the patient's face was performed (Figure 2).
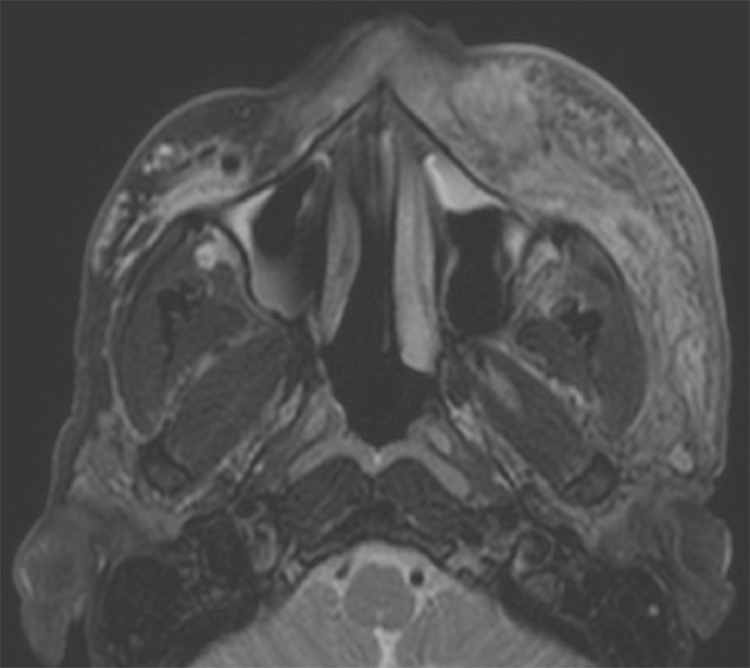
Figure 2.
MR Face demonstrating left midfacial cellulitis and, at the same level, right T2 hyperintense foci, consistent with hyaluronic acid filler.
In addition to the expected finding of midface cellulitis without drainable fluid collections, the MRI demonstrated evidence of hyperintense foci in the contralateral midface on T2-weighted, pre-contrast axial slices. Upon further discussion with the patient, he had undergone cosmetic midface augmentation over the zygomas bilaterally with Voluma 3.5 years and 2.5 years prior to his infection. On both occasions, he received 0.45cc per side with a 27 g sharp needle divided across three positions deposited on bone, all lateral to the midpupillary line (0.1cc in the lateralmost deposit, 0.15cc in the middle deposit, and 0.2cc in the medial deposit). The lack of identifiable filler on the affected side was thought to be due to cellulitic changes overtaking the density of the filler, as the patient reported symmetric filler injections.
A discussion was had with the patient regarding the risks and benefits of hyaluronidase administration in the setting of visible filler on MRI in the region of cellulitis refractory to aggressive medical management. He consented to proceed with hyaluronidase injection.
The dose of hyaluronidase was calculated using the aesthetic complication guidelines from the Journal of Clinical and Aesthetic Dermatology.2 After a patch test of 20 units was nonreactive, a total of 130 units of hyaluronidase were administered to the left midface over the zygoma, where the patient reported the initial filler injection was placed. He was readmitted to the hospital service to restart intravenous ceftriaxone and metronidazole.
The following morning, the patient demonstrated marked improvement his facial edema, erythema, and pain (Figure 1c). An additional 150 units of hyaluronidase were administered, and he was discharged on amoxicillin-clavulanate with close follow up scheduled with an otolaryngologist, and infectious disease specialist, and his primary care provider. The day following discharge, he was injected with two more doses of 150 units of hyaluronidase each by his aesthetic medicine specialist for persistent palpable filler. At his outpatient follow-up with otolaryngology 1.5 weeks after discharge, he had continued to improve with only mild residual edema of the lower eyelid. At his outpatient follow up with infectious disease 2 weeks after discharge, he tested negative for HIV. He has made a full recovery with minimal scarring.
Discussion
The patient described here had the presence of residual HA filler confirmed by MR imaging 2.5 years after his injection. The appearance of the filler identified on MRI was consistent with that described in the literature as T2 hyperintense.9 Moreover, the filler deposits in his contralateral midface did not demonstrate evidence of infection or inflammation, suggesting that the filler was still in a normal resorption process. HA filler may be longer-lasting than previously described, carrying risk for delayed complications while any residual filler remains.
This patient's case illustrates the need to consider the presence of HA filler in all patients, as the use of hyaluronidase earlier in his course would have saved him considerable morbidity and multiple days of hospitalization and work absenteeism. The case also reiterates the importance of the synergy provided by both antibiotics and hyaluronidase to treat filler-associated infections.10
Conclusion
HA filler may be longer-lasting than previously described, and the risk of delayed complications persist until resorption has completed.
Funding
Dr. Olivia Kalmanson receives research funding from the NIH/NIDCD (T32 DC-012280) and from the University of Colorado School of Medicine (internal grant: Enrichment Fund for Women in Surgery).
Patient consent for photo publication
The patient has consented to the publication of his photos in this academic journal. He personally approved the clinical photos as they appear in the manuscript figure.
Ethical approval
Not required.
Declaration of Competing Interest
None.
References
- 1.Park T.H., Seo S.W., Kim J.K., Chang C.H. Clinical experience with hyaluronic acid-filler complications. J Plast Reconstr Aesthet Surg. 2011;64(7):892–896. doi: 10.1016/j.bjps.2011.01.008. Jul. [DOI] [PubMed] [Google Scholar]
- 2.King M., Convery C., Davies E. This month's guideline: The use of hyaluronidase in aesthetic practice (v2.4) J Clin Aesthet Dermatol. 2018;11(6):E61–E68. Jun. [PMC free article] [PubMed] [Google Scholar]
- 3.Verpaele A., Strand A. Restylane SubQ, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006;26(1S):S10–S17. doi: 10.1016/j.asj.2005.09.009. Jan-Feb. [DOI] [PubMed] [Google Scholar]
- 4.Lemperle G., Gauthier-Hazan N., Wolters M., Eisemann-Klein M., Zimmermann U., Duffy D.M. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(6):1842–1863. doi: 10.1097/PRS.0b013e31818236d7. Jun. [DOI] [PubMed] [Google Scholar]
- 5.Dumitraşcu D.I., Georgescu A.V. The management of biofilm formation after hyaluronic acid gel filler injections: A review. Clujul Med. 2013;86(3):192–195. [PMC free article] [PubMed] [Google Scholar]
- 6.Saththianathan M., Johani K., Taylor A., et al. The role of bacterial biofilm in adverse soft-tissue filler reactions: A combined laboratory and clinical study. Plast Reconstr Surg. 2017;139(3):613–621. doi: 10.1097/PRS.0000000000003067. Mar. [DOI] [PubMed] [Google Scholar]
- 7.Alijotas-Reig J., Fernández-Figueras M.T., Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45(1):97–108. doi: 10.1007/s12016-012-8348-5. Aug. [DOI] [PubMed] [Google Scholar]
- 8.Wong Chin-Ho, Khin Lay-Wai, Heng Kien-Seng, Tan Kok-Chai, Low Cheng-Ooi. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Critical Care Medicine. July 2004;32(7):1535–1541. doi: 10.1097/01.ccm.0000129486.35458.7d. MD, MRCSMD, MSCMD, FRCSMD, FRCS. volume. [DOI] [PubMed] [Google Scholar]
- 9.Ginat D.T., Schatz C.J. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34(8):1488–1495. doi: 10.3174/ajnr.A3161. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marusza W., Olszanski R., Sierdzinski J., et al. Treatment of late bacterial infections resulting from soft-tissue filler injections. Infect Drug Resist. 2019;12:469–480. doi: 10.2147/IDR.S186996. Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]