Summary
Pacemaker endocarditis is rare and symptoms may be misleading. If missed, it carries significant morbidity and mortality, particularly in the elderly. Advances in multi-modality imaging in recent years have emphasised its role in clinical decision making. This case highlights the ability of multi-modality imaging techniques to individualise diagnosis, management and prognosis in patients with suspected cardiovascular implantable electronic device (CIED) endocarditis.
Keywords: cardiac pacemaker, endocarditis, computed tomography, three-dimensional imaging, echocardiography
Pacemaker endocarditis is an infrequent but potentially fatal complication of pacemaker implantation.1 The incidence of cardiovascular implantable electronic device (CIED) endocarditis however is increasing, as percutaneous insertion of various devices is on the rise in cardiology practice. Additionally, an aging population with multiple co-morbidities increases the risk for device infections.2 The reported incidence, although varied among studies, is between 0.13 and 7%.3
Case report
We present a case of a 64-year-old woman with a background of poorly controlled type 2 diabetes mellitus, primary systemic hypertension and dyslipidaemia. Her medication included the use of simvastatin 20 mg at night, amlodipine 10 mg daily, enalapril 10 mg twice daily, metformin 850 mg and biphasic insulin twice daily. She was not known to have any resulting target-organ damage.
A Medtronic VDD pacemaker was implanted in 2009 for third-degree heart block, which required a generator change in June 2020. Following the procedure, there was normal ventricular lead impedance (686 Ω). She subsequently presented a month later with an index episode of syncope. Device interrogation showed paroxysmal atrial fibrillation. Relevant clinical examination findings included a tachycardia (112 beats per minute), a normal body temperature (37.6°C) and a normal cardiac examination. There were no features suggestive of infective endocarditis (IE) and her neurological function was normal. A few days into the admission, she developed fever.
Laboratory investigations revealed an elevation in systemic inflammatory markers [C-reactive protein 85 mg/l (normal < 10 mg/l), white cell count 13.24 × 109 cells/l (3.9–12.6 × 109 cells/l), neutrophil count 10.14 × 109 cells/l (1.6–8.3 × 109 cells/l) and procalcitonin 12.81 μg/l (2–10 μg/l is suggestive of systemic infection)]. Serial blood cultures were persistently positive for Enterococcus faecalis, sensitive to ampicillin.
Transthoracic echocardiography (TTE) was unhelpful in the search for infective or CIED endocarditis due to reverberation artefact. The patient underwent two-dimensional (2D) and threedimensional (3D) transoesophageal echocardiography (TEE) while being treated empirically for CIED endocarditis with benzyl penicillin and gentamicin. A large oscillating mass, likely a vegetation, was noted on the atrial aspect of the transvenous pacing lead measuring 20 × 14 mm in length (Figs 1–4).
Due to the development of confusion and dyspnoea, a computed tomography (CT) scan of the brain and chest was performed. There was no acute intracranial pathology and CT scan of the chest confirmed a large vegetation (122 Hounsfield units) along the pacemaker lead, which extended through the tricuspid valve (Fig. 5). Distal septic pulmonary artery emboli were also noted (Fig. 5).
Fig. 1.
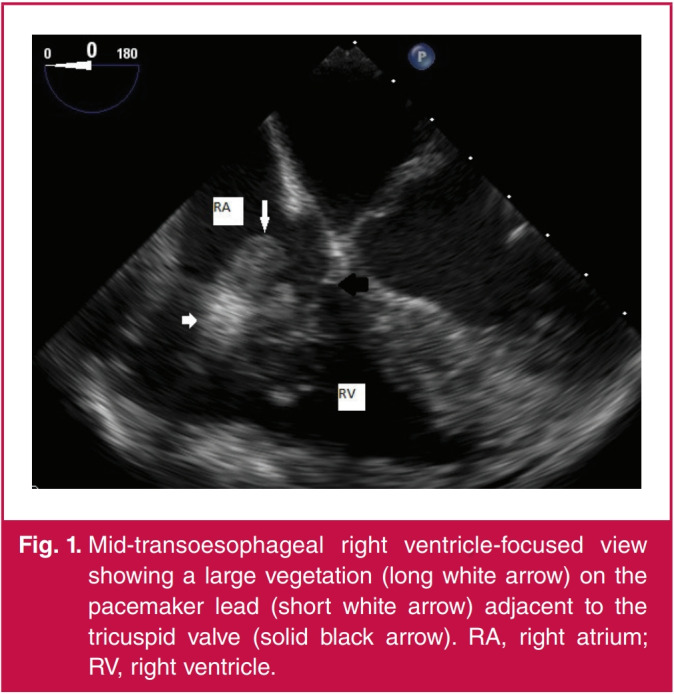
Mid-transoesophageal right ventricle-focused view showing a large vegetation (long white arrow) on the pacemaker lead (short white arrow) adjacent to the tricuspid valve (solid black arrow). RA, right atrium; RV, right ventricle.
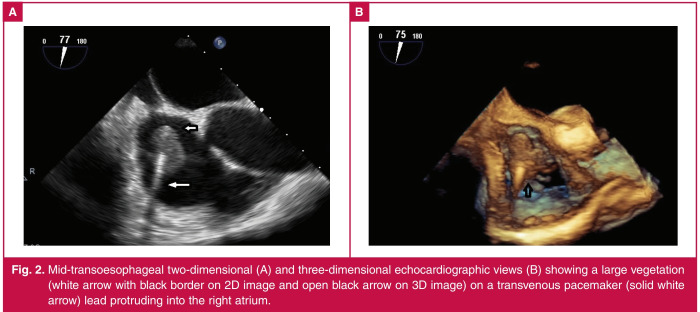
Fig. 2.
Mid-transoesophageal two-dimensional (A) and three-dimensional echocardiographic views (B) showing a large vegetation (white arrow with black border on 2D image and open black arrow on 3D image) on a transvenous pacemaker (solid white arrow) lead protruding into the right atrium.
Fig. 3.
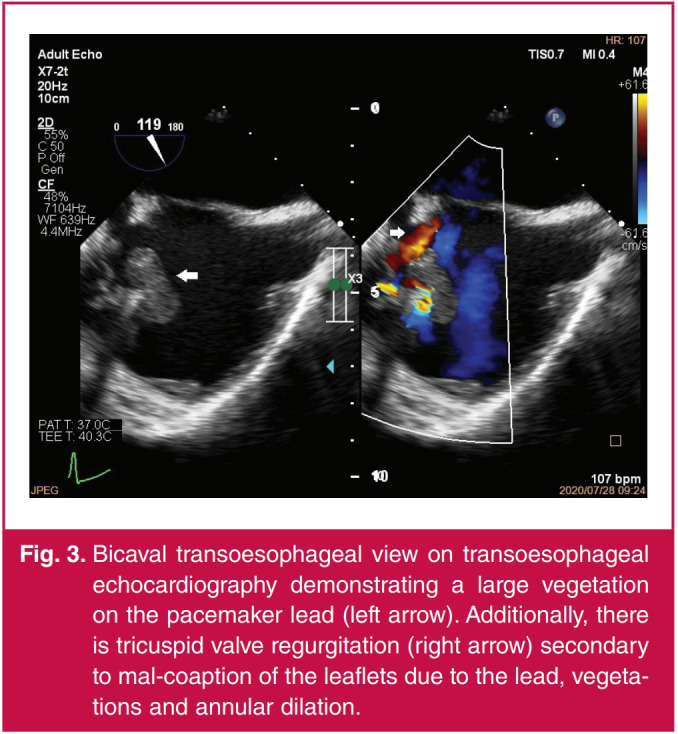
Bicaval transoesophageal view on transoesophageal echocardiography demonstrating a large vegetation on the pacemaker lead (left arrow). Additionally, there is tricuspid valve regurgitation (right arrow) secondary to mal-coaption of the leaflets due to the lead, vegetations and annular dilation.
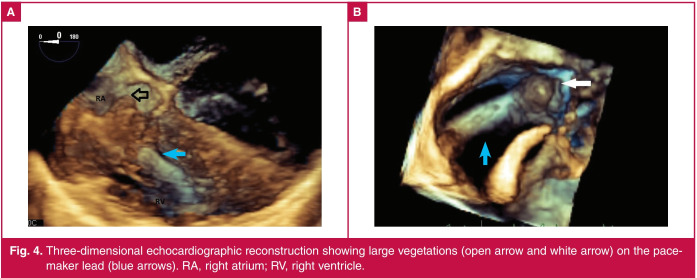
Fig. 4.
Three-dimensional echocardiographic reconstruction showing large vegetations (open arrow and white arrow) on the pacemaker lead (blue arrows). RA, right atrium; RV, right ventricle.
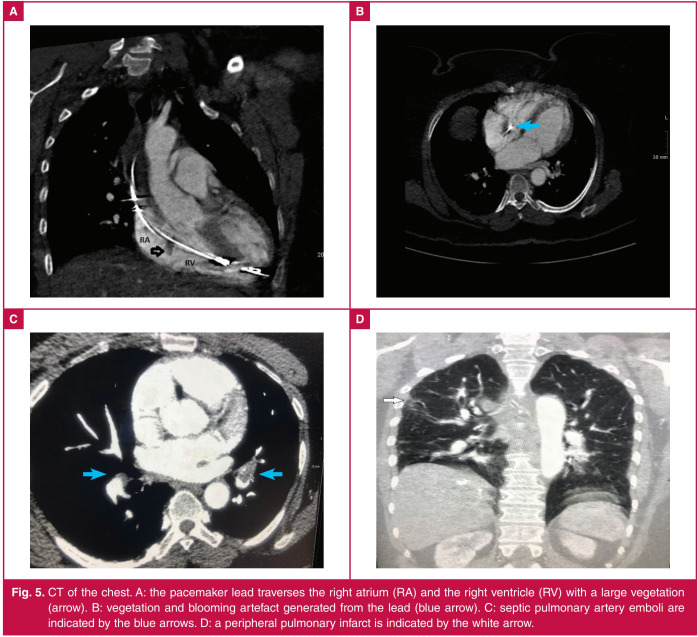
Fig. 5.
CT of the chest. A: the pacemaker lead traverses the right atrium (RA) and the right ventricle (RV) with a large vegetation (arrow). B: vegetation and blooming artefact generated from the lead (blue arrow). C: septic pulmonary artery emboli are indicated by the blue arrows. D: a peripheral pulmonary infarct is indicated by the white arrow.
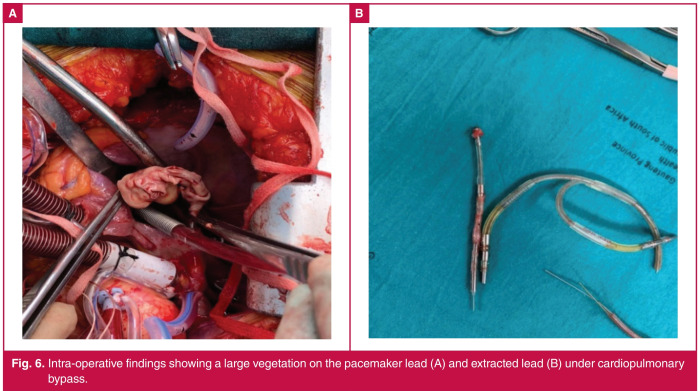
Fig. 6.
Intra-operative findings showing a large vegetation on the pacemaker lead (A) and extracted lead (B) under cardiopulmonary bypass.
Based on the Dukes criteria,3 a definitive diagnosis of CIED endocarditis was made. Following a heart team discussion, the patient was recommended for surgical lead extraction and implantation of an epicardial pacing system (Fig. 6). The patient completed six weeks of intravenous ampicillin and recovered well. She continues to follow up at the pacemaker clinic where her device is interrogated annually. Thus far, there have been no short- to medium-term complications following device extraction and epicardial lead implantation.
Discussion
CIED endocarditis secondary to enterococci is not well described.4 Prior studies have validated that the elderly with multiple underlying co-morbidities are most susceptible and suffer high morbidity and mortality rates.4 Blood-borne seeding of the device, either originating from a genitourinary (GU) or gastrointestinal (GI) source, is associated with a longer time to infection.4 This event however may be spontaneous and asymptomatic, even in the absence of a documented GU or GI procedure in the days preceding enterococci-related CIED endocarditis.4
Diagnosing CIED endocarditis can be challenging, as infection may be localised to the skin pocket, or extending to involve the leads, valve leaflets or endocardium. TTE has poor sensitivity and specificity in detecting CIED endocarditis.5 This is due to the acoustic shadowing created by the metallic components of the leads. Right-sided leads may have superimposed fibrin strands or thrombotic material due to the low-pressure venous system.6 These thrombi are indistinguishable from vegetations on TTE. Additionally, many of the extra-cardiac portions of the leads are not visualised on standard transthoracic echo views.6
The superiority of 2D TEE compared to TTE in the detection of vegetations on pacing leads has been corroborated.7 TEE has a sensitivity of 87–100% and a specificity of 91–100% for the diagnosis of infective endocarditis, but slightly lower for CIED endocarditis.6 Although TTE may diagnose a small proportion of cases, TEE is unequivocally the mainstay of imaging in CIED endocarditis as it is readily available, portable, inexpensive and not associated with radiation exposure.7 It allows for focused views of the right atrial and superior vena cava segments of the lead, as well as defining the size of vegetations that ultimately impacts on embolic risk and decision for early surgery.6 Infrequently, there may still be diagnostic uncertainty following TEE and additional modalities may be required.
Three-dimensional reconstruction of a real-time anatomical image has enabled some of the limitations posed by 2D echocardiography to be overcome.7 Despite poor temporal resolution of 3D echocardiography, excellent frame rates are attained, allowing good image quality as noted in the current case.7 The main limitation of 2D TEE is in selecting the true maximum diameter of vegetations.8 Realtime 3D TEE solves this problem by utilising volumetric reconstruction of masses. This enables an accurate assessment of vegetation size and morphology, thereby better predicting risk of embolism. It also allows visualisation of longer segments and the path that pacemaker leads take through the tricuspid valve.8
Cardiac CT has poor sensitivity in identifying vegetations on pacemaker leads compared to TEE. This is due to blooming, streak and beam-hardening artefacts.5 Electrocardiographgated cardiac CT improves spatial resolution while reducing radiation exposure. Non-gated contrast-enhanced CT plays a role in differentiating pacemaker pocket sepsis from non-infected haematomas or superficial cellulitis. Contrastenhanced CT may be used to search for pulmonary emboli or other vascular complications, such as mycotic aneurysms in the context of complicated endocarditis,5 as in our case.
The management of CIED endocarditis includes prolonged antibiotic therapy (four to six weeks) and device removal, as medical therapy alone is associated with an increased mortality and recurrence rate.3 Parenteral antibiotics should be administered for at least two weeks after device extraction in cases of bacteraemia and blood cultures should be negative for at least 72 hours, prior to re-implantation of a new device. Ultimately, transvenous versus surgical lead removal should be individualised, taking into account vegetation size, patient co-morbidities, expertise of staff, as well as the presence of cardiothoracic surgical support.3
Conclusions
Multi-modality imaging plays a critical role in the diagnostic work-up and clinical decision making of patients with complicated CIED endocarditis. Not only does it enable an accurate diagnosis, but allows for early referral to a tertiary centre for appropriate management. Our case highlights the appropriate and effective use of both cardiac CT and 3D echocardiography when TTE was inconclusive. Additionally, this case highlights maintenance of a high index of suspicion for CIED endocarditis on follow up of elderly patients postpacemaker implantation. This is to ensure early diagnosis and treatment of a condition associated with high morbidity and mortality rates if left untreated.
Acknowledgments
Consent for the write-up of this report was obtained from the chief executive officer of our institution and the Wits University Human Research Ethics Council. Informed patient consent was obtained as per the standard consent form used at our institution. The authors declare that no identifiable patient data appear in this article. Dr P Parbhoo obtained patient consent, collected data and wrote the manuscript. Dr R Meel collected data, obtained and processed 3D echocardiography and conceptualised the article. Dr T Nell acquired and processed cardiac CT images and reviewed the manuscript. Dr Lebohang Molopa ís acknowledged for providing intra-operative images.
Contributor Information
Priya Parbhoo, Email: priya.parbhoo@gmail.com, Department of Internal Medicine, Division of Cardiology, Chris Hani Baragwanath Academic Hospital, University of the Witwatersrand, Johannesburg, South Africa.
Ruchika Meel, Department of Internal Medicine, Division of Cardiology, Chris Hani Baragwanath Academic Hospital, University of the Witwatersrand, Johannesburg, South Africa.
Tamarin Nell, Department of Radiology, Chris Hani Baragwanath Academic Hospital, University of the Witwatersrand, Johannesburg, South Africa.
References
- 1.De Silva K, Fife A, Murgatroyd F, Gall N. Pacemaker endocarditis: an important clinical entity. Br Med J Case Rep. 2009;bcr02 doi: 10.1136/bcr.02.2009.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelstein S, Yahalom M. Cardiac device-related endocarditis: Epidemiology, pathogenesis, diagnosis and treatment – a review. Int J Angiol. 2009;18:167–172. doi: 10.1055/s-0031-1278347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F. et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 4.Oh TS, Le K, Baddour LM, Sohail MR, Vikram HR, Hernandez- Meneses M. et al. Cardiovascular implantable electronic device infections due to enterococcal species: clinical features, management, and outcomes. Pacing Clin Electrophysiol. 2019;42:1331–1339. doi: 10.1111/pace.13783. [DOI] [PubMed] [Google Scholar]
- 5.Galea N, Bandera F, Lauri C, Autore C, Laghi A, Erba PA. Multimodality imaging in the diagnostic work-up of endocarditis and cardiac implantable electronic device (CIED) infection. J Clin Med. 2020;9:2237. doi: 10.3390/jcm9072237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horgan SJ, Mediratta A, Gillam LD. Cardiovascular imaging in infective endocarditis: a multimodality approach. Circ Cardiovasc Imaging. 2020;13:e008956. doi: 10.1161/CIRCIMAGING.120.008956. [DOI] [PubMed] [Google Scholar]
- 7.Galzerano D, Kinsara AJ, Di Michele S, Vriz O, Fadel B, Musci RL. et al. Three dimensional transesophageal echocardiography: a missing link in infective endocarditis imaging? Int J Cardiovasc Imaging. 2020;36:403–413. doi: 10.1007/s10554-019-01747-x. [DOI] [PubMed] [Google Scholar]
- 8.Sordelli C, Fele N, Mocerino R, Weisz SH, Ascione L, Caso P. et al. Infective endocarditis: echocardiographic imaging and new imaging modalities. J Cardiovasc Echogr. 2019;29:149–155. doi: 10.4103/jcecho.jcecho_53_19. [DOI] [PMC free article] [PubMed] [Google Scholar]