Highlights
-
•
Despite recent progress regarding inexpensive medications in clinical settings and, many individuals suffer from moderate to severe pain globally.
-
•
It is thought that understating the close association between these phenomena paves the way to use exosomes as therapeutic bullets to reduce pain under pathological conditions.
Keywords: Autophagy, Neural Exosome, Pain Management, Cell Therapy
Abbreviations: NPCs-Exo, NPCs-derived Exo; CESC-Exo, cartilage endplate stem cell-derived Exo; MMP, matrix metalloproteinase; NPCs, nucleus pulposus cells; HSPA8, heat shock protein family A member 8; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptors; nSMase, ceramide-generating enzyme neutral sphingomyelinases; ESCRT, endosomal sorting complex required for transport; MVBs, multivesicular bodies; LAMP2, lysosomal‑associated membrane protein type 2; TLR4, Toll-like receptor 4; TRAF6, TNF receptor-associated factor 6; MAPK8/JNK, mitogen-activated protein kinase 8p-/c-Jun N-terminal Kinase; NFKB/NF-κB, nuclear factor of kappa light polypeptide gene enhancer in B cells; ER, endoplasmic reticulum; LAT1, large amino acid transporter; LTs, leukotrienes
Abstract
Despite recent progress regarding inexpensive medical approaches, many individuals suffer from moderate to severe pain globally. The discovery and advent of exosomes, as biological nano-sized vesicles, has revolutionized current knowledge about underlying mechanisms associated with several pathological conditions. Indeed, these particles are touted as biological bio-shuttles with the potential to carry specific signaling biomolecules to cells in proximity and remote sites, maintaining cell-to-cell communication in a paracrine manner. A piece of evidence points to an intricate relationship between exosome biogenesis and autophagy signaling pathways at different molecular levels. A close collaboration of autophagic response with exosome release can affect the body’s hemostasis and physiology of different cell types. This review is a preliminary attempt to highlight the possible interface of autophagy flux and exosome biogenesis on pain management with a special focus on neuropathic pain. It is thought that this review article will help us to understand the interplay of autophagic response and exosome biogenesis in the management of pain under pathological conditions. The application of therapies targeting autophagy pathway and exosome abscission can be an alternative strategy in the regulation of pain.
Introduction
The occurrence of pain, especially chronic type with limited therapeutic options, is relatively common among individuals (Hylands-White et al., 2017, Golzari et al., 2014). Pain is generally classified into acute and chronic forms in terms of duration (Soleimanpour et al., 2022, Ghojazadeh et al., 2019). Acute pain can last for a short period and accompanies anxiety and distress while the latter type is associated with a long-term painful condition because of nerve injury (Glare et al., 2020, Ghojazadeh et al., 2019, Dolati et al., 2020). It is believed that pain is a multidimensional phenomenon and occurs because of an injury to the nervous system (neuropathic pain; NP) or tissue damage (nociceptive pain) (Hylands-White et al., 2017). Irrespective of the pain type, this phenomenon is regulated by engaging several cellular and molecular mechanisms (Cho and Huh, 2020). Changes in the activity of glia and neuronal lineages, and delivery of several cytokines within the nervous system can contribute to neuropathic effects (Oh et al., 2018). The increase of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) stimulates phenotype shifting in astrocytes from A2 to A1 types, which per se causes chronic pain. By contrast, astrocyte phenotypic switching from A1 to A2 reduces chronic pain. In response to inflammatory conditions, the increase of glial fibrillary acidic protein (GFAP), a well-known astrocyte hallmark, leads to NP generation (Li et al., 2019). Upon the stimulation of astrocytes, the activation of microglia results in the local release of several biomolecules with a protective potential for neurons and glial cells (Salaffi et al., 2018).
Exosomes (Exo) are a subclass of extracellular vesicles (EVs) released from several cell types and participate in paracrine interaction between the cells (Amini et al., 2021). Regarding their unique size ranging from 40 to 200 nm, Exo can easily distribute in biofluids and transfer signaling biomolecules such as proteins, lipids, and nucleic acids from donor cells to the recipient cells. In line with these statements, it is thought that Exo are key mediators to modulate biochemical reactions and cellular activity during physiological and pathological conditions (Rahbarghazi et al., 2021). Of note, the critical roles of Exo on specific pathological conditions like neurodegenerative disease, inflammatory responses, osteoarthritis, etc., have been shown previously (Lin et al., 2020). The reciprocal Exo exchange between the cells within the central nervous system (CNS) and peripheral nervous system (PNS) makes these nanoparticles a novel therapeutic modality (Bahlakeh et al., 2021). Regarding the ability of Exo to transfer specific cargo, these particles can be used to retrieve several pathological conditions (Ni et al., 2020).
From a biological viewpoint, Exo are originated from the endosomal system consisting of several regulatory mechanisms (Rahbarghazi et al., 2021). Based on numerous studies, there is synergy between Exo biogenesis and other secretory pathways like autophagy machinery, as an early-stage mechanism activated in the host cells to turnover damaged materials (Colletti et al., 2021, Hassanpour et al., 2020). It is also suggested that the close crosstalk between the autophagy-lysosomal pathway and Exo biogenesis is an adaptive response to further preserve cellular hemostasis (Rahbarghazi et al., 2021). Here, we aimed to highlight the synergy of Exo-autophagy in alleviating pathological conditions and reducing pain within the nervous system.
Molecular aspects of peripheral neuropathy
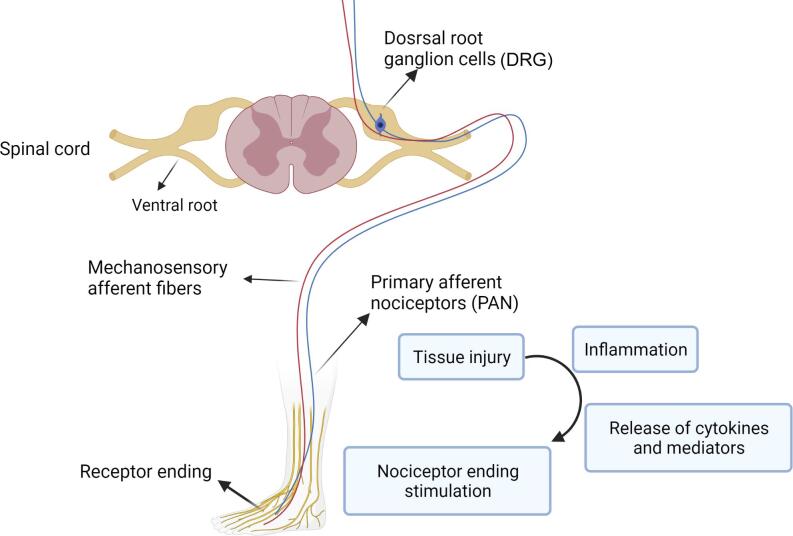
Primary afferent nociceptors (PAN) are touted as the first structures involved in the perception of chemical, mechanical, and thermal stimuli. PAN are peripheral nerve fibers originating from pseudounipolar sensory neurons that locate in the trigeminal ganglion in the face and the dorsal root ganglion (DRG) in the body (Wang et al., 2010) (Fig. 1).
Fig. 1.
Simplified mechanism of pain perception via ascending and descending pathways in peripheral nerves and spinal cord.
Molecular investigations indicated that large amino acid transporter 1 (LAT1) is highly expressed in the DRG region following the spinal cord injury. These data support the crucial role of LAT1/4F2hc/Wnt/frizzled/β-catenin signal transduction pathway in the pain process (Alles et al., 2020). Of note, voltage-gated potassium channels (Kv1.1 and Kv1.2), the voltage-gated sodium channel (Nav1.7), and to a lesser extent, voltage-activated calcium channel auxiliary subunit α2δ-1 are also involved in the pain process. Besides, LAT1 has the potential to stimulate the mTOR signaling pathway, particularly in the inflammatory responses and NP (Alles et al., 2020). In a recently published study, it was suggested that autophagy and apoptosis, as adaptive mechanisms, can interact through various proinflammatory cytokine regulations when peripheral neuropathy occurs. In this era, proinflammatory cytokines can encourage autophagic/apoptotic function and subsequent NP formation. Autophagy per se quenches the proinflammatory cytokine activities and further modulates pain behavior (Liao et al., 2022).
Inflammatory responses in NP
Mounting evidence highlighted the role of neurogenic inflammation and neuroinflammation in developing pain patterns (Matsuda et al., 2019). Inflammatory mediators, including prostaglandins (PGs), pro-inflammatory cytokines, and chemokines are elevated in response to noxious stimuli and concurrently persuade pain perception via direct stimulation of primary sensory neurons, namely nociceptors (Matsuda et al., 2019). The promotion of cytokines can per se stimulate cytokine receptors, resulting in modified nociceptive signaling and neuronal activity. Along with these changes, excitatory synaptic transmission is induced while the basal inhibitory synaptic transmission is prohibited (Vanderwall and Milligan, 2019). During several pathological conditions, specific cytokines such as TNF-α and other interleukins are frequently expressed at the injury site. TNF-α could intensify spontaneous excitatory post-synaptic activity via the elevation of excitatory α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA. Along with these changes, IL-6 blunts spontaneous inhibitory post-synaptic action via the suppression of GABA and glycine (Kawasaki et al., 2008).
Interestingly, the increase of endogenous pyrogen namely IL-1 can bilaterally mediate these features (Kawasaki et al., 2008). Besides the overactivity of neurons during the inflammatory conditions, the critical role of glial cells should not be neglected in the context of pain perception. Given the highly intricate interaction of glial cells such as microglia and astrocytes within the nervous system, it is logical to hypothesize that these cells actively partake in the modulation of painful conditions. In support of this notion, it has been shown that these cells are juxtaposed to neural synapses and eligible to express several types of neurotransmitter receptors as described in neurons (Matejuk and Ransohoff, 2020). For instance, astrocytes and microglia can express neurokinin 1, glutamate, AMPA, and purinergic receptors in presynaptic terminals. The activation of astrocytes and microglia occurs in the shortest time after the exposure to chemokines, colony-stimulating factor- 1, ATP, and substance P. In response to these conditions, the production of specific factors such as IL-1, −6, TNF-α, NO, chemokines, ATP, and other excitatory factors are increased (Zhang et al., 2017). Holmes and co-workers found that the modulation of primary motor and somatosensory areas, changes in nerve divisions, and overexpression of cytokines involved in pain perception of ankle injury in human (Holmes et al., 2020). In this regard, neurogenic inflammation applies catastrophic effects by neuropeptide release with rapid plasma extravasation. On the other hand, neuroinflammation can activate the glial cells located in the DRG or spinal ganglion to propagate pro-inflammatory cytokines and chemokines in both the peripheral nervous system (PNS) and CNS (Matsuda et al., 2019). Of course, extravasation is indicated by the unilateral leakage of plasma factors into the injured tissues, resulting in local pain and swelling. Therefore, NP is the main source of pain markers, but in the later steps, the involvement of supporting vessels can increase the intensity of the pain (Kim et al., 2020).
Autophagy and analgesia
Autophagy definition
Autophagy is also known as macro-autophagy with a ubiquitous catabolic activity that plays an extremely important role in the homeostasis of the nervous system. The activation of the autophagy process can lead to the recycling of all worn-out cellular constituents (Bar-Yosef et al., 2019). Using autophagic response, exhaust cargos are sequestered by double-membrane autophagosomes and finally delivered to lysosomes for degradation and turnover under various pathophysiologic conditions (Rezabakhsh et al., 2019, Nikoletopoulou et al., 2015). Molecular investigations have revealed different autophagic responses, including macro-, micro-autophagy, and chaperone-mediated autophagy. In response to insulting conditions, upstream factors, mainly Beclin-1, are activated, leading to phagophore formation. In the latter phases, autophagy-related genes (ATGs; ATG-5, -12,-16L, and -7) along with other effectors such as P62 and microtubule-associated protein 1A/1B-light chain 3 (LC3)-II accelerate the formation of the autophagosomes. Subsequently, the fusion of autophagosomes with lysosomes contributes to the formation of autophagolysosomes which in turn recycles or efflux the cargo out of the host cells (Hassanpour et al., 2018). Like macroautophagy, chaperone-mediated autophagy can assist the host cells in adapting to the insulting conditions via engaging heat shock protein-70 (HSP70) and lysosome-associated membrane protein type-2A (LAMP2A) (Herpin et al., 2020). The last form of autophagy response, microautophagy, is commonly seen in plants and fungi. In mammalians, this phenomenon acts directly by digestion of exhaust materials via lysosomal activity (Schuck, 2020). It was suggested that the autophagy signaling pathway could be regulated in the early and late stages, generally by targeting PI3K activity or autophagolysosome formation (Zolali et al., 2019, Chicote et al., 2020). Emerging datasets from in vivo and in vitro studies have remarked that constitutive autophagy machinery exerts a notable neuroprotective role in the nervous system (Zha et al., 2021). In line with this claim, autophagy dysregulation can promote neuronal hyperexcitability and predispose to the development of neurodegenerative diseases (Kuijpers et al., 2021). Disruption of autophagy flux leads to an aggravated NP (Yin et al., 2018). Following the downregulation of the ATGs in neurons, the accumulation of injured endoplasmic reticulum (ER) in the neuronal axons results in an inefficient calcium-dependent excitatory transmission, and subsequent neurotransmitters release (Yin et al., 2018). Within the PNS, the stimulation of autophagy-related effectors in Schwann cells can compensate for tissue injury by excluding myelin debris associated with chronic pain (Gomez-Sanchez et al., 2015). Based on the data, activation of autophagy via certain stimulators such as rapamycin and metformin can decrease pain. By contrast, the inhibition of autophagy via blockers such as 3-Methyladenine (3-MA) or genetic manipulation can exacerbate the intensity of the pain (Jang et al., 2016). It has been implied that cellular death occurred in various brain regions, including the cerebellum, hippocampus, cortex, and amygdala, in knock-out Atg7 mice, indicating the protective role of autophagy within the brain parenchyma (Peker and Gozuacik, 2020, Komatsu et al., 2006). Likewise, the accumulation of P62-tagged cytoplasmic inclusion bodies in motor/sensory deficits, with behavioral abnormalities was also reported in Atg5 deficient mice (Hara et al., 2006). Notably, intracellular accumulation of P62 indicates an impaired clearance system with incomplete autophagic response in the host cells (Liu et al., 2016). The suppression and/or accumulation of P62 can sensitize neurons to tauopathy (Blaudin de Thé et al., 2021). These findings indicate that the prohibition of autophagic efflux can reduce the viability of injured neurons during pathological conditions. In this regard, the regulation of autophagic flux can be of particular interest for further research.
Therapeutic potential of autophagy activation following NP
Increasing data have demonstrated that dysregulated autophagy response can lead to several pathological conditions. For example, the suppression of autophagy can accelerate Wallerian degeneration by injuring neurons by reducing the efflux of abnormal myelin. This phenomenon is also known as myelinophagy (Gomez-Sanchez et al., 2015). Similarly, these features in glial cells can contribute to worsening outcomes in NP (Jang et al., 2016). It has been documented that autophagy modulation in the DRG neurons can relieve peripheral nerve pain and promote neuronal healing to some extent (Liao et al., 2022). In a recent study, it was suggested that the production of nerve growth factor substantially accelerates debris clearance and improves axonal regeneration via the stimulation of autophagy in a rat model of sciatic nerve crush injury and in vitro Schwann cell culture (Jadli et al., 2020). This effect is presumably accomplished through the neurotrophin receptor p75NTR and the AMPK/mTOR-dependent axis (Jadli et al., 2020). The results indicated a positive correlation between autophagy induction by rapamycin and the expression of neurofilament (NF)-200 and myelin basic protein (MBP), which participate in major axonal projections and myelination, respectively (Zha et al., 2021). In parallel with these findings, autophagy exerts neuroprotective effects on peripheral nerve and motor function recovery (Huang et al., 2016). According to the earlier data, autophagy inhibition in astrocytes enhanced the maintenance of neuroinflammatory responses through the TRAF6-p-MAPK8-JNK-NF-κB signaling axis, while the combined stimulation of autophagy and NFE2L2-NRF2 pathway has favorable effects on the regulation of NP (Li et al., 2021). Of course, specific conditions can deteriorate the normal activity of autophagy. For instance, it has been implicated that there is a relationship between the activation of microglial Toll-like receptor 4 (TLR4) and autophagy/mitophagy impairment (Piao et al., 2018). The overactivity of TLR4 can induce pro-inflammatory status in cells, resulting in the down-regulation of autophagy-related proteins such as LC3, Beclin-1, and P62. Due to the close relationship between autophagy and mitophagy, the activity of mitophagy marker PINK-1 can lead to chronic constriction injury (CCI)-induced NP progression (Piao et al., 2018). Despite the protective role of autophagy under pathological conditions, it seems that the overactivity of autophagic response or induction of certain factors in the autophagy signaling pathway not only cannot protect cells against noxious stimuli but also promote cell death via necroptosis and apoptosis (Wu and Lipinski, 2019). More recently, underlying signaling pathways involved in the decreased autophagic response were also explored in neuroinflammatory conditions (Liao et al., 2021). The increase of TNF receptor-associated factor 6 (TRAF6) connected to K63 ubiquitinated protein can induce MAPK8-JNK, and NFKB/NF-κB in B lymphocytes, leading to abortion of autophagic response (Li et al., 2021). These features can increase the possibility of neuronal death because of the impairment of antioxidant mechanisms in astrocytes. It was suggested that the activation of nuclear factor, erythroid derived 2, like 2/nuclear erythroid 2-related factor 2 (NFE2L2/NRF2) pathway can return an autophagic response (Li et al., 2021). Therefore, simultaneous activation of autophagy and NFE2L2 seems to be more effective in the restoration of injured neuron function and pain alleviation, indicating synergy and interplay between autophagy and other signaling cascades in the control of pain (Liao et al., 2021). Similarly, it has been reported that the NP is exacerbated in autophagy-defective Ambra1 mice, particularly in sub-clinical diabetic status. Of note, caloric restriction (CR) causes autophagy activation and regulates, in part, metabolic disorder (glucose metabolism) to govern NP. These findings highlight the analgesic, anti-inflammatory, and myelinogenesis properties of autophagy (Coccurello et al., 2018). Furthermore, the influence of autophagy has been indicated in astrocyte activation, allodynia, and hyperalgesia in NP (Chen et al., 2018). In a study conducted by Chen et al., they showed controversial results following the induction of NP by CCI. The expression of autophagy-related factors such as LC3 and Beclin-1, LAMP2, and RAB7 was remarkably increased which coincided with the suppression of P62, indicating autophagy activation accompanied by amelioration of hyperalgesia, allodynia, and the suppression of astrocyte (Chen et al., 2018). They concluded that the activation of autophagy is a compensatory response to alleviate NP in the rat model of CCl. It is postulated that heat shock protein B8 (HSPB8), ATG7, and mammalian target of rapamycin (mTOR), are shared molecules between autophagy and NP (Liu et al., 2019). From the molecular aspects, HSPB8 is recruited during autophagosome and lysosome fusion. As a correlate, situation associated with HSPB8 function can predispose pathological conditions. For example, the mutation of HSPB8 is associated with distal hereditary motor neuropathy (dHMN) and the Charcot-Marie-Tooth disease type 2 (CMT2), a dominant axonal peripheral neuropathy (Liu et al., 2019, Bouhy et al., 2018). Colecchia and his co-workers previously discovered a close relationship between RAB7A mutation in CMT2 disease and autophagy dysregulation. RAB7A possesses a pivotal role in autophagosome maturation. Thus, the deletion of this factor can increase malfunctioned materials and the possibility of neurodegenerative treatment (Colecchia et al., 2018). Among several ATGs, ATG7 functions as a critical factor in maintaining the hemostasis of axons and dendrites. In contrast to ATG7, mTOR activation inhibits autophagy via the regulation of ULK1/2 complex and autophagy flux (Jung et al., 2010). These features show that autophagy machinery consists of several factors with pleiotropic effects. Taken together, the promotion of autophagy response during pathological conditions can exert neuroprotective effects and reduce NP.
Key role of mTOR signaling in progression of NP
As a master regulator of protein synthesis, mTOR and downstream effectors can influence either cognitive or emotional aspects of pain (Cho et al., 2018). It has been shown that mTOR is phosphorylated during spinal cord injury and chronic pain (Wang et al., 2016). Upon the occurrence of nerve injury, mTOR signaling is activated to regulate sensory and cognitive processes, particularly in the intercostal region (Kwon et al., 2017, Megat and Price, 2018). As previously investigated, AKT/TSC2/mTOR axis was stimulated in a rat model of neuropathy induced by spinal nerve ligation (SNL), whereas hyperbaric oxygen (HBO) treatment alleviated NP through the inhibition of the AKT/TSC2/mTOR pathway (Liu et al., 2019). Following the inhibition of the AKT/TSC2/mTOR axis, the expression of LC3II causes an autophagic response in injured cells (Liu et al., 2019).
Interestingly, it was suggested that intracranial injection of metformin, an mTOR inhibitor, to the anterior cingulate cortex led to reduced allodynia in rats (Um et al., 2019). One reason would be that mTOR inhibition suppresses the synthesis of synaptic factors regulating the excitatory pathways via the regulation of the eIF4E complex (Yousuf et al., 2021). Therefore, the application of mTOR blockers seems to be a novel therapeutic approach to controlling synaptic plasticity and managing NP. Despite the protective role of mTOR inhibition in the reduction of NP, prolonged inhibition of mTOR can also increase the sensitivity of neurons to pathological conditions. One reason would be that persistent inhibition of mTOR coincides with the dysregulation of S6K1/2–insulin receptor substrate 1, leading to the activation of MAPK and phosphorylation of eIF4E (Yousuf et al., 2021). In another study, it was indicated that the administration of metformin blunted fibrosis in a rat model induced by silica via the regulation of the AMPK-mTOR axis and induction of P62, Beclin-1, and LC3. Histological examination revealed the phenotype acquisition of pneumocytes within the lung parenchyma (Li et al., 2021). Regarding the close relationship between mTOR and autophagy response, the application of pharmacological agents with the potential to regulate mTOR activity is an appropriate strategy for the regulation of NP.
Therapeutic potential of autophagy following diabetic neuropathy
Diabetic peripheral neuropathy (DPN) is a prevalent long-term complication of diabetes mellitus, leading to NP (Juster-Switlyk and Smith, 2016). Previous results indicated the protective role of autophagy under hyperglycemic conditions (Blaudin de Thé et al., 2021). However, prolonged high glucose conditions can induce autophagy cessation in the peripheral sensory neurons, leading to Schwann cells hypersensitivity and apoptosis (Liu et al., 2020). Besides, these conditions predispose sciatic nerve damage that is mainly characterized by disordered myelin, axonal shrinkage, fibrosis, and inflammation. Recent work on damaged sciatic nerve induced by DPN showed a neuroprotective effect of lipin1, as a well-established enzyme to maintain normal conduction in peripheral nerve by modulating dysregulated autophagy and neutralizing the Schwann cell apoptosis (Wang et al., 2021). This factor is diminished under diabetic conditions. In addition, changes in the transcription of miRNAs associated with the regulation of autophagy are detected during DPN. For instance, Hu et al. showed the negative role of miR-34c on the growth of trigeminal ganglion (TG) tissue by reduction of autophagy flux mediated via the downregulation of ATG4B and LC3-II in a mouse model of type I diabetes mellitus (Hu et al., 2019). In vitro incubation of high-glucose treated Schwann cells (RSC96 cell line) with natural phytocompounds, Salvianolic acid B restored cellular function via the modulation of the JNK pathway, leading to the activation of autophagy and suppression of apoptosis (Wang et al., 2019). In another study, the neuroprotective property of Lycium barbarum polysaccharide was achieved during DPN via the inhibition of mTOR/ribosomal protein S6 kinase I (p70S6K) and autophagy induction in a rat model (Liu et al., 2018). The higher content of glucose has been shown to up-regulate semaphoring 3A via the mTOR activity in human keratinocyte HaCaT cells. Thus, targeting this axis could be an alternative strategy for the reduction of diabetes-induced small fiber neuropathy (Wu et al., 2018). Other factors associated with DPN are the reduction of AMPK-eNOS and induction of oxidative stress, and apoptosis-related factors (Chung et al., 2018). Activating the CaMKKβ-LKB-1-AMPK-eNOS pathway via Cinacalcet improves sensorimotor function and autophagy, leading to reduced DPN in both in vitro and in vivo settings (Chung et al., 2018). In conclusion, the modulation of autophagy-related factors alone or with other signaling cascades causes neuroprotective outcomes during metabolic disorders.
Exo biogenesis
Exo are nano-sized EVs with an endosomal compartment origin. It has been suggested that these biological nano-carriers are secreted from all cell types into biofluids in response to stimuli during physiological and pathological conditions (Coughlan et al., 2020, Datta et al., 2018). Exo enable the transfer of several signaling molecules from donor cells toward acceptor cells to regulate bioactivities and various biological phenomena in a paracrine manner (Isola and Chen, 2017, Henne et al., 2011). Within the host cells, Exo are generated via invagination into the lumen of early endosomes and multivesicular bodies (MVBs) where they are named intraluminal vesicles (ILVs). These compartments are destined for lysosomal degradation or secretion into the extracellular matrix (ECM). Upon fusion of MVBs with the plasma membrane, ILVs are released into the ECM, hereafter known as Exo (Mathivanan and Simpson, 2009). To be specific, MVBs can be formed via early endosomes originating from endocytosis-based mechanisms or trans-Golgi network activity where specific cargos are sequestrated into releasing ILVs (Ge et al., 2012). Several biomolecules such as proteins, lipids, miRNAs, and mRNAs are transferred into recipient cells after the uptake of Exo (Colombo et al., 2013). Given the highly intricate underlying mechanisms involved in Exo biogenesis, the endosomal sorting complex required for transport (ESCRT) machinery has a considerable role in Exo biogenesis. Several studies have demonstrated that the endosomal ESCRT complex accounts for MVB biogenesis, plasma membrane reconstruction, abscission, and virus release from the host cells (Rahbarghazi et al., 2021). ESCRT consisted of five functional subsets: ESCRTs −0, -I, -II, and -III, and AAA ATPase Vps4 complex (Mathivanan and Simpson, 2009). Among these factors, ESCRT-0 functions to classify and sequestrate ubiquitinated proteins into generating ILVs. ESCRT-I, in collaboration with ESCRT–II, accelerates invagination of the MVB membrane, and the addition of ESCRT-III completes this mechanism by cleaving vesicles into the MVB lumen. Molecular investigations have suggested that the ESCRT-0 complex consists of two subunits: Hrs and STAM1/2. Hrs determines the ubiquitinated proteins with STAM, Eps15, and clathrin. Besides, Hrs can recruit TSG101 from the ESCRT-I complex, facilitating the addition of other ESCRT subsets such as ESCRT-II and -III and ALIX (Stuffers et al., 2009).
Beyond these mechanisms, some reports demonstrate the existence of the ESCRT-independent pathway in the biogenesis of MVBs, which were confirmed after the suppression of four main subunits of the ESCRT complex (Juan and Fürthauer, 2018). In support of this notion, it has been shown that the release of Exo in mouse oligodendrocytes is an ESCRT-free mechanism via the activity of ceramide-generating enzyme neutral sphingomyelinases (nSMase) (Heidarzadeh et al., 2021). Tetraspanins, as intracellular effectors, partake in the biogenesis of Exo and cargo sorting (Chairoungdua et al., 2010). These factors are determined based on four transmembrane domains. Different members of Tetraspanins have been supposed to be involved in ILV formation. Among different Tetraspanins, the suppression of CD9 in murine cells can diminish the Exo secretion (Nazarenko et al., 2010). Tetraspanin 8 can also alter the genomic and proteomic contents of Exo (Ghossoub et al., 2020). The precise collaboration of other Tetraspanin members such as Tetraspanin 6 and syndecan can regulate the balance between exosomal secretion and lysosomal degradation, having a pivotal role in the intracellular orientation of Exo. Of note, the close interaction of syntenin-syndecan via ALIX, in turn, increases the exosomal release of CD63, while the recruitment of CD9 and CD81 is syntenin-syndecan independent (Janas et al., 2015).
In contrast to protein loading in Exo, sorting RNAs into the exosomal lumen is closely associated with lipid activity. For instance, certain genomic cargo can be sorted into Exo after interaction with lipid rafts and sphingosine activity (Hessvik and Llorente, 2018). To promote the release of Exo from host cells, the functionality of small GTPases, cytoskeletal proteins, molecular motors such as dyneins and kinesins, and the membrane fusion apparatus soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, including Vamp7, Vti1b, syntaxin 7, and 8 are mandatory (Yue et al., 2020). Rab GTPase family consists of about 70 subtypes that can facilitate the fusion and budding of vesicles (Hsu et al., 2010). Additionally, each Rab type has a critical role in Exo development and biogenesis. For instance, Rab35 regulates the docking of endocytic vesicles with the plasma membrane in a GTP-dependent manner (Fukuda, 2013), whereas Rab27 can activate effectors involved in the transportation and fusion of secretory vesicles to the cell membrane (Villarroya-Beltri et al., 2014). The molecular motors and microtubules support anchorage sites to dispatch MVBs to the plasma membrane (Dingjan et al., 2018). The molecular investigation revealed that SNAREs are indispensable in membrane fusion (Zheng et al., 2019). SNARE complex consists of three subsets in a coiled-coil shape, which interlinks two dissenting membranes in a zipper-like form (Chairoungdua et al., 2010, Hsu et al., 2010).
Upon reaching released Exo to the host cells, general non-specific cell responses such as direct fusion, macropinocytosis, micropinocytosis, phagocytosis, clathrin-dependent endocytosis, lipid rafts, and clathrin-independent endocytosis can help Exo to internalize into the target cell cytosol (van Dongen et al., 2016, Fitzner et al., 2011). In addition, the close interaction of exosomal ligands with host cell receptors can accelerate Exo entry. Therefore, both specific and non-specific mechanisms can manage exosomal internalization. Whether and how these mechanisms dominate is the subject of debate (Kamerkar et al., 2017). The presence of specific peptides on the Exo surface can inhibit the direct ligand-receptor interaction, leading to delayed Exo entrance into the acceptor cells. It has been postulated that exosomal CD47, an integrin-associated protein, blocks direct phagocytosis of reaching Exo and increases circulation time (Mathieu et al., 2019). In contrast to the activity of CD47, it was suggested that the existence of distinct superficial proteins like integrins, lectins/proteoglycans, T cell immunoglobulin, and mucin domain-containing protein 4 (Tim4) support timely internalization of Exo (Kanada et al., 2015). Soon after Exo’s arrival, Exo cargo can be directly targeted for lysosomal degradation or released into the specific sites in the cytosol. However, there is an inconsistent hypothesis about the exact place of delivery. It has been thought that the formation of endosomes is the putative leading site for delivery of content, and membrane assimilation in response to acidic pH has been considered a feasible system comparable to the process used in some viruses (Kanada et al., 2015, Rezaie et al., 2019).
Emerging role of Exo for pain management
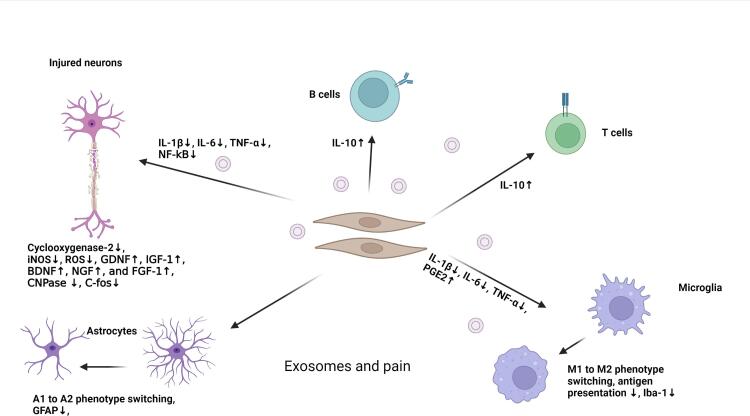
Previous studies have revealed several therapeutic aspects of Exo under pathological conditions (Rezaie et al., 2018) (Table 1; Fig. 3). As mentioned above, these nanoparticles can be released by several cell types, especially stem cells, with the potential to manage NP (Ren et al., 2019). In the context of CNS and PNS, mesenchymal stem cell (MSC)-derived Exo can exert analgesic effects in chronic pain models via the transfer of certain miRNAs (Hmadcha et al., 2020). It is noteworthy that MSCs are the most common stem cell types used to regenerate injured tissue in the different animal models and human counterparts (Martins and Schratt, 2021). It is believed that Exo are eligible to transfer therapeutic factors to injured neurons. These Exo can enhance axonal growth and neuronal viability (Zhang et al., 2021). Besides genetic materials, the existence of several neurotrophic factors, including GDNF, IGF-1, BDNF, NGF, and FGF-1 in Exo lumen, can intensify therapeutic effects (Tofiño-Vian et al., 2017). The promotion of inflammation is touted as the leading cause of pain. Exo can also suppress the production of pro-inflammatory cytokines such as IL-1β, −6, TNF-α, and PGE2 in injured areas and stimulate the release of IL-10, leading to anti-nociceptive effects (Cata et al., 2021).
Table 1.
Role of Exo in the alleviation of pain in the experimental settings.
| Model | Year | Exo source | Target cells or tissues | Outcome | Ref |
|---|---|---|---|---|---|
| In vitro model of osteoarthritis | 2020 | Bone marrow MSCs | IL-1β-treated chondrocytes | Chondrocyte Migration↑, Proliferation↑, COL2A1↑ and ACAN↑, ADAMTS5↓, MMP13↓ | (Console et al., 2019) |
| In vivo rat model of osteoarthritis induced by sodium iodoacetate | 2020 | Bone marrow MSCs | Knee joint | Reduced neuropathic pain via CGRP↓ and iNOS↓, improved PWL values | (Console et al., 2019) |
| Plasma exchange in patients with complex regional pain syndrome | 2019 | Plasma Exo miRNAs | Blood | hsa-miR-338-5p↓, IL-6↓, Inflammation↓ | (Shiue et al., 2019) |
| Administration of alginate-loaded Exo in rats with mechanical allodynia and thermal hyperalgesia | 2020 | Human umbilical MSCs | Right L5/6 spinal nerve | Withdrawal threshold and latency↑, Fos↓, GFAP↓, Iba1↓, TNF-α↓ and IL-1β↓, GDNF↑, Antinociceptive properties↑, Inflammation↓ | (Zhang et al., 2019) |
| Intrathecal injection of Exo in rats with nerve injury-induced neuropathic pain | 2019 | Human umbilical MSCs | L5/6 spinal nerve | Mechanical and thermal hypersensitivities↓, Pain↓, c-Fos↓, CNPase↓, GFAP↓, and Iba1↓, TNF-α↓ and IL-1β↓, IL-10↑, | (Zhou et al., 2021) |
| Application of Exo in rat model of osteoarthritis | 2019 | MSCs | Temporomandibular joint | Pain↓, Degeneration↓, Inflammation↓, Subchondral bone formation↑, | (Jean-Toussaint et al., 2021) |
| In vitro exposure of Exo with chondrocytes | 2019 | MSCs | Chondrocytes | Akt/Erk/AMPK↑, s-GAG synthesis↑, IL-1β↓, iNOS↓, MMP13↓ | (Jean-Toussaint et al., 2021) |
| Different doses of Exo in a rat model of osteoarthritis induced by monoiodoacetate-induced | 2021 | MSCs | knee joint | PWT and PWL values↑, GAP-43↑, ATF-3↓, Dose-dependent regeneration activity↑, Pain relief↑ | (Simeoli et al., 2017) |
| Macrophages Exo | 2021 | mouse RAW 264.7 macrophages-induced by lipopolysaccharide | Cortical neurons, Microglia, and Astrocytes | Pro-inflammatory miRNA↓ | (Moen et al., 2017) |
| Acute mouse model of acute inflammation induced by formalin | 2021 | mouse RAW 264.7 macrophages-induced by lipopolysaccharide | A single intrathecal injection | Mechanical hyperalgesia↓, prophylactic pain relief↑ | (Moen et al., 2017) |
| Dorsal root ganglia sensory neurons treated with capsaicin | 2020 | Exposure of macrophages to miR-21-5p antagomir-loaded Exo | Cell culture | Macrophage NOS2↓, Spry2↓, | (Simeoli et al., 2017) |
| Enhanced spinal cord nociceptive responses in rat model using placing nucleus pulposus onto dorsal nerve roots (Vertebraes Th13-L1, and L3–S1) | 2017 | Nucleus pulposus grafts Exo | Dorsal nerve roots | miR-223↑, Nociceptive spinal signaling↓, | (Moen et al., 2017) |
Mesenchymal stem cells: MSCs; Aggrecan: ACAN; ADAM Metallopeptidase With Thrombospondin Type 1 Motif 5: ADAMTS5; Type II collagen: COL2A1; Matrix metalloproteinases-13: MMP13; Calcitonin Gene-Related Peptide: CGRP; Inducible nitric oxide synthase: iNOS; Paw withdrawal latency: PWL; Glial Cell Derived Neurotrophic Factor: GDNF; Ionized calcium binding adaptor molecule 1: Iba1; Fos Proto-Oncogene, AP-1 Transcription Factor Subunit: FOS; Glial fibrillary acidic protein: GFAP; Sulfated glycosaminoglycan: s-GAG; Growth Associated Protein 43: GAP-43; Cyclic AMP-dependent transcription factor: ATF-3; Nitric Oxide Synthase 2: NOS2; Sprouty RTK Signaling Antagonist 2: Spry2.
Fig. 3.
Modulatory effect of Exo on pain via different mechanisms.
Another study suggested that miRNA-29-enriched Exo originated from stem cells and can alleviate pro-inflammatory responses in osteoarthritis in a rat model (Jin et al., 2020). Xenogenic injection of human MSC Exo enriched with miR-26a-5p can reduce pathological changes via the down-regulation of cyclooxygenase-2 (PTGS2) in rat synovial fibroblasts (Wu and Shen, 2020). Along with these changes, free radical and nitrosative stress production is ipsilaterally diminished by the regulation of inducible nitric oxide synthase activity in dorsal root ganglia (Console et al., 2019). Another analgesic facet of Exo is associated with tightly controlled immune cell response and antigen presentation (Wang et al., 2021). For example, it has been shown that Schwann cell-derived Exo can reduce neuropathic injuries of dorsal root ganglia via the control of neuronal sensitization and macrophage recruitment under pathological conditions (Jin et al., 2020). Likewise, intrathecal injection of Exo originating from hypoxic neurons can diminish NP in a rat model of spinal cord injury via the suppression of IL-1β, −6, TNF-α, and NF-kB, leading to M1 to M2 polarization of microglia (Liu et al., 2020, Hsu et al., 2020).
Based on previous data, sustained release and therapeutic effects of Exo were also investigated in a mouse model of L5/6 spinal nerve ligation. To this end, Jong-Ming and co-workers isolated Exo from human umbilical cord MSCs, loaded them in gelfoam, and implanted them into the vicinity of the injury site. They claimed that this approach could blunt the increase of TNF-α, IL-1β, GFAP, Iba1, and c-Fos coincided with the restoration of myelin formation and up-regulation of IL-10 (Shiue et al., 2019). In a similar work, human umbilical cord MSCs Exo administration led to therapeutic effects in a rat model of nerve ligation–induced pain (Degli Esposti et al., 2021). It was suggested that the transplanted Exo could distribute ipsilaterally in the L5 spinal dorsal horn and dorsal root ganglion and reach IB41+, CGRP+, and NF200+ sensory neurons. Along with these changes, the expression of GFAP, Iba1, c-Fos, and CNPase was diminished (Degli Esposti et al., 2021). These data show that Exo can manage pain by reducing pro-inflammatory cytokines and free radicals and promoting neuronal proliferation and function.
Exo as a pain biomarkers
Emerging data have shown that Exo can be used for early-stage monitoring of pain following several pathologies (Rezaie et al., 2018). Of note, the type of parent cells, epigenetic changes, and insulting conditions can change exosomal cargo (D’Agnelli et al., 2020). Regarding synaptic activity in the nervous system, Exo can be interchanged between neurons and participate in inter-neuronal communication (Ramanathan et al., 2019). It was suggested that the existence of pain-related factors in the Exo lumen is indicative of pathological conditions (Ramanathan et al., 2019). These features help us to detect the type and intensity of pain via monitoring the exosomal cargo, indicating the diagnostic and prognostic values of Exo (Orlova et al., 2011). To be specific, the molecular signature in Exo secreted during the injury of different tissues can be differed. For instance, in complex regional pain syndrome (CRPS) patients Exo carry large amounts of miR-338-5p, indicating pathological conditions (Kowalski et al., 2022). It was suggested that exosomal miR-338-5p could modulate NP after spinal cord injury via the regulation of apoptosis and neuroinflammation (Freger et al., 2021). Molecular analyses have revealed a close relationship between exosomal miRNA and the production of pro-inflammatory cytokines such as IL-6 in response to pathological conditions (Orlova et al., 2011). The occurrence of pathological conditions can result in specific cargo sorting and enrichment with unique signaling molecules. For example, Freger and collaborators demonstrated that in females with endometriosis and pelvic pain, circulating Exo harbor specific long non-coding RNAs (lncRNAs) and proteins that can potentially regulate neurogenesis, and angiogenesis, immune system response, and histone modification (D'Agnelli et al., 2020). These features show that Exo are the key regulators during physiological and pathological conditions with specific factors that give us valuable data about the status and intensity of pathologies. Because Exo can easily distribute in the systemic circulation, studying exosomal profiles in blood samples is helpful in the determination of pain-related factors (Heidarzadeh et al., 2021). Despite the existence of a blood–brain barrier with selective permeability, Exo can engage several strategies to cross this natural barrier from the brain to the blood side and vice versa (van Dijk et al., 2010). Besides, the production of several inflammatory cytokines can deteriorate endothelial barrier integrity and lead to the easier transfer of inflammatory Exo into the circulation system, resulting in the dissemination of Exo from original cells to remote sites (van Dijk et al., 2010). Unlike blood, the collection of Exo from CSF during different CNS pathologies is touted as an invasive approach. Despite this disadvantage, the proximity of CSF to the brain and spinal cord enables us to directly monitor possible molecular alteration and signature (Pegtel et al., 2014). Exo with distinct markers can be produced by a wide range of cells within the nervous system and released into the CSF (Hornung et al., 2020). Therefore, molecular analysis of the Exo proteome and genome can give us valuable diagnostic and prognostic data about various neurodegenerative conditions (Hercher et al., 2021). Like CNS, the occurrence of pain in PNS can contribute to the production of Exo, which are identical to the status of pain and inflammatory response (Gurunathan et al., 2019). In animal models of neuritis, Schwann cells produce and release Exo carrying neural cell adhesion molecules 1 and P75, which can show degenerative conditions related to axonal demyelination (Leoni et al., 2015). These factors are increased in human counterparts with several peripheral neuropathy types (Leoni et al., 2015). In the circumstances associated with chronic pain conditions such as osteoarthritis and other diseases, Exo with large contents of Annexin A-1 can be detected in the blood (Descalzi et al., 2015). It is suggested that Annexin A-1 acts as an anti-nociceptive agent with pro-inflammatory potential (Descalzi et al., 2015). These features demonstrate that Exo are eligible biological nanovesicles that can reflect pathological changes and the pain intensity in different tissue types.
Autophagy and Exo synergy for peripheral neuropathy management
Epigenetic changes in the spinal cord and peripheral nerves have been introduced during chronic pain, which may guide substantial advances in novel therapeutic interventions. In this regard, autophagy can also be an aid for exploring the effects of experimental therapeutic options targeted at epigenetic mechanisms (Jung and Lim, 2015). Moreover, previous literature proposed a correlation between the activation of microRNAs (miRNAs, potent nano-sized regulators of gene expression) and the autophagic flux in the terms of NP (Shi et al., 2013). The underlying mechanisms involved in the modulation of autophagy via epigenetic changes following chronic pain in the brain and spinal cord are yet to be completely deciphered. However, miRNAs are involved in the autophagic regulation in the case of NP (Shi et al., 2013). For instance, the miRNA-195 simultaneously stimulates neuroinflammation and NP via inhibiting autophagy flux following peripheral nerve injury (Jung and Lim, 2015, Xu et al., 2018).
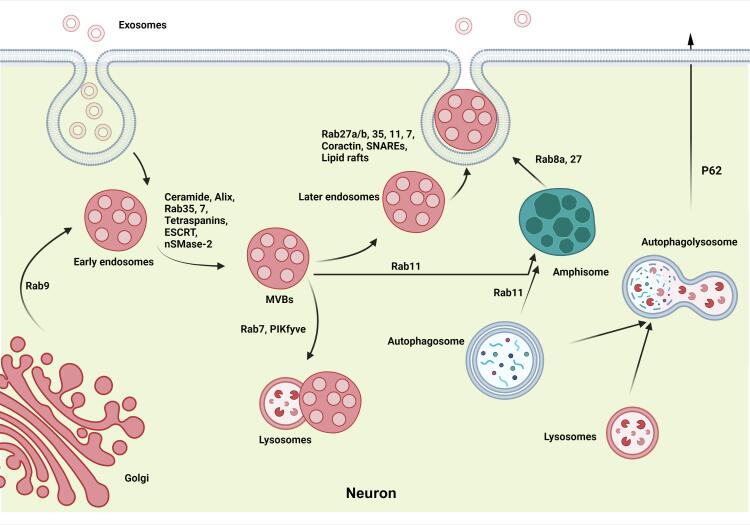
Emerging data have indicated varied intracellular effectors shared between Exo biogenesis and autophagy machinery (Rahbarghazi et al., 2021). In Fig. 2, a presumed cross-talk between autophagy and Exo biogenesis has been depicted to exert a therapeutic potential in the face of pain. As mentioned above, ATGs are the main signaling molecules regulating autophagic procedures. Of these ATGs, ATG5 promotes the fusion of MVBs with the plasma membrane and the release of ILVs (Murrow et al., 2015). On the other hand, the activation of LC3, a well-known autophagy biomarker, can stimulate Alix from the ESCRT complex (Villarroya-Beltri et al., 2016). Further fusion of autophagosomes with MVBs can also lead to the formation of amphisomes. These intracellular vesicles harbor the content of autophagy machinery and MVBs to ECM via engaging certain Rabs such as Rab8a and Rab27 (Murrow et al., 2015, Kuo et al., 2021). Alternatively, amphisomes join the lysosome for further digestion and degradation (Murrow et al., 2015).
Fig. 2.
A cross-talk between endosomal-derived Exo biogenesis and the autophagy process in favor of pain management. Exo are synthesized via entering into the lumen of early endosomes and MVBs. Rab GTPase family (e.g., Rab-11, −27, −35) and SNARE are mainly involved in Exo development and membrane fusions, respectively. Final fusion of autophagosomes with MVBs can lead to the formation of amphisomes encompassing Exo-autophagic cargos, exiting the cell through exocytosis. Abbreviations: (SNARE) Exosomes: Exo; Multivesicular Bodies: MVBs; Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptors: SNARE.
By 2021, increasing evidence has designated a close link between Exo secretion and autophagy flux in the case of pain management, declaring a new field of research for advanced cell therapy in clinics. For example, following the sciatic nerve crush injury model, a novel combination therapy consisting of adipose-derived stem cells-derived Exo (ADSC-F-Exo) and immunosuppressive drug tacrolimus (FK506) was used (Pan et al., 2021). The results showed that local administration of ADSC-F-Exo could alleviate the aberrant autophagy process in the DRG and spinal cord dorsal horn after nerve crush injury (Pan et al., 2021). In addition, a proteome analysis revealed the presence of 22 exosomal proteins, heat shock protein family A member 8 (HSPA8), and eukaryotic translation elongation factor 1 alpha 1 (EEF1A1) in ADSC-derived Exo (Pan et al., 2021). Pan et al. declared that the application of primary Schwann cell-derived Exo (SCDEs) promoted autophagy and inhibited apoptosis to retrieve spinal cord injury and axonal/motor functions by regulation of epidermal growth factor receptor (EGFR)/Akt/mTOR signaling pathway (Luo et al., 2021).
Intervertebral disc degeneration (IDD) is a challenging clinical condition causing back pain and subsequently affects a patient’s quality of life. In this regard, degeneration of the cartilage endplate plays a crucial role in IDD induction. Upon the promotion of IDD, certain factors such as matrix metalloproteinases (MMPs) and proinflammatory cytokines are up-regulated. These features coincide with decreased numbers of functional nucleus pulposus cells (NPCs) and prominent morphological changes. It has been indicated that the Exo-autophagy interface has therapeutic potential in the context of IDD (Zhang et al., 2021). Recently, the potential impacts of normal and degenerated cartilage endplate stem cell-derived Exo (CESC-Exo) on autophagy activation were investigated. Luo et al. claimed that normal CESC-Exo is more effective in modulating autophagy flux and NPC apoptosis rate. Moreover, they highlighted that PI3K/AKT/autophagy axis has a pivotal role in retrieving CEP-induced IDD (Zhang et al., 2021). In parallel with these findings, it has also been proved that autophagy can mitigate the MMPs activity and inflammatory responses to prevent NPCs matrix degradation (Liu et al., 2021). In detail, autophagy-activated NPCs-derived Exo (NPCs-Exo) could recover IDD at least partly by exosomal miR-27 recruitment and targeting MMP-13 (Liu et al., 2021). Long non-coding RNAs (lncRNAs) belong to regulatory ncRNAs that mainly participate in DPN development. Recent research conducted by Liu et al. reported a favorable role of lncRNA X-inactive specific transcript (XIST) in attenuating DPN in diabetic mice (Ramanathan et al., 2019). Data showed that hyperglycemia triggered a significant suppression in the expression of XIST, sirtuin1 (SIRT1), LC3II, and Beclin-1, while the expression of microRNA-30d-5p (miR-30d-5p) was increased in the trigeminal sensory neurons which reversed by XIST administration (Ramanathan et al., 2019).
Conclusion and future direction
Exo secretion from glial cells such as astrocytes and microglia seems to be a critical event in the pathogenesis of pain. As a correlate, these nanoparticles can exchange different biomolecule types between the cells and dictate specific behavior in recipient cells such as neurons. However, there is a long way to underpin the underlying mechanisms behind exosomal delivery of certain factors and pain modulation in several pathologies. It is suggested that autophagy can affect the exocytosis capacity and thus alter the distribution of certain biomolecules within the CNS and PNS via autophagic response. Whether and how autophagy can change exosomal cargo and biogenesis during acute and chronic pain is the subject of debate. It is suggested that different therapeutic agents and compounds can be loaded onto the Exo using different engineering strategies to alleviate the pain. Due to close cross-talk between autophagy and Exo biogenesis, the modulation of these pathways can be touted as an alternative approach after the onset of pain. Despite these availabilities, numerous studies are needed to establish an optimized approach to managing acute and chronic pain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to appreciate of the cooperation of Clinical Research Development Unit, Imam Reza General Hospital, Tabriz, Iran in conducting of this research.
Data availability
No data was used for the research described in the article.
References
- Hylands-White N., Duarte R.V., Raphael J.H. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2017;37(1):29–42. doi: 10.1007/s00296-016-3481-8. [DOI] [PubMed] [Google Scholar]
- Golzari S.E., Soleimanpour H., Mahmoodpoor A., Safari S., Ala A. Lidocaine and pain management in the emergency department: a review article. Anesthesiol. Pain Med. 2014;4 doi: 10.5812/aapm.15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimanpour H, Imani F, Dolati S, Soleimanpour M, Shahsavarinia K. Management of pain using magnesium sulphate: a narrative review. Postgraduate Medicine. 2022(just-accepted). [DOI] [PubMed]
- Ghojazadeh M., Sanaie S., Parsian Z., Najafizadeh R., Soleimanpour H. Use of lidocaine for pain management in the emergency medicine: a systematic review and meta-analysis. Pharm. Sci. 2019;25(3):177–183. [Google Scholar]
- Glare P., Overton S., Aubrey K. Transition from acute to chronic pain: where cells, systems and society meet. Pain Manage. 2020;10(6):421–436. doi: 10.2217/pmt-2019-0039. [DOI] [PubMed] [Google Scholar]
- Ghojazadeh M., Sanaie S., Paknezhad S.P., Faghih S.-S., Soleimanpour H. Using ketamine and propofol for procedural sedation of adults in the emergency department: a systematic review and meta-analysis. Adv. Pharm. Bull. 2019;9(1):5. doi: 10.15171/apb.2019.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolati S., Tarighat F., Pashazadeh F., Shahsavarinia K., Gholipouri S., Soleimanpour H. The role of opioids in pain management in elderly patients with chronic kidney disease: a review article. Anesthesiol. Pain Med. 2020;10(5) doi: 10.5812/aapm.105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Huh Y. Astrocytic calcium dynamics along the pain pathway. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.594216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.-H., Yoon M.H., Lim K.J., Yu B.S., Jee I.G., Jung K.T. Nefopam downregulates autophagy and c-Jun N-terminal kinase activity in the regulation of neuropathic pain development following spinal nerve ligation. BMC Anesthesiol. 2018;18(1):97. doi: 10.1186/s12871-018-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Chen X., Zhang C., Zhang Y., Yao W. An update on reactive astrocytes in chronic pain. J. Neuroinflammation. 2019;16(1):1–13. doi: 10.1186/s12974-019-1524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaffi F., Giacobazzi G., Di Carlo M. Chronic pain in inflammatory arthritis: mechanisms, metrology, and emerging targets—a focus on the JAK-STAT pathway. Pain Res. Manage. 2018;2018 doi: 10.1155/2018/8564215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini H., Rezabakhsh A., Heidarzadeh M., Hassanpour M., Hashemzadeh S., Ghaderi S., et al. An examination of the putative role of melatonin in exosome biogenesis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.686551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbarghazi R., Amini H., Rezabakhsh A., Heidarzadeh M., Hassanpour M., Hashemzadeh S., et al. An examination of the putative role of melatonin in exosome biogenesis. Front. Cell Dev. Biol. 2021;9:1396. doi: 10.3389/fcell.2021.686551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Anderson J.D., Rahnama L.M., Gu S.V., Knowlton A.A. Exosomes in disease and regeneration: biological functions, diagnostics, and beneficial effects. Am. J. Physiol.-Heart Circulatory Physiol. 2020;319(6):H1162–H1180. doi: 10.1152/ajpheart.00075.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlakeh G., Rahbarghazi R., Mohammadnejad D., Abedelahi A., Karimipour M. Current knowledge and challenges associated with targeted delivery of neurotrophic factors into the central nervous system: focus on available approaches. Cell Biosci. 2021;11(1):181. doi: 10.1186/s13578-021-00694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z., Zhou S., Li S., Kuang L., Chen H., Luo X., et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8(1):1–18. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti M., Ceglie D., Di Giannatale A., Nazio F. Autophagy and exosomes relationship in cancer: friends or foes? Front. Cell Dev. Biol. 2021;1802 doi: 10.3389/fcell.2020.614178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour M., Rezaie J., Darabi M., Hiradfar A., Rahbarghazi R., Nouri M. Autophagy modulation altered differentiation capacity of CD146+ cells toward endothelial cells, pericytes, and cardiomyocytes. Stem Cell Res. Ther. 2020;11(1):1–14. doi: 10.1186/s13287-020-01656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.J., Wilkie, D.J., Molokie, R. 2010. Neurobiological mechanisms of pain in sickle cell disease. Hematology 2010, the American Society of Hematology Education Program Book. 2010(1):403-408. [DOI] [PMC free article] [PubMed]
- Alles S.R., Gomez K., Moutal A., Khanna R. Putative roles of SLC7A5 (LAT1) transporter in pain. Neurobiol. Pain. 2020;8 doi: 10.1016/j.ynpai.2020.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M.-F., Lu K.-T., Hsu J.-L., Lee C.-H., Cheng M.-Y., Ro L.-S. The role of autophagy and apoptosis in neuropathic pain formation. Int. J. Mol. Sci. 2022;23(5):2685. doi: 10.3390/ijms23052685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Huh Y., Ji R.-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesthesia. 2019;33(1):131–139. doi: 10.1007/s00540-018-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwall A.G., Milligan E.D. Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.03009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y., Zhang L., Cheng J.-K., Ji R.-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejuk A., Ransohoff R.M. Crosstalk between astrocytes and microglia: an overview. Front. Immunol. 2020;11:1416. doi: 10.3389/fimmu.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-J., Jiang B.-C., Gao Y.-J. Chemokines in neuron–glial cell interaction and pathogenesis of neuropathic pain. Cell. Mol. Life Sci. 2017;74(18):3275–3291. doi: 10.1007/s00018-017-2513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S., Barakat N., Bhasin M., Lopez N., Lebel A., Zurakowski D., et al. Biological and behavioral markers of pain following nerve injury in humans. Neurobiol. Pain. 2020;7 doi: 10.1016/j.ynpai.2019.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.T., Park J.Y., Lee H.J., Cheon Y.J. Guidelines for the management of extravasation. J. Educ. Eval. Health Professions. 2020;17 doi: 10.3352/jeehp.2020.17.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Yosef T., Damri O., Agam G. Dual role of autophagy in diseases of the central nervous system. Front. Cell. Neurosci. 2019;13:196. doi: 10.3389/fncel.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezabakhsh A., Rahbarghazi R., Malekinejad H., Fathi F., Montaseri A., Garjani A. Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine. 2019;56:183–193. doi: 10.1016/j.phymed.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V., Papandreou M., Tavernarakis N. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ. 2015;22(3):398–407. doi: 10.1038/cdd.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour M., Rezabakhsh A., Pezeshkian M., Rahbarghazi R., Nouri M. Distinct role of autophagy on angiogenesis: highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res. Ther. 2018;9(1):1–16. doi: 10.1186/s13287-018-1060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A., Lescat L., Bobe J., Jenny A., Seiliez I. Lighting chaperone-mediated autophagy (CMA) evolution with an ancient LAMP: the existence of a functional CMA activity in fish. Autophagy. 2020;16(10):1918–1920. doi: 10.1080/15548627.2020.1797344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S. Microautophagy–distinct molecular mechanisms handle cargoes of many sizes. J. Cell Sci. 2020;133(17):jcs246322. doi: 10.1242/jcs.246322. [DOI] [PubMed] [Google Scholar]
- Zolali E., Rezabakhsh A., Nabat E., Jaberi H., Rahbarghazi R., Garjani A. Metformin effect on endocan biogenesis in human endothelial cells under diabetic condition. Arch. Med. Res. 2019;50(5):304–314. doi: 10.1016/j.arcmed.2019.08.012. [DOI] [PubMed] [Google Scholar]
- Chicote J., Yuste V.J., Boix J., Ribas J. Cell death triggered by the autophagy inhibitory drug 3-methyladenine in growing conditions proceeds with DNA damage. Front. Pharmacol. 2020;1632 doi: 10.3389/fphar.2020.580343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha H., Fan Y., Yang L., Yin M., Miao W., He J., et al. Autophagy protects against cerebral ischemic reperfusion injury by inhibiting neuroinflammation. Am. J. Transl. Res. 2021;13(5):4726. [PMC free article] [PubMed] [Google Scholar]
- Kuijpers, M., Kochlamazashvili, G., Stumpf, A., Puchkov, D., Swaminathan A, Lucht MT, et al. Neuronal autophagy regulates presynaptic neurotransmission by controlling the axonal endoplasmic reticulum. Neuron. 109(2):299-313. e9. [DOI] [PMC free article] [PubMed]
- Yin Y., Yi M.-H., Kim D.W. Impaired autophagy of GABAergic interneurons in neuropathic pain. Pain Res. Manage. 2018;2018 doi: 10.1155/2018/9185368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez J.A., Carty L., Iruarrizaga-Lejarreta M., Palomo-Irigoyen M., Varela-Rey M., Griffith M., et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015;210(1):153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.Y., Shin Y.K., Park S.Y., Park J.Y., Lee H.J., Yoo Y.H., et al. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia. 2016;64(5):730–742. doi: 10.1002/glia.22957. [DOI] [PubMed] [Google Scholar]
- Peker N., Gozuacik D. Autophagy as a cellular stress response mechanism in the nervous system. J. Mol. Biol. 2020;432(8):2560–2588. doi: 10.1016/j.jmb.2020.01.017. [DOI] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Chiba T., Murata S., Iwata J.-i., Tanida I., et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Liu W.J., Ye L., Huang W.F., Guo L.J., Xu Z.G., Wu H.L., et al. p62 links the autophagy pathway and the ubiqutin–proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016;21(1):29. doi: 10.1186/s11658-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaudin de Thé F.-X., Lassus B., Schaler A.W., Fowler S.L., Goulbourne C.N., Jeggo R., et al. P62 accumulates through neuroanatomical circuits in response to tauopathy propagation. Acta Neuropathol. Commun. 2021;9(1):177. doi: 10.1186/s40478-021-01280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadli A.S., Ballasy N., Edalat P., Patel V.B. Inside (sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol. Cell. Biochem. 2020;467(1):77–94. doi: 10.1007/s11010-020-03703-z. [DOI] [PubMed] [Google Scholar]
- Huang H.-c., Chen L., Zhang H.-x., Li S.-f., Liu P., Zhao T.-y., et al. Autophagy promotes peripheral nerve regeneration and motor recovery following sciatic nerve crush injury in rats. J. Mol. Neurosci. 2016;58(4):416–423. doi: 10.1007/s12031-015-0672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Tian M., Hua T., Wang H., Yang M., Li W., et al. Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy. 2021;17(12):4062–4082. doi: 10.1080/15548627.2021.1900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao Y., Gwon D.H., Kang D.-W., Hwang T.W., Shin N., Kwon H.H., et al. TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice. Mol. Brain. 2018;11(1):1–13. doi: 10.1186/s13041-018-0354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Lipinski M.M. Autophagy in neurotrauma: good, bad, or dysregulated. Cells. 2019;8(7):693. doi: 10.3390/cells8070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M.-F., Yeh S.-R., Lu K.-T., Hsu J.-L., Chao P.-K., Hsu H.-C., et al. Interactions between autophagy, proinflammatory cytokines, and apoptosis in neuropathic pain: granulocyte colony stimulating factor as a multipotent therapy in rats with chronic constriction injury. Biomedicines. 2021;9(5):542. doi: 10.3390/biomedicines9050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R., Nazio F., Rossi C., De Angelis F., Vacca V., Giacovazzo G., et al. Effects of caloric restriction on neuropathic pain, peripheral nerve degeneration and inflammation in normometabolic and autophagy defective prediabetic Ambra1 mice. PLoS One. 2018;13(12):e0208596. doi: 10.1371/journal.pone.0208596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Hu Y., Xie K., Chen Y., Wang H., Bian Y., et al. Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. Int. J. Mol. Med. 2018;42(4):2009–2019. doi: 10.3892/ijmm.2018.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhu M., Ju Y., Li A., Sun X. Autophagy dysfunction in neuropathic pain. Neuropeptides. 2019;75:41–48. doi: 10.1016/j.npep.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Bouhy D., Juneja M., Katona I., Holmgren A., Asselbergh B., De Winter V., et al. A knock-in/knock-out mouse model of HSPB8-associated distal hereditary motor neuropathy and myopathy reveals toxic gain-of-function of mutant Hspb8. Acta Neuropathol. 2018;135(1):131–148. doi: 10.1007/s00401-017-1756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colecchia D., Stasi M., Leonardi M., Manganelli F., Nolano M., Veneziani B.M., et al. Alterations of autophagy in the peripheral neuropathy Charcot-Marie-Tooth type 2B. Autophagy. 2018;14(6):930–941. doi: 10.1080/15548627.2017.1388475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C.H., Ro S.-H., Cao J., Otto N.M., Kim D.-H. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C., Michailidis V., Martin L.J. Revealing brain mechanisms of mTOR-mediated translational regulation: implications for chronic pain. Neurobiol. Pain. 2018;4:27–34. doi: 10.1016/j.ynpai.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li X., Huang B., Ma S. Blocking mammalian target of rapamycin (mTOR) improves neuropathic pain evoked by spinal cord injury. Transl. Neurosci. 2016;7(1):50–55. doi: 10.1515/tnsci-2016-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M., Han J., Kim U.J., Cha M., Um S.W., Bai S.J., et al. Inhibition of mammalian target of rapamycin (mTOR) signaling in the insular cortex alleviates neuropathic pain after peripheral nerve injury. Front. Mol. Neurosci. 2017;10:79. doi: 10.3389/fnmol.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megat S., Price T.J. Therapeutic opportunities for pain medicines via targeting of specific translation signaling mechanisms. Neurobiol. Pain. 2018;4:8–19. doi: 10.1016/j.ynpai.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-D., Wang Z.-B., Han G., Jin L., Zhao P. Hyperbaric oxygen relieves neuropathic pain through AKT/TSC2/mTOR pathway activity to induce autophagy. J. Pain Res. 2019;12:443. doi: 10.2147/JPR.S189353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um S.W., Kim M.J., Leem J.W., Bai S.J., Lee B.H. Pain-relieving effects of mTOR inhibitor in the anterior cingulate cortex of neuropathic rats. Mol. Neurobiol. 2019;56(4):2482–2494. doi: 10.1007/s12035-018-1245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf M.S., Shiers S.I., Sahn J.J., Price T.J. Pharmacological manipulation of translation as a therapeutic target for chronic pain. Pharmacol. Rev. 2021;73(1):59–88. doi: 10.1124/pharmrev.120.000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-X., Li C., Pang X.-R., Zhang J., Yu G.-C., Yeo A.J., et al. Metformin attenuates silica-induced pulmonary fibrosis by activating autophagy via the AMPK-mTOR signaling pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.719589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster-Switlyk, K., Smith, A.G. 2016. Updates in diabetic peripheral neuropathy. F1000Research. 5. [DOI] [PMC free article] [PubMed]
- Liu Y.-p., Shao S.-j., Guo H.-d. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248 doi: 10.1016/j.lfs.2020.117459. [DOI] [PubMed] [Google Scholar]
- Wang M., Xie M., Yu S., Shang P., Zhang C., Han X., et al. Lipin1 alleviates autophagy disorder in sciatic nerve and improves diabetic peripheral neuropathy. Mol. Neurobiol. 2021;58(11):6049–6061. doi: 10.1007/s12035-021-02540-5. [DOI] [PubMed] [Google Scholar]
- Hu J., Hu X., Kan T. MiR-34c participates in diabetic corneal neuropathy via regulation of autophagy. Invest. Ophthalmol. Vis. Sci. 2019;60(1):16–25. doi: 10.1167/iovs.18-24968. [DOI] [PubMed] [Google Scholar]
- Wang Q.-q., Zhai C., Wahafu A., Zhu Y.-t. Liu Y-h, Sun L-q. Salvianolic acid B inhibits the development of diabetic peripheral neuropathy by suppressing autophagy and apoptosis. J. Pharm. Pharmacol. 2019;71(3):417–428. doi: 10.1111/jphp.13044. [DOI] [PubMed] [Google Scholar]
- Liu S.-Y., Chen L., Li X.-C., Hu Q.-K., He L.-J. Lycium barbarum polysaccharide protects diabetic peripheral neuropathy by enhancing autophagy via mTOR/p70S6K inhibition in Streptozotocin-induced diabetic rats. J. Chem. Neuroanat. 2018;89:37–42. doi: 10.1016/j.jchemneu.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Wu L.-y., Li M., Qu M.-L., Li X., Pi L.-H., Chen Z., et al. High glucose up-regulates Semaphorin 3A expression via the mTOR signaling pathway in keratinocytes: a potential mechanism and therapeutic target for diabetic small fiber neuropathy. Mol. Cell. Endocrinol. 2018;472:107–116. doi: 10.1016/j.mce.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Chung Y.C., Lim J.H., Oh H.M., Kim H.W., Kim M.Y., Kim E.N., et al. Calcimimetic restores diabetic peripheral neuropathy by ameliorating apoptosis and improving autophagy. Cell Death Dis. 2018;9(12):1–18. doi: 10.1038/s41419-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan C., Bruce K.D., Burgy O., Boyd T.D., Michel C.R., Garcia-Perez J.E., et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr. Protoc. Cell Biol. 2020;88(1):e110. doi: 10.1002/cpcb.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Kim H., McGee L., Johnson A.E., Talwar S., Marugan J., et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci. Rep. 2018;8(1):8161. doi: 10.1038/s41598-018-26411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola A.L., Chen S. Exosomes: the messengers of health and disease. Curr. Neuropharmacol. 2017;15(1):157–165. doi: 10.2174/1570159X14666160825160421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., Emr S.D. The ESCRT pathway. Dev. Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Mathivanan S., Simpson R.J. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- Ge R., Tan E., Sharghi-Namini S., Asada H.H. Exosomes in cancer microenvironment and beyond: have we overlooked these extracellular messengers? Cancer Microenviron. 2012;5(3):323–332. doi: 10.1007/s12307-012-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- Stuffers S., Sem Wegner C., Stenmark H., Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- Juan T., Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Heidarzadeh M., Gürsoy-Özdemir Y., Kaya M., Eslami Abriz A., Zarebkohan A., Rahbarghazi R., et al. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci. 2021;11(1):142. doi: 10.1186/s13578-021-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chairoungdua A., Smith D.L., Pochard P., Hull M., Caplan M.J. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010;190(6):1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko I., Rana S., Baumann A., McAlear J., Hellwig A., Trendelenburg M., et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- Ghossoub R., Chéry M., Audebert S., Leblanc R., Egea-Jimenez A.L., Lembo F., et al. Tetraspanin-6 negatively regulates exosome production. Proc. Natl. Acad. Sci. USA. 2020;117(11):5913–5922. doi: 10.1073/pnas.1922447117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas T., Janas M.M., Sapoń K., Janas T. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015;589(13):1391–1398. doi: 10.1016/j.febslet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue B., Yang H., Wang J., Ru W., Wu J., Huang Y., et al. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020;53(7):e12857. doi: 10.1111/cpr.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C., Morohashi Y., Yoshimura S., Manrique-Hoyos N., Jung S., Lauterbach M.A., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14(9):949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- Villarroya-Beltri C., Baixauli F., Gutiérrez-Vázquez C., Sánchez-Madrid F., Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin. Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingjan I., Linders P.T.A., Verboogen D.R.J., Revelo N.H., Ter Beest M., van den Bogaart G. Endosomal and phagosomal SNAREs. Physiol. Rev. 2018;98(3):1465–1492. doi: 10.1152/physrev.00037.2017. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Tu C., Zhang J., Wang J. Inhibition of multiple myeloma-derived exosomes uptake suppresses the functional response in bone marrow stromal cell. Int. J. Oncol. 2019;54(3):1061–1070. doi: 10.3892/ijo.2019.4685. [DOI] [PubMed] [Google Scholar]
- van Dongen H.M., Masoumi N., Witwer K.W., Pegtel D.M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. Rev. 2016;80(2):369–386. doi: 10.1128/MMBR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzner D., Schnaars M., van Rossum D., Krishnamoorthy G., Dibaj P., Bakhti M., et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011;124(Pt 3):447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- Kanada M., Bachmann M.H., Hardy J.W., Frimannson D.O., Bronsart L., Wang A., et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA. 2015;112(12):E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie J., Rahbarghazi R., Pezeshki M., Mazhar M., Yekani F., Khaksar M., et al. Cardioprotective role of extracellular vesicles: a highlight on exosome beneficial effects in cardiovascular diseases. J. Cell. Physiol. 2019;234(12):21732–21745. doi: 10.1002/jcp.28894. [DOI] [PubMed] [Google Scholar]
- Rezaie J., Nejati V., Khaksar M., Oryan A., Aghamohamadzadeh N., Shariatzadeh M.A., et al. Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res. 2018;374(3):555–565. doi: 10.1007/s00441-018-2895-x. [DOI] [PubMed] [Google Scholar]
- Ren J., Liu N., Sun N., Zhang K., Yu L. Mesenchymal stem cells and their exosomes: Promising therapeutics for chronic pain. Curr. Stem Cell Res. Ther. 2019;14(8):644–653. doi: 10.2174/1574888X14666190912162504. [DOI] [PubMed] [Google Scholar]
- Hmadcha A., Martin-Montalvo A., Gauthier B.R., Soria B., Capilla-Gonzalez V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front. Bioeng. Biotechnol. 2020;43 doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins H.C., Schratt G. MicroRNA-dependent control of neuroplasticity in affective disorders. Transl. Psychiatry. 2021;11(1):1–16. doi: 10.1038/s41398-021-01379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.-C., Du W.-Q., Zhang J.-Y., Yu S.-X., Lu F.-Z., Ding H.-M., et al. Mesenchymal stem cell treatment for peripheral nerve injury: a narrative review. Neural Regener. Res. 2021;16(11):2170. doi: 10.4103/1673-5374.310941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofiño-Vian M., Guillén M.I., Pérez del Caz M.D., Castejón M.A., Alcaraz M.J. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxidative Med. Cell. Longevity. 2017;(2017) doi: 10.1155/2017/7197598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata J.P., Uhelski M.L., Gorur A., Dougherty P.M. Nociception and pain: new roles for exosomes. Neuroscientist. 2021 doi: 10.1177/10738584211027105. 10738584211027105. [DOI] [PubMed] [Google Scholar]
- Jin Z., Ren J., Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 2020;78 doi: 10.1016/j.intimp.2019.105946. [DOI] [PubMed] [Google Scholar]
- Wu J., Shen Z. Exosomal miRNAs as biomarkers for diagnostic and prognostic in lung cancer. Cancer Med. 2020;9(19):6909–6922. doi: 10.1002/cam4.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console L., Scalise M., Indiveri C. Exosomes in inflammation and role as biomarkers. Clin. Chim. Acta. 2019;488:165–171. doi: 10.1016/j.cca.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Wang H., Chen F.-S., Zhang Z.-L., Zhou H.-X., Ma H., Li X.-Q. MiR-126-3p-enriched extracellular vesicles from hypoxia-preconditioned VSC 4.1 neurons attenuate ischaemia-reperfusion-induced pain hypersensitivity by regulating the PIK3R2-mediated pathway. Mol. Neurobiol. 2021;58(2):821–834. doi: 10.1007/s12035-020-02159-y. [DOI] [PubMed] [Google Scholar]
- Liu W., Rong Y., Wang J., Zhou Z., Ge X., Ji C., et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflammation. 2020;17(1):47. doi: 10.1186/s12974-020-1726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.M., Shiue S.J., Yang K.D., Shiue H.S., Hung Y.W., Pannuru P., et al. Locally applied stem cell exosome-scaffold attenuates nerve injury-induced pain in rats. J. Pain Res. 2020;13:3257–3268. doi: 10.2147/JPR.S286771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue S.-J., Rau R.-H., Shiue H.-S., Hung Y.-W., Li Z.-X., Yang K.D., et al. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury–induced pain in rats. Pain. 2019;160(1):210–223. doi: 10.1097/j.pain.0000000000001395. [DOI] [PubMed] [Google Scholar]
- Degli Esposti C., Iadarola B., Maestri S., Beltrami C., Lavezzari D., Morini M., et al. Exosomes from plasma of neuroblastoma patients contain doublestranded DNA reflecting the mutational status of parental tumor cells. Int. J. Mol. Sci. 2021;22(7):3667. doi: 10.3390/ijms22073667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agnelli S., Gerra M.C., Bignami E., Arendt-Nielsen L. Exosomes as a new pain biomarker opportunity. Mol. Pain. 2020;16 doi: 10.1177/1744806920957800. 1744806920957800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S., Douglas S.R., Alexander G.M., Shenoda B.B., Barrett J.E., Aradillas E., et al. Exosome microRNA signatures in patients with complex regional pain syndrome undergoing plasma exchange. J. Transl. Med. 2019;17(1):1–12. doi: 10.1186/s12967-019-1833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova I.A., Alexander G.M., Qureshi R.A., Sacan A., Graziano A., Barrett J.E., et al. MicroRNA modulation in complex regional pain syndrome. J. Transl. Med. 2011;9(1):1–11. doi: 10.1186/1479-5876-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski J.L., Nguyen N., Battaglino R.A., Falci S.P., Charlifue S., Morse L.R. miR-338-5p levels and cigarette smoking are associated with neuropathic pain severity in individuals with spinal cord injury: preliminary findings from a genome-wide microRNA expression profiling screen. Arch. Phys. Med. Rehabil. 2022;103(4):738–746. doi: 10.1016/j.apmr.2021.09.005. [DOI] [PubMed] [Google Scholar]
- Freger S., Leonardi M., Foster W. Exosomes and their cargo are important regulators of cell function in endometriosis. Reproductive BioMed. Online. 2021;43(3):370–378. doi: 10.1016/j.rbmo.2021.05.022. [DOI] [PubMed] [Google Scholar]
- D'Agnelli S., Gerra M.C., Bignami E., Arendt-Nielsen L. Exosomes as a new pain biomarker opportunity. Mol. Pain. 2020;16 doi: 10.1177/1744806920957800. 1744806920957800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidarzadeh M., Gürsoy-Özdemir Y., Kaya M., Eslami Abriz A., Zarebkohan A., Rahbarghazi R., et al. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021;11(1):1–28. doi: 10.1186/s13578-021-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk K.D., Teunissen C.E., Drukarch B., Jimenez C.R., Groenewegen H.J., Berendse H.W., et al. Diagnostic cerebrospinal fluid biomarkers for Parkinson’s disease: a pathogenetically based approach. Neurobiol. Dis. 2010;39(3):229–241. doi: 10.1016/j.nbd.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel D., Peferoen L., Amor S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. Royal Soc. B: Biol. Sci. 2014;369(1652):20130516. doi: 10.1098/rstb.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung S., Dutta S., Bitan G. CNS-derived blood exosomes as a promising source of biomarkers: opportunities and challenges. Front. Mol. Neurosci. 2020;13:38. doi: 10.3389/fnmol.2020.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercher D., Nguyen M.Q., Dworak H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp. Neurol. 2021 doi: 10.1016/j.expneurol.2021.113968. [DOI] [PubMed] [Google Scholar]
- Gurunathan S., Kang M.-H., Jeyaraj M., Qasim M., Kim J.-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G., Neumann P.-A., Kamaly N., Quiros M., Nishio H., Jones H.R., et al. Annexin A1–containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Investig. 2015;125(3):1215–1227. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descalzi G., Ikegami D., Ushijima T., Nestler E.J., Zachariou V., Narita M. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015;38(4):237–246. doi: 10.1016/j.tins.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K.T., Lim K.J. Autophagy: can it be a new experimental research method of neuropathic pain? Korean J. Pain. 2015;28(4):229. doi: 10.3344/kjp.2015.28.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Shi J., Liu K., Liu N., Wang Y., Fu Z., et al. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia. 2013;61(4):504–512. doi: 10.1002/glia.22451. [DOI] [PubMed] [Google Scholar]
- Xu J., Camfield R., Gorski S.M. The interplay between exosomes and autophagy–partners in crime. J. Cell Sci. 2018;131(15):jcs215210. doi: 10.1242/jcs.215210. [DOI] [PubMed] [Google Scholar]
- Murrow L., Malhotra R., Debnath J. ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 2015;17(3):300–310. doi: 10.1038/ncb3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C., Baixauli F., Mittelbrunn M., Fernández-Delgado I., Torralba D., Moreno-Gonzalo O., et al. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016;7(1):1–11. doi: 10.1038/ncomms13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo P.-J., Rau C.-S., Wu S.-C., Lin C.-W., Huang L.-H., Lu T.-H., et al. Exosomes secreted by adipose-derived stem cells following FK506 stimulation reduce autophagy of macrophages in spine after nerve crush injury. Int. J. Mol. Sci. 2021;22(17):9628. doi: 10.3390/ijms22179628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Zhu S., Zhang W., Wei Z., Yang F., Guo Z., et al. Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in rats. Biotechnol. Lett. 2021;1–14 doi: 10.1007/s10529-021-03198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Jian X., Sun H., Qin J., Wang Y., Zhang J., et al. Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem Cells. 2021;39(4):467–481. doi: 10.1002/stem.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.C., Hu S.Q., Hu A.N., Zhang T.W., Jiang L.B., Li X.L. Autophagy-activated nucleus pulposus cells deliver exosomal miR-27a to prevent extracellular matrix degradation by targeting MMP-13. J. Orthopaedic Res. 2021;39(9):1921–1932. doi: 10.1002/jor.24880. [DOI] [PubMed] [Google Scholar]
- Liu B.-Y., Li L., Bai L.-W., Xu C.-S. Long Non-coding RNA XIST Attenuates diabetic peripheral neuropathy by inducing autophagy through MicroRNA-30d-5p/sirtuin1 axis. Front. Mol. Biosci. 2021;8:655157. doi: 10.3389/fmolb.2021.655157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S., Douglas S.R., Alexander G.M., Shenoda B.B., Barrett J.E., Aradillas E., et al. Exosome microRNA signatures in patients with complex regional pain syndrome undergoing plasma exchange. J. Transl. Med. 2019;17(1):81. doi: 10.1186/s12967-019-1833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue S.-J., Rau R.-H., Shiue H.-S., Hung Y.-W., Li Z.-X., Yang K.D., et al. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury–induced pain in rats. Pain. 2019;160(1) doi: 10.1097/j.pain.0000000000001395. [DOI] [PubMed] [Google Scholar]
- Zhang S., Teo K.Y.W., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang L, Cao Q, Xinhe L, Hu Y, Li J, et al. Intraarticular Injection of Different Doses of Mesenchymal Stem Cell Derived Exosomes Reduces ATF-3 Expression in the Dorsal Root Ganglion in Monoiodoacetate-Induced Rats of Osteoarthritis. 2021.
- Jean-Toussaint R., Lin Z., Tian Y., Gupta R., Pande R., Luo X., et al. Therapeutic and prophylactic effects of macrophage-derived small extracellular vesicles in the attenuation of inflammatory pain. Brain Behav. Immun. 2021;94:210–224. doi: 10.1016/j.bbi.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoli R., Montague K., Jones H.R., Castaldi L., Chambers D., Kelleher J.H., et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017;8(1):1778. doi: 10.1038/s41467-017-01841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen A., Jacobsen D., Phuyal S., Legfeldt A., Haugen F., Røe C., et al. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J. Transl. Med. 2017;15(1):89. doi: 10.1186/s12967-017-1194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.