Abstract
Objectives
Dry powder inhalers (DPIs) and soft mist inhalers have a substantially lower global warming potential than pressurised metered-dose inhalers (pMDIs). To help mitigate climate change, we assessed the potential emission reduction in CO2 equivalents when replacing pMDIs by non-propellant inhalers (NPIs) in Dutch respiratory healthcare and estimated the associated cost.
Design
We performed a descriptive analysis of prescription data from two national databases of two independent governmental bodies. First, we calculated the number of patients with chronic obstructive pulmonary disease (COPD) and asthma that were using inhalation medication (2020). Second, we calculated the number and total of daily defined doses of pMDIs and NPIs including DPIs and soft mist inhalers, as well as the number of dispensed spacers per patient (2020). Third, we estimated the potential emission reduction in CO2 equivalents if 70% of patients would switch from using pMDIs to using NPIs. Fourth, we performed a budget impact analysis.
Setting
Dutch respiratory healthcare.
Primary and secondary outcome measures
The carbon footprint of current inhalation medication and the environmental and financial impact of replacing pMDIs with NPIs.
Results
In 2020, 1.4 million patients used inhalers for COPD or asthma treatment. A total of 364 million defined daily doses from inhalers were dispensed of which 49.6% were dispensed through pMDIs. We estimated that this could be reduced by 70% which would lead to an annual reduction in greenhouse gas emission of 63 million kg.CO2 equivalents saving at best EUR 49.1 million per year.
Conclusions
In the Netherlands, substitution of pMDIs to NPIs for eligible patients is theoretically safe and in accordance with medical guidelines, while reducing greenhouse gas emission by 63 million kg.CO2 equivalents on average and saving at best EUR 49.1 million per year. This study confirms the potential climate and economic benefit of delivering a more eco-friendly respiratory care.
Keywords: Change management, Health policy, Protocols & guidelines, Asthma, Chronic airways disease
Strengths and limitations of this study.
In countries with national administrative databases on drug use, this type of study quickly provides insight in the current CO2 impact and the potential for improvement in respiratory healthcare.
This type of study provides insight in the cost/benefit of a large-scale shift from propellant to non-propellant inhalers.
This type of study may be used to monitor implementation strategies to decrease use of propellant inhalers.
Given availability and reliability of the data, the present analysis could easily be replicated elsewhere which allows for international comparison and aggregation.
Implementation challenges remain underexposed.
Introduction
Climate change is the greatest global health threat of our times, inflicting a range of ill health outcomes including (re)emerging zoonoses such as COVID-19, non-communicable diseases and mental health disorders.1 2 Paradoxically, the healthcare industry contributes substantially to global warming. If global healthcare were a country, it would rank fifth for greenhouse gas emissions and its environmental footprint is substantial.3 4 In the Netherlands, the healthcare sector is responsible for 6%–7% of the total national CO2 equivalents emission.5 Hence, the Dutch healthcare sector could play a significant role in meeting the national climate policy goals, thereby preserving planetary health and human health that depends on it. Public concerns for healthcare and for the ecological crises rank high in consecutive opinion surveys of the national statistical office, Statistics Netherlands (CBS).
Among the impactful solutions to deliver sustainable healthcare is the choice of inhaler type to deliver medication to the lungs of patients with asthma, allergies or chronic obstructive pulmonary disease (COPD). Pressurised metered-dose inhalers (pMDIs) contain propellants known as hydrofluorocarbons (HFCs), potent F gases that account for 15 megaton CO2 equivalents (0.03%) of all greenhouse gas emissions worldwide.5 In the European Union, HFCs will be phased out by two-thirds in 2030 through limiting sale and use of air conditioning and refrigeration equipment. However, their application in metered-dose inhalers is exempted from this regulation.6 pMDIs contain either the propellant HFC-134a or HFC-227ea. Other commonly used inhalers are dry powder inhalers (DPIs) and soft mist inhalers. For the purpose of this paper we label these as non-propellant inhalers (NPIs). These are as safe and effective in most patients but do not contain greenhouse gases which is why the life cycle assessment (LCA) of their environmental impact is substantially lower than those of pMDIs.7
Several studies have assessed the costs and benefits of switching to medication with a lower global warming potential (GWP) (see box 1). Wilkinson et al found considerable reductions in both CO2 emissions and pharmaceutical costs.8 Janson et al recommend that ‘the lower carbon footprint of DPIs should be considered alongside other factors when choosing inhaler devices’.9 In their review, Starup-Hansen et al recommend to update guidelines: ‘guidance should consider the potential benefits of advising DPIs as the device of choice in new diagnoses of asthma and COPD as well as the benefits of switching patients currently using pMDIs to DPIs where clinically appropriate’.10 These recommendations have been recently adopted in the guidelines ‘Asthma in adults’11 and ‘COPD’12 of the Dutch College of General Practitioners (NHG). Among other updates, these guidelines contain the same modest, though historical, reference to considering the environmental impact of the medicine of choice for the prescribing physician (see box 2).
Box 1. Global warming potential (GWP).
The GWP is the heat absorbed by any greenhouse gas in the atmosphere compared with the mass of CO2. The GWP of CO2 is 1.0. The GWPs of HFC-134a and HFC-227ea, hydrofluorocarbons used in metered-dose inhalers, are 1330 and 3220.
Box 2. NHG Guidelines ‘Asthma in adults’ (2020) and ‘COPD’ (2021).
One of the criteria in the decision aid for choosing an inhaler device is:
A general objection against metered-dose inhalers is that they contain a greenhouse gas with a strong environmental impact.
Note: Metered-dose inhalers use hydrofluorocarbon propellants. The F-gas hydrofluorocarbon does not affect the ozone layer but is a strong greenhouse gas. The environmental impact of 1 inhalation is 25 times larger than a dry powder inhalation. Environmental impact of production, transport and waste processing (…) have not been included.
To understand the implications of changing from pMDI to more eco-friendly NPI use for policy, practice and patients in settings, we build on the cost and carbon analysis of Wilkinson et al.8 In this paper, we calculated the environmental impact of this change in Dutch primary and secondary respiratory healthcare and analysed the associated pharmaceutical and device costs.
Methods
We performed a four-step data analysis of prescription data in order to estimate the carbon equivalent footprint of prescribed inhalers over a 1-year period (2020). We determined how much inhalation medication could be attributed to the following patient groups: (1) asthma, (2) COPD, (3) severe COPD and (4) children younger than 7 years of age. Estimations were based on the Genees- en hulpmiddelen Informatie Project (GIP) database (Medicines and medical devices Information Project) of the Dutch National Health Care Institute and the DBC Informatie Systeem (DIS) database (Diagnosis-Treatment Combination Information system) of the Dutch Healthcare Authority, both independent government bodies residing under the Dutch Ministry of Health, Welfare and Sports. GIP is a representative information system containing data on the use and cost of prescription drugs and medical devices.13 DIS contains information of all treatment trajectories in Dutch medical specialist care, including pulmonary medicine, mental healthcare, forensic care and rehabilitation.14 Healthcare providers are legally required to deliver these data for policy-making and regulation. Online supplemental file 1 contains the complete data analysis protocol and additional information regarding methodological details, assumptions and choices made.
bmjopen-2021-055546supp001.pdf (291.2KB, pdf)
First, we calculated the number of patients with asthma or COPD who used inhalation medication in the Netherlands in 2020 by joining diagnoses codes to inhalation medication. Second, we calculated the number of defined daily doses (DDDs) discriminating between pMDIs and NPIs. Nebulizers were excluded from the analysis since they do not contain propellants and due to their size and dependency on electricity, they are not to be considered an alternative to pMDIs for use by patients at home. We included the soft mist inhalers in the NPI group, because they do not contain propellants and may be considered an alternative to pMDIs. Third, we determined the volume of pMDIs that could hypothetically be replaced by NPIs in a safe and medically responsible way. We estimated the size of this volume in DDDs, according to current medical guidelines excluding children younger than 7 years of age and those patients with severe COPD having at least two exacerbations per year. In our data, the subgroups ‘younger than 7 years’ and ‘severe COPD’ consume 13.6% of the total medication delivered by pMDI. Hence, if we would disregard their pMDI use and only replace inhalers of the remaining patients, we could theoretically achieve a 86.4% reduction of pMDI use. In these two subgroups (younger than 7, severe COPD), it is possible to safely replace pMDIs in inhalation corticosteroid (ICS) maintenance therapy for NPIs, without any negative medical impact. Here, breathing is not hampered during maintenance therapy and an immediate effect of ICS is not required. We nonetheless choose a more conservative estimate of change. We used the frequently stated figure of 10% pMDI use in Sweden as a proxy, assuming Sweden and the Netherlands are comparable in terms of a variety of social-epidemiological indicators.15 16 Hence, it is likely that the latter country could approach Sweden’s level of NPI prescription to an again more conservative, putative 15%. From the current level of 49.6% down to 15% pMDI use equals a 70% reduction, which is considerably less than the previous 86.4%. Based on our data, we know how many canisters of each type were prescribed in 2020, and we applied two conversion tables, one published by Wilkinson et al8 and the other one by Jeswani and Azapagic.7 Since they use different resources for quantification we have used a range instead of an average. Finally, we calculated the kg.CO2 equivalents decrease as a consequence of this substantial 70% reduction in pMDI use. In the fourth and last step, we calculated if this potential replacement could be achieved in a cost-neutral way. By determining both the current costs of medication, spacers and estimated replacement costs, we calculated the difference. For the replacement costs, we applied two realistic scenarios, one is the low-cost scenario in which pMDIs are replaced by low-cost NPIs. In the second scenario pMDIs are replaced by average-cost NPIs.
Patient and public involvement
No patients were involved.
Results
In 2020, 1 434 311 patients used inhalation medication in the Netherlands, and they received a total of 364 111 907 DDDs (table 1). In addition, 544 544 spacers were administered to 509 650 (pMDI-using) patients meaning that 60% of 856 425 pMDI-using patients could use their inhaler together with a yearly to-be-replaced spacer, as recommended by Dutch medical guidelines.
Table 1.
Inhalation medication in the Netherlands 2020
| Inhaler type | Number of patients* | Number of DDDs | % of total DDD use |
| Pressured metered-dose inhaler (pMDI) | 856 425 | 178 116 715 | 49.6 |
| Non-propellant inhaler (NPI) | 822 996 | 181 163 394 | 50.4 |
| Nebulizers (excluded in further analysis) | 24 178 | 4 831 798 | |
| pMDI and/or NPI (included) | 1 429 677 | 359 280 109 | 100.0 |
| pMDI and/or NPI and/or nebulizers (total group) | 1 434 311 | 364 111 907 |
*Users may use different types of inhalers at the same time.
DDDs, defined daily doses.
After excluding the use of nebulizers, we focused on the group of 1 429 677 patients using pMDI and/or NPI, who were prescribed over 359 280 109 DDDs in 2020 (table 1). The total amount of medication delivered in 2020 by pMDI is 178 116 715 DDDs. We observed that 49.6% of the medication has been delivered using pMDIs, 50.4% per NPIs (table 1).
Not all inhalation medication is delivered by both types of inhalers and can be switched. Long-acting muscarinic antagonists (LAMA) and the combination of long-acting beta agonists with LAMA were only available as NPI, the combination of short-acting beta agonists (SABA) with short-acting muscarinic-antagonists was only available as pMDI. SABA–ICS has not been analysed as it was not prescribed.
The number of patients who could hypothetically switch safely to NPIs with the same content would be using 121 043 039 DDDs, equal to 3 543 553 canisters. Here, we may safely assume equal bioavailability of pMDIs and NPIs, because their DDD differs which corrects for differences in bioavailability.
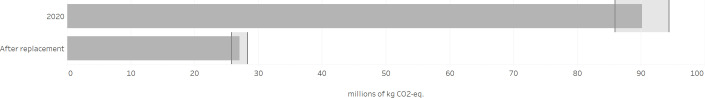
Using the Wilkinson’s conversion table with ‘mg HFC per canister’ delivers a reduction of 66 million kg.CO2 equivalents.8 Using the conversion table from Jeswani and Azapagic yields a reduction of 60 million kg.CO2 equivalents,7 the range being 66 028 669–60 142 156 kg.CO2 equivalents with an average of 63 085 412 kg.CO2 equivalents corresponding to 47 977 kg/HFC, HFC-134a for the better part (figure 1).
Figure 1.
Environmental impact (in kg.CO2 equivalents) of a hypothetical replacement of pressurised metered-dose inhalers in the Netherlands.
We calculated if shifting to NPIs could be achieved in a cost-neutral way. We determined both the current costs of medication and spacers, we estimated replacement costs and we calculated the difference. For the replacement costs we applied two realistic scenarios. One is a low-cost scenario in which pMDIs are replaced by low-cost NPIs. In the second scenario pMDIs are replaced by average-cost NPIs in current market share (table 2).
Table 2.
DDD volumes, costs of medication and spacers
| pMDI use in 2020, in medication groups: SABA, LABA, ICS, SAMA, LABA–ICS, LABA–SAMA–ICS | 70% of pMDI use (part that can theoretically be safely replaced) | Replacement of pMDI by low-cost NPI | Replacement by NPI, in current market share | |
| Volume in DDD | 172 918 633 | 121 043 043 | 121 043 043 | 121 043 043 |
| Medication cost | €129 856 283 | €90 899 398 | €54 419 848 | €107 245 032 |
| Cost of spacers | €18 004 187 | €12 602 931 | €0 | €0 |
| Total cost | €147 860 470 | €103 502 329 | €54 419 848 | €107 245 032 |
| Impact of replacement | €49 082 481 savings |
€3 742 703 increased costs |
DDD, defined daily dose; ICS, inhalation corticosteroids; LABA, long-acting beta agonists; LAMA, long-acting muscarinic antagonists; NPI, non-propellant inhaler; pMDI, pressurised metered-dose inhaler; SABA, short-acting beta agonists; SAMA, short-acting muscarinic antagonists.
If the percentage of DDDs from pMDI could be reduced from 49.6% to 15%, this 70% reduction implies a decrease of 121 043 043 DDDs which equals EUR 103 502 329 (medication+inhalers cost EUR 90 899 398 plus the cost of spacers EUR 12 602 931). Replacing this by low-cost NPIs would incur a cost of EUR 54 419 848 saving approximately EUR 49.1 million annually. The average-cost scenario would result in EUR 3.7 million annual added expenses.
Discussion
The healthcare sector needs to decrease greenhouse gas emissions to help mitigate climate change. This may be viewed as a moral and practical obligation in times of climate crisis and the global health emergency it implies.17 To achieve this, substantiated and medically safe eco-friendly alternatives are necessary. In this study, we assessed the hypothetical impact of converting eligible patients from using pMDIs to using NPIs in the Netherlands, both in terms of greenhouse gas emissions and in cost. With these outcomes we seek to offer insight into the impact of making this change and to inspire healthcare professionals to act climate responsibly which is congruent with announcements of professional organisations such as the British Thoracic Society,18 the European Respiratory Society,19 the International Society for Quality in Health Care20 and the US National Academy of Sciences, Engineering, and Medicine.21
Our results show that a sizeable reduction in greenhouse gas emissions is attainable in the Netherlands with a readily available eco-friendly alternative. The financial impact of this shift depends on the choice for either a low-cost option or a more expensive option, but we demonstrated a cost reduction is feasible. The estimated cost saving does not include financial calculations of patient training or potential drawbacks of substitution such as lower adherence leading to increased GP visits or hospital admissions.
These results are in accordance with earlier studies8 9 22 but we were relatively stringent in our eligibility criteria (which patients are able to change safely) and more selective as to what brands to include for the financial impact estimation. Obviously the outcomes refer to Dutch respiratory healthcare, its specific patient population and medication use.
In estimating the environmental impact of pMDIs, we considered their full amount of propellants. We did not subtract unknown quantities of propellants that may remain in the canister after use, assuming that sooner or later 100% of these gases will be released into the atmosphere. We neither included other environmental impacts of pMDIs nor NPIs, as would have been done in a full LCA. LCAs typically include the whole spectrum of production, packaging, distribution, usage, waste, etc. However, pMDIs’ global warming effect is mainly caused by their use (95%–98%), not by the manufacturing of this class of inhalers.7 8 Though NPIs, as opposed to pMDIs, generate much lower GWP, LCAs imply other harmful impacts that eventually should be included in a comparison such as human toxicity, marine eutrophication or fossil depletion.7 Like Wilkinson et al, we could not perform a full LCA due to the lack of reliable LCA data across all different types of inhalers, spacers, distribution and manufacturing processes. Since the use of propellants represents a major part of the environmental impact, we nonetheless believe this provides a good start for dealing with these issues.8
Our study implies that if medically safe and possible, choosing the medicine or device with the least environmental impact is imperative in times of global climate crisis. This is not just about patients’ choice, as may be suggested by a patient decision aid developed by NICE (National Institute for Health and Care Excellence).23 It could be considered the prescriber’s task as well. Therefore, it should be integrated in medical guidelines and standards as part of healthcare quality improvement trajectories much like Mortimer et al have elegantly proposed and practiced.24 This should not affect the established fact that suitable patient training and monitoring of inhalation techniques are a sine qua non for effective inhaler use for all a patients, especially for children.25 26 In the Netherlands, general practitioners updated their guidelines on the management of asthma and COPD, and included a recommendation to consider the environmental impact of the medicine of choice (see Textbox 2). In view of the health emergency represented by the climate crisis, we recommend that pulmonologists also consider to update national and local guidelines and appreciate the potential benefits of advising green inhalers as the device of choice in new diagnostics of asthma and COPD and the benefits of resetting patients currently using pMDIs to NPIs if safe and possible. In 2019, Belgian pulmonologists recommended the use of DPIs to lung patients not just because they can deliver better treatment results for asthma and COPD but also because they are ‘far less damaging to the environment than traditional propellant driven aerosols’.27
Evidently, the chosen medication should be fitting for the individual patient. It is beyond the scope of this study to include all specific circumstances in which patients cannot use NPIs. Since daily use and emergency use are quite different, there have been reservations about DPIs in case of exacerbations especially since both the expiratory flow and the inspiratory (‘trapped air’) flow of breath are obstructed leading to patients’ preference for pMDIs in such circumstances. In Sweden soft mist inhalers are recently used more often in such cases because they require minimal inspiratory power. Wilkinson et al referring to a data analysis of the NHS Business Services Authority suggest that in England ‘clinicians believe the vast majority of patients can use a DPI effectively’.8
Apart from climate and economic benefits, we identified more advantages of replacing pMDIs with NPIs as suggested by research and practice (table 3).
Table 3.
Plausible advantages of replacing pMDIs with DPIs
| Plausible advantages | References (if present) |
| Less critical errors are made using DPIs as compared with pMDIs. | Chrystyn H, van der Palen J, Sharma R, Barnes N, Delafont B, Mahajan A, Thomas M. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017 Apr 3;27(1):22. |
| Sometimes pMDIs are used when empty, which may lead to poor disease control and less quality of life. | Conner JB, Buck PO. Improving asthma management: the case for mandatory inclusion of dose counters on all rescue bronchodilators. J Asthma. 2013 Aug;50(6):658–63. doi: 10.3109/02770903.2013.789056. Epub 2013 Apr 29. Tsangarides A, Wilkinson A, Mir F. Disadvantages of salbutamol pressurised metered-dose inhalers (pMDIs). Thorax 2018;73:A193-A194. |
| Some pMDIs are unknowingly considered empty and are disposed of leading to unnecessary costs. | Holt S, Holt A, Weatherall M, Masoli M, Beasley R. Metered-dose inhalers: a need for dose counters. Respirology (Carlton, Vic.). 2005 Jan;10(1):105–106. |
| Following Dutch clinical guidelines, pMDI users should yearly receive a new spacer. During 2020 however, only 60% of pMDI-using patients received it which implies suboptimal quality of care. | |
| Changing to DPI may improve guideline adherence because use of a spacer is not required for DPI. | |
| Use of DPI requires no spacers and consequently does at least not generate non-reusable plastics. |
DPIs, dry powder inhalers; pMDIs, pressurised metered-dose inhalers.
The present study does not discuss implementation questions or possible (dis)advantages of pMDI or NPI use. We have assumed a 100% implementation to determine the maximum impact. What level of implementation can be achieved in healthcare practice is yet unknown and depends on a range of contextual factors, for example, does the patient perceive benefits or harm. But if one could estimate what level of implementation can be achieved in practice, the actual impact could easily be calculated with the data from the present paper. It is certainly useful to address the preferences and prejudices of patients and professionals and we know that citizens, patients and professionals are increasingly willing to choose eco-friendly alternatives but there is no knowledge on this specific shift from pMDIs to NPIs.28–30 Next to that, while some practical (dis)advantages of both pMDIs and NPIs are known, we recommend explaining these to patients similar to the NICE decision aid as well as to professionals.23 31 For example, most pMDIs do not have dose counters. While all DPIs have a counter they do not necessarily prevent using an empty device. Without a dose counter it may be hard to know how many doses are left in the device. Unknowingly using empty pMDIs could lead to avoidable exacerbations or even avoidable hospital admissions. Unknowingly replacing pMDIs that still contain medication would incur unnecessary cost.32 Adherence to inhalation instructions may be an issue when it comes to changing, but this is already an issue, for example, not every patient with an pMDI uses the recommended, though bulky, spacer. Also, adherence to inhalation medication therapy should be supported and promoted by repeated inhalation instruction.33 Switching without sufficient instruction may result in uncontrolled, exacerbations and increased use of healthcare services. Uniformity of the devices in case of multiple inhaler use is relevant here. Such questions pertain to responsible implementation, a subject we address in the follow-up study, that has already begun.
The pharmaceutical industry meanwhile continues to develop and study inhalers with lower climate impacts. And new propellants will enter the market. For patients who are dependent on pMDIs, this is meaningful. Given that these developments have not yet entered the market and knowledge of these is still limited, we will not elaborate on this matter. Research should nonetheless include more green metrics into their output and outcome parameters. This would enable meta-analyses and evidence-based climate-responsible innovation in healthcare.
Conclusions
Large-scale replacement of pMDIs with NPIs would have a substantial climate impact in respiratory healthcare. In 2020, about 1.4 million patients using pMDI and/or NPI were prescribed over 364 million DDDs. The use of pMDIs is more or less equally prevalent among patients with COPD and patients with asthma. Half (49.6%) of the medication has been delivered through pMDIs that have a relatively high GWP. The percentage of NPI-delivered inhalation medication that can safely be replaced is estimated to be 70%, resulting in an environmental health benefit of 63 085 412 kg.CO2 equivalents on average, which equals the carbon dioxide emission of just over 8400 Dutch households. This shift could be achieved with low budgetary risk. In the low-cost scenario it may even lead to a cost reduction of approximately EUR 49.1 million per year in Dutch respiratory healthcare. The average-cost scenario would result in EUR 3.7 million annual added costs while still reducing greenhouse gas emission.
Supplementary Material
Acknowledgments
The authors would like to thank the National Health Care Institute for their support in drafting this paper.
Footnotes
Twitter: @hcointox
Contributors: From the beginning, all authors fully participated in all respective steps and are now preparing for the follow-up implementation study. All authors contributed to the conception, cocreated the design of the study, did parts of the analyses, did interpretation of the data, drafted the paper, critically revised and improved the report, gave final approval and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. HCO acts as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JK reports personal fees from BENU Pharmacists, BENU Nederland B.V./Brocacef Groep N.V.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. Both databases used for this article are publicly accessible. DIS database (2021)—DBC Informatie Systeem | Diagnosis-Treatment Combination Information system—https://www.opendisdata.nl/ and GIP database (2021)—Genees- en hulpmiddelen Informatie Project | Medicines and medical devices Information Project—https://www.gipdatabank.nl/. Commercially sensitive information related to brand names cannot be made available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet countdown on health and climate change: from 25 years of inaction to a global transformation for public health. Lancet 2018;391:581–630. 10.1016/S0140-6736(17)32464-9 [DOI] [PubMed] [Google Scholar]
- 2.EASAC | European Academies – Science Advisory Council (2019) . The imperative of climate action to protect human health in Europe. available. Available: https://easac.eu/publications/details/the-imperative-of-climate-action-to-protect-human-health-in-europe/ [Accessed 21 Feb 2021].
- 3.HCWH | Health Care Without Harm (2019) . Health care’s climate footprint. Available. Available: https://noharm-uscanada.org/content/global/health-care-climate-footprint-report [Accessed 20 Jan 2021].
- 4.Lenzen M, Malik A, Li M, et al. Suveges Moreira Chaves L, capon a, Pencheon D The environmental footprint of health care: a global Assessment. Lancet Planet Health 2020;4:e271–9. [DOI] [PubMed] [Google Scholar]
- 5.RIVM | National Institute for Public Health and the Environment (2021) . The win-win effect of sustainable healthcare: measures and their health effects. available. Available: https://www.rivm.nl/documenten/win-win-effect-of-sustainable-health-care-measures-and-their-health-effects [Accessed 15 June 2021].
- 6.Union E. Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on fluorinated greenhouse gases and repealing Regulation (EC) No 842/2006 Text with EEA relevance OJ L 150, 20.5.2014, p. 195–230, 2014. Available: http://data.europa.eu/eli/reg/2014/517/oj [Accessed 21 Jun 2021].
- 7.Jeswani HK, Azapagic A. Life cycle environmental impacts of inhalers. J Clean Prod 2019;237:117733. 10.1016/j.jclepro.2019.117733 [DOI] [Google Scholar]
- 8.Wilkinson AJK, Braggins R, Steinbach I, et al. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open 2019;9:e028763. 10.1136/bmjopen-2018-028763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janson C, Henderson R, Löfdahl M, et al. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax 2020;75:82–4. 10.1136/thoraxjnl-2019-213744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starup-Hansen J, Dunne H, Sadler J, et al. Climate change in healthcare: exploring the potential role of inhaler prescribing. Pharmacol Res Perspect 2020;8:e00675. 10.1002/prp2.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottema JW, Bouma M, Broekhuizen L, et al. NHG | Nederlands Huisartsen Genootschap (2020) NHG-richtlijn Astma bij volwassenen [Dutch College of General Practitioners Guideline Asthma among adults]. Available. Available: https://richtlijnen.nhg.org/standaarden/astma-bij-volwassenen [Accessed 20 Feb 2021].
- 12.Bischoff E, Bouma M, Broekhuizen L, et al. NHG | Nederlands Huisartsen Genootschap (2021) NHG-richtlijn COPD [Dutch College of General Practitioners Guideline COPD]. Available. Available: https://richtlijnen.nhg.org/standaarden/COPD [Accessed 19 Apr 2021].
- 13.database GIP. Genees- en hulpmiddelen Informatie project | medicines and medical devices information project. available, 2021. Available: https://www.gipdatabank.nl/ [Accessed 21 Feb 2021].
- 14.database DIS. DBC Informatie Systeem | Diagnosis-Treatment combination information system. available, 2021. Available: https://www.opendisdata.nl/ [Accessed 21 Feb 2021].
- 15.Lavorini F, Corrigan CJ, Barnes PJ, et al. Aerosol drug management improvement team. retail sales of inhalation devices in European countries: so much for a global policy. Respir Med 2011;105:1099–103. [DOI] [PubMed] [Google Scholar]
- 16.OECD | Organisation for Economic Co-operation and Development, 2020 . OECD health statistics 2020. available. Available: https://www.oecd.org/health/health-data.htm [Accessed 23 May 2021].
- 17.Salas RN, Maibach E, Pencheon D, et al. A pathway to net zero emissions for healthcare. BMJ 2020;371:m3785. 10.1136/bmj.m3785 [DOI] [PubMed] [Google Scholar]
- 18.British Thoracic Society . Position statement, 2020. available. Available: https://networks.sustainablehealthcare.org.uk/sites/default/files/media/BTS%20Environment%20and%20Lung%20Health%20Position%20Statement%202020.pdf [Accessed 25 Feb 2021].
- 19.ERS | European Respiratory Society (2021) . Position statement on asthma and the environment. available. Available: https://www.ersnet.org/news-and-features/news/ers-publishes-position-statement-asthma-environment/ [Accessed 16 May 2021].
- 20.Ossebaard HC. Lachman P climate change, environmental sustainability and health care quality. Int J Quality in Health Care 2020;32:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NASEM | US National Academy of Sciences, Engineering, and Medicine, 2020 . 2030 next steps to healthcare climate leadership. Available: https://www.nationalacademies.org/event/10-13-2020/fall-2020-hcs-meeting [Accessed 16 Mar 2021].
- 22.Braggins R, Smith J. Wilkinson a the true cost of switching to low global-warming potential inhalers. An analysis of NHS prescription data in England. Thorax 2018;73:A196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NICE | National Institute for Health and Care Excellence, 2019 . Patient decision aid. inhalers for asthma. Available: https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patient-decision-aid-pdf-6727144573 [Accessed 16 Mar 2021].
- 24.Mortimer F, Isherwood J, Wilkinson A, et al. Sustainability in quality improvement: redefining value. Future Healthc J 2018;5:88–93. 10.7861/futurehosp.5-2-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lexmond AJ, Kruizinga TJ, Hagedoorn P, et al. Effect of inhaler design variables on paediatric use of dry powder inhalers. PLoS One 2014;9:e99304. 10.1371/journal.pone.0099304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ammari WG, Al-Hyari N, Obeidat N, et al. Mastery of pMDI technique, asthma control and quality-of-life of children with asthma: A randomized controlled study comparing two inhaler technique training approaches. Pulm Pharmacol Ther 2017;43:46–54. 10.1016/j.pupt.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 27.University Hospital Leuven . Belgian lung specialists recommend environmentally friendly inhalers that produce better treatment results for lung patients, 2019. available. Available: https://www.uzleuven.be/en/news/belgian-lung-specialists-recommend-environmentally-friendly-inhalers-produce-better-treatment [Accessed 05 May 2021].
- 28.Eurobarometer . Citizen support for climate action, 2019. Available: https://ec.europa.eu/clima/citizens/support_en [Accessed 12 Mar 2021].
- 29.Souto-Miranda S, Gonçalves A-C, Valente C, et al. Environmental awareness for patients with COPD undergoing pulmonary rehabilitation: is it of added value? Int J Environ Res Public Health 2020;17:7968. 10.3390/ijerph17217968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotcher J, Maibach E, Miller J, et al. Views of health professionals on climate change and health: a multinational survey study. Lancet Planet Health 2021;5:e316–23. 10.1016/S2542-5196(21)00053-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: common errors barriers to use and recommended solutions. Resp Med 2013;107:37–46. [DOI] [PubMed] [Google Scholar]
- 32.Tsangarides A, Wilkinson A, Mir F. Disadvantages of salbutamol pressurised metered-dose inhalers (pMDIs). Thorax 2018;73:A193–4. [Google Scholar]
- 33.Takemura M, Kobayashi M, Kimura K, et al. Repeated instruction on inhalation technique improves adherence to the therapeutic regimen in asthma. Journal of Asthma 2010;47:202–8. 10.3109/02770900903581692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055546supp001.pdf (291.2KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. Both databases used for this article are publicly accessible. DIS database (2021)—DBC Informatie Systeem | Diagnosis-Treatment Combination Information system—https://www.opendisdata.nl/ and GIP database (2021)—Genees- en hulpmiddelen Informatie Project | Medicines and medical devices Information Project—https://www.gipdatabank.nl/. Commercially sensitive information related to brand names cannot be made available.