Abstract
Astrocytes activation in response to stroke results in altered mitochondrial exchange with neurons. Ginsenoside Rb1is a major ginsenoside of Panax ginseng particularly known for its neuroprotective potential. This work aimed to investigate if Rb1 could rescue neurons from ischemic insult via astrocyte inactivation and mitochondrial transfer. We prepared conditioned astrocytes-derived medium for co-culture with neurons and examined the role of Rb1 in mitochondrial transfer from astrocytes to neurons. The neuroprotective potential of Rb1 was further confirmed in vivo using a mouse model of brain ischemia. In response to oxygen-glucose deprivation and reperfusion (OGD/R), astrocytes were reactivated and produced reactive oxygen species (ROS), an action that was blocked by Rb1. Mechanistically, Rb1 inhibited NADH dehydrogenase in mitochondrial complex I to block reverse electron transport-derived ROS production from complex I, and thus inactivated astrocytes to protect the mitochondria. Mitochondrial signal, mitochondrial membrane potential and ATP production detected in conditioned astrocyte-derived medium indicated that Rb1 protected functional mitochondria and facilitated their transfer. When neurons were injured by OGD/R insult, co-culturing with conditioned medium increased mitochondrial membrane potential and oxygen consumption rate within the neurons, indicating the protection conferred on them by Rb1 via mitochondrial transfer from astrocytes. Using the ischemic mouse brain model, CD38 knockdown in the cerebral ventricles diminished the neuroprotective effects of Rb1, providing evidence in support of the role of astrocyte mitochondrial transfer. Transient inhibition of mitochondrial complex I by Rb1 reduced mitochondrial ROS production and consequently avoided astrocyte activation. Astrocyte mitochondrial transfer therefore seemed a means by which Rb1 could promote neuronal survival and function. Different from the neurocentric view, these findings suggest the astrocytes may be a promising target for pharmacological interventions in ischemic brain injury.
Keywords: Astrocyte reactivity, Ginsenoside Rb1, Mitochondrial transfer, Stroke
Abbreviations
- GFAP
glial fibrillary acidic protein
- GS
glutamine synthetase
- LDH
lactate dehydrogenase
- NAC
N-acetyl-l-cysteine
- OCR
oxygen consumption rate
- OGD/R
oxygen-glucose deprivation and reperfusion
- Rb1
ginsenoside Rb1
- RET
reverse electron transfer
- ROS
reactive oxygen species
1. Introduction
The brain has high energy requirement and neurons sustain a high rate of mitochondrial oxidation to fulfill their energy demands. The mitochondria are dynamic organelles that provide energy, coordinate the redox state, buffer intracellular calcium levels, and consolidate survival and death cues to ensure neuronal function [1]. In cerebral ischemia, oxygen supply to the mitochondria is impaired, resulting in production of excessive reactive oxygen species (ROS) in the neurons [2]. Mitochondrial dysfunction is a leading cause of brain injury [3,4]. Results of preclinical evaluations point to neuroprotection as a credible approach to treating acute ischemic stroke [5]. However, this neurocentric view is challenged by the fact that the strategy designed only for neuroprotection fails to provide dramatic benefits in clinical trials [5]. One reason for this is that, neurons are highly differentiated cells, and their limited capability to upregulate glycolysis and to combat oxidative stress renders them more vulnerable to ischemic injury.
In the brain, neurons structurally interact with glial cells. Astrocytes are the most abundant cells that support neuronal survival and functions from different physiological perspectives. Toxic lipid peroxides produced in neurons are transferred to astrocytes wherein these lipids are detoxified by mitochondrial oxidation [6]. Glutamate is an excitatory neurotransmitter released from the neurons and converted to glutamine by the astrocytes. The glutamate-glutamine cycle thus functions to protect the neurons from excitoxicity, especially in the setting of ischemic stroke [7,8]. In addition, astrocytes maintain ionic and osmotic homeostasis, regulate metabolism of neurotransmitters, and provide antioxidant defense in the brain [9]. In these contexts, manipulation of astrocytes is likely to be a more promising therapeutic approach to attenuate ischemic brain injury.
In response to stroke, astrocytes assume a distinct phenotype in terms of morphology, proliferation and gene expression, known as “reactive”, a process termed as reactive astrogliosis [10,11]. The reactive astrocytes are characterized by phenotype change and increased expression of glial fibrillary acidic protein (GFAP) [12]. Astrocyte reactivity occurs in diverse nervous disorders as an early response to injury, and is detectable even in the absence of overt neuronal death [13]. Although reactive astrocytes constitute a border between a focal lesion and surronding tissues, astrocytes in general are increasingly viewed as playing a critical role in neurological disorders [10,13]. Activated microglia secrete IL-1α, TNF-α, and C1q to induce neurotoxic reactive astrocytes, which is indicative of inflammation in astrocyte reactivity [14]. In line with this observation, reactive astrocytes have been shown to amplify ischemic injury via ROS-associated inflammation [15]. These events indicate the impact of astrocyte reactivity on neuronal injury. Neurons can release and transfer damaged mitochondria to astrocytes for disposal and recycling [16]. A recent study showed that, astrocytes can transfer mitochondria to neurons and improve mitochondrial function against cerebral ischemic injury [17]. This finding not only provides new insight into cellular communication within the brain, but also addresses the role of astrocytes in neuroprotection.
Ginsenosides are the main bioactive components of Panax ginseng C. A. Mey, which are widely used for the treatment of diverse cardiovascular diseases. Ginsenoside Rb1(Rb1) is one of the most abundant ginsenosides, with known neuroprotective effects against ischemic brain injury due to its anti-inflammatory, anti-oxidative, and anti-apoptosis effects [18]. We recently reported that by inhibiting NADH dehydrogenase of mitochondrial complex I, Rb1 suppressed ROS production from mitochondria to protect the heart against ischemic insult [19]. Because mitochondrial oxidation is essential for neuronal survival, we theorized that Rb1 might ameliorate mitochondrial dysfunction by combating mitochondrial oxidative stress. We show here that although astrocytes could transfer mitochondria to neurons to confer neuroprotection, the transfer was within the context of maintaining functional mitochondrial integrity. Upon ischemic insult, Rb1 blocked ROS production from the mitochondria to restrain astrocyte reactivity, and ensured functional mitochondrial transfer to neurons. These results indicate that the astrocytes might be an important strategic target for pharmacological interventions aimed at protecting the neurons.
2. Materials and methods
2.1. Materials
Ginsenoside Rb1 (B21050), oligomycin A (S48601) and carbonyl cyanide 3-chlorophenylhydrazone (CCCP, R30080) were obtained from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Rotenone (R105076) was the product of Aladdin Co., Ltd. (Shanghai, China). Dimethyl succinate (492248) and N-acetyl-l-cysteine (NAC, ST1546) were from Sigma-Aldrich (St Louis, MO, USA) and Beyotime Institute of Biotechnology (Shanghai, China), respectively. BAPTA-AM (S7534) was provided by Selleck Chemicals (Houston, TX, USA).
2.2. Animals and treatments
C57BL/6J male mice (6–8 weeks old) were purchased from GemPharmatech (Nanjing, China) and housed in colony cages under 12 h light/dark cycles with free access to standard food and water. Animal care and treatments, as well as the experimental procedures were approved by the Animal Ethics Committee of China Pharmaceutical University.
2.3. Photochemical cerebral thrombosis in mice
For CD38 knockdown in the brain, lateral ventricle injection was performed according to a previous study [17]. Briefly, CD38 siRNA or negative control (NC) siRNA were stereotactically injected slowly into the right lateral ventricle of the mice with the aid of a brain locator. 2 days later, anesthetized mice were placed in a stereotaxic apparatus and their skulls exposed after incising the midline and removing the periosteum. A cold light source (11500 lux) converged by a 2-mm-diameter fiber optic bundle was placed in the position of 1.5 mm to the right of the bregma. After injection of rose bengal (100 mg/kg, i.p.) for 5 min, the indicated position on the skull was illuminated for 15 min. Mice in the sham group received the same surgical procedures, but received 0.9% saline instead of rose bengal. Rb1(100 mg/kg, i.p.) was administered 30 min before the surgery and then administered for another 5 consecutive days. The CD38 siRNA and negative control (NC) siRNA were purchased from Gene Pharma (Shanghai, China). The sequences for mouse CD38 siRNAs were designed as follows; Sequence 1: 5′-GGGCUA CAUUGC UGA UGA UTT-3′, Sequence 2: 5′-CCCAUCGUGUAG ACU UAAUTT-3′, Sequence 3: 5′- GGACCCAAAUAAGGUUCAUTT-3′.
2.4. Cell preparation and culture
Primary astrocytes were prepared from cerebral cortices of 1-day-old neonatal Sprague-Dawley rats. Briefly, cerebral cortices were isolated and digested with 0.25% trypsin at 37 °C for 15 min followed by filtering through a 70 μm cell strainer. The isolated cells were cultured in Dulbecco's modified Eagle medium for 9–11 days until they reached 80% confluence. The astrocytes were identified by GFAP staining to ensure purity.
Primary neurons were prepared from cerebral cortices of 1-day-old neonatal Sprague-Dawley rats. Briefly, the isolated cerebral cortices were minced into small pieces and then digested with 0.16% papain at 37 °C for 30 min. After centrifugation, the harvested cell pellets were resuspended with F12/DMEM supplemented with 10% (v/v) FBS. Four hours later, the culture was replaced with Neurobasal medium (Gibco, 21103–049) supplemented with 2% B27 (Gibco, 17504044) and 10% (v/v) FBS. Cultures were used for experiments from 7 to 10 days after seeding. The purity of cultured neurons was determined by immunostaining for the neuron-specific marker microtubule-associated protein 2.
To mimic ischemia and reperfusion injury during stroke, cells were pretreated with indicated agents at given concentrations and then subjected to hypoxia in a hypoxia incubator chamber in which O2 was replaced by N2 (37 °C, 1% O2, 5% CO2, and 94% N2) for 4 h in glucose-free DMEM (Gibco, 11966), followed by 1 h reoxygenation with glucose supplement. Astrocyte-derived medium was collected as conditioned medium for the incubation of neurons. With reference to our previous study [19], to mimic reverse electron transfer (RET)-ROS in astrocytes, cells were cultured in assay buffer (132 mM NaCl; 10 mM HEPES; 4.2 mM KCl; 1 mM MgCl2; 1 mM CaCl2; 25 μM 2-deoxyglucose; 10 mg/L sodium pyruvate; pH 7.4) in the presence or absence of dimethyl succinate (5 mM) or oligomycin (4 μM) for 2 h.
For experiments with brain slices, the hippocampus of mouse was removed into the artificial cerebro-spinal fluid (ACSF, containing 125 mM NaCl, 3 mM KCl, 1.5 mM MgSO4, 26 mM NaHCO3, 2 mM CaCl2, 1.25 mM NaH2PO4 and 10 mM glucose). The isolated cerebral cortex/hippocampus was transversely sliced and incubated at 37 °C for 30 min before treatment. For oxygen-glucose deprivation/reperfusion (OGD/R) treatment, the brain slices were pretreated with indicated agents and subjected to hypoxia (1% O2) for 4 h in glucose-free ACSF, followed by 1 h reoxygenation in ACSF. 8-hydroxy-2' -deoxyguanosine(8-OHdG) content in the tissues was measured using commercial Kit.
To prepare the conditioned astrocytes-derived medium, the astrocytes were subjected to OGD/R (4/1 h) insult with or without Rb1 or NAC treatment. After washing, astrocytes were re-cultured in fresh medium for another 5 h, and this medium was collected as conditioned medium. For CCCP treatment, the astrocytes were treated first with CCCP for 4 h. After washing, the cells were re-cultured in fresh medium for another 5 h to collect conditioned medium.
2.5. ROS production assay
For cytosolic ROS detection, treated primary astrocytes were loaded with 10 μM DCFH-DA (Beyotime, S0033S) at 37 °C for 30 min. Cells were then washed with PBS three times before scanned by a microplate reader. For mitochondrial ROS detection in astrocytes, cells were loaded with 5 μM MitoSOX™ Red (Invitrogen, M36008) for 10 min and 200 nM Mito-Tracker Green (Beyotime, C1048) at 37 °C for 30 min. To visualize ROS production in the brain tissues, the slices were stained with 10 μM DHE probe (S0063) and DAPI dye (Beyotime, C1006). Images were viewed with a confocal scanning microscope.
2.6. Measurement of intracellular calcium, ATP, GSH, NADPH and mitochondrial complex I activity and released glutamate and LDH in the medium
Cells were loaded with 2 μM Fura-2 AM (Beyotime, S1052) at 37 °C for 30 min, and intracellular calcium contents were determined by scanning with a microplate reader after washing. Intracellular ATP, GSH and NADPH levels and mitochondrial complex I activity were measured using commercial Kits. After treatment, the medium was collected for the assay of the released glutamate and lactate dehydrogenase (LDH) activity using commercial Kits.
2.7. Mitochondrial membrane potential (Δψm), mitochondrial mass and mitochondrial translocation
For Δψm assay, treated cells were loaded with the potentiometric dye 500 nM TMRE (Beyotime, C2001S) at 37 °C for 20 min and the staining was viewed by a confocal scanning microscope after washing. To measure Δψm in extracellular particles, astrocyte-conditioned medium were collected and large debris were excluded by centrifugation at 2000 g for 10 min. Each 100 μl supernatant was added into an opaque-walled 96-well plate and determined by a microplate reader.
For the mitochondrial mass fission, cells were loaded with 200 nM Mito Tracker Red CMXRos (Beyotime, C1035) at 37 °C for 20 min. Cells were then washed with PBS three times before viewed with a confocal scanning microscope, while mitochondrial mass was stained with 200 nM Mito-Tracker Green (Beyotime, C1048), to label mitochondria in a membrane potential-independent manner at 37 °C for 30 min in darkness.
For mitochondrial translocation detection, untreated astrocytes were dyed with 200 nM MitoTracker Red CMXRos (Beyotime, C1035) at 37 °C for 30 min in darkness. Then astrocytes were subjected to OGD/R with or without reagent treatment to prepare the conditioned medium. Neurons were incubated with astrocyte-conditioned medium, washed with PBS three times before viewed with a confocal scanning microscope.
2.8. Flow cytometry analysis of mitochondrial signal
To quantify mitochondrial number, astrocytes were dyed with 200 nM MitoTracker Green (Beyotime, C1048) at 37 °C for 30 min in darkness. Then astrocytes were washed with PBS five times. After being subjected to hypoxia for 4 h followed by reperfusion for 1 h with or without ginsenoside Rb1 or NAC treatment, the supernatant were collected and cell debris were excluded by centrifugation at 20,000 g for 30 min. Each 500 μl fractions from the bottom were used for labeled mitochondrial fraction by flow cytometry (Beckman Coulter, Inc., USA). Flow cytometry analysis was performed using an unstained control to determine appropriate gates, voltages, and compensations required in multivariate flow cytometry.
2.9. Oxygen consumption rate (OCR) measurements
Neurons were seeded at an equal density of 10,000 cells/well and incubated in neurobasal medium. The cells were subjected to hypoxia (1% O2) for 4 h and reoxygenated in conditioned astrocyte-derived medium for 18 h. After washing, neurons were incubated with 10 mM glucose, 1 mM pyruvate and 2 mM l-glutamine -supplemented XF base medium minimal DMEM at 37 °C in a CO2-free incubator for 1 h for the detection of OCR with seahorse XFe96, and the results were analyzed using Wave 2.6.1 software.
2.10. Quantitative real-time PCR
Total RNA was isolated using Trizol reagent (Yeasen, 19201ES60) following the manufacturer's instructions. RNA samples were reverse transcribed to cDNA. The relative gene expression was relatively quantified by Hieff™ qPCR SYBR Green Master Mix (No Rox Plus) kit (Yeasen, 11201ES08) with CFX96TM Realtime system (BIO-RAD, USA). The qPCR primers used in this study are shown in Table S1 of the Supplementary Materials. The 2−ΔΔCt method was used for relative quantification of the target gene.
2.11. Western blot analysis
Primary astrocytes or brain tissues were lysed to collect proteins. Equal amounts of proteins were electrophoresed on SDS-PAGE, transferred to a PVDF membrane, and then blocked at room temperature for 2 h. Membranes were incubated with primary antibodies against anti-GS (1:2000 dilution, Abcam, ab64613), anti-VDAC1 (1:1000 dilution, Abways Technology, CY5416), anti-FLAG (1:8000 dilution, Bioworld Technology, AP0007 M) and anti-GAPDH (1:8000 dilution, Bioworld Technology, AP0063). After blotted with the primary antibodies at 4 °C overnight, all the membranes were incubated for 2 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibodies. The values of band intensities were detected by enhanced chemiluminescence (ECL) and quantized by Image-Pro Plus 6.0 software.
2.12. Transfection
NADH dehydrogenase (Ndi1) plasmid was provided by Genomeditech Biotechnology (Shanghai, China) and yeast genomic DNA was extracted from wild type Saccharomyces Cerevisiae. The Ndi1 gene was PCR-amplified from yeast genomic DNA using primers (F: 5′ TGTAAAACGACGGCCAGT 3′ and R: 5′ CCG CTCGAGTAATCCTTTAAAAAAGTCTCTTTTGAAAAATGCTAATTTAATCC3′). We employed Lipofectamine 3000 reagent (Invitrogen, L3000008) to transfect Ndi1 plasmid into primary astrocytes at 70–80% confluence and the efficiency was confirmed by immunoblotting. The transfected cells were cultured for 24 h and then exposed to OGD/R treatment.
2.13. Immunofluorescence
Treated primary astrocytes were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.2% Triton X-100 for 10min. After blocking with 3% BSA for 2 h, cells were incubated with anti-GFAP antibody (1:200 dilution, Beyotime, AF1177) overnight at 4 °C. After washing, the cells were incubated with Alexa Fluor 488 AffiniPure Goat Anti-Rabbit IgG (H + L) antibody (1:200 dilution, Yeasen, China) for 2 h at 37 °C. They were then washed in PBS three times and incubated in DAPI (Beyotime, C1006) for 15 min at 37 °C. Immunofluorescence signals were visualized by a confocal microscope (LSM 700, Zeiss, Germany).
The brains of the mice were taken out and fixed with 4% paraformaldehyde. After embedding the brain tissues with O.C.T. compound or paraffin, the slide was blocked with goat serum or 1% BSA before being incubated with the primary antibodies (anti-GFAP, 1:100, Abcam, ab53554 and anti-Iba1, 1:500, Abcam, ab178846) overnight at 4 °C in a humidified chamber. After washing, the goat anti-rabbit IgG H&L (FITC) (1:500, Jackson ImmunoResearch, 111-095-003) or donkey anti-goat IgG H&L (Alexa Fluor® 488) (1:500, Abcam, ab150129) was incubated at room temperature for 2 h. The slide was then stained with DAPI (Beyotime, C1006). Protein expression was observed by confocal microscopy.
2.14. Statistical analysis
The data were collected from at least three individual biological replicates and were expressed as the mean ± SD. The significance of differences was analyzed by one-way ANOVA followed by the Bonferroni test. Differences were considered statistically significant at p values of <0.05. Statistical analysis was performed using GraphPad Prism 6.0 software.
3. Results
3.1. Rb1 protected the brain from OGD/R injury
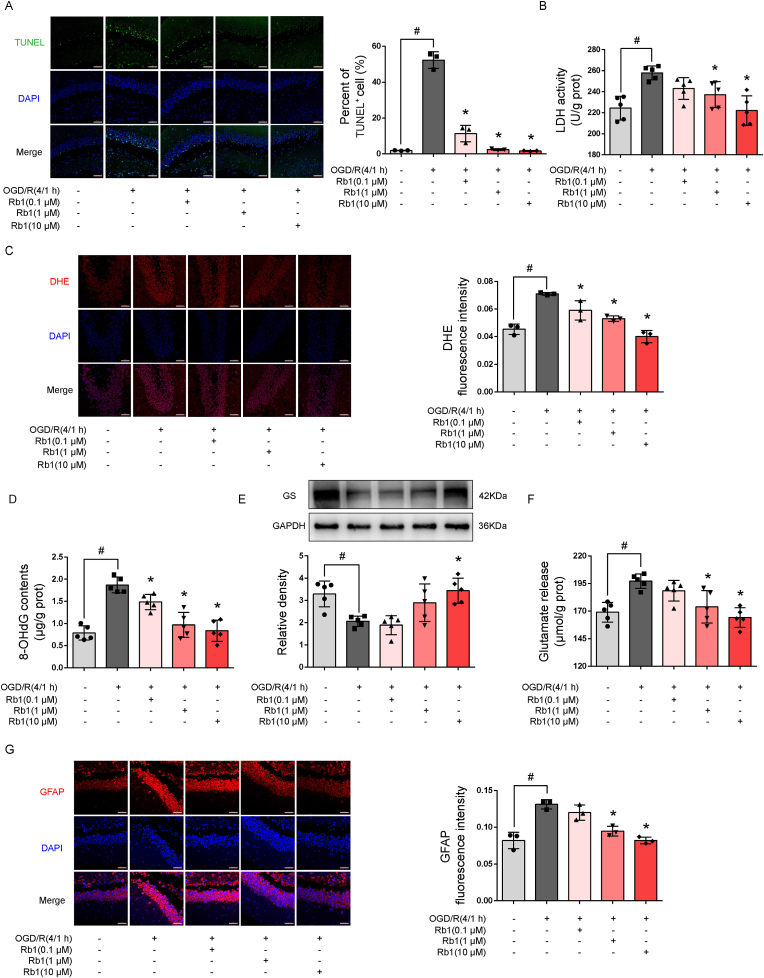
We first examined the effects of Rb1 on neuroprotection in slices of the hippocampus of mouse brain after expsure to OGD/R. Rb1 treatment promoted cell survival and reduced LDH release in a concentration-dependent manner (Fig. 1A and B). ROS production is an initial cause of ischemic brain injury. Rb1 suppressed ROS production and reduced the formation of 8-OHdG to attenuate oxidative damage (Fig. 1C and D). Cerebral ischemia induced glutamate release from neurons to evoke excitotoxicity, but Rb1 increased glutamine synthetase (GS) protein abundance with reduced glutamate accumulation in the medium (Fig. 1E and F), demonstrating its role to promote glutamate clearance, likely owing to improved astrocyte function. As a consequence, Rb1 suppressed astrocyte reactivity, evidenced by attenuated GFAP staining (Fig. 1G). Together, these results demonstrate that Rb1 attenuated brain injury and protected astrocytes.
Fig. 1.
Rb1 protected the brain against oxygen and glucose deprivation and reoxygenation injury (OGD/R). Slices of the hippocampus were exposed to oxygen and glucose deprivation for 4 h, followed by reoxygenation for 1 h. A. Representative pictures and quantification of TUNEL staining (one of three independent experiments, scale bar 50 μm); B. LDH released in the medium (n = 5); C. Representative pictures and quantification of intracellular ROS production (one of three independent experiments, scale bar 50 μm); D. 8-OHdG contents (n = 5); E. Immunoblot of glutamine synthetase (GS, n = 5); F. glutamate released in the medium (n = 5). G. Representative pictures and quantification of GFAP staining (one of three independent experiments, scale bar 50 μm). Data are presented as mean ± SD. *p < 0.05 vs.the OGD/R only treatment; #p < 0.05 vs. indicated treatments. OGD/R, oxygen glucose deprivation/reperfusion; Rb1, ginsenoside Rb1; GS, glutamine synthetase; GFAP, glial fibrillary acidic protein.
3.2. Rb1 inhibited astrocyte activation
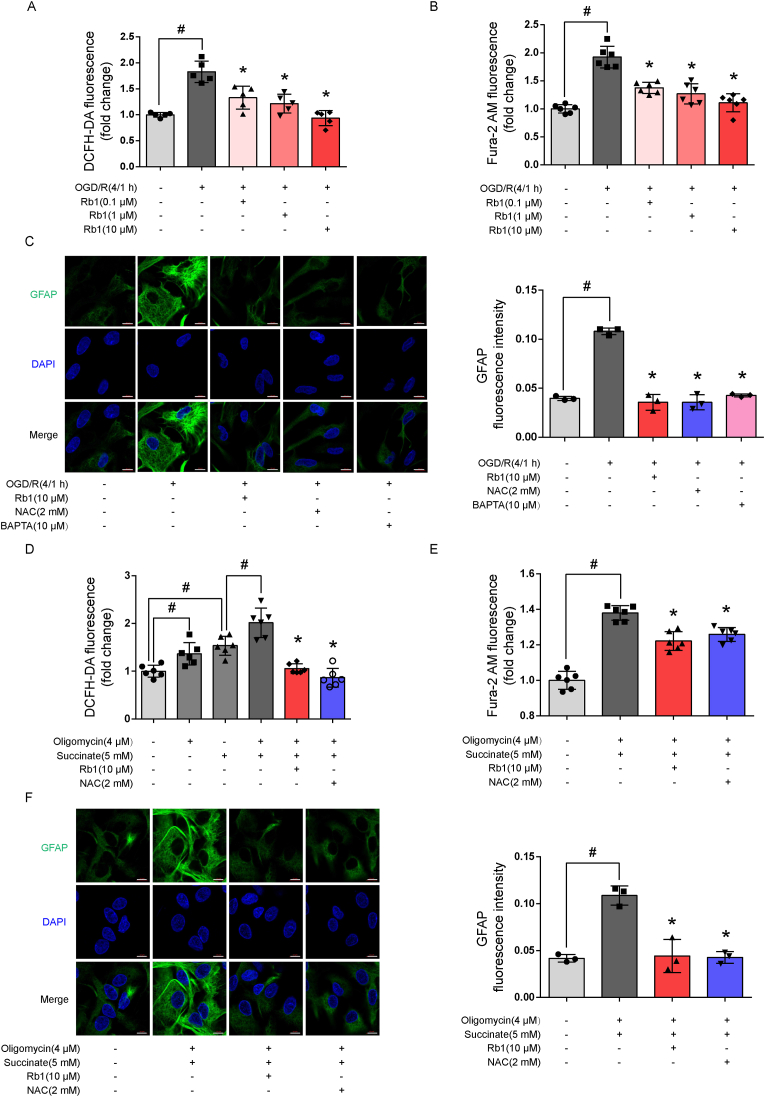
Astrocytes are key homeostatic cells in the brain, and their activation is an early response to injury. Rb1 reduced ROS production and calcium overload in a concentration-dependent manner (Fig. 2A and B), and thus inhibited astrocyte activation in response to OGD/R challenge (Fig. 2C). Similarly, ROS scavenger NAC (2 mM) and calcium chelator BAPTA (10 μM) suppressed GFAP protein induction (Fig. 2C). Our choice of the concentrations of the drugs was based on published literature [20,21]. These results indicate that the reactive astrocytes were associated with ROS production and calcium overload. When ATP production from mitochondria is inhibited during ischemia, succinate dehydrogenase (SDH) is activated to increase succinate accumulation [22]. Upon reperfusion, accumulated succinate drives ROS production from mitochondrial complex I through RET reaction [22]. To mimic ROS production from RET under ischemic conditions, we treated astrocytes with membrane-permeable dimethyl succinate to mimic ROS production from RET when ATP synthase was inhibited by oligomycin A. The results showed that Rb1 effectively suppressed intracellular ROS production in astrocytes exposed to succinate and oligomycin A (Fig. 2D). As expected, Rb1 reduced calcium overload and prevented astrocyte activation (Fig. 2E and F). Together, these results suggest that blocking RET-driven ROS production from the mitochondria might be a means for Rb1 to prevent astrocyte activation. In astrocytes cultured under basal conditions, Rb1, as well as NAC- and BAPTA-only treatments at given concentrations had no influence on ROS level, GFAP expression and did not impair cell survival, hence, excluding potential cytotoxicity in their inhibitory effects (Supple Fig. S1).
Fig. 2.
Rb1 inhibited astrocyte reactivity. A. Intracellular ROS production (n = 5); B. Intracellular calcium signal (n = 6); C. Representative pictures and quantification of GFAP staining in astrocytes (one of three independent experiments, scale bar 10 μm); D. ROS production in astrocytes exposed to succinate and oligomycin A (n = 6); E. Intracellular calcium signal in astrocytes (one of three independent experiments, scale bar 10 μm); F. Representative pictures and quantification of GFAP staining in astrocytes exposed to succinate and oligomycin A (one of three independent experiments, scale bar 10 μm). Data are presented as mean ± SD. *p < 0.05 vs. the OGD/R only treatment or succinate plus oligomycin A treatment; #p < 0.05 vs. indicated treatments. OGD/R, oxygen glucose deprivation/reperfusion; Rb1, ginsenoside Rb1; NAC, n-acetylcysteine; GFAP, glial fibrillary acidic protein.
3.3. Rb1 suppressed astrocyte activation via reversal inactivation of complex 1
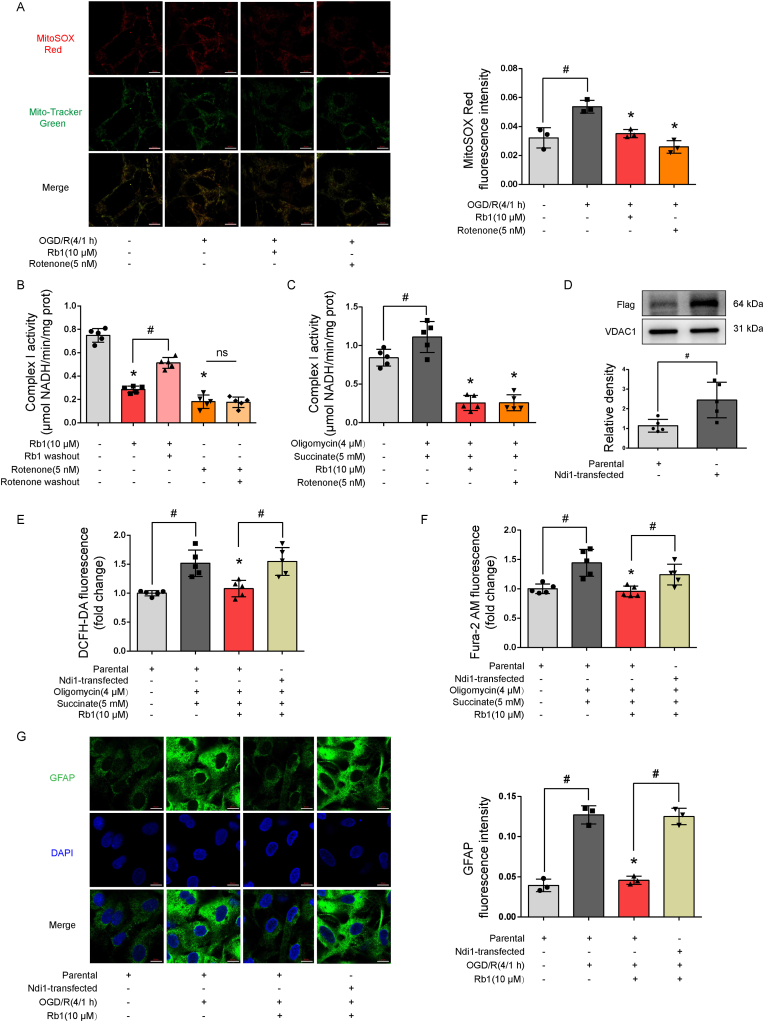
We have previously revealed that Rb1 transiently inhibits mitochondrial complex I in ischemic heart [19], an action that contributes to suppressing mitochondrial ROS production in the setting of oxygen deprivation. Similar to complex I inhibitor rotenone (5 nM, based on published literature [23]), Rb1 reduced ROS production from astrocyte mitochondria exposed to OGD/R (Fig. 3A). In astrocytes, we investigated the effect of Rb1 on mitochondrial complex I by assaying NADH dehydrogenase activity, and found that different from rotenone, Rb1 inhibited complex I activity in a manner that was reversible, as complex I activity was restored when Rb1 was removed (Fig. 3B), without potential neuronal cytotoxicity (Supple Fig. S2). Similarly, Rb1 effectively inhibited RET-driven complex I activation (Fig. 3C). We transfected astrocytes with gene encoding yeast NADH dehydrogenase Ndi1 (Fig. 3D), which is a single polypeptide that works as a replacement molecule for complex I to rescue ROS production by bypassing electron transport [24]. Indeed, the inhibitory effects of Rb1 on ROS production and calcium overload were diminished by Ndi1 expression (Fig. 3E and F). Moreover, suppression of astrocyte activation by Rb1 was reversed by Ndi1 expression (Fig. 3G). These results provided evidence in support of our assertion that Rb1 prevents astrocyte activation by inhibiting mitochondrial ROS production.
Fig. 3.
Rb1 inhibited mitochondrial complex I to prevent astrocytes reactivity. A. Representative pictures and quantification of mitochondrial ROS production in response to oxygen and glucose deprivation and reoxygenation (OGD/R)(one of three independent experiments, scale bar 10 μm); B, C. Mitochondrial complex I activity in astrocytes in the presence or absence of succinate and oligomycin A (n = 5); D. Immunoblot for the efficiency of Ndi1 transfection (n = 5); E, F. Intracellular ROS production and calcium in Ndi1-expressed astrocytes (n = 5); G. Representative pictures and quantification of GFAP staining in Ndi1-expressed astrocytes (one of three independent experiments, scale bar 10 μm). Data are presented as mean ± SD. *p < 0.05 vs. the untreated control or succinate plus oligomycin A treatment; #p < 0.05 vs. indicated treatments; ns: no significant difference. OGD/R, oxygen glucose deprivation/reperfusion; Rb1, ginsenoside Rb1.
3.4. Rb1 protected astrocyte function against ischemic insult
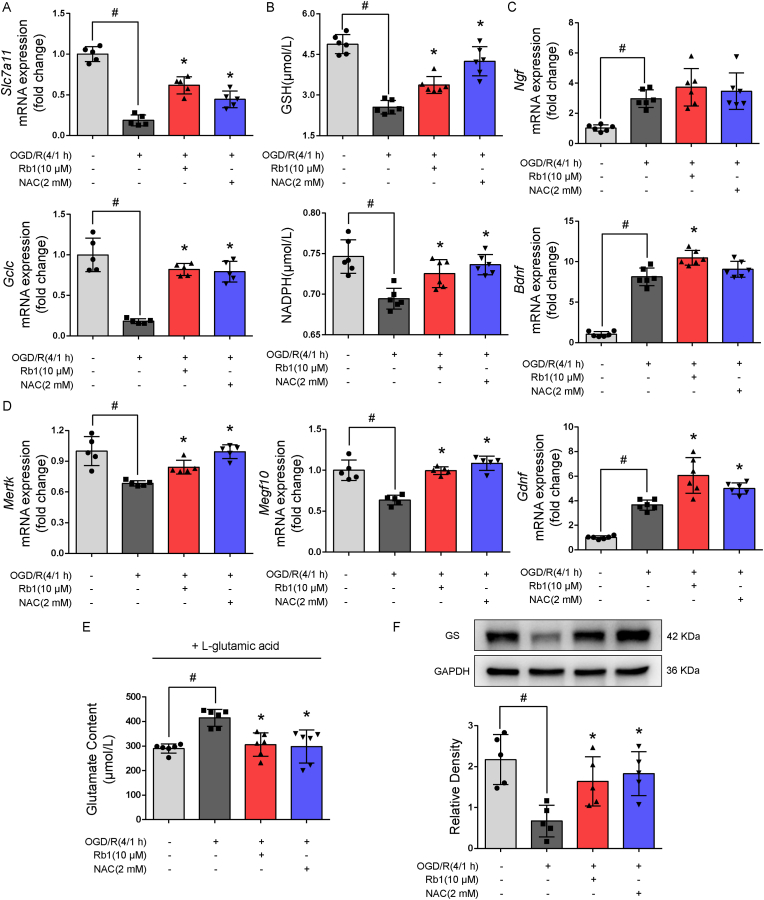
Astrocytes are the predominant source of glutathione (GSH) in the brain that play a key role in neuronal protection against oxidative stress. Slc7a11 is a gene encoding XC-antiporter that mediates the exchange of intracellular glutamate with extracellular cystine to provide substrates for GSH generation. GCLC is a catalytic subunit of glutamate-cysteine ligase that catalyzes glutathione synthesis. Rb1 preserved gene induction of Slc7a11 and Gclc (Fig. 4A), and thus increased GSH and NADPH contents against OGD/R insult in astrocytes (Fig. 4B), well demonstrating its anti-oxidative ability. Astrocytes generate neurotropic factors to support neurons. Gene induction of neurotropic factors was likely to be a compensatory response to ischemic injury, and Rb1 sought to promote this response (Fig. 4C). Meanwhile, Rb1 also effectively upregulated gene expressions of phagocytic factors, Mertk and Megf10 (Fig. 4D). After OGD/R treatment, astrocytes were re-cultured in glutamine-free medium with the addition of glutamate, and Rb1 promoted glutamate clearance (Fig. 4E), largely due to increased glutamate uptake and conversion to glutathione, since the GS protein expression was increased (Fig. 4F). Together, these results demonstrate that Rb1 protected astrocyte function against ischemic damage.
Fig. 4.
Rb1 protected astrocyte function. Astrocytes were exposed to oxygen and glucose deprivation for 4 h, followed by reoxygenation for 1 h (OGD/R). A. Gene expression of Slc7a11 and Gclc (n = 5); B. Intracellular GSH and NADPH contents (n = 6); C. Gene expression of neurotropic factors (n = 6); D. Gene expression of phagocytic factors, Mertk and Megf10 (n = 5); E. Glutamate contents in the medium (n = 6); F. Immunoblot of glutamine synthetase (GS, n = 5). Data are presented as mean ± SD. *p < 0.05 vs. the OGD/R only treatment; #p < 0.05 vs. indicated treatments. OGD/R, oxygen glucose deprivation/reperfusion; Rb1, ginsenoside Rb1; NAC, n-acetylcysteine; GS, glutamine synthetase.
3.5. Rb1 facilitated mitochondria translocation from astrocytes
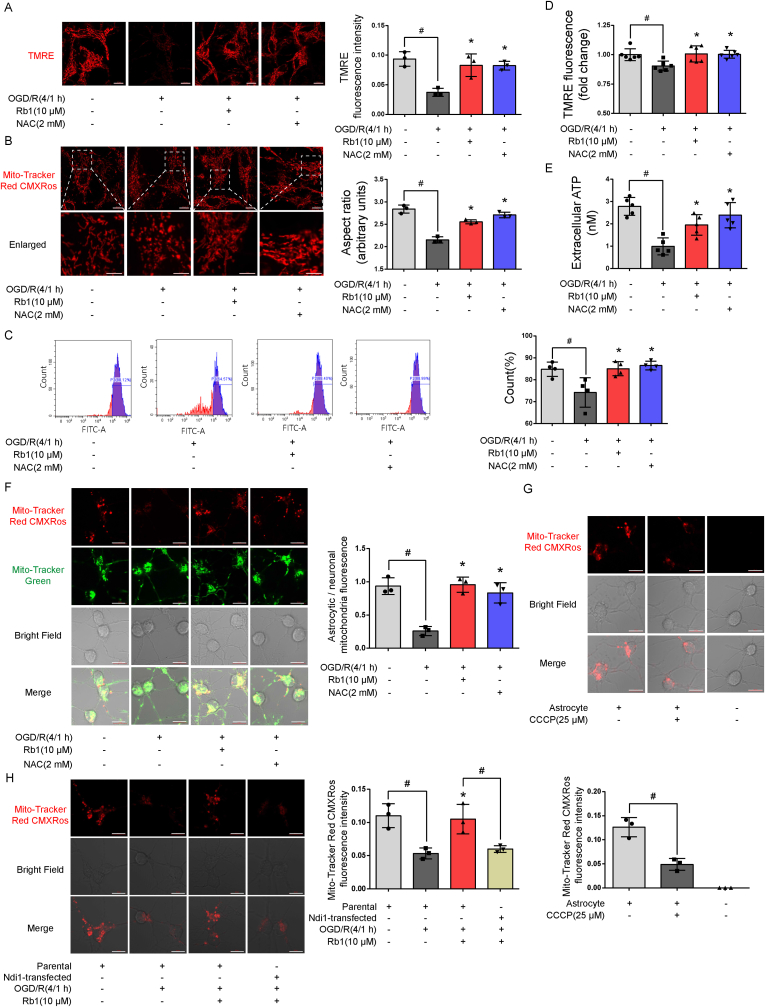
Rb1 protected mitochondrial membrane potential and prevented mitochondrial fragment against OGD/R insult, an indication of its ability to protect and maintain mitochondrial integrity (Fig. 5A and B). Neurons primarily rely on oxidative metabolism to support their function. Because inhibition of mitochondrial function in astrocytes makes neurons vulnerable to excitotoxicity [25], we explored whether promotion of mitochondria translocation from astrocytes is a potential mechanism by which Rb1 protects mitochondrial function within neurons. Mitochondria in astrocytes were labeled with MitoTracker probe. After washing, the astrocytes were re-cultured in fresh medium followed by exposure to OGD/R, and the medium was collected as conditioned medium. Mitochondrial signal was viewed with FACS in the collected medium; Rb1 and NAC increased mitochondria signal in the medium against OGD/R insult (Fig. 5C). In support of the transfer, Rb1 treatment rescued mitochondrial membrane potential and ATP contents in the medium collected (Fig. 5D and E). These results indicate that Rb1 protected astrocytes against I/R insult and facilitated the translocation of functional mitochondria. Neurons were incubated in conditioned astrocyte-derived medium and endogenous (neuronal, labeled with Mito-Tracker Green) and exogenous (astrocytic, labeled with Tracker Red CMXRos) mitochondrial signals observed within the neurons. Quantitative assessment of mitochondrial transfer showed that culture with conditional medium derived from OGD/R-treated astrocytes induced an approximate 3.0-fold decrease of astrocytic/neuronal mitochondrial signal (Fig. 5F). Rb1 protected astrocytes against OGD/R injury and effectively restored mitochondrial signal in neurons after co-culturing in conditioned medium (Fig. 5F). CCCP (25 μM, chosen based on published literature [26]) uncouples mitochondrial oxidation from phosphorylation and this treatment in astrocytes reduced neuronal mitochondrial signal (Fig. 5G), indicating that mitochondria translocation from astrocytes to neurons was within the context of maintaining functional integrity. ROS scavenger NAC showed similar results as Rb1, indicating that combating oxidative stress ensured mitochondria transfer. Indeed, Rb1 treatment in Ndi1 expressing astrocytes failed to promote mitochondrial transfer from astrocytes to neurons (Fig. 5H).
Fig. 5.
Rb1 facilitated mitochondrial translocation from astrocytes. A, B: Representative pictures and quantification of mitochondrial membrane potential (scale bar 10 μm) and structure (up, scale bar 10 μm; down, magnified images, scale bar 5 μm) in astrocytes subjected to oxygen and glucose deprivation (4 h) and reoxygenation (1 h) (OGD/R) (one of three independent experiments); C, D. Mitochondrial signal (n = 4) and mitochondrial membrane potential (n = 6) in astrocyte-conditioned medium; E. ATP generation in astrocyte-conditioned medium (n = 5); F, G, H. Representative pictures and quantification of mitochondrial signal in neurons cultured in conditioned medium after indicated treatments (one of three independent experiments, scale bar 10 μm); Data are presented as mean ± SD. *p < 0.05 vs. the OGD/R only treatment; #p < 0.05 vs. indicated treatments. OGD/R, oxygen glucose deprivation/reperfusion; Rb1, ginsenoside Rb1; NAC, n-acetylcysteine; TMRE, tetramethylrhodamine ethyl ester.
3.6. Rb1 protected neurons by manipulation of astrocytes
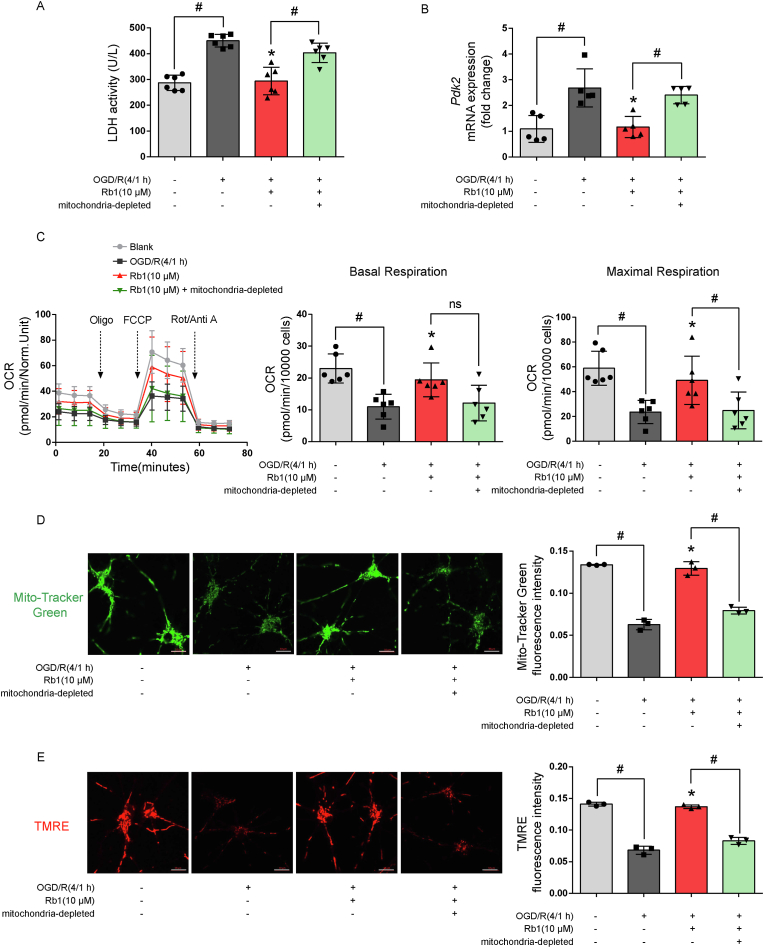
After exposure to oxygen and glucose depletion for 4 h, neurons were re-cultured in conditioned astrocyte-derived medium for another 18 h with reoxygenation. Co-culturing with conditioned Rb1-pretreated medium reduced LDH release from neurons. This effect was however lost when mitochondria were removed by filtering conditioned medium through 0.2 μm filters (Fig. 6A), indicating that mitochondria transfer from astrocytes conferred neuroprotection against OGD/R insult. OGD/R impaired mitochondrial oxidation in neurons, indicated by increasing PDK2 gene expression and reduced OCR, whereas these alternations were reversed by incubation with conditioned medium prepared from Rb1-treated astrocytes in a manner dependent on the mitochondria content (Fig. 6B and C). Consistently, culturing with conditioned medium increased astrocyte-mitochondrial signal and membrane potential within neurons in a similar pattern (Fig. 6D and E). Although astrocytes support neurons in diverse ways, these results demonstrate that facilitating mitochondrial translocation from astrocytes is a means by which Rb1 promotes neuronal recovery from ischemic injury.
Fig. 6.
Rb1 treatment in astrocytes conferred neuroprotection. After exposure to oxygen and glucose deprivation for 4 h, neurons were re-cultured in conditioned astrocyte-derived medium for 18 h with reoxygenation. A. LDH release from neurons (n = 6); B. Gene expression of Pdk2 in neurons (n = 5); C. Oxygen consumption rate (OCR) in neurons (n = 6); D, E. Representative pictures and quantification of mitochondrial signal and membrane potential in neurons (one of three independent experiments, scale bar 10 μm); Data are presented as mean ± SD. *p < 0.05 vs. the OGD/R only treatment; #p < 0.05 vs. indicated treatments; ns: no significant difference. OGD/R, oxygen glucose deprivation/reperfusion; Rb1, ginsenoside Rb1; Oligo, oligomycin; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; Rot, rotenone; Anti A, antimycin A; TMRE, tetramethylrhodamine ethyl ester.
3.7. Blocking mitochondrial translocation attenuated the neuroprotective effects of Rb1 against cerebral ischemic injury
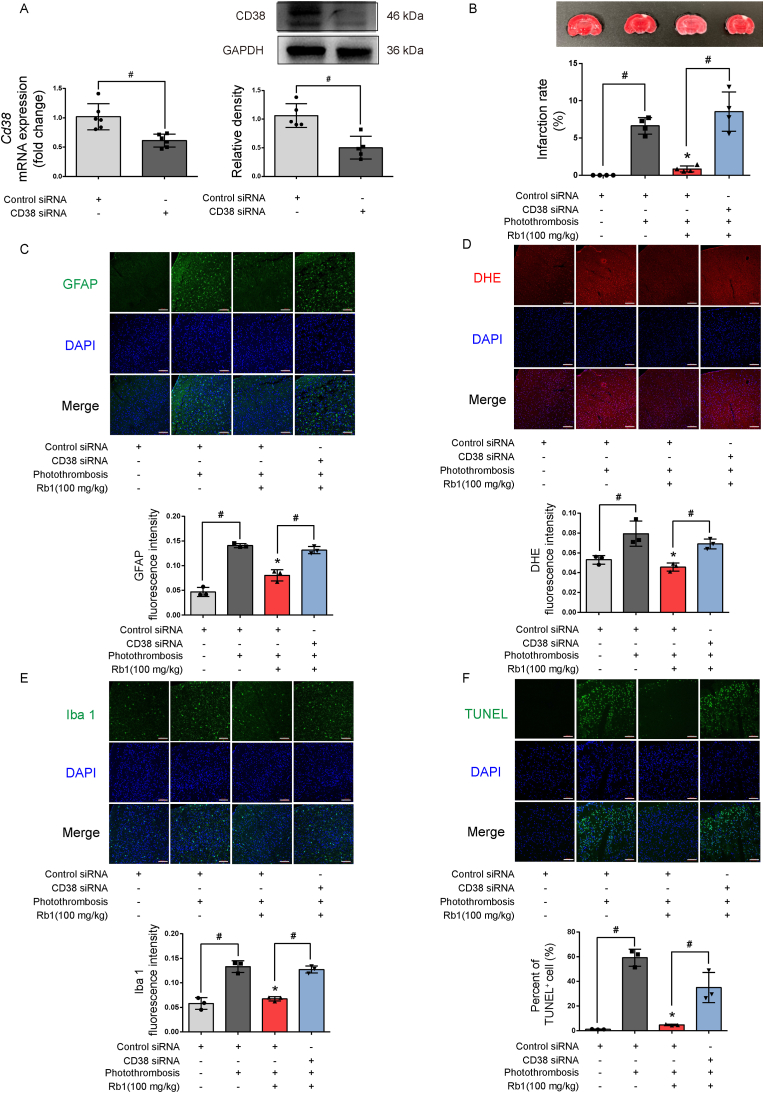
We prepared an ischemic brain model in mice by photothrombosis of cortical microvessels to observe the protective effects of Rb1 in vivo. CD38 catalyzes the synthesis of a calcium messenger, cyclic ADP-ribose (cADPR) in mitochondrial membranes to mediate mitochondrial transfer from astrocytes to neurons [17]. CD38 siRNA or control siRNA were injected into cerebral ventricles to confirm the role of mitochondria transfer in neuroprotection (Fig. 7A). Rb1 administration reduced brain infarct size and astrocyte reactivity with concomitant suppression of ROS production in a CD38-dependent manner (Fig. 7B, C, D). Ionized calcium binding adapter molecule 1 (Iba 1) is a protein marker of microglia; Rb1 treatment reduced microglia recruitment in the brain (Fig. 7E). Together, these results indicate that facilitation of mitochondrial transfer from astrocytes contributed to the attenuation of ischemic brain injury by Rb1. In support of this, TUNEL staining confirmed that Rb1 promoted cell survival against ischemia (Fig. 7F).
Fig. 7.
The neuroprotective effects of Rb1 in mouse stroke model. A. Gene and protein expression of Cd38 knockdown in cerebral ventricles (n = 6); B. Brain infarct size and quantification of infarction area (one of four independent experiments); C. Representative pictures and quantification of immunofluorescence staining of GFAP in the brain (one of three independent experiments, scale bar 100 μm). D. Representative pictures and quantification of ROS production in the brain (one of three independent experiments, scale bar 100 μm); E. Representative pictures and quantification of immunofluorescence staining of Iba 1 in the brain (one of three independent experiments, scale bar 100 μm); F. Representative pictures and quantification of TUNEL staining in the brain (one of three independent experiments, scale bar 100 μm). Data are presented as mean ± SD. *p < 0.05 vs. the photothrombosis only treatment; #p < 0.05 vs. indicated treatments. Rb1, ginsenoside Rb1; GFAP, glial fibrillary acidic protein; Iba 1, ionized calcium binding adaptor molecule 1.
4. Discussion
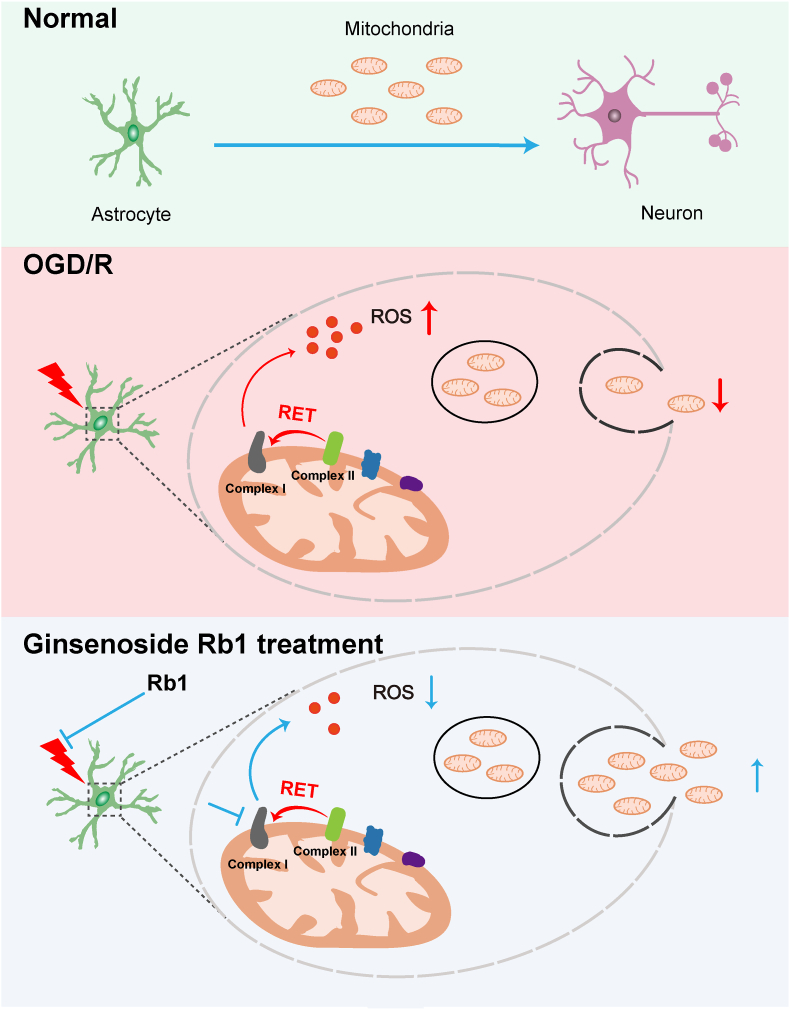
Neuroglia are a highly heterogeneous population of nonexcitable cells while astrocytes are the most abundant cells that protect and support neuronal function. Compared to neurons, astrocytes are more resistant to nutrient depletion and oxidative stress, suggesting a therapeutic window to rescue neurons from ischemic injury. Rb1 promoted neuronal function and survival by enhancing mitochondrial transfer from astrocytes, providing a new insight into the cellular communication from the perspective of mitochondrial coupling (Fig. 8).
Fig. 8.
Schematic representation of the neuroprotective effect of Rb1. Ischemic stroke affects astrocytes and impairs mitochondrial transfer. Transient inhibition of mitochondrial complex I by ginsenoside Rb1 blocks reverse electron transfer-driven ROS production from complex I and prevents astrocyte activation and mitochondrial dysfunction, resultantly facilitating mitochondrial function, functional mitochondrial transfer, and neuronal survival.
In view of the special cytoarchitectural organization of the brain, we first examined the effects of Rb1 in brain slices subjected to ischemic injury, since the results observed were the combined effects of different pathological factors. Under ischemic conditions, extracellular glutamate accumulation mediates excitotoxicity. GS protein level of astrocytes decreased sharply in response to OGD/R insult via ROS-induced 20S proteasomal degradation [8]. Rb1 inhibited astrocyte reactivity and reduced GS loss to promote glutamate disposal, which is indicative of astrocyte function protection. Moreover, its neuroprotective role was also confirmed in cultured astrocytes. Although the function of reactive astrocytes has been a subject of some debate, it is generally accepted that astrocyte reactivity is a response to brain injury such as trauma, ischemic damage, neuro-inflammation, or neurodegeneration [10,13]. The hallmark of reactive astrogliosis is the change in the morphologies of astrocytes and the induction of GFAP. Consistent with a previous study [27], we have demonstrated that OGD/R insult rapidly led to increased GFAP staining in reactive astrocytes. Reactive astrocytes appear to exhibit increased ROS and calcium dysregulation in aging mice [28]. We have shown that ROS-associated mitochondrial dysfunction contributed to astrocyte reactivity under ischemic conditions.
Though ischemia and reperfusion impair normal brain function via diverse mechanisms, excessive ROS production from the mitochondria is considered as the initial factor that mediates a cascade of events that result in tissue damage and dysregulation of iron homeostasis [22]. Mitochondrial complex I drives ROS production and the potential candidate site is flavin mononucleotide (FMN) in NADH dehydrogenase [29]. Complex I is a NADH:ubiquinone oxidoreductase that uses NADH oxidation and ubiquinone reduction to build the proton motive force to drive ATP synthesis. It is generally known that the inhibition of complex I leads to electron leakage, resulting in excessive ROS production and cell damage [30]. Notably, this occurs during conventional forward electron transport along the ETC. If complex I inhibitor rotenone blocks the CoQ-binding site, electrons cannot be transferred to CoQ, causing over-reduction of FMN to generate ROS. This is the mechanism by which rotenone induces ROS production under basal conditions. Distinct from rotenone, we observed that Rb1 had no influence on ROS level and did not impair cell survival under basal conditions, excluding potential cytotoxicity. Under ischemic conditions, ATP synthesis is inhibited due to limited oxygen availability, and high mitochondrial membrane potential forces electrons backward from the CoQ pool onto the FMN to produce superoxide at mitochondrial complex I [2,30]. Inhibition of mitochondrial complex I can therefore block RET-ROS production, which in turn can be reversed by bypassing complex I using Ndi1. Under conventional electron transport settings, Ndi1 protein is reported to limit ROS production, and alleviate neurodegeneration in rotenone-induced parkinson's disease due to its action as a shuttle from NADH to the downstream respiratory chain [31]. However, in the RET process, electrons may also be pushed backward from ubiquinone to NADH dehydrogenase site in Ndi1 and contribute to producing excessive ROS in Ndi1-expressing cells. Mitochondrial complex I inhibitor rotenone has been shown to irreversibly reduce ROS production upon reperfusion [32]. Rb1 on the otherhand reversibly suppressed ROS production within the mitochondria by transient inhibition of complex I; an observation that was similarly made in the instance of cardioprotection (against ischemic injury) [19]. In support of this, S-nitrosation of mitochondrial complex I at special sites slowed the reactivation of mitochondria to limit ROS production upon reperfusion [33]. In cultured astrocytes, Rb1 suppressed mitochondrial ROS production to attenuate oxidative damage, and contributed to preventing astrocyte activation.
The astrocytes heavily rely on glycolysis for their energy needs. They also consume a lot oxygen and exhibit a high rate of oxidative metabolism [34]. Mitochondrial dynamics within the fine-tuned processes of astrocytes are critical for oxidation, metabolism, calcium signaling and neurotransmitter recycle. Mitochondrial dysfunction in reactive astrocytes impacts on neuronal homeostasis, survival, and ROS production; the release of cytokines and chemokines is involved in neuroinflammation [34,35]. Although astrocytes contain higher levels of endogenous antioxidants, we found that OGD/R insult impaired innate antioxidative defense and hampered mitochondrial integrity, leading to functional disorders. Reversible inhibition of complex I by Rb1 suppressed mitochondrial ROS production and conferred protection on the mitochondria. Moreover, Rb1 improved astrocytes function, including glutamate clearance, phagocytic ability and neurotrophic effects. Though neurons can synthesize GSH, the precursors from astrocytes are dependent on the glutamate-glutamine cycle. Rb1 treatment increased astrocytic GSH and NADPH contents, showing an alternative means by which Rb1 protects neurons from ischemic injury. In view of the intense cooperativity between astrocytes and neurons [1], we reasoned that protection of astrocytes by Rb1 contributed to promoting neuron survival and facilitating recovery from ischemic stroke. Indeed, inhibition of astrocyte mitochondria was previously shown to increase the vulnerability of neurons to glutamate toxicity [36].
Mitochondria are dynamic organelles as they move, divide and fuse with one another, and are removed if damaged. Mitochondrial transfer has emerged as an additional mechanism for the replacement of damaged mitochondria [37]. Mitochondria within endothelial progenitor cells are shown to support brain endothelial energetics and barrier integrity against ischemic damage through extracellular transfer [38]. Injection of unaffected mitochondria into the heart ischemic zone just before reperfusion could improve postischemic heart function and cell viability [39]. Interestingly, a study demonstrated that mitochondrial transfer from astrocytes to injured neurons after stroke can improve recovery from ischemic damage [17]. Neurons metabolically rely on mitochondrial oxidation for functional support, and they are vulnerable to ischemic damage when oxygen supply is blocked. Stroke affects all cellular elements of the brain, including astrocytes. We found that the transfer of astrocyte mitochondria to neurons was within the context of maintaining functional integrity, and hint at the significance of astrocyte manipulation in neuroprotection. After ischemic stroke, astrocytes in the peri-infarct region are reactive and are characterized by intracellular calcium overload, inflammation as well as the activation of signaling pathways involving p38 MAPK, Notch and STAT3 [40,41]. Importantly, the morphology and location within the brain is ideal for astrocytes to integrate signaling cascades and maintain homeostasis between the nervous, immune and vascular components. Rb1 protected astrocytes against ischemic injury, ensured mitochondrial transfer, and provided metabolic support for neuronal survival and function. Ginsenosides are known to elicit beneficial effects in astrocytes [[42], [43], [44]]. We have shown that mitochondrial protection was important to prevent astrocytes activation. Although ginsenosides are shown to attenuate ischemic brain damage via different mechanisms in ischemic mouse brain models, we have demonstrated that astrocyte inactivation and mitochondrial transfer are important means by which Rb1 rescues neurons from ischemic damage.
5. Conclusion
Ischemic insult affects astrocytes, and ROS-associated mitochondrial dysfunction is a cause of astrocyte activation. By blocking RET-ROS production to restrain astrocyte reactivity, Rb1 protected mitochondrial integrity, facilitated the transfer of functional mitochondria from astrocytes to neurons, and contributed to rescuing neurons from ischemic damage.
Author contributions
Feng-Qing Huang, Jia Li and Baolin Liu conceived the original idea and designed the study. Xue-Chun Ni, Hong-Fei Wang, and Dai Yang performed the experiments. Xue-Chun Ni and Yuan-Yuan Cai conducted data analysis and data interpretation. Baolin Liu, Feng-Qing Huang and Xue-Chun Ni prepared the manuscript. Raphael N. Alolga edited the language. All authors approved the final version of the manuscript.
Declaration of competing interest
No competing financial interest declared.
Acknowledgments
This work was financially supported in part by the National Natural Science Foundation of China (No. 82003979, 82003978) and the China Postdoctoral Science Foundation (No. 2020M671660).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102363.
Contributor Information
Jia Li, Email: 460184@njucm.edu.cn.
Feng-Qing Huang, Email: 1620194561@cpu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabol. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Chouchani E.T., Pell V.R., Gaude E., et al. Ischemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi R.K., Beal M.F. Mitochondrial diseases of the brain. Free Radic. Biol. Med. 2013;63:1–29. doi: 10.1016/j.freeradbiomed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Yin F., Boveris A., Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxidants Redox Signal. 2014;20:353–371. doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel R.A.G., McMullen P.W. Neuroprotection in the treatment of acute ischemic stroke. Prog. Cardiovasc. Dis. 2017;59:542–548. doi: 10.1016/j.pcad.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Ioannou M.S., Jackson J., Sheu S.H., et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177:1522–1535. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Sonnewald U., Qu H., Aschner M. Pharmacology and toxicology of astrocyte- neuron glutamate transport and cycling. J. Pharmacol. Exp. Therapeut. 2002;301:1–6. doi: 10.1124/jpet.301.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Song X., Gong Z., Liu K., et al. Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkhratsky A., Nedergaard M., Hertz L. Why are astrocytes important? Neurochem. Res. 2015;40:389–401. doi: 10.1007/s11064-014-1403-2. [DOI] [PubMed] [Google Scholar]
- 10.Pekny M., Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol. Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury G.R., Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol. Dis. 2016;85:234–244. doi: 10.1016/j.nbd.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hol E.M., Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015;32:121–130. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Escartin C., Galea E., Lakatos A., et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liddelow S.A., Guttenplan K.A., Clarke L.E., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson R.A., Ying W., Kauppinen T.M. Astrocyte influences on ischemic neuronal death. Curr. Mol. Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 16.Davis C.O., Kim K.Y., Bushong E.A., et al. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayakawa K., Esposito E., Wang X., et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y.H., Li Y., Wang Y., et al. Ginsenoside-Rb1 for ischemic stroke: a systematic review and meta-analysis of preclinical evidence and possible mechanisms. Front. Pharmacol. 2020;11:285. doi: 10.3389/fphar.2020.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L., Yin X., Chen Y.H., et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. 2021;11:1703–1720. doi: 10.7150/thno.43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Su Y., Richmond A. Antioxidants tiron and N-acetyl-L-cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-kappaB pathway. Free Radic. Biol. Med. 2007;42:1369–1380. doi: 10.1016/j.freeradbiomed.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao K., Wang C.R., Jiang F., et al. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia. 2013;61:2063–2077. doi: 10.1002/glia.22577. [DOI] [PubMed] [Google Scholar]
- 22.Chouchani E.T., Pell V.R., James A.M., et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabol. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Testa C.M., Sherer T.B., Greenamyre J.T. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res. Mol. Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Seo B.B., Kitajima-Ihara T., Chan E.K., et al. Molecular remedy of complex I defects: rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9167–9171. doi: 10.1073/pnas.95.16.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voloboueva L.A., Suh S.W., Swanson R.A., et al. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J. Neurochem. 2007;102:1383–1394. doi: 10.1111/j.1471-4159.2007.04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmukler E., Solomon S., Simonovitch S., et al. Altered mitochondrial dynamics and function in APOE4-expressing astrocytes. Cell Death Dis. 2020;11:578. doi: 10.1038/s41419-020-02776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugliese A.M., Traini C., Cipriani S., et al. The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices. Br. J. Pharmacol. 2009;157:818–830. doi: 10.1111/j.1476-5381.2009.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii T., Takanashi Y., Sugita K., et al. Endogenous reactive oxygen species cause astrocyte defects and neuronal dysfunctions in the hippocampus: a new model for aging brain. Aging Cell. 2017;16:39–51. doi: 10.1111/acel.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirst J., King M.S., Pryde K.R. The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 30.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marella M., Seo B.B., Nakamaru-Ogiso E., et al. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson's disease. PLoS One. 2008;3:e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesnefsky E.J., Chen Q., Moghaddas S., et al. Blockade of electron transport during ischemia protects cardiac mitochondria. J. Biol. Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 33.Chouchani E.T., Methner C., Nadtochiy S.M., et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gollihue J.L., Norris C.M. Astrocyte Mitochondria: central players and potential therapeutic targets for neurodegenerative diseases and injury. Ageing Res. Rev. 2020;59 doi: 10.1016/j.arr.2020.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson J.G., Robinson M.B. Regulation of mitochondrial dynamics in astrocytes: mechanisms, consequences, and unknowns. Glia. 2018;66:1213–1234. doi: 10.1002/glia.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voloboueva L.A., Suh S.W., Swanson R.A., et al. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J. Neurochem. 2007;102:1383–1394. doi: 10.1111/j.1471-4159.2007.04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Y. Qin, X. Jiang, Q. Yang, et al., The functions, methods, and mobility of and mitochondrial transfer between cells, Front. Oncol. 11 (2021) 672781. doi: 10.3389/ fonc.2021.672781. [DOI] [PMC free article] [PubMed]
- 38.Hayakawa K., Chan S.J., Mandeville E.T., et al. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cell. 2018;36:1404–1410. doi: 10.1002/stem.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCully J.D., Cowan D.B., Pacak C.A., et al. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H94–H105. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhury G.R., Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol. Dis. 2016;85:234–244. doi: 10.1016/j.nbd.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cekanaviciute E., Buckwalter M.S. Astrocytes: integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics. 2016;13:685–701. doi: 10.1007/s13311-016-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun C., Lai X., Huang X., et al. Protective effects of ginsenoside Rg1 on astrocytes and cerebral ischemic-reperfusion mice. Biol. Pharm. Bull. 2014;37:1891–1898. doi: 10.1248/bpb.b14-00394. [DOI] [PubMed] [Google Scholar]
- 43.Hou J., Kim S., Sung C., et al. Ginsenoside Rg3 prevents oxidative stress-induced astrocytic senescence and ameliorates senescence paracrine effects on glioblastoma. Molecules. 2017;22:1516. doi: 10.3390/molecules22091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lou Y.X., Wang Z.Z., Xia C.Y., et al. The protective effect of ginsenoside Rg1 on depression may benefit from the gap junction function in hippocampal astrocytes. Eur. J. Pharmacol. 2020;882 doi: 10.1016/j.ejphar.2020.173309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.