Abstract
Background
Chronic kidney disease‐mineral and bone disorder (CKD‐MBD) is a systemic dysfunction of mineral and bone metabolism in people with CKD. Recent research shows that phosphate retention plays a significant role in the development of CKD‐MBD. Compared with drug therapies, dietary interventions may be simple, inexpensive and feasible for phosphate retention. However, there is little evidence to support these interventions.
Objectives
Our objective was to assess the benefits and harms of any dietary intervention for preventing and treating CKD‐MBD.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register to 27 August 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. We also searched the Chinese Biomedicine Database (CBM) (1976 to August 2015), China Knowledge Resource Integrated Database (CNKI) (1979 to August 2015), and VIP (1989 to August 2015).
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs looking at dietary interventions for prevention or treatment of CKD‐MBD were eligible for inclusion.
Data collection and analysis
Two authors independently assessed the eligibility, methodological quality, and extracted data. Continuous outcomes (serum calcium level, serum phosphorus level, calcium × phosphate product, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF‐23) and alkaline phosphatase) were expressed as mean difference (MD) with 95% confidence interval (CI). Dichotomous outcomes (mortality) were expressed as risk ratio (RR) with 95% CI. We used a random‐effects model to meta‐analyse studies.
Main results
Nine studies were included in this review which analysed 634 participants. Study duration ranged from 4 to 24 weeks. The interventions included calcium‐enriched bread, low phosphorus intake, low protein intake, very low protein intake, post haemodialysis supplements and hypolipaemic diet. Only one study reported death; none of the included studies reported cardiovascular events or fractures. There was insufficient reporting of design and methodological aspects among the included studies to enable robust assessment of risk of bias.
There was limited and low‐quality evidence to indicate that calcium‐enriched bread increased serum calcium (1 study, 53 participants: MD ‐0.16 mmol/L, 95% CI ‐0.51 to ‐0.31), decreased serum phosphorus (53 participants: MD ‐0.41 mmol/L, 95% CI ‐0.51 to ‐0.31) and decreased the calcium × phosphate product (53 participants: MD ‐0.62 mmol²/L², 95% CI ‐0.77 to ‐0.47).
Very low protein intake was not superior to conventional low protein intake in terms of effect on serum phosphorus (2 studies, 41 participants: MD ‐0.12 mmol/L, 95% CI ‐0.50 to 0.25), serum calcium (MD 0.00 mmol/L, 95% CI ‐0.17 to 0.17), or alkaline phosphatase (MD ‐22.00 U/L, 95% CI ‐78.25 to 34.25). PTH was significantly lower in the very low protein intake group (2 studies, 41 participants: MD ‐69.64 pmol/L, 95% CI ‐139.83 to 0.54).
One study reported no significant difference in the number of deaths between low phosphorus intake and normal diet (279 participants: RR 0.18, 95% CI 0.01 to 3.82). Low phosphorus intake decreased serum phosphorus (2 studies, 359 participants: MD ‐0.18 mmol/L, 95% CI ‐0.29 to ‐0.07; I2 = 0%).
One study reported post‐haemodialysis supplements did not increase serum phosphorus compared to normal diet (40 participants: MD 0.12 mmol/L, 95% CI ‐0.24 to 0.49).
One study reported low phosphorus intake plus lanthanum carbonate significantly decreased FGF‐23 (19 participants: MD ‐333.80 RU/mL, 95% CI ‐526.60 to ‐141.00), but did not decrease serum phosphorus (19 participants: MD ‐0.10 mg/dL, 95% CI ‐0.38 to 0.58) or PTH (19 participants: MD 31.60 pg/mL, 95% CI ‐29.82 to 93.02).
Authors' conclusions
There was limited low quality evidence to indicate that dietary interventions (calcium‐enriched bread or low phosphorus/protein intake) may positively affect CKD‐MBD by increasing serum calcium, decreasing serum phosphorus, the calcium × phosphate product and FGF‐23. Large and well‐designed RCTs are needed to evaluate the effects of various interventions for people with CKD‐MBD.
Keywords: Humans; Bread; Acetates; Acetates/administration & dosage; Alkaline Phosphatase; Alkaline Phosphatase/blood; Bone Density; Bone Diseases, Metabolic; Bone Diseases, Metabolic/blood; Bone Diseases, Metabolic/etiology; Bone Diseases, Metabolic/therapy; Calcium; Calcium/blood; Calcium Compounds; Calcium Compounds/administration & dosage; Calcium Phosphates; Calcium Phosphates/blood; Calcium, Dietary; Calcium, Dietary/administration & dosage; Dietary Proteins; Dietary Proteins/administration & dosage; Fibroblast Growth Factor‐23; Hydroxymethylglutaryl‐CoA Reductase Inhibitors; Hydroxymethylglutaryl‐CoA Reductase Inhibitors/administration & dosage; Phosphorus; Phosphorus/administration & dosage; Phosphorus/blood; Randomized Controlled Trials as Topic; Renal Insufficiency, Chronic; Renal Insufficiency, Chronic/complications
Plain language summary
Are changes to diet effective to manage mineral and bone abnormalities in people with chronic kidney disease?
Problems with mineral and bone metabolism are very common in people with chronic kidney disease (CKD) which can lead to broken bones (fracture), heart and blood circulation (cardiovascular) problems, and sometimes death. Many pharmaceutical treatments used to treat mineral‐bone disease can have side effects and cause problems for patients. We wanted to find out if specific diets (such as low protein or phosphorus intake) were better or worse than normal diets or pharmaceutical treatments.
We searched the literature to August 2015 and included nine studies that analysed 634 participants; durations of studies ranged from 4 and 24 weeks. The interventions included calcium‐enriched bread, low phosphorus intake, low protein intake, very low protein intake, post‐haemodialysis supplements and low lipid diet. Only one study reported death; none of the included studies reported cardiovascular events or fractures. One study reported adverse events. There was insufficient reporting of design and methodological aspects among the included studies to enable robust assessment of risk of bias.
We found scant evidence to suggest that restricting protein or phosphorus in the diet may have positive effects for people with CKD. Evidence from one small, low quality study suggested that calcium‐enriched bread may help to increase calcium and decrease phosphorus and the calcium × phosphate product.
Evidence was assessed as low quality, and was insufficient to inform clinical decision‐making about the value of dietary modification for people with CKD‐MBD. None of the included studies reported our primary outcomes of cardiovascular events or fracture; only one study reported adverse events.
Summary of findings
Summary of findings for the main comparison. Calcium‐enriched bread versus calcium acetate for people with CKD‐MBD.
| Calcium‐enriched bread versus calcium acetate for people with CKD‐MBD | ||||||
| Patient or population: people with CKD‐MBD Settings: outpatient dialysis unit Intervention: calcium‐enriched bread Comparison: calcium acetate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Calcium acetate | Calcium‐enriched bread | |||||
| Serum phosphorus Follow‐up: mean 14 weeks | Mean serum phosphorus (control) 2.08 mmol/L | Mean serum phosphorus (intervention) 0.41 mmol/L lower (0.51 to 0.31 lower) |
53 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Serum calcium Follow‐up: mean 14 weeks | Mean serum calcium (control) 2.11 mmol/L | Mean serum calcium (intervention) 0.16 mmol/L higher (0.09 to 0.23 higher) | 53 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Calcium × phosphate product Follow‐up: mean 14 weeks | Mean calcium × phosphate product (control) 4.42 mmol²/L² | Mean calcium × phosphate product (intervention) 0.62 mmol²/L² lower (0.77 to 0.47 lower) | 53 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Alkaline phosphatase activity Follow‐up: mean 14 weeks | Mean alkaline phosphatase activity (control) 95 IU/L | Mean alkaline phosphatase activity (intervention) 10 IU/L higher (2.7 lower to 22.7 higher) | 53 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Mortality | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Cardiovascular events | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Fracture | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The study reported using randomised controlled methods, but details of random sequence generation, allocation concealment and blinding were not reported 2 Only one published study was included.
Summary of findings 2. Very low versus low protein diet for people with CKD‐MBD.
| Very low versus low protein diet for people with CKD‐MBD | ||||||
| Patient or population: people with CKD‐MBD Settings: outpatient clinic Intervention: very low protein intake Comparison: low protein intake | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Very low protein diet | Low protein diet | |||||
| Serum phosphorus Follow‐up: 9 to 18 months | Mean serum phosphorus (control) 1.2 to 1.32 mmol/L | Mean serum phosphorus (intervention) 0.12 mmol/L lower (0.5 lower to 0.25 higher) | 41 (2) | ⊕⊝⊝⊝ very low1,2,3 | ||
| PTH Follow‐up: 9 to 18 months | Mean PTH (control) 23.11 to 139 pmol/L | Mean PTH (intervention) 9.98 pmol/L lower (12.85 to 7.1 lower) | 41 (2) | ⊕⊝⊝⊝ very low1,2,3 | ||
| Serum calcium Follow‐up: mean 9 months | Mean serum calcium (control) 2.3 mmol/L | Mean serum calcium (intervention) No higher (0.17 lower to 0.17 higher) | 22 (1) | ⊕⊝⊝⊝ very low1,4 | ||
| Alkaline phosphatase Follow‐up: mean 9 months | Mean alkaline phosphatase (control) 123 U/L | Mean alkaline phosphatase (intervention) 22 U/L lower (78.25 lower to 34.25 higher) | 22 (1) | ⊕⊝⊝⊝ very low1,4 | ||
| Mortality | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Cardiovascular events | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Fracture | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The study reported using randomised controlled methods, but details of random sequence generation, allocation concealment and blinding were not reported 2 Very low protein intake (0.3 g/kg/d) showed a positive effect; low protein diet (0.4 g/kg/d) showed a negative effect. 3 Only published, small studies were included. Some negative results were reported. 4 Only one published study was included.
Summary of findings 3. Low phosphorus versus normal diet for people with CKD‐MBD.
| Low phosphorus versus normal diet for people with CKD‐MBD | ||||||
| Patient or population: people with CKD‐MBD Settings: multicentre Intervention: low phosphorus diet Comparison: normal diet | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Normal diet | Low phosphorus diet | |||||
| Serum phosphorus Follow‐up: mean 6 months | Mean serum phosphorus (control) 2 mmol/L | Mean serum phosphorus (intervention) 0.22 mmol/L lower (0.41 to 0.03 lower) | 80 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Mortality | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Cardiovascular events | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Fracture | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The study reported using randomised controlled methods, but details of random sequence generation, allocation concealment and blinding were not reported 2 Only one published study was included.
Summary of findings 4. Post‐haemodialysis dietary supplement versus normal diet for people with CKD‐MBD.
| Post‐haemodialysis dietary supplement versus normal diet for people with CKD‐MBD | ||||||
| Patient or population: people with CKD‐MBD undergoing haemodialysis Settings: HD unit Intervention: post‐haemodialysis dietary supplement Comparison: normal diet | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Normal diet | Post‐haemodialysis dietary supplement | |||||
| Serum phosphorus Follow‐up: mean 1 month | Mean serum phosphorus (control) 2.1 mmol/L | Mean serum phosphorus (intervention) 0.12 mmol/L higher (0.24 lower to 0.49 higher) | 54 (2) | ⊕⊝⊝⊝ Very low1,2 | ||
| Serum phosphorus ‐ home‐prepared dietary supplement versus normal diet Follow‐up: mean 1 month | Mean serum phosphorus (control) 2.1 mmol/L | Mean serum phosphorus (intervention) 0.06 mmol/L higher (0.45 lower to 0.57 higher) | 30 (1) | ⊕⊝⊝⊝ very low1,3 | ||
| Serum phosphorus ‐ commercial dietary supplement versus normal diet Follow‐up: mean 1 month | Mean serum phosphorus (control) 2.1 mmol/L | Mean serum phosphorus (intervention) 0.19 mmol/L higher (0.34 lower to 0.72 higher) | 24 (1) | ⊕⊝⊝⊝ very low1,3 | ||
| Mortality | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Cardiovascular events | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Fracture | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Studies reported using randomised controlled methods, but did not report details of random sequence generation, allocation concealment, or blinding 2 Only published, small studies were included. Some negative results were reported. 3 Only one published study was included.
Summary of findings 5. Low phosphorus intake (avoiding food additives) versus normal diet for people with CKD‐MBD.
| low phosphorus intake (avoiding food additives) versus normal diet for people with CKD‐MBD | ||||||
| Patient or population: patients with CKD‐MBD Settings: multicentre Intervention: low phosphorus intake (avoiding food additives) Comparison: normal diet | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Normal diet | Low phosphorus intake (avoiding food additives) | |||||
| Serum phosphorus Follow‐up: mean 3 months | Mean serum phosphorus (control) 20.7 mmol/L |
Mean serum phosphorus (intervention) 1.7 mmol/L lower (3.01 to 0.39 lower) |
279 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Mortality Follow‐up: mean 3 months | Study population | RR 0.18 (0.01 to 3.82) | 279 (1) | ⊕⊕⊕⊝ moderate2 | ||
| 15 per 1000 | 3 per 1000 (0 to 55) | |||||
| Medium risk population | ||||||
| 15 per 1000 | 3 per 1000 (0 to 55) | |||||
| Cardiovascular events | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Fracture | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 It was assessed as unclear risk of selection bias, performance bias and detection bias. 2 Only one published study was included.
Summary of findings 6. Low phosphorus intake plus placebo versus ad libitum diet plus placebo for people with CKD‐MBD.
| Low phosphorus intake plus placebo versus ad libitum diet plus placebo for people with CKD‐MBD | ||||||
| Patient or population: patients with CKD‐MBD Settings: clinical research centre Intervention: low phosphorus intake plus placebo Comparison: ad libitum diet plus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ad libitum diet plus placebo | Low phosphorus intake plus placebo | |||||
| Serum phosphorus Follow‐up: mean 3 months | Mean serum phosphorus (control) 3.5 mg/dL |
Mean serum phosphorus (intervention) 0.1 mg/dL higher (0.48 lower to 0.68 higher) |
20 (1) | ⊕⊝⊝⊝ very low1 | ||
| FGF‐23 Follow‐up: mean 3 months | MeanFGF‐23 (control) ‐5.6 RU/mL |
Mean FGF‐23 (intervention) 2.3 RU/mL higher (13.18 lower to 17.78 higher) |
20 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| PTH Follow‐up: mean 3 months | Mean PTH (control) 49.8 pg/mL |
Mean PTH (intervention) 25.6 pg/mL higher (5.13 to 46.07 higher) |
20 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Mortality | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Cardiovascular events | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Fracture | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Only one published study was included. However, the result was negative. 2 The study declared to have using randomised controlled methods, but no details of random sequence generation or allocation concealment. Performance bias, attrition bias and reporting bias were assessed as high risk.
Summary of findings 7. Low phosphorus intake plus lanthanum carbonate versus ad libitum diet plus lanthanum carbonate for people with CKD‐MBD.
| Low phosphorus intake plus lanthanum carbonate versus ad libitum diet plus lanthanum carbonate for people with CKD‐MBD | ||||||
| Patient or population: patients with CKD‐MBD Settings: clinical research centre Intervention: Low phosphorus intake plus lanthanum carbonate Comparison: ad libitum diet plus lanthanum carbonate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ad libitum diet plus lanthanum carbonate | Low phosphorus intake plus lanthanum carbonate | |||||
| Serum phosphorus Follow‐up: mean 3 months | Mean serum phosphorus (control) 3.3 mg/dL |
Mean serum phosphorus (intervention) 0.1 mg/dL higher (0.38 lower to 0.58 higher) |
19 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| FGF‐23 Follow‐up: mean 3 months | Mean FGF‐23 (control) 24.3 RU/mL |
Mean FGF‐23 (intervention) 333.80 RU/mL lower (141.00 lower to 526.6 higher) |
19 (1) | ⊕⊝⊝⊝ very low1,3 | ||
| PTH Follow‐up: mean 3 months | Mean PTH (control) 68.9 pg/mL |
Mean PTH (intervention) 31.6 pg/mL higher (29.82 lower to 93.02 higher) |
19 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| Mortality | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Cardiovascular events | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| Fracture | Not reported | Not reported | Not estimable | ‐ | Not estimable | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The study declared to have using randomised controlled methods, but no details of random sequence generation or allocation concealment. Performance bias, attrition bias and reporting bias were assessed as high risk 2 Only one published study was included. However, the result was negative 3 Only one published study was included
Background
Description of the condition
Chronic kidney disease‐mineral and bone disorder (CKD‐MBD) is a systemic dysfunction of mineral and bone metabolism in people with chronic kidney disease (CKD). CKD‐MBD results from abnormalities in calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism levels; bone turnover, mineralization, volume, linear growth or strength, vascular or other soft tissue calcification (KDIGO 2009). In its early stages, CKD‐MBD is characterised by bone fractures, bone pain, skeletal deformities in growing children, reduced velocity in bone growth, abnormal height, vascular and other soft tissue calcification (Mejía 2011). Developments in dialysis technology have meant that fewer patients with CKD die from uraemia and have longer rates of survival. However, CKD‐MBD is a significant contributor to decreased quality of life and increased mortality and morbidity risks, and progression of CKD (Moe 2006; Moe 2007).
Phosphate retention plays an important role in the development of CKD‐MBD. As kidney function declines, excretion of phosphate becomes more difficult. Phosphate retention stimulates PTH and fibroblast growth factor (FGF)‐23 function before hyperphosphataemia is detected during the early stages of CKD. In general, FGF‐23 and PTH can suppress renal reabsorption of phosphorus. However as CKD develops, kidney response to these hormones decreases (Razzaque 2011). In contrast, residual kidney function is challenged in converting 25(OH)D to 1,25(OH)₂D, which may reduce intestinal calcium absorption and increase PTH and FGF‐23 levels (Komaba 2008).
Recent research has also indicated that the Klotho gene, which encodes a transmembrane co‐receptor specific for FGF‐23, declines in people with CKD. The Klotho gene also causes FGF‐23 resistance and stimulates PTH (Kuro‐O 2011). Both FGF‐23 and PTH increase as CKD progresses, eventually leading to renal osteodystrophy, cardiovascular and soft issue calcification. CKD‐MBD has been associated with both renal bone disease and higher mortality (Moe 2007; Tentori 2008).
Description of the intervention
Phosphate retention usually begins early in the course of CKD.
Dietary phosphate restriction and use of phosphate binders are two principal measures for the management of elevated phosphate levels. It has been shown that if serum phosphorus can be decreased in relation to the glomerular filtration rate (GFR), plasma PTH elevation could be prevented (Slatopolsky 1973).
Small sample research has also demonstrated that prolonged limiting of dietary phosphate intake is effective in suppressing secondary hyperparathyroidism, and was recommended for implementation at all stages of kidney disease (McCrory 1987; Takeda 2007).
However, challenges persist in the treatment of hyperphosphataemia. At present, calcium‐containing and non‐calcium containing phosphate binders, such as sevelamer and lanthanum, are the major drugs used to lower phosphate levels. Calcium‐containing phosphate binders may increase the risk of positive calcium balance, and lead to cardiovascular and soft tissue calcification, particularly when associated with vitamin D therapy. Sevelamer for reducing serum phosphorus has been demonstrated to decrease progression of coronary artery calcification compared with calcium salts. However, high treatment cost of sevelamer limits its use, and the same is true for lanthanum.
Pelletier 2010 compared older and younger haemodialysis patients and reported better control of serum phosphorus with less phosphate binder and cinacalcet. This study indicated that phosphate binders may not be the determinant in maintaining serum phosphorus. Moreover, the increasing number of patients with CKD requires a large number of conventional drugs which imposes a significant burden for both patients and society (Navaneethan 2009). Thus, searching for interventions that are both efficient and affordable is a pivotal target for preventing and treating CKD‐MBD.
Compared with drug therapies, dietary interventions seem to be simple, inexpensive and feasible. Dietary phosphate restriction is recommended in many guidelines. The KDOQI 2003 guidelines suggest that dietary phosphorus should be restricted to 800 to 1000 mg/day when plasma levels of intact PTH are elevated above the target range of the CKD stage. The KDIGO 2009 guidelines recommend that patients with CKD stages 3 to 5D limit their dietary phosphate intake for the treatment of hyperphosphataemia, alone or in combination with other treatments; however, there is currently little evidence to support this recommendation.
Because phosphate intake usually parallels protein intake, dietary phosphate restriction is often achieved by restricting protein intake. Cianciaruso 2008 and Klahr 1994 conducted studies to investigate the effects of different protein diets on metabolic control and CKD progression. Sullivan 2009 focused on the effects of food additives on hyperphosphataemia in people with end‐stage kidney disease and reported benefits when phosphorus‐containing food additives were avoided. Soroka 1998 investigated feasibility low phosphate diets and showed that a low‐phosphorus vegan diet in which only an appropriate cereal‐legume mixture was consumed could achieve the same goal as a conventional low‐protein diet. Patients not only avoided protein malnutrition, but also reduced phosphate intake, which is an abundant mineral in animal‐based foods.
How the intervention might work
Phosphate retention plays a significant role in the development of CKD‐MBD. Lowering dietary phosphate by restricting food additives, processed foods and protein, and sometimes in combination with phosphate binders, should therefore be the first step to protect people with CKD from developing mineral and bone disorder.
Why it is important to do this review
Although dietary interventions are well recognised as an important way to help prevent and treat CKD‐MBD, there has been no systematic review of these interventions. The safety and efficacy of dietary interventions for people with CKD‐MBD remain unknown.
Objectives
Our objective was to assess the benefits and harms of any dietary intervention for preventing and treating CKD‐MBD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at dietary interventions for preventing or treating CKD‐MBD were included. The first periods of randomised cross‐over studies were also eligible for inclusion.
Types of participants
People with CKD stages 3 to 5D as defined by the KDOQI 2003 guidelines (stage 3: GFR 30 to 59 mL/min/1.73 m²; stage 4: GFR 15 to 29 mL/min/1.73 m², stage 5: GFR < 15 mL/min/1.73 m² or dialysis) were included. Children and kidney transplant recipients were also included.
Types of interventions
Any dietary intervention versus placebo, no treatment, another dietary intervention or any other interventions
Any dietary intervention in combination with other interventions versus placebo, no treatment, another dietary intervention or any other interventions.
Dietary interventions included protein restricted diets and phosphate restricted diets.
Types of outcome measures
Outcome data of four weeks intervention or longer were included because it seemed impossible to evaluate the effect of dietary interventions over a shorter time
Fracture at any site measured by radiographic examination
Cardiovascular events measured by records of symptoms, any ultrasonic, electrocardiogram or heart intervention
Vascular calcification or soft tissue calcification measured by CT, X‐ray or ultrasonic imaging
Incidence of calciphylaxis measured by symptoms, X‐ray or biopsy
Bone density (assessed by dual‐energy X‐ray absorptiometry using Z‐scores at the lumbar spine, femoral neck or radius)
Bone turnover (by bone histomorphometry)
Potential adverse events included protein energy malnutrition, gastrointestinal symptoms, hypophosphataemia, hyper‐ or hypocalcaemia
Other outcomes measured by blood examination at the end of the interventions.
Primary outcomes
Mortality
Cardiovascular events
Fracture.
Secondary outcomes
-
Biochemical parameters
Serum phosphorus (mmol/L, mg/dL)
Serum calcium (mmol/L, mg/dL)
Calcium × phosphate product
PTH (iPTH) (pmol/L, pg/mL)
Alkaline phosphatase (μkat/L, U/L)
Urinary phosphorus excretion (mmol/L, mg/dL)
Serum FGF‐23 (pg/mL)
Vascular calcification
Soft tissue calcification
Left ventricular mass
Incidence of calciphylaxis
Bone density (assessed by dual‐energy X‐ray absorptiometry using Z‐scores at the lumbar spine, femoral neck or radius)
Bone turnover (by bone histomorphometry)
Bone micro‐architecture by high‐resolution peripheral computed tomography (HR‐pQCT)
Longitudinal growth in children
Adverse events.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 27 August 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
We also searched:
The Chinese Biomedicine Database (CBM) (1976 to August 2015)
Chinese National Knowledge Infrastructure (CNKI) (1979 to August 2015)
VIP Database for Chinese Technical Periodicals (VIP) (1989 to August 2015).
See Appendix 1 for search terms.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that might relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. However, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfy the inclusion criteria. There were no disagreements.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. We grouped reports of the same study together and only the publication with the most complete data was used in the analyses. There were no disagreements.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
There were no reports of dichotomous outcomes (such as mortality, cardiovascular events, fracture, adverse events and so forth). Where continuous scales of measurement was used to assess the effects of treatment (such as serum phosphorus, serum calcium, Ca × P product, PTH (iPTH), alkaline phosphatase), the mean difference (MD) with 95% confidence intervals (CI) were used. We analysed final measurement outcomes data for meta‐analysis if available.
Unit of analysis issues
Only data from the first period of cross‐over studies were included. For multiple intervention groups, we pooled all relevant experimental intervention groups into a single group, and likewise pooled all relevant control intervention groups into a single control group.
Dealing with missing data
Further information required from the original author was requested by written correspondence (e‐mailing and/or writing to corresponding author/s) and relevant information obtained in this manner was included in the review.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We were unable to construct funnel plots to assess and presence of reporting bias because of the small number of included studies.
Data synthesis
Only data from Di Iorio 2003 and Herselman 1995 were pooled using the random‐effects model. We were unable to conduct pooled analyses because of the range of interventions reported.
Subgroup analysis and investigation of heterogeneity
We were unable to perform subgroup analysis because of the small number of included studies.
Sensitivity analysis
We were unable to perform sensitivity analyses because of the small number of included studies.
Results
Description of studies
Results of the search
We identified 1077 records. After initial screening we excluded 911 records (duplicates, animal studies, not randomised, wrong population). After title and abstract review we excluded an additional 129 records. We obtained the full‐text of 37 records. We included nine studies (12 records) and excluded 12 studies (15 records). Six studies are awaiting assessment and there are three ongoing studies (Figure 1).
1.
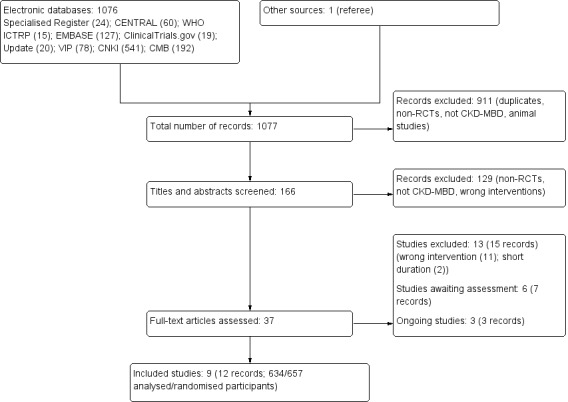
Study flow diagram
Included studies
We included nine studies (634/657 analysed/randomised patients) that investigated six types of dietary interventions (Babarykin 2004;Bunio 2004;Di Iorio 2003;Herselman 1995;Isakova 2013; Li 2011c; Lou 2012; Sharma 2002c; Sullivan 2009).
Babarykin 2004 compared calcium‐enriched bread diet with calcium acetate. Di Iorio 2003 and Herselman 1995 compared very low protein intake with low protein intake. Herselman 1995 compared 0.6 g protein/kg/day with 0.4 g protein/kg/day supplemented with essential amino acids. Di Iorio 2003 compared 0.6 g protein/kg/day with 0.3 g protein/kg/day of vegetable origin, supplemented with a mixture of keto analogues and essential amino acids. Bunio 2004 compared hypolipaemic diet with statin/lovastatin 20 mg/day. Li 2011c compared low protein intake (0.8 g/kg ideal body weight/day, with keto acid‐supplementation) with normal protein intake (1 to 1.2 g/kg ideal body weight/day). Isakova 2013, Lou 2012 and Sullivan 2009 studied phosphorus restricted diets for CKD. Isakova 2013 compared low phosphorus intake (900 mg phosphorus/day) plus lanthanum carbonate/placebo with ad libitum plus lanthanum carbonate/placebo. Lou 2012 compared low phosphorus diet (800 to 900 mg phosphorus/day) with normal diet. Sullivan 2009 also compared low phosphorus diet (education on avoiding foods with phosphorus additives) with usual diet. Sharma 2002c compared post‐haemodialysis supplementation (home‐prepared or commercially available formula providing 500 Kcal and 15 g protein) with normal diet.
Excluded studies
We excluded 13 studies. Of these, 11 investigated non‐dietary interventions (ACTRN12611000500954; Ambrus 2003; Ashurst 2003; Cheng 2008; Chertow 2003; Clark 2010; Morey 2008; NCT01665651; Olivero 2006; Padhi 2007; Young 2009a) and two were of shorter duration than specified in our inclusion criteria (Moe 2011; Spiegel 2012). (See Characteristics of excluded studies).
Studies awaiting assessment
Karavetian 2012 was available only as an abstract, and the details of the nutritional therapy investigated were unclear. Garini 1992 was published in Italian. We unable to translate it and the details of the study were unknown.
Prior to publication a search of the Specialised Register identified four potential studies (Akizawa 2014a; Block 2013; Hill 2013; Karavetian 2013). These studies will be assessed in a future update of this review.
Ongoing studies
Three studies are ongoing and will be assessed in a future update of this review (NCT00755690; NCT01865526; NCT02005302).
Risk of bias in included studies
Overall, study quality was suboptimal. There was insufficient reporting of design and methodological aspects among the included studies to enable robust assessment of risk of bias. (See Characteristics of included studies; Figure 2; Figure 3).
2.
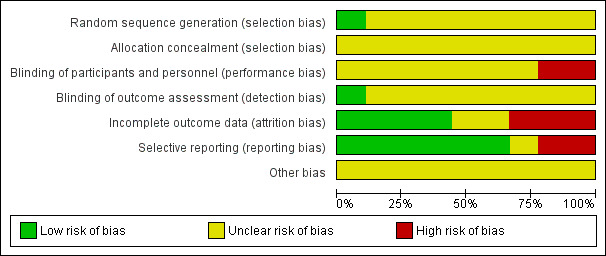
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
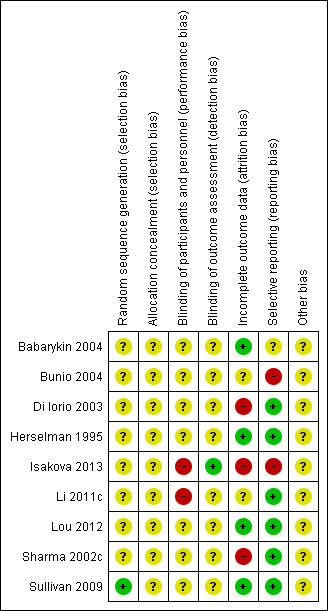
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Although the included studies reported applying randomised controlled methods, only one study reported specific randomisation method(Sullivan 2009). None adequately reported random sequence generation or allocation concealment.
Blinding
Li 2011c, an open‐label study, was assessed at high risk of performance bias. Isakova 2013 was assessed as low risk of detection bias because the investigators remained blinded to the dietary group. However, dietitian and participants were unblinded to the assigned dietary counselling group and the performance bias was assessed as high risk. All other studies (Babarykin 2004; Bunio 2004; Di Iorio 2003; Herselman 1995; Lou 2012; Sharma 2002c; Sullivan 2009) were assessed as unclear risk of performance and detection bias.
Incomplete outcome data
Missing data did not balance between groups in Sharma 2002c and Isakova 2013. In Di Iorio 2003 follow‐up duration differed significantly between intervention and control groups; and most control group participants withdrew after 18 months, which meant that participant data did not balanced at the end of the study (24 months). We assessed Di Iorio 2003, Isakova 2013 and Sharma 2002c at high risk of attrition bias. Sullivan 2009 was assessed as low risk of attrition bias because missing outcome data had similar reasons and balanced in numbers across intervention groups. Multiple imputations were also used to account for missing data. Bunio 2004 was abstract only and details of outcome data were unknown. Li 2011c was assessed as unclear risk of attrition bias because detailed control group data were not reported. Babarykin 2004, Herselman 1995 and Lou 2012 were assessed at low risk of attrition bias.
Selective reporting
Bunio 2004 and Isakova 2013 did not report on specified outcomes, and we therefore assessed these studies at high risk of reporting bias. There were insufficient data to assess reporting bias in Babarykin 2004. All other included studies were assessed at low risk of reporting bias (Di Iorio 2003; Herselman 1995; Li 2011c; Lou 2012; Sharma 2002c; Sullivan 2009).
Other potential sources of bias
There was insufficient information to determine if there were any other potential sources of bias present.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Primary outcomes
Only Sullivan 2009 reported death. None of the included studies reported cardiovascular events or fracture.
Death
Sullivan 2009 reported no significant difference in the number of patients who died between low phosphorus intake and normal diet (Analysis 3.1 (279 participants): RR 0.18, 95% CI 0.01 to 3.82).
3.1. Analysis.
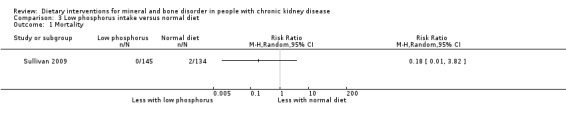
Comparison 3 Low phosphorus intake versus normal diet, Outcome 1 Mortality.
We inferred from end‐of‐study data that no deaths occurred in four studies (Babarykin 2004; Herselman 1995; Li 2011c; Sharma 2002c). We were unsure about mortality rates in the other studies because of participant withdrawals and poor reporting of losses to follow‐up (Bunio 2004; Di Iorio 2003; Isakova 2013; Lou 2012).
Secondary outcomes
Serum phosphorus
Serum phosphorus was reported in seven studies (Babarykin 2004; Di Iorio 2003; Herselman 1995; Isakova 2013; Lou 2012; Sharma 2002c; Sullivan 2009).
Babarykin 2004 reported calcium‐enriched bread significantly reduced serum phosphorus levels compared to calcium acetate (Analysis 1.1 (53 participants): MD ‐0.41 mmol/L, 95% CI ‐0.51 to ‐0.31).
1.1. Analysis.

Comparison 1 Calcium‐enriched bread versus calcium acetate, Outcome 1 Serum phosphorus.
There was no significant difference in serum phosphorus between low protein and very low protein intake (Analysis 2.1 (2 studies, 41 participants): MD ‐0.12 mmol/L, 95% CI ‐0.50 to 0.25; I2 = 80%) (Di Iorio 2003; Herselman 1995). Heterogeneity was high and this may be due to the different amounts of protein given to the very low protein group (0.3 g/kg/d versus 0/4 g/kg/d) and the duration of treatment (24 months versus 9 months)
2.1. Analysis.
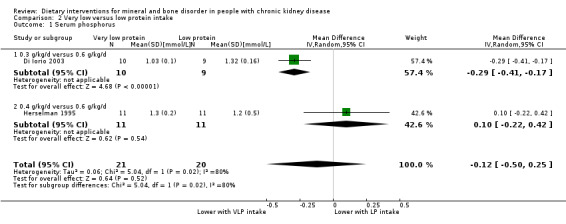
Comparison 2 Very low versus low protein intake, Outcome 1 Serum phosphorus.
Serum phosphorus was significantly reduced with a low phosphorus intake compared to a normal diet (Analysis 3.2 (2 studies, 359 participants): MD ‐0.18 mmol/L, 95% CI ‐0.29 to ‐0.07; I2 = 0%) (Lou 2012; Sullivan 2009).
3.2. Analysis.
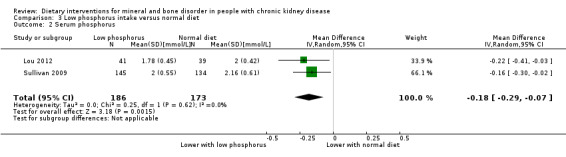
Comparison 3 Low phosphorus intake versus normal diet, Outcome 2 Serum phosphorus.
Sharma 2002c reported neither home‐prepared (Analysis 4.1.1 (23 participants): MD 0.06 mmol/L, 95% CI ‐0.57 to 0.69) nor commercially available diet supplements (Analysis 4.1.2 (17 participants): MD 0.19 mmol/L, 95% CI ‐0.46 to 0.84) showed significant changes in serum phosphorus levels compared with normal diet (pooled result ‐ Analysis 4.1 (40 participants): MD 0.12 mmol/L, 95% CI ‐0.33 to 0.58; I2 = 0%).
4.1. Analysis.
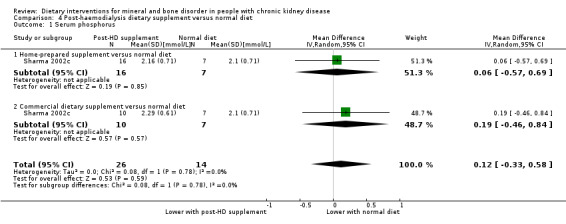
Comparison 4 Post‐haemodialysis dietary supplement versus normal diet, Outcome 1 Serum phosphorus.
Isakova 2013 reported no significant difference in serum phosphorus between either low phosphorus intake plus placebo compared with ad libitum diet plus placebo (Analysis 5.1.1 (20 participants): MD 0.10 mg/dL, 95% CI ‐0.48 to 0.68) or low phosphorus intake plus lanthanum carbonate compared with ad libitum diet plus placebo (Analysis 5.1.2 (19 participants): MD 0.10 mg/dL, 95% CI ‐0.38 to 0.58).
5.1. Analysis.
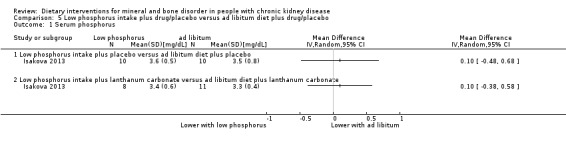
Comparison 5 Low phosphorus intake plus drug/placebo versus ad libitum diet plus drug/placebo, Outcome 1 Serum phosphorus.
Serum calcium
Babarykin 2004 reported calcium‐enriched bread significantly increased serum calcium levels compared to calcium acetate (Analysis 1.2 (53 participants): MD 0.16 mmol/L, 95% CI 0.09 to 0.23).
1.2. Analysis.

Comparison 1 Calcium‐enriched bread versus calcium acetate, Outcome 2 Serum calcium.
Herselman 1995 reported no significant difference in calcium levels between very low protein intake (0.4 g protein/kg/d) and low protein intake (0.6 g protein/kg/d) (Analysis 2.2 (22 participants): MD 0.00 mmol/L, 95% CI ‐0.17 to 0.17).
2.2. Analysis.
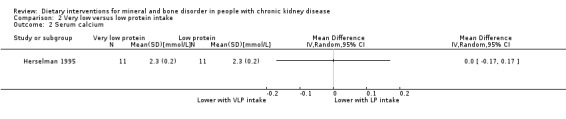
Comparison 2 Very low versus low protein intake, Outcome 2 Serum calcium.
Calcium × phosphate product
Babarykin 2004 reported calcium‐enriched bread significantly reduced calcium × phosphate product compared to calcium acetate (Analysis 1.3 (53 participants): MD ‐0.62 mmol²/L², 95% CI ‐0.77 to ‐0.47).
1.3. Analysis.

Comparison 1 Calcium‐enriched bread versus calcium acetate, Outcome 3 Calcium × phosphate product.
Alkaline phosphatase activity
Babarykin 2004) reported no significant difference in alkaline phosphatase activity between calcium‐enriched bread and calcium acetate (Analysis 1.4 (53 participants): MD 10.00 IU/L, 95% CI ‐2.70 to 22.70).
1.4. Analysis.
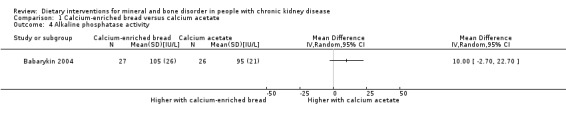
Comparison 1 Calcium‐enriched bread versus calcium acetate, Outcome 4 Alkaline phosphatase activity.
Herselman 1995 reported no significant difference in alkaline phosphatase activity between very low and low protein intake (Analysis 2.3 (22 participants): MD ‐22.00 U/L, 95% CI ‐78.25 to 34.25).
2.3. Analysis.
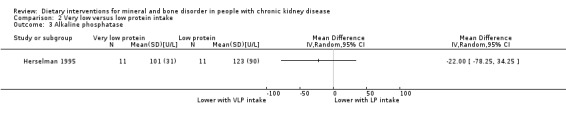
Comparison 2 Very low versus low protein intake, Outcome 3 Alkaline phosphatase.
Parathyroid hormone
PTH was significantly lower with very low protein intake compared to low protein intake (Analysis 2.4 (2 studies, 41 participants): MD ‐69.64 pmol/L, 95% CI ‐139.83 to 0.54; I2 = 57%) (Di Iorio 2003; Herselman 1995).
2.4. Analysis.
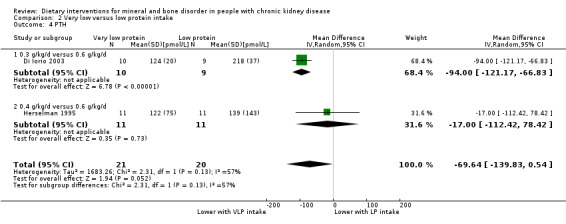
Comparison 2 Very low versus low protein intake, Outcome 4 PTH.
Isakova 2013 reported PTH was significantly lower with low phosphorous intake compared to ad libitum diet (Analysis 5.2.1 (20 participants): MD 25.60 pg/mL, 95% CI 5.13 to 46.07), however there was no significant difference in PTH between low phosphorous intake plus lanthanum carbonate compared to ad libitum diet plus lanthanum carbonate (Analysis 5.2.2 (19 participants): MD 31.60 pg/mL, 95% CI ‐29.82 to 93.02).
5.2. Analysis.
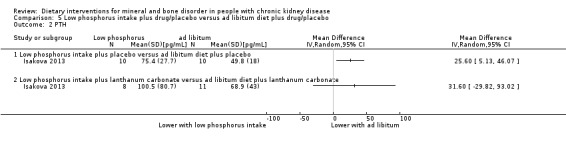
Comparison 5 Low phosphorus intake plus drug/placebo versus ad libitum diet plus drug/placebo, Outcome 2 PTH.
Fibroblast growth factor 23
Isakova 2013 reported no significant difference in FGF‐23 with low phosphorous intake compared to ad libitum diet (Analysis 5.3.1 (20 participants): MD 2.30 RU/mL, 95% CI ‐13.18 to 17.78), however there was a significant decrease in FGF‐23 in the low phosphorous intake plus lanthanum carbonate group compared to ad libitum diet plus lanthanum carbonate (Analysis 5.3.2 (19 participants): (MD ‐333.80 RU/mL, 95% CI ‐526.60 to ‐141.00).
5.3. Analysis.
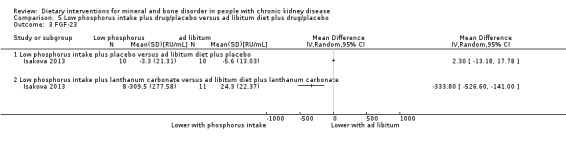
Comparison 5 Low phosphorus intake plus drug/placebo versus ad libitum diet plus drug/placebo, Outcome 3 FGF‐23.
Adverse events
Lou 2012 reported clinical complications (3) and kidney transplantation (3), but participants' groups were not reported.
Isakova 2013 reported five participants (three who received lanthanum carbonate and two who received lanthanum carbonate placebo) had gastrointestinal adverse effects. Two participants in low phosphate diet plus lanthanum carbonate group had nausea and vomiting.
The included studies did not report on any other of the secondary outcomes of interest for this review.
Discussion
Summary of main results
Evidence from the included studies was low quality and insufficiently powered to inform clinical decision making about the value of dietary modification for people with CKD‐MBD. None of the included studies reported on the primary outcomes of cardiovascular events or fracture; only one study reported adverse events and another reported mortality. Most studies focused on chemical parameters, particularly serum phosphorus levels.
There was limited, low quality evidence to indicate that calcium‐enriched bread may increase serum calcium, decrease serum phosphorus and the calcium × phosphate product (Babarykin 2004). Elsewhere, it was reported that reduced phosphorus intake may decrease serum phosphorus level (Lou 2012; Sullivan 2009). Low phosphorus intake plus lanthanum carbonate showed benefit in decreasing FGF‐23 level compared with ad libitum diet plus lanthanum carbonate (Isakova 2013). Very low protein intake was not superior to conventional low protein diet in terms of effect on serum phosphorus, serum calcium, and alkaline phosphatase levels (Di Iorio 2003; Herselman 1995), however PTH levels were significantly lower with very low protein diets. Low protein intake supplemented with keto‐acids may decrease serum phosphorus compared with normal protein intake in people undergoing haemodialysis (Li 2011c). No changes in PTH and alkaline phosphatase were observed when haemodialysis patients adopted a hypolipaemic diet compared with statins (Bunio 2004). Compared with a normal diet, post‐haemodialysis diet supplements did not increase serum phosphorus levels (Sharma 2002c).
Restricting protein or phosphorus, taking calcium‐enriched bread in the diet may have positive effects for people with CKD. It mainly showed in chemical parameters. However, none of the included studies reported cardiovascular events and fracture, and only one study reported mortality. CKD‐MBD guidelines currently suggest not exceeding dietary phosphorus intake of 800 to 1000 mg/day (Bellorin‐Font 2013; Goldsmith 2010), but little practical information about how to assess and alter dietary phosphate intake was provided, the same as the included studies. It is worth noting that combined interventions, like calcium‐enriched bread served as a phosphate binder, showed another way of decreasing phosphorus level.
Overall completeness and applicability of evidence
The included studies were conducted in America, China, India, Italy, Latvia, Poland, Spain and South Africa. Participant ethnicity was not reported. Herselman 1995, Isakova 2013 and Lou 2012 reported clear age definitions for inclusion (≥ 18 years); all other studies provided mean age. It is unknown if the dietary interventions investigated had similar effects on children. CKD stages among the included studies differed; six included people undergoing haemodialysis (Babarykin 2004;Bunio 2004; Li 2011c; Lou 2012; Sharma 2002c; Sullivan 2009).
Isakova 2013 reported estimated GFR of 15 to 59 mL/min/1.73 m2. Herselman 1995 reported serum creatinine (150 to 700 μmol/L) but Di Iorio 2003 analysed creatinine clearance (≤ 25 mL/min). A robust conclusion therefore could not be made about the CKD stage at which people may derive benefits from any of the interventions.
Pooled analysis was not conducted because of the range and diversity of interventions explored in the studies. Some dietary interventions were home‐prepared and others were commercial preparations (Sharma 2002c); content and manufacturing methods were not reported. Only calorie and calcium content were reported.
Although results showed some positive outcomes, the studies were underpowered and provided low quality evidence. Outcomes should be interpreted with caution.
Quality of the evidence
The methodological quality of the included studies was poor. Although all studies reported assigning randomised controlled methods, only one study reported specific randomisation method (Sullivan 2009). None reported allocation concealment. Isakova 2013 was assessed as at low risk of detection bias because the investigators remained blinded to the dietary group. Overall, the quality of the evidence was assessed as low and insufficiently powered to inform clinical decision making.
Potential biases in the review process
The small number of included studies meant that we were unable to construct a funnel plot to investigate publication bias.
Agreements and disagreements with other studies or reviews
Di Iorio 2012 reported that intensive restriction of protein and phosphate intake decreased FGF‐23 and serum phosphorus level compared to low protein diet. Fouque 2009 found that reducing protein intake in people with CKD could reduce renal death rate (defined as dialysis, death, or kidney transplantation) by 32% compared with higher or unrestricted protein intake. These studies supported protein restriction in people with CKD. Other dietary phosphate control interventions included reducing food additives and boiling (Cupisti 2013).
Restricting protein may be contraindicated for people with uraemia. Klahr 1994 found that very low protein diets did not significantly slow progression of kidney disease compared with low protein diets among people with CKD stage 3 (GFR 25 to 55 mL/min/1.73 m²). Johnson 2006 considered that therapeutic effects of low protein diets were unclear, because of the poor evidence and the high prevalence of malnutrition in people with CKD.
Authors' conclusions
Implications for practice.
There was limited, low powered and suboptimal quality evidence to suggest that consumption of calcium‐enriched bread or low phosphorus and protein intake may provide some benefit for people with CKD‐MBD. Very low protein intake, post‐haemodialysis diet supplements and hypolipaemic diets were conferred no significant benefit compared with controls. There was insufficient evidence to support the use of these interventions.
Implications for research.
Large, well‐designed RCTs are needed to evaluate the effect of dietary interventions or combination interventions (including diet) for people with CKD‐MBD that report mortality, cardiovascular and fracture‐related outcomes and measure impact on quality of life. Adverse events, reasons for participants' withdrawals, and losses to follow‐up should be reported.
What's new
| Date | Event | Description |
|---|---|---|
| 16 February 2016 | Amended | Clarification of stage of chronic kidney disease in 'Agreements and disagreements with other studies' section |
Notes
16 February 2016: Clarification of stage of chronic kidney disease in Agreements and disagreements with other studies section
Acknowledgements
We would like to thank Ruth Mitchell, Trials Search Co‐ordinator, Cochrane Kidney and Transplant, for her help with search strategies and advice. We would also like to thank the referees for their feedback and suggestions. We also want to thank Dr Mao Wei and Dr Paul from the Guangdong Provincial Hospital of Chinese Medicine, and Professor Wu Taixiang of the Chinese Cochrane Centre for suggesting the need for this review.
Appendices
Appendix 1. Electronic Search Strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Calcium‐enriched bread versus calcium acetate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum phosphorus | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Serum calcium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Calcium × phosphate product | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Alkaline phosphatase activity | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Very low versus low protein intake.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum phosphorus | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.50, 0.25] |
| 1.1 0.3 g/kg/d versus 0.6 g/kg/d | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.41, ‐0.17] |
| 1.2 0.4 g/kg/d versus 0.6 g/kg/d | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.22, 0.42] |
| 2 Serum calcium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Alkaline phosphatase | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 PTH | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐69.64 [‐139.83, 0.54] |
| 4.1 0.3 g/kg/d versus 0.6 g/kg/d | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐94.0 [‐121.17, ‐66.83] |
| 4.2 0.4 g/kg/d versus 0.6 g/kg/d | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐17.0 [‐112.42, 78.42] |
Comparison 3. Low phosphorus intake versus normal diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Serum phosphorus | 2 | 359 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.29, ‐0.07] |
Comparison 4. Post‐haemodialysis dietary supplement versus normal diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum phosphorus | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.33, 0.58] |
| 1.1 Home‐prepared supplement versus normal diet | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.57, 0.69] |
| 1.2 Commercial dietary supplement versus normal diet | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.46, 0.84] |
Comparison 5. Low phosphorus intake plus drug/placebo versus ad libitum diet plus drug/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum phosphorus | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Low phosphorus intake plus placebo versus ad libitum diet plus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Low phosphorus intake plus lanthanum carbonate versus ad libitum diet plus lanthanum carbonate | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PTH | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Low phosphorus intake plus placebo versus ad libitum diet plus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Low phosphorus intake plus lanthanum carbonate versus ad libitum diet plus lanthanum carbonate | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FGF‐23 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Low phosphorus intake plus placebo versus ad libitum diet plus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Low phosphorus intake plus lanthanum carbonate versus ad libitum diet plus lanthanum carbonate | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Babarykin 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Bunio 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | iPTH, bone specific alkaline phosphatase were listed in the methods, but no detailed information provided in the results |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Di Iorio 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Most control group participants withdrew after 18 months; missing data were therefore imbalanced at the end of the study (24 months) |
| Selective reporting (reporting bias) | Low risk | Published reports included all prespecified outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Herselman 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Patients received the same standard of counselling. Each patient was supplied with food scales for the weighing of food, and was visited at home to optimise education. Following the training period of 8 weeks, patients were matched for underlying nephropathy, SCr, creatinine clearance, known duration of disease, age, sex and dietary knowledge, then randomised. Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data at nine months follow‐up |
| Selective reporting (reporting bias) | Low risk | Published report included all specified outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Isakova 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | All participants met with the dietitian, who provided personalized dietary recommendations during a 60‐minute session at the randomisation visit and at 30‐minute follow‐up visits during weeks 2, 8, and 12, when adherence with the dietary intervention was reassessed with 3‐day food records. Dietitian used the food records to counsel participants to follow a diet tailored to their randomisation group. Treatment group A
Control group A
Treatment group B
Control group B
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Dietitian and participants were unblinded to the assigned dietary counselling group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators remained blinded to the dietary group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers and reasons for missing data across intervention groups |
| Selective reporting (reporting bias) | High risk | Data did not show all of the study's pre‐specified primary outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Li 2011c.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Phosphorus and calcium data from control group participants were not provided in detail at the end of 8 weeks |
| Selective reporting (reporting bias) | Low risk | Published reports included all prespecified outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lou 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data at the end of the study |
| Selective reporting (reporting bias) | Low risk | Published reports included all prespecified outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Sharma 2002c.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven patients did not complete the study; data for these participants were not reported |
| Selective reporting (reporting bias) | Low risk | The published reports included all expected outcomes that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Sullivan 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention
Control
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | a random number generator was used |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data had similar reasons and balanced in numbers across intervention groups. Multiple imputation was used to account for missing data. |
| Selective reporting (reporting bias) | Low risk | The published reports included all expected outcomes that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement |
CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; FGF‐23 fibroblast growth factor 23; HD ‐ haemodialysis; M/F ‐ male/female; PTH ‐ parathyroid hormone; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12611000500954 | Wrong intervention |
| Ambrus 2003 | Wrong intervention |
| Ashurst 2003 | Wrong intervention |
| Cheng 2008 | Wrong intervention |
| Chertow 2003 | Wrong intervention |
| Clark 2010 | Wrong intervention |
| Moe 2011 | Study duration was shorter than our inclusion criteria |
| Morey 2008 | Wrong intervention |
| NCT01665651 | Wrong intervention |
| Olivero 2006 | Wrong intervention |
| Padhi 2007 | Wrong intervention |
| Spiegel 2012 | Study duration was shorter than our inclusion criteria |
| Young 2009a | Wrong intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
Akizawa 2014a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Block 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Garini 1992.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Hill 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Karavetian 2012.
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
| Outcomes |
|
| Notes |
|
Karavetian 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Contributions of authors
Draft the protocol: LZZ
Study selection: MWZ, ZC, GXF
Extract data from studies: ZL, XY, LXS
Enter data into RevMan: WYF
Carry out the analysis: YQC
Interpret the analysis: LXS, ZC
Draft the final review: LZZ, SGB
Disagreement resolution: LXS, GXF
Update the review: LZZ, SGB
Sources of support
Internal sources
Methodological Research Unit, Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, China.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Babarykin 2004 {published data only}
- Babarykin D, Adamsone I, Amerika D, Spudass A, Moisejev V, Berzina N, et al. Calcium‐enriched bread for treatment of uremic hyperphosphatemia. Journal of Renal Nutrition 2004;14(3):149‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bunio 2004 {published data only}
- Bunio A, Kwiecinski R, Steciwko A, Migas A. Effect of lipid lowering treatment on direct markers of bone metabolism in hemodialysis patients [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:320‐1. [CENTRAL: CN‐00509112]
Di Iorio 2003 {published data only}
- Iorio BR, Bellizzi V, Minutolo R, Nicola L, Iodice C, Conte G. Supplemented very low‐protein diet in advanced CRF: is it money saving?. Kidney International 2004;65(2):742. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Iorio BR, Minutolo R, Nicola L, Bellizzi V, Catapano F, Iodice C, et al. Supplemented very low protein diet ameliorates responsiveness to erythropoietin in chronic renal failure. Kidney International 2003;64(5):1822‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herselman 1995 {published data only}
- Herselman MG, Albertse EC, Lombard CJ, Swanepoel CR, Hough FS. Supplemented low‐protein diets ‐ are they superior in chronic renal failure?. South African Medical Journal (Suid Afrikaanse Tydskrif vir Geneeskunde) 1995;85(5):361‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Isakova 2013 {published data only}
- Isakova T, Barchi‐Chung A, Enfield G, Smith K, Vargas G, Houston J, et al. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(6):1009‐18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakova T, Barchi‐Chung A, Enfield G, Smith KT, Vargas GS, Houston J, et al. Effects of dietary phosphate restriction and lanthanum carbonate on fibroblast growth factor 23 in chronic kidney disease [abstract no: FR‐OR068]. Journal of the American Society of Nephrology 2012;23:45A. [Google Scholar]
Li 2011c {published data only}
- Li H, Long Q, Shao C, Fan H, Yuan L, Huang B, et al. Effect of short‐term low‐protein diet supplemented with keto acids on hyperphosphatemia in maintenance hemodialysis patients. Blood Purification 2011;31(1‐3):33‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lou 2012 {published data only}
- Garcia‐Mena M, Caverni A, Miguel Lou L, Gimeno JA, Perez J, Alvarez R, et al. Dietary intervention focused on phosphate intake in hemodialysis patients with hyperphosphoremia [abstract]. NDT Plus 2010;3:iii512. [EMBASE: 70484082] [PubMed] [Google Scholar]
- Lou LM, Caverni A, Gimeno JA, Moreno R, Perez J, Alvarez R, et al. Dietary intervention focused on phosphate intake in hemodialysis patients with hyperphosphoremia. Clinical Nephrology 2012;77(6):476‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Sharma 2002c {published data only}
- Sharma M, Rao M, Jacob S, Jacob CK. A controlled trial of intermittent enteral nutrient supplementation in maintenance hemodialysis patients. Journal of Renal Nutrition 2002;12(4):229‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sullivan 2009 {published data only}
- Sullivan C, Sayre SS, Leon JB, Machekano R, Love TE, Porter D, et al. Effect of food additives on hyperphosphatemia among patients with end‐stage renal disease: a randomized controlled trial. JAMA 2009;301(6):629‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12611000500954 {published data only}
- Tan KS. The effect of slow release nicotinic acid on serum phosphorus in dialysis patients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000500954 (accessed 27 August 2015).
Ambrus 2003 {published data only}
- Ambrus C, Marton A, Almasi C, Berta K, Deak G, Horvath C, et al. Native vitamin D supplementation may slow down bone loss in patients on maintenance hemodialysis [abstract no: SU‐FC219]. Journal of the American Society of Nephrology 2003;14:48A. [CENTRAL: CN‐00550502] [Google Scholar]
- Marton A, Ambrus C, Almasi C, Berta k, Csaktornyai E, Vezekenyi Z, et al. Effects of native Vitamin D supplementation in patients on maintenance hemodialysis [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):146. [CENTRAL: CN‐00446637] [Google Scholar]
Ashurst 2003 {published data only}
- Ashurst Ide B, Dobbie H. A randomized controlled trial of an educational intervention to improve phosphate levels in hemodialysis patients. Journal of Renal Nutrition 2003;13(4):267‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheng 2008 {published data only}
- Cheng SC, Young DO, Delmez JA, Coyne DW. The effect of oral niacinamide on serum phosphorus levels in hemodialysis patients [abstract no: PUB430]. Journal of the American Society of Nephrology 2007;18(Abstracts):924A. [Google Scholar]
- Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW. A randomized, double‐blind, placebo‐controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(4):1131‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chertow 2003 {published data only}
- Chertow GM. Slowing the progression of vascular calcification in hemodialysis. Journal of the American Society of Nephrology 2003;14(Suppl 4):S310‐4. [DOI] [PubMed] [Google Scholar]
Clark 2010 {published data only}
- Clark B, Patel A, Groves‐Seaman L, Palombine J, Marcus R. Strategies to improve phosphorus control in patients with chronic severe hyperphosphatemia [abstract]. American Journal of Kidney Diseases 2010;55(4):A49. [EMBASE: 70124728] [Google Scholar]
Moe 2011 {published data only}
- Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(2):257‐64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morey 2008 {published data only}
- Morey B, Walker R, Davenport A. More dietetic time, better outcome? A randomized prospective study investigating the effect of more dietetic time on phosphate control in end‐stage kidney failure haemodialysis patients. Nephron 2008;109(3):c173‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT01665651 {published data only}
- Wang Y. Effect of kidney yin/yang replenishment on bone metabolism in patients with renal osteodystrophy. www.clinicaltrials.gov/ct2/show/NCT01665651 (accessed 27 August 2015).
Olivero 2006 {published data only}
- Olivero J, Garney P, Armentrout B, Mays L, Holland R. Niacin used for the treatment of hyperphosphatemia in hemodialysis patients [abstract no: F‐PO104]. Journal of the American Society of Nephrology 2006;17(Abstracts):358A. [Google Scholar]
Padhi 2007 {published data only}
- Padhi D, Salfi M, Harris RZ. The pharmacokinetics of cinacalcet are unaffected following consumption of high‐ and low‐fat meals. American Journal of Therapeutics 2007;14(3):235‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spiegel 2012 {published data only}
- Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low‐ and high‐calcium diets. Kidney International 2012;81(11):1116‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2009a {published data only}
- Young DO, Cheng SC, Delmez JA, Coyne DW. The effect of oral niacinamide on plasma phosphorus levels in peritoneal dialysis patients. Peritoneal Dialysis International 2009;29(5):562‐7. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Akizawa 2014a {published data only}
- Akizawa T, Tsuruta Y, Okada Y, Miyauchi Y, Suda A, Kasahara H, et al. Effect of chitosan chewing gum on reducing serum phosphorus in hemodialysis patients: a multi‐center, randomized, double‐blind, placebo‐controlled trial. BMC Nephrology 2014;15(1):98. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Block 2013 {published data only}
- Block GA, Persky MS, Shamblin BM, Baltazar MF, Singh B, Sharma A, et al. Effect of salivary phosphate‐binding chewing gum on serum phosphorus in chronic kidney disease. Nephron 2013;123(1‐2):93‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garini 1992 {published data only}
- Garini G, Arisi L, Borrini A, Fiaccadori E, Guariglia A, Magnati G, et al. Adherence to dietetic treatment, the nutritional metabolic status and the progression of chronic kidney failure. Annali Italiani di Medicina Interna 1992;7(2):71‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Hill 2013 {published data only}
- Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3‐4 chronic kidney disease. Kidney International 2013;83(5):959‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karavetian 2012 {published data only}
- Karavetian M, Vries N, Elzein H. Nutritional intervention for management of osteodystrophy (NIMO) program in hemodialysis patients, Lebanon and baseline data [abstract no: 107]. Kidney Research & Clinical Practice 2012;31(2):A43. [EMBASE: 70814816] [Google Scholar]
Karavetian 2013 {published data only}
- Karavetian M, Ghaddar S. Nutritional education for the management of osteodystrophy (NEMO) in patients on haemodialysis: a randomised controlled trial. Journal of Renal Care 2013;39(1):19‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saade A, Karavetian M, ElZein H, Vries N. Nutritional education for management of osteodystrophy (NEMO) trial: Impact on quality of life [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i119. [EMBASE: 71075331] [Google Scholar]
References to ongoing studies
NCT00755690 {published data only}
- Levin A. Study of dietary phosphate and mineral homeostasis in early chronic kidney disease. www.clinicaltrials.gov/ct2/show/NCT00755690 (accessed 27 August 2015).
NCT01865526 {published data only}
- Riccio E. Effects of the 6‐point diet on the metabolic control, the compliance and the nutritional status of CKD Patients Stage 3b‐5. www.clinicaltrials.gov/ct2/show/NCT01865526 (accessed 27 August 2015).
NCT02005302 {published data only}
- Liu W. Study of vitamin D2 virus 1,25(OH)2‐vitamin D3 and normal protein diet virus low protein diet in the treatment of CKD‐MBD and malnutrition for progressive CKD. www.clinicaltrials.gov/ct2/show/NCT02005302 (accessed 27 August 2015).
Additional references
Bellorin‐Font 2013
- Bellorin‐Font E, Ambrosoni P, Carlini RG, Carvalho AB, Correa‐Rotter R, Cueto‐Manzano A, et al. Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation and Treatment of Mineral and Bone Disorders in Chronic Kidney Disease (CKD‐MBD) in Adults. Nefrología 2013;33 Suppl 1:1‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cianciaruso 2008
- Cianciaruso B, Pota A, Pisani A, Torraca S, Annecchini R, Lombardi P, et al. Metabolic effects of two low protein diets in chronic kidney disease stage 4‐5‐‐a randomized controlled trial. Nephrology Dialysis Transplantation 2008;23(2):636‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cupisti 2013
- Cupisti A, Gallieni M, Rizzo MA, Caria S, Meola M, Bolasco P. Phosphate control in dialysis. International Journal of Nephrology & Renovascular Disease 2013;6:193‐205. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Iorio 2012
- Iorio B, Micco L, Torraca S, Sirico ML, Russo L, Pota A, et al. Acute effects of very‐low‐protein diet on FGF23 levels: a randomized study. Clinical Journal of The American Society of Nephrology: CJASN 2012;7(4):581‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fouque 2009
- Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001892.pub3] [DOI] [PubMed] [Google Scholar]
Goldsmith 2010
- Goldsmith DJ, Covic A, Fouque D, Locatelli F, Olgaard K, Rodriguez M, et al. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) Chronic Kidney Disease–Mineral and Bone Disorder (CKD‐MBD) Guidelines: a European Renal Best Practice (ERBP) commentary statement. Nephrology Dialysis Transplantation 2010;25:3823‐31. [DOI: 10.1093/ndt/gfq513] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnson 2006
- Johnson DW. Dietary protein restriction as a treatment for slowing chronic kidney disease progression: the case against. Nephrology 2006;11(1):58‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2009
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD‐MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease‐mineral and bone disorder (CKD‐MBD). Kidney International ‐ Supplement 2009, (113):S1‐130. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDOQI 2003
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease In chronic kidney disease. American Journal of Kidney Diseases 2003;42(4 Suppl 3):S1‐201. [MEDLINE: ] [PubMed] [Google Scholar]
Klahr 1994
- Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood pressure control on the progression of chronic renal disease. New England Journal of Medicine 1994;330(13):877‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Komaba 2008
- Komaba H, Tanaka M, Fukagawa M. Treatment of chronic kidney disease‐mineral and bone disorder (CKD‐MBD). Internal Medicine 2008;47(11):989‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuro‐O 2011
- Kuro‐O M. Phosphate and Klotho. Kidney International ‐ Supplement 2011, (121):S20‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCrory 1987
- McCrory WW, Gertner JM, Burke FM, Pimental CT, Nemery RL. Effects of dietary phosphate restriction In children with chronic renal failure. Journal of Pediatrics 1987;111(3):410‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mejía 2011
- Mejía N, Roman‐García P, Miar AB, Tavira B, Cannata‐Andía JB. Chronic kidney disease‐mineral and bone disorder: a complex scenario. Nefrologia 2011;31(5):514‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moe 2006
- Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney International 2006;69(11):1945‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moe 2007
- Moe SM, Drüeke T, Lameire N, Eknoyan G. Chronic kidney disease‐mineral bone disorder: a new paradigm. Advances In Chronic Kidney Disease 2007;14(1):3‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navaneethan 2009
- Navaneethan S, Palmer SC, Schreiber MJ, Strippoli GF. Weighing the evidence for treatment of chronic kidney disease mineral and bone disorder. Nephrology 2009;14(4):363‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pelletier 2010
- Pelletier S, Roth H, Bouchet JL, Drüeke T, London G, Fouque D, et al. Mineral and bone disease pattern in elderly haemodialysis patients. Nephrology Dialysis Transplantation 2010;25(9):3062‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Razzaque 2011
- Razzaque MS. Osteo‐renal regulation of systemic phosphate metabolism. IUBMB Life 2011;63(4):240‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slatopolsky 1973
- Slatopolsky E, Bricker NS. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism In chronic renal disease. Kidney International 1973;4(2):141. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Soroka 1998
- Soroka N, Silverberg DS, Greemland M, Birk Y, Blum M, Peer G, et al. Comparison of a vegetable‐based (soya) and an animal‐based low‐protein diet in predialysis chronic renal failure patients. Nephron 1998;79(2):173‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Takeda 2007
- Takeda E, Yamamoto H, Nishida Y, Sato T, Sawada N, Taketani Y. Phosphate restriction In diet therapy. Contributions to Nephrology 2007;155:113‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tentori 2008
- Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). American Journal of Kidney Diseases 2008;52(3):519‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liu 2013
- Liu Z, Su G, Guo XF, Wu Y, Liu X, Zou C, et al. Dietary interventions for mineral and bone disorder in people with chronic kidney disease. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010350] [DOI] [PMC free article] [PubMed] [Google Scholar]


