Abstract
Background and aims: Post-operative atrial fibrillation (POAF), defined as new-onset AF in the immediate period after surgery, is associated with poor adverse cardiovascular events and a higher risk of permanent AF. Mechanisms leading to POAF are not completely understood and epicardial adipose tissue (EAT) inflammation could be a potent trigger. Here, we aim at exploring the link between EAT-secreted interleukin (IL)-1β, atrial remodeling, and POAF in a population of coronary artery disease (CAD) patients.
Methods: We collected EAT and atrial biopsies from 40 CAD patients undergoing cardiac surgery. Serum samples and EAT-conditioned media were screened for IL-1β and IL-1ra. Atrial fibrosis was evaluated at histology. The potential role of NLRP3 inflammasome activation in promoting fibrosis was explored in vitro by exposing human atrial fibroblasts to IL-1β and IL-18.
Results: 40% of patients developed POAF. Patients with and without POAF were homogeneous for clinical and echocardiographic parameters, including left atrial volume and EAT thickness. POAF was not associated with atrial fibrosis at histology. No significant difference was observed in serum IL-1β and IL-1ra levels between POAF and no-POAF patients. EAT-mediated IL-1β secretion and expression were significantly higher in the POAF group compared to the no-POAF group. The in vitro study showed that both IL-1β and IL-18 increase fibroblasts’ proliferation and collagen production. Moreover, the stimulated cells perpetuated inflammation and fibrosis by producing IL-1β and transforming growth factor (TGF)-β.
Conclusion: EAT could exert a relevant role both in POAF occurrence and in atrial fibrotic remodeling.
Keywords: epicardial adipose tissue, cytokines, inflammation, atrial fibrillation, fibrosis, cardiac remodeling
Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia, is the result of electrical and structural remodeling of the atria encompassing interactions among cellular and neurohormonal mediators (Hu et al., 2015). Post-operative AF (POAF), defined as new-onset AF in the immediate period after surgery, is associated with hemodynamic instability, increased risk of stroke, an eightfold increase in the risk of subsequent AF (Ahlsson et al., 2010), and cardiovascular death (Ahlsson et al., 2010; Lee et al., 2014; Melduni et al., 2015).
Mechanisms leading to POAF are not completely understood, but it is probably the consequence of both pre-existing factors, related to atrial remodeling, and peri-operative triggers that induce AF when a vulnerable substrate is present. Inflammation could be one of the most potent AF triggers and the NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome myocardial activation, through the production of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18, is associated with the pathogenesis of AF by promoting atrial structural and electrical remodeling (Yao et al., 2018). IL-1β increased gene expression levels are associated with atrial remodeling and sustained AF (Matsushita et al., 2019). IL-1β plays an important role in the inflammatory cascade and coordinates the cellular response to tissue injury, promoting the recruitment of inflammatory cells and the increased production of other cytokines (Dinarello, 2011). Animal studies suggest that IL-1β contributes to myocardial electrophysiological remodeling and its inhibition reduces arrhythmogenesis (De Jesus et al., 2017). Epicardial adipose tissue (EAT), the visceral fat depot of the heart, has been proposed as a local source of inflammatory mediators with a potential role in AF (Arai et al., 2011; Wong et al., 2017). Potential arrhythmogenic mechanisms of EAT in AF include structural remodeling of the atria and arrhythmic trigger by infiltrations of adipose tissue, fibrosis modulation, myocardial inflammation, and oxidative stress (Wong et al., 2017). IL-1β stimulates activin A expression (Arai et al., 2011) that has been reported to be produced by epicardial adipose tissue (EAT) of patients evolving POAF after cardiac surgery (de Kretser et al., 2012; Wang et al., 2019). We recently reported an increased inflammatory status of EAT also in POAF (Petraglia et al., 2022). However, further evidence is required to confirm all these hypothetic mechanisms in order to clarify the role of EAT in cardiovascular disease and AF.
In the present manuscript, we aim at exploring the link between EAT-secreted IL-1β, atrial remodeling, and POAF in a population of coronary artery disease (CAD) patients undergoing cardiac surgery.
Materials and Methods
Patient Enrollment and Tissue Collection
Forty patients with coronary artery disease (CAD) undergoing elective coronary artery bypass grafting (CABG) were enrolled at the cardiac surgery unit of the University of Naples “Federico II.”
Exclusion criteria were the following: chronic inflammatory diseases that could interfere with the systemic or local inflammatory profile, history of AF. Before cardiac surgery, all patients underwent clinical and echocardiographic assessment. Clinical and demographic data were recorded, including cardiovascular risk factors and drug therapies. According to standard techniques, echocardiograms were performed by a Vivid E9 (GE Healthcare) machine. EAT was visualized in a parasternal long-axis view between the free wall of the right ventricle and the anterior surface of the ascending aorta. Once visualized the EAT deposit, the maximum EAT thickness was measured at end-systole, as previously described (Parisi et al., 2020a). The average value from three cardiac cycles was used for the statistical analysis.
Before cardiac surgery, we collected blood samples for serum collection. Intraoperative EAT biopsies (average 0.1–0.5 g) and right atrial appendages were carried out before the initiation of cardiopulmonary bypass. EAT biopsies were taken between the free wall of the right ventricle and the anterior surface of the ascending aorta, just after the opening of the pericardial sac. Each tissue sample was stored at −80°C until analysis. All patients were postoperatively monitored by ECG telemetry and data on POAF occurrence were recorded during the hospital stay.
Informed consent was obtained from every subject before the surgical procedure. Protocols were approved by the ethical committee of the University of Naples (prot. no. 301/19). All procedures performed in the study were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and conformed to the Declaration of Helsinki on human research.
Conditioned Media Preparation and Cytokines Assessment
According to tissue weight, serum-free Dulbecco modified Eagle medium (DMEM)-F12 (1:1) containing 0.25% BSA (1 ml medium/0.1 g tissue) was added to the well and incubated at 37°C in a CO2 incubator. After 24 h, the medium was collected, centrifuged at 14,000 × g to remove debris, and then stored as aliquots at –80°C until further use. Serum samples and conditioned media were screened for the concentration of interleukin (IL)-1ra, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, basic fibroblast growth factor (FGF), eotaxin, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), interferon-γ (IFN-γ), interferon-γ inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 (MIP-1) α, MIP-1β, C–C motif chemokine ligand 5 (CCL5)/RANTES, TNF-α, platelet-derived growth factor (PDGF-BB) and vascular endothelial growth factor (VEGF) using the Bio-Plex Pro Human Cytokine Grp I Panel 27-Plex kit (cat. no. M500KCAF0Y) according to the supplier’s instructions. The magnetic bead-based assay was performed on a Bio-Plex 200 analyzer with Bio-Rad Bio-Plex Manager (Bio-Rad, Hercules, CA, United States).
Atrial Fibrosis Assessment at Histology
Fresh tissue from atrial biopsies was formalin-fixed and paraffin-embedded. For each case 4 µm-thick sections were cut and stained with hematoxylin/eosin and observed on a light microscope to evaluate fibrosis, graded as 0, 1+, 2+ and 3+, respectively when absent or observed in <10%, 10–50% and in >50% of the sample. The volume fraction of collagen (CVF) was analyzed by staining with hematoxylin/eosin. Four separate views were selected (magnification = original ×400) and the CVF was calculated using the following formula: CVF = collagen area/total visual area × 100%, to assess the degree of cardiac fibrosis. Each sample was independently evaluated by two different pathologists.
Cell Culture and Treatments
Human fibroblasts were isolated from the right atrial auricle by mechanical and enzymatic digestion (collagenase type II) of the tissue and cultured in Advanced Dulbecco’s modified eagle’s medium (Ad DMEM; Life Technologies) with 10% fetal bovine serum (FBS; Microtech, IT), 1% penicillin (Life Technologies), 1% streptomycin (Life Technologies), and 1% L-glutamine (Life Technologies). Primary cells were immortalized by transduction with commercial lentiviral particles carrying out the encoding sequences for SV40 large T antigen and human telomerase reverse transcriptase (hTERT) enzyme, under cytomegalovirus promoter (GeneCopoeia). Interleukin (IL) treatments were performed using human recombinant IL-1β (R&D Systems, Inc., Minneapolis, MN, United States) at 0.1, 1, and 10 ng/ml and IL-18 (R&D Systems, Inc., Minneapolis, MN, United States) at 1, 10, and 100 ng/ml. FBS at 10% was used as a positive control for migration assay.
MTT Assay
To evaluate the proliferation rate, 3000 cells per well were plated in a 96 well plate and followed by Incucyte (Essen BioScience) every 8 h for 5 days. Cells were treated with recombinant ILs (as previously reported) every other day. At the end of the survey, cell viability was assessed by 3-(4, 5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich). Briefly, MTT solution (0.5 mg/ml) was added into each well and incubated at 37°C, 5% CO2 for 4 h. The supernatant was discarded and dimethylsulfoxide (DMSO; Sigma-Aldrich) was added into each well. The corresponding absorbance value was observed using the microplate reader (TECAN pro infinite M200) at a dual-wavelength of 590 and 620 nm (reference). Cell viability was expressed as a percentage with respect to the mean of untreated, referred to as 100%.
Migration Assay
To evaluate the migration, 5,000 cells per well were plated in a 96 well plate and starved overnight in Ad DMEM without FBS. The day after, a scratch was performed using wound maker 96 (Essen BioScience), treatments with ILs were added and wound healing was evaluated by Incucyte (Essen BioScience) every 2 h for 1 day. Relative wound density was expressed as a percentage referred to the same well at time 0.
RNA Isolation and Expression Analysis
Total RNA was isolated from EAT biopsies and cell culture, using TRIzol solution (Life Technologies, CA, United States), quantified (NanoDrop spectrophotometer, Life Technologies, Carlsbad, CA, United States), and reverse-transcribed using SuperScript III Reverse Transcriptase according to the manufacturer’s instructions. qPCR was performed by iTaq Universal SYBR Green Supermix (Biorad). Absolute quantification of gene expression (Arbitrary Units—AU) was measured by using the 2−ΔCt method (EAT biopsies). Relative quantification (AU) of gene expression was measured by using 2−ΔΔCt method (cell culture). Expression levels of IL-1β (IL-1β primer pairs: F-5′-ACTGAAAGCTCTCCACCTCC-3’; R-5′-CATCTTTCAACACGCAGGAC-3′), Collagen 1A1 (COL1A1 primer pairs: F-5′-GGACACAGAGGTTTCAGTGG-3’; R-5′-CCAGTAGCACCATCATTTCC-3′), Collagen 3A1 (COL3A1 primer pairs: F-5′-CTACTTCTCGCTCTGCTTCATC-3’; R-5′-TTGGCATGGTTCTGGCTT-3′), Transforming Growth Factor β1 (TGFβ1 primer pairs: F-5′-GTTCAGGTACCGCTTCTCG-3’; R-5′-CCGACTACTACGCCAAGGA-3′) were normalized for the reference sample using Peptidylprolyl Isomerase A (PPIA) as housekeeping gene (PPIA primer pairs: F-5′-TACGGGTCCTGGCATCTTGT-3’; R-5′-GGTGATCTTCTTGCTGGTC-3′).
Statistical Analysis
Statistical analyses were performed with R statistical platform and with GraphPad Prism 8.0 software (GraphPad Software Inc., La Jolla, CA). D’Agostino-Pearson normality test was used to evaluate whether the continuous data were normally distributed, and according to the results, a Welch’s two-tailed t-test for independent samples (for normally distributed data) or a Mann-Whitney U test (for not normally distributed data) was used. Categorical values were described by the number of occurrences and percentages and compared by the chi-square test. One-way analysis of variance (ANOVA) followed by Tukey’s multi comparison test was used for the in vitro data.
Receiver-operating characteristics (ROC) curves were used to evaluate IL-1β′s ability to classify POAF and no-POAF patients.
Results
Patient Clinical Characteristics and Atrial Fibrosis
Forty CAD patients were selected for the study (mean age 61 years, male gender 95%). No differences in surgical procedures were noted in the two groups, and in all patients extracorporeal circulation was performed. Table 1 illustrates the demographic, clinical, and echocardiographic characteristics of overall patients. Sixteen patients out of 40 (40%) developed AF during the 7 days following the cardiac surgery (POAF group), while 60% exhibited constant sinus rhythm in the postoperative period (no-POAF). The two groups were homogeneous concerning age, clinical and echocardiographic parameters, including left atrial volume and EAT thickness (Table 1). In a subgroup of 20 patients, we assessed atrial fibrosis at histology. The 60% of patients has grade 0 (no fibrosis), the 5% had grade 1 (<10% fibrosis), the 25% had grade 2 (10–50% fibrosis) and 10% had grade 3 (>50%) (Supplementary Figure S1). Overall atrial fibrosis was present in eight patients. Of note, no association was observed between POAF and the presence of atrial fibrosis (p = 0.582, data not shown).
TABLE 1.
Patient clinical characteristics in No-POAF and POAF groups.
| No-POAF (n = 24) | POAF (n = 16) | p-value (Test) | |
|---|---|---|---|
| Sex (M) (%) | 22 (91.67%) | 16 (100%) | 0.236 a |
| Age (years) | 61 ± 10.5 | 62.1 ± 9.51 | 0.717 b |
| BMI (Kg/m2) | 29.4 (27.7; 31.5) | 29.4 (27.4; 30.4) | 0.445 b |
| Diabetes (%) | 4 (20%) | 6 (37.5%) | 0.136 a |
| LVEF (%) | 55 (51; 60) | 55 (48; 59.5) | 0.445 c |
| E/A | 0.72 (0.68; 0.83) | 0.805 (0.61; 0.975) | 0.712 c |
| E/E′ | 8.8 (6.52; 10) | 9.76 (7.22; 11.4) | 0.595 b |
| EAT thickness (mm) | 14.5 (12.3; 16.8) | 14.5 (12; 17) | 0.904 b |
| Left Atrial Volume (ml/m2) | 32.5 (28.5; 45.8) | 37 (31.3; 43.8) | 0.439c |
| Creatinine (mg/dl) | 0.89 (0.80; 1.04) | 0.96 (0.78; 1.27) | 0.677 c |
| GFR (ml/min) | 87 (75.25; 99.75) | 80 (59; 93) | 0.250 b |
| Hypertension (%) | 21 (87.5%) | 16 (100%) | 0.141 a |
| CRP (mg/L) | 1.75 (0.825; 4.85) | 1.2 (0.75; 4.1) | 0.617 c |
| Blood Glucose (mg/dl) | 97 (89.25; 115) | 107.5 (96; 168.8) | 0.068 b |
| Sodium (mmol/L) | 141 (140; 142) | 140 (138; 141) | 0.066 b |
| Potassium (mmol/L) | 4.5 (4.125; 4.7) | 4.3 (4.025; 4.7) | 0.745 b |
| Calcium (mg/dl) | 8.95 (8.8; 9.375) | 9.2 (8.925; 9.4) | 0.367 c |
| INR | 1.06 (0.96; 1.10) | 1.06 (1.02; 1.07) | 0.178 b |
| aPTT (sec) | 29.95 (28.18; 32.65) | 30 (28.18; 33.18) | 0.736 b |
| Serum IL-1β (pg/ml) | 3.14 (2.83; 3.53) | 3.24 (2.86; 3.34) | 0.767 c |
| Serum IL-1ra (pg/ml) | 1072 (1025; 1176) | 1132 (1025; 1191) | 0.527 c |
| EAT IL-1β (pg/ml) | 0.86 (0.76; 0.985) | 1.04 (0.873; 1.43) | 0.009 c |
| EAT IL-1ra (pg/ml) | 452 (326; 623) | 654 (361; 781) | 0.121 b |
Results are expressed as median and range (25% percentile; 75% percentile) or as the number of cases (%). Age is expressed as media ±SD. BMI, body mass index; LVEF, left ventricular ejection fraction; EAT, epicardial adipose tissue; IL, interleukine; GFR, glomerular filtration rate; CRP, C-reactive protein; INR, international normalized ratio; aPTT, activated partial thromboplastin time.
Statistically significative values (p < 0.05) are reported in bold.
Chi Squared test.
Unpaired t-test.
Mann-Whitney U test.
Circulating and EAT-Derived Interleukins
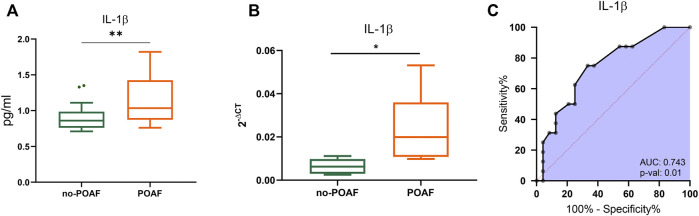
No significant difference was observed in serum levels of IL-1β, IL-1ra and other cytokines, chemokines, and growth factors between POAF and no-POAF groups [IL-1β: 3.24 pg/ml (2.86; 3.34) vs. 3.14 pg/ml (2.83; 3.53); IL-1ra: 1132 pg/ml (1025; 1191) vs. 1072 pg/ml (1025; 1176), Table 1 and Supplementary Table S1]. To gain insight into a possible involvement of local inflammation in POAF occurrence, we obtained conditioned media from EAT biopsies and analyzed a panel of inflammatory mediators. IL-1β local levels were significantly higher in the POAF group compared to the no-POAF group (p = 0.009) (Figure 1A; Table 1). In parallel, no significant differences were observed in EAT-derived IL-1ra between the two groups (Table 1). Consistently, EAT-derived IL-2, IL-5, IL-6, IL-8, and IP-10 levels were significantly higher in the POAF group (Supplementary Table S1). To note, according to secretion, also IL-1β mRNA expression was significantly higher in EAT biopsies from POAF patients compared to no-POAF (p = 0.0308) (Figure 1B).
FIGURE 1.
IL-1β in POAF subgroups. (A) Boxplots denote IL-1β concentration distributions in conditioned media from EAT biopsies of subjects with the no-POAF outcome (“no-POAF”—green) and with POAF outcome (“POAF”—orange); IL-1β concentration is expressed as pg/ml. Box plots denote median and 25th–75th percentiles (boxes) and tukey whiskers. (B) Boxplots denote IL-1β absolute expression in EAT biopsies for 6 subjects with no POAF outcome (“no-POAF”—green) and 6 with POAF outcome (“POAF”—orange). Box plots denote median and 25th–75th percentiles (boxes) and min-to-max whiskers. (C) AUC of ROC analysis indicates the performance of IL-1β. p-value refers to the significant difference from the AUC basal level of 0.5 (red dotted line). *p < 0.05; **p < 0.01.
ROC analyses revealed that IL-1β provides valuable discrimination between the two groups (AUC 0.743, 95% CI: 0.588–0.899, p = 0.01) (Figure 1C).
Interleukins’ Effects on Human Cardiac Fibroblasts
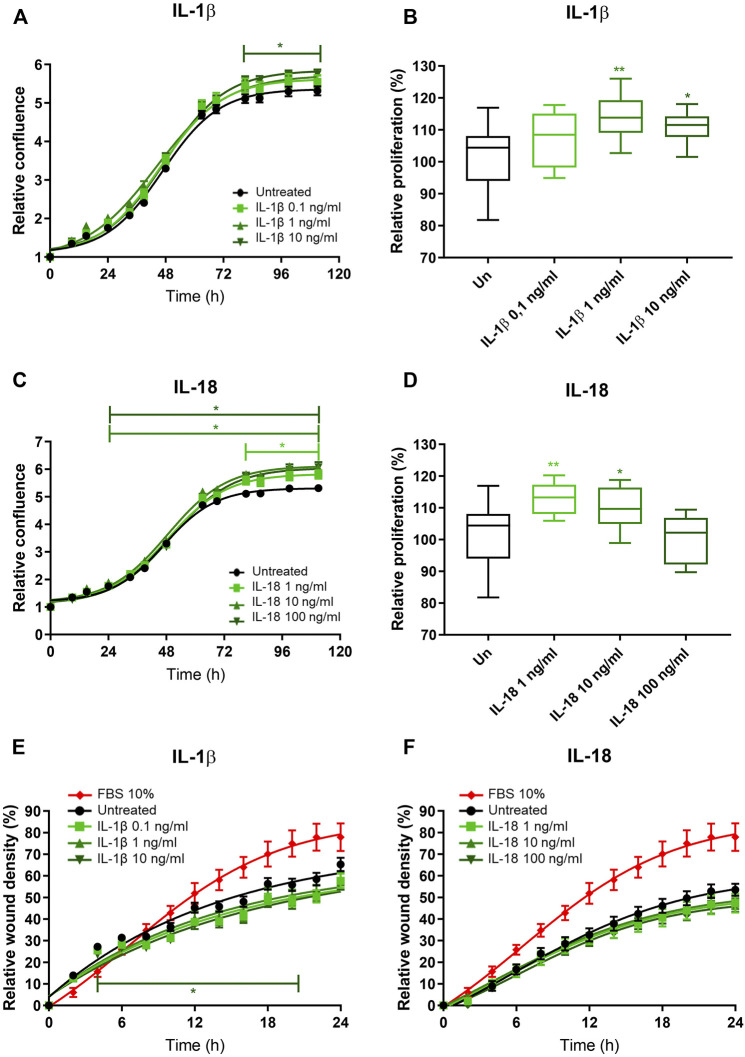
To investigate the potential role of EAT-secreted IL-1β on atrial remodeling and fibrosis, we in vitro tested the effects of IL-1β on human immortalized fibroblasts obtained from the right atrium of patients undergoing cardiac surgery. The relative confluence of fibroblasts exposed to increasing concentrations of recombinant IL-1β was higher compared to the untreated, at the highest tested concentration (10 ng/ml) starting from the third day (p < 0.05; Figure 2A). MTT assay revealed that the proliferation rate of fibroblasts exposed to IL-1β was higher than the control at the concentration of 1 ng/ml (114.0% ± 7.1% vs. 101.6% ± 9.8%; p = 0.003) and 10 ng/ml (111.0% ± 5.2%; p = 0.03), while the lower tested concentration (0.1 ng/ml) had no effect (107.7% ± 8.7%; p = 0.13; ANOVA p = 0.004; Figure 2B). As IL-1β and IL-18 are members of the same structural family and are involved in the NLRP3 inflammasome, we also tested the in vitro effects of IL-18 on fibroblasts. IL-18 induced an increased relative confluence than control, even at the lowest tested concentration (1 ng/ml; Figure 2C). Indeed, the proliferation rate of cells exposed to IL-18 was higher than control (1 ng/ml 112.9% ± 5.0% vs. 10 ng/ml 109.8% ± 6.8% vs. 100 ng/ml 99.9% ± 7.5% vs. control 101.6% ± 9.8%; ANOVA p = 0.003; Figure 2D). To better characterize the effects of these ILs on human fibroblasts, we analyzed the migration rate. IL-1β reduced the invasion capacity of fibroblasts at the highest tested concentration (10 ng/ml; p = 0.05; Figure 2A), while IL-18 did not have any effects on wound healing capacity at any analyzed concentration (Figures 2E,F).
FIGURE 2.
IL-1β and IL-18 effects on cardiac fibroblast proliferation and migration. (A–C) Relative confluence normalized on T0 and (B–D) relative proliferation with respect to the untreated (setted to 100%) and (E,F) relative wound healing density normalized on T0 of human immortalized fibroblasts exposed to different concentrations of (A,B–E) IL-1β and (C,D–F) IL-18 (n = 8). Data are expressed by mean ± SEM. *p < 0.05; **p < 0.01 vs. untreated.
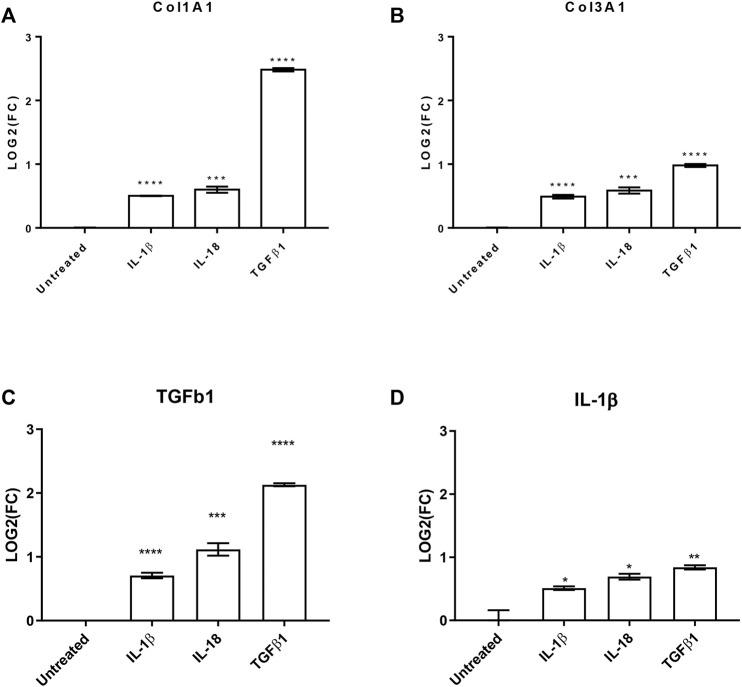
To further evaluate the effects of these interleukins on pro-fibrotic and pro-inflammatory effects on cardiac fibroblast, we investigated by qPCR the production of collagen 1A1 (COL1A1), COL3A1, and transforming growth factor β 1 (TGFβ1) as well as the production of IL-1β. All the analyzed transcripts were significantly upregulated by both the used interleukins (Figure 3). Interestingly, the treatments increased TGFβ1 production, which is the most powerful pro-fibrotic inducer, and at the same time potentiate the pro-inflammatory effects.
FIGURE 3.
IL-1β and IL-18 effects on pro-fibrotic and pro-inflammatory genes. Bar graphs reporting the relative gene expression of (A) collagen 1A1 (Col1A1), (B) collagen 3A1 (Col3A1), (C) transforming growth factor β1 (TGFβ1), and (D) interleukin 1β (IL-1β) of human cardiac fibroblasts under IL-1β, IL-18, and TGFβ1 (as positive control) treatments (n = 3). Data are expressed by mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs. untreated.
In conclusion, IL-1β and IL-18 improved the proliferation rate of human cardiac fibroblasts potentiating the fibrosis machinery with a rebound on the inflammation itself.
Discussion
The present study explored the potential role of EAT-secreted IL-1β in atrial remodeling and POAF occurrence in patients undergoing CABG, without a history of AF. We observed: 1) POAF onset is not associated with clinical nor echocardiographic parameters; 2) In patients with and without POAF there are no differences in EAT thickness, atrial dilation, and fibrosis; 3) EAT-IL1β secreted levels, but not circulating IL-1β levels are associated with POAF occurrence; 4) NLRP3 inflammasome cytokines, IL1β and IL-18 could promote atrial fibrosis.
In patients undergoing cardiac surgery, POAF is a frequent complication and occurs approximately in 20–40% of cases (Dobrev et al., 2019). It is a clinically relevant event, being associated with hemodynamic instability, increased risk of stroke, lengthened hospital and intensive care unit stays, and greater hospital costs (Dobrev et al., 2019). POAF is also a predictor of permanent AF. However, the mechanisms underlying AF and POAF are probably different as POAF is mainly driven by periprocedural triggers. Cardiac surgery itself contributes to the pathophysiology of AF, both in the initiation and maintenance of arrhythmia (Hu et al., 2015). In particular, POAF occurs in presence of factors inducing atrial arrhythmogenic remodeling before surgery and is promoted by peri-operative triggers. In some studies, POAF is associated with preoperative structural alterations including interstitial fibrosis (Maesen et al., 2012). However, in the present study neither left atrial volume, evaluated at echocardiography nor atrial fibrosis, evaluated at histology, were associated with POAF occurrence. Similarly, in our population, we didn’t observe differences in demographic, clinical, and echocardiographic parameters among patients with and without POAF. Thus, here, we can exclude a possible association of POAF with clear predisposing factors.
Inflammation is considered a major trigger for POAF, even if the association between levels of inflammatory cytokines and the occurrence of POAF is not well defined. Recently, it has been reported a strong association between EAT-derived activin A expression and atrial remodeling (Venteclef et al., 2015; Wang et al., 2019). Of note, it has been shown that there is a direct link between activin A and IL-1β in the contest of inflammation and fibrosis. Moreover, this link is bidirectional since the former is able to stimulate the latter expression and vice versa (de Kretser et al., 2012). In this context, our study, focusing on IL-1β, is in accordance with the literature and adds a brick to the wall of knowledge regarding inflammatory cytokines involvement and POAF onset. Of note, in our population, there was no significant differences in circulating levels of cytokines, chemokines and growth factors evaluated. In particular, no variation in circulating levels of IL-1β and IL-1ra between POAF and no-POAF patients, raising the hypothesis of a specific role of EAT and local inflammation in POAF.
EAT is the visceral fat depot of the heart, and it is located between the myocardium and the visceral layer of the pericardium. In physiological conditions, EAT has several protective functions on the heart; however, it is now well established that it can also have undesirable effects on cardiac tissue. In absence of fascial boundaries, EAT directly influences the myocardial electrical and structural remodeling and it is associated with cardiovascular diseases (Mazurek et al., 2003; Chaldakov et al., 2014; Parisi et al., 2015; Ghenev et al., 2016). The release of proinflammatory cytokines by EAT is initiated by the innate inflammatory response through Toll-like receptors (TLRs) located in the macrophages, B cells, dendritic cells, and adipocyte membranes. The receptors recognize antigens such as lipopolysaccharide (LPS) and saturated fatty acids, promoting nuclear factor kappa-beta (NF-κβ) translocation into the nucleus of epicardial adipocytes, with transcription of inflammatory mediators such as IL-1, IL-8, IL-6, and TNF-α, linking innate immunity and chronic inflammatory responses. We previously described that EAT is a source of IL-1β in CAD patients, and the imbalance between levels of IL‐1β and its receptor antagonist (IL‐1ra) drives cardiovascular events (Parisi et al., 2020b).
Our data indicate that EAT inflammatory profile is increased with POAF occurrence. In particular, EAT-mediated IL-1β secretion and mRNA expression are higher in POAF patients and IL-1ra secretion doesn’t differ in the two groups, indicating an increased pro-inflammatory effect of IL-1β in POAF patients. To note, all biopsies were collected at the surgical time, before extracorporeal circulation to avoid possible inflammation of tissues mediated by surgical manipulation. Along with the evidence that local inflammatory status could give rise to abnormal atrial conduction (Mariscalco and Engström, 2008), we have hypothesized that POAF patients may have a pre-existing local inflammatory state characterized by enhanced IL-1β, an essential component of the NLRP3-inflammasome signaling. The NLRP3- inflammasome is a deeply rooted signaling pathway responsible for IL-1β and IL-18 releases from innate immune cells (Heijman et al., 2020).
Patients with POAF are considered to be at higher risk of permanent AF (Dobrev et al., 2019), thus it is plausible that factors that trigger POAF could promote atrial remodeling and fibrosis over time. To verify this hypothesis, we mimicked post-operative inflammation by in vitro stimuli of NLRP3-related cytokines, IL-1β and IL-18, on human fibroblasts isolated from atrial myocardium. We observed that both IL-1β and IL-18 promote fibroblasts proliferation. In patients with AF undergoing left atrial appendage excision, EAT-derived IL-1β was associated with the total atrial collagen content (Abe et al., 2018). However, no evidence is available on POAF patients. Animal studies conducted on mice suggested that IL-18 stimulates fibroblast migration and proliferation (Fix et al., 2011). Moreover, recent evidence supports the hypothesis that pre-existing activation of the atrial cardiomyocyte NLRP3-inflammasome contributes to the POAF-predisposing substrate (Heijman et al., 2020). Here, we provide the first evidence that the paracrine release of IL-1β from EAT could promote POAF, even in absence of pre-operative predisposing factors. Furthermore, we observed that inflammatory stimuli on atrial fibroblasts promote the production of collagen and TGFβ1, the most powerful pro-fibrotic inducer, and at the same time potentiate the pro-inflammatory effects. All the way, our observations give convincing proof that IL-1β could be an important mediator of the pro-fibrotic effect of EAT on atrial remodeling as already described for Activin A (Wang et al., 2019) and Galectin-3 (Rhodes et al., 2013; Fashanu et al., 2017).
This intensive crosstalk between EAT-derived molecules and the surrounding myocardium provides novel insights into the potential establishment of a pro-fibrotic milieu and supports promoting changes in the atrial myocardium. These results pay the way for new studies exploring the potential benefits of anti-inflammatory drugs in the perioperative period and suggest that EAT could mediate the antiarrhythmic effects observed by using anti-inflammatory therapies in animal studies (Wu et al., 2020).
Study Limitations
The present study evaluated the predictive value of pre-operative circulating inflammatory markers in POAF occurrence. We didn’t explore the predictive value of post-operative inflammatory markers. However, since cardiac intervention is an important inflammatory trigger, a dedicated study should be designed to appropriately identify the impact of post-operative inflammatory markers in POAF occurrence.
We in vitro tested the effects of single cytokines on fibroblast proliferation and collagen production. Further studies are required to test the effects of the entire EAT-secretome on cardiac cells and the potential benefits of IL-1β inhibition.
Genetic variation and heritability take part in AF onset (Ellinor et al., 2012; Kolek et al., 2015). Genome-wide association studies have identified several common variant loci associated with AF and functionally implicated in cardiac development, electrophysiology, and cardiomyocyte behavior (Roselli et al., 2020). Surely, adding information on AF genetic variability and/or on common risk Single Nucleotide Polymorphisms (SNPs) would improve insight in our population.
Acknowledgments
We sincerely appreciated the technical help of D. Liguoro and A. D’Andrea.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Naples Federico II. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SC and MC designed research, interpreted the data, and wrote the manuscript. LP and PC enrolled patients, collected clinical/anamnestic data, performed echocardiograms. GC and EP performed epicardial adipose tissue and atrial biopsies during cardiac surgery. ADM and MoC performed histology. SR analyzed serum samples and epicardial fat biopsies and analyzed the data. DM and VV carried out in vitro experiments. MFDT performed the statistical analysis. VD’E and PP critically reviewed the manuscript. PF, DL, and VP conceived the study, critically reviewed the manuscript, and edited it. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2022.893729/full#supplementary-material
References
- Abe I., Teshima Y., Kondo H., Kaku H., Kira S., Ikebe Y., et al. (2018). Association of Fibrotic Remodeling and Cytokines/Chemokines Content in Epicardial Adipose Tissue with Atrial Myocardial Fibrosis in Patients with Atrial Fibrillation. Heart Rhythm 15 (11), 1717–1727. 10.1016/j.hrthm.2018.06.025 [DOI] [PubMed] [Google Scholar]
- Ahlsson A., Fengsrud E., Bodin L., Englund A. (2010). Postoperative Atrial Fibrillation in Patients Undergoing Aortocoronary Bypass Surgery Carries an Eightfold Risk of Future Atrial Fibrillation and a Doubled Cardiovascular Mortality. Eur. J. Cardio-Thoracic Surg. 37 (6), 1353–1359. 10.1016/j.ejcts.2009.12.033 [DOI] [PubMed] [Google Scholar]
- Arai K. Y., Ono M., Kudo C., Fujioka A., Okamura R., Nomura Y., et al. (2011). IL-1β Stimulates Activin βA mRNA Expression in Human Skin Fibroblasts through the MAPK Pathways, the Nuclear Factor-κB Pathway, and Prostaglandin E2. Endocrinology 152 (10), 3779–3790. 10.1210/en.2011-0255 [DOI] [PubMed] [Google Scholar]
- Chaldakov G. N., Fiore M., Ghenev P. I., Beltowski J., Ranćić G., Tunçel N., et al. (2014). Triactome: Neuro-Immune-Adipose Interactions. Implication in Vascular Biology. Front. Immunol. 5, 130. 10.3389/fimmu.2014.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus N. M., Wang L., Lai J., Rigor R. R., Francis Stuart S. D., Bers D. M., et al. (2017). Antiarrhythmic Effects of Interleukin 1 Inhibition after Myocardial Infarction. Heart Rhythm 14 (5), 727–736. 10.1016/j.hrthm.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kretser D. M., O’Hehir R. E., Hardy C. L., Hedger M. P. (2012). The Roles of Activin A and its Binding Protein, Follistatin, in Inflammation and Tissue Repair. Mol. Cell Endocrinol. 359 (1-2), 101–106. 10.1016/j.mce.2011.10.009 [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. (2011). Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 117 (14), 3720–3732. 10.1182/blood-2010-07-273417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev D., Aguilar M., Heijman J., Guichard J.-B., Nattel S. (2019). Postoperative Atrial Fibrillation: Mechanisms, Manifestations and Management. Nat. Rev. Cardiol. 16 (7), 417–436. 10.1038/s41569-019-0166-5 [DOI] [PubMed] [Google Scholar]
- Ellinor P. T., Lunetta K. L., Albert C. M., Glazer N. L., Ritchie M. D., Smith A. V., et al. (2012). Meta-Analysis Identifies Six New Susceptibility Loci for Atrial Fibrillation. Nat. Genet. 44 (6), 670–675. 10.1038/ng.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fashanu O. E., Norby F. L., Aguilar D., Ballantyne C. M., Hoogeveen R. C., Chen L. Y., et al. (2017). Galectin-3 and Incidence of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 192, 19–25. 10.1016/j.ahj.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix C., Bingham K., Carver W. (2011). Effects of Interleukin-18 on Cardiac Fibroblast Function and Gene Expression. Cytokine 53 (1), 19–28. 10.1016/j.cyto.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghenev P. I., Kitanova M., Popov H., Evtimov N., Stoev S., Tonchev A., et al. (2016). Neuroadipobiology of Arrhythmogenic Right Ventricular Dysplasia. An Immunohistochemical Study of Neurotrophins. Adipobiology 8, 55–58. 10.14748/adipo.v8.2214 [DOI] [Google Scholar]
- Heijman J., Muna A. P., Veleva T., Molina C. E., Sutanto H., Tekook M., et al. (2020). Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Postoperative Atrial Fibrillation. Circ. Res. 127 (8), 1036–1055. 10.1161/CIRCRESAHA.120.316710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.-F., Chen Y.-J., Lin Y.-J., Chen S.-A. (2015). Inflammation and the Pathogenesis of Atrial Fibrillation. Nat. Rev. Cardiol. 12 (4), 230–243. 10.1038/nrcardio.2015.2 [DOI] [PubMed] [Google Scholar]
- Kolek M. J., Muehlschlegel J. D., Bush W. S., Parvez B., Murray K. T., Stein C. M., et al. (2015). Genetic and Clinical Risk Prediction Model for Postoperative Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 8 (1), 25–31. 10.1161/CIRCEP.114.002300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Kang D. R., Uhm J.-S., Shim J., Sung J.-H., Kim J.-Y., et al. (2014). New-Onset Atrial Fibrillation Predicts Long-Term Newly Developed Atrial Fibrillation after Coronary Artery Bypass Graft. Am. Heart J. 167 (4), 593–600. 10.1016/j.ahj.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Maesen B., Nijs J., Maessen J., Allessie M., Schotten U. (2012). Post-Operative Atrial Fibrillation: A Maze of Mechanisms. Europace 14 (2), 159–174. 10.1093/europace/eur208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariscalco G., Engström K. G. (2008). Atrial Fibrillation after Cardiac Surgery: Risk Factors and Their Temporal Relationship in Prophylactic Drug Strategy Decision. Int. J. Cardiol. 129 (3), 354–362. 10.1016/j.ijcard.2007.07.123 [DOI] [PubMed] [Google Scholar]
- Matsushita N., Ishida N., Ibi M., Saito M., Takahashi M., Taniguchi S., et al. (2019). IL-1β Plays an Important Role in Pressure Overload-Induced Atrial Fibrillation in Mice. Biol. Pharm. Bull. 42 (4), 543–546. 10.1248/bpb.b18-00363 [DOI] [PubMed] [Google Scholar]
- Mazurek T., Zhang L., Zalewski A., Mannion J. D., Diehl J. T., Arafat H., et al. (2003). Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 108 (20), 2460–2466. 10.1161/01.CIR.0000099542.57313.C5 [DOI] [PubMed] [Google Scholar]
- Melduni R. M., Schaff H. V., Bailey K. R., Cha S. S., Ammash N. M., Seward J. B., et al. (2015). Implications of New-Onset Atrial Fibrillation after Cardiac Surgery on Long-Term Prognosis: A Community-Based Study. Am. Heart J. 170 (4), 659–668. 10.1016/j.ahj.2015.06.015 [DOI] [PubMed] [Google Scholar]
- Parisi V., Petraglia L., Cabaro S., D’Esposito V., Bruzzese D., Ferraro G., et al. (2020). Imbalance between Interleukin-1β and Interleukin-1 Receptor Antagonist in Epicardial Adipose Tissue is Associated with Non ST-Segment Elevation Acute Coronary Syndrome. Front. Physiol. 11, 42. 10.3389/fphys.2020.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi V., Petraglia L., Formisano R., Caruso A., Grimaldi M. G., Bruzzese D., et al. (2020). Validation of the Echocardiographic Assessment of Epicardial Adipose Tissue Thickness at the Rindfleisch Fold for the Prediction of Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 30 (1), 99–105. 10.1016/j.numecd.2019.08.007 [DOI] [PubMed] [Google Scholar]
- Parisi V., Rengo G., Pagano G., D'Esposito V., Passaretti F., Caruso A., et al. (2015). Epicardial Adipose Tissue has an Increased Thickness and is a Source of Inflammatory Mediators in Patients with Calcific Aortic Stenosis. Int. J. Cardiol. 186, 167–169. 10.1016/j.ijcard.2015.03.201 [DOI] [PubMed] [Google Scholar]
- Petraglia L., Conte M., Comentale G., Cabaro S., Campana P., Russo C., et al. (2022). Epicardial Adipose Tissue and Postoperative Atrial Fibrillation. Front. Cardiovasc. Med. 9, 810334. 10.3389/fcvm.2022.810334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. H., Pini M., Castellanos K. J., Montero-Melendez T., Cooper D., Perretti M., et al. (2013). Adipose Tissue-Specific Modulation of Galectin Expression in Lean and Obese Mice: Evidence for Regulatory Function. Obesity 21 (2), 310–319. 10.1002/oby.20016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli C., Rienstra M., Ellinor P. T. (2020). Genetics of Atrial Fibrillation in 2020: GWAS, Genome Sequencing, Polygenic Risk, and Beyond. Circ. Res. 127 (1), 21–33. 10.1161/CIRCRESAHA.120.316575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteclef N., Guglielmi V., Balse E., Gaborit B., Cotillard A., Atassi F., et al. (2015). Human Epicardial Adipose Tissue Induces Fibrosis of the Atrial Myocardium through the Secretion of Adipo-Fibrokines. Eur. Heart J. 36 (13), 795–805. 10.1093/eurheartj/eht099 [DOI] [PubMed] [Google Scholar]
- Wang Q., Min J., Jia L., Xi W., Gao Y., Diao Z., et al. (2019). Human Epicardial Adipose Tissue Activin A Expression Predicts Occurrence of Postoperative Atrial Fibrillation in Patients Receiving Cardiac Surgery. Heart Lung Circ. 28 (11), 1697–1705. 10.1016/j.hlc.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Wong C. X., Ganesan A. N., Selvanayagam J. B. (2017). Epicardial Fat and Atrial Fibrillation: Current Evidence, Potential Mechanisms, Clinical Implications, and Future Directions. Eur. Heart J. 38 (17), 1294–1302. 10.1093/eurheartj/ehw045 [DOI] [PubMed] [Google Scholar]
- Wu Q., Liu H., Liao J., Zhao N., Tse G., Han B., et al. (2020). Colchicine Prevents Atrial Fibrillation Promotion by Inhibiting IL-1β-induced IL-6 Release and Atrial Fibrosis in the Rat Sterile Pericarditis Model. Biomed. Pharmacother. 129, 110384. 10.1016/j.biopha.2020.110384 [DOI] [PubMed] [Google Scholar]
- Yao C., Veleva T., Scott L., Cao S., Li L., Chen G., et al. (2018). Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 138 (20), 2227–2242. 10.1161/CIRCULATIONAHA.118.035202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.