Abstract
Low pH and salt are two factors contributing to the inactivation of bacterial pathogens during a 60-day curing period for cheese. The kinetics of inactivation for Mycobacterium avium subsp. paratuberculosis strains ATCC 19698 and Dominic were measured at 20°C under different pH and NaCl conditions commonly used in processing cheese. The corresponding D values (decimal reduction times; the time required to kill 1 log10 concentration of bacteria) were measured. Also measured were the D values for heat-treated and nonheated M. avium subsp. paratuberculosis in 50 mM acetate buffer (pH 5.0, 2% [wt/vol] NaCl) and a soft white Hispanic-style cheese (pH 6.0, 2% [wt/vol] NaCl). Samples were removed at various intervals until no viable cells were detected using the radiometric culture method (BACTEC) for enumeration of M. avium subsp. paratuberculosis. NaCl had little or no effect on the inactivation of M. avium subsp. paratuberculosis, and increasing NaCl concentrations were not associated with decreasing D values (faster killing) in the acetate buffer. Lower pHs, however, were significantly correlated with decreasing D values of M. avium subsp. paratuberculosis in the acetate buffer. The D values for heat-treated M. avium subsp. paratuberculosis ATCC 19698 in the cheese were higher than those predicted by studies done in acetate buffer. The heat-treated M. avium subsp. paratuberculosis strains had lower D values than the nonheated cells (faster killing) both in the acetate buffer (pH 5, 2% [wt/vol] NaCl) and in the soft white cheese. The D value for heat-treated M. avium subsp. paratuberculosis ATCC 19698 in the cheese (36.5 days) suggests that heat treatment of raw milk coupled with a 60-day curing period will inactivate about 103 cells of M. avium subsp. paratuberculosis per ml.
Paratuberculosis is a chronic, incurable, inflammatory bowel disease affecting ruminants. Also called Johne's disease, it is caused by infection with Mycobacterium avium subsp. paratuberculosis. The symptoms in cattle include chronic diarrhea and weight loss (37). Investigators have reported the possible etiological role of M. avium subsp. paratuberculosis in Crohn's disease, a human inflammatory bowel disease. The possible link between M. avium subsp. paratuberculosis and Crohn's disease is based on reports of sporadic isolation of the organism from Crohn's disease patient tissues (intestines and lymph nodes) and detection of a DNA insertion sequence (IS900) unique to M. avium subsp. paratuberculosis in these tissues (5–9, 12, 21, 23–26).
Dairy products such as milk and cheese have been proposed as possible sources of M. avium subsp. paratuberculosis infection. Taylor et al. (36) and Sweeney et al. (35) reported that M. avium subsp. paratuberculosis was cultured from 9 of 26 milk samples (35%) obtained from cows with clinically advanced Johne's disease, as well as 9 of 77 milk samples (11.6%) obtained from infected but clinically normal cows. Streeter et al. (32) also isolated M. avium subsp. paratuberculosis from milk samples taken from 126 clinically normal but M. avium subsp. paratuberculosis-infected cows; 2.4% of the samples collected yielded M. avium subsp. paratuberculosis.
In 1993, Chiodini and Hermon-Taylor (4) demonstrated that bovine and human strains of M. avium subsp. paratuberculosis survive pasteurization and that human strains were generally more heat resistant at a temperature of 72°C than were bovine strains. Grant et al. (14) showed that M. avium subsp. paratuberculosis strains were inactivated neither by low-temperature holding (63.5°C for 30 min) nor by high-temperature, short-time (HTST) (71.7°C for 15 s) pasteurization methods. Using standardized laboratory conditions, we previously reported the D value (decimal reduction time: the time required to kill 1 log10 concentration of bacteria) for M. avium subsp. paratuberculosis in milk (34). The thermal D value for clinical strains of M. avium subsp. paratuberculosis in milk at 71°C (D71°C) was 11.67 s, indicating that the M. avium subsp. paratuberculosis strains tested may survive HTST pasteurization methods when the initial concentration of the mycobacteria in raw milk is greater than 101 cells/ml, although the study of Stabel et al. (31) using a laboratory scale pasteurizer contradicts these findings.
Standards promulgated by the Food and Drug Administration in 1949 provided two options for producing safe cheese products: either pasteurization of milk or holding the finished cheese for at least 60 days at temperatures of 2°C (curing). A survey of cheese production in the United States 1987 (5.4 billion pounds) found that 62% were cheese varieties for which pasteurization is not mandated, albeit it is “frequently” used. It is likely, therefore, that consumers can purchase cheese products prepared using the curing process only (16).
Low pH and salt are two of most important factors contributing to inactivation of bacterial pathogens during the 60-day curing process (11, 16). Therefore, we evaluated the rates of inactivation of M. avium subsp. paratuberculosis, expressed as D values, under low-pH and various salt conditions (pH 4 to 6, NaCl concentration of 0 to 6% [wt/vol]) independently and in combination. We prepared a soft white Hispanic-style cheese using pasteurized retail milk spiked with 106 M. avium subsp. paratuberculosis cells/ml to determine inactivation rates in this type of cheese and to contrast the results with D values of M. avium subsp. paratuberculosis determined under low-pH and various salt conditions. In addition, we examined whether heat-treated M. avium subsp. paratuberculosis cells responded differently from nonheated cells in the acetate buffer (pH 5, 2% [wt/vol] NaCl) and the soft white cheese.
MATERIALS AND METHODS
M. avium subsp. paratuberculosis strains.
Two M. avium subsp. paratuberculosis strains (ATCC 19698 and Dominic) were tested. They were prepared for testing as described previously (34). Briefly, they were cultured in 100 ml of 7H9 broth medium containing 10% (vol/vol) Middlebrook OADC (oleic acid-dextrose-catalase; Difco, Detroit, Mich.), 0.5% (vol/vol) Tween 80 (Sigma, St. Louis, Mo.), and 0.0002% (wt/vol) mycobactin J (Allied Monitor Inc., Fayette, Mo.) for 4 months. The M. avium subsp. paratuberculosis cultures were centrifuged at 15,000 × g for 1 h, and the cell pellets were suspended with 30 ml of 7H9 broth and homogenized with an overhead stirrer (Wheaton Instruments, Milville, N.J.) for 4 min on ice to break up large clumps of M. avium subsp. paratuberculosis cells. As a result, the suspensions were comprised predominantly of small clumps and single cells. The homogenized cell pellet suspensions were stored at −70°C and used as the inoculum. The average time that the cell suspensions were frozen was about 1 month before use.
Menstruum.
To obtain the three desired pH levels (4.0, 5.0, and 6.0), acetate buffer (50 mM) was used as a base menstruum with starting pHs of 4.0, 5.0, and 6.0. Various NaCl concentrations (0, 2, 4, and 6% [wt/vol]) and 1% (vol/vol) lactic acid (50 mM) were added to the acetate buffers to more closely mimic conditions in cheese. The pHs of the acetate buffers were then measured and adjusted to the desired levels with 10 mM acetic acid and 10 mM sodium acetate to yield working menstruum. Changes of pH in the working menstruums after autoclaving were <0.1 pH unit. The pHs of the working menstruums were monitored with a pH meter and a pH electrode (pH meter 125; Corning, Charlotte, N.C.); the pH remained constant (to within 0.2 pH unit of the desired pH levels) throughout the duration of the survival study. The factorial experimental design used was four NaCl concentration levels times three pH levels.
Survival experiments after pH and NaCl treatments.
In order to test the rate of inactivation of M. avium subsp. paratuberculosis due to acidity (pH) and/or salt, 9 ml of the working menstruum was placed in sterilized Wheaton glass vials (3 by 6 cm) in duplicate. One milliliter of M. avium subsp. paratuberculosis culture was added to the Wheaton vial to achieve a final concentration of 106 cells/ml, and the vials were sealed. The vials were incubated at 20°C, and after thorough vortexing for 1 min, aliquots (0.5 ml) were sampled after 0, 7, 14, 28, 56, 112, 224, and 336 days of incubation from each vial for viable M. avium subsp. paratuberculosis enumeration. The identity of M. avium subsp. paratuberculosis strains was verified by testing for IS900 both before and after pH and salt survival experiments.
Survival experiments after heat, pH, and NaCl combination treatments.
M. avium subsp. paratuberculosis strains (ATCC 19698 and Dominic) were heat treated as described previously (34). In brief, after preheating lactate solution (50 mM, pH 6.8) for 30 min in a water bath adjusted to the target temperature (71 or 62°C), M. avium subsp. paratuberculosis strains (0.2 ml) were suspended with 1.3 ml of the preheated lactate solution in a Wheaton vial (13 by 20 mm) in triplicates. The final concentration of bacteria was 106 to 107 M. avium subsp. paratuberculosis cells/ml. Each vial was sealed, immersed in the water bath, and held 4 cm below the water's surface. After heating for the specific intervals at the target temperature (0, 240, 480, 720, and 960 s at 62°C and 0, 20, 40, and 60 s at 71°C), the vials were removed from the water bath and immediately chilled by immersion in ice water for 5 min. Lactate solution (1.0 ml) from each vial was collected for subsequent pH and NaCl treatment as described below, and 0.1 ml of lactate solution from each vial was inoculated into BACTEC bottles to estimate the number of M. avium subsp. paratuberculosis cells surviving the heat treatment.
The lactate solution (1.0 ml) from each heat-treated vial was transferred into a Wheaton bottle (32 by 58 mm) containing 9 ml of the acetate buffer (pH 5, 2% [wt/vol] NaCl). From each Wheaton bottle, 0.5 ml was sampled after 0, 5, 10, 15, 23, 27, and 33 days and inoculated into BACTEC bottles in triplicate to enumerate the number of heat-treated M. avium subsp. paratuberculosis cells surviving in the acetate buffer. The heat-treated M. avium subsp. paratuberculosis cells were held in the acetate buffer at 20°C.
Manufacture and sampling of cheese.
Hispanic-style soft white cheese (Queso Fresco) was made according to a traditional procedure obtained from the available literature (29) and described below. The cheese was made three times with nonheated M. avium subsp. paratuberculosis strain ATCC 19698 and three times with strain ATCC 19698 heat treated at 62°C for 240 s, resulting in six independent trials. On each trial, 1 gallon (3.8 liters) of milk was used.
One gallon of pasteurized 2% fat milk was purchased from a retail market. A portion (100 ml) was centrifuged at 15,000 × g for 1 h, and the pellet was cultured in BACTEC bottles to check for contamination with mycobacteria. Either nonheated or heat-treated M. avium subsp. paratuberculosis ATCC 19698 culture was added to the remaining milk to achieve a final concentration of 106 cells/ml. The milk was then placed in a stainless steel vat, gently agitated, and warmed to 31°C in a water bath. A 3% (vol/vol) Streptococcus thermophilus starter culture (kindly provided by Charles W. Kaspar), grown in 100 ml of 10% (wt/vol) nonfat dry milk for 24 h at 37°C, was added. After 30 min, 20 ml of distilled water and 0.4 ml of rennet was added and the agitation was stopped. The coagulum was cut with an autoclaved knife 45 min after the rennet was added. The curds and whey were heated to 37.7°C and held for 40 min, after which the whey on top of the curd was poured off. After another 30 min, the curds were placed in sterile strainers to further drain the whey. Two percent (vol/vol) salt was added to the curd, and the curds were placed in a sterilized mold and stored at 4°C for 24 h to drain the remaining whey. The cooled cheese was cut into approximately 15 pieces, and each cheese piece (about 10 g) was packed in a sterile plastic bag and stored at 4°C for up to 4 weeks. Two cheese samples each were removed at 0, 7, 14, 21, and 28 days, and 100 ml of 2% (wt/vol) sterile sodium citrate solution was added to each sample. The plastic bags were placed in a water bath adjusted to 37°C to liquify the cheese. After 2 h, the liquified cheese was centrifuged at 15,000 × g for 1 h at 4°C, and the pellets were added to 30 ml of 1% (wt/vol) HPC (cetylpyridinium chloride; Sigma) solution. The HPC solution was vortexed for 1 min, stored at room temperature overnight, and centrifuged at 15,000 × g for 1 h at 4°C. The pellets were then inoculated into BACTEC bottles to enumerate viable M. avium subsp. paratuberculosis cells. The M. avium subsp. paratuberculosis cells recovered from the cheese were identified by PCR for IS900.
Enumeration methods.
Viable M. avium subsp. paratuberculosis cell numbers were estimated using a radiometric culture method (BACTEC). At each sampling time, 0.5 ml was removed from each vial and inoculated into commercial BACTEC 12B bottles (Becton Dickinson Microbiologic Systems, Sparks, Md.) containing 1.0 ml of egg yolk suspension (Difco), 0.1 ml of a 40-μg/ml mycobactin J solution (Allied Monitor Inc.), and 0.1 ml of an antibiotic cocktail containing vancomycin, amphotericin B, and nalidixic acid. The final concentrations of these antibiotics in the radiometric broth (BACTEC 12B bottles) were 8.4, 16.8, and 25.2 μg/ml, respectively. The bottles were incubated at 37°C without agitation and read on a BACTEC 460 instrument without CO2 daily for 45 days. The BACTEC 460 instrument measures 14CO2 gas produced by metabolism of [14C]palmitate in the medium. From the total amount of CO2 gas produced (cumulative growth index), the number of M. avium subsp. paratuberculosis cells is estimated by comparison to a standard growth model described by the following equation (17):
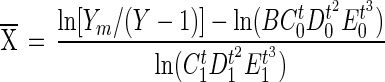 |
where Y is the cumulative growth response in units of 14CO2 released; Ym is a fixed value bounded by the maximum cumulative growth response, which is 12,950; X is the inoculum size; t is the incubation time; and B through E are regression coefficient constants determined to have the following values: B = 10,340, C0 = 1.2217, C1 = 0.84345, D0 = 0.98959, D1 = 1.004644, E0 = 1.00008339, and E1 = 0.99996559.
D value.
The D value was calculated from the slope of the best-fit line graphically determined by plotting the log10 of M. avium subsp. paratuberculosis survivors/ml versus either exposure time for each pH and salt combination tested or storage time of the cheese (33). The best-fit lines were calculated with Minitab regression analysis software (MINITAB Inc., State College, Pa.).
Statistical analysis.
Linear regressions of inactivation curves for D-value determinations were based on the concepts presented by Chatterjee and Price (3) and Draper and Smith (10). Differences among slopes of inactivation curves were analyzed using the SAS program (release 6.10. SAS Institute, Inc., Cary, N.C.). P values of <0.05 were considered significant.
RESULTS
Effect of pH and NaCl without prior heat treatment.
Figure 1 shows the inactivation curves for two M. avium subsp. paratuberculosis strains in acetate buffer (pH 4) with various salt concentrations (0, 2, 4, and 6% [wt/vol]). All inactivation curves for M. avium subsp. paratuberculosis strains were linear (r2 > 0.90) with narrow 95% confidence intervals. The addition of salt did not result in significantly different D values for strain Dominic at pH 4 (P > 0.1) (Fig. 1A; Table 1). However, D values for the other strain (ATCC 19698) were significantly higher in the complete absence of salt (P < 0.001). The addition of NaCl, at any of the concentrations tested, reduced the D value (Fig. 1B; Table 1). M. avium subsp. paratuberculosis strains were not viable beyond 56 days in all salt concentrations tested, but ATCC 19698 in the absence of salt was viable for up to 112 days at pH 4 (Fig. 1B).
FIG. 1.
Inactivation curves for M. avium subsp. paratuberculosis in acetate buffer (50 mM, pH 4) with various salt concentrations (0, 2, 4, and 6% [wt/vol]) at 20°C. (A) Dominic. (B) ATCC 19698.
TABLE 1.
D values for M. avium subsp. paratuberculosis strains tested at pH 4, 5, and 6 at 20°C
| pH | NaCl concn (%) |
D value (days) for strain:
|
|
|---|---|---|---|
| ATCC 19698 | Dominic | ||
| 4 | 0 | 16.1a | 9.2 |
| 2 | 7.9 | 9.7 | |
| 4 | 7.8 | 9.9 | |
| 6 | 8.7 | 11.3 | |
| 5 | 0 | 27.2a | 19.7 |
| 2 | 17.4 | 19.6 | |
| 4 | 19.3 | 19.7 | |
| 6 | 17.5 | 11.8a | |
| 6 | 0 | 32.5 | 31.9 |
| 2 | 38.2 | 32.0 | |
| 4 | 35.2 | 24.0 | |
| 6 | 39.1 | 33.9 | |
Significantly different from D values for other individual NaCl concentrations at the same pH.
For pH 5, as with pH 4, the inactivation curves for Dominic (r2 > 0.90) were linear, and D values for this strain were the same in the presence and absence of salt (P > 0.1). Strain ATCC 19698 showed lower r2 values, in the range of 0.78 to 0.97 (data not shown), and had higher D values in the absence of salt than in the presence of salt (P = 0.0075) (Table 1). That is, viable counts of strain ATCC 19698 at pH 5 declined more rapidly in the menstruum with 2, 4, and 6% salt than without salt. Viable M. avium subsp. paratuberculosis ATCC 19698 cells were detected for up to 112 days in the presence of salt and up to 196 days in the absence of salt at pH 5. Strain Dominic was totally inactivated after 112 days in 0, 2, and 4% salt and after 56 days in 6% salt at pH 5.
Inactivation curves for strain Dominic were linear at pH 6 (r2 > 0.93) in the absence and presence of salt, except at 4% salt (r2 = 0.78), but the ATCC 19698 strain showed less-linear inactivation than Dominic in both the presence and absence of salt (r2 > 0.60) (data not shown). The presence of salt did not affect survival time; neither M. avium subsp. paratuberculosis strain survived beyond 196 days irrespective of the presence or absence of salt. The D values for both strains were virtually unaffected by any level of salt tested at pH 6 (P > 0.1) (Table 1).
Effect of heat treatment followed by exposure to pH 5.0 and 2% NaCl.
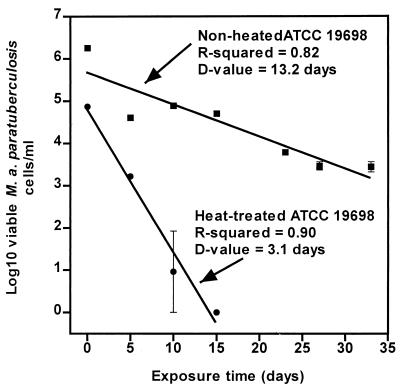
Table 2 shows the D values for all heat-treated and nonheated M. avium subsp. paratuberculosis strains tested when exposed to the acetate buffer (pH 5, 2% NaCl). The r2 values for all inactivation curves ranged from 0.81 to 0.98. After heat treatment alone at 62°C for 0, 240, 480, 720, and 960 s, the population of M. avium subsp. paratuberculosis ATCC 19698 was reduced to 6.26 ± 0.07, 4.87 ± 0.21, 4.26 ± 0.04, 3.86 ± 0.09, and 2.27 ± 1.01 log10 cells/ml, respectively. The thermal D62°C was 267.4 s, with a linear regression r2 value of 0.85. Heat-treated M. avium subsp. paratuberculosis ATCC 19698 cells were more rapidly inactivated when exposed to the acetate buffer (pH 5, 2% NaCl) than were cells not treated with heat. D values for M. avium subsp. paratuberculosis ATCC 19698 heat treated at 62°C for 240, 480, and 720 s were 3.1, 2.4, and 1.4 days, respectively, in the acetate buffer, while the D value for cells not treated with heat was 13.2 days in this buffer. The D value for M. avium subsp. paratuberculosis ATCC 19698 heat treated at 62°C for 960 s could not be obtained, since the number of M. avium subsp. paratuberculosis cells was low after heat treatment and the cells were completely inactivated at the first sampling time. Strain Dominic also showed the same inactivation pattern as strain ATCC 19698. Thus, heat treatment in this buffer (at 62 or 71°C) resulted in D values for both strains that were lower than the D values for cells not exposed to heat.
TABLE 2.
D values for heat-treated M. avium subsp. paratuberculosis strains at pH 5, 2% (vol/vol) NaCl, and 20°C
| Temp (°C) | Heating time (s) | Log10 cell no. (mean ± SD) after heat treatment of strain:
|
D value (days) for strain:
|
||
|---|---|---|---|---|---|
| ATCC 19698a | Dominicb | ATCC 19698 | Dominic | ||
| 62 | 0 | 6.26 ± 0.07 | 4.60 ± 0.05 | 13.2 | 12.3 |
| 240 | 4.87 ± 0.21 | 2.48 ± 0.20 | 3.1 | 4.3 | |
| 480 | 4.26 ± 0.04 | 0.17 ± 0.23 | 2.4 | NAb | |
| 720 | 3.86 ± 0.09 | 0 | 1.3 | NA | |
| 960 | 2.27 ± 1.01 | 0 | NA | NA | |
| 71 | 0 | 6.65 ± 0.02 | 4.67 ± 0.13 | 16.3 | 17.4 |
| 20 | 6.03 ± 0.16 | 3.94 ± 0.04 | 5.9 | 4.7 | |
| 40 | 5.11 ± 0.12 | 2.49 ± 0.26 | 2.7 | 1.4 | |
| 60 | 3.83 ± 0.49 | 0 | 0.8 | NA | |
The D62°C and D71°C were 267.4 and 21.3 s, respectively.
The D62°C and D71°C were 108.4 and 13.5 s, respectively.
NA, D values were not available, since the number of M. avium subsp. paratuberculosis cells was low after heat treatment and the cells were completely inactivated at the first sampling time.
As an example, Fig. 2 shows the inactivation curves for heat-treated (at 62°C for 240 s) and nonheated ATCC 19698 in the acetate buffer (pH 5, 2% NaCl). The inactivation curves for heat-treated and nonheated M. avium subsp. paratuberculosis were linear (r2 = 0.90 and 0.82, respectively). The heat-treated M. avium subsp. paratuberculosis had a significantly lower D value than the nonheated cells (P < 0.001).
FIG. 2.
Inactivation curves for nonheated and heat-treated (at 62°C for 240 s) M. avium subsp. paratuberculosis strain ATCC 19698 in the acetate buffer (pH 5, 2% [wt/vol] NaCl) at 20°C.
Inactivation of M. avium subsp. paratuberculosis in soft white cheese.
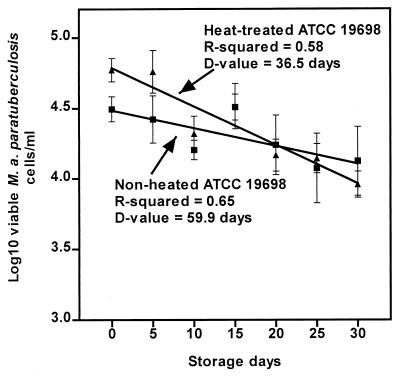
Figure 3 shows the inactivation curves for heat-treated and nonheated M. avium subsp. paratuberculosis ATCC 19698 in soft white Hispanic-style cheese. When the cheese was made using milk spiked with nonheated M. avium subsp. paratuberculosis, the pHs for three independent trials were 6.24, 6.05, and 6.16. The mean pH was 6.15 ± 0.08, and the corresponding r2 values were 0.64, 0.59, and 0.71, respectively. The data for the three independent trials were pooled since they were statistically the same (P > 0.5). At time zero, 4.64 ± 0.06 log10 M. avium subsp. paratuberculosis cells/ml were recovered from the cheese. After the cheese was held for 30 days at 4°C, 4.12 ± 0.55 log10 M. avium subsp. paratuberculosis cells/ml were recovered. The D value for the pooled data was 59.9 days, and the r2 value for the regression line was 0.65.
FIG. 3.
Inactivation curves for nonheated and heat-treated (at 62°C for 240 s) M. avium subsp. paratuberculosis strain ATCC 19698 in the soft white cheese at 4°C.
When heat-treated M. avium subsp. paratuberculosis was used, the pHs of the cheese in three independent trials were 6.02, 6.11, and 6.05 at the r2 values of 0.68, 0.72, and 0.86, respectively; the mean pH was 6.06 ± 0.04. The data for these three independent trials were also pooled since they were not significantly different (P > 0.6). At time zero, 4.79 ± 0.14 log10 M. avium subsp. paratuberculosis cells/ml were recovered from the cheese, and after 30 days, 3.96 ± 0.16 log10 M. avium subsp. paratuberculosis cells/ml were recovered. The D value for heat-treated M. avium subsp. paratuberculosis in the cheese was 36.5 days, and the r2 value for the regression line was 0.58.
Holding the cheese for more than 4 weeks was not possible due to the increasing level of sample contamination.
DISCUSSION
In 1949, the Food and Drug Administration promulgated rules that provided two options for producing safe cheese products: (i) pasteurization of milk or (ii) 60-day holding of cheese at 2°C if raw or subpasteurized milk was used to produce the cheese (16). The 60-day holding period was offered as an alternative to pasteurization based on the assumption that any bacterial pathogens present in fresh cheese would die within this period. Two characteristics of most cheeses are critical to the accuracy of this assumption: acidity (pH 4.5 to 5.3) and concentration of salt (1 to 5%) (11). For this reason, we evaluated the effects of low pH and NaCl alone and in combination on the inactivation of M. avium subsp. paratuberculosis.
We have found that a lower pH is associated with faster inactivation of M. avium subsp. paratuberculosis (Table 1). The average D values for two M. avium subsp. paratuberculosis strains tested at pH 4.0, 5.0, and 6.0 were 10.0 ± 2.5, 19.0 ± 3.9, and 33.3 ± 4.4 days, respectively. As the pH increased, so did the D values: a 1-unit increase in pH resulted in an approximate doubling of the D values.
The D values for the M. avium subsp. paratuberculosis strains tested are higher than those previously reported for other bacterial pathogens. Little et al. (22) reported that the D values for Yersinia enterocolitica 0:3 in tryptone soy broth acidified to pH 4.0 with lactic or acetic acid (1 M) were 0.4 and 0.6 days, respectively. Buchanan et al. (2) obtained D values of 0.5, 1.2, and 3.6 days for Listeria monocytogenes in brain heart infusion broth acidified with acetic acid (50 mM) to pH levels of 4.0, 5.0, and 6.0, respectively. Thus, M. avium subsp. paratuberculosis is far more resistant to inactivation by low pH than these other milk-borne pathogens, displaying 10-fold higher D values.
The present study showed that at one pH level (pH 6.0), the D values for both strains were unaffected by the presence of NaCl at any concentration (0 to 6%). Strain Dominic showed the same pattern of being unaffected by NaCl at pH 4 and 5. For trials with strain ATCC 19698 at pH 4 and 5, however, the addition of NaCl at any concentration (≥2%) reduced the D values (Fig. 1 and Table 1). We conclude that the D values for the M. avium subsp. paratuberculosis strains tested were not significantly different with low (2%) and high (6%) concentrations of NaCl at the same pH.
The survival time for M. avium subsp. paratuberculosis at the NaCl concentrations tested is much longer than that for L. monocytogenes. For example, Cole et al. (8) reported that none of a starting inoculum of 104 L. monocytogenes cells/ml survived after 21 days at pH 4.19 with 0 to 6% NaCl. Based on the average D value (10.0 ± 2.5 days) for M. avium subsp. paratuberculosis at pH 4.0, the estimated time to inactivate 104 cells/ml would be 40 days at all NaCl concentrations tested, which is twice as long as for L. monocytogenes.
The D values for M. avium subsp. paratuberculosis in acetate buffer did not reflect the inactivation rate obtained in the soft cheese. The D value (36.5 days) for heat-treated M. avium subsp. paratuberculosis in Queso Fresco (pH 6.06 ± 0.04, 2% NaCl) was about 12 times higher than the D value (3.1 days) in the acetate buffer (pH 6.0, 2% NaCl), and the D value (59.9 days) for nonheated M. avium subsp. paratuberculosis in Queso Fresco (pH 6.15 ± 0.08, 2% NaCl) was roughly twice the D value (38.2 days) in the acetate buffer (pH 6.0, 2% NaCl) (Table 2 and Fig. 3). We interpret this to mean that the actual rate of inactivation of M. avium subsp. paratuberculosis in the soft white cheese was less than what was predicted by studies done with acetate buffer.
This phenomenon was also observed previously with another bacterium of concern in cheese, L. monocytogenes. Glass et al. (13) reported that populations of L. monocytogenes exhibited a decrease of 0.6 log10 CFU/g in cheese (pH 5.6 to 5.84, 2.3 to 2.6% salt) stored for 4 days at 30°C. However, Buchanan et al. (2) published a D value for L. monocytogenes in brain heart infusion broth (pH 5.0, acidified with 50 mM acetic acid) of 1.2 days at 28°C. That means the organism should have exhibited a decrease of 4 log10 CFU/ml in the brain heart infusion broth during 4 days of storage based on the D value reported by Buchanan et al., but a reduction of only 0.6 log10 CFU/g was found in cheese. Therefore, just as previous reports indicate that survival of L. monocytogenes was dependent on both medium composition and incubation temperature (28, 40), so too the rate of inactivation of M. avium subsp. paratuberculosis may be affected by a combination of factors in cheese, such as proteins, lipids, and water content, etc.
We recovered 104 to 105 M. avium subsp. paratuberculosis cells/ml from the cheese immediately after inoculation, although the predicted initial concentration was 106 cells/ml. We also observed greater variability in counts (low r2 value [Fig. 3]) made in cheese compared to the acetate buffer. Apparently, some unknown combination of factors in cheese interferes with efficient recovery of M. avium subsp. paratuberculosis. Alternatively, the HPC treatment of cheese samples, which is required to control contaminating microflora, could impair recovery of M. avium subsp. paratuberculosis. However, HPC is routinely used to isolate M. avium subsp. paratuberculosis from fecal samples (39). Also, controlled trials have shown that 1% (wt/vol) HPC does not affect M. avium subsp. paratuberculosis viability for up to 1 week (unpublished data).
Heat treatment rendered M. avium subsp. paratuberculosis strains more easily inactivated in both the acetate buffer (Table 2 and Fig. 2) and a soft cheese (Fig. 3). In acetate buffer (pH 5 and 2% NaCl), M. avium subsp. paratuberculosis strains sublethally heat treated at either 62 or 71°C were more rapidly inactivated than nonheated strains; the D values for heat-treated M. avium subsp. paratuberculosis strains were significantly (about three times) lower than those for nonheated strains. In the soft cheese, the D values for heat-treated and nonheated M. avium subsp. paratuberculosis ATCC 19698 in the soft white Hispanic-style cheese (Queso Fresco; pH 6.15 ± 0.08, 2% NaCl) were 36.5 and 59.9 days, respectively. This finding is interesting, since other bacterial pathogens (such as Escherichia coli O157, Salmonella spp., and Shigella flexneri) appear to have stress resistance systems that are able to enhance their survival during the curing period after having been heat shocked (1, 18–20, 30, 38). The strains of M. avium subsp. paratuberculosis used in this study did not appear to display comparable stress resistance responses.
Extrapolation from data presented in this paper and from our previous pasteurization study (34) supports the following: if a cheese (pH 6, 2% salt) is made from HTST-pasteurized milk and cured for 60 days, approximately 3 log10 M. avium subsp. paratuberculosis cells/ml would be inactivated. That is, previously we along with others (4, 14) showed that a 1-log10-unit reduction of the M. avium subsp. paratuberculosis population would occur through pasteurization. Based on the thermal D values for clinical strains of M. avium subsp. paratuberculosis suspended in raw milk, which were 228.8, 47.8, 21.8, and 11.6 s at 62, 65, 68, and 71°C, respectively, we reported that the HTST pasteurization method may effectively kill 100% of M. avium subsp. paratuberculosis cells if the initial concentration is ≤101 cells/ml under laboratory conditions (34). This finding supported the conclusion of Grant et al. (15). The present study showed that there would be about a 2-log10-unit reduction by the 60-day curing, since the D value for heat-treated M. avium subsp. paratuberculosis ATCC 19698 in the soft cheese (pH 6, 2% NaCl) was 36.5 days (Fig. 3). Therefore, we conclude that if cheese is made from pasteurized milk and undergoes 60-day curing, there will be a reduction of approximately 3 log10 M. avium subsp. paratuberculosis cells/ml: a 1-log10-unit reduction through pasteurization and a 2-log10-unit reduction through the 60-day curing period.
The concentration of M. avium subsp. paratuberculosis in raw milk has not been well defined. Sweeney et al. (35) found 2 to 8 CFU/ml of milk in nine culture-positive milk samples collected from clinically normal fecal culture-positive cows. Grant et al. (14) suggested that M. avium subsp. paratuberculosis contamination rates could reach as high as 104 CFU/ml of milk due to fecal contamination. In a modeling study, Nauta and van der Giessen (27) found that contamination of milk with feces of cows with clinical paratuberculosis is the greatest contributor to the potential M. avium subsp. paratuberculosis burden in raw milk. Our data indicate that cheese production using pasteurized milk and a 60-day curing period will largely eliminate the predicted level of M. avium subsp. paratuberculosis contamination. However, much more study is needed to quantify the amount of M. avium subsp. paratuberculosis in raw milk under farm conditions in different localities and dairy production systems.
In summary, NaCl had little to no effect on M. avium subsp. paratuberculosis inactivation rates. Decreasing D values were associated with decreasing menstruum pH for the M. avium subsp. paratuberculosis strains tested. The D value for M. avium subsp. paratuberculosis in a soft white Hispanic cheese was twice as high as the values obtained in acetate buffer with comparable pH and NaCl conditions. Heat-treated M. avium subsp. paratuberculosis cells were inactivated faster than nonheated cells in both acetate buffer (pH 5.0 and 2% NaCl) and the cheese. The 60-day curing period resulted in a 2-log10-unit reduction in the number of heat-treated M. avium subsp. paratuberculosis cells per milliliter in cheese. Therefore, the present study has shown that the mandatory 60-day curing periods, along with a required heat treatment, are important elements in reducing the population of any contaminating M. avium subsp. paratuberculosis cells during production of cheese products.
ACKNOWLEDGMENTS
We thank Charles W. Kaspar and Susan E. Ansay, Food Research Institute, University of Wisconsin—Madison, for their advice and critical review of the manuscript and Rodrick J. Chiodini, Rehoboth, Mass., for providing M. avium subsp. paratuberculosis strain Dominic. We express our thanks to Murray Clayton, Department of Statistics, University of Wisconsin—Madison, for statistical analysis.
This research was funded in part by the Wisconsin Milk Marketing Board (project UW 9507).
REFERENCES
- 1.Benjamin M M, Datta A R. Acid tolerance of enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1995;61:1669–1672. doi: 10.1128/aem.61.4.1669-1672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan R L, Golden M H, Whiting R C. Differentiation of the effects of pH and lactic or acetic acid concentration on the kinetics of Listeria monocytogenes inactivation. J Food Prot. 1993;56:474–478. doi: 10.4315/0362-028X-56.6.474. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee S, Price B. Regression analysis by example. 2nd ed. New York, N.Y: John Wiley and Sons, Inc.; 1991. pp. 107–116. [Google Scholar]
- 4.Chiodini R J, Hermon-Taylor J. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. J Vet Diagn Invest. 1993;5:629–631. doi: 10.1177/104063879300500424. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini R J, Rossiter C A. Paratuberculosis: a potential zoonosis? Vet Clin N Am Food Anim Pract. 1996;12:457–467. doi: 10.1016/s0749-0720(15)30417-5. [DOI] [PubMed] [Google Scholar]
- 6.Chiodini R J, Van Kruiningen H J, Merkal R S, Thayer W R, Jr, Coutu J A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984;20:966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiodini R J, Van Kruiningen H J, Thayer W S, Coutu J A. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J Clin Microbiol. 1986;24:357–363. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole M B, Jones M V, Holyoak C. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J Appl Bacteriol. 1990;69:63–72. doi: 10.1111/j.1365-2672.1990.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 9.Dell'Isola B, Poyart C, Goulet O, Mougenot J F, Sadoun-Journo E, Brousse N, Schmitz J, Ricour C, Berche P. Detection of Mycobacterium paratuberculosis by polymerase chain reaction in children with Crohn's disease. J Infect Dis. 1994;169:449–451. doi: 10.1093/infdis/169.2.449. [DOI] [PubMed] [Google Scholar]
- 10.Draper N R, Smith H. Applied regression analysis. 2nd ed. New York, N.Y: John Wiley and Sons, Inc.; 1981. pp. 241–257. [Google Scholar]
- 11.Foster E M. Microbiology of cheese. In: Foster E M, Nelson F E, Speck M L, Doetsch R N, Olson J C, editors. Dairy microbiology. Atascadero, Calif: Ridgeview Publishing Company; 1983. pp. 334–413. [Google Scholar]
- 12.Gitnick G, Collins J, Beaman B, Brooks D, Arthur M, Imaeda T, Palieschesky M. Preliminary report on isolation of mycobacteria from patients with Crohn's disease. Digest Dis Sci. 1989;34:925–932. doi: 10.1007/BF01540280. [DOI] [PubMed] [Google Scholar]
- 13.Glass K A, Kaufman K M, Johnson E A. Survival of bacterial pathogens in pasteurized process cheese slices stored at 30°C. J Food Prot. 1998;61:290–294. doi: 10.4315/0362-028x-61.3.290. [DOI] [PubMed] [Google Scholar]
- 14.Grant I R, Ball H J, Neill S D, Rowe M T. Inactivation of Mycobacterium paratuberculosis in cows' milk at pasteurization temperatures. Appl Environ Microbiol. 1996;62:631–636. doi: 10.1128/aem.62.2.631-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant I R, Ball H J, Rowe M T. Effect of high-temperature, short-time (HTST) pasteurization on milk containing low numbers of Mycobacterium paratuberculosis. Lett Appl Microbiol. 1998;26:166–170. doi: 10.1046/j.1472-765x.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson E A, Nelson J H, Johnson M. Microbiological safety of cheese made from heat-treated milk, part I. Executive summary, introduction and history. J Food Prot. 1990;53:441–452. doi: 10.4315/0362-028X-53.5.441. [DOI] [PubMed] [Google Scholar]
- 17.Lambrecht R S, Carriere J F, Collins M T. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl Environ Microbiol. 1988;54:910–916. doi: 10.1128/aem.54.4.910-916.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leyer G J, Johnson E A. Acid adaptation promotes survival of Salmonella spp. in cheese. Appl Environ Microbiol. 1992;58:2075–2080. doi: 10.1128/aem.58.6.2075-2080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyer G J, Wang L L, Johnson E A. Acid adaptation of Escherichia coli O157:H7 increase survival in acidic foods. Appl Environ Microbiol. 1995;61:3752–3755. doi: 10.1128/aem.61.10.3752-3755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Lee I S, Slonczewski J L, Foster J W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol. 1995;177:4097–4104. doi: 10.1128/jb.177.14.4097-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisby G, Andersen J, Engbaek K, Binder V. Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn's disease demonstrated by a nested primer polymerase chain reaction. Scand J Gastroenterol. 1994;29:923–929. doi: 10.3109/00365529409094864. [DOI] [PubMed] [Google Scholar]
- 22.Little C L, Adams M R, Easter M C. The effect of pH, acidulant and temperature on the survival of Yersinia enterocolitica. Lett Appl Microbiol. 1992;14:148–152. [Google Scholar]
- 23.McFadden J J, Butcher P D, Chiodini R J, Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987;25:796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFadden J J, Collins J, Baeman B, Arthur M, Gitnick G. Mycobacteria in Crohn's disease: DNA probes identify the Wood Pigeon strain of Mycobacterium avium and Mycobacterium paratuberculosis from human tissue. J Clin Microbiol. 1992;30:3070–3073. doi: 10.1128/jcm.30.12.3070-3073.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishna D, Katsel P, Brown S T, Gilberts E C A M, Greenstein R J. On the etiology of Crohn disease. Proc Natl Acad Sci USA. 1996;93:9816–9820. doi: 10.1073/pnas.93.18.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray A, Oliaro J, Schlup M M T, Chadwick V S. Mycobacterium paratuberculosis and inflammatory bowel disease: frequency distribution in serial colonoscopic biopsies using the polymerase chain reaction. Microbios. 1995;83:217–228. [PubMed] [Google Scholar]
- 27.Nauta M J, van der Giessen J W. Human exposure to Mycobacterium paratuberculosis via pasteurized milk: a modeling approach. Vet Rec. 1998;143:293–296. doi: 10.1136/vr.143.11.293. [DOI] [PubMed] [Google Scholar]
- 28.Parish M E, Higgins D P. Survival of Listeria monocytogenes in low pH model broth system. J Food Prot. 1989;52:144–147. doi: 10.4315/0362-028X-52.3.144. [DOI] [PubMed] [Google Scholar]
- 29.Path J. Hispanic cheese: a promising new market for the specialty cheese maker. UW Dairy Pipeline. 1991;3:1–4. [Google Scholar]
- 30.Reitsma C J, Henning D R. Survival of enterohemorrhagic Escherichia coli O157:H7 during the manufacture and curing of cheddar cheese. J Food Prot. 1996;59:460–464. doi: 10.4315/0362-028X-59.5.460. [DOI] [PubMed] [Google Scholar]
- 31.Stabel J R, Steadham E M, Bolin C A. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl Environ Microbiol. 1997;63:4975–4977. doi: 10.1128/aem.63.12.4975-4977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streeter R N, Hoffsis G F, Bech-Nielsen S, Shulaw W P, Rings M. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am J Vet Res. 1995;56:1322–1324. [PubMed] [Google Scholar]
- 33.Stumbo C R. Thermal resistance of bacteria. In: Stumbo C R, editor. Thermobacteriology in food processing. 2nd ed. New York, N.Y: Academic Press; 1973. pp. 93–120. [Google Scholar]
- 34.Sung N, Collins M T. Thermal tolerance of Mycobacterium paratuberculosis. Appl Environ Microbiol. 1998;64:999–1005. doi: 10.1128/aem.64.3.999-1005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney R W, Whitlock R H, Rosenberger A E. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J Clin Microbiol. 1992;30:166–171. doi: 10.1128/jcm.30.1.166-171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor T K, Wilks C R, McQueen D S. Isolation of Mycobacterium paratuberculosis from milk of a cow with Johne's disease. Vet Rec. 1981;109:532–533. [PubMed] [Google Scholar]
- 37.Thompson D E. The role of mycobacteria in Crohn's disease. J Med Microbiol. 1994;41:74–94. doi: 10.1099/00222615-41-2-74. [DOI] [PubMed] [Google Scholar]
- 38.Wang G, Doyle M P. Heat shock response enhances acid tolerance of Escherichia coli O157:H7. Lett Appl Microbiol. 1998;26:31–34. doi: 10.1046/j.1472-765x.1998.00264.x. [DOI] [PubMed] [Google Scholar]
- 39.Whipple D L, Callihan D L, Jarnagin J L. Cultivation of Mycobacterium paratuberculosis from bovine fecal specimens and a suggested standardized procedure. J Vet Diagn Invest. 1991;3:368–373. doi: 10.1177/104063879100300424. [DOI] [PubMed] [Google Scholar]
- 40.Wilkins P O, Bourgeois R, Murray R G E. Psychrotrophic properties of Listeria monocytogenes. Can J Microbiol. 1972;18:543–551. doi: 10.1139/m72-087. [DOI] [PubMed] [Google Scholar]