Abstract
A complete human fecal flora and cultures of defined species obtained from fecal flora were investigated in vitro to determine their ability to ferment the dietary fiber pectin. Bacteroides thetaiotaomicron was tested as a pectin-degrading microorganism alone and in coculture with Escherichia coli. Macromolecular pectins with different degrees of esterification were used as substrates in microbial degradation studies. The levels of oligogalacturonic acids formed in batch cultures were estimated during a 24- or 48-h incubation period by using high-performance thin-layer chromatography and high-performance anion-exchange chromatography. The spectrum and the amount of unsaturated oligogalacturonic acids formed as intermediate products of pectin fermentation changed permanently in the culture media during incubation with the complete fecal flora. After 24 h, no oligogalacturonic acids were detected. The pectin-degrading activities of pure cultures of B. thetaiotaomicron were lower than the pectin-degrading activity of a complete fecal flora. Cocultures of B. thetaiotaomicron and E. coli exhibited intermediate levels of degradation activity. In pure cultures of E. coli no pectin-degrading activity was found. Additionally, the rate of pectin degradation was affected by the degree of esterification of the substrate. Saturated oligogalacturonic acids were not found during pectin fermentation. The disappearance of oligogalacturonic acids in the later stages of fermentation with both the complete fecal flora and B. thetaiotaomicron was accompanied by increased formation of short-chain fatty acids.
In human nutrition, pectin is one of the most important sources of dietary fiber. It is present in vegetables and fruits as a component of the plant cell wall. Pectin consists mainly of long linear chains of α-1,4-glycoside-linked d-galacturonic acid (homogalacturonan; “smooth” regions) which are partially esterified with methanol. In addition, branched and complex pectic substances are present in the cell wall (rhamnogalacturonans I and II; “hairy” regions) (45). Like other types of dietary fiber, pectin is not depolymerized by endogenous gastrointestinal enzymes during passage through the stomach and the small intestine. A number of physiological effects of pectin or pectin-containing diets have been described; these effects include decreasing serum cholesterol levels (17), increasing fecal excretion of steroids (24), interacting with metal ions (25), and interacting with bile acids in vitro (11). These effects depend on the macromolecular state of pectin.
In the colon, pectin is fermented more or less completely by the microflora, as shown previously (8, 9, 18, 28, 48). However, there have been only a few studies in which the intermediate steps in pectin degradation by gastrointestinal microorganisms have been examined. On the other hand, the end products of bacterial fermentation of pectin are well known; a spectrum of short-chain fatty acids (SCFA) and different gases (CO2, H2, H2S, CH4) are formed. In some studies it was shown that feeding rats pectin can decrease the number of colon tumors (36). Likewise, it was found that the SCFA butyrate may inhibit the growth of different colon cancer lines (4, 27, 44) or may decrease the total number of tumors induced by 1,2-dimethylhydrazine in rats (33). However, the results of other studies did not support these conclusions (26).
In some cases, physiological effects of pectin are independent of the polymer state but dependent on the intermediate cleavage products. Thus, it has been shown that oligogalacturonic acids (oligoGalA) bind heavy metal ions, especially lead, with high efficiency. These acids seem to play an important role in the mechanism which results in increased excretion of lead into urine after pectin is eaten or pectin-rich diets are used (13, 51). Such an effect is directly related to the chemical nature, the amount, and the stability of cleavage products formed in the gut. Therefore, besides the end products of fermentation, the intermediate products of bacterial pectin degradation may have physiological importance.
In a previous pilot study, it was shown that pectin is fragmented into a spectrum of oligoGalA that are intermediate products during incubation in vitro with the human fecal flora. Mixtures of unsaturated di-, tri-, and tetragalacturonic acids were the end products of pectate lyase activity in the cultures examined. Later, these compounds disappeared as a result of further fermentation by the gastrointestinal microflora. Low-methoxyl pectins were depolymerized and fermented faster than high-methoxyl pectins. Furthermore, it was found that a mixture of unsaturated oligoGalA prepared from pectic acid by using pectate lyase from Erwinia carotovora was completely fermented by human fecal flora in vitro (14).
In this study, the time course of pectin degradation, the transient formation of oligoGalA, and the conversion of these acids to SCFA catalyzed by human fecal flora, pure cultures of Bacteroides thetaiotaomicron, or a defined coculture containing B. thetaiotaomicron and Escherichia coli isolated from human feces were investigated in relation to the degree of esterification of pectin.
MATERIALS AND METHODS
Pectin preparations.
In all experiments, high-molecular-weight citrus pectin preparations with different degrees of esterification were used. Pectins B and C were commercial low- and high-methoxyl citrus pectin preparations without additives produced by Copenhagen Pectin A/S (Lille Skensved, Denmark). These preparations were purified further by extraction with acidified 60% ethanol. Very highly esterified pectin D was prepared by treating pectin C with methanol-concentrated H2SO4 at 4°C (14). Pectin A (pectic acid) was prepared by alkaline deesterification of pectin C (11). The pectin preparations used contained no acetyl or amide groups.
Pectin analysis.
The galacturonan (“anhydro”-galacturonic acid) contents of the pectin preparations and fractions were determined colorimetrically by the m-hydroxydiphenyl method (7). The methyl ester group contents were determined by the chromotropic acid method (6). Intrinsic viscosity was determined by using an Ubbelohde viscosimeter at 25.0°C and pH 6.0 in 0.155 M NaCl (high-methoxyl pectins) or in 0.05 M NaCl–0.005 M sodium oxalate (low-methoxyl pectins). The relationship between intrinsic viscosity and the average molecular weight of pectins is described by the Mark-Houwink equation (2).
Bacterial strains and culture conditions.
An inoculum was prepared from fresh feces collected from a healthy female volunteer who ingested a normal Western diet, had no digestive diseases, and had not taken antibiotics for the previous 6 months. The feces were collected anaerobically. For batch cultures, 5-g portions of fresh human feces were incubated in 150-ml portions of nutritive medium in 200-ml bottles without aeration at 37°C in a water bath. The medium used for the batch cultures contained 0.25 or 0.5% galacturonan in 0.067 M Sörensen phosphate buffer (pH 7.8) (medium A) or in Sörensen phosphate buffer enriched with 1% pancreatic peptone (Merck, Darmstadt, Germany) (medium B). The pectin-containing medium was sterilized by filtration with a Sartobran PH minicartridge (pore size, 0.45 μm; Sartorius, Göttingen, Germany).
B. thetaiotaomicron and E. coli were identified as pectin-degrading microorganisms obtained from human feces by using the modified method of Jensen and Canale-Parola (22) and blood agar plates containing pectin; the organisms were isolated and rinsed by repeated plating on selective agar plates, as described below for enumeration of viable cells. Organisms were identified by the VITEK automatic identification method (BioMerieux, Nürtlingen, Germany). The numbers of viable cells in batch cultures were determined by using 0.5-ml samples for serial dilution and subsequent plating on Columbia blood agar plates (BioMerieux) that were incubated aerobically and anaerobically. Organisms were differentiated on the following selective media: aerobically incubated Endo agar (Merck) for E. coli and coliforms, aerobically incubated MRS agar (Merck) for members of the Lactobacillus group, and anaerobically incubated neomycin-blood agar for B. thetaiotaomicron and members of the gram-negative anaerobic group. Plates were incubated anaerobically by using the Anaerocult System (Merck). Starter cultures of E. coli and B. thetaiotaomicron were preincubated for 18 and 40 h, respectively, in nutrient broth (Difco, Augsburg, Germany) without aeration. Starter cultures (4 ml) containing 5 × 108 cells/ml were used as inocula for the batch cultures in 150-ml portions of medium B. Samples (4 ml) were taken immediately after inoculation and then periodically after 2 to 48 h of incubation.
Characterization of pectin degradation.
To determine the amounts of high-molecular-weight and low-molecular-weight pectin fractions and oligoGalA in batch cultures, 4-ml samples were mixed immediately with 1 ml of 0.2 M HCl and 5 ml of ethanol to stop bacterial growth and enzymatic reactions. After centrifugation (6,000 × g, 30 min, 4°C), some of the supernatant was used to determine the galacturonan content (low-molecular-weight pectin fraction content. The remaining supernatant was concentrated in a vacuum, dissolved in 1.5 ml of H2O, centrifuged (10,000 × g, 30 min, 4°C), and used for chromatographic determination of the oligoGalA content.
To remove low-molecular-weight substances, the residues (coagulates) that remained after centrifugation were extracted three times with 50% ethanol and once with 96% ethanol with stirring. During the second extraction, enzymes were inactivated by heating the preparation at 85°C for 15 min. After extraction of the residues with 0.5% EDTA (pH 6.0) and coagulation at pH 2 in 50% ethanol, the macromolecular pectin contents of batch cultures were determined colorimetrically (7).
The oligoGalA content and the oligoGalA composition were determined by using a combination of two chromatographic techniques with different detection methods.
A high-performance thin-layer chromatography (HPTLC) apparatus obtained from Camag (Muttenz, Switzerland) included a model III automatic thin-layer chromatography sampler, an automatic development chamber, a dipping device, and a model II thin-layer chromatography scanner with CATS evaluation software. Up to 3 μl of a sample was applied to an activated Silica Gel 60 F254 plate (Merck). The chromatograms were developed four times with n-propanol–water mixtures (7:4.50 to 7:2.75) by using the following conditions: run distance, 40 to 80 mm; drying time, 10 min; heating time, 1.5 min; and precondition time, 5 min. The delta-4,5 double bonds of unsaturated oligoGalA that formed were detected at 235 nm. Then the plates were dipped twice for 3 s in a 0.5% solution of m-hydroxydiphenyl in acetone, heated for 10 min at 100°C, and scanned at 525 nm to obtain information concerning the total amounts of substances in individual spots (12).
Additionally, oligoGalA were analyzed by high-performance anion-exchange chromatography (HPAEC) by using a chromatography system obtained from Kontron (Neufahrn, Germany) and equipped with a UV (250-nm) chiralyser and a pulsed amperometric detector. A CarboPac PA1 column (250 by 9 mm) from Dionex (Idstein, Germany) with a precolumn and a nonlinear gradient consisting of 40 to 100% 1 M sodium acetate in 0.15 M NaOH and 60 to 0% 0.15% NaOH for 50 min (flow rate, 2 ml/min) were used.
Both chromatographic methods were calibrated by using a mixture of oligoGalA with degrees of polymerization between 2 and >7 prepared from pectic acid by using pectate lyase from E. carotovora (12).
Determination of SCFA contents.
The concentration of SCFA was determined by gas-liquid chromatography by using a Carbowax 20M column (25 m by 0.32 mm [inside diameter]) attached to a Hewlett-Packard model 5890A chromatograph equipped with a flame ionization detector and a split injector. Helium was used as the carrier gas. The column temperature was maintained at 125°C, and the injector port and detector temperatures were 200°C.
Isobutyrate (internal standard), perchloric acid, and an NaOH solution were added to 1 ml of each sample. After freeze-drying, the material was homogenized in a mixture containing 100 μl of 5 M formic acid and 400 μl of acetone. A 1-μl sample of the organic phase was injected into the gas-liquid chromatograph.
RESULTS
Pectin substrates.
The galacturonan concentrations of the pectin preparations used were between 59 and 73%. The degrees of esterification of the substrates were as follows: pectin A, 0%; pectin B, 34.4%; pectin C, 66.0%; and pectin D, 94.7%. The free and esterified carboxyl groups in the pectin macromolecules were distributed in a random (statistical) manner. All of the pectins used were high-molecular-weight preparations; their intrinsic viscosities were between 270 and 920 ml/g of galacturonan.
Microbiology.
Table 1 shows the effects of incubation time and degree of esterification of the substrate on selected groups of intestinal microbes, as indicated by the number of viable cells under the experimental conditions used. The viable cell content increased slightly or, in some cases, significantly for the Bacteroides group. The number of bacteria belonging to the Enterobacteriaceae increased. The colony counts for lactobacilli (log 3 to 4) and the total anaerobic cell counts (log 8.5 to 9.2) were nearly constant. Furthermore, the degree of esterification of pectin did not have a significant effect on the viable cell counts.
TABLE 1.
Numbers of viable cells of members of the Enterobacteriaceae and the Bacteroides group after a 24 h of incubation of fecal flora with pectins with different degrees of esterification
| Degree of esterification (%) | Log viable cells/ml
|
|||||
|---|---|---|---|---|---|---|
| Zero time
|
8 h
|
24 h
|
||||
| Enterobacteriaceae | Bacteroides group | Enterobacteriaceae | Bacteroides group | Enterobacteriaceae | Bacteroides group | |
| 0.0 | 4.8 ± 0.5a | 7.8 ± 0.3 | 8.5 ± 0.4b | 8.3 ± 0.4 | 7.8 ± 0.4b | 8.5 ± 0.3 |
| 34.4 | 4.8 ± 0.3 | 7.5 ± 0.4 | 7.6 ± 0.5b | 8.2 ± 0.3 | 7.8 ± 0.3b | 9.2 ± 0.4b |
| 66.0 | 4.5 ± 0.2 | 8.2 ± 0.2 | 8.5 ± 0.5b | 8.3 ± 0.4 | 7.8 ± 0.2b | 9.3 ± 0.3b |
| 94.7 | 4.8 ± 0.3 | 7.7 ± 0.4 | 8.2 ± 0.3b | 7.3 ± 0.3 | 7.3 ± 0.6b | 7.6 ± 0.4 |
Values are means ± standard deviations (n = 3).
Significantly different from the zero-time value (P < 0.05, as determined by Student's t test).
After 24 h, the numbers of viable cells were similar for pure cultures of E. coli or B. thetaiotaomicron and for mixed cultures, as well as for complete feces cultures (Table 2).
TABLE 2.
Numbers of viable E. coli and B. thetaiotaomicron cells after 24-h of incubation of different microbial cultures with pectin B (degree of esterification, 34.4%)
| Culture | Log viable cells/ml
|
|||||
|---|---|---|---|---|---|---|
| Zero time
|
8 h
|
24 h
|
||||
| E. coli | B. thetaiotaomicron | E. coli | B. thetaiotaomicron | E. coli | B. thetaiotaomicron | |
| E. coli | 7.3 ± 0.4a | 7.5 ± 0.3 | 7.7 ± 0.4 | |||
| B. thetaiotaomicron | 7.8 ± 0.4 | 7.8 ± 0.2 | 8.3 ± 0.4 | |||
| E. coli + B. thetaiotaomicron | 6.8 ± 0.3 | 6.9 ± 0.4 | 7.9 ± 0.5b | 7.8 ± 0.3b | 8.2 ± 0.2b | 8.3 ± 0.4b |
Values are means ± standard deviations (n = 3).
Significantly different from the zero-time value (P < 0.05, as determined by Student's t test).
The pH of the culture medium decreased from 7.7 at the beginning of incubation to 7.2 to 6.7 at the end of incubation.
Pectin degradation with complete fecal flora.
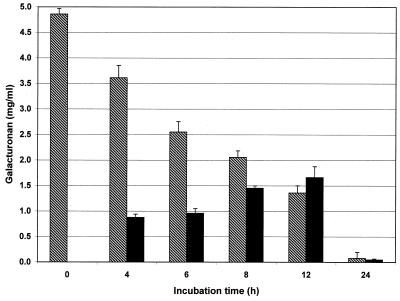
During incubation of pectin with a complete feces culture in vitro the following effects were observed. The portion of macromolecular pectin that was not soluble in 50% ethanol decreased during incubation, whereas the portion of galacturonan in the low-molecular-weight fraction that was soluble in 50% ethanol increased continuously during the first phase of fermentation. When high-methoxyl pectin C was used as a substrate, the amount of the low-molecular-weight fraction reached a maximum value after 10 to 12 h (Fig. 1). Then it decreased almost like the amount of the macromolecular fraction decreased. After incubation for 24 h, only very small amounts of both pectin fractions were present in the experimental culture. Generally, the high-methoxyl pectins were degraded more slowly than the minimally esterified substrates by the total fecal flora (14).
FIG. 1.
Concentrations of macromolecular (cross-hatched bars) and low-molecular-weight (solid bars) pectin fractions during in vitro fermentation of high-methoxyl pectin C (degree of esterification, 66.0%) with complete human fecal flora (n = 6).
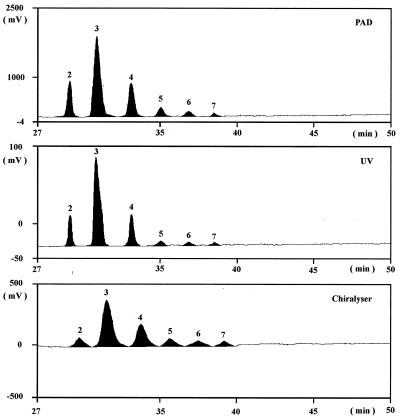
The changes in oligoGalA composition were measured during incubation by HPTLC and HPAEC. The multiple development technique used with HPTLC improved separation of the individual oligomers by a “pseudogradient.” HPAEC with combined UV, pulsed amperometric, and chirality detection was a suitable technique for determining oligoGalA contents. The UV absorption values which were related to the number of double bonds in the unsaturated oligoGalA were affected by the presence of acetate in the elution buffer. This effect could be suppressed by obtaining UV measurements at 250 nm instead of 235 nm. The sensitivity of detection decreased with the degree of polymerization of oligomers. In contrast, pulsed amperometric detection was closely related to the reducing end groups of the oligomers, and the analytical sensitivity decreased with chain length. Chirality detection was related to monomer units in the chain, and except for very low degrees of polymerization, the response was not related to chain length. A typical HPAEC chromatogram is shown in Fig. 2. Determinations of the qualitative and the quantitative compositions of oligoGalA were optimized by using both chromatographic techniques with different detection methods.
FIG. 2.
Determination of oligoGalA contents by HPAEC with pulsed amperometric (PAD), UV (250-nm), and chirality detection after incubation of low-methoxyl pectin B with complete human fecal flora (peaks 2 through 7 correspond to degrees of polymerization of 2 through 7, respectively).
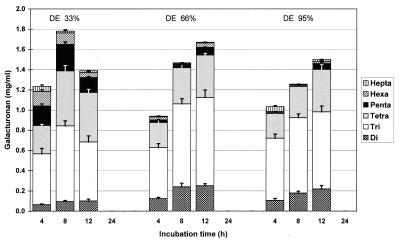
A broad spectrum of oligoGalA was found in cultures during incubation. The quantity of oligoGalA also depended on the degree of esterification of the substrate. Maximal formation of these oligomers occurred after approximately 8 h if low-methoxyl pectin B was used as the substrate. No maximum was observed for up to 12 h when high-methoxyl pectins C and D were fermented. After 24 h, oligoGalA were absent in the culture inoculated with the complete fecal flora (Fig. 3). Saturated oligoGalA were not formed as a result of bacterial action.
FIG. 3.
Unsaturated oligoGalA contents and compositions during incubation of pectins with different degrees of esterification (DE) with complete human fecal flora (n = 4).
The oligoGalA formed must have been depolymerized further to the unstable monomer, which was rearranged to 4-deoxy-l-threo-5-hexoseulose uronic acid (37) by unidentified enzymes. It was not possible to detect this monomer chromatographically, perhaps because it was formed intracellularly. The end products of fermentation of pectin are the SCFA, which are physiologically important metabolites (40).
The concentration of SCFA (acetic acid, propionic acid, and butyric acid, as well as low concentrations of valeric acids) increased continuously during incubation (Table 3). In accordance with the rate of formation of oligoGalA, the amount of SCFA produced was significantly larger when low-methoxyl pectins were used as substrates. The lower concentration of SCFA obtained after 24 h when high-methoxyl substrates were used was related to incomplete depolymerization of the pectins to monomeric units (Fig. 1). The preferred SCFA formed was acetic acid, which accounted for more than 75 mol%. The molar concentrations of propionic acid and butyric acid were relatively similar for incubation times up to 12 h. It was remarkable that significantly more butyrate than propionate was found after 24 h of incubation with all of the pectin substrates (Table 3). Only very small amounts of n-valerate and isovalerate were present. Additionally, the pH decreased continuously in the media as a result of formation of SCFA.
TABLE 3.
Formation of SCFA and pH values in cultures during fermentation of pectins with different degrees of esterification when the complete human fecal flora was used
| Degree of esterification of pectin (%) | Incubation time (h) | pH | Total SCFA concn (μmol/ml)a | % of SCFA
|
||
|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | ||||
| 0.0 | 4 | 6.76 | 7.06 ± 0.22b | 82.1 ± 0.3 | 8.6 ± 0.2 | 9.3 ± 0.1 |
| 6 | 6.70 | 12.88 ± 0.29 | 83.2 ± 0.4 | 8.1 ± 0.1 | 8.6 ± 0.1 | |
| 8 | 6.54 | 17.27 ± 0.27 | 80.7 ± 0.2 | 9.8 ± 0.3 | 9.5 ± 0.2 | |
| 12 | 6.20 | 40.58 ± 0.17 | 84.2 ± 0.3 | 7.7 ± 0.1 | 8.1 ± 0.2 | |
| 24 | 6.21 | 50.81 ± 0.33 | 77.3 ± 0.2 | 8.4 ± 0.4 | 14.2 ± 0.4 | |
| 34.4 | 4 | 6.96 | 7.22 ± 0.22 | 76.3 ± 0.2 | 10.3 ± 0.1 | 13.4 ± 0.3 |
| 6 | 6.91 | 10.13 ± 0.26c | 78.6 ± 0.3 | 9.0 ± 0.4 | 12.3 ± 0.1 | |
| 8 | 6.76 | 16.23 ± 0.25c | 80.5 ± 0.4 | 9.3 ± 0.1 | 10.2 ± 0.2 | |
| 12 | 6.48 | 27.70 ± 0.39c | 79.5 ± 0.5 | 10.3 ± 0.2 | 10.3 ± 0.2 | |
| 24 | 6.34 | 47.20 ± 0.31c | 76.1 ± 0.4 | 10.2 ± 0.2 | 13.7 ± 0.1 | |
| 66.0 | 4 | 7.29 | 7.36 ± 0.40 | 84.9 ± 0.5 | 8.1 ± 0.2 | 7.0 ± 0.3 |
| 6 | 7.19 | 9.80 ± 0.19c | 84.1 ± 0.1 | 8.4 ± 0.3 | 7.5 ± 0.3 | |
| 8 | 7.04 | 11.76 ± 0.24cd | 85.3 ± 0.4 | 7.1 ± 0.1 | 7.6 ± 0.2 | |
| 12 | 6.82 | 20.39 ± 0.58cd | 84.5 ± 0.2 | 8.5 ± 0.7 | 7.0 ± 0.6 | |
| 24 | 6.17 | 45.20 ± 0.46cd | 80.5 ± 0.5 | 8.1 ± 0.6 | 11.5 ± 0.2 | |
| 94.7 | 4 | 7.33 | 6.79 ± 0.17 | 81.7 ± 0.4 | 7.3 ± 0.1 | 11.0 ± 0.2 |
| 6 | 7.24 | 8.19 ± 0.23cde | 81.8 ± 0.1 | 8.3 ± 0.4 | 9.9 ± 0.1 | |
| 8 | 7.14 | 10.28 ± 0.47cde | 80.3 ± 0.3 | 10.5 ± 0.7 | 9.1 ± 0.4 | |
| 12 | 6.77 | 18.87 ± 0.42cde | 82.2 ± 0.6 | 8.0 ± 0.1 | 9.8 ± 0.3 | |
| 24 | 6.32 | 41.40 ± 0.28cde | 76.6 ± 0.2 | 9.9 ± 0.2 | 13.3 ± 0.5 | |
Sum of acetate, propionate, and butyrate concentrations.
Values are means ± standard deviations (n = 3).
Significantly different from the value for pectic acid (degree of esterification, 0%) (P < 0.05, as determined by Student's t test).
Significantly different from the value for pectin with a degree of esterification of 34.4% (P < 0.05, as determined by Student's t test).
Significantly different from the value for pectin with a degree of esterification of 66.0% (P < 0.05, as determined by Student's t test).
Pectin degradation with defined bacterial cultures.
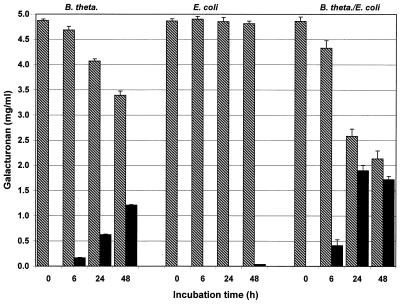
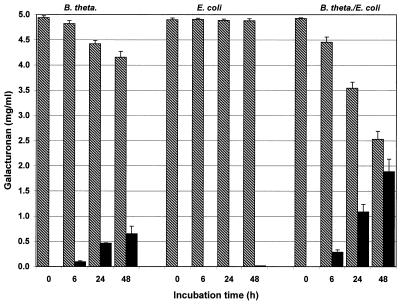
Compared with the complete fecal flora cultures, development of the different pectin fractions in pure cultures of B. thetaiotaomicron was similar, but the reaction was slower. This effect was found to be independent of the degree of esterification of pectin, but it was less pronounced when high-methoxyl substrates were used (Fig. 4 and 5). Distinct degradation did not begin before a culture had been incubated for approximately 6 h. Therefore, maximum formation of oligoGalA occurred later than it occurred in experiments in which the complete fecal flora was used. A reduction in the amount of the low-molecular-weight pectin fraction did not occur in any of these cultures until after 48 h of incubation.
FIG. 4.
Variation in macromolecular (cross-hatched bars) and low-molecular-weight (solid bars) fractions during in vitro fermentation of low-methoxyl pectin B with B. thetaiotaomicron (B. theta.), E. coli, and a mixed culture containing B. thetaiotaomicron and E. coli (n = 6).
FIG. 5.
Variation in macromolecular (cross-hatched bars) and low-molecular-weight (solid bars) fractions during in vitro fermentation of very highly esterified pectin D with B. thetaiotaomicron (B. theta.), E. coli, and a mixed culture containing B. thetaiotaomicron and E. coli (n = 6).
In the case of an E. coli strain, the agar plate test for pectin degradation activity resulted in a positive reaction on pectin-blood agar but no reaction on pectin-Endo agar. Likewise, no pectin degradation was observed in batch cultures (medium B) of E. coli. However, in cocultures with B. thetaiotaomicron, the degradation activity was greater than the degradation activity in pure Bacteroides cultures (Fig. 4 and 5). When the mixed bacterial culture was used, low-methoxyl pectin was degraded more pronounced than high-methoxyl pectin. After 48 h of incubation, a decrease in the oligomer content was observed if low-methoxyl pectin was used.
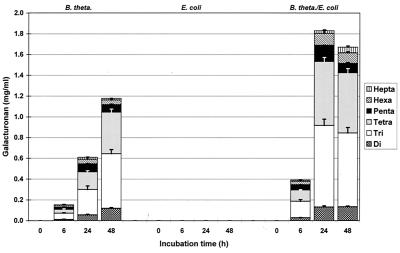
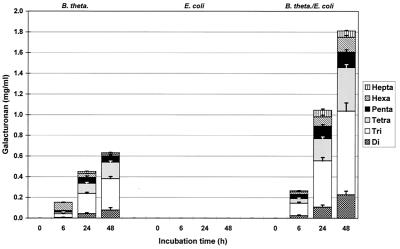
In pure B. thetaiotaomicron cultures, a broad spectrum of oligoGalA was detected (Fig. 6 and 7). This spectrum was similar to the spectrum obtained in experiments performed with a complete fecal flora. Also, in all cases only unsaturated oligoGalA were detected.
FIG. 6.
Unsaturated oligoGalA contents during incubation of low-methoxyl pectin B with B. thetaiotaomicron (B. theta.), E. coli, and a mixed culture containing B. thetaiotaomicron and E. coli (n = 4).
FIG. 7.
Unsaturated oligoGalA contents during incubation of very highly esterified pectin D with B. thetaiotaomicron (B. theta.), E. coli, and a mixed culture containing B. thetaiotaomicron and E. coli (n = 4).
The influence of the degree of esterification of pectins on the formation of oligoGalA suggests that pectate lyase and pectin esterase activities occurred in the bacterial cultures from feces. Although we could not directly detect pectin esterase activity, we hypothesized that this enzyme was involved in pectin degradation, especially if more highly esterified pectins were used. The degrees of esterification of low- and high-methoxyl substrates were stable under the conditions used in the absence of fecal bacteria for up to 48 h. However, a slight decrease in the degree of esterification was observed if the very highly esterified pectin D was treated under the same conditions.
Degradation of oligoGalA to SCFA took place throughout incubation. Approximately 15 μmol of acetic acid per ml was formed during the first 8 h of incubation with pectin acid or low-methoxyl pectin. On the other hand, only approximately 10 μmol/ml was formed when high-methoxyl or very highly esterified pectin was used. In the case of high-methoxyl pectin, the amount of acetic acid increased continuously during incubation. Compared with other substrates used, more acetic acid was formed between 8 and 12 h. Relatively small amounts of propionic and butyric acids were formed continuously with all of the pectins.
DISCUSSION
It is well known that pectin (which is consumed mostly in the form of fruits and vegetables but also as hydrocolloid in “functional foods,” jellies, milk products, etc.) is fermented in the large intestine to SCFA and gases. Decomposition of the polysaccharide pectin occurs during the following main steps: (i) macromolecular pectin, (ii) (unsaturated) oligoGalA, (iii) monogalacturonic acid (or its rearrangement products), and (iv) SCFA (and gases).
Formation of oligoGalA as intermediate products during pectin degradation by human or animal microflora has not been analyzed systematically previously. Our results show that the velocity of oligoGalA formation depends on the structure of the pectin used (especially the degree of esterification), although all pectins are fermented more or less completely by microflora in vitro and in vivo in the end. The velocity of formation of the oligoGalA also acts as a limiting factor for later formation of SCFA.
Previously, the fate and fermentation of the dietary fiber pectin during passage through the gastrointestinal tracts of humans and animals were investigated in vivo and in vitro. Cummings et al. (9) observed no increase in fecal excretion of pectin after intake of 36 g of pectin per day for 6 weeks by male volunteers. This was because there was intense bacterial fermentation of pectin in the colon. Different human intestinal bacteria are known to degrade pectin; these bacteria include Bacteroides strains, eubacteria, clostridia, and Bifidobacteria (15, 20, 30, 41, 42). Dekker and Palmer (10) observed that a Bacteroides strain from human feces contained constitutive polysaccharidases, especially polygalacturonic acid-degrading activities. Jensen and Canale-Parola (22, 23) described Bacteroides sp. strains as pectin-degrading microbes in the human intestinal flora. When B. thetaiotaomicron was grown in a pectic acid-containing medium, cell-associated polygalacturonic acid lyase (EC 4.2.2.2) and hydrolase (EC 3.2.1.15) were present (29). A Clostridium butyricum-Clostridium beijerinckii strain isolated from human feces was able to produce pectate lyase and to decompose pectic acid (31). Recently, Matsuura (32) identified a pectate lyase of the endotype which splits pectic acid into unsaturated oligoGalA in human feces extracts. It is well known that B. thetaiotaomicron can utilize a wide variety of plant polysaccharides (50). For instance, Reeves et al. characterized an outer membrane protein which is essential for utilization of maltooligosaccharides and starch by this Bacteroides species (38). Tomlin et al. (49) determined that pectin was completely fermented by human fecal bacteria within 21 h, that the viscosity of the pectin was lost, and that the culture pH declined. In some of these studies, the workers investigated the disappearance of pectin, the enzymes or microorganisms involved, and/or the formation of SCFA. Sometimes, the fermentation of pectin-containing complex substrates, like apple fiber, by human fecal bacteria was studied (19). Recently, Tierny et al. (47) found pectate lyase, pectin esterase, and polygalacturonase activities in B. thetaiotaomicron 217 grown on pectin as the sole carbon source. These authors investigated molecular cloning and expression of genes encoding the pectate lyase and pectin esterase activities in E. coli.
Only limited information is available concerning the variation in pectin molecules or the dynamics of pectin degradation during bacterial activity in the colon.
Consistent with the results of in vivo experiments performed with rats (35), low-methoxyl pectins were fermented in vitro more efficiently than high-methoxyl pectins were fermented. Therefore, Dongowski and Lorenz concluded that low-methoxyl pectins are the preferred substrates of the pectin-depolymerizing enzymes of the human microflora (14).
The mechanism of oligoGalA formation from ingested pectin has not been sufficiently investigated. In the experiments described here, macromolecular pectin was depolymerized and enzymatically degraded to a spectrum of unsaturated oligoGalA. The pattern of oligoGalA formed as intermediate products showed that the key enzyme during this pectin degradation is pectate lyase. It is well known that high-methoxyl and very highly esterified pectins are degraded at decreased rates by pectate lyases (or polygalacturonases) (39). Pectin lyase is the only enzyme which is able to split all pectins independent of the degree of esterification. However, this enzyme is preferentially present in fungi (39). Distinct chemical deesterification was not detected under the conditions used. The influence of the degree of esterification of pectin on fermentation, the formation of oligoGalA, and the decrease in the pH of the medium indicated that both pectate lyase activity and pectin esterase activity were present. However, we did not detect pectin esterase activities in in vitro experiments.
There are at least two processes that occur side by side, depolymerization of galacturonan macromolecules and decomposition of the oligomers formed to the end products of fermentation, SCFA and gases. The results of depolymerization are increases in the oligoGalA content and the presence of relatively high concentrations of these oligomers in batch cultures after 4 to 6 h. Later, the level of oligoGalA formation decreases in connection with a decrease in the pectin content of the culture media under the conditions which we used. Formation of SCFA starts immediately after oligoGalA appear, but the reaction speed seems to be lower than the reaction speed of the pectin degradation process.
The effect of pectin on excretion of heavy metals, such as lead, via kidneys has been discussed previously (13, 51). OligoGalA were described as the active substances. Anger et al. (3) found that 9 to 45% of oligoGalA directly injected into the ceca of rats were recovered in the urine within 16 h.
Based on our results obtained in vitro, we propose the following hypothesis. The pectin content in a continuous-flow culture (like the gut) may not decrease for a long time. Therefore, formation of oligoGalA may continue with a high yield over a relatively long period. With this background, the results suggest the possibility that some of the bioactive molecules formed from ingested pectin may be absorbed in the colon.
The increasing numbers of E. coli cells in feces cultures and positive reactions on blood agar plates indicate that these microbes may participate in pectin degradation. This hypothesis is not supported by the results obtained with pure cultures of E. coli. On the other hand, the pectin-degrading activity was greater in mixed cultures containing E. coli and B. thetaiotaomicron than in pure Bacteroides cultures. This indicates that E. coli may participate in degradation of pectin.
There are two possible ways to interpret these results: (i) E. coli is able to promote the degrading activity of B. thetaiotaomicron (e.g., by deesterification) without affecting the viable cells in the culture; or (ii) E. coli has an inducible pectate lyase which requires external stimulation or support. Further investigations may answer the questions raised here.
Although some of the oligoGalA formed from pectin in the colon may be absorbed (3), most of them are fully degraded to the main fermentation end products of dietary fiber, gases and SCFA, which are detected in feces. The SCFA butyrate plays an important physiological role (46). It is the major energy source for colonic epithelial cells (40) and acts as a regulator in the cell cycle. The effects of butyrate on normal and neoplastic cells may be different or opposite (21). Butyrate may have a role in preventing certain types of colitis (43). However, the hypothesis that butyrate protects against colon cancer was not supported by all of the studies performed (26). Barry et al. (5) described an interlaboratory study in which it was found that pectin was almost completely fermented (97.4%) within 24 h in vitro. Compared with cellulose, sugar beet fiber, soybean fiber, or maize bran, greater total SCFA production (67.7 mmol/g after 24 h), a molar ratio of acetic acid to propionic acid to butyric acid of 74.4:8.9:16.9, and a decrease in pH of 0.93 U were estimated. Other authors found between 2 and 17% butyrate in the SCFA during fermentation of pectin by human fecal bacteria in vitro (1, 16, 34).
In conclusion, we found that distinct amounts of unsaturated oligoGalA are formed as metabolites during pectin fermentation. The rate of the enzymatic reactions is influenced by both molecular parameters of the substrate, such as the degree of esterification, and the synergistic effects of bacteria. Later formation of oligoGalA due to the use of highly esterified pectins as substrates resulted in later formation of SCFA. Therefore, it seems to be possible to extend the region of intense formation of SCFA into the distal parts of the colon by using dietary fibers (like pectin) with special structural parameters.
ACKNOWLEDGMENTS
We thank Ingrid Vogel and Horst Maischack for their skillful technical assistance.
This study was supported by the German Federal Ministry for Education, Science, Research and Technology.
REFERENCES
- 1.Adiotomre J, Eastwood M A, Edwards C A, Brydon W G. Dietary fiber: in vitro methods that anticipate nutrition and metabolic activity in humans. Am J Clin Nutr. 1990;52:128–134. doi: 10.1093/ajcn/52.1.128. [DOI] [PubMed] [Google Scholar]
- 2.Anger H, Berth G. Gel permeation chromatography and the Mark-Houwink relation for pectins with different degrees of esterification. Carbohydr Res. 1986;6:193–202. [Google Scholar]
- 3.Anger H, Walzel E, Kahrmann B. About the absorption of oligogalacturonides from caecum of rats. FASEB J. 1994;8:A152. [Google Scholar]
- 4.Archer S, Meng S, Wu J, Johnson J, Tang R, Hodin R. Butyrate inhibits colon carcinoma cell growth through two distinct pathways. Surgery. 1998;124:248–253. [PubMed] [Google Scholar]
- 5.Barry J-L, Hoebler C, Macfarlane G T, Macfarlane S, Mathers J C, Reed K A, Mortensen P B, Nordgaard I, Rowland I R, Rumney C J. Estimation of the fermentability of dietary fiber in vitro: a European interlaboratory study. Br J Nutr. 1995;74:303–322. doi: 10.1079/bjn19950137. [DOI] [PubMed] [Google Scholar]
- 6.Bäuerle G, Otterbach G, Gierschner K, Baumann G. Bestimmung des Polyuronidgehaltes und des Veresterungsgrades des Pektins in Handelspräparaten, Apfelsäften und Apfelmaceraten. Dtsch Lebensm Rundsch. 1977;73:281–286. [Google Scholar]
- 7.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 8.Cummings J H, Englyst H N. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr. 1987;45:1243–1255. doi: 10.1093/ajcn/45.5.1243. [DOI] [PubMed] [Google Scholar]
- 9.Cummings J H, Southgate D A T, Branch W J, Wiggins H S, Houston H, Jenkins D J A, Jivraj T, Hill M J. The digestion of pectin in the human gut and its effect on calcium absorption and large bowel function. Br J Nutr. 1979;41:477–485. doi: 10.1079/bjn19790062. [DOI] [PubMed] [Google Scholar]
- 10.Dekker J, Palmer J K. Enzymatic degradation of plant cell wall by a Bacteroides of human fecal origin. J Agric Food Chem. 1981;29:480–484. doi: 10.1021/jf00105a010. [DOI] [PubMed] [Google Scholar]
- 11.Dongowski G. Influence of pectin structure on the interaction with bile acids under in vitro conditions. Lebensm Unters Forsch. 1995;201:390–398. doi: 10.1007/BF01192740. [DOI] [PubMed] [Google Scholar]
- 12.Dongowski G. Determination of saturated and unsaturated oligogalacturonic acids by means of thin-layer chromatography. J Chromatogr A. 1996;756:211–217. [Google Scholar]
- 13.Dongowski G, Walzel E, Ozierenski B, Stark C, Kroll J, Lorenz A. Effects of pectin and oligogalacturonic acids on excretion and incorporation of lead in subchronic lead exposed rats. In: Hartemink R, editor. Non-digestible oligosaccharides: healthy food for the colon? Wageningen, The Netherlands: Graduate School VLAG; 1997. p. 151. [Google Scholar]
- 14.Dongowski G, Lorenz A. Unsaturated oligogalacturonic acids are generated by in vitro treatment of pectin with human faeces flora. Carbohydr Res. 1998;314:237–244. doi: 10.1016/s0008-6215(98)00304-8. [DOI] [PubMed] [Google Scholar]
- 15.Edwards C A, Rowlands I R. Bacterial fermentation in the colon and its measurement. In: Schweizer T F, Edwards C A, editors. Dietary fibre—a component of food. Nutritional function in health and disease. London, United Kingdom: Springer-Verlag; 1992. pp. 121–136. [Google Scholar]
- 16.Englyst H N, Hay S, Macfarlane G T. Polysaccharide breakdown by mixed populations of human faecal bacteria. Microb Ecol. 1987;95:163–171. [Google Scholar]
- 17.Fernandez M L. Distinct mechanisms of plasma LDL lowering by dietary fiber in guinea pig: specific effects of pectin, guar gum, and psyllium. J Lipid Res. 1995;36:2394–2404. [PubMed] [Google Scholar]
- 18.Gibson G R, Macfarlane S, Cummings J H. The fermentability of polysaccharides by mixed faecal bacteria in relation to their suitability as bulk-forming laxatives. Lett Appl Microbiol. 1990;11:251–254. [Google Scholar]
- 19.Guillon F, Renard C M G C, Hospers J, Thibault J-F, Barry J-L. Characterisation of residual fibres from fermentation of pea and apple fibres by human faecal bacteria. J Sci Food Agric. 1995;68:521–529. [Google Scholar]
- 20.Hill M J. Bacterial fermentation of complex carbohydrate in the human colon. Eur J Cancer Prevent. 1995;4:353–358. doi: 10.1097/00008469-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Jacobasch G, Schmiedl D, Schmehl K. Darmprävention durch resistente Stärke? Ernaehr Umsch. 1997;44:318–326. , 369–373. [Google Scholar]
- 22.Jensen N S, Canale-Parola E. Nutritionally limited pectinolytic bacteria from the human intestine. Appl Environ Microbiol. 1985;50:172–173. doi: 10.1128/aem.50.1.172-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen N S, Canale-Parola E. Bacteroides pectinophilus sp. nov. and Bacteroides galacturonicus sp. nov.: two pectinolytic bacteria from the human intestinal tract. Appl Environ Microbiol. 1986;52:880–887. doi: 10.1128/aem.52.4.880-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kay R M, Truswell A S. Effect of citrus pectin on blood lipids and fecal steroid excretion in man. Am J Cli Nutr. 1977;30:171–175. doi: 10.1093/ajcn/30.2.171. [DOI] [PubMed] [Google Scholar]
- 25.Kim M, Atallah M T, Amarasiriwardena C, Barnes R. Pectin with low molecular weight and high degree of esterification increases absorption of 58Fe in growing rats. J Nutr. 1996;126:1883–1890. doi: 10.1093/jn/126.7.1883. [DOI] [PubMed] [Google Scholar]
- 26.Lupton J R. Butyrate and colonic cytokinetics: differences between in vitro and in vivo studies. Eur J Cancer Prevent. 1995;4:373–378. doi: 10.1097/00008469-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 27.McBain J A, Eastman A, Nobel C S, Mueller G C. Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone acetylase inhibitors. Biochem Pharmacol. 1997;53:1357–1368. doi: 10.1016/s0006-2952(96)00904-5. [DOI] [PubMed] [Google Scholar]
- 28.McBurney M I, Thompson L U. In vitro fermentabilities of purified fiber supplements. J Food Sci. 1989;54:347–350. [Google Scholar]
- 29.McCarthy R E, Kotarsky S F, Salayers A A. Location and characteristics of enzymes involved in the breakdown of polygalacturonic acid by Bacteroides thetaiotaomicron. J Bacteriol. 1985;161:493–499. doi: 10.1128/jb.161.2.493-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macfarlane G T, Macfarlane S. Factors affecting fermentation reactions in the large bowel. Proc Nutr Soc. 1993;52:367–373. doi: 10.1079/pns19930072. [DOI] [PubMed] [Google Scholar]
- 31.Matsuura Y. Decomposition of pectic acid by transeliminase from Clostridium butyricum-Clostridium beijerinckii isolated from human feces. Nippon Nogeikagaku Kaishi. 1987;61:1583–1588. [Google Scholar]
- 32.Matsuura Y. Pectic acid degrading enzymes from human feces. Agric Biol Chem. 1991;55:885–886. [Google Scholar]
- 33.Medina V, Afonso J J, Alvarez Arguelles H, Hernandez C, Gonzalez F. Sodium butyrate inhibits carcinoma development in a 1,2-dimethylhydrazine-induced rat colon cancer. JPEN J Parenter Enteral Nutr. 1998;22:14–17. doi: 10.1177/014860719802200114. [DOI] [PubMed] [Google Scholar]
- 34.Mortensen P B, Nordgaard-Andersen I. The dependence of the in vitro fermentation of dietary fibre to short-chain fatty acids on the contents of the soluble non-starch polysaccharides. Scand J Gastroenterol. 1993;28:418–422. doi: 10.3109/00365529309098242. [DOI] [PubMed] [Google Scholar]
- 35.Nyman M, Asp N-G. Fermentation of dietary fibre components in the rat intestinal tract. Br J Nutr. 1982;47:357–366. doi: 10.1079/bjn19820047. [DOI] [PubMed] [Google Scholar]
- 36.Ohkami H, Tazawa K, Yamashita I, Shimizu T, Murai K, Tobashi K, Fujimaki M. Effects of apple pectin on fecal bacterial enzymes in azoxymethane-induced rat colon carcinogenesis. Jpn J Cancer Res. 1995;86:523–529. doi: 10.1111/j.1349-7006.1995.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preiss J, Ashwell G. Polygalacturonic acid metabolism in bacteria. I. Enzymatic formation of 4-deoxy-l-threo-5-hexoseulose uronic acid. J Biol Chem. 1963;238:1571–1576. [PubMed] [Google Scholar]
- 38.Reeves A R, D'Elia J N, Frias J, Salayers A A. A Bacteroides thetaiotaomicron outer membrane protein that is essential for utilization of maltooligosaccharides and starch. J Bacteriol. 1996;178:823–830. doi: 10.1128/jb.178.3.823-830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rexová-Benková L, Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- 40.Roediger W E W. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- 41.Salayers A A, Leedle J A Z. Carbohydrate metabolism in the human colon. In: Hentges D J, editor. Human intestinal microflora in health and disease. London, United Kingdom: Academic Press; 1983. pp. 129–146. [Google Scholar]
- 42.Salayers A A, Panjeau M. Competitiveness of different polysaccharide utilization mutants of Bacteroides thetaiotaomicron in the intestinal tract of germfree mice. Appl Environ Microbiol. 1989;55:2572–2578. doi: 10.1128/aem.55.10.2572-2578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35(1Suppl.):S35–S38. doi: 10.1136/gut.35.1_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheppach W, Bartram H-P, Richter F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur J Cancer. 1995;31A:1077–1080. doi: 10.1016/0959-8049(95)00165-f. [DOI] [PubMed] [Google Scholar]
- 45.Schols H A, Voragen A G J. Complex pectins: structure elucidation using enzymes. In: Visser J, Voragen A G J, editors. Pectins and pectinases. Amsterdam, The Netherlands: Elsevier Science; 1996. pp. 3–19. [Google Scholar]
- 46.Smith J G, Yokoyama W H, German J B. Butyric acid from the diet: actions at the level of gene expression. Crit Rev Food Sci. 1998;38:259–297. doi: 10.1080/10408699891274200. [DOI] [PubMed] [Google Scholar]
- 47.Tierny Y, Béchet M, Joncquiert J-C, Dubourguler H-C, Guillaume J B. Molecular cloning and expression in Escherichia coli of genes encoding pectate lyase and pectin methylesterase activities from Bacteroides thetaiotaomicron. J Appl Bacteriol. 1994;75:592–602. doi: 10.1111/j.1365-2672.1994.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 48.Titgemeyer E C, Bouquin L D, Fahey G C, Garleb K A. Fermentability of various fiber sources by human fecal bacteria in vitro. Am J Clin Nutr. 1991;53:1418–1424. doi: 10.1093/ajcn/53.6.1418. [DOI] [PubMed] [Google Scholar]
- 49.Tomlin J, Taylor J S, Read N W. The effects of mixed bacteria on a selection of viscous polysaccharides in vitro. Nutr Rep Int. 1989;39:121–135. [Google Scholar]
- 50.Vince A J, McNeil N I, Wager J D, Wrong O M. The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria. Br J Nutr. 1990;63:17–26. doi: 10.1079/bjn19900088. [DOI] [PubMed] [Google Scholar]
- 51.Walzel E, Anger H, Bleyl D, Bock W, Kohn R, Kujawa M, Malovikova A, Raab M. Wirkungen delta-4,5-ungesättigter Oligogalakturonate auf die Bleieliminierung sowie ausgewählte essentielle Mineralstoffe bei der bleiexponierten Ratte. In: Anke M, Brückner C, Groppel B, Gürtler H, Grün M, Lombeck I, Schneider H-J, editors. Mengen- und Spurenelemente (10. Arbeitstagung, Leipzig). Oberlungwitz, Germany: VEB Kongreß- und Werbedruck; 1990. pp. 156–167. [Google Scholar]