Abstract
The ability of evolution to shape organic form involves the interactions of multiple systems of constraints, including fabrication, phylogeny and function. The tendency to place function above everything else has characterized some of the historical biological literature as a series of ‘Just-So’ stories that provided untested explanations for individual features of an organism. A similar tendency occurs in biomaterials research, where features for which a mechanical function can be postulated are treated as an adaptation. Moreover, functional adaptation of an entire structure is often discussed based on the local characterization of specimens kept in conditions that are far from those in which they evolved. In this work, environmental- and frequency-dependent mechanical characterization of the shells of two cephalopods, Nautilus pompilius and Argonauta argo, is used to demonstrate the importance of multi-scale environmentally controlled characterization of biogenic materials. We uncover two mechanistically independent strategies to achieve deformable, stiff, strong and tough highly mineralized structures. These results are then used to critique interpretations of adaptation in the literature. By integrating the hierarchical nature of biological structures and the environment in which they exist, biomaterials testing can be a powerful tool for generating functional hypotheses that should be informed by how these structures are fabricated and their evolutionary history.
Keywords: structure–function, biominerals, mechanical properties, adaptation
Significance statement
The impressive material properties of biomineralized tissues have motivated a wealth of research into the characterization of their macro-, meso- and nano-scale features. Traditionally, an isolated feature set at one scale is investigated under dehydrated conditions. These results are then combined with some postulated function to frame these features as adaptations of the animal. We demonstrate that the properties at one scale cannot always be predicted using the properties from a different scale and the necessity of testing biological tissues in environments similar to their natural state. To understand the origins of these features one needs to consider not just the potential functions but also the growth of the structure and the phylogeny and ecology of the organism.
1. Introduction
For over 500 million years, starting—perhaps—as early as the Cryogenian [1], a planet-wide set of iterative experiments have been running, modifying the composition and morphology of biomineralized hard parts of living organisms into a diverse array of structures: from spicules, plates and sclerites, to teeth, shells and skeletons. These structures share a similar organization in the sense of integrating a mineral component in an organic matrix, with more derived architectures exhibiting a hierarchical system of morphology [2,3]. From a functional perspective, the combination of a compliant phase and a stiff phase, coupled with the hierarchical nature of these structures, contributes to a variety of stiff, tough and stable end-products with the capability to deform, creep, recover, undergo stress relaxation, absorb energy, filter frequencies and more. These are often well beyond what would be expected based on a simple homogeneous mixture of the constituent parts [4]. Unsurprisingly, the structure–function relationship of these tissues comprises a significant fraction of the literature on biomaterials, with a focus on detailing the nano-, micro- and macro-scale features that could contribute towards the development of these impressive properties. A common trend easily observable in much of this literature is the effort to paint all such features, necessarily, as outcomes of adaptive evolution—with adaptation here referring both to the process by which an organism becomes fitted to its environment and to the outcomes of this process.
Through much of the 1900s, rooted in traditions such as the idea of the Allmacht of natural selection [5], there was a tendency among biologists to view animals as atomized parts and search for adaptive explanations of these parts divorced from either a view of the organism as a cohesive whole, or any constraints on the outcomes of the evolutionary process [6,7]. Although the goal of biomaterials research is not necessarily to probe evolution, as stated previously, the way evolution is evoked in the research often echoes the trends of this early, atomized view of organisms. Are local gradients in crystal aspect ratio [8], mineral bridges between crystals [9] and screw-like dislocations [10,11] adaptations to increase strength and toughness or are they necessarily formed during the self-assembly of the mineral [12–14]? Of course, these two aspects are not mutually exclusive; the function of a part and how that part forms are two different topics. Although there is no a priori reason to invoke functionality if fabrication presents a sufficient explanation, especially when functionality is used as ‘window dressing’ rather than the topic being explored. Even more so when the functionality of the feature in question is viewed without accounting for rest of the organism, assuming that the mechanical effect of these small-scale features simply scales up to the organismal level and overlooking modularity [15]—the potential interactions between different components of the organism both in growth and in function. This assumption is even more glaring in cases where the environment of the biomaterial during life is ignored when measuring its material properties; biomaterials are often tested in dehydrated states under simple quasi-static loading conditions.
Consider, for example, one of the most studied biomaterials: nacre. Nacre has long been thought remarkable for its strength and toughness relative to pure aragonite largely due to the interplay of several different features including: nanoasperities [16,17], dove tailing [18] and tablet interlocking [19]. Yet most of the experiments on nacre of molluscan shells have been performed on dehydrated samples, with rare exceptions [20,21], and despite the knowledge that moisture content has a significant effect on measured material properties [22–24]. Furthermore, the mechanical ‘superiority’ of nacre is commonly discussed while ignoring that it is only one part and, in many cases, only a small part of the entire shell [25,26].
The goal of this contribution is to examine the adaptationist narrative as it manifests in the field of biomaterials and suggests some ways such narratives can be framed in a way that does not present evolution as a simple optimization process. Largely drawing on broader evolutionary frameworks, such as constructional morphology [7,27,28], that integrate multiple factors that shape organismal form, such as the environment, phylogeny, fabrication and function. In the light of the stated goal, much of the discussion here will focus on function as it is the aspect of our broader framework that biomaterials research can directly address. As we can see in the examples from the previous paragraph, this adaptationist narrative commonly manifests from discussions resulting from measuring material properties and then relating those properties to some adaptive scenario. How these properties are measured, the scale at which the measurements are done, and the connection to functional morphology are, therefore, all of primary concern. The questions then are as follows: How do moisture and scale affect the results of some common tests? How do they scale up to reflect the performance of an entire biomaterial? Can these be put into the perspective of adaptive evolution? To explore this, we compared environmentally dependent static and dynamic properties of two different but related biogenic mineralized structures at different length scales.
2. Results
The shells of molluscs present an excellent medium to test these material properties due to several decades of research into their structure and mechanics [20,29–31]. The aforementioned nacre ultrastructure is taken from the external shell of the cephalopod Nautilus pompilius (figure 1a–d). The nautilid shell is predominately nacre sandwiched between two thin prismatic layers [32,33]. This shell grows uni-directionally in thickness, starting at the homogeneous zone of the outer prismatic layer [13,34] and progressing to the columnar zone, which then transforms into nacre (figure 1c). The columnar nacre layer eventually transforms into the inner prismatic layer. This architecture is standard for externally shelled cephalopods as fossil ammonoids, nautiloids and basal coleoids also share this three-layered shell structure [33,35,36]. A notable exception to this, and the second structure studied for this work, is the shells of the pelagic octopus genus Argonauta (figure 1e–h).
Figure 1.
Overview of the two animal shells used in this study: the aragonitic shell of Nautilus pompilius (a–d) and the calcitic shell of Argonauta argo (e–h). The shell of N. pompilius (a) is composed of three primary layers (b): the outer prismatic layer that transitions into the nacre layer (c), which then transitions into the inner prismatic layer. EBSD of N. pompilius shows a clear increase in texture going from the homogeneous zone (top of the image) down to the columnar and finally the nacreous zones (d). The shell of A. argo (e), in contrast with N. pompilius, grows bi-directionally from a central organic layer (f). Most of the thickness of the shell is formed by acicular calcite crystals that grow in conical clusters. These clusters begin as spherulites in the organic layer (g). The conical crystal clusters that make up the shell of A. argo are visible within the EBSD map and show a co-orientation within the clusters, the blue/green clusters near the image centre (h). Much of the variation in orientation seen in the image is due to neighbouring clusters going into and out of the plane. The colour-coded inverse pole figures have their reference direction normal to the image plane.
Well described by Aristotle, these pelagic octopuses were once thought to have been parasitic in the sense that they would steal the shell of some other Carinaria-like animal to live in [37]. It was not until the pioneering work of Villepreux-Power, who was able to raise argonauts in aquariums, that the ability of female argonauts to construct the shell themselves was discovered [38]. Unlike other mollusc shells that are formed by the mantle, this shell is formed through two membranes on the dorsal arm pair in female argonauts [38]. This observation emphasizes the fact that the shell is non-homologous to the shells of other molluscs. This purely calcitic shell is derived through a different developmental pathway [38] and possesses different shell matrix proteins compared to other cephalopod shells while also lacking chitin in the organic shell matrix [39]. Unlike the uni-directional construction exhibited by the nautilid shell, the argonaut constructs its shell bi-directionally in thickness from a central organic layer (figure 1f). The long, thin crystals that form the bulk of the shell thickness begin as spherulites within the organics-rich layer (figure 1g) that grow outwards in conical clusters. Though the ultrastructure, crystallography and geochemistry of the shell have been previously investigated [40–46], the mechanics of the shell are, to our knowledge, unexplored beyond notes of the shell being fairly flexible when wet [38]. The unique construction of the argonaut shell, with a large central organics-rich layer, and the relative simplicity of the organization of the mineral structure compared to Nautilus make for an interesting comparison between the two architectures. Previous work done by the authors has shown a minimal effect of humidity on indentation properties of N. pompilius, which further provides an opportunity to compare the effects of moisture and if the shell of A. argo indeed becomes flexible when wet.
2.1. Shell structure and texture
The different architecture of the two shells arises from fundamentally different growth modes discussed previously. These different growth modes also explain the differences in crystallographic texture seen in the electron backscatter diffraction (EBSD) images (figure 1d,h). EBSD results taken from A. argo agree with previous work [44]. The coherence appears lower at the immediate edges of the central organic zone while the fully developed acicular crystals appear co-oriented (figure 1h). Much of the variation in crystal orientation seen in the shell wall is due to neighbouring fibre clusters growing into/out of the image plane. Unlike in N. pompilius, where the organics are largely dispersed throughout the shell thickness between the mineral units, A. argo shows a concentration of organics in the centre of the shell (figure 1g,h).
Thermogravimetric analysis (TGA) was performed to test whether this central organic layer contributed to an overall higher weight per cent of the organic phase in the argonaut shell. TGA results show slightly different levels of organics between the two samples; A. argo has a higher relative organic content: 7.4% compared to N. pompilius at 6.1%. In order to test whether the differences between N. pompilius and A. argo described here lead to differences in their mechanical performance, both shells were subject to a series of quasi-static and dynamic mechanical tests at multiple length scales to characterize potential variations in trends and sensitivities to moisture.
2.2. Nano-scale analysis
Quasi-static nanoindentation line-scan tests were performed on the entire cross-sections of N. pompilius (figure 2a,b) and A. argo (figure 2d,e) at a range of relative humidities, from 30% to 90%. The obtained results show a general decrease of reduced modulus and hardness with increasing moisture content, though the effect is structure dependent in N. pompilius compared to A. argo. A. argo shows a higher sensitivity to water content with a greater change in reduced modulus and hardness at higher humidities compared to N. pompilius. For example, the global average reduced modulus decreases by 10% in N. pompilius and 26% in A. argo when comparing the results at 30% RH with 90% RH.
Figure 2.
Mechanical characterization on the nano-scale. Reduced modulus (Er) and indentation hardness graphs were calculated from two indentation maps from Nautilus pompilius (a,b), covering an area of 1300 × 200 µm2 and Argonauta argo (d,e) covering an area of 96 × 36 µm2. The mean values presented in the graphs were made by averaging indentation results across a row of indents made at the same height. Shaded regions represent ±1 s.d. of the averaged data. NanoDMA experiments performed on a cross-section of the shell of N. pompilius (c) and A. argo (f) at a relative humidity of 90%.
Nano-dynamic mechanical analysis (nanoDMA) was then employed to characterize potential differences in local dynamic properties at high humidity. Dynamic nanoindentation tests performed on a cross-section of N. pompilius (figure 2c) and A. argo (figure 2f) at 90% RH show a similar variation in stiffness in major ultrastructural parts of the shell. Like in the quasi-static tests, the organics-rich layer in A. argo shows lower modulus values compared to the mineral phase while the prismatic layer shows higher maximal values compared to nacre in N. pompilius. An interesting, and unexpected, observation is the emergence of a slight frequency-dependent response mostly visible in the argonaut mineral phase (figure 2f) but not present in the nautilid shell.
2.3. Macro-scale analysis
Classical DMA experiments were performed to see how this frequency dependence scales up to the macro-scale and how it is affected by the internal architecture and the morphology of the shell. Rectangular samples were cut from the edge of the aperture from both N. pompilius (figure 3a–c) and A. argo (figure 3d–f) for dynamic three-point bending tests under ambient ‘dry’ conditions and while fully immersed in water. In the case of the nautilid, two types of samples were tested: an intact shell and segments where the prismatic layers were gently polished away. Furthermore, due to the wavy morphology of the argonaut shell (figure 1e), the samples were bent on both sides. This geometric irregularity resulted in the high spread of the results (figure 3d–f). It is important to mention that calcium carbonate, both calcite and aragonite, are mechanically anisotropic materials [47,48] and, therefore, the direction of load application has a significant effect on the obtained mechanical results on all scales. Hence, in this work, indentation experiments were performed on shell cross-sections, which is also the direction of stress generation during macroscopic bending experiments during macro-scale analysis.
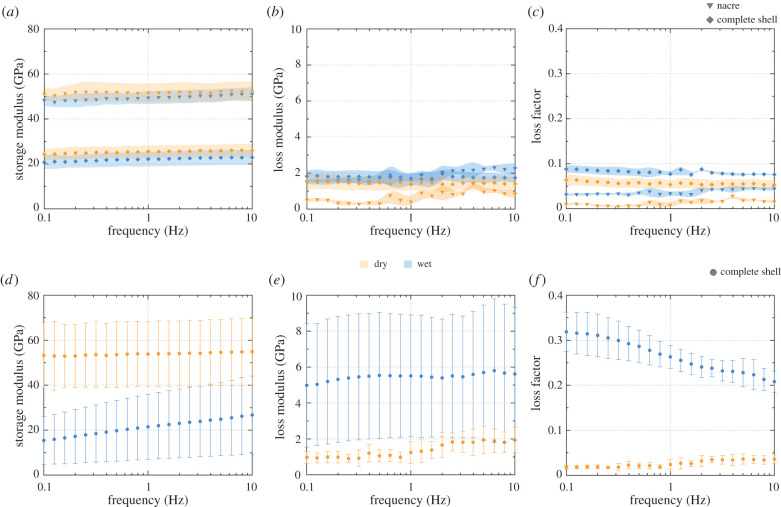
Figure 3.
Mechanical characterization on the macro-scale. Storage modulus, loss modulus and loss factor versus frequency graphs for Nautilus pompilius (a–c) and Argonauta argo (d–f), respectively. Inverted triangles, diamonds and circles represent data obtained from N. pompilius nacre only, complete N. pompilius shell and complete A. argo shell, respectively. For the data of N. pompilius, the shaded regions represent ±1 s.d. of the averaged data. The error bars for the A. argo plots, while also calculated as ±1 s.d., show a greater spread compared to N. pompilius due to the geometric variation of the beams cut from the shell. In this regard, most of the ‘error’ for A. argo is due to geometric differences between the two sides of the same beam that were averaged together for each point.
The most striking outcome of this set of experiments is that the storage modulus of pure nautilid nacre is two- to three-fold larger than that of the complete shell, having the outer prismatic architectures intact (figure 3a). Similar to the nano-scale, all specimens show a decrease in storage modulus (figure 3a,d) and an increase in loss modulus (figure 3b,e) under wet conditions. Comparing the change in storage modulus between ‘dry’ and wet conditions averaged across all frequencies: N. pompilius nacre shows an average decrease of 4.5%, the complete N. pompilius shell shows an average decrease of 13.0%, while the argonaut samples show an average decrease of 60.6%. Similar averaged comparisons of loss factor (tan delta) also show the separation of these three groups (figure 3c,f). The loss factor increases under wet conditions. The argonaut shell shows a very large increase in loss factor when wet, with an average increase of 922.0%. N. pompilius nacre shows a higher increase of 180.8% compared to the complete shell with an increase of 43.6%. Nevertheless, the absolute value of loss factor is significantly higher in wet argonaut than in any other structure, wet or dry.
The frequency-dependent response previously seen in nanoDMA for A. argo is recreated at this length scale but with a much more pronounced effect despite being measured at much smaller frequencies range. In N. pompilius, both nacre and the complete shell also show a slight frequency-dependent response on the macro-scale though not as noticeable compared to A. argo. While both shells show a general stiffening with increasing frequency and a higher dependence on strain rate while immersed, the observed effect is greater in the argonaut shell compared to any nautilid sample.
3. Discussion
Decades of research has undoubtedly advanced our knowledge on morphogenesis, structure and mechanical performance of biological materials. Nacre is an excellent example of how furthering our understanding of fine-scale morphology can also advance our understanding of material properties [16,18,49,50]. This greater understanding has been used to create increasingly more sophisticated and robust biomimetic structures by incorporating features, such as asperities and mineral bridges [51,52]. Indeed, this is a major goal of this field of research. However, the common invocation of functional adaptation leads to an inevitable question: how has research in this vein improved our understanding of nacre evolution and biomineralization, or even of any biomaterial?
While nacre is clearly well characterized, the argonaut shell represents a significant unknown in this study. The ultrastructure and crystallography are known [41,43,53], but its mechanics are poorly understood. The argonaut shell also does not share the more common molluscan structural motifs, such as nacre, crossed-lamellar or prisms; nor does it grow in a similar manner. The spherulitic-fibrous structure of the argonaut shell exhibits notably different mechanical behaviour compared to the familiar spherulitic-prismatic/nacreous shell, specifically in response to high humidity and water immersion. While on the nano-scale the N. pompilius shell shows some reduction of reduced modulus in certain regions (figure 2a), A. argo shows a systematic reduction as a function of relative humidity (figure 2d). At the macro-scale, this difference is significantly enhanced (figure 3a,d). The more extreme dependence in the argonaut shell suggests either a greater water absorption capacity or perhaps some different interaction with water owing to a different organic matrix compared to the nautilid shell [39]. However, if one had compared the properties of these shell structures in their ‘dry’ state [8,54–57], almost no difference in their mechanical performance would have been reported. It should be noted, however, that the rehydrated state does not necessarily capture the original properties of the shell in life due to the potential impact of soft-tissue degradation on the mechanics of the tissues [58,59], though the actual potential impact of this degradation is unknown.
An even more intriguing difference between the two shells is revealed by a comparison at different frequencies. The emergence of a strong frequency-dependent response in the argonaut shell during macroscopic DMA (figure 3), despite the lack of a significant response in nanoDMA (figure 2), indicates some additional mechanism not present at the nano-scale. Also here, if one were to report frequency-dependent mechanical properties of the argonaut based on nano-scale measurements only—a common practice in biomaterials study—a vital aspect of the mechanical performance of this shell would have been overlooked. Surprisingly, combining two ultrastructures that show almost no frequency-dependent behaviour on the nano-scale (figure 2f) results in the formation of a material with a pronounced dependency on load application rate (figure 3d,f). In the nautilid shell, however, frequency-related effects are slight enough to be potentially overlooked on any scale.
We cannot determine the exact mechanisms behind the observed mechanical behaviours; however, we can speculate about their possible dynamics. Both shells have a similar organic content, the mechanical properties of which are assumed to be strongly dependent on moisture acting as a plasticizer. It is expected to contribute to an increased deformability and viscoelasticity of these biomineralized structures [24]. Nevertheless, the nature of the ultrastructures and the arrangement of the constituent materials in space are key to their performance. While nacre has a number of features that limit inter-tablet movement, such as nanoasperities, mineral bridges and dove tailing [60,61], these reinforcing mechanisms were not observed in the prismatic ultrastructure nor the argonaut ultrastructure. Furthermore, the organic phase in the nautilid shell is largely spread throughout thin lamellae in nacre, whereas in the argonaut shell, it is concentrated in relatively large volumes, both in between crystal units [45] and at the centre of the shell (figure 1g). These characteristics likely permit a relatively large deformability of the argonaut shell at the macro-scale compared to nacre that has a fairly limited range of motion regardless of the properties of the organic phase. In both cases, these effects cannot possibly be registered by nanoindentation where mostly the mineral phase is probed.
Indentation of hydrated nacreous and prismatic ultrastructures in N. pompilius yields reduced modulus values of around 70 GPa and upwards of 80 GPa, respectively (figure 2a). Macroscopic DMA measurements are performed in three-point bending mode, meaning that the largest stresses develop along the outer edges of the sample. In nacre specimens, these stresses would be borne by the nacre tablets, with all of their reinforcing mechanisms that limit deformation. However, in the complete shell samples, these stresses are borne by the prismatic layers (figure 1b) that, similarly to the shell of the argonaut, lack these reinforcing mechanisms. Therefore, bending of the complete shell shows a storage modulus in the range of 20–25 GPa despite most of the bending stresses occurring within the prismatic layer, which has a higher storage and reduced modulus compared to nacre when measured on the nano-scale (figure 3a). Bending of pure nacre shows a significantly higher storage modulus of approx. 50 GPa (figure 3a).
When summarizing the experimental mechanical data, the two shells, having very similar elastic modulus values of approximately 20 GPa in their hydrated state demonstrate very different scale-, humidity- and frequency-dependent characteristics. The shell of N. pompilius appears to be a ‘perfect’ architecture that combines stiffness, toughness and strength provided by the inner nacreous ultrastructure with deformability provided by the outer prismatic ultrastructures. The shell of A. argo seems to dissipate mechanical energy through its viscoelastic response and therefore is extremely sensitive to relative humidity and load application rate, whereas the properties of N. pompilius are only slightly sensitive to both. We can also argue, following the tradition discussed in the Introduction, that this study provides a paradigm example of functional adaptation where two externally shelled cephalopods evolved to provide the organisms with mechanical stability and protection using two very different but thoroughly ‘designed’ strategies. However, this leads back to the question posed at the beginning of the discussion: how or even if the obtained data really improved our understanding of the evolution of these shells.
To address this question, we will draw on two examples from the literature to show how the invocation of evolution and the assumption of adaptation have shaped this discussion. The first example deals with the shell of the familiar Nautilus. In this study, samples taken from the Nautilus sp. shell were indented and subjected to three-point bending experiments to measure bending strength, study crack propagation and calculate the fracture toughness for the organic/mineral components. The authors argue that the Nautilus sp. shell ‘exhibits an outstanding environment adaptability in the deep sea’ [55]. However, there are two points to make with regard to the conclusions of this paper. The first is that it is unclear how representative the reported values are of the actual shell during life since all of the experiments were performed on dehydrated specimens. The second concerns the assertion built upon these data: that the spherulitic-prismatic/nacreous shell is an adaptation of extant nautilids to the deep sea.
In a critique of the panselectionist argument that natural selection is wholly sufficient to explain form, Gould & Lewontin [6] invoke the ‘Just-So’ stories of Rudyard Kipling to describe the manifested narrative of these arguments. These stories are framed such that adaptation to some aspect of the current ecology is the ultimate cause of the organism's phenotype. In the case of Nautilus, the prismatic/nacre architecture is common in nautiloids older than the genus Nautilus [62–64] as well as other externally and internally shelled cephalopods [36]; it seems more likely that the species of Nautilus inherited this ultrastructure from a nacre bearing ancestor. While this ultrastructure may not be an adaptation formed by Nautilus specifically, we can ask: is there is a connection between a deep-water habitat and the spherulitic-prismatic/nacreous architecture? However, the fossil record argues against a possible connection. The habitats of nautiloids and ammonoids span from shallow, coastal environments to open ocean and deeper water environments without any variation in the basic shell ultrastructure [65–69]. How can the spherulitic-prismatic/nacreous shell be an adaptation to deep water if it is also commonly present in shallow-water cephalopods as well? We can continue to ask questions in this vein, for example, does this shell architecture originate in a deep sea group or could the utility of the shell in the deep sea be a case of exaptation? We can also ask more targeted questions related to the results of the mechanical tests: does the high fracture toughness or bending strength improve the shell resistance relative to some other possible ultrastructure if we consider the actual strain caused by water pressure or the bite force of a known predator? The point of these questions is not to answer them here but to illustrate the kind of queries that should be asked when trying to meaningfully discuss the potential adaptational value of these structures. Simply because improved performance, such as higher bending strength or toughness, of a structure is shown compared to other ultrastructures does not automatically mean that this is the function of the structure or even that the structure is an adaptation at all. To address these questions one has to have an understanding of the ecology of the animal, i.e. the forces the organism is subjected to, as well as its evolutionary history. Finally, the origin of morphology extends beyond functional constraints and also has to include other factors, such as fabrication, phylogeny and environment [7]. This type of approach can be applied to our second example from the literature.
An interesting observation to be made about biomineralized structures across all clades is that their smallest building blocks tend to be on the nanometre-sized scale [70,71]. The potential importance of this was modelled under tension by treating the organic components embedded within mineral units as flaws and calculating the fracture strength of the ‘flawed’ crystal [70]. By calculating the fracture strength at different sizes of the mineral units, the authors concluded that there is a critical length at which the mineral building blocks become insensitive to flaws and their fracture strength is near that of a perfect crystal. This impressive result is then used to suggest that the basic nano-scale theme of biomineralized structures is driven by adaptation towards maximizing fracture strength and flaw tolerance [70]. The authors note that there are other constraints at play, such as the volume fraction of the components, molecular size and other biochemical factors. However, we would like to expand this discussion of constraints by considering the same types of questions we did with the previous example.
The assumption that the nano-granular texture of biominerals is driven by adaptation implies the existence of non-nano-granular textures in early biominerals that are then, due to some external forces, driven by functional demands to nano-granularity. This begs the question: what kind of texture did early biomineralizers possess? This question is not trivial due to the incomplete nature of the fossil record and diagenesis of early shells. That being said, some of the earliest biomineralized structures, such as those from the Ediacaran Cloudina, show evidence of a nano-granular texture [71]. If the earliest examples of biomineralized structures already possess a nanometre-scale basic unit it is difficult to make a case for adaptation as there is no non-nano-granular texture to select against. Combining this observation of early structures with the likely independent acquisition of biomineralization among phyla [72,73], the origin of this nano-granular texture may also be explained by fabricational constraints related to the fundamental mechanisms of biologically controlled mineralization via particle attachment [74].
With these two examples in mind, we can return to a previous question: do the data presented here tell us anything about the evolution of the shell of N. pompilius or A. argo? Realistically, the answer is no. We do not delve into the evolutionary history of either species, nor do we discuss the ecology of neither animal nor the forces acting on the shell as a result of that ecology. Though remarkable, some of the properties of the shells discussed here, especially the frequency dependence of the argonaut shell, are not known to be functional.
4. Conclusion
The insights gained into the mechanics of not just the previously untested Argonauta argo shell but also the well tested nacreous shell of Nautilus pompilius demonstrate the importance of both multi-scale experiments and the incorporation of moisture control in testing biominerals. We uncover two very different approaches to achieve a structure that exhibits a combination of high deformability, stiffness, strength and toughness. In both cases, the performance of the shells on the scale of the entire animal is almost impossible to predict using local nanomechanical characterization methods. Furthermore, the energy dissipation mechanism of the argonaut was successfully demonstrated only by probing it under a habitat-like environment—in fully hydrated conditions. The results presented here emphasize the importance of scale and environment when attempting to understand the function of biological structures; how can we fully understand a structure while ignoring the environment, in which the structure evolved and performs? Moreover, how can we meaningfully hypothesize about function when removing the context of ecology?
Furthermore, we claim that although the studied organisms produce shells that provide them with sufficient mechanical support and protection against predation, the morphological, structural and crystallographic properties of the ultrastructures that comprise them are not necessarily the product of functional adaptation. In nacre, the nanoasperities can simply be the consequence of precursor nanoparticle accretion [75], mineral bridges—the result of epitaxial growth [76] and dove tailing—an outcome of space-filling requirements [77].
It is important to note that we are not trying to say that fabrication is the explanation for the commonality of this texture, nor are we saying that it is impossible for function to explain its origin either. Rather, if the case for adaptation is to be made it has to weighted against other potential explanations rather than assumed a priori. The tendency to explain all phenotypic traits of mineralized structures by adaptation, not as a hypothesis but as an obvious conclusion, does not provide meaningful insight into the actual evolution of these structures nor do such statements motivate further research into the potential morphogenetic constraints that might have actually been responsible in shaping their form. If future research wishes to address the question of functionality of the tissues being studied or their evolution, we should move away from the selectionist assumption that all observed features are, by default, products of adaptation.
5. Material and methods
5.1. Specimens and imaging
Two shells of Nautilus pompilius and one shell of Argonauta argo were used for this project. The shell of Argonauta argo was collected in 2012 during a mass stranding in Yoichi Bay. See [46] for further details. Both shells of N. pompilius were collected from the Philippines. Cryo-fractured samples were broken off from near the aperture of the shell, immersed in liquid nitrogen and manually fractured. Scanning electron microscopy images were made using an FEI Scios Dual Beam FIB/SEM. A. argo images were made using a voltage of 5 kV and a current of 50 pA. N. pompilius images were made using a voltage of 2 kV and a current of 25 pA. Samples of A. argo were embedded in poly(methyl methacrylate) and polished along their cross-section for EBSD analysis. EBSD was performed using an EDAX Hikari Plus EBSD system in low-vacuum conditions (0.2 mbar) at 1.6 nA and 15/20 kV.
5.2. Thermogravimetric analysis
The thermal stability of the samples was measured with a TGA system (SETARAM, SENSYS EVO TG-DSC). The shell samples were finely ground and the obtained powder was then used for measurement. At the outset, the sample was equilibrated at 25°C for 10 min to remove any absorbed moisture. After that, a heating ramp from 25 to 830°C was applied at a rate of 5°C min−1 under oxygen atmosphere to monitor the sequential decomposition of the sample contents.
5.3. Nanoindentation and nano-dynamic mechanical analysis
Indentation and nanoDMA experiments were performed on embedded and finely polished plane samples using a Hysitron/Bruker TI950 TriboIndenter equipped with xSol High-Temperature and Humidity Control Stage. A Berkovich diamond tip was used to measure the hardness and reduced modulus. The loading/unloading rate was set to 200 µN s−1, with a 5 s holding period at peak load of 1000 µN. The Oliver and Pharr approach [78] was used to analyse load–displacement curves in order to derive reduced modulus and hardness. A grid of indents was performed across a 96 × 36 µm2 and 1300 × 200 µm2 area, across the entire cross-sectional area of the shell wall of A. argo and N. pompilius, respectively. Indentation measurements were performed at four relative humidities: 30, 50, 70 and 90%. Additional nanoDMA measurements were performed on both shell sections at a relative humidity of 90% on two different regions of the shell: prismatic and nacre for N. pompilius, and mineral and organic rich areas for A. argo. The measurements were done with a set force of 800 µN and an oscillating force of 20 µN during a frequency sweep from 1 to 300 Hz.
5.4. Dynamic mechanical analysis
Rectangular bars were cut from each shell from the lateral area of the aperture. Several N. pompilius samples were also polished from the top and bottom to remove the inner and outer prismatic areas producing only a section of nacre. DMA was performed using an Anton Paar twin drive rheometer with an MCR502 rheometer drive and an MCR702 linear drive. Experiments were performed in three-point bending mode with a free length of 10 mm. Amplitude sweeps were performed for the three samples to compare the change in storage modulus values and displacements/applied forces relevant to the frequency sweep experiments to ensure the samples were within the linear viscoelastic range. All analyses were performed using a set force of 0.15 N and an oscillating force of 0.1 N. Frequency sweeps were performed between 0.1 and 10 Hz with 10 measurements per decade. ‘Dry’ measurements were made in air at ambient conditions (22°C, 60% RH). ‘Wet’ measurements were made in a full immersion cup after soaking each sample for a period of 8–14 h. Soaking time was determined after a series of tests on the N. pompilius shell as this shell is thicker than that of the argonaut and would require a longer rehydration time. First, dry measurements of the N. pompilius shell were taken. Second, the shell was soaked for 8–14 h and then measured in full immersion. These samples were allowed to air-dry and then measured again to ensure they returned to their pre-soaked parameters. Then the samples were soaked in water for two weeks and measured again. No significant difference was found when comparing data between the 8 and 14 h/two week rehydration times.
Each N. pompilius sample was measured four times and the results were averaged together with error bars calculated as 1 s.d. in the data. A. argo samples were also measured four times but this was divided between two orientations. Each A. argo sample was measured twice and then flipped over and measured twice again. This was done because the geometry of the argonaut shell is not flat and cannot be machined into a flat surface at the scale necessary for bending experiments.
Acknowledgements
We acknowledge Luca Bertinetti (B CUBE, Dresden) for assistance in procuring and analysing the thermogravimetric data. We also acknowledge Yasuhiro Iba (Hokkaido University) and Akihiko Suzuki (Hokkaido University of Education) for providing the argonaut shells. The authors are grateful to Prof. Peter Fratzl (Max-Planck Institute of Colloids and Interfaces), Prof. Benny Bar-On (Ben-Gurion University) and Dr Nicole Poulsen (Technische Universität Dresden) for their evaluation of the manuscript.
Contributor Information
Robert Lemanis, Email: robert_evan.lemanis@tu-dresden.de.
Igor Zlotnikov, Email: igor.zlotnikov@tu-dresden.de.
Data accessibility
This article has no additional data.
Authors' contributions
R.L.: conceptualization, formal analysis, investigation, methodology and writing—original draft; K.T.: formal analysis, investigation and methodology; E.R.: investigation and methodology; G.J.: investigation and methodology; R.J.B.: investigation and methodology; K.S.: investigation and methodology; I.Z.: conceptualization, formal analysis, funding acquisition, investigation, methodology, supervision and writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
I.Z. acknowledges the financial support provided by Bundesministerium für Bildung und Forschung (BMBF) through grant no. 03Z22EN11. I.Z. and K.T. acknowledge the financial support provided by Deutsche Forschungsgemeinschaft (DFG) through grant no. 443727504. R.L. acknowledges the financial support provided by DFG through grant no. 462708234.
References
- 1.Maloof AC, Rose CV, Beach R, Samuels BM, Calmet CC, Erwin DH, Poirier GR, Yao N, Simons FJ. 2010. Possible animal-body fossils in pre-Marinoan limestones from South Australia. Nat. Geosci. 3, 653-659. ( 10.1038/ngeo934) [DOI] [Google Scholar]
- 2.Wood R, Ivantsov AY, Zhuravlev AY. 2017. First macrobiota biomineralization was environmentally triggered. Proc. R. Soc. B 284, 20170059. ( 10.1098/rspb.2017.0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eder M, Amini S, Fratzl P. 2018. Biological composites—complex structures for functional diversity. Science 362, 543-547. ( 10.1126/science.aat8297) [DOI] [PubMed] [Google Scholar]
- 4.Wegst UGK, Bai H, Saiz E, Tomsia AP, Ritchie RO. 2015. Bioinspired structural materials. Nat. Mater. 14, 23-36. ( 10.1038/nmat4089) [DOI] [PubMed] [Google Scholar]
- 5.Weismann A. 1893. The all-sufficiency of natural selection. Contemp. Rev. 64, 309-338. [Google Scholar]
- 6.Gould SJ, Lewontin RC. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B 205, 581-598. ( 10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- 7.Seilacher A, Gishlick AD. 2014. Morphodynamics. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Liang SM, Ji HM, Li XW. 2021. A high-strength and high-toughness nacreous structure in a deep-sea Nautilus shell: critical role of platelet geometry and organic matrix. J. Mater. Sci. Technol. 88, 189-202. ( 10.1016/j.jmst.2021.01.082) [DOI] [Google Scholar]
- 9.Ritchie RO. 2011. The conflicts between strength and toughness. Nat. Mater. 10, 817-822. ( 10.1038/nmat3115) [DOI] [PubMed] [Google Scholar]
- 10.Yao N, Epstein A, Akey A. 2006. Crystal growth via spiral motion in abalone shell nacre. J. Mater. Res. 21, 1939-1946. ( 10.1557/jmr.2006.0252) [DOI] [Google Scholar]
- 11.Yao N, Epstein AK, Liu WW, Sauer F, Yang N. 2009. Organic–inorganic interfaces and spiral growth in nacre. J. R. Soc. Interface 6, 367-376. ( 10.1098/rsif.2008.0316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Checa AG, Cartwright JHE, Willinger MG. 2011. Mineral bridges in nacre. J. Struct. Biol. 176, 330-339. ( 10.1016/j.jsb.2011.09.011) [DOI] [PubMed] [Google Scholar]
- 13.Schoeppler V, Lemanis R, Reich E, Pusztai T, Gránásy L, Zlotnikov I. 2019. Crystal growth kinetics as an architectural constraint on the evolution of molluscan shells. Proc. Natl Acad. Sci. USA 116, 20 388-20 397. ( 10.1073/pnas.1907229116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beliaev M, Zöllner D, Pacureanu A, Zaslansky P, Zlotnikov I. 2021. Dynamics of topological defects and structural synchronization in a forming periodic tissue. Nat. Phys. 17, 410-415. ( 10.1038/s41567-020-01069-z) [DOI] [Google Scholar]
- 15.Melo D, Porto A, Cheverud JM, Marroig G. 2016. Modularity: genes, development and evolution. Annu. Rev. Ecol. Evol. Syst. 47, 463-486. ( 10.1146/annurev-ecolsys-121415-032409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barthelat F, Li CM, Comi C, Espinosa HD. 2006. Mechanical properties of nacre constituents and their impact on mechanical performance. J. Mater. Res. 21, 1977-1986. ( 10.1557/jmr.2006.0239) [DOI] [Google Scholar]
- 17.Katti DR, Pradhan SM, Katti KS. 2004. Modeling the organic-inorganic interfacial nanoasperities in a model bio-nanocomposite, nacre. Rev. Adv. Mater. Sci. 6, 162-168. [Google Scholar]
- 18.Barthelat F, Tang H, Zavattieri PD, Li CM, Espinosa HD. 2007. On the mechanics of mother-of-pearl: a key feature in the material hierarchical structure. J. Mech. Phys. Solids 55, 306-337. ( 10.1016/j.jmps.2006.07.007) [DOI] [Google Scholar]
- 19.Katti KS, Katti DR, Pradhan SM, Bhosle A. 2005. Platelet interlocks are the key to toughness and strength in nacre. J. Mater. Res. 20, 1097-1100. ( 10.1557/JMR.2005.0171) [DOI] [Google Scholar]
- 20.Currey JD, Taylor JD. 1974. The mechanical behaviour of some molluscan hard tissues. J. Zool. 173, 395-406. ( 10.1111/j.1469-7998.1974.tb04122.x) [DOI] [Google Scholar]
- 21.Currey JD. 1976. Further studies on the mechanical properties of mollusc shell material. J. Zool. 180, 445-453. ( 10.1111/j.1469-7998.1976.tb04690.x) [DOI] [Google Scholar]
- 22.Shen CH, Springer GS. 1977. Effects of moisture and temperature on the tensile strength of composite materials. J. Compos. Mater. 11, 2-16. ( 10.1177/002199837701100102) [DOI] [Google Scholar]
- 23.Bertinetti L, Hangen UD, Eder M, Leibner P, Fratzl P, Zlotnikov I. 2015. Characterizing moisture-dependent mechanical properties of organic materials: humidity-controlled static and dynamic nanoindentation of wood cell walls. Phil. Mag. 95, 1992-1998. ( 10.1080/14786435.2014.920544) [DOI] [Google Scholar]
- 24.Bayerlein B, Bertinetti L, Bar-On B, Blumtritt H, Fratzl P, Zlotnikov I. 2016. Inherent role of water in damage tolerance of the prismatic mineral–organic biocomposite in the shell of Pinna nobilis. Adv. Funct. Mater. 26, 3663-3669. ( 10.1002/adfm.201600104) [DOI] [Google Scholar]
- 25.Jiao D, Liu ZQ, Zhu YK, Weng ZY, Zhang ZF. 2016. Mechanical behavior of mother-of-pearl and pearl with flat and spherical laminations. Mater. Sci. Eng. C 68, 9-17. ( 10.1016/j.msec.2016.05.089) [DOI] [PubMed] [Google Scholar]
- 26.Dutta A, Vanderklok A, Tekalur SA. 2012. High strain rate mechanical behavior of seashell-mimetic composites: analytical model formulation and validation. Mech. Mater. 55, 102-111. ( 10.1016/j.mechmat.2012.08.003) [DOI] [Google Scholar]
- 27.Seilacher A. 1970. Arbeitskonzept Zur Konstruktions-Morphologie. Lethaia 3, 393-396. ( 10.1111/j.1502-3931.1970.tb00830.x) [DOI] [Google Scholar]
- 28.Reify WE, Thomas RDK, Fischer MS. 1985. Constructional morphology: the analysis of constraints in evolution. Acta Biotheor. 34, 233-248. ( 10.1007/BF00046787) [DOI] [PubMed] [Google Scholar]
- 29.Lemanis R, Zlotnikov I. 2018. Finite element analysis as a method to study molluscan shell mechanics. Adv. Eng. Mater. 20, 24. ( 10.1002/adem.201700939) [DOI] [Google Scholar]
- 30.Barthelat F, Rim JE, Espinosa HD. 2009. A review on the structure and mechanical properties of mollusk shells: perspectives on synthetic biomimetic materials. In Applied scanning probe methods XIII (eds Bhushan B, Fuchs H), pp. 17-44. Berlin, Germany: Springer. [Google Scholar]
- 31.Taylor John D, Layman M. 1972. The mechanical properties of bivalve (Mollusca) shell strucutres. Palaeontology 15, 73-87. [Google Scholar]
- 32.Appellöf A. 1893. Die Schalen von Sepia, Spirula und Nautilus. Studien über den Bau und das Wachstum. Kongl Svenska Vetensk -Akad Handl 25, 1-106. [Google Scholar]
- 33.Mutvei H. 1964. Remarks on the anatomy of recent and fossil Cephalopoda: with description of the minute shell structure of belemnoids. Stockholm Contrib. Geol. 11, 79-102. [Google Scholar]
- 34.Mitchell PR, Phakey PP. 1995. Notes on the microstructure of the Nautilus shell. Scanning Microsci. 9, 215-230. [Google Scholar]
- 35.Mutvei H. 1967. On the microscopic shell structure in some Jurassic ammonoids. Neues Jahrbuch Fur Geologie Und Palaontologie-Abhandlungen 129, 157-166. [Google Scholar]
- 36.Kulicki C, Tanabe K, Landman NH, Kaim A. 2015. Ammonoid shell microstructure. In Ammonoid paleobiology: from anatomy to ecology (eds Klug C, Korn D, Baets KD, Kruta I, Mapes RH), pp. 321-357. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 37.Dall WH. 1869. Notes on the argonaut. Am. Nat. 3, 236-239. ( 10.1086/270419) [DOI] [Google Scholar]
- 38.Villepreux-Power J. 1856. Observations physiques sur le poulpe de l'Argonauta argo: commencées en 1832 et terminées en 1843. Paris, France: Charles de Mourges frères. [Google Scholar]
- 39.Oudot M, Shir IB, Schmidt A, Plasseraud L, Broussard C, Neige P, Marin F. 2020. A nature's curiosity: the argonaut ‘Shell’ and its organic content. Crystals 10, 839. ( 10.3390/cryst10090839) [DOI] [Google Scholar]
- 40.Bandel K, Dullo WC. 1984. Zur Schalenstruktur fossiler und rezenter Argonauta-Gehaeuse (Octopoda, Cephalopoda). Natur und Mensch 1984, 33-38. [Google Scholar]
- 41.Mitchell PR, Phakey PP, Rachinger WA. 1994. Ultrastructural observations of the argonaut shell. Scanning Microsci. 8, 35-46. [Google Scholar]
- 42.Finn JK. 2013. Taxonomy and biology of the argonauts (Cephalopoda: Argonautidae) with particular reference to Australian material. Molluscan Res. 33, 143-222. ( 10.1080/13235818.2013.824854) [DOI] [Google Scholar]
- 43.Wolfe K, Smith AM, Trimby P, Byrne M. 2013. Microstructure of the paper nautilus Argonauta nodosa shell and the novel application of electron backscatter diffraction (EBSD) to address effects of ocean acidification. Mar. Biol. 160, 2271-2278. ( 10.1007/s00227-012-2032-4) [DOI] [Google Scholar]
- 44.Stevens K, Griesshaber E, Schmahl W, Casella LA, Iba Y, Mutterlose J. 2017. Belemnite biomineralization, development, and geochemistry: the complex rostrum of Neohibolites minimus. Palaeogeogr. Palaeoclimatol. Palaeoecol. 468, 388-402. ( 10.1016/j.palaeo.2016.12.022) [DOI] [Google Scholar]
- 45.Checa AG, Linares F, Grenier C, Griesshaber E, Rodríguez-Navarro AB, Schmahl WW. 2021. The argonaut constructs its shell via physical self-organization and coordinated cell sensorial activity. iScience 24, 103288. ( 10.1016/j.isci.2021.103288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens K, Iba Y, Suzuki A, Mutterlose J. 2015. Biological and environmental signals recorded in shells of Argonauta argo (Cephalopoda, Octobrachia) from the Sea of Japan. Mar. Biol. 162, 2203-2215. ( 10.1007/s00227-015-2750-5) [DOI] [Google Scholar]
- 47.Chen CC, Lin CC, Liu LG, Sinogeikin SV, Bass JD. 2001. Elasticity of single-crystal calcite and rhodochrosite by Brillouin spectroscopy. Am. Mineral. 86, 1525-1529. ( 10.2138/am-2001-11-1222) [DOI] [Google Scholar]
- 48.Liu L, Chen C, Lin CC, Yang Y. 2005. Elasticity of single-crystal aragonite by Brillouin spectroscopy. Phys. Chem. Minerals 32, 97-102. ( 10.1007/s00269-005-0454-y) [DOI] [Google Scholar]
- 49.Tushtev K, Murck M, Grathwohl G. 2008. On the nature of the stiffness of nacre. Mater. Sci. Eng. C 28, 1164-1172. ( 10.1016/j.msec.2007.10.039) [DOI] [Google Scholar]
- 50.Sullivan M, Chen Y, Prorok B. 2015. New strengthening mechanisms of nacre in the abalone shell. Int. J. Exp. Comp. Biomech. 3, 236-249. ( 10.1504/IJECB.2015.073926) [DOI] [Google Scholar]
- 51.Jia Z, Wang L. 2019. 3D printing of biomimetic composites with improved fracture toughness. Acta Mater. 173, 61-73. ( 10.1016/j.actamat.2019.04.052) [DOI] [Google Scholar]
- 52.Launey ME, Munch E, Alsem DH, Barth HB, Saiz E, Tomsia AP, Ritchie RO. 2009. Designing highly toughened hybrid composites through nature-inspired hierarchical complexity. Acta Mater. 57, 2919-2932. ( 10.1016/j.actamat.2009.03.003) [DOI] [Google Scholar]
- 53.Kobayashi I. 1971. Internal microstructure of the shell of Argonauta argo. Venus 30, 103-111. ( 10.18941/venusjjm.30.3_103) [DOI] [Google Scholar]
- 54.Kuhn-Spearing LT, Kessler H, Chateau E, Ballarini R, Heuer AH, Spearing SM. 1996. Fracture mechanisms of the Strombus gigas conch shell: implications for the design of brittle laminates. J. Mater. Sci. 31, 6583-6594. ( 10.1007/BF00356266) [DOI] [Google Scholar]
- 55.Liang SM, Ji HM, Li YY, Li XW. 2021. An ingenious microstructure arrangement in deep-sea Nautilus shell against the harsh environment. ACS Biomater. Sci. Eng. 7, 4819-4827. ( 10.1021/acsbiomaterials.1c00956) [DOI] [PubMed] [Google Scholar]
- 56.Alghamdi S, Tan T, Hale-Sills C, Vilmont F, Xia T, Yang J, Huston D, Dewoolkar M. 2017. Catastrophic failure of nacre under pure shear stresses of torsion. Sci. Rep. 7, 13123. ( 10.1038/s41598-017-13492-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strąg M, et al. 2020. Anisotropy of mechanical properties of Pinctada margaritifera mollusk shell. Nanomaterials 10, 634. ( 10.3390/nano10040634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aldred N, Wills T, Williams Dn, Clare AS. 2007. Tensile and dynamic mechanical analysis of the distal portion of mussel (Mytilus edulis) byssal threads. J. R. Soc. Interface 4, 1159-1167. ( 10.1098/rsif.2007.1026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreri S, Hu B, Qin YX. 2013. Dehydration and rehydration alter elastic and viscoelastic nanomechanical properties of cortical and trabecular bone. Am. Soc. Mech. Eng. Digital Collection 44038, 331-332. ( 10.1115/SBC2010-19059) [DOI] [Google Scholar]
- 60.Lin AYM, Meyers MA. 2009. Interfacial shear strength in abalone nacre. J. Mech. Behav. Biomed. Mater. 2, 607-612. ( 10.1016/j.jmbbm.2009.04.003) [DOI] [PubMed] [Google Scholar]
- 61.Barthelat F, Espinosa HD. 2007. An experimental investigation of deformation and fracture of nacre-mother of pearl. Exp. Mech. 47, 311-324. ( 10.1007/s11340-007-9040-1) [DOI] [Google Scholar]
- 62.Teichert C, Matsumoto T. 1987. The ancestry of the genus nautilus. In Nautilus: the biology and paleobiology of a living fossil, reprint with additions (eds Saunders WB, Landman NH), pp. 25-32. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 63.Mutvei H. 1983. Flexible nacre in the nautiloid Isorthoceras, with remarks on the evolution of cephalopod nacre. Lethaia 16, 233-240. ( 10.1111/j.1502-3931.1983.tb00660.x) [DOI] [Google Scholar]
- 64.Landman NH, Rye DM, Shelton KL. 1983. Early ontogeny of Eutrephoceras compared to recent Nautilus and Mesozoic ammonites: evidence from shell morphology and light stable isotopes. Paleobiology 9, 269-279. ( 10.1017/S0094837300007685) [DOI] [Google Scholar]
- 65.Westermann GEG. 1999. Life habits of nautiloids. In Functional morphology of the invertebrate skeleton (ed. Savazzi E), pp. 263-298. New York, NY: John Wiley & Sons. [Google Scholar]
- 66.Westermann GEG. 1996. Ammonoid life and habitat. In Ammonoid paleobiology (eds Landman NH, Tanabe K, Davis RA), pp. 607-707, New York, NY: Springer. [Google Scholar]
- 67.Lukeneder A. 2015. Ammonoid habitats and life history. In Ammonoid paleobiology: from anatomy to ecology (eds Klug C, Korn D, Baets KD, Kruta I, Mapes RH), pp. 689-791. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 68.Stevens K, Mutterlose J, Wiedenroth K. 2015. Stable isotope data (delta O-18, delta C-13) of the ammonite genus Simbirskites: implications for habitat reconstructions of extinct cephalopods. Paleogeogr. Paleoclimatol. Paleoecol. 417, 164-175. ( 10.1016/j.palaeo.2014.10.031) [DOI] [Google Scholar]
- 69.Landman NH, Grier JW, Cochran JK, Grier JC, Petersen JG, Towbin WH. 2018. Nautilid nurseries: hatchlings and juveniles of Eutrephoceras dekayi from the lower Maastrichtian (Upper Cretaceous) Pierre Shale of east-central Montana. Lethaia 51, 48-74. ( 10.1111/let.12222) [DOI] [Google Scholar]
- 70.Gao H, Ji B, Jäger IL, Arzt E, Fratzl P. 2003. Materials become insensitive to flaws at nanoscale: lessons from nature. Proc. Natl Acad. Sci. USA 100, 5597-5600. ( 10.1073/pnas.0631609100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilbert PUPA, Porter SM, Sun CY, Xiao S, Gibson BM, Shenkar N, Knoll AH. 2019. Biomineralization by particle attachment in early animals. Proc. Natl Acad. Sci. USA 116, 17 659-17 665. ( 10.1073/pnas.1902273116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murdock DJE. 2020. The ‘biomineralization toolkit’ and the origin of animal skeletons. Biol. Rev. 95, 1372-1392. ( 10.1111/brv.12614) [DOI] [PubMed] [Google Scholar]
- 73.Murdock DJE, Donoghue PCJ. 2011. Evolutionary origins of animal skeletal biomineralization. Cells Tissue Organs 194, 98-102. ( 10.1159/000324245) [DOI] [PubMed] [Google Scholar]
- 74.De Yoreo JJ, et al. 2015. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760. ( 10.1126/science.aaa6760) [DOI] [PubMed] [Google Scholar]
- 75.Dauphin Y, Ball AD, Cotte M, Cuif JP, Meibom A, Salomé M, Susini J, Williams CT. 2008. Structure and composition of the nacre–prisms transition in the shell of Pinctada margaritifera (Mollusca. Bivalvia). Anal. Bioanal. Chem. 390, 1659-1669. ( 10.1007/s00216-008-1860-z) [DOI] [PubMed] [Google Scholar]
- 76.Olson IC, Blonsky AZ, Tamura N, Kunz M, Pokroy B, Romao CP, White MA, Gilbert PUPA. 2013. Crystal nucleation and near-epitaxial growth in nacre. J. Struct. Biol. 184, 454-463. ( 10.1016/j.jsb.2013.10.002) [DOI] [PubMed] [Google Scholar]
- 77.Beliaev M, Zöllner D, Pacureanu A, Zaslansky P, Bertinetti L, Zlotnikov I. 2020. Quantification of sheet nacre morphogenesis using X-ray nanotomography and deep learning. J. Struct. Biol. 209, 107432. ( 10.1016/j.jsb.2019.107432) [DOI] [PubMed] [Google Scholar]
- 78.Oliver WC, Pharr GM. 1992. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 7, 1564-1583. ( 10.1557/JMR.1992.1564) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.