Abstract
Background
African swine fever (ASF), a highly contagious disease affecting both domestic and wild pigs, has been having a serious impact on the swine industry worldwide. This important transboundary animal disease can be spread by animals and ticks via direct transmission and by contaminated feed and fomites via indirect transmission because of the high environmental resistance of the ASF virus. Thus, the prevention of the introduction of ASF to areas free of ASF is essential. After an outbreak was reported in China, intensive import policies and biosecurity measures were implemented to prevent the introduction of ASF to pig farms in Thailand.
Objective
Enhancing prevention and control, this study aims to identify the potential areas for ASF introduction and transmission in Thailand, develop a tool for farm assessment of ASF risk introduction focusing on smallholders, and develop a spatial analysis tool that is easily used by local officers for disease prevention and control planning.
Methods
We applied a multi-criteria decision analysis approach with spatial and farm assessment and integrated the outputs with the necessary spatial layers to develop a spatial analysis on a web-based platform.
Results
The map that referred to potential areas for ASF introduction and transmission was derived from 6 spatial risk factors; namely, the distance to the port, which had the highest relative importance, followed by the distance to the border, the number of pig farms using swill feeding, the density of small pig farms (<50 heads), the number of pigs moving in the area, and the distance to the slaughterhouse. The possible transmission areas were divided into 5 levels (very low, low, medium, high, and very high) at the subdistrict level, with 27 subdistricts in 10 provinces having very high suitability and 560 subdistricts in 34 provinces having high suitability. At the farm level, 17 biosecurity practices considered as useful and practical for smallholders were selected and developed on a mobile app platform. The outputs from the previous steps integrated with necessary geographic information system layers were added to a spatial analysis web-based platform.
Conclusions
The tools developed in this study have been complemented with other strategies to fight against the introduction of ASF to pig farms in the country. The areas showing high and very high risk for disease introduction and transmission were applied for spatial information planning, for example, intensive surveillance, strict animal movement, and public awareness. In addition, farms with low biosecurity were improved in these areas, and the risk assessment developed on a mobile app in this study helped enhance this matter. The spatial analysis on a web-based platform helped facilitate disease prevention planning for the authorities.
Keywords: African swine fever, multi-criteria decision analysis, risk-based surveillance, risk assessment, spatial analysis
Introduction
Background
African swine fever (ASF) is a highly contagious disease affecting both domestic and wild pigs of all ages. It is highly contagious because the morbidity and mortality in exposed pig herds are possibly up to 100% [1]. The ASF virus (ASFV) is a large, enveloped, double-stranded DNA virus with a size of 170 to 190 kbp, and its genome includes >50 structural proteins and several nonstructural proteins [2,3]. Owing to the large size and complex structure of the virus, there is no effective vaccine producible at the moment [2]. The virus is also highly survivable, allowing the virus to spread via various sources, including infected live and dead pigs, infected pork products, contaminated feed, and contaminated fomites [3]. The ASFV can survive for up to 18 months in serum at room temperature and for several months in raw pork products such as raw ham or sausage and treated products [2]. It survives for a longer time in frozen material and resists a pH level between 4 and 13 [4]. Hence, it is a serious disease in the swine industry worldwide, causing serious economic and production losses [3].
The World Organization for Animal Health identifies ASF as an important transboundary animal disease that has spread across almost all continents and in many countries [3]. After the ASF genotype I was first detected in Kenya in 1910, it circulated in several countries in Africa [1]. The virus was then introduced to Europe, where it was first found in Portugal in 1957 and spread to many countries in Europe and also in South and Central America [3,5,6]. Besides Sardinia [7], ASF type I has been successfully eradicated in Europe and America [1,2]. Subsequently, ASF genotype II emerged in Africa and was first introduced to Europe in 2007 and spread to many countries, beginning with Georgia [8] to its neighbors [5,6] and across to the west [9]. The virus was first introduced to Asia in 2018 in China and, since then, the disease has spread to many countries in Asia and the Pacific [10]. In 2019, 11 countries encountered the ASF, including Mongolia, Vietnam, Cambodia, Hong Kong, the Democratic People’s Republic of Korea, Laos, Myanmar, the Philippines, the Republic of Korea, East Timor, and Indonesia. In 2020, 2 more countries were affected by the disease: Papua New Guinea and India. The latest outbreak was detected in the north of east Malaysia in 2021 on the Borneo island, which it shares with Brunei and Indonesia [9].
The transmission cycle of ASF worldwide includes 3 main hosts: wild pigs, soft ticks, and domestic pigs. The ASFV can replicate in the soft ticks of the genus Ornithodoros, which are mainly found in Africa and some parts of Europe [11]. This type of tick has been identified as a biological vector of the ASFV and has spread the virus to wild and domestic pigs [1,11]. Pig-to-pig transmission occurs via direct and indirect contact. Direct contact between infected and susceptible pigs has been identified as a very effective transmission route [12]. Indirect contact shows that the ASFV is introduced to susceptible pigs through people, contaminated feed, infected boar semen, and contaminated fomites [11,13]. Experimental studies have found that the Stomoxys flies can be a mechanical vector transmitting ASFV to domestic pigs for a limited time [14,15]; however, ASFV tested in flies collected on ASF-affected farms in Lithuania produced negative results [11]. Feeding pigs with contaminated pork products or fodder is considered to play a major role in the transmission of ASF across countries. Although the import of pigs and pork products from ASF-infected countries was officially banned, it was found that the first outbreak in Europe occurred in a pig farm near the Lisbon airport in Portugal in 1957 caused by feeding pigs waste from airline flights [16]. The same occurred in 2007; the ASF genotype II was first introduced to Georgia through contaminated pork carried by international ships that was then fed to pigs [8].
ASF has a high impact not only on the commercial pig industry but also on smallholders. The introduction of ASF to countries has resulted in many impacts, for example, the loss of up to 50% of the pig population, affecting food security, the cost of disease control, and the loss of status for international trade [16]. The greatest losses occur in countries where most pig farmers are smallholders or practice backyard farming [16]. This sector usually relates to low farm biosecurity, poor knowledge of disease prevention, and a lack of financial resources for farm improvement [17-20]. Europe has experience in ASF spread and successful eradication, the lessons learned including, for example, that (1) pig holders with poor biosecurity usually facilitate the first occurrence of the outbreak [16]; (2) in the areas dominated by commercial pig production, strict animal movement and implementation of culling policies successfully prevented the spread of the disease [16]; and (3) in endemic areas mostly dominated by poor biosecurity farming, apart from both aforementioned measures, the eradication program emphasized improving farm biosecurity, increased disease awareness in pig farmers, and extensive monitoring activities [16].
Objectives
Southeast Asia, where Thailand is located, has been facing the spread of ASF [10]. Immediately after ASF was reported in China in 2018 [21], Thailand has been intensively preventing the introduction of ASF to pig farms in the country by implementing the control measures learned from other countries, in particular European countries [5,6,16,22]. We conducted this study to enhance the measures for preventing the introduction of the ASFV to high-risk farms, focusing on high-risk areas in the country, as well as for assisting responsible officers in spatial information planning. Therefore, the objectives of this study were 3-fold: (1) to identify the potential areas for ASF introduction and transmission in Thailand, (2) to develop a tool for farm assessment of ASF risk introduction focusing on smallholders, and (3) to develop a spatial analysis tool that is easily used by local officers for disease control planning.
Methods
We developed tools for ASF prevention and control, including (1) a suitability map for ASF introduction and transmission in the first stage of virus introduction; (2) a mobile app for farm assessment of ASF risk introduction; and (3) a web application for spatial analysis of ASF prevention and control by combining a layer of suitability map, locations with risk level of farm assessment, and other relevant layers. The methods for each step are detailed in the following sections.
Developing a Suitability Map for ASF Introduction and Transmission
We applied a knowledge-driven model called a spatial multi-criteria decision analysis (MCDA) to determine the suitability areas for ASF introduction and transmission. The analytical hierarchy process, one of the MCDA methods, was used in this study for its power and simplicity [23]. The analysis consisted of four steps: (1) defining and standardizing risk factors, (2) assigning relative importance to the risk factors, (3) combining all layers of risk factors, and (4) assessing the sensitivity and uncertainty of the analysis.
We used a participatory approach [24] by inviting 20 experts in relevant fields, including 12 (60%) epidemiologists, 2 (10%) virologists, and 6 (30%) stakeholders in pig production, to define, standardize, and assign the relative importance of the risk factors of ASF introduction and transmission in the country, with emphasis on the first stage of virus introduction. Each expert initially assigned individual outputs, and then all experts assigned the final outputs together. The defined factors were standardized using fuzzy membership functions [25] in which the relationship between the values of each factor and the suitability for ASF introduction and transmission ranging from 0 (unsuitable) to 1 (highly suitable) was defined. There were 4 types of relationships proposed to the experts—namely, linear, sigmoidal (s-shaped), j-shaped, and user-defined—with increasing, decreasing, or symmetrical functions [25]. The Fuzzy tool from the IDRISI software (Clark Labs) [26] was used to implement this standardization step. The Fuzzy tool requires the position along the x-axis of each risk factor of 4 parameters (a, b, c, and d) governing the shape of the fuzzy membership function [25].
A pairwise comparison technique was used to define the relative importance of each factor. The procedure consisted of comparing each pair of factors using a 9-point continuous comparison scale (Table 1). The weight value for each factor (Wi) was calculated by taking the eigenvector corresponding to the largest eigenvalue of the pairwise score matrix and then normalizing the sum of the components to a unity [27-29]. The consistency ratio (CR), which is calculated as the consistency index divided by a random index, was used to verify the consistency of the matrix. The random index, derived from the study by Saaty [30], depends on the number of analyzed factors (3 factors=0.58, 4 factors=0.90, 5 factors=1.12, 6 factors=1.24, 7 factors=1.32, 8 factors=1.41, 9 factors=1.46, 10 factors=1.49, 11 factors=1.51, 12 factors=1.54, 13 factors=1.56, 14 factors=1.57, and 15 factors=1.58). The consistency index is calculated as
Table 1.
The 9-point scale values used in the pairwise comparison of factors.
| Intensity of importance | Description |
| 1 | Equal importance |
| 3 | Moderate importance |
| 5 | Strong or essential importance |
| 7 | Very strong or demonstrated importance |
| 9 | Extreme importance |
| 2, 4, 6, 8 | Intermediate values |
| Reciprocals | Values for inverse comparison |
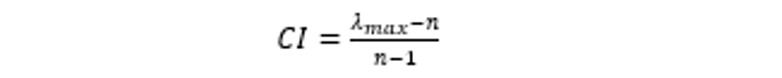 |
where λmax is the maximum eigenvalue of the judgment matrix and n is the number of factors. If the CR is >0.10, then some pairwise values need to be reconsidered, and the process is repeated until the desired value of CR of <0.10 is reached [30].
The suitability map was produced by incorporating all standardized factor layers using the weighted linear combination (WLC) [31] method in the R software (R Foundation for Statistical Computing). The packages raster, maptools, and fields in R were used. In the WLC, each standardized factor is multiplied by its corresponding weight, these are summed, and then the sum is divided by the number of factors. Its equation is as follows:
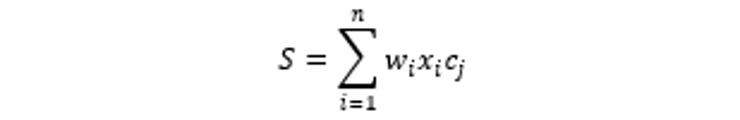 |
where wi is the weight of criterion i, xi is the criterion score of criterion i (value of the corresponding raster cell in the criterion raster map), n is the number of criteria, and cj is the criterion score (1 or 0) of constraint j.
With regard to the sensitivity analysis, we applied the one-at-a-time method, which works by changing 1 input factor at a time and evaluating the effect of the change on the output [32]. It was selected for its simplicity and good comparability results. The sensitivity analysis was carried out for each factor by setting 2 parameters: a step size of 1% and a range of 50% (–25% to +25%) [33]. By changing 1 factor at a time, all other factors can be fixed, at least to a great extent, to their central or baseline value. The sum of all criteria weights at any percent change (PC) level should always be equal to 1. The weight of the main changing criterion (W(cm, pc)) at a certain PC level can be calculated as follows: W(cm, pc) = W(cm, 0) + (W(cm, 0) * pc), 1≤m≤n, where W(cm, 0) is the weight of the main changing criterion cm at the base run (the original weights). The weights of the other criteria W(ci, pc) are adjusted proportionally in accordance with W(cm, pc) to maintain the sum of all criteria weights at any PC of 1 with the following equation:
where W(ci, 0) is the weight of the ith criterion ci at the base run.
We evaluated this step using the mean of the absolute change rate (MACR) [34]. In each simulation, the original suitability map (the original weights) and the output map of the alternative model (changing criterion weights) were quantitatively matched through a pixel-by-pixel comparison. The MACR was calculated using the following equation:
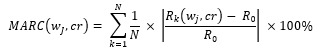 |
where MARC(wJ, cr) is the mean absolute value of the change rate, with wJ as the change rate, and N is the number of pixels. In addition, an uncertainty surface resulting from the changes in weights was produced for the study area representing the SD of the different suitability maps [35,36].
The spatial data used in this part are listed in Table 2. The distance risk factors were processed using the cost distance tool in ArcGIS (version 10.2; Esri) [37], in which the objects from the nearest distances, including the border, the ports, and the slaughterhouses, were estimated. Pig movement data in 2018 were obtained from a web-based movement registration system [38] (e-Movement), through which the movements of pigs and other animals are required to be registered. Pig population data were obtained from the animal census data operated by the Department of Livestock Development (DLD) officers annually, in which pig farms with <50 pigs were included for analysis. Surveys of pig farms using swill feeding were conducted by local DLD officers between September 2018 and December 2018. All geographical data were converted into raster data sets with a 100-meter resolution using ArcGIS.
Table 2.
Spatial risk factors, standardized methods, and relative importance of each factor.
| Spatial risk factors | Fuzzy membership functions |
Inflection points | Weights | ||||
|
|
|
a | b | c | d |
|
|
| Distance to border, m | Sigmoidal monotonically decreasing | 10,000 | 10,000 | 10,000 | 100,000 | 0.2295 | |
| Distance to port, m | Sigmoidal monotonically decreasing | 10,000 | 10,000 | 10,000 | 100,000 | 0.3567 | |
| Distance to slaughterhouse, m | Sigmoidal monotonically decreasing | 10,000 | 10,000 | 10,000 | 100,000 | 0.0580 | |
| Pigs moving in the area, n | Sigmoidal monotonically increasing | 1 | 5 | 5 | 5 | 0.0668 | |
| Density of small pig farms (<50 heads), farms/km2 | Sigmoidal monotonically increasing | 0.1 | 1 | 1 | 1 | 0.1219 | |
| Pig farms using swill feeding, farms/km2 | Sigmoidal monotonically increasing | 0.01 | 0.5 | 0.5 | 0.5 | 0.1670 | |
Developing a Mobile App for Farm Assessment of ASF Risk Introduction
We also applied the analytical hierarchy process to develop a set of factors and algorithms for risk assessment, which was performed through a platform in a mobile app. Publications on ASF risk factors were reviewed and proposed to the experts, who then selected and standardized the factors. First, risk factors for ASF introduction at the farm level such as biosecurity measures and characteristics of the farms’ environments were selected by the experts. The selected factors were also standardized by defining the relationship between each of the factors and suitability using a 5-point scale (1=very low, 2=low, 3=medium, 4=high, and 5=very high). The relative importance of each factor was then defined using a pairwise comparison technique.
We designed questionnaires and coded algorithms on a mobile app for both iOS and Android operating systems by applying the outputs obtained from the previous steps. The combination of all factors to produce a final weighted estimate of suitability was implemented using a mobile app. The final score of each farm was obtained from the WLC method categorized into 5 suitability levels (<1.5=very low, >1.5-2.5=low, >2.5-3.5=medium, >3.5-4.5=high, and >4.5=very high). We set the provinces that found high and very high suitability for ASF distribution areas (analyzed in the previous step) as the targeted areas for farm assessment. District livestock officers were trained on how to install and use the app and then evaluated all non–Good Agricultural Practice pig farms in their responsible areas between April 2019 and July 2019.
Developing a Spatial Analysis of ASF Prevention and Control on a Web Application
We developed a spatial analysis of ASF prevention and control on a web-based platform. The necessary geodata for prevention and control planning were provided, including a suitability map for ASF introduction and transmission (step 1), the farm locations with ASF risk level (step 2), and other relevant layers such as locations of slaughterhouses (collected by DLD staff). The buffer rings surrounding selected pig farms were also developed, which allows users to download the important data in spreadsheet files, such as the number and details of neighboring farms and the distance to selected farms.
Ethics Approval
This study was approved by the Research Committee of the Bureau of Disease Control and Veterinary Services, DLD, Thailand (permit 64(2)-0105-110).
Results
A Suitability Map for ASF Introduction and Transmission
Table 2 shows the results of the defined factors, the standardized methods, and the relative importance of each factor, where distances are measured in meters and areas are measured in square kilometers (km2). Six spatial risk factors were identified by the experts: (1) the distance to the border, (2) the distance to the port, (3) the distance to the slaughterhouse, (4) the number of pigs moving in the area, (5) the density of small pig farms (<50 heads), and (6) the number of pig farms using swill feeding. The results showed that, according to the experts, the distance to the port had the highest weight, followed by the distance to the border, the number of pig farms using swill feeding, the density of small pig farms (<50 heads), the number of pigs moving in the area, and the distance to the slaughterhouse. Figure 1 shows the standardized risk factors used to produce the final suitability map for ASF distribution.
Figure 1.
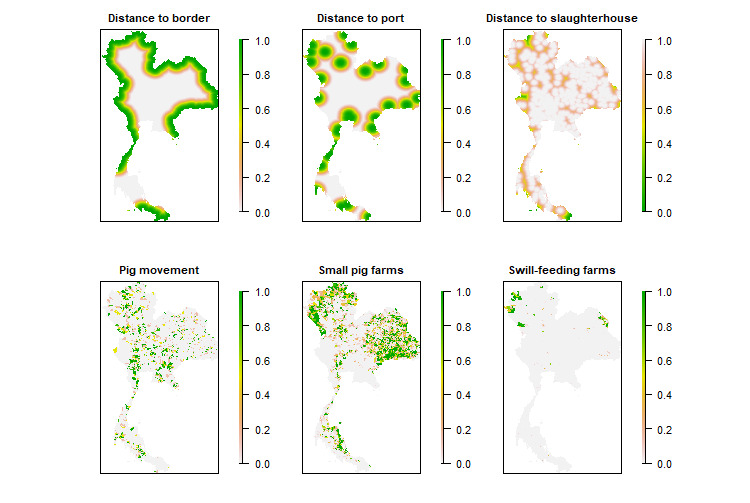
Maps of the standardized risk factors used to analyze the suitability for African swine fever introduction and transmission in Thailand. From top left to bottom right: the distance to the border, the distance to the port, the distance to the slaughterhouse, the number of pigs moving in the area, the density of small pig farms (<50 heads), and the number of pig farms using swill feeding.
Figure 2 shows the suitability map for ASF introduction and transmission in Thailand if it were first introduced to the country. The resulting most potential areas were clustered near the north and northeast borders. The entire area was extracted and aggregated into 5 levels (very low to very high) at the subdistrict level, as shown in Table 3. There were 27 subdistricts in 10 provinces with very high suitability and 560 subdistricts in 34 provinces with high suitability.
Figure 2.
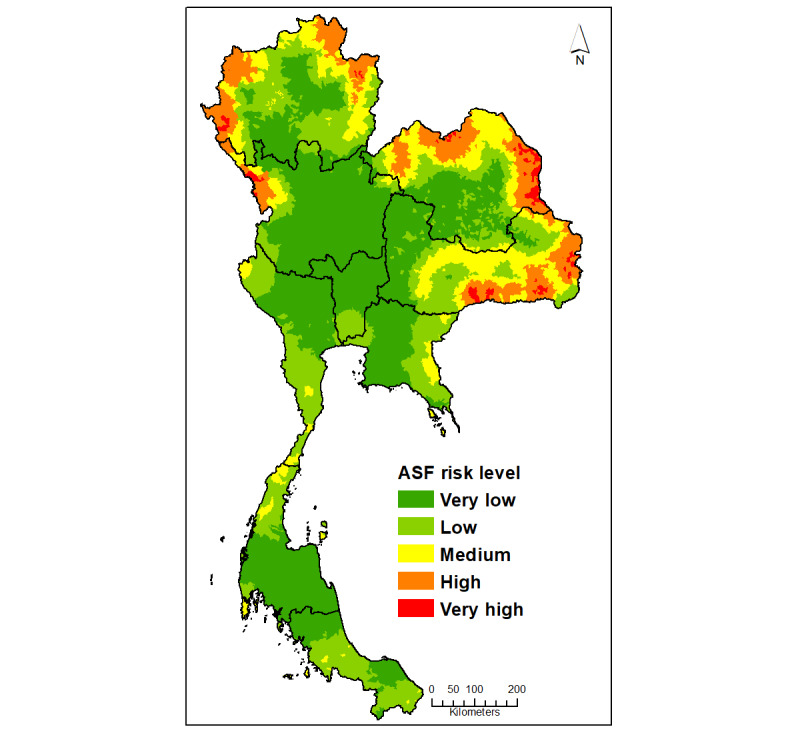
The suitability map for African swine fever (ASF) introduction and transmission in Thailand.
Table 3.
Number of subdistricts, districts, and provinces according to African swine fever (ASF) risk levels.
| ASF risk levels | Subdistricts (N=7416), n (%) | Districts (N=926), n (%) | Provinces (N=77), n (%) |
| Very high | 27 (0.4) | 17 (1.8) | 10 (12.9) |
| High | 560 (7.6) | 144 (15.6) | 34 (44.2) |
| Medium | 1408 (18.9) | 353 (38.1) | 52 (67.5) |
| Low | 2693 (36.3) | 490 (52.9) | 75 (97.4) |
| Very low | 5833 (78.7) | 478 (51.6) | 55 (71.4) |
Figure 3 shows the results of the one-at-a-time sensitivity analysis, in which the simulated suitability maps for ASFV transmission in pigs in Thailand were generated with the weight of each factor changed from −25% to 25% with a step size of 1%. The MACRs were used to display the sensitivity of each factor, with a high gradient indicating a greater change in the values of the output maps (high sensitivity). It appeared that the most sensitive factor was the distance to the port followed by the distance to the border, the distance to the slaughterhouse, the number of pigs moving in the area, the density of small pig farms, and the number of pig farms using swill feeding.
Figure 3.
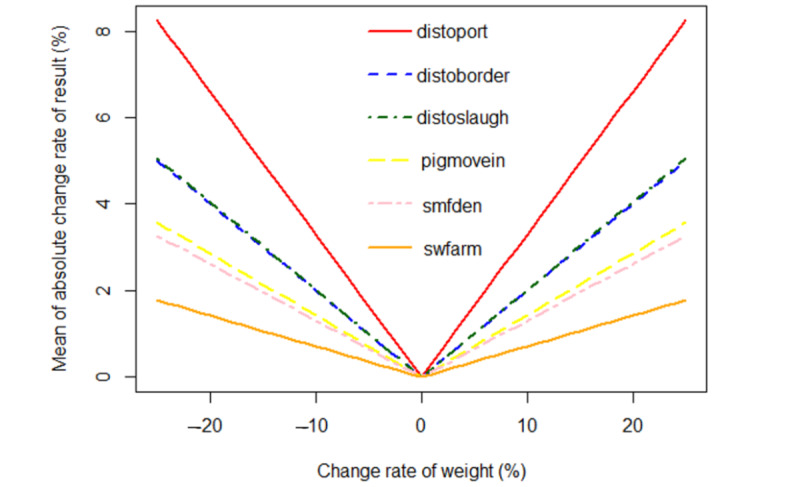
The results of the one-at-a-time sensitivity analysis. Distoborder: the distance to the border; distoport: the distance to the port; distoslaugh: the distance to the slaughterhouse; pigmovein: the number of pigs moving in the area; smfden: the density of small pig farms; swfarm: the number of pig farms using swill feeding.
The uncertainty analysis showed fairly robust results and a spatial heterogeneity. The uncertainty surface remained stable, with the maximum SD value being <0.1 (Figure 4) even though the risk factors were varied. This implies that the predicted suitability areas for ASFV transmission in pigs in Thailand according to the suitability index are fairly robust.
Figure 4.
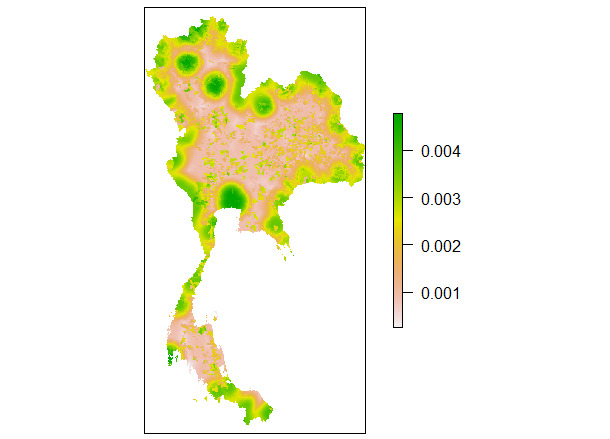
Uncertainty map: SD of the suitability maps for African swine fever introduction and transmission in pigs in Thailand.
Farm Assessment of ASF Risk Introduction Using a Mobile App
The defined risk factors of ASF introduced to pig farms, standardized risk factors, and relative importance of risk factors (weight) are shown in Multimedia Appendix 1 [39-54]. The experts defined 17 risk factors that would be important at the farm level, which were categorized into 3 groups: farm biosecurity, farm management, and farm location. The 3 most important identified factors were the pig feed, the farm location on an ASF risk level, and the breeding practices on the farm.
The results of the farm assessment of ASF introduction are presented in Table 4. There were 61,747 pig farms in 34 provinces evaluated using the developed app on a mobile platform. Of the 61,747 evaluated farms, 4 (0%) and 4380 (7.09%) were found to have very high and high risk of ASF introduction, respectively.
Table 4.
The results of pig farms assessed using an app developed on a mobile platform.
| Risk assessment level | Farms (N=61,747), n (%) | Provinces (N=34), n (%) |
| Very low | 3919 (6.4) | 32 (94.1) |
| Low | 23,604 (38.2) | 33 (97.1) |
| Medium | 29,840 (48.3) | 32 (94.1) |
| High | 4380 (7.1) | 28 (82.4) |
| Very high | 4 (0) | 4 (11.8) |
A Spatial Analysis of ASF Prevention and Control on a Web Application
Figure 5 shows a spatial analysis on a web application in which the components are composed of the outputs obtained from the previous processes, including the suitability map (step 1) and the locations of pig farms with risk-assessed level (step 2). In addition, we included other important layers useful for disease control planning, including the locations of the slaughterhouses.
Figure 5.
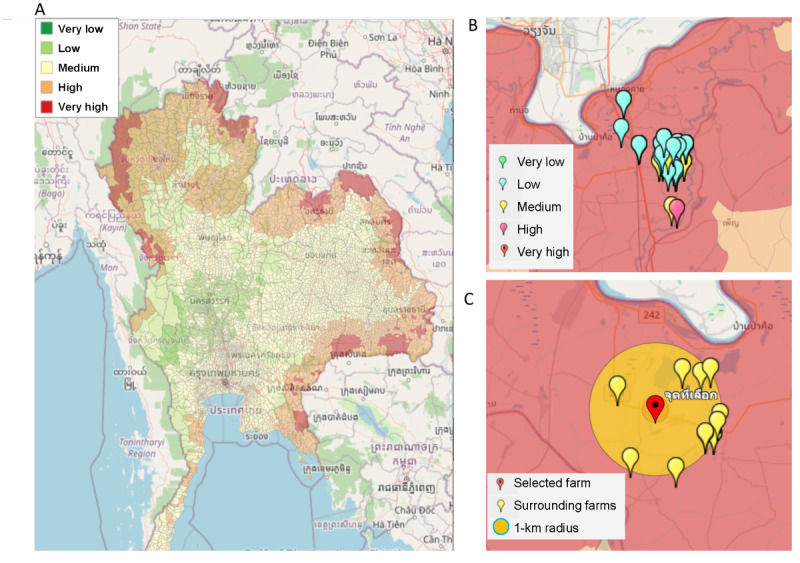
A spatial analysis of African swine fever (ASF) prevention and control on a web application. A spatial analysis conducted by integrating all relevant layers for ASF prevention and control, such as (A) an ASF risk map, (B) farm locations with ASF risk at the farm level, and (C) the buffer zones and farms surrounding a selected farm.
Discussion
Principal Findings
The prevention of the introduction of ASF to Thailand started with the questions of how and where. As learned from the infected countries, the ASFV was introduced to the country possibly through infected live and dead pigs, infected pork products, infected boar semen, contaminated feed, and contaminated fomites [55,56]. Banning pigs, pork products, boar semen, animal feeds, and feed ingredients from affected countries; strictly checking pork products carried by travelers from affected countries; and encouraging pig farmers not to feed pigs with swill were immediately implemented by the Thai authorities [32]. However, with the increasing efficiency of virus detection and prevention, specific areas with a high possibility of virus introduction should be more focused on disease prevention activities [57,58]. Hence, a geographic information system–based MCDA was used in this study to evaluate the suitability for ASFV introduction and transmission in pigs in Thailand in the absence of actual data on ASFV occurrence in the country [59-61], where the outputs of the MCDA were required to help convert the current state of knowledge into a visualization. MCDA is a static, knowledge-driven model that ranks the best choices of a set of weighted rules based on existing publications and expert knowledge [61]. However, the quality of the predictions may be compromised by the misidentification of some unknown factors. It has been applied in many countries to predict suitability maps for ASF [59] and other animal diseases [24,36,62,63].
The 6 spatial risk factors identified and prioritized by the experts were the distance to the port, which had the highest weight; the distance to the border; the number of pig farms using swill feeding; the density of small pig farms (<50 heads); the number of pigs moving in the area; and the distance to the slaughterhouse. This consideration was based on the risk pathways of ASFV transmission by separating the factors into 3 groups in the offensive line. First, if the ASFV were introduced to the country through various sources [1,2,16], as a first line, it would officially and unofficially pass through international and border ports as well as border lines through smuggling [16,58]. Therefore, the distance to the port and to the border was initially included in the analysis. Second, if the ASFV passed through the first line to the territory by not being detected, as a second line, it would initially occur in pig farms that fed pigs with swill feed [16] or it would attack smallholders with poor farm biosecurity, as shown in Europe [16,64,65]. Finally, if the ASFV infected local pig farms, pig movement would facilitate the spread [16], and the virus would be present in slaughterhouses and adjacent areas [49,66].
Prevention of ASFV introduction to pig farms requires good farm biosecurity [16,67]. Biosecurity comprises the measures aimed at reducing the risk of introduction and spread of disease agents or, more simply, “to keep disease agents away from pigs” or “to keep pigs away from disease agents” [68]. Biosecurity practiced by large-scale commercial pig farms mostly corresponds to large investments in infrastructure and equipment that are hardly implemented by smallholders. However, biosecurity improvements in the smallholder sector can already be achieved through very simple and low-cost precautionary measures [69]. This study was focused on selecting biosecurity that is practical for smallholders (as described in Multimedia Appendix 1) and using a simple way for local officers or farmers to be able to evaluate pig farms through a mobile app platform. Risk management [58] can also be communicated through the app, in which biosecurity practices with low scores would be suggested for improvement. Moreover, the outputs of the spatial risk analysis part were added to the farm evaluation part, allowing farmers to be more concerned with biosecurity improvement if their farms are located in high-risk areas [70].
This study developed a spatial analysis web-based platform that can facilitate disease prevention and outbreak control implemented by the responsible officers. Spatial epidemiological analysis plays an important role in planning for disease prevention and outbreak control [61] and has been used to describe and visualize the spatial distribution of hosts [71,72] and diseases [73,74], identify clusters of diseases [73,75], and predict disease risk [36,62]. The outputs of the analyses can be applied for disease prevention [16,59,76], for example, conducting intensive surveillance in high-risk areas, mitigating the risk of disease transmission in high-risk areas by strict animal movement, improving biosecurity, and minimizing the number of susceptible hosts. Spatial analysis is also applied for outbreak control. As guided by the World Organization for Animal Health [77], following the confirmation of an outbreak, control areas based on epidemiological factors may be established around the affected premises. Control measures basically include restriction of animal movement, intensive surveillance, and other specific measures applied to the affected premises. Implementation of these activities requires knowledge of the extent of these areas, the number of animals and farms within the areas, the exact locations of the farms, and the exact locations for setting checkpoints. However, working on spatial analysis is limited by things such as computers with high capacity, geographic information system software, geodata, technicians, and time-consuming processes [61].
Although MCDA is a fast and easy approach to be applied for developing tools for risk assessment at the spatial and farm levels, the limitations may be caused by the approach itself. Knowledge-driven models such as MCDA provide an interesting alternative to model the suitability for ASFV distribution in space or at the farm level as a way to prioritize surveillance and improve prevention [78], but the quality of the predictions may be compromised by the misidentification of some unknown factors. For instance, the spatial risk factors used in this study were focused on the pig-to-pig transmission cycle; therefore, the outputs may not be suitable for the sylvatic transmission cycle as analyzed in Africa [59]. Regarding farm evaluation, the accuracy of the evaluation may cause doubt and needs to be further tested.
Conclusions
This study developed tools by integrating a spatial risk assessment, a farm assessment on a mobile app, and a spatial analysis on a web-based platform aiming for the prevention of ASFV introduction to pig farms in Thailand. The high-risk areas of ASF transmission in Thailand extracted from a risk map were used for disease prevention, such as intensive surveillance, strict movement control, biosecurity improvement if possible or not raising pigs in the farm if not possible, and public awareness. The risk assessments developed on a mobile app were used to evaluate pig farms focusing on smallholders in the most prioritized areas based on a spatial risk assessment. A spatial analysis on a web-based platform was used by local authorities for spatial planning of disease prevention and could be used for outbreak control if an outbreak occurred. The tools developed in this study have been complemented with other strategies to fight against the introduction of the ASFV to pig farms in the country.
Acknowledgments
The authors thank the staff of the Department of Livestock Development for enhancing the tool development; the Center of Excellence for Biomedical and Public Health Informatics, Faculty of Tropical Medicine, Mahidol University, for developing the electronic platforms; and the Faculty of Veterinary Medicine, Kasetsart University, for facilitating the project. The authors would like to express their special thanks to Dr Sumeth Subchukul (Huvepharma [Thailand] Co, Ltd) and the US Agency for International Development and Food and Agriculture Organization for providing funds. Part of this work was supported by the Huvepharma Fund; the Faculty of Veterinary Medicine, Kasetsart University; and a US Agency for International Development and Food and Agriculture Organization grant (LOA/RAP/2018/04).
Abbreviations
- ASF
African swine fever
- ASFV
African swine fever virus
- CR
consistency ratio
- DLD
Department of Livestock Development
- MACR
mean of the absolute change rate
- MCDA
multi-criteria decision analysis
- PC
percent change
- WLC
weighted linear combination
The defined risk factors of African swine fever introduced to farms, standardized factors, and weight of each factor.
Data Availability
The data that support the findings of this study are available from the Department of Livestock Development, but restrictions apply to the availability of these data, which were used under license for this study and so are not publicly available. Data are available from the authors upon reasonable request and with permission from the Department of Livestock Development.
Footnotes
Authors' Contributions: WT conceived and designed the study. WT, VW, WS, and KT generated the raw data and performed the statistical analysis. AK developed the mobile app and web application platforms. WT drafted the paper, which KL and JK critically reviewed. SK and NR provided data and facilitated the project. All authors read and approved the final manuscript.
Conflicts of Interest: None declared.
References
- 1.Costard S, Mur L, Lubroth J, Sanchez-Vizcaino J, Pfeiffer D. Epidemiology of African swine fever virus. Virus Res. 2013 Apr;173(1):191–7. doi: 10.1016/j.virusres.2012.10.030.S0168-1702(12)00420-0 [DOI] [PubMed] [Google Scholar]
- 2.Schulz K, Staubach C, Blome S. African and classical swine fever: similarities, differences and epidemiological consequences. Vet Res. 2017 Nov 28;48(1):84. doi: 10.1186/s13567-017-0490-x. https://veterinaryresearch.biomedcentral.com/articles/10.1186/s13567-017-0490-x .10.1186/s13567-017-0490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.African swine fever. OIE Terrestrial Manual. [2022-04-23]. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.09.01_ASF.pdf .
- 4.Mebus C, Arias M, Pineda J, Tapiador J, House C, Sánchez-Vizcaíno J. Survival of several porcine viruses in different Spanish dry-cured meat products. Food Chemistry. 1997 Aug;59(4):555–9. doi: 10.1016/s0308-8146(97)00006-x. [DOI] [Google Scholar]
- 5.Cwynar P, Stojkov J, Wlazlak K. African swine fever status in Europe. Viruses. 2019 Mar 30;11(4):310. doi: 10.3390/v11040310. https://www.mdpi.com/resolver?pii=v11040310 .v11040310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Vizcaíno JM, Mur L, Martínez-López B. African swine fever (ASF): five years around Europe. Vet Microbiol. 2013 Jul 26;165(1-2):45–50. doi: 10.1016/j.vetmic.2012.11.030.S0378-1135(12)00663-3 [DOI] [PubMed] [Google Scholar]
- 7.Mur L, Atzeni M, Martínez-López B, Feliziani F, Rolesu S, Sanchez-Vizcaino JM. Thirty-five-year presence of African swine fever in Sardinia: history, evolution and risk factors for disease maintenance. Transbound Emerg Dis. 2016 Apr 12;63(2):e165–77. doi: 10.1111/tbed.12264. [DOI] [PubMed] [Google Scholar]
- 8.Rowlands RJ, Michaud V, Heath L, Hutchings G, Oura C, Vosloo W, Dwarka R, Onashvili T, Albina E, Dixon LK. African swine fever virus isolate, Georgia, 2007. Emerg Infect Dis. 2008 Dec;14(12):1870–4. doi: 10.3201/eid1412.080591. doi: 10.3201/eid1412.080591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World animal health information system. World Organisation for Animal Health. [2022-04-23]. https://www.oie.int/en/what-we-do/animal-health-and-welfare/disease-data-collection/world-animal-health-information-system/
- 10.Situational updates of ASF In Asia and the Pacific. World Organisation for Animal Health. [2022-03-07]. https://rr-asia.oie.int/en/projects/asf/situational-updates-of-asf/
- 11.Guinat C, Gogin A, Blome S, Keil G, Pollin R, Pfeiffer DU, Dixon L. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet Rec. 2016 Mar 12;178(11):262–7. doi: 10.1136/vr.103593. http://europepmc.org/abstract/MED/26966305 .vr.103593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallardo C, Soler A, Nieto R, Sánchez MA, Martins C, Pelayo V, Carrascosa A, Revilla Y, Simón A, Briones V, Sánchez-Vizcaíno JM, Arias M. Experimental transmission of African swine fever (ASF) low virulent isolate NH/P68 by surviving pigs. Transbound Emerg Dis. 2015 Dec 03;62(6):612–22. doi: 10.1111/tbed.12431. [DOI] [PubMed] [Google Scholar]
- 13.Maes D, Nauwynck H, Rijsselaere T, Mateusen B, Vyt P, de Kruif A, Van Soom A. Diseases in swine transmitted by artificial insemination: an overview. Theriogenology. 2008 Nov;70(8):1337–45. doi: 10.1016/j.theriogenology.2008.06.018.S0093-691X(08)00402-0 [DOI] [PubMed] [Google Scholar]
- 14.Baldacchino F, Muenworn V, Desquesnes M, Desoli F, Charoenviriyaphap T, Duvallet G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite. 2013 Aug 29;20:26. doi: 10.1051/parasite/2013026. http://publications.edpsciences.org/10.1051/parasite/2013026 .parasite130035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellor P, Kitching R, Wilkinson P. Mechanical transmission of capripox virus and African swine fever virus by Stomoxys calcitrans. Res Vet Sci. 1987 Jul;43(1):109–12. doi: 10.1016/s0034-5288(18)30753-7. [DOI] [PubMed] [Google Scholar]
- 16.Costard S, Wieland B, de Glanville W, Jori F, Rowlands R, Vosloo W, Roger F, Pfeiffer DU, Dixon LK. African swine fever: how can global spread be prevented? Philos Trans R Soc Lond B Biol Sci. 2009 Sep 27;364(1530):2683–96. doi: 10.1098/rstb.2009.0098. http://europepmc.org/abstract/MED/19687038 .364/1530/2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dione MM, Dohoo I, Ndiwa N, Poole J, Ouma E, Amia WC, Wieland B. Impact of participatory training of smallholder pig farmers on knowledge, attitudes and practices regarding biosecurity for the control of African swine fever in Uganda. Transbound Emerg Dis. 2020 Nov 17;67(6):2482–93. doi: 10.1111/tbed.13587. http://europepmc.org/abstract/MED/32311216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chilundo AG, Mukaratirwa S, Pondja A, Afonso S, Alfredo Z, Chato E, Johansen MV. Smallholder pig farming education improved community knowledge and pig management in Angónia district, Mozambique. Trop Anim Health Prod. 2020 May 22;52(3):1447–57. doi: 10.1007/s11250-019-02148-x.10.1007/s11250-019-02148-x [DOI] [PubMed] [Google Scholar]
- 19.Dione MM, Akol J, Roesel K, Kungu J, Ouma EA, Wieland B, Pezo D. Risk factors for African swine fever in smallholder pig production systems in Uganda. Transbound Emerg Dis. 2017 Jun 12;64(3):872–82. doi: 10.1111/tbed.12452. [DOI] [PubMed] [Google Scholar]
- 20.Izzati UZ, Inanaga M, Hoa NT, Nueangphuet P, Myint O, Truong QL, Lan NT, Norimine J, Hirai T, Yamaguchi R. Pathological investigation and viral antigen distribution of emerging African swine fever in Vietnam. Transbound Emerg Dis. 2021 Jul 07;68(4):2039–50. doi: 10.1111/tbed.13851. http://europepmc.org/abstract/MED/32979250 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao D, Liu R, Zhang X, Li F, Wang J, Zhang J, Liu X, Wang L, Zhang J, Wu X, Guan Y, Chen W, Wang X, He X, Bu Z. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg Microbes Infect. 2019 Mar 22;8(1):438–47. doi: 10.1080/22221751.2019.1590128. https://www.tandfonline.com/doi/full/10.1080/22221751.2019.1590128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woźniakowski G, Fraczyk M, Niemczuk K, Pejsak Z. Selected aspects related to epidemiology, pathogenesis, immunity, and control of African swine fever. J Vet Res. 2016;60:119–25. doi: 10.1515/jvetres-2016-0017. https://www.sciendo.com/article/10.1515/jvetres-2016-0017# . [DOI] [Google Scholar]
- 23.Huang IB, Keisler J, Linkov I. Multi-criteria decision analysis in environmental sciences: ten years of applications and trends. Sci Total Environ. 2011 Sep 01;409(19):3578–94. doi: 10.1016/j.scitotenv.2011.06.022.S0048-9697(11)00646-2 [DOI] [PubMed] [Google Scholar]
- 24.Gerber PJ, Carsjens GJ, Pak-uthai T, Robinson TP. Decision support for spatially targeted livestock policies: diverse examples from Uganda and Thailand. Agricultural Syst. 2008 Mar;96(1-3):37–51. doi: 10.1016/j.agsy.2007.05.004. [DOI] [Google Scholar]
- 25.IDRISI Kilimanjaro : Guide to GIS and Image Processing. Worcester, Mass: Clark University; 2003. [Google Scholar]
- 26.IDRISI Image Processing in TerrSet 2020. Clark Labs. [2022-04-23]. https://clarklabs.org/terrset/idrisi-image-processing/
- 27.Ishizaka A, Labib A. Review of the main developments in the analytic hierarchy process. Expert Syst App. 2011;38(11):14336–45. doi: 10.1016/j.eswa.2011.04.143. [DOI] [Google Scholar]
- 28.Chen Y, Yu J, Khan S. Spatial sensitivity analysis of multi-criteria weights in GIS-based land suitability evaluation. Environ Model Softw. 2010 Dec;25(12):1582–91. doi: 10.1016/j.envsoft.2010.06.001. [DOI] [Google Scholar]
- 29.Rosenbloom E. A probabilistic interpretation of the final rankings in AHP. Eur J Operation Res. 1997 Jan;96(2):371–8. doi: 10.1016/s0377-2217(96)00049-5. [DOI] [Google Scholar]
- 30.Decision Aids for Selection Problems. New York: Springer; 1996. The analytic hierarchy process. [Google Scholar]
- 31.Malczewski J. GIS-based land-use suitability analysis: a critical overview. Progress Planning. 2004 Jul;62(1):3–65. doi: 10.1016/j.progress.2003.09.002. [DOI] [Google Scholar]
- 32.Frey DD, Engelhardt F, Greitzer EM. A role for "one-factor-at-a-time" experimentation in parameter design. Res Eng Design. 2003 Feb 1;14(2):65–74. doi: 10.1007/s00163-002-0026-9. [DOI] [Google Scholar]
- 33.ASF Contingency Plan and Clinical Practice Guideline. Bangkok, Thailand: Department of Livestock Development; 2019. [Google Scholar]
- 34.Xu E, Zhang H. Spatially-explicit sensitivity analysis for land suitability evaluation. Applied Geography. 2013 Dec;45:1–9. doi: 10.1016/j.apgeog.2013.08.005. [DOI] [Google Scholar]
- 35.Ligmann-Zielinska A, Jankowski P. Spatially-explicit integrated uncertainty and sensitivity analysis of criteria weights in multicriteria land suitability evaluation. Environ Model Softw. 2014 Jul;57:235–47. doi: 10.1016/j.envsoft.2014.03.007. [DOI] [Google Scholar]
- 36.Paul MC, Goutard FL, Roulleau F, Holl D, Thanapongtharm W, Roger FL, Tran A. Quantitative assessment of a spatial multicriteria model for highly pathogenic avian influenza H5N1 in Thailand, and application in Cambodia. Sci Rep. 2016 Aug 04;6(1):31096. doi: 10.1038/srep31096. doi: 10.1038/srep31096.srep31096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Introducing StoryMaps. Esri. [2022-04-23]. http://www.esri.com/
- 38.Log In. e-Movement. [2022-05-06]. https://tinyurl.com/bdf4uh6b .
- 39.Asambe A, Sackey AK, Tekdek LB. Sanitary measures in piggeries, awareness, and risk factors of African swine fever in Benue State, Nigeria. Trop Anim Health Prod. 2019 May 19;51(4):997–1001. doi: 10.1007/s11250-018-1764-7. http://europepmc.org/abstract/MED/30569230 .10.1007/s11250-018-1764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Understanding and combatting African Swine Fever A European perspective. The Netherlands: Wageningen Academic Publishers; 2021. Biosecurity measures against African swine fever in domestic pigs. [Google Scholar]
- 41.Kabuuka T, Kasaija P, Mulindwa H, Shittu A, Bastos A, Fasina F. Drivers and risk factors for circulating African swine fever virus in Uganda, 2012-2013. Res Vet Sci. 2014 Oct;97(2):218–25. doi: 10.1016/j.rvsc.2014.07.001.S0034-5288(14)00202-1 [DOI] [PubMed] [Google Scholar]
- 42.Nantima N, Ocaido M, Ouma E, Davies J, Dione M, Okoth E, Mugisha A, Bishop R. Risk factors associated with occurrence of African swine fever outbreaks in smallholder pig farms in four districts along the Uganda-Kenya border. Trop Anim Health Prod. 2015 Mar 24;47(3):589–95. doi: 10.1007/s11250-015-0768-9. [DOI] [PubMed] [Google Scholar]
- 43.Kukielka EA, Jori F, Martínez-López B, Chenais E, Masembe C, Chavernac D, Ståhl K. Wild and domestic pig interactions at the wildlife-livestock interface of Murchison Falls national park, Uganda, and the potential association with African swine fever outbreaks. Front Vet Sci. 2016 Apr 14;3:31. doi: 10.3389/fvets.2016.00031. doi: 10.3389/fvets.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laddomada A, Rolesu S, Loi F, Cappai S, Oggiano A, Madrau MP, Sanna ML, Pilo G, Bandino E, Brundu D, Cherchi S, Masala S, Marongiu D, Bitti G, Desini P, Floris V, Mundula L, Carboni G, Pittau M, Feliziani F, Sanchez-Vizcaino JM, Jurado C, Guberti V, Chessa M, Muzzeddu M, Sardo D, Borrello S, Mulas D, Salis G, Zinzula P, Piredda S, De Martini A, Sgarangella F. Surveillance and control of African swine fever in free-ranging pigs in Sardinia. Transbound Emerg Dis. 2019 May 25;66(3):1114–9. doi: 10.1111/tbed.13138. http://europepmc.org/abstract/MED/30715791 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fasina FO, Kissinga H, Mlowe F, Mshang'a S, Matogo B, Mrema A, Mhagama A, Makungu S, Mtui-Malamsha N, Sallu R, Misinzo G, Magidanga B, Kivaria F, Bebay C, Nong'ona S, Kafeero F, Nonga H. Drivers, risk factors and dynamics of African swine fever outbreaks, Southern Highlands, Tanzania. Pathogens. 2020 Feb 25;9(3):155. doi: 10.3390/pathogens9030155. https://www.mdpi.com/resolver?pii=pathogens9030155 .pathogens9030155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.European Food Safety Authority Evaluation of possible mitigation measures to prevent introduction and spread of African swine fever virus through wild boar. EFSA J. 2014 Mar;12(3):3616. doi: 10.2903/j.efsa.2014.3616. [DOI] [Google Scholar]
- 47.Dione M, Ouma E, Opio F, Kawuma B, Pezo D. Qualitative analysis of the risks and practices associated with the spread of African swine fever within the smallholder pig value chains in Uganda. Prev Vet Med. 2016 Dec 01;135:102–12. doi: 10.1016/j.prevetmed.2016.11.001.S0167-5877(16)30518-9 [DOI] [PubMed] [Google Scholar]
- 48.Bellini S, Rutili D, Guberti V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet Scand. 2016 Nov 29;58(1):82. doi: 10.1186/s13028-016-0264-x. https://actavetscand.biomedcentral.com/articles/10.1186/s13028-016-0264-x .10.1186/s13028-016-0264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fasina F, Agbaje M, Ajani F, Talabi O, Lazarus D, Gallardo C, Thompson P, Bastos A. Risk factors for farm-level African swine fever infection in major pig-producing areas in Nigeria, 1997-2011. Prev Vet Med. 2012 Nov 01;107(1-2):65–75. doi: 10.1016/j.prevetmed.2012.05.011.S0167-5877(12)00167-5 [DOI] [PubMed] [Google Scholar]
- 50.Heilmann M, Lkhagvasuren A, Adyasuren T, Khishgee B, Bold B, Ankhanbaatar U, Fusheng G, Raizman E, Dietze K. African swine fever in Mongolia: course of the epidemic and applied control measures. Vet Sci. 2020 Feb 17;7(1):24. doi: 10.3390/vetsci7010024. https://www.mdpi.com/resolver?pii=vetsci7010024 .vetsci7010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kouakou K, Michaud V, Biego H, Gnabro H, Kouakou A, Mossoun A, Awuni J, Minoungou G, Aplogan G, Awoumé FK, Albina E, Lancelot R, Couacy-Hymann E. African and classical swine fever situation in Ivory-Coast and neighboring countries, 2008-2013. Acta Trop. 2017 Feb;166:241–8. doi: 10.1016/j.actatropica.2016.10.027.S0001-706X(16)30610-6 [DOI] [PubMed] [Google Scholar]
- 52.Gulenkin V, Korennoy F, Karaulov A, Dudnikov S. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Prev Vet Med. 2011 Dec 01;102(3):167–74. doi: 10.1016/j.prevetmed.2011.07.004.S0167-5877(11)00230-3 [DOI] [PubMed] [Google Scholar]
- 53.Oganesyan A, Petrova O, Korennoy F, Bardina N, Gogin A, Dudnikov S. African swine fever in the Russian Federation: spatio-temporal analysis and epidemiological overview. Virus Res. 2013 Apr;173(1):204–11. doi: 10.1016/j.virusres.2012.12.009.S0168-1702(12)00471-6 [DOI] [PubMed] [Google Scholar]
- 54.Martínez-López B, Perez AM, Feliziani F, Rolesu S, Mur L, Sánchez-Vizcaíno JM. Evaluation of the risk factors contributing to the African swine fever occurrence in Sardinia, Italy. Front Microbiol. 2015 Apr 14;6:314. doi: 10.3389/fmicb.2015.00314. doi: 10.3389/fmicb.2015.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guinat C, Gubbins S, Vergne T, Gonzales JL, Dixon L, Pfeiffer DU. Experimental pig-to-pig transmission dynamics for African swine fever virus, Georgia 2007/1 strain. Epidemiol Infect. 2015 May 20;144(1):25–34. doi: 10.1017/s0950268815000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dixon LK, Stahl K, Jori F, Vial L, Pfeiffer DU. African swine fever epidemiology and control. Annu Rev Anim Biosci. 2020 Feb 15;8(1):221–46. doi: 10.1146/annurev-animal-021419-083741. [DOI] [PubMed] [Google Scholar]
- 57.Stärk KD, Regula G, Hernandez J, Knopf L, Fuchs K, Morris RS, Davies P. Concepts for risk-based surveillance in the field of veterinary medicine and veterinary public health: review of current approaches. BMC Health Serv Res. 2006 Feb 28;6(1):20. doi: 10.1186/1472-6963-6-20. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-6-20 .1472-6963-6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Risk analysis. OIE - Terrestrial Animal Health Code. [2022-04-23]. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_import_risk_analysis.pdf .
- 59.de Glanville WA, Vial L, Costard S, Wieland B, Pfeiffer DU. Spatial multi-criteria decision analysis to predict suitability for African swine fever endemicity in Africa. BMC Vet Res. 2014 Jan 09;10(1):9. doi: 10.1186/1746-6148-10-9. https://bmcvetres.biomedcentral.com/articles/10.1186/1746-6148-10-9 .1746-6148-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spatial risk assessment of ASF in Thailand using MCDA technique. Spatial risk assessment of ASF in Thailand. [2022-03-07]. https://drive.google.com/file/d/1zgS3R6v2_0Eilqdl26RMASopoWPxvPye/view .
- 61.Spatial Analysis in Epidemiology. Online: Oxford Scholarship Online; 2008. [Google Scholar]
- 62.Thanapongtharm W, Paul MC, Wiratsudakul A, Wongphruksasoong V, Kalpravidh W, Wongsathapornchai K, Damrongwatanapokin S, Schar D, Gilbert M. A spatial assessment of Nipah virus transmission in Thailand pig farms using multi-criteria decision analysis. BMC Vet Res. 2019 Mar 04;15(1):73. doi: 10.1186/s12917-019-1815-y. https://bmcvetres.biomedcentral.com/articles/10.1186/s12917-019-1815-y .10.1186/s12917-019-1815-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran A, Trevennec C, Lutwama J, Sserugga J, Gély M, Pittiglio C, Pinto J, Chevalier V. Development and assessment of a geographic knowledge-based model for mapping suitable areas for rift valley fever transmission in Eastern Africa. PLoS Negl Trop Dis. 2016 Sep 15;10(9):e0004999. doi: 10.1371/journal.pntd.0004999. https://dx.plos.org/10.1371/journal.pntd.0004999 .PNTD-D-16-00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bech-Nielsen S, Perez Bonilla Q, Sanchez-Vizcaino J. Benefit-cost analysis of the current African swine fever eradication program in Spain and of an accelerated program. Prev Vet Med. 1993 Nov;17(3-4):235–49. doi: 10.1016/0167-5877(93)90032-o. [DOI] [Google Scholar]
- 65.Bech-Nielsen S, Arias ML, Panadero J, Escribano JM, Gomez-Tejedor C, Perez Bonilla Q, Sanchez-Vizcaino J. Laboratory diagnosis and disease occurrence in the current African swine fever eradication program in Spain, 1989–1991. Prev Vet Med. 1993 Nov;17(3-4):225–34. doi: 10.1016/0167-5877(93)90031-n. [DOI] [Google Scholar]
- 66.Lichoti JK, Davies J, Maru Y, Kitala PM, Githigia SM, Okoth E, Bukachi SA, Okuthe S, Bishop RP. Pig traders' networks on the Kenya-Uganda border highlight potential for mitigation of African swine fever virus transmission and improved ASF disease risk management. Prev Vet Med. 2017 May 01;140:87–96. doi: 10.1016/j.prevetmed.2017.03.005.S0167-5877(17)30224-6 [DOI] [PubMed] [Google Scholar]
- 67.Penrith M, Vosloo W. Review of African swine fever: transmission, spread and control. J S Afr Vet Assoc. 2009 Jun 22;80(2):58–62. doi: 10.4102/jsava.v80i2.172. [DOI] [PubMed] [Google Scholar]
- 68.Biosecurity for highly pathogenic avian influenza. FAO Animal Production and Health Paper. [2022-05-06]. https://www.fao.org/3/i0359e/i0359e00.htm .
- 69.Good practices for biosecurity in the pig sector. The Pig Site. [2022-04-23]. https://www.thepigsite.com/articles/good-practices-for-biosecurity-in-the-pig-sector .
- 70.Commission Decision of 2 May 2005 approving the plan for the eradication of African swine fever in feral pigs in Sardinia, Italy (notified under document number C(2005) 1255) (Only the Italian text is authentic) (Text with EEA relevance) (2005/362/EC) European Union. [2022-04-23]. https://www.legislation.gov.uk/eudn/2005/362/introduction .
- 71.Thanapongtharm W, Linard C, Chinson P, Kasemsuwan S, Visser M, Gaughan AE, Epprech M, Robinson TP, Gilbert M. Spatial analysis and characteristics of pig farming in Thailand. BMC Vet Res. 2016 Oct 06;12(1):218. doi: 10.1186/s12917-016-0849-7. https://bmcvetres.biomedcentral.com/articles/10.1186/s12917-016-0849-7 .10.1186/s12917-016-0849-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Boeckel TP, Thanapongtharm W, Robinson T, D'Aietti L, Gilbert M. Predicting the distribution of intensive poultry farming in Thailand. Agric Ecosyst Environ. 2012 Mar 01;149:144–53. doi: 10.1016/j.agee.2011.12.019. http://europepmc.org/abstract/MED/22323841 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thanapongtharm W, Linard C, Pamaranon N, Kawkalong S, Noimoh T, Chanachai K, Parakgamawongsa T, Gilbert M. Spatial epidemiology of porcine reproductive and respiratory syndrome in Thailand. BMC Vet Res. 2014 Aug 05;10(1):174. doi: 10.1186/s12917-014-0174-y. https://bmcvetres.biomedcentral.com/articles/10.1186/s12917-014-0174-y .s12917-014-0174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thanapongtharm W, Van Boeckel TP, Biradar C, Xiao X, Gilbert M. Rivers and flooded areas identified by medium-resolution remote sensing improve risk prediction of the highly pathogenic avian influenza H5N1 in Thailand. Geospat Health. 2013 Nov 01;8(1):193–201. doi: 10.4081/gh.2013.66. doi: 10.4081/gh.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfeiffer DU, Minh PQ, Martin V, Epprecht M, Otte MJ. An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. Vet J. 2007 Sep;174(2):302–9. doi: 10.1016/j.tvjl.2007.05.010.S1090-0233(07)00174-8 [DOI] [PubMed] [Google Scholar]
- 76.Ma J, Chen H, Gao X, Xiao J, Wang H. African swine fever emerging in China: distribution characteristics and high-risk areas. Prev Vet Med. 2020 Feb;175:104861. doi: 10.1016/j.prevetmed.2019.104861.S0167-5877(19)30068-6 [DOI] [PubMed] [Google Scholar]
- 77.Guidelines for animal disease control. OIE World Organisation for Animal Heath. [2022-04-23]. https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/A_Guidelines_for_Animal_Disease_Control_final.pdf .
- 78.Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P, Stockton P, Rupprecht CE, Ksiazek TG, Hemachudha T. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005 Dec;11(12):1949–51. doi: 10.3201/eid1112.050613. https://wwwnc.cdc.gov/eid/article/11/12/05-0613_article.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The defined risk factors of African swine fever introduced to farms, standardized factors, and weight of each factor.
Data Availability Statement
The data that support the findings of this study are available from the Department of Livestock Development, but restrictions apply to the availability of these data, which were used under license for this study and so are not publicly available. Data are available from the authors upon reasonable request and with permission from the Department of Livestock Development.


