Graphical abstract
Keywords: Ovarian cancer, Carcinogenesis, Inflammation, Aging, Inflammaging
Highlights
-
•
Ovarian cancer (OvCa) is a multifactorial disease.
-
•
Several factors are involved in age-related increases in carcinogenesis.
-
•
Exposure to inflammatory mediators contributes to increased cell division and genetic and epigenetic changes.
-
•
We discuss the current carcinogenic hypotheses, sites of origin, and etiological factors of OvCa.
Abstract
Ovarian cancer is one of the most common gynecologic cancers and has the highest mortality rate. The risk/protective factors of ovarian cancer suggest that its etiology is multifactorial. Several factors are involved in age-related increases in carcinogenesis, including the accumulation of senescent cells, inflammaging (a chronic inflammatory state that persists in the elderly), and immunosenescence (aging of the immune system) changes associated with poor immune surveillance. At sites of inflammation, exposure to high levels of inflammatory mediators, such as reactive oxygen species, cytokines, prostaglandins, and growth factors, contributes to increased cell division and genetic and epigenetic changes. These exposure-induced changes promote excessive cell proliferation, increased survival, malignant transformation, and cancer development. Furthermore, the proinflammatory tumor microenvironment contributes to ovarian cancer metastasis and chemoresistance.
This narrative review of the literature was carried out to delineate the possible role of inflammaging in the etiopathogenesis of ovarian cancer development. We discuss the current carcinogenic hypotheses, sites of origin, and etiological factors of ovarian cancer. Treatment of inflammation may represent an attractive strategy for both the prevention and therapy of ovarian cancer.
1. Introduction
Ovarian cancer (OvCa) is the second most common gynecological cancer in the United States and Western Europe (Khazaei et al., 2021); it causes more deaths than all other gynecological cancers combined (Menon et al., 2021). Therefore, prevention strategies are being established to reduce the risk of OvCa (Pérez-López et al., 2017). Such strategies include opportunistic salpingectomy, which is the most effective intervention (Hanley et al., 2022); genetic testing for individuals who are at high risk, risk reducing surgery for individuals with inherited genetic testing, effective clinical management of endometriosis, policies to prevent the onset of tobacco use and promote smoking cessation (Yarmolinsky et al., 2019), and the use of most contraceptive methods (Sánchez-Borrego and Sánchez-Prieto, 2021).
The incidence of OvCa increases with age and reaches its peak in the seventh decade of life (Momenimovahed et al., 2019). Aging leads to numerous changes that affect many components of the immune system, called “immunosenescence” (Lian et al., 2020). Immunosenescence is a process of immune dysfunction that occurs with age and includes remodeling of lymphoid organs, leading to changes in the immune function of the elderly, which is closely related to the development of infections, autoimmune diseases, and malignant tumors (Lian et al., 2020). Indeed, elderly individuals exhibit increased susceptibility to many diseases, including cancer, autoimmune disorders, and other chronic inflammatory diseases (Feehan et al., 2021).
Inflammation has been considered a key mechanism for carcinogenesis after detecting leukocytes in cancer tissues. Inflammation can mediate and stimulate the production of tumor-promoting compounds, including cytokines, especially interleukin (IL) 6 and 8 and chemokines, as well as reactive oxygen species (ROS), tumor necrosis factor (TNF)-α and IL-1β, and lipid hydroperoxides (Browning et al., 2018). These compounds can contribute to cell proliferation, malignant transformation, and cancer development (Savant et al., 2018, Zhou et al., 2021, Crusz and Balkwill, 2015).
Among other factors, such as hereditary, environmental, and lifestyle factors, inflammation is an important risk factor for OvCa. Several studies have suggested that factors related to ovarian surface epithelium (OSE) inflammation, such as ovulation, endometriosis, and pelvic inflammatory diseases (PIDs), are associated with an increased risk of OvCa (Trabert et al., 2020, Tanha et al., 2021). Mucin 16 (MUC16) is a membrane-associated gene that encodes cancer antigen 125 (CA125), and the upregulation of its expression is another mechanism underlying the effects of inflammatory factors in OvCa (Liu et al., 2021). The inflammation-associated factors lipopolysaccharides, IL-6, IL-8 and TNF- α increase the expression levels of MUC16 and enhanced CA125 release in OvCa (Liu et al., 2021).
The term “inflammaging” is used to define the systemic and sterile (in the absence of infection) low-grade chronic inflammation status that is currently considered a central biological mainstay of the aging process (Franceschi, 2007). Inflammaging is a condition induced by the senescence-associated secretory phenotype (SASP), which is produced by the innate immune, parenchymal, and nonparenchymal cells within the organs (Shirasuna and Iwata, 2017). Importantly, once cells become senescent, they produce and secrete the so-called SASP. SASP is thought to be the main driver of age-related inflammation (Franceschi, 2007). Broadly, SASP is composed of pro-inflammatory cytokines and chemokines, growth factors, proteases, and angiogenic factors that can act in a paracrine and autocrine manner to spread and strengthen senescence (Lopes-Paciencia et al., 2019). The presence of SASP-producing senescent cells can have negative long-term effects; such as chronic inflammation, that can produce tissue dysfunction and/or carcinogenesis (Rubio-Tomás et al., 2021).
Several theories have been postulated to explain the etiology of OvCa. Notably, these theories are likely not mutually exclusive, as they all converge on the role of inflammaging in promoting ovarian tumorigenesis and cancer progression.
We briefly discuss how each of these processes evokes or involves a persistent inflammatory response. This response leads to an environment rich in cytokines and growth factors in the internal genitalia and peritoneum and contributes to OvCa initiation, progression, and metastasis.
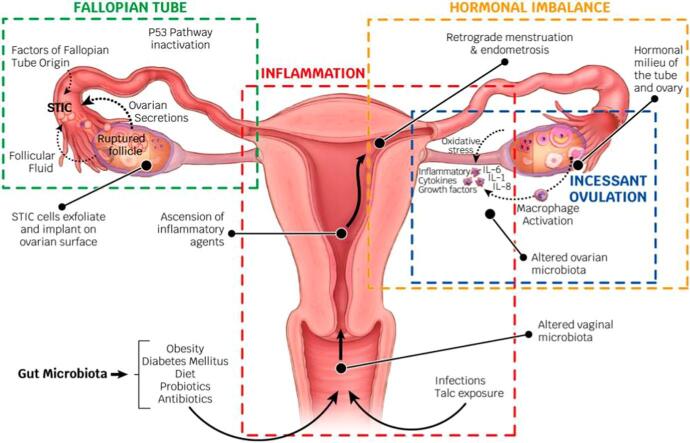
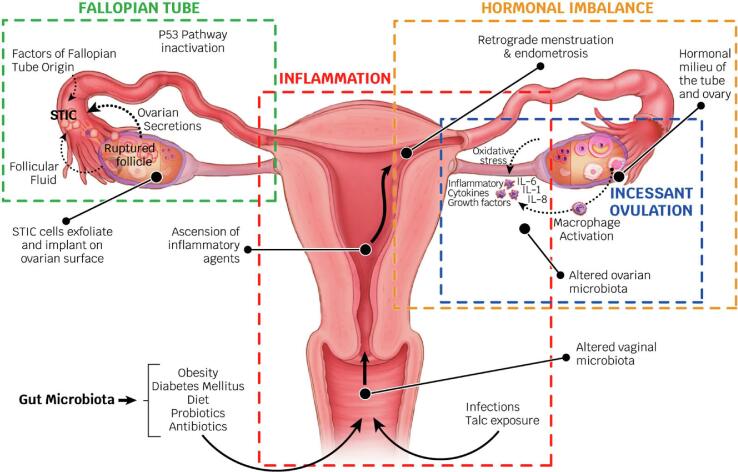
2. Inflammaging hypotheses of ovarian cancer (Fig. 1)
Fig. 1.
Inflammaging hypotheses of ovarian cancer. Abbreviations: STIC, serous tubal intraepithelial carcinoma; ILs, interleukins.
The most important hypothesis regarding OvCa carcinogenesis is the ovulation theory, which relates the risk of OvCa to incessant ovulation (Fathalla, 1971) and the etiological role of the inflammation that accompanies each ovulation (Huang et al., 2020). The tissue adjacent to the site of ovulation may be continually exposed to an inflammatory and oxidative environment; resulting in an increased risk of malignant transformation. Intriguingly, the ovulatory process and the repair steps following liberation of the ovum are characterized by the generation of an enormous amount of cytokines/chemokines and matrix-remodeling enzymes, including prostaglandins, bioactive eicosanoids, plasminogen activators, collagenases, ILs, TNF-α and various growth factors. It is also characterized by the recruitment of activated immune cells to the wounded epithelial surface, which represents the global activation of the proinflammatory network (Macciò and Madeddu, 2012).
Menstruation allows for retrograde menstruation and the possibility of ectopic implantation of endometrial tissue (Critchley et al., 2020). The risk of OvCa, particularly nonserous types, decreases after the retrograde route is blocked by tubal ligation (Pérez-López et al., 2017). The menstruating endometrium is rich in cytokines and prostaglandins. Iron metabolites in menstrual blood exposed to the fallopian tube epithelium (FTE) and ovary may exert the Fenton reaction upon iron oxides by interacting with the H2O2 released from ovulation (Rockfield et al., 2019, Shigeta et al., 2016). Fenton reaction is an advanced oxidation process in which highly reactive hydroxyl radicals are produced. This is done under acidic environment conditions, with ambient pressure and temperature, using hydrogen peroxide (H2O2) that is catalyzed with transition metals, generally iron (Shigeta et al., 2016). Thus, ovulation and retrograde menstruation produce a repeated tolerable Fenton reaction in FTE and OSE, which can promote the development of OvCa (Rockfield et al., 2019, Shigeta et al., 2016).
The gonadotropin stimulation hypothesis proposes that high gonadotropin levels can influence OSE cells and promote carcinogenesis (Mertens-Walker et al., 2012) by increasing the proliferation and mitotic activity of the ovarian epithelium (Han et al., 2021). This fact could explain why the majority of patients present with OvCa after menopause (American Cancer Society). The damage associated with the rupture of follicles during ovulation resulting from high levels of follicle stimulating hormone (FSH), luteinizing hormone (LH) and estrogen are also risk factors and may lead to OvCa (Mertens-Walker et al., 2012). Progesterone plays a protective role in follicular fluid during pregnancy or exogenously (Wu et al., 2020). A cleansing effect of progesterone has been confirmed specifically on p53-defective tubal epithelial cells and on the progesterone receptor (PR) by inducing necroptosis, which is an inflammatory cell death that contributes to innate immunity (Wu et al., 2020). Therefore, endogenous or exogenous progesterone protects against carcinogenesis induced by ovulation and retrograde menstruation (Wu et al., 2020, Wheeler et al., 2019).
The fallopian tube, particularly the fimbriae, has emerged as another possible site of origin according to findings related to prophylactic surgery for reducing the risk of OvCa in women with a genetic predisposition to the disease (Hanley et al., 2022). Based on these observations, the tubal inflammation hypothesis was proposed by Salvador (Salvador et al., 2009). High-grade serous carcinoma (HGSC) and clear cell and endometrioid cancers have been hypothesized to arise from the epithelium of the fallopian tubes (Kurman and IeM, 2010) and share a common pathogenetic mechanism (Zhang et al., 2019), i.e., iron-induced oxidative stress derived from retrograde menstruation. The fallopian tube is regularly exposed to a variety of inflammatory agents and can show signs of acute and chronic inflammation. This inflammation is activated by constituents of the ovulatory follicular fluid (Hsu et al., 2019) and through the process of retrograde bleeding from the endometrial cavity during menstruation. Fimbriae floating in bloody peritoneal fluid is exposed to the action of catalytic iron and to the genotoxic effect of ROS generated from the hemolysis of erythrocytes by activated pelvic macrophages and by secreting cytokines (Macciò and Madeddu, 2012).
Emerging and, for some cancers, strong experimental and translational data support the contribution of the microbiome to cancer biology and disease progression (Knippel et al., 2021). The emergence of high-throughput technologies, such as genomics and transcriptomics, has afforded the generation of large volumes of data in relation to specific pathologies, such as different cancer types (Wei et al., 2021). A link has been demonstrated between alterations in the composition of the microbiota (oncobiosis, when referring to dysbiosis in neoplastic diseases) and the development of gynecological cancer (Borella et al., 2021). In OvCa, oncobiosis has been identified in numerous compartments, including the upper and lower female genital tract, serum, peritoneum, and intestines, as well as tumor tissue itself (Borella et al., 2021). Local inflammation probably participates in the initiation and continuation of carcinogenesis (Cheng et al., 2020).
Infection also induces inflammation in the fallopian tube (Salvador et al., 2009). Vaginal infections (for example, Neisseria gonorrhoeae or Chlamydia trachomatis) increase the risk of developing OvCa (Idahl et al., 2020). PIDs are also associated with an increased ovarian cancer risk, as indicated by an updated meta-analysis (Piao et al., 2020). The role of viruses, such as human papillomavirus, in the initiation, promotion, and progression of OvCa is unknown (Ibragimova et al., 2021). With the use of highly sensitive techniques, several groups have detected the presence of a viral genome in OvCa tissues (Pathak et al., 2020), but it is not sufficient to prove that viruses are a causal factor in the genesis of the disease (Cherif et al., 2021).
Another example of an inflammatory factor involved in the carcinogenesis of OvCa is the use of talcum powder in the genital area. Talc, along with associated components such as asbestos or quartz, which are known carcinogens and can contaminate talc products, might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries. This could possibly trigger an inflammatory response that may promote carcinogenesis. Taken together, epidemiological data suggest that there may be a small positive association between the use of genital powder and OvCa (Wentzensen and O'Brien, 2021).
The tumor microenvironment (TME) is composed of a variety of immune cells and stromal cells and is crucial in the occurrence and development of OvCa (Shan and Liu, 2009). Oncogenic RAS-transformed ovarian epithelial cells generate a proinflammatory secretome that diffuses into the stroma and causes fibroblast senescence. Senescence of ovarian stromal fibroblasts creates a secondary proinflammatory phenotype and converts the ovarian epithelial microenvironment into a field filled with inflammatory mediators that advance tumor progression. The inflammatory network contributes to the communication between the ovarian tumor epithelium and the underlying stroma (Shan and Liu, 2009).
3. Other potential origins (Fig. 1)
Metabolic disorders, such as diabetes, dyslipidemia, hypertension and obesity, have been determined to overactivate the immune system by causing leukocyte activation. An increase in the number of leukocytes is associated with proinflammatory phenotypes and thus explains the predisposition to chronic inflammatory diseases (Garn et al., 2016). Oxidative stress mediated by toxic habits (such as smoking), excessive pollution, allergies, sleep disorders and increasing age has also been demonstrated to predispose patients to a chronic inflammatory state (Garn et al., 2016).
Metaflammation (the metabolic inflammation that accompanies metabolic diseases) is believed to be a form of chronic inflammation that is driven by an excess of nutrients (Franceschi et al., 2018). Consequently, nutrition influences the incidence, natural progression and therapeutic response of malignant diseases by modulating chronic inflammation (Zitvogel et al., 2017).
Obesity is associated with a higher incidence of and poorer survival in OvCa (Craig et al., 2016). A positive association has been found between the dietary inflammatory potential measured by the Dietary Inflammatory Index (DII) and the OvCa, and there is a significantly higher risk of OvCa in postmenopausal women (Yang et al., 2022). Most of the recent studies on epithelial OvCa have suggested a relationship between increasing body mass index (BMI) and nonserous histologies (Wichmann and Cuello, 2021). Additionally, obesity increases the risk of serous histologies that arise from the peritoneum (Wichmann and Cuello, 2021).
It has been proven that adipose tissue not only serves as calorie storage but also as a source of both proinflammatory and anti-inflammatory factors, known as adipocytokines (Craig et al., 2016). Adipocytes promote OvCa epithelial growth, invasion, metastasis, and angiogenesis through adipokine secretion, metabolic remodeling, and modulation of the immune microenvironment (Dai et al., 2020). Therefore, it has been suggested that organs rich in adipocytes, such as the omentum, may be sites of both metastasis and origin. That is, they may serve as a site of the initial seeding and transformation of malignant precursor cells that originate in other parts of the abdomen (Iyoshi et al., 2021). This could explain why apparently cancer-free women are diagnosed with advanced-stage disease within a few months.
Studies have shown a strong correlation between diabetes mellitus (DM) and carcinogenesis, and the most evident correlation that has been reported is between carcinogenesis and type 2 diabetes mellitus (T2DM). T2DM has been reported to cause T cell senescence in both prediabetic and diabetic patients (Broadway et al., 2021). Hyperglycemia causes epigenetic alterations through several mechanisms; including DNA methylation and chromatin remodeling, and results in abnormal gene expression. A systematic review and meta-analysis evaluated the association between DM and the incidence of OvCa and suggested that women with DM had a 20% elevated risk of OvCa compared with those without a history of DM (Wang et al., 2020).
4. Discussion
OvCa is a multifactorial disease, including demographic, reproductive, gynecologic, hormonal, genetic, and lifestyle factors (Tanha et al., 2021).
Ovulation has a significant contribution to the etiology of OvCa. However, the peak incidence of OvCa occurs at approximately 63 years of age, which is more than a decade after the mean age of menopause (Gibson et al., 2016). Following depletion of the ovarian follicles, the remaining ovary is reduced to collagen scar tissue (Jia et al., 2018). The migration of malignant cells from the oviduct to the ovary via a cytokine/chemokine gradient formed from the surface of the wounded ovary could potentially explain why menopausal women are at a higher risk of OvCa (Pakuła et al., 2020). This phenomenon might also explain the protective effect of hysterectomy, salpingectomy, and tubal ligation against the development of OvCa (Pérez-López et al., 2017).
Several other factors, such as senescent cells, are involved in age-related increases in carcinogenesis (Pakuła et al., 2020), but inflammation is one of the most prominent factors. In fact, inflammaging is characterized by the establishment of a systemic proinflammatory state with increased levels of circulating interleukins, such as IL-6, IL-1 and TNF-α, and inflammatory markers, such as C-reactive protein (CRP) (Leonardi et al., 2018). These factors have been shown to be associated with the risk of OvCa (Peres et al., 2019). This results from the activation of signaling networks critical to inflammation, along with a variety of different sources of the inflammatory stimuli triggering and sustaining inflammaging. Such sources include those that occur in the external genitalia (bacterial and viral infections, changes in the vulvovaginal microbiome, exposure to external toxins, etc.) and, above all, in the internal genitalia (ovulation, endometriosis, PID). However, they can also occur from extragenital causes, such as meta-inflammation (Craig et al., 2016), the gut microbiota (Rubio-Tomás et al., 2021), and nutrition (Yang et al., 2022).
Unlike most other tumor types that metastasize via the vasculature, OvCa metastasizes predominantly via the transcoelomic route within the peritoneal cavity. Selectins are glycan-binding molecules that facilitate the initiation of tumor-mesothelial adhesion in the peritoneal cavity, and the important role of selectins in peritoneal carcinomatosis of OvCa has emerged (Hassan et al., 2020). Their role in inflammation is recognized, expressing P- and E-selectins at sites of inflammation (Hassan et al., 2020).
Discovering which cellular and molecular processes promote and inhibit the seeding of malignant cells to the ovary could facilitate the development of markers for early detection as well as the identification of rate-limiting events in the early stages of OvCa development, when cures are more viable (Kotsopoulos et al., 2016).
In conclusion, this narrative review adds to the existing evidence that inflammaging and inflammatory mediators play a role in ovarian carcinogenesis. However, we need to better understand the mechanisms by which the inflammaging process culminates in ovarian tumor initiation, as it does in other tumors. Thus, we can develop ways to target inflammatory mediators and reduce the risk of OvCa.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Glossary
- CA125
Cancer antigen 125
- CRP
C-reactive protein
- DII
Dietary inflammatory index
- DM
Diabetes mellitus
- DNA
deoxyribonucleic acid
- FTE
Fallopian-tube-epithelium
- FSH
Follicle stimulating hormone
- HPV
Human papillomavirus
- IISE
Impaired inflammatory state of the endometrium
- IL
Interleukin
- LH
Luteinizing hormone
- NF-κB
Nuclear factor-κB
- OSE
Ovarian surface epithelium
- OvCa
Ovarian cancer
- PID
Pelvic inflammatory disease
- PR
Progesterone receptor
- ROS
Reactive oxygen species
- SASP
Senescence-associated secretory phenotype
- T2DM
Type 2 diabetes mellitus
- TME
Tumor microenvironment
- TNF
Tumor necrosi factor
- Tregs
Regulatory T cells
References
- American Cancer Society. Ovarian Cancer. https://www.cancer.org/content/dam/crc/pdf/public/8773.00.pdf.
- Borella F., Carosso A.R., Cosma S., Preti M., Collemi G., Cassoni P., Bertero L., Benedetto C. Gut Microbiota and Gynecological Cancers: A Summary of Pathogenetic Mechanisms and Future Directions. ACS Infect. Dis. 2021;7(5):987–1009. doi: 10.1021/acsinfecdis.0c00839. [DOI] [PubMed] [Google Scholar]
- Broadway R., Patel N.M., Hillier L.E., El-Briri A., Korneva Y.S., Zinovkin D.A., Pranjol M.Z.I. Potential Role of Diabetes Mellitus-Associated T Cell Senescence in Epithelial Ovarian Cancer Omental Metastasis. Life (Basel). 2021 Aug 4;11(8):788. doi: 10.3390/life11080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning L., Patel M.R., Horvath E.B., Tawara K., Jorcyk C.L. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag. Res. 2018 Dec;5(10):6685–6693. doi: 10.2147/CMAR.S179189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Wang Z., Cui L., Wen Y., Chen X., Gong F., Yi H. Opportunities and Challenges of the Human Microbiome in Ovarian Cancer. Front. Oncol. 2020 Feb;10 doi: 10.3389/fonc.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif S., Amine A., Thies S., Taube E.T., Braicu E.I., Sehouli J., Kaufmann A.M. Prevalence of human papillomavirus detection in ovarian cancer: a meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(9):1791–1802. doi: 10.1007/s10096-021-04282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E.R., Londoño A.I., Norian L.A., Arend R.C. Metabolic risk factors and mechanisms of disease in epithelial ovarian cancer: A review. Gynecol. Oncol. 2016 Dec;143(3):674–683. doi: 10.1016/j.ygyno.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.O.D., Babayev E., Bulun S.E., Clark S., Garcia-Grau I., Gregersen P.K., Kilcoyne A., Kim J.-Y., Lavender M., Marsh E.E., Matteson K.A., Maybin J.A., Metz C.N., Moreno I., Silk K., Sommer M., Simon C., Tariyal R., Taylor H.S., Wagner G.P., Griffith L.G. Menstruation: science and society. Am. J. Obstet. Gynecol. 2020;223(5):624–664. doi: 10.1016/j.ajog.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusz S.M., Balkwill F.R. Inflammation and cancer: advances and new agents. Nat. Rev. Clin. Oncol. 2015 Oct;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- Dai L., Song K., Di W. Adipocytes: active facilitators in epithelial ovarian cancer progression? J. Ovarian Res. 2020 Sep 23;13(1):115. doi: 10.1186/s13048-020-00718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathalla M.F. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971 Jul 17;2(7716):163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- Feehan J., Tripodi N., Apostolopoulos V. The twilight of the immune system: The impact of immunosenescence in aging. Maturitas. 2021 May;147:7–13. doi: 10.1016/j.maturitas.2021.02.006. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr. Rev. 2007 Dec;65(12 Pt 2):S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018 Oct;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- Garn H., Bahn S., Baune B.T., Binder E.B., Bisgaard H., Chatila T.A., Chavakis T., Culmsee C., Dannlowski U., Gay S., Gern J., Haahtela T., Kircher T., Müller-Ladner U., Neurath M.F., Preissner K.T., Reinhardt C., Rook G., Russell S., Schmeck B., Stappenbeck T., Steinhoff U., van Os J., Weiss S., Zemlin M., Renz H. Current concepts in chronic inflammatory diseases: Interactions between microbes, cellular metabolism, and inflammation. J. Allergy Clin. Immunol. 2016;138(1):47–56. doi: 10.1016/j.jaci.2016.02.046. [DOI] [PubMed] [Google Scholar]
- Gibson S.J., Fleming G.F., Temkin S.M., Chase D.M. The Application and Outcome of Standard of Care Treatment in Elderly Women with Ovarian Cancer: A Literature Review over the Last 10 Years. Front. Oncol. 2016 Mar;24(6):63. doi: 10.3389/fonc.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G.H., Hwang I., Cho H., Ylaya K., Choi J.-A., Kwon H., Chung J.-Y., Hewitt S.M., Kim J.-H. Clinical Significance of Tumor Infiltrating Lymphocytes in Association with Hormone Receptor Expression Patterns in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2021 May 27;22(11):5714. doi: 10.3390/ijms22115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley G.E., Pearce C.L., Talhouk A., Kwon J.S., Finlayson S.J., McAlpine J.N., Huntsman D.G., Miller D. Outcomes From Opportunistic Salpingectomy for Ovarian Cancer Prevention. JAMA Netw. Open. 2022;5(2):e2147343. doi: 10.1001/jamanetworkopen.2021.47343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.A., Artemenko M., Tang M.K.S., Wong A.S.T. Selectins: An Important Family of Glycan-Binding Cell Adhesion Molecules in Ovarian Cancer. Cancers (Basel). 2020 Aug 10;12(8):2238. doi: 10.3390/cancers12082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.F., Huang H.S., Chen P.C., Ding D.C., Chu T.Y. IGF-axis confers transformation and regeneration of fallopian tube fimbria epithelium upon ovulation. EBioMedicine. 2019 Mar;41:597–609. doi: 10.1016/j.ebiom.2019.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Shafrir A.L., Eliassen A.H., Rexrode K.M., Tworoger S.S. Estimated Number of Lifetime Ovulatory Years and Its Determinants in Relation to Levels of Circulating Inflammatory Biomarkers. Am. J. Epidemiol. 2020 Jul 1;189(7):660–670. doi: 10.1093/aje/kwz264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragimova M.K., Kokorina E.V., Tsyganov M.M., Churuksaeva O.N., Litviakov N.V. Human papillomavirus and ovarian cancer (review of literature and meta-analysis) Infect. Genet. Evol. 2021 Nov;95 doi: 10.1016/j.meegid.2021.105086. [DOI] [PubMed] [Google Scholar]
- Idahl A., Le Cornet C., González Maldonado S., et al. Serologic markers of Chlamydia trachomatis and other sexually transmitted infections and subsequent ovarian cancer risk: Results from the EPIC cohort. Int. J. Cancer. 2020 Oct 15;147(8):2042–2052. doi: 10.1002/ijc.32999. [DOI] [PubMed] [Google Scholar]
- Iyoshi S., Yoshihara M., Nakamura K., Sugiyama M., Koya Y., Kitami K., Uno K., Mogi K., Tano S., Tomita H., Kajiwara K., Taki M., Yamaguchi S., Nawa A., Kajiyama H. Pro-tumoral behavior of omental adipocyte-derived fibroblasts in tumor microenvironment at the metastatic site of ovarian cancer. Int. J. Cancer. 2021;149(11):1961–1972. doi: 10.1002/ijc.33770. [DOI] [PubMed] [Google Scholar]
- Jia D., Nagaoka Y., Katsumata M., Orsulic S. Inflammation is a key contributor to ovarian cancer cell seeding. Sci. Rep. 2018;8(1):12394. doi: 10.1038/s41598-018-30261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaei Z., Namayandeh S.M., Beiranvand R., Naemi H., Bechashk S.M., Goodarzi E. Worldwide incidence and mortality of ovarian cancer and Human Development Index (HDI): GLOBOCAN sources and methods 2018. J. Prev. Med. Hyg. 2021 Apr 29;62(1):E174–E184. doi: 10.15167/2421-4248/jpmh2021.62.1.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippel R.J., Drewes J.L., Sears C.L. The Cancer Microbiome: Recent Highlights and Knowledge Gaps. Cancer Discov. 2021 doi: 10.1158/2159-8290.CD-21-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsopoulos J., Rosen B., Fan I., Moody J., McLaughlin J.R., Risch H., May T., Sun P., Narod S.A. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol. Oncol. 2016;140(1):42–47. doi: 10.1016/j.ygyno.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Kurman R.J., IeM S. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am. J. Surg. Pathol. 2010 Mar;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi G.C., Accardi G., Monastero R., Nicoletti F., Libra M. Ageing: from inflammation to cancer. Immun. Ageing. 2018 Jan;19(15):1. doi: 10.1186/s12979-017-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J., Yue Y., Yu W., Zhang Y. Immunosenescence: a key player in cancer development. J. Hematol. Oncol. 2020 Nov 10;13(1):151. doi: 10.1186/s13045-020-00986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li L.i., Luo N., Liu Q.i., Liu L.i., Chen D., Cheng Z., Xi X. Inflammatory signals induce MUC16 expression in ovarian cancer cells via NF-κB activation. Exp. Ther. Med. 2021 Feb;21(2) doi: 10.3892/etm.2020.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Paciencia S., Saint-Germain E., Rowell M.-C., Ruiz A.F., Kalegari P., Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. doi: 10.1016/j.cyto.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Macciò A., Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012 May;58(2):133–147. doi: 10.1016/j.cyto.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Menon U., Gentry-Maharaj A., Burnell M., Singh N., Ryan A., Karpinskyj C., Carlino G., Taylor J., Massingham S.K., Raikou M., Kalsi J.K., Woolas R., Manchanda R., Arora R., Casey L., Dawnay A., Dobbs S., Leeson S., Mould T., Seif M.W., Sharma A., Williamson K., Liu Y., Fallowfield L., McGuire A.J., Campbell S., Skates S.J., Jacobs I.J., Parmar M. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182–2193. doi: 10.1016/S0140-6736(21)00731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens-Walker I., Baxter R.C., Marsh D.J. Gonadotropin signalling in epithelial ovarian cancer. Cancer Lett. 2012 Nov 28;324(2):152–159. doi: 10.1016/j.canlet.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Momenimovahed Z., Tiznobaik A., Taheri S., Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int. J. Womens Health. 2019 Apr;30(11):287–299. doi: 10.2147/IJWH.S197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakuła M., Mały E., Uruski P., Witucka A., Bogucka M., Jaroszewska N., Makowska N., Niklas A., Moszyński R., Sajdak S., Tykarski A., Mikuła-Pietrasik J., Książek K. Deciphering the Molecular Mechanism of Spontaneous Senescence in Primary Epithelial Ovarian Cancer Cells. Cancers (Basel). 2020 Jan 27;12(2):296. doi: 10.3390/cancers12020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S., Wilczyński J.R., Paradowska E. Factors in Oncogenesis: Viral Infections in Ovarian Cancer. Cancers (Basel). 2020 Feb 29;12(3):561. doi: 10.3390/cancers12030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres L.C., Mallen A.R., Townsend M.K., Poole E.M., Trabert B., Allen N.E., Arslan A.A., Dossus L., Fortner R.T., Gram I.T., Hartge P., Idahl A., Kaaks R., Kvaskoff M., Magliocco A.M., Merritt M.A., Quirós J.R., Tjonneland A., Trichopoulou A., Tumino R., van Gils C.H., Visvanathan K., Wentzensen N., Zeleniuch-Jacquotte A., Tworoger S.S. High Levels of C-Reactive Protein Are Associated with an Increased Risk of Ovarian Cancer: Results from the Ovarian Cancer Cohort Consortium. Cancer Res. 2019;79(20):5442–5451. doi: 10.1158/0008-5472.CAN-19-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-López F.R., Ceausu I., Depypere H., Kehoe S., Lambrinoudaki I., Mueck A., Senturk L.M., Simoncini T., Stevenson J.C., Stute P., Rees M. Interventions to reduce the risk of ovarian and fallopian tube cancer: A European Menopause and Andropause Society Position Statement. Maturitas. 2017;100:86–91. doi: 10.1016/j.maturitas.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Piao J., Lee E.J., Lee M. Association between pelvic inflammatory disease and risk of ovarian cancer: An updated meta-analysis. Gynecol. Oncol. 2020 May;157(2):542–548. doi: 10.1016/j.ygyno.2020.02.002. [DOI] [PubMed] [Google Scholar]
- Rockfield S., Kee Y., Nanjundan M. Chronic iron exposure and c-Myc/H-ras-mediated transformation in fallopian tube cells alter the expression of EVI1, amplified at 3q26.2 in ovarian cancer. Oncogenesis. 2019;8(9) doi: 10.1038/s41389-019-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Tomás T., Rueda-Robles A., Plaza-Díaz J., Álvarez-Mercado A.I. Nutrition and cellular senescence in obesity-related disorders. J. Nutr. Biochem. 2021 Sep;11(99) doi: 10.1016/j.jnutbio.2021.108861. [DOI] [PubMed] [Google Scholar]
- Salvador S., Gilks B., Köbel M., Huntsman D., Rosen B., Miller D. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int. J. Gynecol. Cancer. 2009 Jan;19(1):58–64. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- Sánchez-Borrego R., Sánchez-Prieto M. What are the mechanisms of action of the different contraceptive methods to reduce the risk of ovarian cancer? Eur. J. Contracept. Reprod. Health Care. 2021 Feb;26(1):79–84. doi: 10.1080/13625187.2020.1849617. [DOI] [PubMed] [Google Scholar]
- Savant S.S., Sriramkumar S., O'Hagan H.M. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers (Basel) 2018 Jul 30;10(8):251. doi: 10.3390/cancers10080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W., Liu J. Inflammation: a hidden path to breaking the spell of ovarian cancer. Cell Cycle. 2009 Oct 1;8(19):3107–3111. doi: 10.4161/cc.8.19.9590. [DOI] [PubMed] [Google Scholar]
- Shigeta S., Toyoshima M., Kitatani K., Ishibashi M., Usui T., Yaegashi N. Transferrin facilitates the formation of DNA double-strand breaks via transferrin receptor 1: the possible involvement of transferrin in carcinogenesis of high-grade serous ovarian cancer. Oncogene. 2016 Jul 7;35(27):3577–3586. doi: 10.1038/onc.2015.425. [DOI] [PubMed] [Google Scholar]
- Shirasuna K., Iwata H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017 Oct;3(2):23. doi: 10.1186/s40834-017-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanha K., Mottaghi A., Nojomi M., Moradi M., Rajabzadeh R., Lotfi S., Janani L. Investigation on factors associated with ovarian cancer: an umbrella review of systematic review and meta-analyses. J. Ovarian Res. 2021;14(1) doi: 10.1186/s13048-021-00911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabert B., Tworoger S.S., O'Brien K.M., Townsend M.K., Fortner R.T., Iversen E.S., Hartge P., White E., Amiano P., Arslan A.A., Bernstein L., Brinton L.A., Buring J.E., Dossus L., Fraser G.E., Gaudet M.M., Giles G.G., Gram I.T., Harris H.R., Bolton J.H., Idahl A., Jones M.E., Kaaks R., Kirsh V.A., Knutsen S.F., Kvaskoff M., Lacey J.V., Lee I.-M., Milne R.L., Onland-Moret N.C., Overvad K., Patel A.V., Peters U., Poynter J.N., Riboli E., Robien K., Rohan T.E., Sandler D.P., Schairer C., Schouten L.J., Setiawan V.W., Swerdlow A.J., Travis R.C., Trichopoulou A., van den Brandt P.A., Visvanathan K., Wilkens L.R., Wolk A., Zeleniuch-Jacquotte A., Wentzensen N. Ovarian Cancer Cohort Consortium (OC3). The Risk of Ovarian Cancer Increases with an Increase in the Lifetime Number of Ovulatory Cycles: An Analysis from the Ovarian Cancer Cohort Consortium (OC3) Cancer Res. 2020;80(5):1210–1218. doi: 10.1158/0008-5472.CAN-19-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhong L., Xu B., Chen M., Huang H. Diabetes mellitus and the risk of ovarian cancer: a systematic review and meta-analysis of cohort and case-control studies. BMJ Open. 2020 Dec 29;10(12) doi: 10.1136/bmjopen-2020-040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.Q., Cheong I.H., Yang G.H., Li X.G., Kozlakidis Z., Ding L., Liu N.N., Wang H. The Application of High-Throughput Technologies for the Study of Microbiome and Cancer. Front. Genet. 2021 Jul;12 doi: 10.3389/fgene.2021.699793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen N., O'Brien K.M. Talc, body powder, and ovarian cancer: A summary of the epidemiologic evidence. Gynecol. Oncol. 2021 doi: 10.1016/j.ygyno.2021.07.032. S0090-8258(21)00598-9. [DOI] [PubMed] [Google Scholar]
- Wheeler L.J., Desanto K., Teal S.B., Sheeder J., Guntupalli S.R. Intrauterine Device Use and Ovarian Cancer Risk: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2019 Oct;134(4):791–800. doi: 10.1097/AOG.0000000000003463. [DOI] [PubMed] [Google Scholar]
- Wichmann I.A., Cuello M.A. Obesity and gynecological cancers: A toxic relationship. Int. J. Gynaecol. Obstet. 2021 Oct;155(Suppl 1):123–134. doi: 10.1002/ijgo.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N.Y., Fang C., Huang H.S., Wang J., Chu T.Y. Natural history of ovarian high-grade serous carcinoma from time effects of ovulation inhibition and progesterone clearance of p53-defective lesions. Mod. Pathol. 2020 Jan;33(1):29–37. doi: 10.1038/s41379-019-0370-1. [DOI] [PubMed] [Google Scholar]
- Yang J., Ma J., Jin Y., Cheng S., Huang S., Wang Y.u. Dietary Inflammatory Index and Ovarian Cancer Risk: A Meta-Analysis. Nutr. Cancer. 2022;74(3):796–805. doi: 10.1080/01635581.2021.1931366. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky J., Relton C.L., Lophatananon A., Muir K., Menon U., Gentry-Maharaj A., Walther A., Zheng J., Fasching P., Zheng W., Yin Ling W., Park S.K., Kim B.-G., Choi J.-Y., Park B., Davey Smith G., Martin R.M., Lewis S.J., Minelli C. Appraising the role of previously reported risk factors in epithelial ovarian cancer risk: A Mendelian randomization analysis. PLoS Med. 2019 Aug 7;16(8):e1002893. doi: 10.1371/journal.pmed.1002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Dolgalev I., Zhang T., Ran H., Levine D.A., Neel B.G. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat. Commun. 2019 Nov 26;10(1):5367. doi: 10.1038/s41467-019-13116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Wang X., Ren X., Zhou L., Wang N., Kang H. Disease Burden and Attributable Risk Factors of Ovarian Cancer From 1990 to 2017: Findings From the Global Burden of Disease Study 2017. Front. Public Health. 2021 Sep;17(9) doi: 10.3389/fpubh.2021.619581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Pietrocola F., Kroemer G. Nutrition, inflammation and cancer. Nat. Immunol. 2017 Jul 19;18(8):843–850. doi: 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]