Abstract
Oxidative stress is one of the major factors that contributes to brain deterioration in the elderly. Oxidation causes molecular alterations, structural damage, and brain dysfunction, which includes cognitive impairment. Memory loss can begin in middle-aged individuals, so prevention of brain deterioration before aging is important. Several studies have reported that curcumin and γ-oryzanol exhibits anti-oxidant and anti-inflammatory properties. However, curcumin and γ-oryzanol exhibit low aqueous solubility. Thus, a solid dispersion technique was used to prepare curcumin and γ-oryzanol to enhance their solubility and stability. This study aims to evaluate the effects and mechanisms of γ-oryzanol solid dispersion (GOSD) and curcumin solid dispersion (CURSD) on learning and memory in six groups of male rats (n=5/group). Group one was the adult control consisting of 6-week old male rats, and the remaining five groups consisted of 42-week (middle-aged) male rats. The groups were labeled as the control group, the GO group (GOSD 10 mg/kg·BW), the Cur group (CURSD 50 mg/kg·BW), the GO-LCur group (GOSD 10 mg/kg·BW plus CURSD 25 mg/kg·BW), and the GO-HCur group (GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW). Substances were administrated by oral gavage once daily for 42 consecutive days. The GO-HCur group exhibited significantly increased learning and memory performance in a Morris water maze and in reacting to a spontaneous tendency novel object test. The rats also exhibited decreased levels of lipid peroxidation, increased superoxide dismutase levels, glutathione peroxidase levels, catalase activity, and enhanced c-Fos expression both in the hippocampus and prefrontal cortex. The results indicated that GOSD 10 mg/kg plus CURSD 50 mg/kg was able to enhance learning and memory performance in the middle-aged rats.
Keywords: γ-oryzanol, curcumin, solid dispersion, antioxidant activity, learning and memory
Introduction
Physiological and morphological changes that occur during aging impact the functional process related to an individual quality of life. The brain irreversibly and progressively declines in function, and is subject to several debilitating ailments such as neurodegenerative diseases, cognitive impairment disorders, neuroinflammatory disorders, or Alzheimer's disease (1-3). The major risk factor concerning aging is an increase in free radical levels, with a concurrent decrease in antioxidant levels. These are the two major aspects that lead to oxidative stress, an increase in reactive oxygen species (ROS) related to the diminishing antioxidant defenses, and an increase in chronic low-grade inflammation (4-6). Previous studies have shown that a diet rich in antioxidants may decrease the risk of developing various neurodegenerative diseases (7,8). Prevention of brain deterioration before aging is important. Curcumin and γ-oryzanol have been reported to reduce ROS, oxidative stress, and inflammation. Moreover, these two elements have been identified as compounds that increase antioxidant levels such as those of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (9-12). However, studies have found that the beneficial effects of curcumin and γ-oryzanol are limited by their low water-solubility, leading to low bioavailability (13-16). Therefore, curcumin and γ-oryzanol were prepared in a solid dispersion to enhance their water solubility. The aim of this study was to evaluate the effects of γ-oryzanol solid dispersion (GOSD) and curcumin solid dispersion (CURSD) on learning and memory of middle-aged rats. In order to measure the level of improvement, rats were tasked with performing a series of behavioral evaluations: The Morris water maze (MWM) test and a novel object recognition (NOR) test. Subsequently, the c-Fos activity, the levels of malondialdehyde (MDA), the levels of tumor necrosis factor-α (TNF-α), and antioxidant activities (including SOD, CAT, and GPx) in the hippocampus and prefrontal cortex area of middle-aged rats were determined.
Materials and methods
Experimental animals
A total of 30 Sprague-Dawley male rats were obtained; 5 rats were aged 6-weeks old and 25 rats were aged 42-weeks old. All rats were obtained from Nomura Siam International Co., Ltd. The animals were acclimatized for 1 week prior to initiation of the experiments. Dependent on the size of the rat, 2 or 3 rats were housed per cage under control condition (12-h light/dark cycle, room temperature of 22±1˚C, 55±10% humidity) and provided ad libitum access to standard rat chow and tap water. All animal welfare considerations were accounted for under veterinary care. The animals were observed by well-trained technicians at least once daily for clinical signs of illness. This observation allowed prompt reporting and appropriate actions to be performed as necessary by the animal veterinarian. Death or 20% weight loss were considered as endpoints. The experimental protocols were in compliance with the standards of animal care and use established under the ethical guidelines and policies of Naresuan University, and were approved by the Ethics Committee of the Centre for Animal Research of Naresuan University (Phitsanulok, Thailand) (approval no. NU-AE620514).
Preparation of GOSD and CURSD
GOSD and CURSD were prepared using a melting solvent method with PVPK30 as a carrier and lecithin as a co-carrier. GOSD appeared as a white powder with γ-oryzanol content of 12%, while CURSD appeared as a yellow powder with curcumin content of 10%. Upon dilution in water, the water solubility of γ-oryzanol and curcumin increased to 1.3 and 14 mg/ml, respectively.
Experimental design
In this experiment, the sample population was divided into two clusters. One cluster with 5 rats aged 6-weeks old that were assigned as the adult control group (n=5); and the second cluster consisted of 25 rats aged 42-weeks old (middle-aged) that were randomly divided into 5 groups (n=5/group). Those clusters were then labeled as the control group, the GO group (GOSD 10 mg/kg·BW), the Cur group (CURSD 50 mg/kg·BW), the GO-LCur group (GOSD 10 mg/kg·BW plus CURSD 25 mg/kg·BW), and the GO-HCur group (GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW). Rats were administered the substance orally once daily for 42 consecutive days. On days 40-42, learning and memory performance was evaluated using NOR and MWM tests. Once the behavioral assessments had been completed, all rats were euthanized by intraperitoneal injection of thiopental sodium overdose (100 mg/kg) and death was verified by cessation of the heartbeat and respiration, and a lack of response to a noxious stimulus (hind paw pinch). Rats were sacrificed and the brains were removed. The right hippocampus and prefrontal cortex were stored at -80˚C and subsequently used to analyze MDA, SOD, CAT, GPx, and TNF-α, levels as well as being used for histopathological analysis.
MWM test
The MWM test was used to assess spatial learning and referent memory using a circular pool (diameter 130 cm, height 60 cm) filled with water (26±1˚C) with a depth of 40 cm, and was made to appear opaque by mixing in additional powder. The platform (diameter 10 cm) was submerged 1 cm below the surface and placed in a stable motionless position. Animals were placed in the pool and allowed 60 sec to swim to the hidden platform (17). During the trials, each animal underwent three trials on 7 consecutive days. During the test, the platform was removed from the pool and the time to swim in the former target quadrant (retention time) was recorded. The videos were analyzed using tracking software (Smart 3.0; Panlab).
NOR test
The NOR test was used to assess the recognition memory of familiar and novel objects (18). The test used in this study was performed using a contained open field (100x100x40 cm) enclosure that included three objects. The object used were of different shapes but of similar sizes. In the familiarization phase, the rat was placed in an open field with two objects, a triangle and sphere (object A and B, respectively) for 10 min. The objects were placed in the center, ~20 cm apart. For the test phase, the rat was placed in the open field again; however, one of the objects was changed, the sphere was changed to a square (object C), to test the recognition memory of the rat. Between each phase, the rat was placed back in their cages for 10 min. The time spent exploring the object was considered when the rat's nose pointed towards an object at a distance of ≤1 cm. The percentage recognition index of each rat was calculated based on the ratio (TAx100)/(TA+TC), where TA and TC are the time span each animal spent at object A and C, respectively.
Histopathological study
The left hippocampus and prefrontal cortex were fixed in 10% formaldehyde buffer at 22±1˚C for 3 days. Next the tissue samples were transferred to a 30% sucrose solution in PBS (0.1 M, pH 7.4) at 4˚C for 2-3 days (until submerged); transferred to a tissue freezing medium (Leica GmbH, cat no. 3801480); and then frozen in dry ice and stored at -80˚C until required for sectioning. The brain coronal was sectioned at 30 µm on a cryostat machine. The sections were stored in cryoprotectant solution at -20˚C until used for immunohistochemical processing.
c-Fos immunohistochemistry
The immunohistochemical staining used to detect c-Fos activity was performed using the free-floating section technique. The sections were washed three times in PBS (pH 7.4), immersed in a 3% solution of H2O2 in PBS for 5 min, washed three times in PBS, incubated in BSA (Capricorn scientific, cat no. BSA-1S) for 1 h at 22±1˚C and then incubated with a Rabbit polyclonal antibody (anti-c-Fos, hippocampus 1:400, prefrontal cortex 1:500, Abcam, cat no. Ab190289) for 15 h at 4˚C. The sections were washed three times in PBS, and then the sections were incubated with goat anti-rabbit IgG H&L (HRP) (1:500, Abcam, cat no. Ab205718) for 1 h at 22±1˚C. Subsequently, they were washed three times in PBS, and next incubated with DAB (Abcam, cat no. Ab64238) for 5 min and then they were washed three times in PBS. Finally, the sections were mounted on slides, air dried, dehydrated in ethanol solutions and xylene, and a cover slip placed on top with Mounting Medium (Thermo Fisher Scientific, Inc., cat no. SP15-500). c-Fos was detected using dark-brown staining. The number of c-Fos positive cells were counted in three regions of the dorsal hippocampus (CA1, CA3, and Dentate gyrus; DG) and the medial prefrontal cortex (mPFC). Images of the two sections per animal brains were counted using ImageJ (Version 1.53; National Institutes of Health).
Tissue preparation
The hippocampus and prefrontal cortex were homogenized in 0.1 M PBS (pH 7.4) and centrifuged at 9,000 x g for 20 min at 4˚C. The supernatants were collected and stored at -80˚C until required for biochemical processing.
Estimation of glutathione and TNF-α
The glutathione activity and the levels of the inflammatory cytokine TNF-α in the brain were measured using a glutathione peroxidase assay kit (Abcam, cat no. Ab102530) and rat TNF-α ELISA kit (Abcam, cat no. Ab100785) according to the manufacturer's protocol.
Measurement of lipid peroxide levels
MDA levels were determined as a measure of lipid peroxidation in the brain, based on the formation of thiobarbituric acid reactive substances (TBARS) as described by Liu et al (19). TBARS are formed as a result of the reaction between one molecule of MDA with 2 molecules of 2-thiobarbituric (TBA) at high temperatures under acidic conditions. The experiment consisted of 100 µl sample or standard (1,1',3,3' tetramethoxy propane), 1.5 ml 20% acetic acid solution (pH 3.5), 200 µl 8.1% SDS, and a 1.5 ml 0.8% sodium thiobarbiturate solutions. The mixtures were incubated at 95˚C for 60 min in a heat-box to induce initiation of the chemical reaction. Once the incubation process had completed, the mixtures were cooled and centrifuged at 2,000 x g for 20 min at 22±1˚C, and then the absorbance was measured at 532 nm using a spectrophotometer. The protein content was measured using a bicinchoninic acid protein assay reagent kit (Thermo Fisher Scientific, cat no. 23225) according to the manufacture's protocol.
Superoxide dismutase (SOD) activity assay
SOD activity was determined by measuring the ability of SOD to inhibit the pyrogallol autoxidation according as described by Marklund and Marlund (20). The reaction mixture consisted of 50 mM Tris-EDTA (pH 8.2), 0.2 mM pyrogallol in 50 mM Tris-HCl (pH 7.4), and sample. The reaction kinetics were measured, and the results showed that there was an optical density change in absorbance at 420 nm, 25˚C for 5 min. The percentage of inhibition was calculated by using a comparison with a blank assay system. One unit of SOD activity was defined as the amount of SOD in the sample needed to inhibit pyrogallol oxidation by 50%. Results are reported as U/mg protein.
Catalase (CAT) activity assay
CAT activity was determined by measuring the reaction of the decomposition of hydrogen peroxide (H2O2) to water and oxygen following the method established by Beers and Sizer (21). The reaction mixture consisted of 0.05 M sodium phosphate buffer (pH7), 0.059 M H2O2 in a buffer and the sample. The reaction kinetics were measured based on the changes in absorbance at 240 nm, 25˚C for 5 min. The CAT activity was calculated using the molar absorbance coefficient of H2O2 (represented as 43.6) and reported as U/mg protein.
Statistical analysis
The data were analyzed using GraphPad Prism version 9 (GraphPad Software, Inc.) and are presented as the mean ± SEM. Data were compared using a Student's-test or a one-way ANOVA with a post-hoc Bonferroni test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of age on performance and biochemical parameters
The middle-aged group (42-weeks old) showed a significantly shorter retention time in the MWM test (P<0.05) and a lower number of c-Fos positive cells in the mPFC (P<0.05), DG (P<0.0001), CA3 (P<0.0001), and CA1 (P<0.0001) regions of the brain compared with the adult group (Fig. 1). Moreover, the results found that the levels of MDA (P<0.01) and TNF-α (P<0.05) in the hippocampus of the middle-aged group were significantly higher than the adult group (Table I).
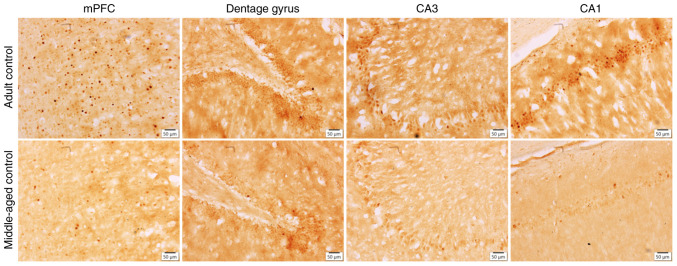
Figure 1.
Expression of c-Fos was evaluated by immunohistochemical staining. Middle-aged control rats showed lower c-Fos expression than the adult controls in the mPFC, dentate gyrus, CA3, and CA1 areas. Magnification, x20; scale bar, 50 µm. mPFC, medial prefrontal cortex.
Table I.
Effect of age on the assessed variables in rats.
| Variable | Adult control | Middle-aged control | P-value |
|---|---|---|---|
| Retention time, sec | 28.76±1.06 | 18.11±3.45 | 0.0185a |
| Percentage recognition index | 57.72±1.75 | 55.05±2.78 | 0.4401 |
| MDA in hippocampus, µmol/mg protein | 1.57±0.08 | 2.98±0.40 | 0.0092b |
| MDA in prefrontal cortex, µmol/mg protein | 2.46±0.40 | 1.97±0.22 | 0.3206 |
| TNF-α in hippocampus, pg/mg | 160.99±10.50 | 193.21±3.78 | 0.0203a |
| TNF-α in prefrontal cortex, pg/mg | 168.38±3.46 | 172.11±4.46 | 0.5266 |
| c-Fos positive cells in mPFC, cells/mm2 | 290.16±16.37 | 198.15±30.81 | 0.0167a |
| c-Fos positive cells in dentate gyrus, cells/mm2 | 422.99±11.79 | 190.67±9.24 | <0.0001c |
| c-Fos positive cells in CA3, cells/mm2 | 186.50±8.23 | 105.00±3.49 | <0.0001c |
| c-Fos positive cells in CA1, cells/mm2 | 228.75±6.49 | 106.53±4.71 | <0.0001c |
aP<0.05,
bP<0.01,
cP<0.0001 vs. adult control group. Data are presented as the mean ± SEM. MDA, malondialdehyde; TNF-α, tumor necrosis factor-α; mPFC, medial prefrontal cortex.
Effect of GOSD and CURSD on learning and memory
Spatial learning and reference memory were evaluated. The time that each rat spent in the target quadrant in the testing period (retention time) was recorded in order to observe the behavior of the rats. The data showed that GO-HCur group significantly increased retention time when compared with the control group (P<0.01). The results indicated that GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW exhibited the largest improvement in learning and memory performance in middle-aged rats (Fig. 2).
Figure 2.

Effect of GOSD and CURSD on spatial learning and reference memory in the Morris water maze test. Data are presented as the mean ± SEM. **P<0.01 vs. control. GOSD, γ-oryzanol solid dispersion; CURSD, curcumin solid dispersion.
Effect of GOSD and CURSD on NOR memory
The percentage recognition index was calculated from the time spent exploring the novel object to the time whereby the rats were able to distinguish familiar objects during the testing period. GO-HCur group showed a significant increase in the percentage recognition index when compared with the control group (P<0.001). As the rats remembered the familiar objects, which became mundane, they began to become more interested in exploring the novel object. The results indicated that GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW exhibited the largest improvement in recognition memory performance in the middle-aged rats (Fig. 3).
Figure 3.
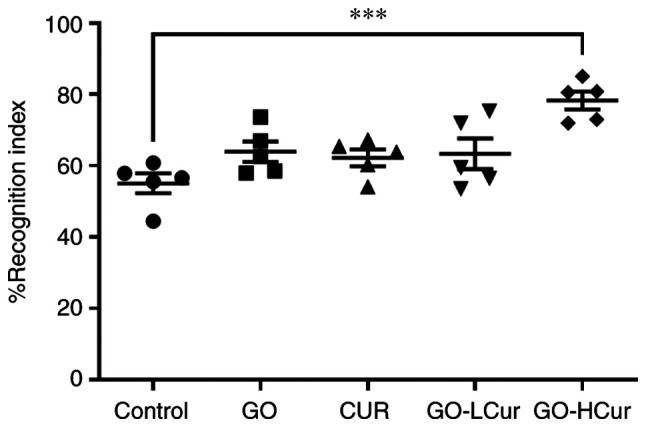
Effect of GOSD and CURSD on recognition memory in the novel object recognition test. Data are presented as the mean ± SEM. ***P<0.001 vs. control.
Effect of GOSD and CURSD on c-Fos expression
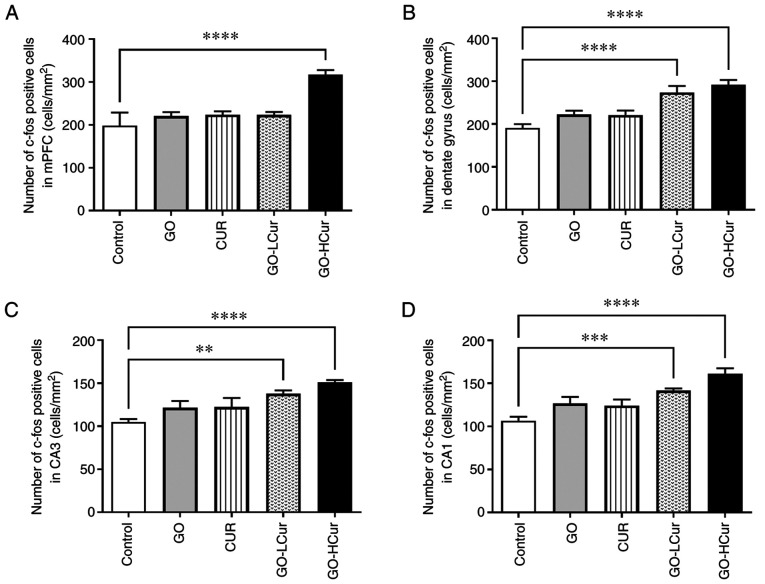
c-Fos expression was evaluated using immunohistochemistry to stain the prefrontal cortex and hippocampal areas of the brain. The data from the prefrontal cortex showed that the GO-HCur group exhibited significantly increased numbers of c-Fos positive cells in the mPFC compared to the control group (P<0.0001). This was especially apparent in the hippocampal areas, which include the DG, CA3, and CA1 regions. The number of c-Fos positive cells was significantly increased in GO-LCur (DG, P<0.0001; CA3, P<0.01; and CA1, P<0.001, respectively), and the GO-HCur (P<0.0001) compared to the control group. The results indicated that GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW enhanced the c-Fos activity in both the prefrontal cortex and the hippocampus in middle-aged rats (Figs. 4 and 5).
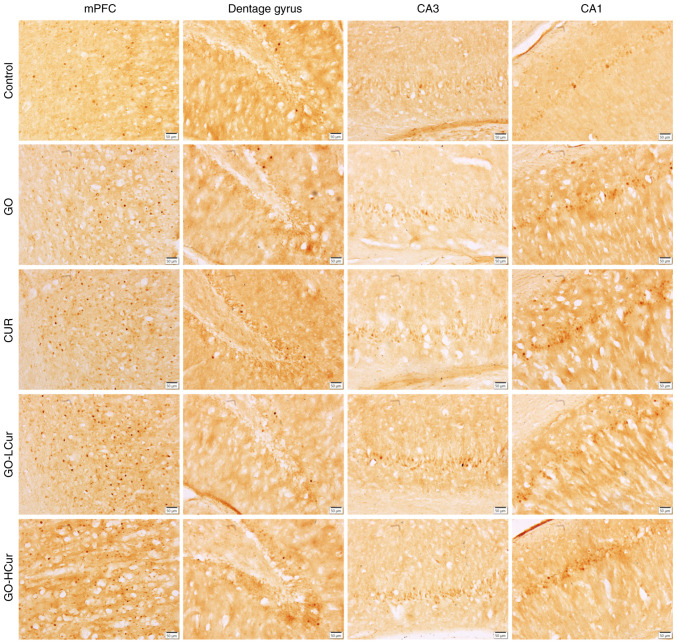
Figure 4.
Expression of c-Fos was evaluated by immunohistochemical staining. GOSD and CURSD increased c-Fos expression in the prefrontal cortex and hippocampus. Magnification, x20; scale bar, 50 µm. mPFC, medial prefrontal cortex; GOSD, γ-oryzanol solid dispersion; CURSD, curcumin solid dispersion.
Figure 5.
Effect of GOSD and CURSD on the expression of c-Fos in (A) the mPFC, (B) the dentate gyrus, and (C) the CA3 and (D) CA1 regions. Data are presented as the mean ± SEM. **P<0.01, ***P<0.001, ****P<0.0001 vs. control. mPFC, medial prefrontal cortex; GOSD, γ-oryzanol solid dispersion; CURSD, curcumin solid dispersion.
Determination of GOSD and CURSD on oxidative stress and antioxidant status. Effect of GOSD and CURSD on lipid peroxidation
Lipid peroxidation was used to evaluate the levels of MDA in the prefrontal cortex and hippocampus using a TBARs assay. The data showed that the GO-HCur group exhibited significantly decreased levels of MDA in both the prefrontal cortex and hippocampus compared with the control group (P<0.01 and P<0.05, respectively). These results indicated that the administration of GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW reduced lipid peroxidation in both the prefrontal cortex and the hippocampus in middle-aged rats (Fig. 6).
Figure 6.
Effect of GOSD and CURSD on oxidative stress in (A) the prefrontal cortex, and (B) the hippocampal areas. Data are presented as the mean ± SEM. *P<0.05, **P<0.01 vs. control. MDA, malondialdehyde; GOSD, γ-oryzanol solid dispersion; CURSD, curcumin solid dispersion.
Effect of GOSD and CURSD on SOD activity
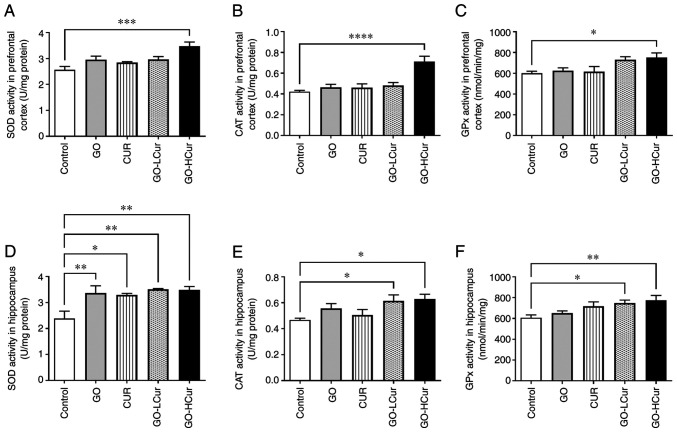
The data showed that the GO-HCur group exhibited significantly increased SOD activity in the prefrontal cortex when compared with the control group (P<0.001). Moreover, the data demonstrated that the SOD activity in the hippocampus significantly increased in middle-aged rats administered with GO (P<0.01), Cur (P<0.05), GO-LCur (P<0.01), and GO-HCur (P<0.01) compared with the control group. The results indicated that GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW increased the activity of SOD in both the prefrontal cortex and the hippocampus in the middle-aged rats (Fig. 7).
Figure 7.
Effect of GOSD and CURSD on antioxidant status. (A-C) Antioxidant status in the prefrontal cortex and (D-F) in the hippocampus. (A and D) SOD activity, (B and E) CAT activity, and (C and F) GPx activity. Data are presented as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. control. SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GOSD, γ-oryzanol solid dispersion; CURSD, curcumin solid dispersion.
Effect of GOSD and CURSD on CAT activity
The data showed that CAT activity in the prefrontal cortex was significantly increased in the GO-HCur group when compared with the control group (P<0.0001). In the hippocampus, the activity of CAT was significantly increased in the GO-LCur and GO-HCur groups when compared with the control group (both P<0.05). The results indicated that GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW enhanced CAT activity in both the prefrontal cortex and the hippocampus in the middle-aged rats (Fig. 7).
Effect of GOSD and CURSD on GPx activity
The data showed that GO-HCur group exhibited significantly increased GPx activity in the prefrontal cortex when compared with the control group (P<0.05). Moreover, the data showed that GO-LCur and GO-HCur groups exhibited significantly increased GPx activity in the hippocampus when compared with the control group (P<0.05 and P<0.01, respectively). The results indicated that GOSD 10 mg/kg·BW plus CURSD 50 mg/kg·BW enhanced the activity of GPx in both the prefrontal cortex and the hippocampus in the middle-aged rats (Fig. 7).
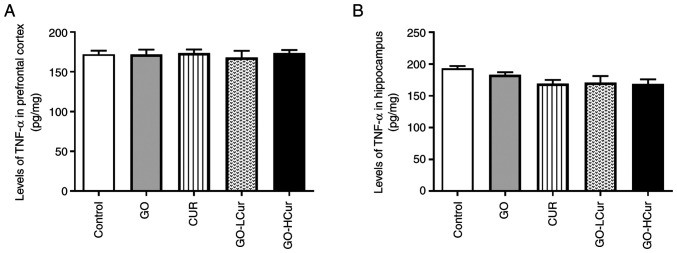
Effect of GOSD and CURSD on the levels of TNF-α
The proinflammatory cytokine levels were assessed based on the levels of TNF-α in the prefrontal cortex and hippocampus. The data showed that levels of TNF-α did not significantly differ among the five groups in both regions (Fig. 8).
Figure 8.
Effect of GOSD and CURSD on levels of TNF-α in (A) the prefrontal cortex, and (B) hippocampus areas. Data are presented as the mean ± SEM. TNF-α, tumor necrosis factor-α; GOSD, γ-oryzanol solid dispersion; CURSD, curcumin solid dispersion.
Discussion
The aim of this study was to evaluate the effects of GOSD and CURSD on learning and memory in middle-aged rats. The results showed an association between age with brain functions and behavioral changes. Several studies reported that oxidative stress and inflammation associated with age affect the brain's structure and function, leading to a cognitive decline in an age-dependent manner (22-24). MDA and TNF-α are well-known indicators for the study of oxidative stress and inflammation. The middle-aged rats used in this study may not have shown the prominent alterations in the recognition memory or the molecular changes of the prefrontal cortex yet. However, some parameters such as the molecular changes in the hippocampus including MDA, TNF-a, and c-Fos expression that are associated with learning and memory, specifically in spatial memory, were observed. The molecular changes in middle-aged involved oxidative stress (6), which is caused by the accumulation of free radicals and ROS, the levels of which increase with age. Although free radicals and ROS may be beneficial at optimal levels, excessive generation and accumulation leads to oxidative stress (25). This can induce lipid peroxidation, inflammation, and neurodegeneration in the brain (26). Furthermore, previous studies have suggested that damage in the hippocampus leads to a reduction in learning and memory functions in rats (27,28). Immunohistochemical staining was used to detect c-Fos expression, a marker of activated neurons in the brain. The expression of c-Fos in the middle-aged rat group was lower than that in the adult rats in both the hippocampus and prefrontal cortex. These results indicated that middle-aged rats exhibited less neuronal activity than the adult rats, which corresponds to the results of the MWM test, which showed that the learning and memory performance declined. These results also suggest that oxidative stress also affected the structural alterations of neurons, which may have led to a reduction in excitatory postsynaptic potential and neuronal activity in the brain (2,29).
According to previous studies, the intake of dietary antioxidants can prevent neurodegenerative diseases associated with oxidative stress and aging (7,8). Curcumin and γ-oryzanol compounds exhibit a wide range of pharmacological properties, including antioxidant, anti-inflammatory, anti-tumor, and anti-diabetic properties (9-12,30). Nevertheless, both compounds have been reported to be limited with regard to their use as a nutritional product given their low water-solubility, resulting in low absorption and bioavailability (13-16). The present study prepared curcumin and γ-oryzanol as a solid dispersion to enhance their water solubility. Several studies have reported that solid dispersion techniques enhance the absorption and bioavailability by improving the water solubility and dissolvability of poor water-soluble drugs (31,32). In addition, previous studies have found that curcumin in solid dispersion showed stronger pharmacological properties than native curcumin (33). The present study demonstrated GOSD and CURSD prevented the elevation of oxidative stress in middle-aged rats by acting as an antioxidant, which involved the elimination or neutralization of free radicals and/or ROS. The increase in the levels of antioxidant enzymes, including that of SOD, CAT, and GPx have been shown to associated with the reduction of malondialdehyde in both the hippocampus and the prefrontal cortex. Nevertheless, the results of the present study did not show significantly reduced levels of TNF-α in any of the treatment groups. Treatment with GOSD 10 mg/kg combined with CURSD 50 mg/kg was more effective than the GOSD, CURSD, or GOSD 10 mg/kg combined with CURSD 25 mg/kg with regard to the defense against oxidative stress in middle-aged rats. These results are in agreement with previous studies that showed the ability of curcumin and γ-oryzanol in reducing the oxidative stress and lipid peroxidation by increasing the antioxidant activity (9,34). The elevation in antioxidant levels played a key role in protecting neurons from free radicals and ROS by decreasing lipid peroxidation (35,36) and reducing any structural alterations in neurons, in-turn maintaining neuronal activity and improving learning and memory (34,37). This was consistent with the results that administration of GOSD 10 mg/kg combined with CURSD 50 mg/kg significantly increased expression the c-Fos, and this is associated with a significant increase in reference memory and recognition memory in behavioral tests (38).
In conclusion, the present study demonstrated that GOSD and CURSD could protect against and slow down the aging of the brain during the early stages of aging through attenuation of oxidative stress by decreasing MDA levels and increasing antioxidant enzyme activity, and this resulted in memory enhancement. In addition, GOSD and CURSD did not exert any noticeable neurotoxic effects on the central nervous system.
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was supported by Agricultural Research Development Agency (Public Organization), the National Research Council of Thailand (grant no. 2564/4, TP), and The Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
TP performed the experiments. TP and OK analyzed the results and wrote the manuscript. WT, ST and OK designed the study, and wrote and edited the manuscript. All authors read and approved the final manuscript. TP and OK confirmed the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved by the by the Ethics Committee of the Centre for Animal Research of Naresuan University (Phitsanulok, Thailand) (approval no. NU-AE620514).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 2.Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 3.Gómez-Gonzalo M, Martin-Fernandez M, Martínez-Murillo R, Mederos S, Hernández-Vivanco A, Jamison S, Fernandez AP, Serrano J, Calero P, Futch HS, et al. Neuron-astrocyte signaling is preserved in the aging brain. Glia. 2017;65:569–580. doi: 10.1002/glia.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandran R, Kumar M, Kesavan L, Jacob RS, Gunasekaran S, Lakshmi S, Sadasivan C, Omkumar RV. Cellular calcium signaling in the aging brain. J Chem Neuroanat. 2019;95:95–114. doi: 10.1016/j.jchemneu.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Monti DM, Rigano MM, Monti SM, Peixoto HS. Role of antioxidants in the protection from aging-related diseases. Oxid Med Cell Longev. 2019;2019(7450693) doi: 10.1155/2019/7450693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikhra V. The aging brain: Recent research and concepts. Gerontol Geriatr Stud. 2017;1:1–11. [Google Scholar]
- 7.Vaiserman A, Koliada A, Zayachkivska A, Lushchak O. Curcumin: A therapeutic potential in ageing-related disorders. PharmaNutrition. 2020;14(100226) [Google Scholar]
- 8.Benameur T, Soleti R, Panaro MA, La Torre ME, Monda V, Messina G, Porro C. Curcumin as prospective anti-aging natural compound: Focus on brain. Molecules. 2021;26(4794) doi: 10.3390/molecules26164794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother. 2017;87:223–229. doi: 10.1016/j.biopha.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 10.Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit. 2007;13:BR286–BR292. [PubMed] [Google Scholar]
- 11.Rungratanawanich W, Abate G, Serafini MM, Guarienti M, Catanzaro M, Marziano M, Memo M, Lanni C, Uberti D. doi: 10.1155/2018/2987249. Characterization of the antioxidant effects of γ-oryzanol: Involvement of the Nrf2 pathway. Oxid Med Cell Longev: Mar 14, 2018 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YX, Li Y, Sun AM, Wang FJ, Yu GP. Hypolipidemic and antioxidative effects of aqueous enzymatic extract from rice bran in rats fed a high-fat and -cholesterol diet. Nutrients. 2014;6:3696–3710. doi: 10.3390/nu6093696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam A, Rebello L, Chepyala S. Review on nanoformulations of curcumin (Curcuma longa Linn.): Special emphasis on Nanocurcumin®. Int J Nat Life Sci. 2019;3:1–12. [Google Scholar]
- 14.Hettiarachchi SS, Dunuweera SP, Dunuweera AN, Rajapakse RMG. Synthesis of curcumin nanoparticles from raw turmeric rhizome. ACS Omega. 2021;6:8246–8252. doi: 10.1021/acsomega.0c06314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawal T, Mishra N, Jha A, Bhatt A, Tyagi RK, Panchal S, Butani S. Chitosan nanoparticles of gamma-oryzanol: Formulation, optimization, and in vivo evaluation of anti-hyperlipidemic activity. AAPS PharmSciTech. 2018;19:1894–1907. doi: 10.1208/s12249-018-1001-8. [DOI] [PubMed] [Google Scholar]
- 16.Rodsuwan U, Pithanthanakul U, Thisayakorn K, Uttapap D, Boonpisuttinant K, Vatanyoopaisarn S, Thumthanaruk B, Rungsardthong V. Preparation and characterization of gamma oryzanol loaded zein nanoparticles and its improved stability. Food Sci Nutr. 2020;9:616–624. doi: 10.1002/fsn3.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 18.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Edamatsu R, Kabuto H, Mori A. Antioxidant action of guilingji in the brain of rats with FeCl3-induced epilepsy. Free Radic Biol Med. 1990;9:451–454. doi: 10.1016/0891-5849(90)90023-c. [DOI] [PubMed] [Google Scholar]
- 20.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 21.Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 22.Hamezah HS, Durani LW, Ibrahim NF, Yanagisawa D, Kato T, Shiino A, Tanaka S, Damanhuri HA, Ngah WZW, Tooyama I. Volumetric changes in the aging rat brain and its impact on cognitive and locomotor functions. Exp Gerontol. 2017;99:69–79. doi: 10.1016/j.exger.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Garg G, Singh S, Singh AK, Rizvi SI. N-acetyl-l-cysteine attenuates oxidative damage and neurodegeneration in rat brain during aging. Can J Physiol Pharmacol. 2018;96:1189–1196. doi: 10.1139/cjpp-2018-0209. [DOI] [PubMed] [Google Scholar]
- 24.Khairy EY, Attia MM. Protective effects of vitamin D on neurophysiologic alterations in brain aging: Role of brain-derived neurotrophic factor (BDNF) Nutr Neurosci. 2021;24:650–659. doi: 10.1080/1028415X.2019.1665854. [DOI] [PubMed] [Google Scholar]
- 25.Pyo IS, Yun S, Yoon YE, Choi JW, Lee SJ. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules. 2020;25(4649) doi: 10.3390/molecules25204649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid Med Cell Longev. 2015;2015(610813) doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anyanwu EC. Neurochemical changes in the aging process: Implications in medication in the elderly. ScientificWorldJournal. 2007;7:1603–1610. doi: 10.1100/tsw.2007.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang TT, Leu D, Zou Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch Biochem Biophys. 2015;576:2–7. doi: 10.1016/j.abb.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastinu A, Bonini SA, Rungratanawanich W, Aria F, Marziano M, Maccarinelli G, Abate G, Premoli M, Memo M, Uberti D. Gamma-oryzanol prevents LPS-induced brain inflammation and cognitive impairment in adult mice. Nutrients. 2019;11(728) doi: 10.3390/nu11040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Xing H, Zhao Y, Ma Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics. 2018;10(74) doi: 10.3390/pharmaceutics10030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jara MO, Warnken ZN, Williams RO III. Amorphous solid dispersions and the contribution of nanoparticles to in vitro dissolution and in vivo testing: Niclosamide as a case study. Pharmaceutics. 2021;13(97) doi: 10.3390/pharmaceutics13010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira VDS, de Almeida AS, Albuquerque IDS, Duarte FÍC, Queiroz BCSH, Converti A, Lima ÁAND. Therapeutic applications of solid dispersions for drugs and new molecules: In vitro and in vivo activities. Pharmaceutics. 2020;12(933) doi: 10.3390/pharmaceutics12100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rungratanawanich W, Cenini G, Mastinu A, Sylvester M, Wilkening A, Abate G, Bonini SA, Aria F, Marziano M, Maccarinelli G, et al. γ-Oryzanol improves cognitive function and modulates hippocampal proteome in mice. Nutrients. 2019;11(753) doi: 10.3390/nu11040753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui X, Song H, Su J. Curcumin attenuates hypoxic-ischemic brain injury in neonatal rats through induction of nuclear factor erythroid-2-related factor 2 and heme oxygenase-1. Exp Ther Med. 2017;14:1512–1518. doi: 10.3892/etm.2017.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV. Immediate early genes, memory and psychiatric disorders: Focus on c-Fos, Egr1 and Arc. Front Behav Neurosci. 2018;12(79) doi: 10.3389/fnbeh.2018.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.