Key Points
Long-term RESONATE-2 data show sustained PFS and OS benefits (medians not reached) for first-line ibrutinib treatment in patients with CLL.
Forty-two percent of patients continued ibrutinib for up to 8 years; dose management for AEs allowed patients continue to benefit from ibrutinib.
Visual Abstract
Abstract
We report long-term follow-up from the RESONATE-2 phase 3 study of the once-daily Bruton’s tyrosine kinase inhibitor ibrutinib, which is the only targeted therapy with significant progression-free survival (PFS) and overall survival (OS) benefit in multiple randomized chronic lymphocytic leukemia (CLL) studies. Patients (≥65 years) with previously untreated CLL, without del(17p), were randomly assigned 1:1 to once-daily ibrutinib 420 mg until disease progression/unacceptable toxicity (n = 136) or chlorambucil 0.5-0.8 mg/kg ≤12 cycles (n = 133). With up to 8 years of follow-up (range, 0.1-96.6 months; median, 82.7 months), significant PFS benefit was sustained for ibrutinib vs chlorambucil (hazard ratio [HR], 0.154; 95% confidence interval [CI], 0.108-0.220). At 7 years, PFS was 59% for ibrutinib vs 9% for chlorambucil. PFS benefit was also observed for ibrutinib- vs chlorambucil-randomized patients with high-risk genomic features: del(11q) (HR, 0.033; 95% CI, 0.010-0.107) or unmutated immunoglobulin heavy chain variable region (HR, 0.112; 95% CI, 0.065-0.192). OS at 7 years was 78% with ibrutinib. Prevalence of adverse events (AEs) was consistent with previous 5-year follow-up. Ibrutinib dosing was held (≥7 days) for 79 patients and reduced for 31 patients because of AEs; these AEs resolved or improved in 85% (67 of 79) and 90% (28 of 31) of patients, respectively. With up to 8 years of follow-up, 42% of patients remain on ibrutinib. Long-term RESONATE-2 data demonstrate sustained benefit with first-line ibrutinib treatment for CLL, including for patients with high-risk genomic features. These trials were registered at www.clinicaltrials.gov as #NCT01722487 and #NCT01724346.
Introduction
Prior to the introduction of novel targeted agents, the standard-of-care for patients older than 65 years with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) included the chemotherapy chlorambucil.1 Primary results from the pivotal RESONATE-2 phase 3 study demonstrated superior efficacy and tolerability of ibrutinib, a once-daily Bruton’s tyrosine kinase (BTK) inhibitor, vs standard-of-care chemotherapy, and supported the initial approval in the United States and European Union2 for patients with CLL/SLL treated in the first-line setting.3 Additionally, ibrutinib is the only therapy to demonstrate both a significant progression-free survival (PFS)3-8 and overall survival (OS)3,4,7,8 benefit in multiple randomized phase 3 studies for patients with first-line CLL/SLL.
Evidence from RESONATE-2 also showed that patients with high-risk genomic features, such as chromosome 11q deletion (del[11q]) or unmutated immunoglobulin heavy chain variable region (IGHV), that predict inferior outcomes with chemotherapy and chemoimmunotherapy, experienced significantly improved long-term efficacy outcomes with single-agent ibrutinib vs chlorambucil.9 At the last update for RESONATE-2, the median PFS for ibrutinib was not reached with a median follow-up of 5 years; 70% of patients with CLL treated with first-line ibrutinib remained progression-free and alive.9
Long-term data on targeted agents in the treatment of patients with CLL/SLL are limited. Therefore, reporting extended follow-up data on patient outcomes and safety is essential to inform clinical decision making. Currently, ibrutinib is the BTK inhibitor with the longest follow-up data in first-line CLL/SLL and other B-cell malignancies.9-13 Herein we report up to 8 years of efficacy and safety data for patients with previously untreated CLL/SLL from the phase 3 RESONATE-2 study.
Methods
Study design and population
RESONATE-2 is a phase 3, open-label, multicenter, international, randomized study (PCYC-1115/PCYC-1116; #NCT01722487 and #NCT01724346) comparing the efficacy and safety of ibrutinib vs chlorambucil in first-line CLL/SLL. Detailed methods have been previously reported.3 Previously untreated patients aged ≥ 65 years requiring therapy per the 2008 International Workshop on CLL criteria14 for CLL/SLL and without chromosome 17p deletion [del(17p)] were randomly assigned 1:1 to once-daily ibrutinib 420 mg until progressive disease (PD) or unacceptable toxicity or to up to 12 cycles of chlorambucil 0.5 mg/kg, increased up to 0.8 mg/kg as tolerated, on days 1 and 15 of each 28-day cycle. Patients randomly assigned to chlorambucil were eligible to cross over to second-line treatment with ibrutinib after confirmed PD. After a median follow-up of 5 years, the protocol was amended to provide up to 10 years of PFS follow-up for both arms; up to 10 years of OS follow-up were planned for the ibrutinib arm only.
This study was conducted according to principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice, and it was approved by the institutional review boards of participating institutions. All patients provided written informed consent.
End points and assessments
End points included PFS, overall response rate (ORR), improvement in hematologic parameters, and safety. Per follow-up, OS is reported here only for patients randomly assigned to ibrutinib. Long-term response was investigator-assessed per International Workshop on CLL 2008 criteria.14 PFS and OS were analyzed according to the Kaplan-Meier method. Hazard ratios (HRs) were estimated using a stratified Cox regression model with treatment as the only covariate; stratification factors used in the model were Eastern Cooperative Oncology Group score (0-1 vs 2) and Rai stage (0/I/II vs III/IV) at baseline. Long-term safety data are reported for patients receiving ongoing treatment in the ibrutinib arm only. Standardized Medical Dictionary for Regulatory Activities queries from MedDRA version 24.0 were used to assess adverse effects (AEs) of clinical interest: major hemorrhage (terms included vitreous hemorrhage, gastrointestinal hemorrhage, postprocedural hemorrhage, subdural hematoma, traumatic hematoma, cerebral hemorrhage, subarachnoid hemorrhage, hematuria) and hypertension (narrow; terms included blood pressure increased, hypertension).15
Results
Patients
In RESONATE-2, 269 patients were randomly assigned to receive ibrutinib (n = 136) or chlorambucil (n = 133; supplemental Figure 1). Baseline patient characteristics were well matched between treatment arms (supplemental Table 1).3 Among enrolled patients, 53% (143 of 269) had 1 or more high-risk genomic features (TP53 mutation, del[11q], and/or unmutated IGHV). Of evaluable patients with available data, 22% (54 of 251) had del(11q) and 58% (118 of 204) had unmutated IGHV.
At the current median follow-up of 7.4 years (88.5 months; range, 0.1-96.6) for patients in the ibrutinib arm, 57 patients (42%) continued first-line ibrutinib treatment (supplemental Table 2). One patient (<1%) randomly assigned to chlorambucil continues to be followed for PFS. Seventy-eight patients (59%) treated with chlorambucil subsequently crossed over to receive second-line ibrutinib treatment after PD (1 patient crossed over to receive ibrutinib without documented PD).
PFS and OS
With up to 8 years of follow-up, the median PFS has not yet been reached for patients in the ibrutinib arm (95% confidence interval [CI], 82.1 months-not estimable [NE]) and was 15 months (95% CI, 10.2-19.4) for patients in the chlorambucil arm (Figure 1A). Ibrutinib treatment led to an 85% reduction in risk of PD or death vs chlorambucil (HR, 0.154; 95% CI, 0.108-0.220). At 7 years, 59% of ibrutinib-randomized patients were estimated to be progression free and alive vs 9% of chlorambucil-randomized patients. Thirty-one patients (23%) in the ibrutinib arm progressed at any point during study follow-up, but only 18 patients (13%) progressed while on active ibrutinib treatment, and the remaining 13 patients (10%) progressed after drug discontinuation.
Figure 1.
Investigator-assessed PFS. (A) PFS with single-agent ibrutinib vs chlorambucil in first-line CLL/SLL in the intent-to-treat population. PFS by (B) del(11q) status and (C) IGHV mutational status. Survival analyses are from randomization until event or censoring at last evidence of non-PD; vertical tick marks indicate censored patients. NE, not estimable; NR, not reached.
Sustained benefit for ibrutinib vs chlorambucil was maintained across subgroup analyses of baseline clinical characteristic factors such as advanced stage and bulky disease (Figure 2). A significant PFS benefit with ibrutinib was also observed for patients with high-risk genomic features, that is, TP53 mutation, del(11q), and/or unmutated IGHV (HR, 0.098; 95% CI, 0.060-0.161). A similar benefit was observed when del(11q) and unmutated IGHV were assessed individually. Patients with del(17p) were excluded from the study, and the small numbers of patients with TP53 mutation (ibrutinib, n = 11; chlorambucil, n = 3) precluded meaningful PFS comparison and analysis.
Figure 2.
Subgroup analysis of PFS. Forest plot of PFS in baseline factor subgroups of interest. ECOG, Eastern Cooperative Oncology Group.
Ibrutinib led to a 97% reduction in risk of PD or death in patients with del(11q) and 81% for those without del(11q) vs chlorambucil (Figure 1B). At 7 years, PFS rates were higher for ibrutinib vs chlorambucil for patients with del(11q) (52% vs 0%) and without del(11q) (61% vs 12%). PFS follow-up was complete for all chlorambucil-randomized patients with del(11q) (n = 25) as of 4 years on study, including 3 patients with censored observations. PFS was similar in ibrutinib-randomized patients with and without del(11q) (HR, 1.228; 95% CI, 0.672-2.241). Ibrutinib also led to an 89% reduction in the risk of PD or death in patients with unmutated IGHV and 83% for patients with mutated IGHV vs chlorambucil (Figure 1C). At 7 years, PFS rates were higher for ibrutinib vs chlorambucil for patients with unmutated IGHV (58% vs 2%) or with mutated IGHV (68% vs 17%). Importantly, PFS was similar in ibrutinib-randomized patients with mutated vs unmutated IGHV (HR, 0.858; 95% CI, 0.437-1.686).
Median OS was not reached for ibrutinib-randomized patients, and the 7-year survival estimate was 78% (Figure 3; HR vs chlorambucil: 0.453; 95% CI, 0.276–0.743). Patients with the high-risk genomic features TP53 mutation, del(11q), and/or unmutated IGHV had improved OS with ibrutinib treatment consistent with the intent-to-treat population (HR vs chlorambucil: 0.461; 95% CI, 0.236–0.900).
Figure 3.
Long-term OS. OS with single-agent ibrutinib vs chlorambucil in first-line CLL/SLL for intent-to-treat population. Brackets indicate that OS was not captured for chlorambucil arm of patients with PD after the median 5 years of complete follow-up. Survival analyses are from randomization until event or censoring at last follow-up; vertical tick marks indicate censored patients. NR, not reached.
Overall response
With this extended follow-up, the proportion of ibrutinib-randomized patients with a best response of complete response (CR) or CR with incomplete bone marrow recovery (CRi) continued to increase up to 34% from 30% observed at 5-year follow-up (Figure 4). ORR was 92% for patients randomly assigned to ibrutinib (including partial response with lymphocytosis) and 37% for patients randomly assigned to chlorambucil, which is consistent with prior follow-ups. Responses with ibrutinib treatment were durable, with the median duration of response not reached (95% CI, 83.8 months-NE) compared with chlorambucil treatment (29.7 months; 95% CI, 15.2-40.4). The median duration of CR was not reached (95% CI, NE) with ibrutinib and was 48.8 months (95% CI, 3.3-NE) with chlorambucil. For ibrutinib-randomized patients who had attained CR/CRi (n = 46), the median PFS was not reached (95% CI: NE); the median PFS was 85 months (95% CI: 63.3 months-NE) for those who had PR (nodular PR, PR, and/or PR with lymphocytosis; n = 79 patients; HR, 0.291; 95% CI, 0.141-0.604). For ibrutinib-randomized patients, ORR was similar in patients with vs without del(11q) (100% vs 90%, respectively), whereas the CR/CRi rate was slightly higher for patients with del(11q) vs without del(11q) (45% vs 31%). ORR and CR/CRi rates were similar for ibrutinib-randomized patients with mutated vs unmutated IGHV (ORR: 88% vs 95%; CR: 33% vs 34%, respectively).
Figure 4.
Investigator-assessed ORR. Cumulative best response over time in all ibrutinib-randomized patients. Percentages of patients in each category of response may not add up to the overall proportion with a response because of rounding. nPR, nodular partial response; PR-L, partial response with lymphocytosis; SD, stable disease.
Safety
The median duration of ibrutinib treatment was 74 months (range, 0.7-96.6 months; supplemental Table 2), and median relative dose intensity was 98% at current follow-up. Chlorambucil safety data are unchanged as reported previously3 with respect to that limited-duration treatment. The most frequent AEs of any grade with ibrutinib were diarrhea (50%), cough (37%), and fatigue (37%). The most common any-grade and grade ≥ 3 AEs over time are shown in Figure 5.
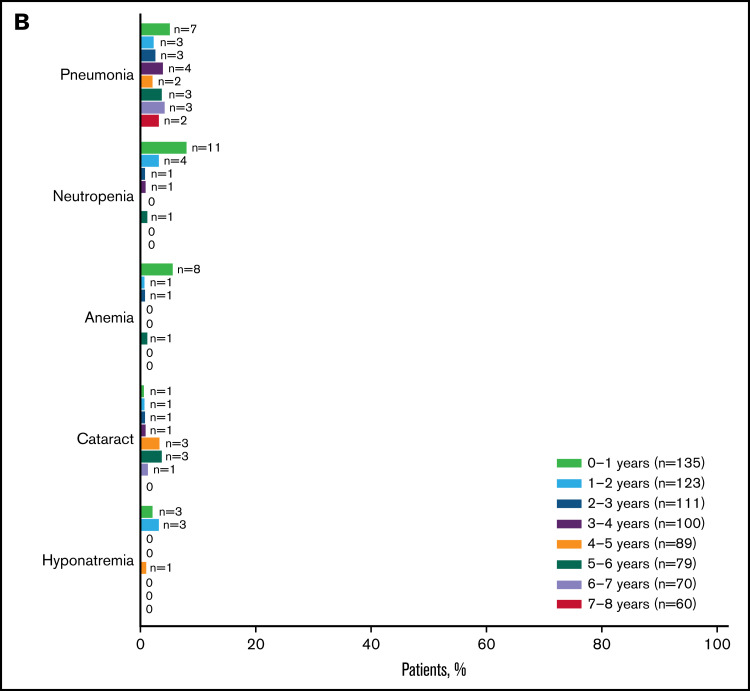
Figure 5.
Summary of AEs for ibrutinib-treated patients. The most common any-grade (A) and grade ≥ 3 AEs (B) are shown by yearly interval. Prevalence was determined by the proportion of patients with a given AE (existing event or new onset of an event) during each yearly interval. Multiple onsets of the same AE term within a specific yearly interval were counted once, and the same AE term continuing across several yearly intervals was counted in each of the intervals. Atrial fibrillation and hypertension are shown in Figure 6. URTI, upper respiratory tract infection; UTI, urinary tract infection.
For AEs of clinical interest, the prevalence rates of hypertension for the current follow-up (grades 1-3; no grade 4 or 5 events occurred) were 25%, 23%, and 25% of patients in years 5-6, 6-7, and 7-8, respectively (Figure 6). Overall, grade 3 hypertension occurred in 17 (12%) patients. Prevalence rates of atrial fibrillation (grades 1-3; no grade 4 or 5 events occurred) over time were 9%, 7%, and 7% of patients in years 5-6, 6-7, and 7-8, respectively (Figure 6). Overall, grade 3 atrial fibrillation occurred in 8 (6%) patients. No patients had any-grade major hemorrhage events in years 5-6 and 6-7; 3% of patients had any-grade major hemorrhage events in years 7-8 (Figure 6). Overall, grade 3 major hemorrhage occurred in 10 (7%) patients, grade 4 in 1 (<1%) patient, and no grade 5 events occurred. Two of the 10 patients (20%) with grade 3 major hemorrhage were also taking concomitant anticoagulation medications. With up to 8 years of follow-up, fatal cardiac events occurred in 4 (3%) patients: cardiac failure (n = 2), myocardial infarction (n = 1), and cardiopulmonary failure (n = 1).
Figure 6.
AEs of clinical interest for ibrutinib-treated patients. Any-grade AEs of clinical interest are shown by yearly interval. Prevalence was determined by the proportion of patients with a given AE (existing event or new onset of an event) during each yearly interval. Multiple onsets of the same AE term within a specific yearly interval were counted once, and the same AE term continuing across several yearly intervals was counted in each of the intervals. aCombined terms.
Discontinuations and dose management with ibrutinib treatment
The primary reason for discontinuation was AEs for 32 patients (24%). Treatment discontinuations because of AEs generally decreased over time, with 7% of patients (9 of 135) discontinuing in years 0 to 1, 6% (7 of 121) in years 1 to 2, 5% (6 of 111) in years 2 to 3, 6% (6 of 99) in years 3 to 4, 1% (1 of 88) in years 4 to 5, 3% (2 of 79) in years 5 to 6, no patients in years 6 to 7, and 2% (1 of 60) in years 7 to 8 (supplemental Figure 2A). AEs leading to discontinuations occurring in >1 patient were atrial fibrillation (n = 5), pneumonia (n = 3), and palpitations (n = 2; supplemental Table 2).
Thirty-one patients (23%) had dose reductions on study because of AEs; 28 of these patients (90%) had improvement or resolution of the AE following dose reduction. The rate of dose reductions because of AEs was highest in years 0 to 1 (9%, 12 of 135) and lower in subsequent years: 5% (6 of 121) in years 1 to 2, 5% (5 of 111) in years 2 to 3, 4% (4 of 99) in years 3 to 4, 5% (4 of 88) in years 4 to 5, 4% (3 of 79) in years 5 to 6, 4% (3 of 70) in years 6 to 7, and no patients (0 of 60) in years 7 to 8 (supplemental Figure 2B). Ibrutinib discontinuation following dose reduction occurred in 20 patients because of AEs (n = 12; 2 patients had dose reduction and subsequent discontinuation because of the same AE) or because of study withdrawal (n = 4), PD (n = 3), or physician decision (n = 1). AEs leading to dose reduction in >1 patient were thrombocytopenia (n = 3) and anemia, arthralgia, diarrhea, fatigue, and palpitations (n = 2 each). After dose reductions related to AEs (lasting a median of 92 days), the ibrutinib dose was re-escalated back to the previous dose in 8 of the 31 patients with dose reduction (26%). Re-escalated ibrutinib treatment continued for a median of 731 days (24.0 months).
Ibrutinib dose-holds (for ≥7 consecutive days) because of AEs of any grade were reported for 79 patients, with most patients (85%, 67 of 79) having improved or resolved AEs following their dose-hold. For the 79 patients with dose-holds, the median duration between first dose-hold of ibrutinib to study treatment discontinuation or last known date alive for those still on treatment was 49 months (maximum, 92+ months). Following dose-hold, ibrutinib was restarted at the same dose in 50 of 79 patients (63%) and at a reduced dose in 23 of 79 patients (29%).
Concomitant medications
Concomitant medication data were summarized during the treatment period, which was a median of 74 months for ibrutinib and a median of 7 months for chlorambucil. Concomitant medications of clinical interest are shown in Table 1. Anticoagulants and/or antiplatelet agents were frequently used (73% and 54%) during treatment with ibrutinib and chlorambucil, respectively, as were antihypertensive medications (73% and 61%), including agents acting on the renin-angiotensin system (56% and 42%), and medications to treat acid-related disorders (64% and 41%), including proton pump inhibitors (56% and 36%). The rate of neutrophil growth factor use was similar between the arms (10% and 12%). In ibrutinib-treated patients who received concomitant medications for acid-related disorders, the estimated proportion of patients who were progression free and alive at 7 years (any anti-acid agent, 61%; proton pump inhibitors, 61%) was similar to that observed in all ibrutinib-randomized patients (59%; supplemental Figure 3).
Table 1.
Concomitant medications of clinical interest
| Ibrutinib, n = 135 | Chlorambucil, n = 132 | |
|---|---|---|
| Antithrombotics, n (%) | 99 (73) | 71 (54) |
| Antiplatelets | 82 (61) | 67 (51) |
| Anticoagulants | 49 (36) | 13 (10) |
| Antihypertensives, n (%) | 98 (73) | 80 (61) |
| Agents acting on the renin-angiotensin system | 76 (56) | 55 (42) |
| β-Blocking agents | 62 (46) | 45 (34) |
| Calcium channel blockers | 49 (36) | 15 (11) |
| Other* | 15 (11) | 6 (5) |
| Acid-related disorders, n (%) | 87 (64) | 54 (41) |
| H2-receptor antagonists | 23 (17) | 10 (8) |
| Proton pump inhibitors | 75 (56) | 47 (36) |
| Other† | 17 (13) | 3 (2) |
| Neutrophil growth factors, n (%) | 13 (10) | 16 (12) |
Excluding agents acting on the renin-angiotensin system, β-blocking agents, and calcium channel blockers.
Excluding proton pump inhibitors or H2-blockers.
Outcomes after ibrutinib discontinuation
For patients who discontinued ibrutinib because of AEs (n = 32), OS estimate rate at 7 years from the time of randomization was 60%. Discontinuations of ibrutinib because of PD occurred in 18 patients. As previously reported,9 this included 2 patients with Richter’s transformation. Of patients who discontinued because of PD, 72% (13 of 18) remain alive or had exited the study with no known death as of data cutoff. The median OS following discontinuation of ibrutinib because of PD (n = 18) was not reached (95% CI, 3.3 months-NE).
First subsequent therapy after ibrutinib discontinuation was reported for 22 patients and included chemoimmunotherapy (n = 9; including fludarabine plus cyclophosphamide and rituximab, bendamustine plus rituximab, vincristine plus rituximab, and obinutuzumab plus chlorambucil), chemotherapy (n = 3; chlorambucil, bendamustine), novel agents (n = 7; including 4 patients on venetoclax), immunotherapy (n = 1), investigational agent (n = 1), and radiation (n = 1). Thirteen patients had the best overall response to the first subsequent drug reported, with 9 who responded, 2 who had stable disease, and 2 who had PD. At the current data cut, of the 22 patients with subsequent therapy, 13 remained on study follow-up, 3 patients withdrew consent, 4 patients died, and 2 patients exited the study at PCYC-1115 closure. In the second or later line of therapy after ibrutinib, 3 patients received venetoclax or venetoclax plus rituximab.
Discussion
These unprecedented long-term data among phase 3 studies of a targeted agent in the first-line treatment of patients with CLL/SLL provide important data for informed decision making in the current treatment landscape. With up to 8 years of follow-up, the median PFS was not reached for patients in the ibrutinib arm, and the majority of patients remain progression free. Only 18 patients have had PD with continuous long-term use of single-agent ibrutinib to date, and rates of CR/CRi continue to increase with further follow-up (34%) compared with 11% at primary analysis with a median follow-up of 18 months.3 This is similar to what was observed previously with long-term follow-up in the phase 1b/2 PCYC-1102/1103 study: previously untreated patients achieved a CR/CRi rate of 35% with up to 8 years of follow-up.11
Ibrutinib continues to be effective for patients with 1 or more high-risk genomic features (TP53 mutation, del[11q], and/or unmutated IGHV), with a 90% reduction in the risk of progression or death overall compared with chlorambucil treatment. Of note, RESONATE-2 excluded patients with del(17p), and as such, the population with TP53 mutation is limited. del(11q) and unmutated IGHV have been shown to be predictors of poor outcomes,16 particularly for patients treated with chemotherapy or chemoimmunotherapy.17-19 At 7 years, RESONATE-2 patients with del(11q) or unmutated IGHV who were randomly assigned to ibrutinib had a significant benefit, with PFS rates of 52% and 58% of patients, respectively. Importantly, patients in the ibrutinib arm who had del(11q) or unmutated IGHV experienced PFS benefits similar to those in patients without del(11q) or with mutated IGHV, respectively. Taken together, our results continue to demonstrate ibrutinib’s effectiveness regardless of genomic risk status. This confirms prior analyses demonstrating that high-risk prognostic risk factors such as del(11q) or unmutated IGHV have less prognostic significance with ibrutinib treatment.20,21
At 7 years, ibrutinib-randomized patients in RESONATE-2 demonstrated an unprecedented OS rate of 78%, confirming the long-term value of first-line ibrutinib treatment, including for patients with high-risk disease features. OS benefit for ibrutinib vs chlorambucil was previously established in analyses both with and without censoring for crossover, with HRs of 0.376 (95% CI, 0.180-0.786) and 0.450 (95% CI, 0.266-0.761), respectively.9 However, these analyses are confounded by the high number of patients in the chlorambucil arm who crossed over to receive ibrutinib (n = 78), with ibrutinib use likely prolonging survival of the patients who crossed over consistent with data in relapsed/refractory populations.10 After a median follow-up of 5 years, patients in the chlorambucil arm who had experienced PD were exited from the study, and a protocol amendment to extend the study to 10 years focused on PFS for both arms and OS for ibrutinib only. Eligible exiting patients could continue ibrutinib in a long-term extension study (PCYC-1145-LT; #NCT03229200).
In terms of concomitant medications, the use of neutrophil growth factor was similar between the 2 treatment arms despite a longer reporting period for patients in the ibrutinib arm vs the chlorambucil arm. In patients treated with ibrutinib, the use of antithrombotic agents was frequent (73%); however, major hemorrhage events were generally rare and decreased over time. Recently, analyses of fatal cardiac events evaluated the impact of cardiovascular disease or angiotensin-converting enzyme inhibitor use in patients treated with ibrutinib plus rituximab in the FLAIR study.22 By contrast, the safety profile of ibrutinib has now been well established with up to 8 years of follow-up, and although use of agents acting on the renin-angiotensin system (angiotensin-converting enzyme inhibitors or angiotensin II receptor antagonists) was frequent (56%) in ibrutinib-treated patients on RESONATE-2, the incidence of cardiac events was consistent with previous reports.23,24 Furthermore, more than half of patients in this study received medications for acid-related disorders while on study treatment, including proton pump inhibitors, with no apparent impact on PFS in ibrutinib-treated patients. Ibrutinib co-administration with acid-altering medications is not contraindicated. Absorption of the BTK inhibitor acalabrutinib is impacted by co-administration with these classes of medications,25 and thus use should be avoided in such patients, an important consideration for treatment selection.26
Ibrutinib remains well tolerated, with no new safety signals observed with long-term follow-up. Indeed, nearly half of patients remain on ibrutinib at up to 8 years of follow-up. The rate of discontinuation because of AEs was most frequent during the first year of ibrutinib treatment and generally decreased over time, which is consistent with previously published studies.27,28 Overall, with longer follow-up, rates of discontinuation because of AEs remain low. Active dose management (dose-holds and reductions) to address AEs enabled most patients who required such dose management to continue benefiting from ibrutinib treatment, and dose re-escalation after AE resolution was feasible. Real-world evidence indicates that practicing dose management (using dose reductions or modifications) resulted in improvement or resolution of AEs without evident impact on disease outcomes.29
The breadth of experience with targeted agents is still expanding; however, ibrutinib has the longest demonstrated efficacy across multiple phase 3 studies,6,7 whereas other targeted agents, including follow-on BTK inhibitors approved or in development for CLL, lack comparable long-term data.30-32 Here we demonstrated in the longest follow-up to date from a phase 3 study of first-line novel agent therapy in patients with CLL/SLL that nearly half of patients with CLL/SLL were able to receive long-term continuous first-line treatment with single-agent ibrutinib. With up to 8 years of follow-up, single-agent ibrutinib continues to confer sustained PFS benefit vs chlorambucil. This persistent efficacy extended to patients with the high-risk genomic features TP53 mutation, del[11q], and/or unmutated IGHV. Superior benefit for ibrutinib vs chlorambucil was also maintained across subgroup analyses of baseline clinical characteristic factors such as advanced stage and bulky disease. The tolerability and safety of ibrutinib observed with long-term follow-up was consistent with previous reports, and no new safety signals emerged.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who participated in the study and their supportive families, as well as the investigators and clinical research staff from the study centers. This study was sponsored by Pharmacyclics LLC, an AbbVie Company. Editorial support was provided by Emily Chastain, PhD, an employee of Pharmacyclics LLC, an AbbVie Company. This study is in memory of Steven Coutre, who died during the writing of the manuscript.
Authorship
Contribution: P.M.B. designed the study in collaboration with the study sponsor; C.O., T.R., A.T., O.B., J.A.B., P.H., S.E.C., C.D., S.G., H.M., J.-Y.L., F.O., C.M., T.J.K., and P.G. contributed to data collection; C.Z. performed the data analyses; A.S., E.H., and C.Z. confirmed the accuracy of the data and compiled it for analysis; and all authors had access to the data and were involved in the interpretation of data, contributed to manuscript review and revisions, and approved the final version for submission.
Conflict-of-interest disclosure: P.M.B. has had a consultancy/advisory role with AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, MEI Pharma, Merck, Morphosys, Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, and TG Therapeutics and received research funding from AstraZeneca and TG Therapeutics. C.O. received honoraria from AbbVie, AstraZeneca, Gilead, Incyte, Janssen, Merck, Roche, and Teva. T.R. received honoraria from AstraZeneca and Janssen and had a consultancy/advisory role with and research funding from Acerta, AstraZeneca, and Janssen. A.T. has had a consultancy/advisory and speakers bureau role for AbbVie, AstraZeneca, BeiGene, and Janssen. O.B. has had a consultancy/advisory role with AbbVie, AstraZeneca, and Janssen and received research funding from Janssen. J.A.B. has received honoraria from and has had a consulting/advisory role with Janssen; received research funding from AstraZeneca, BeiGene, and Pharmacyclics LLC, an AbbVie Company; and has been on the speakers bureau for and received travel expenses from Gilead, Janssen, Novartis, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics. P.H. has had a consultancy/advisory role with AbbVie, AstraZeneca, and Janssen; received research funding from AbbVie, AstraZeneca, Gilead, Janssen, Novartis/GSK, Pharmacyclics LLC, an AbbVie Company, and Roche; has been on the speakers bureau for AbbVie, AstraZeneca, Janssen, and Roche; and received travel expenses from AbbVie and Janssen. S.E.C. received honoraria from AbbVie, Janssen, and Pharmacyclics LLC, an AbbVie Company; had a consultancy/advisory role with AbbVie, Adaptive, AstraZeneca, BeiGene, Celgene, Genentech, Janssen, Novartis, and Pharmacyclics LLC, an AbbVie Company; received research funding from AbbVie, AstraZeneca, Janssen, and Pharmacyclics LLC, an AbbVie Company; and provided expert testimony for Genentech and Janssen. H.M. received honoraria and travel expenses from Janssen and has had a consultancy/advisory role with AbbVie and Janssen. C.M. has had consultancy/advisory role with AbbVie, AstraZeneca, BeiGene, and Janssen and received research funding from and sat on the speakers’ bureau for AbbVie and Janssen. C.Z. E.H., and A.S. are employed by Pharmacyclics LLC, an AbbVie Company, and received stock ownership in AbbVie. T.J.K. has had a consultancy/advisory role with AbbVie, Celgene, Genentech-Roche, Gilead, and Pharmacyclics LLC, an AbbVie Company, and received research funding from AbbVie, Genentech-Roche, Oncternal Therapeutics, and Pharmacyclics LLC, an AbbVie Company. P.G. received honoraria from and had a consultancy/advisory role with AbbVie, Acerta/AstraZeneca, ArQule/MSD, Celgene/Juno/Bristol Myers Squibb, Janssen, Lilly/Loxo, MEI Pharma, and Roche and received research funding from AbbVie, AstraZeneca, Janssen, and Sunesis. The remaining authors declare no competing financial interests.
Correspondence: Paul M. Barr, Wilmot Cancer Institute, University of Rochester Medical Center, 601 Elmwood Ave, #704, Rochester, NY 14642; e-mail: paul_barr@urmc.rochester.edu.
References
- 1.Galton DA, Wiltshaw E, Szur L, Dacie JV. The use of chlorambucil and steroids in the treatment of chronic lymphocytic leukaemia. Br J Haematol. 1961;7(1):73-98. [DOI] [PubMed] [Google Scholar]
- 2.Pharmacyclics LLC, an AbbVie Company. Imbruvica (ibrutinib) prescribing information. Sunnyvale, CA: Pharmacyclics LLC, an AbbVie Company; 2020. [Google Scholar]
- 3.Burger JA, Tedeschi A, Barr PM, et al. ; RESONATE-2 Investigators . Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Brown JR, O’Brien S, et al. ; RESONATE Investigators . Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kipps TJ, Fraser G, Coutre SE, et al. Long-term studies assessing outcomes of ibrutinib therapy in patients with del(11q) chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2019;19(11):715-722. [DOI] [PubMed] [Google Scholar]
- 6.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser G, Cramer P, Demirkan F, et al. Updated results from the phase 3 HELIOS study of ibrutinib, bendamustine, and rituximab in relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma. Leukemia. 2019;33(4):969-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Furman RR, Coutre SE, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase 1b/2 PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trotman J, Buske C, Tedeschi A, et al. Long-term follow-up of ibrutinib treatment for rituximab-refractory Waldenström’s macroglobulinemia: final analysis of the open-label substudy of the phase 3 iNNOVATETM trial. Blood. 2020;136(suppl 1):38-39. [Google Scholar]
- 13.Rule S, Dreyling M, Goy A, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica. 2019;104(5):e211-e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, et al. ; International Workshop on Chronic Lymphocytic Leukemia . Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Introductory Guide MedDRA Version 24.0. Available at: https://admin.new.meddra.org/sites/default/files/guidance/file/intguide_%2024_0_English.pdf. Accessed 15 November 2021.
- 16.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26): 1910-1916. [DOI] [PubMed] [Google Scholar]
- 17.Hallek M, Fischer K, Fingerle-Rowson G, et al. ; German Chronic Lymphocytic Leukaemia Study Group . Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164-1174. [DOI] [PubMed] [Google Scholar]
- 18.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840-1847. [PubMed] [Google Scholar]
- 19.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848-1854. [PubMed] [Google Scholar]
- 20.Kipps TJ, Hillmen P, Demirkan F, et al. 11q deletion (del11q) is not a prognostic factor for adverse outcomes for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) treated with ibrutinib: pooled data from 3 randomized phase 3 studies. Blood. 2016;128(22):2042-2042. [Google Scholar]
- 21.Burger JA, Robak T, Demirkan F, et al. . Outcomes of first-line ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and high-risk genomic features with up to 6.5 years follow-up: Integrated analysis of two phase 3 studies (RESONATE-2 and iLLUMINATE). Blood. 2020;136(suppl_1):25-26. [Google Scholar]
- 22.Munir T, Pitchford A, Bloor A, et al. Sudden or cardiac deaths on ibrutinib-based therapy were associated with a prior history of hypertension or cardiac disease and the use of ACE-inhibitors at study entry: analysis from the phase III NCRI FLAIR trial. Blood. 2021;138(Suppl 1):2636. [Google Scholar]
- 23.Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129(18):2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien S, Hillmen P, Coutre S, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18(10):648-657. [DOI] [PubMed] [Google Scholar]
- 25.de Jong J, Haddish-Berhane N, Hellemans P, Jiao J, Sukbuntherng J, Ouellet D. The pH-altering agent omeprazole affects rate but not the extent of ibrutinib exposure. Cancer Chemother Pharmacol. 2018;82(2):299-308. [DOI] [PubMed] [Google Scholar]
- 26.AstraZeneca Pharmaceuticals. Calquence® (acalabrutinib) Prescribing Information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019. [Google Scholar]
- 27.UK CLL Forum. Follows GA. UK CLL forum 5-year update on 315 relapsed refractory CLL patients treated with ibrutinib in 66 UK and Ireland centres. Blood. 2019;134(suppl_1):1768. [Google Scholar]
- 28.Coutre SE, Byrd JC, Hillmen P, et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3(12):1799-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhtar OS, Attwood K, Lund I, Hare R, Hernandez-Ilizaliturri FJ, Torka P. Dose reductions in ibrutinib therapy are not associated with inferior outcomes in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 2019;60(7):1650-1655. [DOI] [PubMed] [Google Scholar]
- 30.Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16(10):517-523. [DOI] [PubMed] [Google Scholar]
- 32.Tam CS, Robak T, Ghia P, et al. Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica. 2020;106(9):2354-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.