Key Points
Question
Is digital breast tomosynthesis (DBT) associated with lower risk of interval invasive and advanced breast cancer (prognostic pathologic stage II or higher) compared with digital mammography among women with dense breasts?
Findings
In this cohort study that included 504 427 women undergoing 1 379 089 screening mammograms, interval cancer rates were not significantly different for DBT vs digital mammography. Among women with extremely dense breasts and high risk of breast cancer (3.6% of the study population), the advanced cancer rates for DBT vs digital mammography were 0.27 vs 0.80 per 1000 examinations over 12 months, respectively; the difference was statistically significant. Among women with nondense breasts, heterogeneously dense breasts, or with extremely dense breasts but at low to average risk of breast cancer (96.4% of the study population), there were no significant differences in advanced cancer rates.
Meaning
Among women with extremely dense breasts and at high risk of breast cancer, screening with DBT compared with digital mammography was associated with a lower risk of advanced breast cancer.
Abstract
Importance
Digital breast tomosynthesis (DBT) was developed with the expectation of improving cancer detection in women with dense breasts. Studies are needed to evaluate interval invasive and advanced breast cancer rates, intermediary outcomes related to breast cancer mortality, by breast density and breast cancer risk.
Objective
To evaluate whether DBT screening is associated with a lower likelihood of interval invasive cancer and advanced breast cancer compared with digital mammography by extent of breast density and breast cancer risk.
Design, Setting, and Participants
Cohort study of 504 427 women aged 40 to 79 years who underwent 1 003 900 screening digital mammography and 375 189 screening DBT examinations from 2011 through 2018 at 44 US Breast Cancer Surveillance Consortium (BCSC) facilities with follow-up for cancer diagnoses through 2019 by linkage to state or regional cancer registries.
Exposures
Breast Imaging Reporting and Data System (BI-RADS) breast density; BCSC 5-year breast cancer risk.
Main Outcomes and Measures
Rates per 1000 examinations of interval invasive cancer within 12 months of screening mammography and advanced breast cancer (prognostic pathologic stage II or higher) within 12 months of screening mammography, both estimated with inverse probability weighting.
Results
Among 504 427 women in the study population, the median age at time of mammography was 58 years (IQR, 50-65 years). Interval invasive cancer rates per 1000 examinations were not significantly different for DBT vs digital mammography (overall, 0.57 vs 0.61, respectively; difference, −0.04; 95% CI, −0.14 to 0.06; P = .43) or among all the 836 250 examinations with BCSC 5-year risk less than 1.67% (low to average-risk) or all the 413 061 examinations with BCSC 5-year risk of 1.67% or higher (high risk) across breast density categories. Advanced cancer rates were not significantly different for DBT vs digital mammography among women at low to average risk or at high risk with almost entirely fatty, scattered fibroglandular densities, or heterogeneously dense breasts. Advanced cancer rates per 1000 examinations were significantly lower for DBT vs digital mammography for the 3.6% of women with extremely dense breasts and at high risk of breast cancer (13 291 examinations in the DBT group and 31 300 in the digital mammography group; 0.27 vs 0.80 per 1000 examinations; difference, −0.53; 95% CI, −0.97 to −0.10) but not for women at low to average risk (10 611 examinations in the DBT group and 37 796 in the digital mammography group; 0.54 vs 0.42 per 1000 examinations; difference, 0.12; 95% CI, −0.09 to 0.32).
Conclusions and Relevance
Screening with DBT vs digital mammography was not associated with a significant difference in risk of interval invasive cancer and was associated with a significantly lower risk of advanced breast cancer among the 3.6% of women with extremely dense breasts and at high risk of breast cancer. No significant difference was observed in the 96.4% of women with nondense breasts, heterogeneously dense breasts, or with extremely dense breasts not at high risk.
This cohort study evaluates whether digital breast tomosynthesis compared with digital mammography is associated with a lower likelihood of interval invasive cancer, advanced breast cancer, and false alarms among women undergoing routine screening.
Introduction
Digital breast tomosynthesis (DBT), available at 81% of US breast imaging facilities as of January 2022,1 was developed with the expectation that it would decrease recall rate and improve breast cancer detection in women with dense breasts (heterogeneously or extremely dense) by decreasing interval invasive cancer rates. Interval invasive and advanced breast cancer diagnoses are considered screening failures.2 Reducing screening failures is important because women with screening failures have worse survival and morbidity from treatment.3
DBT studies report modest decreases in recall rate4 among screened women with dense and nondense breasts (scattered fibroglandular densities and almost entirely fatty), and modest increases in invasive cancer detection5 and biopsy rates.6 To our knowledge, no studies have shown a statistically significant decrease in interval cancer rate with DBT vs digital mammography screening overall or when stratified by women with dense vs nondense breasts.5,7,8,9,10 One study found the interval cancer rate increased in successive DBT rounds11 while another cross-sectional study found a decreased rate.8
Few studies have examined the cancer types diagnosed among women undergoing DBT. A study reported a higher proportion of advanced cancers detected with DBT vs digital mammography7 whereas another reported no difference in tumor characteristics or molecular subtypes between the 2 modalities.12 One study reported a decrease in stage II or higher cancers with DBT on subsequent screens,13 and another reported a lower proportion of lymph node–positive cancer.8
Previous studies of digital mammography have shown that screening failures were highest among women with dense breasts and elevated breast cancer risk.14,15 DBT studies to date have not examined screening failures by breast density combined with breast cancer risk. The purpose of this study was to evaluate whether DBT compared with digital mammography is associated with a lower likelihood of interval invasive cancer, advanced breast cancer, and false alarms among women undergoing routine screening by extent of breast density and breast cancer risk.
Methods
Study Setting and Data Sources
Data were obtained from 5 Breast Cancer Surveillance Consortium (BCSC) mammography registries.16 Women undergoing screening at participating BCSC facilities have demographic characteristics comparable with the US population.17,18 Data on women’s characteristics and mammography assessments were collected at the time of breast imaging. Breast cancer diagnoses were obtained by linking women’s imaging data to pathology databases and regional or state tumor registries or Surveillance, Epidemiology, and End Results (SEER) programs, with completeness of cancer reporting estimated at more than 94.3%.19 Registries and a central statistical coordinating center received institutional review board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analyses. All procedures were Health Insurance Portability and Accountability Act compliant, and registries and the coordinating center received a federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities.
Participants
We included women aged 40 to 79 years with no history of breast cancer or mastectomy who had a screening digital mammogram, DBT mammogram, or both from January 2011 through December 2018. A screening examination was defined based on the radiologist classification of the clinical indication for the mammogram. We restricted examinations to 44 facilities with at least 100 DBT and 100 digital mammography examinations, of which 45% (20 of 44) changed to 75% to 100% DBT examinations within 2 years of implementation. We excluded first screening examinations (which diagnose prevalent rather than incident cancer) and screening mammography that was unilateral or preceded by mammography within 9 months, performed with a screening ultrasound within 3 months, or for which screening breast magnetic resonance imaging (MRI) occurred within 12 months.
Measures and Definitions
Demographic and breast health history information were from self-administered paper or electronic questionnaires completed at each mammogram screening and/or extracted from electronic health records. Women self-reported race and ethnicity according to SEER and US vital statistics categories (Asian or Pacific Islander, Black non-Hispanic, Hispanic, Native American or Alaska Native, White non-Hispanic, other or multiple races). Radiologists categorized breast density at each mammogram during clinical interpretation using the Breast Imaging Reporting and Data System (BI-RADS) density categories: almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense with strong agreement on density reads between digital mammography and DBT.20 Breast biopsy results were abstracted from clinical pathology reports. We grouped prior benign diagnoses based on the highest level of risk as lobular carcinoma in situ greater than proliferative with atypia greater than proliferative without atypia greater than nonproliferative using published taxonomy21,22 or as unknown if a woman reported a prior biopsy with no available pathology result. Mammograms were classified as positive (BI-RADS 4 or 5 assessment) or negative (BI-RADS 1 or 2 assessment) based on final assessments after complete imaging workup.2,23,24 BI-RADS 3 final assessments were classified as positive because when cancer is present, short-interval follow-up imaging mostly leads to image-detected cancers.2
Mammograms were linked to invasive breast cancer and ductal carcinoma in situ (DCIS) diagnoses within 12 months after mammography. If more than 1 screening mammogram occurred 12 months before a breast cancer diagnosis, we associated the diagnosis date with the mammogram closest to diagnosis. If multiple breast cancer diagnoses occurred 1 year after the screening mammogram, we took the most severe stage. However, if both DCIS and invasive cancer were diagnosed within 1 year of the screening examination, but more than 6 months apart, each cancer type contributed to their respective cancer rates, but only 1 cancer was included for other rates. We calculated interval invasive rates as the number of invasive cancers within 12 months following a negative mammogram result over the total number of mammograms. We calculated screen-detected DCIS rates as the number of DCIS diagnoses within 12 months following a positive mammogram over the total number of mammograms. False-positive recall rates were calculated as the number of mammograms with an initial positive assessment (BI-RADS 0, 3, 4, or 5) without invasive cancer or DCIS within 12 months of the examination, over the total number of mammograms. False-positive short-interval follow-up recommendation rates were calculated as the number of mammograms with final assessment of BI-RADS 3 without invasive cancer or DCIS diagnosed within 12 months divided by the total number of mammograms. False-positive biopsy recommendation rates were calculated as the number of mammograms with a final assessment of BI-RADS 4 or 5 without invasive cancer or DCIS diagnosed within 12 months divided by the total number of mammograms.
BCSC 5-year invasive cancer risk was calculated using the BCSC risk calculator version 2 (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and categorized as low (0-<1.00%), average (1.00%-1.66%), intermediate (1.67%-2.49%), high (2.50%-3.99%), or very high (>3.99%).25
Outcomes
Invasive breast cancers were classified according to the American Joint Committee on Cancer (AJCC) staging system, 8th edition.26 We defined early-stage invasive cancer as prognostic pathologic stage I and advanced cancer as prognostic pathologic stages II, III, or IV.3 If pathologic stage was missing (11.4%), we classified based on AJCC anatomic stage that has a similar risk of breast cancer death as pathologic stage (ie, early invasive cancer, stage I or IIa and advanced cancer, stage IIb, III, or IV).3 We calculated screen–detected stage I cancer rates as the number of stage I cancers diagnosed within 12 months of a positive mammogram result divided by the total number of mammograms. We calculated advanced cancer rates as the number of advanced cancers diagnosed within 12 months of a screening mammogram (positive or negative) divided by the total number of mammograms.
We also classified tumors according to the Tomosynthesis Mammographic Imaging Screening Trial (TMIST) definition of advanced breast cancer that requires at least 1 of the following criteria: (1) tumor 20 mm or larger, (2) tumor larger than 10 mm and either human epidermal growth factor receptor 2 (ERBB2, formerly HER2)–positive or triple-negative, (3) cancer that spread from the breast to at least 1 nearby lymph node, or (4) cancer that spread from the breast to a distant organ.27 We calculated TMIST advanced cancer rates as the number of TMIST advanced cancers diagnosed within 12 months of a screening mammogram (positive or negative) divided by the total number of mammograms.
Statistical Analysis
All analyses were performed using the screening mammogram as the unit of analysis; women could have more than 1 mammogram during the study period. We used descriptive statistics to characterize screening mammograms by imaging modality (DBT vs digital mammography).
We estimated screening outcome rates per 1000 mammograms, absolute differences in screening outcomes by modality (see eMethods in the Supplement), and 95% CIs using log-binomial regression estimated via generalized estimating equations with a working independence correlation structure to account for clustering within facilities. Separate screening outcomes were calculated by breast density and BCSC 5-year risk stratified by low to average risk (<1.67%) and high risk (≥1.67%) based on prior work showing high interval cancer rates for women with extremely dense breasts and 5-year risk of 1.67% or higher.14
To account for differences in the women’s characteristics associated with DBT vs digital mammography screening examinations, we incorporated inverse probability weighting (IPW) derived from propensity scores for receiving DBT. The propensity model used logistic regression adjusting for age at examination, BCSC registry, facility academic or not, calendar year, race and ethnicity, breast density, first-degree family history, time since last mammogram, and the most severe prior benign biopsy result. Race and ethnicity were included in the propensity score model to account for the varied diffusion of DBT by race and ethnicity during the study period.28 Examinations with missing information for a categorical covariate were included in the propensity model and assigned to a separate category of missing. We used stabilized weights29 to balance the distributions of covariates between DBT and digital mammography examinations. To evaluate the propensity model, we compared the weighted descriptive statistics of covariates between DBT and digital mammography examinations by standardized difference in means30 and examined the estimated associations after weighting between covariates and screening modality obtained from the weighted log-binomial model. We also compared the distribution of the propensity scores between DBT and digital mammography examinations to verify common support (see the eFigure in the Supplement).
To test the robustness of our results, we performed a sensitivity analysis using a leave-one-out approach comparing results after removal of each registry, one at a time, and the results were similar. The eMethods including eTable 1 in the Supplement describe imputation methods for missing tumor characteristics used in calculating the TMIST definition of advanced cancer.
Statistical analyses used SAS version 9.4 (SAS Institute Inc). All tests were 2-sided, and statistical significance was defined as P < .05. Because of the potential for type I error due to multiple comparisons, findings for analyses should be interpreted as exploratory.
Results
A total of 308 141 women had only digital mammograms (mean, 2.2 per woman); 56 939 had only DBT mammograms (mean, 1.6 per woman); and 139 347 had both digital and DBT mammograms (mean, 2.3 digital and 2.0 DBT per woman). Women undergoing DBT were more likely to be White non-Hispanic and have a first-degree breast cancer family history, history of breast biopsy, and BCSC 5-year risk of 1.67% or higher (Table 1). Distributions of characteristics at the time of examination were similar for DBT vs digital examinations after IPW. Examination proportions with positive initial (7.4% vs 8.7%) and final (2.9% vs 3.4%) assessment were lower with DBT vs digital mammography.
Table 1. Characteristics of 1 377 902 of 1 379 089 Screening Mammograms From 504 150 of 504 427 Women After Excluding 1187 Digital Breast Tomosynthesis Examinations With Extreme Inverse Probability Weights (>50) by Modality and With and Without Inverse Probability Weighting.
| Digital breast tomosynthesis | Digital mammography | Standardized difference in means | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Raw | IPWa | |||
| Raw | IPWa | Raw | IPWa | |||||
| Screening mammograms | 374 002 | 1 003 900 | ||||||
| Age, y | ||||||||
| No. | 374 002 | 1 003 900 | ||||||
| 40-49 | 81 808 | 21.9 | 26.9 | 230 258 | 22.9 | 23.0 | −0.03 | 0.07 |
| 50-59 | 126 446 | 33.8 | 34.7 | 334 798 | 33.3 | 33.7 | 0.01 | 0.02 |
| 60-69 | 112 483 | 30.1 | 26.4 | 291 157 | 29.0 | 28.9 | 0.02 | −0.06 |
| 70-79 | 53 265 | 14.2 | 11.9 | 147 687 | 14.7 | 14.4 | −0.01 | −0.07 |
| Screening at an academic facility | ||||||||
| No. | 374 002 | 1 003 900 | ||||||
| No | 259 573 | 69.4 | 86.0 | 830 499 | 82.7 | 81.2 | −0.32 | 0.10 |
| Yes | 114 429 | 30.6 | 14.0 | 173 401 | 17.3 | 18.8 | 0.32 | −0.13 |
| Race and ethnicity | ||||||||
| No. | 366 721 | 971 409 | ||||||
| Asian, non-Hispanic | 14 131 | 3.9 | 9.4 | 107 685 | 11.1 | 9.3 | −0.27 | −0.01 |
| Black, non-Hispanic | 24 095 | 6.6 | 5.9 | 125 230 | 12.9 | 11.1 | −0.21 | −0.20 |
| Hispanic | 11 170 | 3.0 | 6.3 | 60 477 | 6.2 | 5.4 | −0.15 | 0.02 |
| White, non-Hispanic | 311 545 | 85.0 | 76.0 | 660 510 | 68.0 | 72.4 | 0.41 | −0.03 |
| Other or multiple racesb | 5780 | 1.6 | 2.4 | 17 507 | 1.8 | 1.8 | −0.02 | 0.03 |
| Menopausal status | ||||||||
| No. | 325 316 | 922 815 | ||||||
| Premenopausal or perimenopausal | 78 551 | 24.1 | 30.6 | 268 138 | 29.1 | 28.4 | −0.13 | −0.04 |
| Postmenopausal | 230 945 | 71.0 | 64.8 | 607 844 | 65.9 | 66.5 | 0.02 | −0.18 |
| Surgical menopausal | 15 820 | 4.9 | 4.7 | 46 833 | 5.1 | 5.1 | −0.02 | −0.05 |
| First-degree family history of breast cancerc | ||||||||
| No. | 352 987 | 974 555 | ||||||
| No | 281 329 | 79.7 | 82.9 | 811 716 | 83.3 | 82.6 | −0.14 | −0.13 |
| Yes | 71 658 | 20.3 | 17.1 | 162 839 | 16.7 | 17.4 | 0.08 | −0.05 |
| BI-RADS breast densityd | ||||||||
| No. | 364 694 | 958 685 | ||||||
| Almost entirely fat | 39 059 | 10.7 | 12.8 | 101 842 | 10.6 | 11.2 | 0.01 | 0.05 |
| Scattered fibroglandular densities | 173 534 | 47.6 | 43.4 | 428 513 | 44.7 | 45.1 | 0.07 | −0.02 |
| Heterogeneously dense | 127 740 | 35.0 | 36.9 | 357 811 | 37.3 | 36.4 | −0.03 | 0.02 |
| Extremely dense | 24 361 | 6.7 | 6.9 | 70 519 | 7.4 | 7.3 | −0.02 | −0.01 |
| BCSC 5-y risk, %e | ||||||||
| No. | 346 601 | 902 710 | ||||||
| 0 to <1.00 | 84 236 | 24.3 | 32.1 | 258 190 | 28.6 | 28.2 | −0.07 | 0.09 |
| 1.00-1.66 | 134 199 | 38.7 | 38.4 | 359 625 | 39.8 | 39.6 | 0 | 0 |
| 1.67-2.49 | 81 491 | 23.5 | 20.2 | 189 943 | 21.0 | 21.3 | 0.07 | −0.01 |
| 2.50-3.99 | 39 628 | 11.4 | 8.1 | 82 467 | 9.1 | 9.4 | 0.08 | −0.03 |
| ≥4.00 | 7047 | 2.0 | 1.2 | 12 485 | 1.4 | 1.5 | 0.05 | −0.02 |
| History of benign breast disease | ||||||||
| No. | 374 002 | 1 003 900 | ||||||
| No prior biopsy | 287 105 | 76.8 | 79.2 | 782 160 | 77.9 | 77.7 | −0.03 | 0.04 |
| Biopsy, pathology unknown | 41 152 | 11.0 | 10.9 | 133 622 | 13.3 | 12.7 | −0.07 | −0.06 |
| Nonproliferative disease | 31 525 | 8.4 | 7.6 | 62 489 | 6.2 | 6.9 | 0.08 | 0.03 |
| Proliferative with atypia | 11 448 | 3.1 | 1.9 | 21 061 | 2.1 | 2.2 | 0.06 | −0.03 |
| Proliferative without atypia | 2342 | 0.6 | 0.4 | 3855 | 0.4 | 0.4 | 0.03 | −0.01 |
| LCIS | 430 | 0.1 | 0.1 | 713 | 0.1 | 0.1 | 0.01 | 0 |
| Time since last mammogram, yf | ||||||||
| No. | 370 143 | 961 039 | ||||||
| 1 (8-18 mo) | 271 099 | 73.2 | 72.7 | 691 320 | 71.9 | 72.5 | 0.08 | 0.02 |
| 2 (19-30 mo) | 55 501 | 15.0 | 15.5 | 155 329 | 16.2 | 15.7 | −0.02 | 0 |
| ≥3 (>30 mo) | 43 543 | 11.8 | 11.8 | 114 390 | 11.9 | 11.8 | 0.01 | 0.01 |
| Initial assessmentg | ||||||||
| No. | 374 002 | 1 003 900 | ||||||
| Negative | 243 274 | 65.0 | 69.2 | 634 990 | 63.3 | 63.7 | 0.04 | 0.11 |
| Benign finding | 102 684 | 27.4 | 23.7 | 279 520 | 27.8 | 27.5 | −0.01 | −0.09 |
| Need additional imaging evaluation | 27 862 | 7.4 | 7.1 | 88 859 | 8.9 | 8.7 | −0.05 | −0.06 |
| Probably benign finding | 124 | 0 | 0.1 | 377 | 0 | 0 | 0 | 0.01 |
| Suspiciously abnormal | 42 | 0 | 0 | 124 | 0 | 0 | 0 | 0 |
| Highly suggestive of malignancy | 16 | 0 | 0 | 30 | 0 | 0 | 0 | 0 |
| Final assessmenth | ||||||||
| No. | 374 002 | 1 003 900 | ||||||
| Negative | 248 242 | 66.4 | 70.5 | 654 748 | 65.2 | 65.7 | 0.02 | 0.10 |
| Benign finding | 113 416 | 30.3 | 26.5 | 310 684 | 30.9 | 30.6 | −0.01 | −0.09 |
| Need additional imaging evaluation | 1621 | 0.4 | 0.4 | 4589 | 0.5 | 0.4 | 0.00 | −0.01 |
| Probably benign finding | 5425 | 1.5 | 1.1 | 17 737 | 1.8 | 1.8 | −0.03 | −0.06 |
| Suspiciously abnormal | 4889 | 1.4 | 1.4 | 15 065 | 1.5 | 1.4 | −0.02 | 0 |
| Highly suggestive of malignancy | 409 | 0.1 | 0.1 | 1077 | 0.1 | 0.1 | 0 | 0 |
Abbreviations: BCSC, Breast Cancer Surveillance Consortium; BI-RADS, Breast Imaging Reporting and Data System; IPW, inverse probability weighting; LCIS, lobular cancer in situ; Raw, raw numbers.
Variables in propensity model: age at mammogram, BCSC registry, indicator if academic facility, examination year, race and ethnicity, breast density, first-degree family history of breast cancer, time since last mammogram, history of benign breast disease.
Other racial category included Native American, Alaska Native, non-Hispanic with 2 or more reported races, and other.
Defined as first-degree relative (mother, sister, or daughter) with breast cancer.
Breast density assessed for each screening mammogram.
Five-year risk calculated using age, race, first-degree family history of breast cancer, history of breast biopsy, BI-RADS density. Risk categories are defined as low (0% to <1.00%), average (1.00%-1.66%), intermediate (1.67%-2.49%), high (2.50%-3.99%), and very high (>3.99%).
One-year median, 13 months; 2-year median, 24 months; ≥3-year median, 45 months.
Assessment based on initial mammography interpretation.
Assessment based after complete imaging workup; a small proportion (0.45%) of examinations had a final recommendation for additional imaging but no additional imaging was recorded.
Table 2 shows the joint distribution of breast density and BCSC 5-year risk. Among DBT screened women, 33 692 (9.7%) were at low to average risk with fatty breasts and 13 291 (3.8%) at high risk with extremely dense breasts.
Table 2. Distribution of Breast Cancer Surveillance Consortium 5-Year Risk by Breast Density and by Modality.
| BI-RADS breast density, No. (%) | ||||
|---|---|---|---|---|
| Almost entirely fat | Scattered fibroglandular densities | Heterogeneously dense | Extremely dense | |
| Digital breast tomosynthesis | ||||
| No. of examinations | 36 402 | 163 307 | 122 990 | 23 902 |
| BCSC 5-y riska | ||||
| 0% to <1.66% | 33 692 (9.7) | 117 322 (33.9) | 56 810 (16.4) | 10 611 (3.1) |
| ≥1.67% | 2710 (0.78) | 45 985 (13.3) | 66 180 (19.1) | 13 291 (3.8) |
| Digital mammography | ||||
| No. of examinations | 93 570 | 397 770 | 342 274 | 69 096 |
| BCSC 5-y riska | ||||
| 0% to <1.66% | 88 143 (9.8) | 306 387 (33.9) | 185 489 (20.6) | 37 796 (4.2) |
| ≥1.67% | 5427 (0.60) | 91 383 (10.1) | 156 785 (17.4) | 31 300 (3.5) |
Abbreviations: BCSC, Breast Cancer Surveillance Consortium; BI-RADS, Breast Imaging Reporting and Data System.
Five-year risk calculated using age, race, first-degree family history of breast cancer, history of breast biopsy, BI-RADS density. Risk categories are defined as low to average (0-<1.66%) and high (≥1.67%).
Screening Outcomes Overall
For overall screening outcomes per 1000 examinations, (Figure 1 and Figure 2 and eTable 2 in the Supplement), DBT vs digital mammography had significantly fewer advanced cancers (0.36 vs 0.45; difference, −0.09; 95% CI, −0.18 to −0.01), false-positive recalls (66.2 vs 83.4; difference, −17.2; 95% CI, −25.2 to −9.2), and false-positive short-interval follow-up recommendations (11.2 vs 17.9; difference, −6.7; 95% CI, −11.2 to −2.2), respectively. Stage I–detected cancers occurred in 3.45 vs 2.99 per 1000 examinations, respectively (difference, 0.46; 95% CI, −0.01 to 0.93; P = .06). Interval invasive cancers occurred in 0.57 vs 0.61 (difference, −0.04; 95% CI, −0.14 to 0.06; P = .43).
Figure 1. Rates and Absolute Risk Differences per 1000 Examinations for Breast Cancer Screening Benefits and Failures.
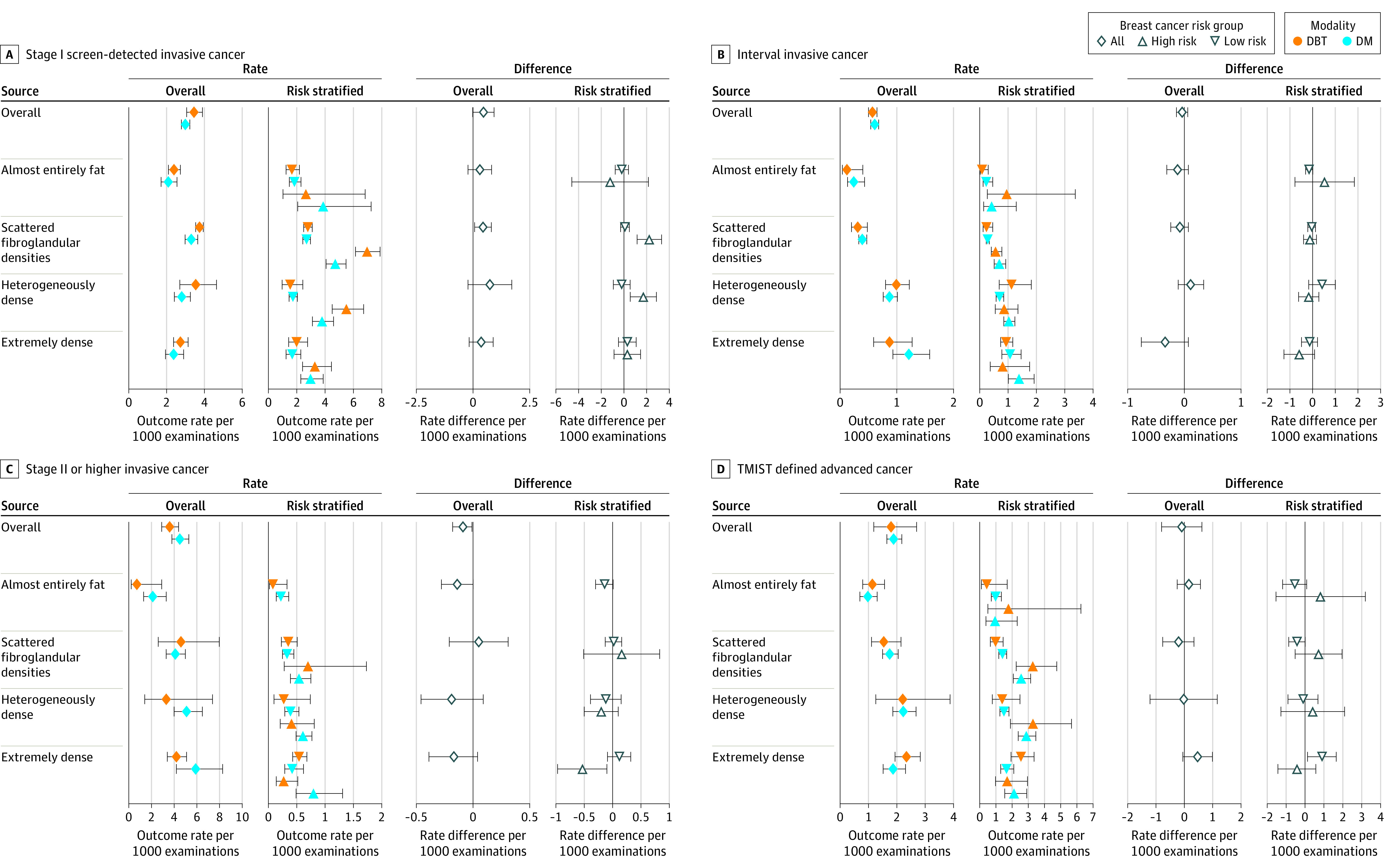
Data by breast density categories and 5-year risk categories (low risk <1.67%; high risk ≥1.67%). DM indicates digital mammography; DBT, digital breast tomosynthesis; and TMIST, Tomosynthesis Mammographic Imaging Screening Trial. See eTables 3 and 5 in the Supplement for exact values. 95% CIs represented by whiskers.
Figure 2. Rates and Absolute Risk Differences per 1000 Breast Cancer Screening Examinations .
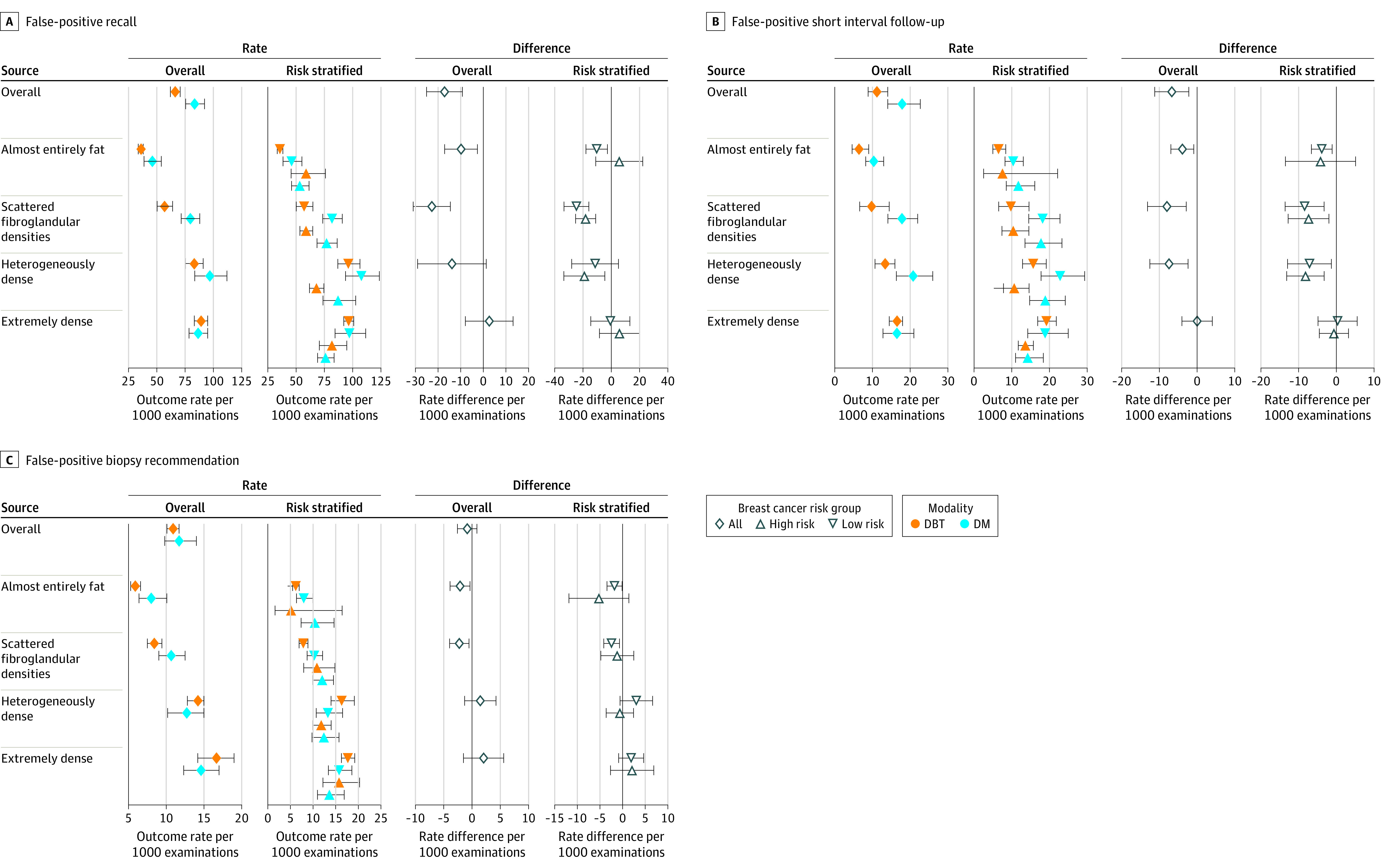
Data by breast density categories and 5-year risk categories (low risk <1.67%; high risk ≥1.67%). DM indicates digital mammography; DBT, digital breast tomosynthesis. See eTables 4 and 6 in the Supplement for exact values. 95% CIs represented by whiskers.
Screening Outcomes by Breast Density
Screen–detected stage I cancer rates were significantly higher for DBT vs digital mammography for women with scattered fibroglandular densities (Figure 1; eTable 3 in the Supplement). Rates of interval invasive cancer, advanced cancer, and TMIST advanced cancer were not significantly different for DBT vs digital mammography across breast density categories (Figure 1; eTable 3 in the Supplement).
False-positive recall, short-interval follow-up recommendation, and biopsy recommendation rates were significantly lower for DBT than digital mammography for women with nondense breasts. False-positive short-interval follow-up rates were significantly lower for DBT than digital mammography among women with heterogeneously dense breasts (Figure 2; eTable 4 in the Supplement).
Screening Outcomes by Breast Density and BCSC 5-Year Risk
Screen–detected stage I cancer rates were significantly higher for DBT vs digital mammography among women with BCSC 5-year risk of 1.67% or higher (high risk) and scattered fibroglandular densities or heterogeneously dense breasts (Figure 1; eTable 5 in the Supplement). Interval invasive rates were not significantly different for DBT vs digital mammography across breast density categories among women with BCSC 5-year risk of less than 1.67% (low to average risk) or high risk (Figure 1; eTable 5 in the Supplement). Advanced cancer rates were significantly lower for DBT vs digital mammography for women with extremely dense breasts at high risk (0.27 vs 0.80; difference, −0.53; 95% CI, −0.97 to −0.10) but not low to average-risk (0.54 vs 0.42; difference, 0.12; 95% CI, −0.09 to 0.32; Figure 1; eTable 5 in the Supplement). TMIST advanced cancer rates were significantly higher for low-risk women with extremely dense breasts with DBT vs digital mammography (2.55 vs 1.66; difference, 0.90; 95% CI, 0.14 to 1.70).
False-positive recall and short-interval follow-up recommendation rates were significantly lower for DBT vs digital mammography for low- to average-risk women with nondense breasts and for high-risk women with scattered fibroglandular densities and heterogeneously dense breasts (Figure 2; eTable 6 in the Supplement). False-positive short-interval follow-up recommendations rates were significantly lower for DBT vs digital mammography for low- to average-risk women with heterogeneously dense breasts. False-positive biopsy recommendation rates were significantly lower for DBT vs digital mammography for low- to average-risk women with nondense breasts (Figure 2; eTable 6 in the Supplement).
Discussion
In this cohort study, screening with DBT vs digital mammography was associated with a significantly lower risk of advanced breast cancer (prognostic pathologic stage II or higher) among women with extremely dense breasts and high risk of breast cancer, defined as BCSC 5-year invasive cancer risk 1.67% or higher. No significant difference was observed in risk of prognostic pathologic stage II or higher in women with extremely dense breasts not at high risk. Screening with DBT vs digital mammography was not associated with a significant difference in risk of interval invasive cancer. A difference may not have been observed because only 25% of interval cancers were advanced cancers and only 7.4% were among women with extremely dense breasts and at high risk.
Women undergoing screening mammography in the US may undergo digital mammography or DBT depending on the facility they attend and on their insurance coverage. Although 81% of accredited facilities offer DBT, only 45% of all mammography units are DBT.1 Thus, facilities may offer digital mammography only, both digital mammography and DBT, or only offer DBT.31 At some facilities, when DBT was introduced, women with dense breasts were triaged to DBT machines when possible.32 This study’s findings suggest for facilities with both DBT and digital mammography available, triaging women with extremely dense breasts and at high risk to undergo DBT may be clinically indicated. For other women, there were no significant differences between DBT and digital mammography in the ability to detect interval and advanced cancers. However, digital mammography may be associated with a higher false-positive recall and short-interval follow-up recommendation rate. Women should be informed of the likelihood of screening failures and harms for both modalities.
Studies in the US have reported an elevated breast cancer detection rate with DBT vs digital mammography,31,33 in particular, in women with scattered fibroglandular densities and heterogeneously dense breasts.34,35 In the current study, the significantly higher likelihood of screen–detected stage I cancers with DBT vs digital mammography in high-risk women with scattered fibroglandular densities or heterogeneously dense breasts demonstrates only about a third of screened women (29% of study population) may have significantly higher cancer detection with DBT. The lack of a significant increase in screen-detected early-stage disease concomitant with the reduction in advanced cancer rates with use of DBT among high-risk women with extremely dense breasts could be because an increase in screen-detected early-stage cancer rates may not be observed until multiple, consecutive rounds with reduced screening failures. Future studies will need to examine early-stage and advanced-stage rates with DBT by screening round, breast density, and risk to determine in which groups early-stage cancer rates increase as advanced stage cancer rates decrease indicating that more aggressive tumors are being diagnosed earlier.
Studies have reported an increase in biopsy rates with DBT vs digital mammography among women with heterogeneously dense breasts.34 This study showed that false-positive biopsy recommendation rates were significantly lower among the 43.7% of low- to average-risk women with nondense breasts undergoing DBT vs digital mammography. Also, undergoing DBT vs digital mammography was associated with a significantly lower risk of false-positive recall and short-interval follow-up recommendation rates among low- to average-risk women with nondense breasts and heterogeneously dense breasts, and high-risk women with scattered fibroglandular densities and heterogeneously dense breasts. To our knowledge, this is the first study to document an association of lower likelihood of false-positive short-interval follow-up and false-positive biopsy with DBT.
TMIST is an ongoing large randomized trial designed to compare the effectiveness of screening digital mammography vs DBT, which uses a new definition of advanced cancer as a primary study outcome.27 Evidence is conflicting on whether DBT decreases TMIST–defined advanced cancer rates compared with digital mammography in cohort studies. In the current study that examined outcomes by density and risk, TMIST–defined advanced cancer rates were not significantly different for most women undergoing DBT vs digital mammography except for women with extremely dense breasts and at low to average risk, among whom DBT was associated with a significantly higher risk of TMIST–defined advanced cancer rates. This finding is consistent with a cohort study that reported a higher proportion of TMIST–defined advanced breast cancers among women undergoing DBT vs digital mammography7 whereas another cohort study found a nonsignificant, lower proportion.8 Neither study reported results by density and risk. Of the study’s cancers that were classified as advanced by the TMIST definition, 79% would be classified as stage I according to the prognostic pathologic definition among women with extremely dense breasts and low breast cancer risk undergoing DBT. Thus, the TMIST definition of advanced cancer includes a high proportion of breast cancers that are likely slow growing and readily detected by DBT, which may explain the increase in TMIST advanced cancer for low-risk women with extremely dense breasts.
Limitations
The study had several limitations. First, women receiving DBT may have been at higher risk, although IPW was used to account for these differences. Second, even with a large study cohort and multiple observations per woman, some estimated CIs were wide due to small samples such that a clinically important effect size may have been missed. Third, the association of synthetic DBT views with screening outcomes was not assessed because this information was not available at the examination level. However, most studies have not reported a difference in screening outcomes with synthetic vs digital mammography views plus DBT.36,37,38,39 Fourth, the reasons women underwent DBT vs digital mammography were not recorded or specified. Selection of DBT vs digital mammography is likely dependent upon the machine available at the time of imaging at facilities that have both modalities, women’s insurance coverage, or both.
Conclusions
Screening with DBT vs digital mammography was not associated with a significant difference in risk of interval invasive cancer and was associated with a significantly lower risk of advanced breast cancer (prognostic pathologic stage II or higher) among the 3.6% of women with extremely dense breasts and at high risk of breast cancer. No significant difference was observed in the 96.4% of women with nondense breasts, heterogeneously dense breasts, or with extremely dense breasts not at high risk.
eMethods. Absolute difference in screening out come
eFigure. Propensity scores for digital mammography and digital breast tomosynthesis examinations
eTable 1. Summary of variables used to input tumor characteristics used to calculate TMIST
eTable 2. Outcomes from screening with digital breast tomosynthesis vs. digital mammography
eTable 3. Rate of screening benefits and failures by breast density for digital breast tomosynthesis vs digital mammography
eTable 4. Rate of screening false-alarms by breast density for digital breast tomosynthesis vs. digital mammography
eTable 5. Rate of screening benefits and failures by breast density for digital mammography and digital breast tomosynthesis
eTable 6. Rate of screening harms by breast density and BCSC 5-year risk for digital breast tomosynthesis vs. digital mammography
References
- 1.Mammography Quality Standards Act and Program. US Food and Drug Administration . Published January 3, 2022. Accessed January 17, 2022. https://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm113858.htm
- 2.Sprague BL, Miglioretti DL, Lee CI, Perry H, Tosteson AAN, Kerlikowske K. New mammography screening performance metrics based on the entire screening episode. Cancer. 2020;126(14):3289-3296. doi: 10.1002/cncr.32939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerlikowske K, Bissell MCS, Sprague BL, et al. Advanced breast cancer definitions by staging system examined in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2021;113(7):909-916. doi: 10.1093/jnci/djaa176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marinovich ML, Hunter KE, Macaskill P, Houssami N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J Natl Cancer Inst. 2018;110(9):942-949. doi: 10.1093/jnci/djy121 [DOI] [PubMed] [Google Scholar]
- 5.Giampietro RR, Cabral MVG, Lima SAM, Weber SAT, Dos Santos Nunes-Nogueira V. Accuracy and effectiveness of mammography versus mammography and tomosynthesis for population-based breast cancer screening: a systematic review and meta-analysis. Sci Rep. 2020;10(1):7991. doi: 10.1038/s41598-020-64802-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald ES, Oustimov A, Weinstein SP, Synnestvedt MB, Schnall M, Conant EF. Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol. 2016;2(6):737-743. doi: 10.1001/jamaoncol.2015.5536 [DOI] [PubMed] [Google Scholar]
- 7.Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295(2):285-293. doi: 10.1148/radiol.2020191751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durand MA, Friedewald SM, Plecha DM, et al. False-negative rates of breast cancer screening with and without digital breast tomosynthesis. Radiology. 2021;298(2):296-305. doi: 10.1148/radiol.2020202858 [DOI] [PubMed] [Google Scholar]
- 9.Houssami N, Zackrisson S, Blazek K, et al. Meta-analysis of prospective studies evaluating breast cancer detection and interval cancer rates for digital breast tomosynthesis versus mammography population screening. Eur J Cancer. 2021;148:14-23. doi: 10.1016/j.ejca.2021.01.035 [DOI] [PubMed] [Google Scholar]
- 10.Hofvind S, Moshina N, Holen AS, et al. Interval and subsequent round breast cancer in a randomized controlled trial comparing digital breast tomosynthesis and digital mammography screening. Radiology. 2021;300(1):66-76. doi: 10.1148/radiol.2021203936 [DOI] [PubMed] [Google Scholar]
- 11.Bahl M, Mercaldo S, Dang PA, McCarthy AM, Lowry KP, Lehman CD. Breast cancer screening with digital breast tomosynthesis: are initial benefits sustained? Radiology. 2020;295(3):529-539. doi: 10.1148/radiol.2020191030 [DOI] [PubMed] [Google Scholar]
- 12.Johnson K, Zackrisson S, Rosso A, et al. Tumor characteristics and molecular subtypes in breast cancer screening with digital breast tomosynthesis: The Malmo Breast Tomosynthesis Screening Trial. Radiology. 2019;293(2):273-281. doi: 10.1148/radiol.2019190132 [DOI] [PubMed] [Google Scholar]
- 13.Caumo F, Montemezzi S, Romanucci G, et al. Repeat screening outcomes with digital breast tomosynthesis plus synthetic mammography for breast cancer detection: results from the prospective Verona pilot study. Radiology. 2021;298(1):49-57. doi: 10.1148/radiol.2020201246 [DOI] [PubMed] [Google Scholar]
- 14.Kerlikowske K, Zhu W, Tosteson ANA, et al. ; Breast Cancer Surveillance Consortium . Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673-681. doi: 10.7326/M14-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerlikowske K, Sprague BL, Tosteson ANA, et al. Strategies to identify women at high risk of advanced breast cancer during routine screening for discussion of supplemental imaging. JAMA Intern Med. 2019;179(9):1230-1239. doi: 10.1001/jamainternmed.2019.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breast Cancer Screening Consortium home page. National Cancer Institute. Published 2022. Accessed April 13, 2022. https://www.bcsc-research.org/.
- 17.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235(3):775-790. doi: 10.1148/radiol.2353040738 [DOI] [PubMed] [Google Scholar]
- 18.Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49-58. doi: 10.1148/radiol.2016161174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94(20):1546-1554. doi: 10.1093/jnci/94.20.1546 [DOI] [PubMed] [Google Scholar]
- 20.Tice JA, Gard CC, Miglioretti DL, et al. Comparing mammographic density assessed by digital breast tomosynthesis or digital mammography: the Breast Cancer Surveillance Consortium. Radiology. 2022;302(2):286-292. doi: 10.1148/radiol.2021204579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast: a long-term follow-up study. Cancer. 1985;55(11):2698-2708. doi: [DOI] [PubMed] [Google Scholar]
- 22.Page DL, Schuyler PA, Dupont WD, Jensen RA, Plummer WD Jr, Simpson JF. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361(9352):125-129. doi: 10.1016/S0140-6736(03)12230-1 [DOI] [PubMed] [Google Scholar]
- 23.American College of Radiology . Breast Imaging Reporting and Data System (BI-RADS). 5th ed. American College of Radiology; 2013. [Google Scholar]
- 24.Yankaskas BC, Taplin SH, Ichikawa L, et al. Association between mammography timing and measures of screening performance in the United States. Radiology. 2005;234(2):363-373. doi: 10.1148/radiol.2342040048 [DOI] [PubMed] [Google Scholar]
- 25.Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137-3143. doi: 10.1200/JCO.2015.60.8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hortobagyi G, Connolly J, Edge SB, et al. Breast. In: Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:589-636. doi: 10.1007/978-3-319-40618-3_48 [DOI] [Google Scholar]
- 27.Lee C, McCaskill-Stevens W. Tomosynthesis mammographic Imaging Screening Trial (TMIST): an invitation and opportunity for the National Medical Association Community to shape the future of precision screening for breast cancer. J Natl Med Assoc. 2020;112(6):613-618. doi: 10.1016/j.jnma.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 28.Lee CI, Zhu W, Onega T, et al. Comparative access to and use of digital breast tomosynthesis screening by women’s race, ethnicity, and socioeconomic status. JAMA Netw Open. 2021;4(2):e2037546. doi: 10.1001/jamanetworkopen.2020.37546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550-560. doi: 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprague BL, Coley RY, Kerlikowske K, et al. Assessment of radiologist performance in breast cancer screening using digital breast tomosynthesis vs digital mammography. JAMA Netw Open. 2020;3(3):e201759. doi: 10.1001/jamanetworkopen.2020.1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alsheik NH, Dabbous F, Pohlman SK, et al. Comparison of resource utilization and clinical outcomes following screening with digital breast tomosynthesis versus digital mammography: findings from a learning health system. Acad Radiol. 2019;26(5):597-605. doi: 10.1016/j.acra.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 33.Miglioretti DL, Abraham L, Lee CI, et al. ; Breast Cancer Surveillance Consortium . Digital breast tomosynthesis: radiologist learning curve. Radiology. 2019;291(1):34-42. doi: 10.1148/radiol.2019182305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowry KP, Coley RY, Miglioretti DL, et al. Comparison of screening performance of digital breast tomosynthesis vs. digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3(7):e2011792. doi: 10.1001/jamanetworkopen.2020.11792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Østerås BH, Martinsen ACT, Gullien R, Skaane P. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293(1):60-68. doi: 10.1148/radiol.2019190425 [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman SP, Conant EF, Keller BM, et al. Implementation of synthesized two-dimensional mammography in a population-based digital breast tomosynthesis screening program. Radiology. 2016;281(3):730-736. doi: 10.1148/radiol.2016160366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caumo F, Zorzi M, Brunelli S, et al. Digital breast tomosynthesis with synthesized two-dimensional images versus full-field digital mammography for population screening: outcomes from the Verona screening program. Radiology. 2018;287(1):37-46. doi: 10.1148/radiol.2017170745 [DOI] [PubMed] [Google Scholar]
- 38.Yoon JH, Kim EK, Kim GR, et al. Comparing recall rates following implementation of digital breast tomosynthesis to synthetic 2D images and digital mammography on women with breast-conserving surgery. Eur Radiol. 2020;30(11):6072-6079. doi: 10.1007/s00330-020-06992-6 [DOI] [PubMed] [Google Scholar]
- 39.Zuckerman SP, Sprague BL, Weaver DL, Herschorn SD, Conant EF. Multicenter evaluation of breast cancer screening with digital breast tomosynthesis in combination with synthetic versus digital mammography. Radiology. 2020;297(3):545-553. doi: 10.1148/radiol.2020200240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Absolute difference in screening out come
eFigure. Propensity scores for digital mammography and digital breast tomosynthesis examinations
eTable 1. Summary of variables used to input tumor characteristics used to calculate TMIST
eTable 2. Outcomes from screening with digital breast tomosynthesis vs. digital mammography
eTable 3. Rate of screening benefits and failures by breast density for digital breast tomosynthesis vs digital mammography
eTable 4. Rate of screening false-alarms by breast density for digital breast tomosynthesis vs. digital mammography
eTable 5. Rate of screening benefits and failures by breast density for digital mammography and digital breast tomosynthesis
eTable 6. Rate of screening harms by breast density and BCSC 5-year risk for digital breast tomosynthesis vs. digital mammography


