Abstract
This systematic review assessed the long-term outcomes for patients treated with intravitreal antivascular endothelial growth factor or dexamethasone for macular oedema (MO) secondary to retinal vein occlusion (RVO). Studies investigating patients of all ages with MO due to RVO only were included. The review was deliberately broad in scope, including comparative and non-comparative studies to ensure inclusion of real-world type evidence. Risk of bias was assessed. In total, 76 data sets were included (10 775 participants). Overall, mean best-corrected visual acuity (BCVA) improved from baseline to 5 years by 16.1 letters (p<0.01). BCVA improved from baseline in both central RVO (CRVO) and branch RVO (BRVOs) at 2 years, by 9.1 (p<0.01) (difference from baseline in CRVOs) and 9.1 (p<0.01) letters, respectively. At 5 years, BCVA improved from baseline in CRVOs by 15.6 letters and in BRVOs by 16.2; the difference between RVO types was not significant (p=0.18). Two studies had 5-year data for ranibizumab, and improvement was evident. There was no significant difference between outcomes in randomised controlled trials (RCTs) compared with non RCTs. These results suggest a benefit to receiving long-term intravitreal treatments for MO due to RVO.
Keywords: macula, retina, drugs, treatment medical, vision
Introduction
Retinal vein occlusion (RVO) is the second most common retinal cause of vision loss after diabetic retinopathy. In 2019, global prevalence of RVO was estimated to be 0.77% in adults aged 30–89 years.1 RVO is caused by thrombus formation, thought to occur due to compression from an adjacent arteriosclerotic artery, where artery and vein cross and share a common adventitial sheath.2 Central RVO (CRVO) has been associated with a significantly lower quality of life has been reported.3 Macular oedema (MO) affects 75% of patients with branch RVO (BRVO) and 85% patients with CRVO in England and Wales2 and is the most common cause of visual loss in RVO. MO secondary to RVO is thought to occur due to increased hydrostatic pressure, inflammatory cytokines and increased capillary permeability causing leakage into the extracellular space.4 Vascular endothelial growth factor (VEGF) is a key cytokine mediating capillary leakage and subsequent MO and is therefore targeted by several intravitreal therapies (bevacizumab, ranibizumab and aflibercept). Dexamethasone is also used as an intravitreal treatment.2
Although the outcomes of these treatments are well described in the literature, their efficacy after two or more years of use is less well established. Landmark studies have had outcomes at 52 weeks (BRAVO), 24 (VIBRANT),5 6 52 (CRUISE),7 100 (COPERNICUS),8 76 (GALILEO)8 and 24 (GENEVA)9 weeks. Anecdotally, patients want to know longer-term outcomes and an evidence based, comprehensive answer is lacking. This systematic review aimed to evaluate treatment outcomes assessed after 2 years or more of intravitreal injection for patients with MO caused by RVO.
Methods
This was a systematic review. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used to guide the conduct and report of this review.10
The aim was to investigate the long-term outcomes for patients with MO due to RVO, treated with intravitreal injections of (1) anti-VEGFs, specifically aflibercept, bevacizumab or ranibizumab or (2) the dexamethasone implant or (3) any combination of these, described as ‘combination treatment’ throughout. Long term was defined as outcomes assessed at 2–5 years. It was planned to accept comparative and non-comparative studies and retrospective and prospective studies. Studies including using laser treatment as the comparator arm were excluded. The outcomes of interest were best-corrected visual acuity (BCVA) and central retinal thickness (CRT) in µm.
An electronic search was conducted in the Medline, Embase, Cochrane and Web of Science databases to identify potentially eligible publications. Search filters were English language studies only, and no time limits were set on publication dates. The search strategy used is summarised in online supplemental tables 1, 2.
bmjophth-2022-001010supp001.pdf (40.7KB, pdf)
bmjophth-2022-001010supp002.pdf (39.7KB, pdf)
After application of the search term a list of ‘potentially eligible studies’ resulted. One reviewer (AH) screened each title and abstract. If eligibility was unclear, studies were included at this stage. Duplicates were identified and the resulting papers were examined again to produce a list of ‘definitely eligible’ studies. This list was used for data extraction. Variables extracted were patient age (mean, median and range), the percentage male, country of study and ethnicity of participants if given, study design, RVO type and drug used. Although there is no generally accepted consensus on whether hemiretinal and hemispheric vein occlusions (HRVOs) are comparable to CRVO or BRVOs, for this study HRVOs were grouped with CRVOs, given the clinical implications of involvement of half the retina, that is, the likely poorer prognosis than with involvement of a single quadrant or less. If more than one drug was given to a patient, this was classified as ‘combination treatment’. Baseline BCVA and CRT were recorded at baseline and if available at 2 years, 3 years, 4 years and 5 years after initiation of treatment. Any BCVA values recorded in the log(MAR) scale or Snellen chart were converted to Early Treatment Diabetic Retinopathy Study (ETDRS) letters. The study-level risk of bias was assessed using the Cochrane Risk of Bias Tool11 for RCTs, the Critical Appraisal Skills Programme cohort checklist12 for cohort studies, and the Joanna Briggs Institute Critical Appraisal case series checklist13 for retrospective studies. Studies included were categorised into low risk, high risk or unclear categories based on selection, detection, attrition and reporting bias domains.14 If information needed to judge the risk of bias was lacking, studies were classified as ‘unclear risk of bias’.
Data were analysed using SPSS V.27. The mean, SD, range and 95% CIs were compared at each year of follow-up with baseline for BCVA and CRT for all papers and for each RVO type, treatment used and study type (divided into RCTs and ‘other’ study types). The means for BCVA and CRT were compared at each time point using independent samples t-test for comparing two groups (for example RCT vs non-RCT study types) and one-way analysis of variance (ANOVA) test for comparing greater than two groups (drug type). The significance value was set at p<0.05.
Results
There were 4104 potentially eligible studies (figure 1). After elimination of duplicates, 3050 studies were left, which were screened using the title and abstracts. In total, 2880 studies were excluded at this stage; 38.9% had a follow-up time less than 2 years, including studies with a 2-year mean follow-up but range starting less than 2 years. Then 170 studies were left to screen using full text if available: two studies had no full text available. A further 120 were excluded for reasons which included the follow-up time totalling less than 2 years, absolute BCVA or CRT values not given, or full text not available in English. Overall, 48 studies were eligible for analysis (online supplemental table 3). Studies were classified as ‘CRVO’, ‘BRVO’ or ‘mixed RVO type’. If articles investigated patients with CRVO and BRVO and presented separate outcomes by RVO type, the studies’ cohorts were analysed separately based on RVO type; thus, there were 76 cohorts in total from 48 studies. Eight studies included both RVO types, however, did not present data separately; therefore, these were recorded as one cohort and classified as ‘mixed’ RVO type’. Eight studies separated RVO type into ischaemic and non-ischaemic,15 however, due to the small numbers and inconsistent definitions, ischaemic status was not recorded. Three studies were deemed to have a high risk of bias and 11 studies had moderate risk of bias (online supplemental tables 4, 5).
Figure 1.
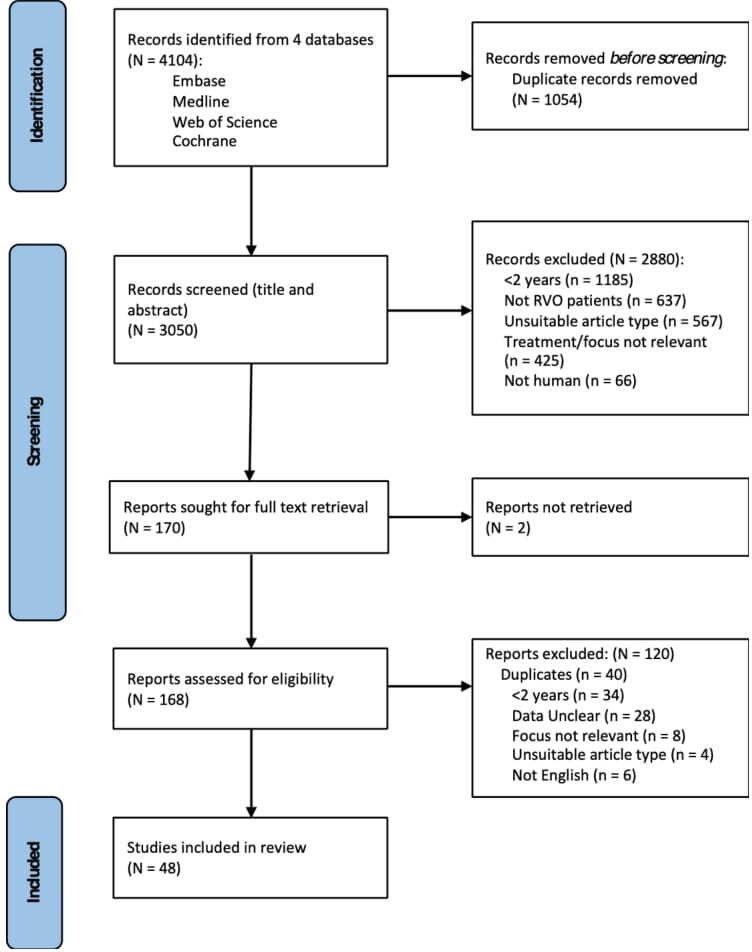
Flow chart of selection and screening process. Adapted from Page et al.10
bmjophth-2022-001010supp003.pdf (3.6MB, pdf)
bmjophth-2022-001010supp004.pdf (48.4KB, pdf)
bmjophth-2022-001010supp005.pdf (71.5KB, pdf)
The 76 cohorts with BCVA at baseline constituted 10 775 participants. At 2 years, there were data for 65 cohorts (10 304 participants), at 3 years 25 cohorts (5775 participants), at 4 years 11 cohorts (501 participants) and at 5 years, 8 cohorts (402 participants) (online supplemental table 6).
bmjophth-2022-001010supp006.pdf (39.1KB, pdf)
For CRVOs, the mean baseline BCVA was 48.2 (95% CI 44.0 to 52.4) letters and for BRVOs 55.4 (95% CI 51.7 to 59.1) letters. After 2 years, BCVA improved in CRVOs by 9.1 letters and in BRVOs also by 9.1 letters, to 57.3 (95% CI 51.9 to 62.7, p<0.01) and 64.5 (95% CI 58.2 to 70.7, p<0.01) letters, respectively. After 3 years BCVA declined for CRVOs to 53.7 (95% CI 48.4 to 59.1, p<0.01) letters and improved from baseline for BRVOs to 67.3 (95% CI 61.4 to 73.1, p<0.01) letters. BCVA improved from baseline at 5 years for CRVOs by 15.6 letters to 63.8 (95% CI 53.3 to 74.2, p<0.01) letters and for BRVOs by 16.2 to 71.6 (95% CI: 59.0 to 84.3, p=0.01) letters, respectively (figure 2).
Figure 2.
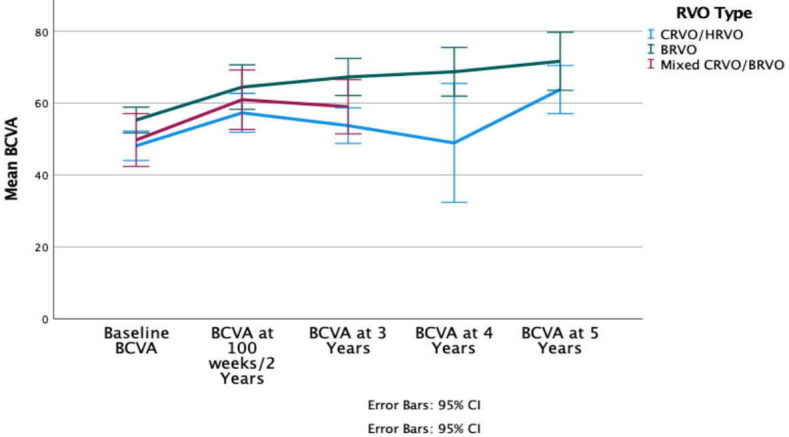
Mean BCVA from baseline to 5 years after initial treatment for each RVO type. BCVA, best-corrected visual acuity; BRVO, branch retinal vein occlusion; CRVO central retinal vein occlusion; HRVO, hemiretinal and hemispheric vein occlusion; RVO, retinal vein occlusion.
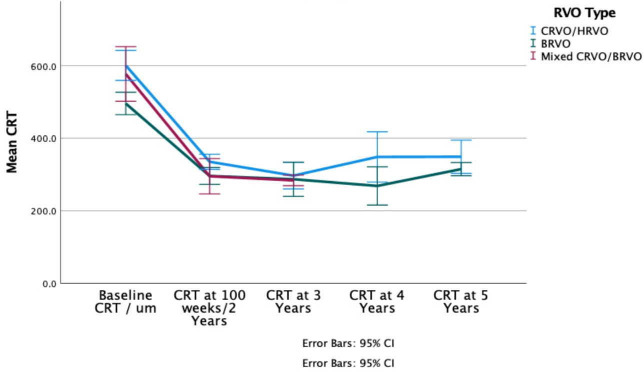
Mean baseline CRT was 603.1 µm (95% CI 560.4 to 645.8) for CRVOs and 496.7 µm (95% CI 464.6 to 528.8) for BRVOs. For all patients, regardless of RVO type, mean CRT decreased from 554.3 µm (95% CI 527.1 to 581.6) to 314.4 µm (95% CI 299.2 to 329.7) (p<0.01 vs baseline) after 2 years. At 5 years follow-up, the mean decrease in CRT for CRVOs was 254.2 µm (p=0.01) and 147.8 µm for BRVOs (p=0.02) (figure 3).
Figure 3.
Mean central retinal thickness (CRT) from baseline to 5 years after initial treatment for each RVO type. BRVO, branch retinal vein occlusion; CRVO central retinal vein occlusion; HRVO, hemiretinal and hemispheric vein occlusion; RVO, retinal vein occlusion.
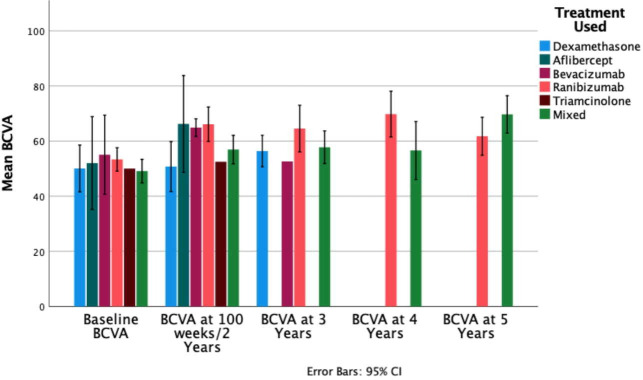
When drugs were compared (figure 4) mean baseline BCVA was lowest in patients who received combination treatment during the study (49.1 letters, 95% CI 44.8 to 53.4), however, this improved to 56.9 (95% CI 51.7 to 62.2, p<0.01 difference from baseline) letters and 69.7 (95% CI 61.2 to 78.2, p=0.01 difference from baseline) letters after 2 and 5 years, respectively. Not all treatment types had follow-up periods beyond 2 years. Ranibizumab had 28 cohorts at baseline (2262 participants) and 8 cohorts (280 participants) with data at 3 years. BCVA for ranibizumab improved 11.2 letters from 53.4 at baseline to 3 years (95% CI 49.1 to 57.6, p<0.01). Dexamethasone had 8 cohorts at baseline with 1165 participants and 5 cohorts (703 participants) at 3 years. BCVA for dexamethasone improved 6.3 letters from 50.1 at baseline at 3 years (95% CI 41.6 to 58.6, p=0.55). Bevacizumab had 8 cohorts at baseline with 582 participants and 1 cohort (57 participants) at 3 years. BCVA with bevacizumab decreased by 2.5 letters from 55.1 letters baseline at 3 years (95% CI 40.7 to 69.2) to 52.6 at 3 years. Two of the studies with ‘ranibizumab only’ cohorts had 5-year follow-up: mean BCVA decreased from 3 years to 61.8 (95% CI 19.2 to 104.3) letters (p=0.077), though this still was an improvement from baseline.
Figure 4.
Mean BCVA at each timepoint according to treatment type. BCVA, best-corrected visual acuity.
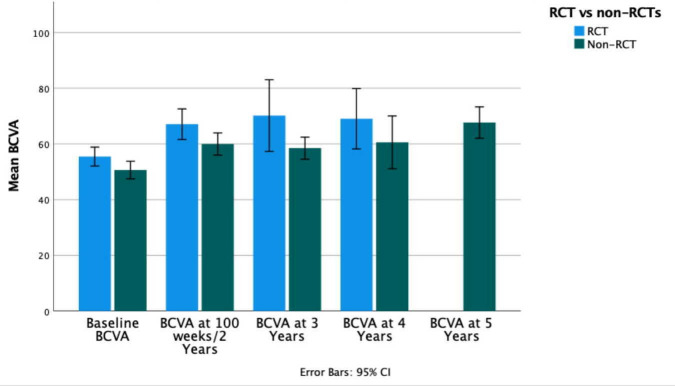
There were 30 retrospective case series, 28 prospective cohort studies, 15 RCTs and 3 prospective case series. When comparing study types, studies were categorised into two groups, RCTs and non-RCTs. At 4 years (there was no 5-year data available for RCTs) in RCTs, BCVA improved by 13.6 letters from 55.5 letters (95% CI 52.1 to 58.9) at baseline to 69.1 letters (95% CI 4.9 to 133, p=0.11) compared with a 10-letter improvement in non-RCTs from 50.6 letters at baseline (95% CI 47.5 to 53.8) to 60.6 (95% CI 48.9 to 72.2, p<0.01) (figure 5). The CRT results similarly varied between RCT and non-RCT studies. At 4 years, in RCTs, there was a decrease in CRT of 366 µm, from 570.7 µm (95% CI 472.4 to 668.9) at baseline to 204.7 µm (95% CI 1.9 to 407.3, p=0.13), and in non-RCTs, CRT decreased by 196.8 µm from 549.6 µm (95% CI 525.7 to 573.5) at baseline to 352.8 µm (95% CI 293.2 to 412.5, p<0.01).
Figure 5.
Bar graph comparing mean BCVA in RCT against non-RCT studies at each time point. BCVA, best-corrected visual acuity. RCT, randomised controlled trials.
The heterogeneity of studies led to varied study characteristics. Thirty-three studies followed patients for up to 2 years whereas others had up to 5 years’ follow-up. Studies varied according to the data source used, for example, most retrospective studies reviewed electronic medical records,16 whereas in RCTs and prospective cohort studies data were recorded contemporaneously.17 Twenty-six studies included only treatment naïve patients. Whereas in six studies, although baseline BCVA prior to original treatment was not recorded, patients with previous treatment for MO due to RVO were not excluded.18 Some studies gave a baseline BCVA and CRT measurement for inclusion.18 19 Ten studies gave separate data for both CRVOs and BRVOs20 and 10 had CRVO data only,21 while 12 had BRVO data only.22 The remaining six studies either combined data or didn’t specify RVO type, and therefore, were assumed to contain both CRVO and BRVO patients.23 Studies varied on use of equipment to measure CRT. Nineteen studies used the Heidelberg Spectralis OCT machine.24 Thirteen studies used the Cirrus HD-OCT, Carl Zeiss Meditec OCT machine25 and two used the 3D-OCT 2000 OCT machine.26 27 The other studies did not specify what equipment was used. One RCT randomised patients to receive a certain drug against sham28; the outcomes for patients in the sham arm were disregarded. Three RCTs randomised patients to receive different doses of the same drug,29 which were recorded as one drug type, and three randomised patients into different treatment groups and results were recorded separately for the purposes of our study.20
Discussion
This systematic review revealed clinically meaningful improvement in BCVA and CRT for up to 5 years for patients treated with intravitreal anti-VEGF or dexamethasone for MO secondary to RVO. It was deliberately the case that data were synthesised from disparate studies, so while formal meta-analysis would have been inappropriate, our review gives a picture of long-term outcomes, combining clinical trial and real-world settings.
Overall, mean BCVA improved from baseline up to 5 years by 16.1 ETDRS letters. At 5 years, BCVA improved from 48.2 letters at baseline to 63.8 in CRVOs and from 55.4 letters to 71.7 letters in BRVOs. CRT decreased by 254.2 µm from 603.1 µm at baseline in CRVOs, and by 181.9 µm from 496.7 µm at baseline in BRVOs. Thus, patients can be encouraged that sustained benefits are possible up to at least 5 years with ongoing treatment. At 5 years, though BCVA and CRT were better for BRVOs than CRVOs, there was no significant difference between CRVO and BRVO patients in BCVA (p=0.18) or CRT (p=0.23), but this may be due to smaller numbers followed up for this long.
The inclusion criteria were set to include various study designs. RCTs are the gold standard, with guaranteed scheduled visits, and strict eligibility criteria, often excluding patients with poor baseline vision or comorbidities. RCTs might be expected to provide potentially better results than real-world studies.30 However, in this review, there was no significant difference between BCVA and CRT results at 4 years between RCTs and non-RCTs. Injection frequency was rarely and inconsistently reported, and so was not analysed in this review. Although biases may have been a factor, for example, in selection of participants into non controlled prospective studies that outcomes in non-RCTs were as good as those in RCTs may give encouragement to those managing RVOs in the ‘real world’.
The study numbers for aflibercept and bevacizumab were too small to draw any meaningful conclusions. For the 28 studies and 2262 participants with ‘ranibizumab only cohorts’, BCVA improved from 53.4 letters at baseline to 61.8 after 5 years (2 cohorts with 21 participants at 5 years). For dexamethasone implant only cohorts, BCVA improved from baseline to 3 years by 6.3 letters to 56.4 letters. Repeated steroid injections will cause cataract,31 which may have blunted absolute BCVA results. For patients treated with a combination treatment, the necessity to switch patients from one drug to another probably resulted from a suboptimal response with the original drug, and so a poorer prognosis may have been expected in this cohort, perhaps reflected in the lower baseline BCVA for combination treatment, though BCVA improved in a clinically meaningful way up to 5 years.
Intravitreal anti-VEGF injections have been proven to be effective and are used first line in treating MO secondary to other diseases including diabetic MO (DMO).32 33 The Protocol T Extension study34 was an RCT investigating long term outcomes of patients with MO secondary to DMO treated with either aflibercept, bevacizumab or ranibizumab over 5 years. BCVA improved from baseline after 5 years, though had fallen from year 2 to 5. Studies and reviews cannot be compared, especially across indications: all that can be said is that sustained improvements have been demonstrated with DMO too, though reasons for the fall after year 2 warrant consideration.
The strengths of this review included the broad inclusion criteria. This review aimed to capture the totality of evidence, including real-world practice as represented, in many cases, by retrospective and prospective uncontrolled studies. Analysing according to type of RVO, drug used and study type (RCT and non-RCT) was informative: although no formal analysis was performed on the interaction of these categories, the likelihood of interaction and confounding of results in clinical practice is low.
In this review, studies had various primary outcomes and methods to measure BCVA and CRT results. For example, some recorded percentage of patients with an improvement in BCVA of 15 letters or greater, while other studies presented absolute values or graphs only. Indeed, data was lost due to the exclusion of 27 studies which included data which was either unclear, in the form of graphs or only provided change in BCVA or CRT rather than absolute values.
Long-term prospective studies are needed to investigate patient outcomes beyond the first few years of treatment. Furthermore, it is important to consider the impact these treatments have on patients: only one study presented QoL data.15 Also, studies investigating the long-term cost-effectiveness of intravitreal anti-VEGF or steroid therapy, balanced with the long-term benefits to patients should be conducted. This systematic review provides evidence of long-term benefits of treatment for patients with MO due to both CRVO and BRVO.
bmjophth-2022-001010supp007.pdf (213KB, pdf)
Footnotes
Twitter: @AlexHunter9808
Contributors: MW conceived the idea, guided the review method and supervised its conduct. He checked and interpreted the analysis and edited the manuscript. AH conducted the search, screened and selected papers, conducted data extraction and analysis and performed statistical analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Song P, Xu Y, Zha M, et al. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J Glob Health 2019;9:010427. 10.7189/jogh.09.010427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Royal College of Ophthalmologists . Retinal vein occlusion (RVO) guidelines, 2015. Available: https://www.rcophth.ac.uk/wp-content/uploads/2021/08/Retinal-Vein-Occlusion-RVO-Guidelines-July-2015.pdf [Accessed August 2021].
- 3.Awdeh RM, Elsing SH, Deramo VA, et al. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item national eye Institute visual function questionnaire. Br J Ophthalmol 2010;94:319–23. 10.1136/bjo.2007.135913 [DOI] [PubMed] [Google Scholar]
- 4.Noma H, Yasuda K, Shimura M. Cytokines and pathogenesis of central retinal vein occlusion. J Clin Med 2020;9:3457. 10.3390/jcm9113457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102–12. 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 6.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the Vibrant study. Ophthalmology 2015;122:538–44. 10.1016/j.ophtha.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 7.Rubio R, Genentech I. A study of the efficacy and safety of ranibizumab injection in patients with macular edema secondary to central retinal vein occlusion (cruise). U.S National Library of Medicine 2017;2011 10.1016/j.ophtha.2010.02.022 [DOI] [Google Scholar]
- 8.Pielen A, Clark WL, Boyer DS, et al. Integrated results from the copernicus and galileo studies. Clin Ophthalmol 2017;11:1533–40. 10.2147/OPTH.S140665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haller JA, Bandello F, Belfort R, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117:1134–46. 10.1016/j.ophtha.2010.03.032 [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;2:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 12.Critical appraisal skills programme cohort study checklist, 2018. Available: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf [Accessed 05/08/21].
- 13.Methodological quality of case series studies. JBI evid synth 2020;18:2127–33. 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korobelnik J-F, Kodjikian L, Delcourt C, et al. Two-year, prospective, multicenter study of the use of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in France. Graefes Arch Clin Exp Ophthalmol 2016;254:2307–18. 10.1007/s00417-016-3394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajric J, Bakri SJ. Outcomes of patients initially treated with intravitreal bevacizumab for central retinal vein occlusion: long-term follow-up. Semin 2016;31:542–7. 10.1080/08820538.2016.1230637 [DOI] [PubMed] [Google Scholar]
- 17.Sen P, Gurudas S, Ramu J, et al. Predictors of visual acuity outcomes after anti-vascular endothelial growth factor treatment for macular edema secondary to central retinal vein occlusion. Ophthalmol Retina 2021;5:1115–24. 10.1016/j.oret.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansour AM, Ashraf M, Charbaji A, et al. Two-year outcomes of intravitreal ziv-aflibercept. Br J Ophthalmol 2018;102:1387–90. 10.1136/bjophthalmol-2017-311591 [DOI] [PubMed] [Google Scholar]
- 19.Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the copernicus study. Ophthalmology 2014;121:1414–20. 10.1016/j.ophtha.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 20.Hykin P, Prevost AT, Vasconcelos JC, et al. Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion. JAMA Ophthalmol 2019;137:1256–64. 10.1001/jamaophthalmol.2019.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Călugăru D, Călugăru M. Intravitreal bevacizumab in acute central/hemicentral retinal vein occlusions: three-year results of a prospective clinical study. J Ocul Pharmacol Ther 2015;31:78–86. 10.1089/jop.2014.0037 [DOI] [PubMed] [Google Scholar]
- 22.Hikichi T, Higuchi M, Matsushita T, et al. Two-year outcomes of intravitreal bevacizumab therapy for macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol 2014;98:195–9. 10.1136/bjophthalmol-2013-303121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkmann I, Knoll K, Wiezorrek M, et al. Individualized treat-and-extend regime for optimization of real-world vision outcome and improved patients' persistence. BMC Ophthalmol 2020;20:122. 10.1186/s12886-020-01397-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spooner K, Fraser-Bell S, Hong T, et al. Five-year outcomes of retinal vein occlusion treated with vascular endothelial growth factor inhibitors. BMJ Open Ophthalmol 2019;4:e000249. 10.1136/bmjophth-2018-000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Arevalo JF, Berrocal MH, et al. Comparison of two doses of intravitreal bevacizumab as primary treatment for macular edema secondary to branch retinal vein occlusions: results of the pan American collaborative retina study group at 24 months. Retina 2009;29:1396–403. 10.1097/IAE.0b013e3181bcef53 [DOI] [PubMed] [Google Scholar]
- 26.Horner F, Lip PL, Mushtaq B, et al. Combination therapy for macular oedema in retinal vein occlusions: 3-year results from a real-world clinical practice. Clin Ophthalmol 2020;14:955–65. 10.2147/OPTH.S241044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maggio E, Mete M, Maraone G, et al. Intravitreal injections for macular edema secondary to retinal vein occlusion: long-term functional and anatomic outcomes. J Ophthalmol 2020;2020:1–8. 10.1155/2020/7817542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAllister IL, Smithies LA, Chen FK, et al. Two-year efficacy of ranibizumab plus laser-induced chorioretinal anastomosis vs ranibizumab monotherapy for central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol 2018;136:1391–7. 10.1001/jamaophthalmol.2018.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tadayoni R, Waldstein SM, Boscia F, et al. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-month results of the brighter study. Ophthalmology 2017;124:1778–87. 10.1016/j.ophtha.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 30.Ziemssen F, Feltgen N, Holz FG, et al. Demographics of patients receiving intravitreal anti-VEGF treatment in real-world practice: healthcare research data versus randomized controlled trials. BMC Ophthalmol 2017;17:7. 10.1186/s12886-017-0401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol 2004;122:336–40. 10.1001/archopht.122.3.336 [DOI] [PubMed] [Google Scholar]
- 32.Downey L, Acharya N, Devonport H, et al. Treatment choices for diabetic macular oedema: a guideline for when to consider an intravitreal corticosteroid, including adaptations for the COVID-19 era. BMJ Open Ophthalmol 2021;6:e000696. 10.1136/bmjophth-2020-000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott AW, Bressler SB. Long-term follow-up of vascular endothelial growth factor inhibitor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol 2013;24:190–6. 10.1097/ICU.0b013e32835fefee [DOI] [PubMed] [Google Scholar]
- 34.Glassman AR, Wells JA, Josic K, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (protocol T extension study). Ophthalmology 2020;127:1201–10. 10.1016/j.ophtha.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2022-001010supp001.pdf (40.7KB, pdf)
bmjophth-2022-001010supp002.pdf (39.7KB, pdf)
bmjophth-2022-001010supp003.pdf (3.6MB, pdf)
bmjophth-2022-001010supp004.pdf (48.4KB, pdf)
bmjophth-2022-001010supp005.pdf (71.5KB, pdf)
bmjophth-2022-001010supp006.pdf (39.1KB, pdf)
bmjophth-2022-001010supp007.pdf (213KB, pdf)