Abstract
In randomised, placebo- or active-controlled trials in patients with heart failure with reduced ejection fraction (HFrEF), each of the combination of a neprilysin inhibitor and an angiotensin-receptor blocker (i.e. sacubitril/valsartan), a beta blocker, a mineralocorticoidreceptor antagonist and a sodium-glucose co-transporter 2 (SGLT2) inhibitor have been shown to reduce morbidity and mortality, firmly establishing the role of these five agents, prescribed as four pills, as foundational therapy for HFrEF. Traditionally, the guideline-advocated strategy for the initiation of these therapies was based on the historical order in which the landmark clinical trials were performed, and the requirement to uptitrate each individual drug to the target dose (or maximally tolerated dose below this) prior to initiation of another therapy. This process could take six months or more to complete, during which time patients would not be taking one or more of these life-saving drugs. Recently an alternative, evidence-based, rapid three-step sequencing strategy has been proposed with the aim of establishing HFrEF patients on low-doses of all four foundational treatments within four weeks. This strategy is based on the premise that the benefits of each of these therapies are independent and additive to the others, the benefits are apparent at low doses early following initiation, and a specific ordering of therapies may increase likelihood of tolerance of others. This article will outline this novel rapid-sequencing strategy and provide an evidence-based framework to support its adoption into clinical practice.
Key words: angiotensin-receptor blocker/neprilysin inhibitor, beta blocker, clinical trials, heart failure with reduced ejection fraction (HFrEF), mineralocorticoid-receptor antagonist, sodium-glucose co-transporter 2 (SGLT2) inhibitor
Introduction
To date, five pharmacological approaches have been demonstrated to significantly reduce the risk of mortality and prevent hospitalisation for worsening heart failure (HF) in patients with HF with reduced ejection fraction (HFrEF): the combination of a neprilysin inhibitor and an angiotensin-receptor blocker (i.e. sacubitril/ valsartan), a beta blocker, a mineralocorticoid-receptor antagonist (MRA) and a sodium-glucose co-transporter 2 (SGLT2) inhibitor. Hereafter, these five agents, which can be prescribed as four pills, will be referred to as the ‘four foundational therapies for HFrEF’.1-11 The combination of these four therapies has been shown to provide clinically meaningful gains in survival free from worsening HF and prolong life-expectancy in patients with HFrEF.12 Until recently, based on the order in which the landmark trials demonstrating the benefit of these drugs were performed, the guideline-advocated approach when initiating these therapies was to introduce one treatment at a time in the order in which they were studied in clinical trials. Moreover, each new drug was only added when the dose of the prior therapy had been up-titrated to the dose shown to be efficacious in the relevant trials (or maximally tolerated dose below that).13,14 In practice, this would mean starting with an angiotensin-converting enzyme (ACE) inhibitor (or angiotensin-receptor blocker [ARB]) and up-titrating to the target dose, then adding a beta blocker and up-titrating the dose, followed by a MRA, a neprilysin inhibitor (added by switching from an ACE inhibitor or ARB) and, finally, an SGLT2 inhibitor. In most patients, this process could take six months or more to complete, during which they would not be taking one or more of these life-saving drugs for much of the time. In a challenge to this widely accepted paradigm, it has been recently suggested that a novel rapid-sequencing strategy should be adopted in which the aim should be to establish patients on low doses of each of the four foundational treatments within four weeks of initiation.15,16 This alternative strategy means that patients are exposed to the individual additive benefits of each class of drug, which are evident within weeks of starting, even at the initiating or sub-target dose. This article will outline this novel rapid-sequencing strategy and provide evidence to support its adoption into clinical practice.
Foundational therapy for HFrEF
Each of the four foundational pharmacological therapies for HFrEF has been shown in large, randomised, placebo- or active-controlled trials to reduce the risk of cardiovascular and/or all-cause mortality, and the risk of hospitalisation for worsening HF.1-11 These benefits have been demonstrated in two or more trials (i.e. providing external validity) or, in the case of sacubitril/valsartan, in one trial with a degree of statistical significance (p<0.00125) that is equivalent to that of at least two individual confirmatory trials. Furthermore, many of these treatments have been shown to have similar benefits in reducing mortality and the development of chronic HF in patients with left ventricular systolic dysfunction and/or HF at the time of acute myocardial infarction, a common pathological precursor to the development of HFrEF.17-22
Along with the four foundational treatments for HFrEF, other medications, including digoxin, ivabradine, vericiguat, omecamtiv mecarbil and the combination of hydralazine and isosorbide dinitrate, are considered second-line therapies. Indeed, in the recently updated European Society of Cardiology (ESC) guidelines for the management of HF, each of these medications are given a class II indication.23 Reasons for this weaker indication (rather than a class I indication as for the four foundational treatments) include a relatively small treatment effect, an effect on HF hospitalisation with no accompanying cardiovascular mortality benefit, or the suggestion of benefit only in certain subgroups of HFrEF patients.
Conventional sequencing of foundational drugs for HFrEF
Until recently, international guidelines for the management of HFrEF advocated a stepwise sequencing strategy when establishing patients on foundational HFrEF treatments.13,14 The recommended order of therapy initiation (renin–angiotensin system [RAS] inhibitor and beta blocker, followed by a MRA, a neprilysin inhibitor and a SGLT2 inhibitor) was based, not on clinical trial evidence supporting that order, but simply on the historical sequence in which the landmark evidence-generating trials were conducted. This strategy assumes, incorrectly, that the treatments studied earlier are more efficacious (and their initiation be prioritised) than those discovered more recently. This can be challenged by the observation that the use of digitalis, taken by >90% of patients at baseline in CONSENSUS (Cooperative North Scandinavian Enalapril Survival Study), has significantly declined and is no longer considered as a foundational treatment for HFrEF based on the results of the Digitalis Investigation Group (DIG) trial.24
A second aspect of the conventional approach was prioritising the up-titration of the dose of each individual drug class to the target dose (or maximally tolerated dose below that) before adding a new medication. During this process, the delay in initiating all four life-saving treatments potentially results in unnecessary hospitalisations and deaths.
The assumption that the recommended drug classes do not exert their benefits until they are up-titrated to maximum doses is contrary to the body of evidence demonstrating that low or starting doses of ACE inhibitors, beta blockers, MRAs and sacubitril/valsartan provide protection from death and reduce the risk of worsening HF. Indeed, each of the four classes of foundational therapies has been shown to exert their benefit within 30 days of initiation (i.e. when the patients were taking a low dose or before any up-titration).25-29 Furthermore, the additive benefits of the dose up-titration of a drug are relatively modest and limited to a reduction in the risk of hospitalisation compared with the benefits of adding a new medication; in the Assessment of Treatment with Lisinopril and Survival (ATLAS) and Heart failure Endpoint evaluation of Angiotensin II Antagonist Losartan (HEAAL) trials, a three-to seven-fold higher dose of a RAS inhibitor did not confer a mortality benefit, unlike the addition of a beta blocker, a MRA, a neprilysin inhibitor or a SGLT2 inhibitor in their respective landmark trials.30,31
Finally, the conventional strategy is based on the assumption that the benefits of new therapies were demonstrated in landmark trials when given to patients who were taking all other background therapies at the maximal dose, therefore, mandating this as a prerequisite before adding in any new medication. We know this not to be the case, as a significant proportion of patients in the randomised-controlled trials detailed above were not on the maximum doses of background therapy when each new additional foundational treatment was studied.32,33 Furthermore, not all patients in PARADIGM-HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) were taking a MRA and a relatively small proportion of patients in the SGLT2 inhibitor trials were taking a neprilysin inhibitor.9-11 By examining the totality of evidence (figure 1) it is quite clear that the benefits of each foundational drug class are independent and additive to the others, i.e. the benefit of each foundational therapy is not contingent on the patient taking the other treatments at target doses. This is perhaps no surprise given that each drug class has a distinct mechanism of action; RAS inhibitors, beta blockers and MRA independently antagonise the maladaptive neurohumoral activation that occurs in HF, neprilysin inhibitors augment endogenous levels of vasoactive peptides, including the natriuretic peptides, and SGLT2 inhibitors have a wide range of proposed mechanisms of action.34,35 A further limitation of the conventional strategy described, is that it is both time and labour intensive, requiring multiple clinic visits. This may explain why, despite a wealth of evidence supporting their benefits, few patients either receive or are on maximally tolerated doses of all four foundational therapies.36,37
Figure 1. Evidence for the independent benefits of foundational drugs for heart failure with reduced ejection fraction (HFrEF).
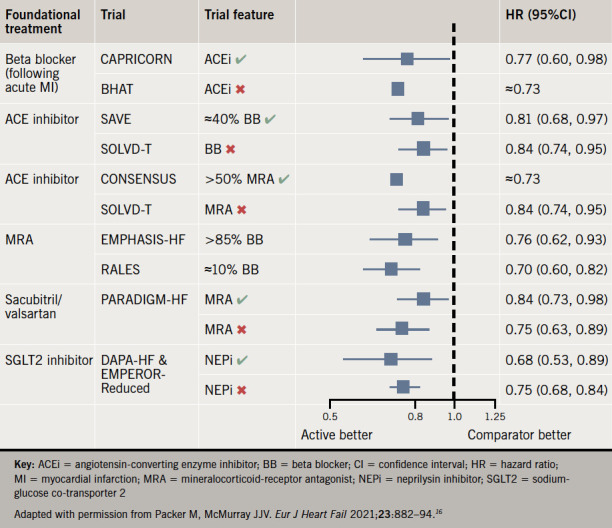
Key: ACEi = angiotensin-converting enzyme inhibitor; BB = beta blocker; CI = confidence interval; HR = hazard ratio; MI = myocardial infarction; MRA = mineralocorticoid-receptor antagonist; NEPi = neprilysin inhibitor; SGLT2 = sodiumglucose co-transporter 2
Adapted with permission from Packer M, McMurray JJV. Eur J Heart Fail 2021;23:882–94.16
Proposed new rapid-sequencing strategy
A novel sequencing strategy (figure 2) has recently been proposed that aims to establish patients with HFrEF on all four foundational guideline-recommended treatments within 30 days.15,16 This proposal is based on a series of evidence-based principles. First, that the order of therapy initiation should not be dictated by historical precedent but rather by factors including the magnitude of reduction in risk of mortality and the ability of one treatment to enhance the tolerability and safety of others. Second, that the foundational treatments exert clinically important benefits early following initiation, and when taken at a low dose. Third, a previously commenced treatment does not require to be up-titrated to the target dose before initiation of subsequent therapies, as the additive benefit provided by dose up-titration is not greater (and may even be less) than that of the addition of a new medication. Finally, that the benefit of each treatment is independent and additive to that of the others.
Figure 2. Conventional and rapid sequencing of foundational drugs16.
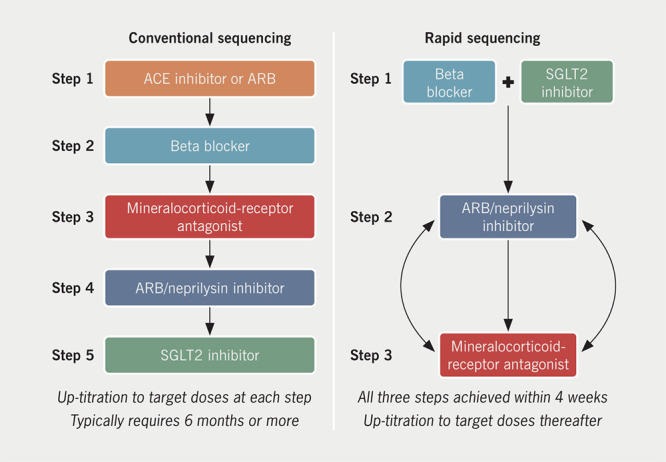
Key: ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker; SGLT2 = sodium-glucose co-transporter 2
Reproduced with permission from Packer M, McMurray JJV. Eur J Heart Fail 2021;23:882–94.16
Based on these underlying principles, the proposed new rapid-sequencing strategy suggests a three-step process aiming to achieve the target of all four foundational treatments initiated within four weeks, with further dose up-titration occurring after this. In other words, starting all four treatments is prioritised over the up-titration of any single treatment.
Step 1
Initiation of a low dose of an evidence-based beta blocker (bisoprolol, metoprolol succinate or carvedilol) along with a SGLT2 inhibitor (dapagliflozin or empagliflozin) in patients who are assessed to be clinically euvolaemic. Beta blockers are suggested as one of the two first-line medications due to the early and large magnitude of benefit in reducing the risk of sudden death.27 The CIBIS (Cardiac Insufficiency Bisoprolol Study) III trial, which reported that initiation of treatment with a beta blocker was safe and non-inferior to initiation of an ACE inhibitor, provides support to this strategy.38 CIBIS III also provides a rationale for combining a beta blocker and a SGLT2 inhibitor as the first step in the algorithm; the increased risk of non-fatal HF hospitalisation seen early after initiation of a beta blocker in CIBIS III may be offset by the substantial effect that SGLT2 inhibitors have in reducing the risk of worsening HF, as well as SGLT2 inhibitor’s potential short-term diuretic effect.39,40 A further, attractive benefit of the early initiation of a SGLT2 inhibitor is their renoprotective effect in attenuating the decline in renal function over time, which is seen in patients with HF.11,41
Step 2
Initiation of a combined ARB/neprilysin inhibitor in the form of sacubitril/valsartan, one to two weeks following Step 1. Sacubitril/ valsartan has an additive effect to RAS inhibition alone, with a comparable effect on reducing the risk of mortality to beta blockers, and a significant effect on both major modes of death in HFrEF, death from progressive HF and sudden cardiac death.9,42 Furthermore, compared with an ACE inhibitor alone, sacubitril/valsartan attenuates the decline in renal function over time; this, along with the observation that both sacubitril/ valsartan and SGLT2 inhibitors reduce the risk of hyperkalaemia and increase tolerance of a MRA, means that establishing patients on both of these medications may increase the subsequent likelihood of safely introducing and maintaining a patient on a MRA.43-45 In a subset of patients with low systolic blood pressure (<100 mmHg), tolerance with regards to hypotension can be first assessed with a low dose of an ARB with the addition of a neprilysin inhibitor once tolerance is established (a test of an ARB is preferred to an ACE inhibitor as the latter requires a minimum 36-hour washout before starting sacubitril/valsartan to minimise the risk of angioedema). Early hypotensive effects frequently resolve with repeated dosing and may sometimes require de-escalation of diuretic therapy.46
Step 3
Introduction of a MRA a further one to two weeks following Step 2 providing that updated renal function and serum potassium measurements are within the acceptable limits for commencing a MRA, i.e. potassium ≤5.0 mmol/L and estimated glomerular filtration rate (eGFR) ≥30 ml/min/1.73 m2. The initiation of a MRA once patients are established on a neprilysin inhibitor and a SGLT2 inhibitor is expected to increase the likelihood of tolerance, for the reasons detailed above, regarding attenuation of worsening renal function and the risk of hyperkalaemia (both of which can be exacerbated by a MRA). In select patients in whom Step 2 consists of adding a neprilysin inhibitor to a longstanding RAS inhibitor, then Steps 2 and 3 can be consolidated into a single step as the initiation of a neprilysin inhibitor and a MRA at the same time is not expected to result in an increase in adverse effects in patients who are already established on a RAS inhibitor. Furthermore, in patients who have difficulties with symptomatic hypotension, Step 2 and Step 3 can be reversed (i.e. a MRA introduced before a neprilysin inhibitor).
Following the completion of these three steps, the four foundational drugs should be up-titrated to the target doses studied in clinical trials or the maximally tolerated dose below (this does not apply to SGLT2 inhibitors as these are used in a single dose and do not require titration).
Conclusion
There is now unequivocal evidence that an ARB/neprilysin inhibitor, a beta blocker, a MRA and a SGLT2 inhibitor should be considered as foundational treatments for HFrEF. Each of these four drug classes provides independent and additive benefits to the others, which are realised early following the initiation of treatment. The onus is now on members of the HF multi-disciplinary team to ensure rapid and safe implementation of these four foundational HFrEF treatments. Although the evidence-based strategy described above may not be possible in all patients, it can be employed in many, and has the potential to prolong survival, prevent hospitalisations, reduce symptom burden, and improve quality of life
Key messages
The foundational pharmacological treatments for heart failure with reduced ejection fraction (HFrEF) consist of the combination of a neprilysin inhibitor and an angiotensin-receptor blocker, i.e. sacubitril/valsartan, a beta blocker, a mineralocorticoid-receptor antagonist (MRA) and a sodium-glucose cotransporter 2 (SGLT2) inhibitor, i.e. five agents given in four tablets
Each of these therapies offers independent and additive benefits in reducing the risk of death and hospitalisation for heart failure, which are realised early following initiation of therapy and at low doses
During the initiation of these treatments we should, therefore, prioritise establishing patients on low doses of all four foundational treatments within four weeks rather than the traditional sequencing strategy of starting each individual medication and up-titrating to maximally tolerated/target dose prior to adding a new therapy
Funding Statement
Sponsorship statement AstraZeneca has provided a sponsorship grant towards this independent programme.
Funding AstraZeneca has provided a sponsorship grant towards this independent programme.
Footnotes
Conflicts of interest
KFD has received personal lecture fees from AstraZeneca and his employer, the University of Glasgow, has received payment from AstraZeneca for his work on the DAPA-HF trial. JJVM is supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217; his employer, Glasgow University, has received payment for his work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Cardurion, Cytokinetics, GlaxoSmithKline, Novartis, Pfizer, Theracos; and he has received personal lecture fees from the Corpus, Abbott, Hickma, Sun Pharmaceuticals, and Medscape.
Contributor Information
Kieran F Docherty, Cardiology Specialist Registrar and Clinical Lecturer Institute of Cardiovascular and Medical Sciences, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, G12 8TA.
John J V McMurray, Professor of Medical Cardiology Institute of Cardiovascular and Medical Sciences, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, G12 8TA.
References
- 1.CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 2.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, McMurray JJV, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 4.Dargie HJ, Lechat P. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. doi: 10.1016/S0140-6736(05)74355-5. [DOI] [PubMed] [Google Scholar]
- 5.MERIT-HF Study Group Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. doi: 10.1016/S0140-6736(99)04440-2. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–168. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 8.Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 12.Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128. doi: 10.1016/S0140-6736(20)30748-0. [DOI] [PubMed] [Google Scholar]
- 13.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 15.McMurray JJV, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction? Circulation. 2021;143:875–877. doi: 10.1161/CIRCULATIONAHA.120.052926. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, McMurray JJV. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23:882–894. doi: 10.1002/ejhf.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 18.The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet. 1993;342:821–828. doi: 10.1016/0140-6736(93)92693-N. [DOI] [PubMed] [Google Scholar]
- 19.Køber L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 21.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/S0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 22.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 23.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 24.Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 25.Lam P, Packer M, Fonarow GC, et al. Early effects of starting doses of enalapril in patients with chronic heart failure in the SOLVD treatment trial. Am J Med. 2020;133:e25–e31. doi: 10.1016/j.amjmed.2019.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitt B, White H, Nicolau J, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–431. doi: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS study. JAMA. 2003;289:712–718. doi: 10.1001/jama.289.6.712. [DOI] [PubMed] [Google Scholar]
- 28.Packer M, McMurray JJV, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]
- 29.Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/ valsartan or enalapril in the PIONEER-HF trial. Circulation. 2019;139:2285–2288. doi: 10.1161/CIRCULATIONAHA.118.039331. [DOI] [PubMed] [Google Scholar]
- 30.Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100:2312–2318. doi: 10.1161/01.CIR.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 31.Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 32.Okumura N, Jhund PS, Gong J, et al. Effects of sacubitril/valsartan in the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Heart Fail. 2016;9:e003212. doi: 10.1161/CIRCHEARTFAILURE.116.003212. [DOI] [PubMed] [Google Scholar]
- 33.Docherty KF, Jhund PS, Inzucchi SE, et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J. 2020;41:2379–2392. doi: 10.1093/eurheartj/ehaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Docherty KF, Vaduganathan M, Solomon SD, McMurray JJV. Sacubitril/valsartan: neprilysin inhibition 5 years after PARADIGM-HF. JACC Heart Fail. 2020;8:800–810. doi: 10.1016/j.jchf.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 36.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 37.Tay WT, Shimizu W, Anand I, et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. 2018;6:e1008–e1018. doi: 10.1016/S2214-109X(18)30306-1. [DOI] [PubMed] [Google Scholar]
- 38.Willenheimer R, Veldhuisen DJ van, Silke B, et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence. Circulation. 2005;112:2426–2435. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- 39.Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure. Circulation. 2020;142:1713–1724. doi: 10.1161/CIRCULATIONAHA.120.048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boorsma EM, Beusekamp JC, ter Maaten JM, et al. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail. 2021;23:68–78. doi: 10.1002/ejhf.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction. Circulation. 2021;143:298–309. doi: 10.1161/CIRCULATIONAHA.120.050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desai AS, McMurray JJV, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–1997. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- 43.Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Desai AS, Vardeny O, Claggett B, et al. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/ valsartan compared with enalapril: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. 2017;2:79–85. doi: 10.1001/jamacardio.2016.4733. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen SL, Docherty KF, Jhund PS, et al. Dapagliflozin reduces the risk of hyperkalaemia in patients with heart failure and reduced ejection fraction: a secondary analysis DAPA-HF. Eur Heart J. 2020;41(suppl 2):ehaa946.0939. doi: 10.1093/ehjci/ehaa946.0939. [DOI] [Google Scholar]
- 46.Vardeny O, Claggett B, Kachadourian J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail. 2019;21:337–341. doi: 10.1002/ejhf.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]


