Abstract
A normal inflammatory response is essential in protecting the body from foreign substances. However, excessive inflammation contributes to diseases such as oxidative stress, heart disease, and cancer. In this study, we evaluated the anti-inflammatory effects of RAPA (red ginseng marc, Artemisia scoparia Waldst.et Kit, Paeonia japonica Miyabe & Takeda, and Angelica gigas Nakai extract mixture) in LPS-stimulated RAW 264.7 cells (macrophages). RAPA suppressed the expression of inflammatory factors such as iNOS and COX-2 and decreased the production of nitric oxide. In addition, RAPA decreased the expression of the inflammatory cytokines TNF-α and IL-6. Furthermore, RAPA inhibited the phosphorylation of MAPKs such as JNK and ERK as well as IκB and NF-κB. In conclusion, RAPA inhibited production of inflammatory mediators via downregulation of the MAPK and NF-κB signaling pathways in LPS-stimulated RAW 264.7 cells. The results of this study demonstrated that RAPA regulates excessive inflammatory responses at the cellular level. Therefore, it is necessary to investigate whether the same effect is observed in vivo through further research.
Keywords: anti-inflammatory, red ginseng marc, Artemisia scoparia, Paeonia japonica, Angelica gigas
Introduction
Inflammation is a protective mechanism against harmful stimuli, which is necessary for cells to maintain physiological conditions (1). The inflammatory response is essential in protecting the body; however, continuous inflammation can cause diseases such as cancer, high blood pressure, arteriosclerosis, allergies, and asthma (2,3). Macrophages play a role in cytokine production, antigen presentation, phagocytosis, and immune regulation in inflammation. Macrophages produce IL-6, TNF-α, IFN-γ, and nitric oxide (NO) following LPS stimulation (4,5). NO protects cells from pathogenically induced DNA damage; however, excessive production of iNOS exacerbates inflammation by promoting the production of inflammatory mediators (6). COX-2 induced by LPS and cytokines is involved in prostaglandin production in acute inflammatory responses (7). High levels of NO, COX-2, and cytokines in the blood are typical features of chronic inflammation, leading to cancer, rheumatic diseases, and endocrine diseases (8-10). Therefore, the control of these inflammatory mediators is a key topic in the treatment of chronic inflammation. Drugs such as Aspirin, Ibuprofen, and Dexibuprofen are used to suppress excessive inflammation (11,12). However, anti-inflammatory drugs cause hypotension, decreased gastrointestinal function, and mucosal damage bleeding due to a decrease in cortisol levels (13,14). Therefore, studies on inflammation control through natural products with relatively fewer side effects are being increasingly studied (15). Red ginseng has been widely used as a traditional medicine in Korea, China, and Japan (16). It contains saponins, ginsenosides, polysaccharides, and fatty acids, which have anti-inflammatory, anti-cancer, and antioxidant effects (17).
Red ginseng (steamed and dried panax ginseng C.A. Meyer) marc, a by-product of processed red ginseng products, is usually thrown away or used as feed for livestock. However, red ginseng marc contains the active ingredients of red ginseng, thus it has potential use (18). Paeonia japonica Miyabe & Takeda is a plant of the Angelica family, known for its antioxidant and immunomodulatory activities, and is widely used in oriental medicine (19). The paeoniflorin and paeonol contained in P. japonica possess analgesic, antipyretic, anti-inflammatory and anti-ulcer effects (20). Angelica gigas Nakai belongs to the Umbelliferae family and is a traditional medicinal plant distributed throughout North Asia, and it has been reported to be efficacious in the treatment of anemia and cancer, and possess anti-inflammatory properties (21). Artemisia scoparia Waldst.et Kit, a perennial plant in the Asteraceae family, grows on sandy beaches and is known to possess anti-inflammatory and antioxidant effects (22-24). Although natural products each have their own health benefits, several studies have been reported that show synergistic effects when they are used as a mixture (25,26).
Therefore, the aim of this study is to evaluate the anti-inflammatory effects of red ginseng marc, P. japonica, A. gigas, and A. scoparia complexes in LPS-stimulated RAW 264.7 cells.
Materials and methods
Materials
Antibodies against JNK (cat. no. sc-7345), p-JNK (cat. no. sc-293136), ERK (cat. no. sc-514302), p-ERK (cat. no. sc-81492), NF-κB (cat. no. sc-8008), p-NF-κB (cat. no. sc-136548), p-IκB (cat. no. sc-8404), and β-actin (cat. no. sc-8432) were purchased from Santa Cruz Biotechnology, Inc. Antibodies against iNOS (cat. no. 13120) and COX-2 (cat. no. 4842) were acquired from Cell Signaling Technology, Inc. The Qunati-MAX™ WST-8 Cell Viability Assay Kit and WestGlow™ Chemiluminescent substrate were obtained from Biomax FBS, RIPA buffer and DMEM were purchased from Gibco; Thermo Fisher Scientific, Inc. Penicillin/streptomycin antibiotics, carboxy-H2DCFDA and Goat anti-Mouse IgG Alexa Fluor 488 (cat. no. A-11001) were purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Thermo Fisher Scientific, Inc.). Griess reagent and lipopolysaccharide (LPS, cat. no. L2630) were procured from Sigma-Aldrich; Merck KGaA. ELISA kits for IL-6 and TNF-α were obtained from R&D Systems, Inc. The Bradford assay reagent and SDS-PAGE sample loading buffer were purchased from Bio-Rad Laboratories, Inc.
Plant extract preparation
Red ginseng marc, A. scoparia, P. japonica and A. gigas were purchased from Jinandang Farming Association Corporation (Jeollabuk-do). The plants were authenticated by Prof Kim Hong-Jun at the College of Oriental Medicine, Woosuk University. Red ginseng marc, A. scoparia, P. japonica and A. gigas (100 g each) were boiled in distilled water (2l) for 2 h at 121˚C, 15 psi. After incubation, these extracts were filtered thrice using an 7 µm filter paper (cat. no. AD.01511110; Advantec) concentrated, dried and further stored at -20˚C. Each extract was mixed in a ratio of red ginseng marc 50: A. scoparia 20: P. japonica 20: A. gigas 10. The mixture was termed RAPA.
Cell culture
RAW 264.7 cells derived from mice were purchased from ATCC and cultured in DMEM containing 10% FBS and 1% penicillin & streptomycin in a CO2 incubator (5% CO2 and 95% atmosphere) with sufficient humidity at 37˚C.
Cell cytotoxicity
RAW 264.7 cells were aliquoted to a final concentration of 2x105 cells/ml in a 96-well plate and cultured for 24 h at 37˚C in an incubator under 5% CO2 conditions. Cultured cells were treated with RAPA extract at a concentration of 40-400 µg/ml, and after 24 h of incubation, cytotoxicity was measured using Qunati-MAX™ WST-8 Cell Viability Assay Kit (Biomax).
NO assay
NO assays were performed as described previously (25).
ELISA
ELISA for IL-6 and TNF-α cytokines were performed using the ELISA Kits on the collected culture medium according to the manufacturer's protocol.
Western blot
A total of 2x105 cells/ml RAW264.7 cells were inoculated into a 60 mm dish for 24 h, pretreated with RAPA mixture (0, 100, or 400 µg/ml) for 1 h, and stimulated with LPS (1 µg/ml) for 30 min or 24 h. To collect the cells, 1 ml PBS was added to the cells, collected with a scraper, and centrifuged twice using PBS. RIPA buffer (100 µl) was added, the cells were incubated on ice for 15 min and the total protein was extracted by centrifugation at 14,000 x g. The protein content was quantified using a Bradford assay, and the extracted proteins from the cell lysate were resolved using a 10% SDS-gel and SDS-PAGE. Resolved proteins were transferred to PVDF membranes, which were subsequently blocked with 5% skimmed milk [0.01 M Tris-HCL buffer (TBST)] for 60 min at room temperature. Next, the PVDF membranes were washed 5 times with TBST and then incubated with the antibodies (iNOS 1:1,000; COX-2 1:1,000; p-JNK 1:200; JNK 1:200; p-ERK 1:200; ERK 1:200; p-NF-κB 1:200; NF-κB 1:200; p-IκB 1:200; IκB 1:200; and β-actin 1:1,000) for 16 h at 4˚C. After 16 h of incubation, the membranes were washed 5 times for 5 min with TBST, incubated with horseradish peroxidase-conjugated secondary primary antibodies for 2 h at room temperature, washed 5 times for 5 min with TBST, and examined using a UVItec Chemiluminescence Imaging System. The protein band densities were analyzed using ImageJ version 1.53 (National Institutes of Health).
Intracellular ROS measurements
A total of 2x105 cells/ml RAW 264.7 were inoculated into a 60 mm dish, and treated with 400 µg/ml RAPA mixture after 24 h. After 1 h, the cells were stimulated with LPS (1 µg/ml) and cultured for 24 h. The cells were treated with carboxy-H2DCFDA (5 µM) for 30 min, harvested, and counted at a FITC wavelength (488 nm) in a flow cytometer (Cyto FLEX LX; Beckman Coulter, Inc.).
Immunofluorescence assay
RAW264.7 cells were aliquoted in 4-well cell culture slides at a concentration of 2x105 cells/ml and cultured for 24 h. After 24 h of incubation, the cells were mixed with RAPA (0, 100, or 400 µg/ml) for 30 min and stimulated with 1 µg/ml LPS for 24 h. The cells were fixed with methanol, blocked with PBS containing 1% BSA, and then incubated at 4˚C for 16 h after addition of the p65 NF-κB antibody (1:50). After washing 3 times for 10 min with PBST, the secondary antibody was reacted with goat anti-Rabbit IgG Alexa Fluor 488, followed by mounting with a ProLong® Gold Antifade Reagent (cat. no. #9071; Cell Signaling Technology Inc.). After drying the slides overnight, images were captured using a fluorescence microscope (Carl Zeiss GmbH; x100 magnification).
Statistical analysis
Data are presented as the mean ± SD. Differences between groups were compared using a one-way ANOVA followed by a Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results and discussion
Effect of RAPA on NO production in LPS-treated RAW 264.7 cells
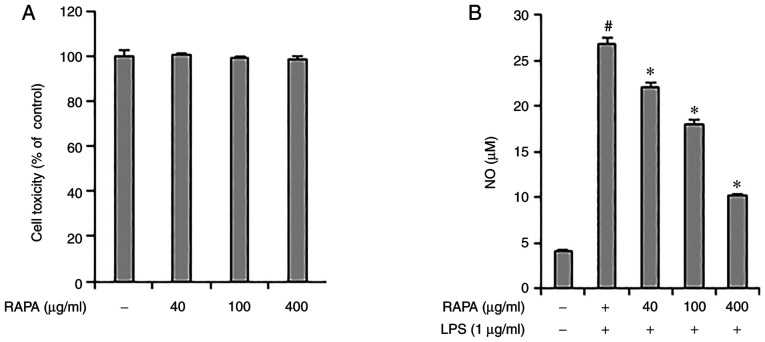
The effect of RAPA on the viability of RAW 264.7 cells was investigated. RAPA did not show any notable toxicity between 40-400 µg/ml (Fig. 1A). Therefore, in this study, cells were treated with <400 µg/ml RAPA. Next, the effect of RAPA on NO production by LPS was investigated using an NO assay. The results showed that treatment with RAPA suppressed NO production. In particular, 400 µg/ml RAPA treatment reduced NO production by 62% compared with the positive control group (Fig. 1B). NO protects cells from pathogens; however, excessive NO production has a cytotoxic effect on the surrounding tissues (27). Therefore, regulation of NO production is essential in the control of chronic inflammation. Based on the above results, it is suggested that RAPA may be useful in managing chronic inflammation as it effectively suppressed NO production in LPS-treated RAW 264.7 cells.
Figure 1.
Effect of RAPA on cell viability and NO production in RAW 264.7 cells. (A) RAW 264.7 cells were pretreated with the indicated concentrations of RAPA for 24 h. (B) Cells were pretreated with RAPA at the indicated concentrations and stimulated with 1 µg/ml LPS for 16 h. The levels of NO in the culture supernatants were analyzed using a Griess assay. Data are presented as the mean ± SD. #P<0.05 vs. untreated cells; *P<0.05 vs. LPS treated cells. RAPA, red ginseng marc, Artemisia scoparia Waldst.et Kit, Paeonia japonica Miyabe & Takeda, and Angelica gigas Nakai extract mixture.
Effect of RAPA on iNOS and COX-2 production in LPS-treated RAW 264.7 cells
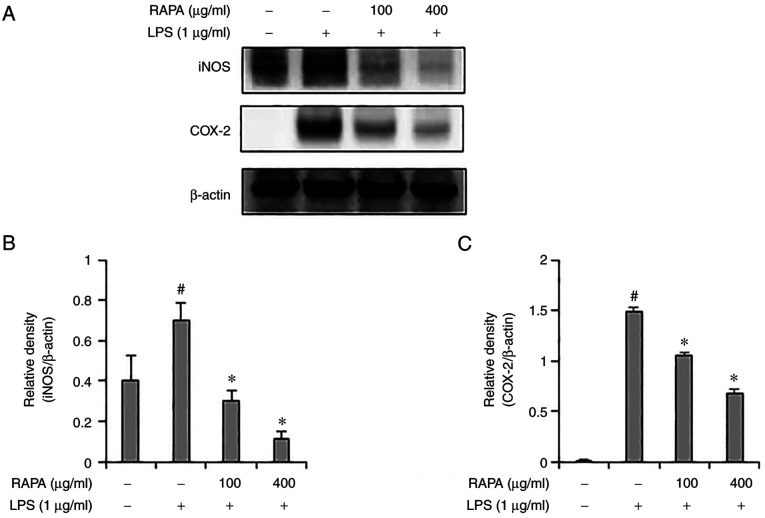
iNOS and COX-2 induce inflammation through the production of NO and PGE2. According to previous studies, natural products such as red ginseng marc, A. scoparia, and P. japonica, A. gigas have been reported to exert an anti-inflammatory effect via regulation of iNOS and COX-2 expression. (25,28). Therefore, in this study, the expression of iNOS and COX-2 were determined in the LPS-treated RAW 264.7 cells. iNOS and COX-2 expression levels were significantly increased in the group treated with LPS alone, but their expression was suppressed in the cells pretreated with RAPA (Fig. 2). These results suggest that RAPA suppresses inflammation by suppressing iNOS and COX-2 expression.
Figure 2.
Effect of RAPA on iNOS and COX-2 expression in LPS-treated RAW 264.7 cells. Cells were retreated with the indicated concentrations of RAPA for 1 h and subsequently stimulated with 1 µg/ml LPS for 24 h. The expression of iNOS and COX-2 were determined by western blot analysis (A) and analyzed using ImageJ software (B) and (C). Data are presented as the mean ± SD. #P<0.05 vs. untreated cells; *P<0.05 vs. LPS treated cells. RAPA, red ginseng marc, Artemisia scoparia Waldst.et Kit, Paeonia japonica Miyabe & Takeda, and Angelica gigas Nakai extract mixture.
Effect of RAPA on IL-6 and TNF-α production in LPS-treated RAW 264.7 cells
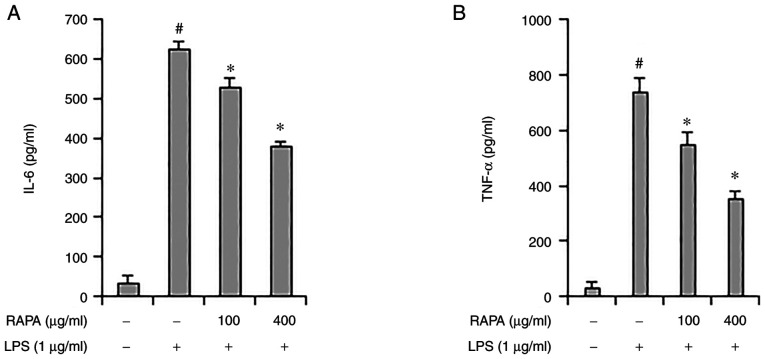
The role of cytokines in inflammation is very important, and TNF-α and IL-6 are considered representative pro-inflammatory cytokines, as they both regulate the inflammatory response (29). Herbs such as Rehmannia glutinosa and Suaeda japonica are known to inhibit inflammation by regulating IL-6 and TNF-α expression (30,31). ELISA was performed to investigate the effect of RAPA on IL-6 and TNF-α expression LPS-treated RAW 264.7 cells. IL-6 and TNF-α expression increased significantly in the positive control group, and their expression decreased in the RAPA-pretreatment group (Fig. 3A and B). These results show that RAPA suppresses inflammation by suppressing cytokine expression.
Figure 3.
Effect of RAPA on IL-6 and TNF-α expression in LPS-treated RAW 264.7 cells. The cells were treated with 100 or 400 µg/ml RAPA and stimulated with 1 µg/ml LPS. IL-6 (A) and TNF-α (B) were measured by ELISA. Data are presented as the mean ± SD. #P<0.05 vs. untreated cells; *P<0.05 vs. LPS treated cells. RAPA, red ginseng marc, Artemisia scoparia Waldst.et Kit, Paeonia japonica Miyabe & Takeda, and Angelica gigas Nakai extract mixture.
Effect of RAPA on the MAPK signaling pathway in LPS-treated RAW 264.7 cells
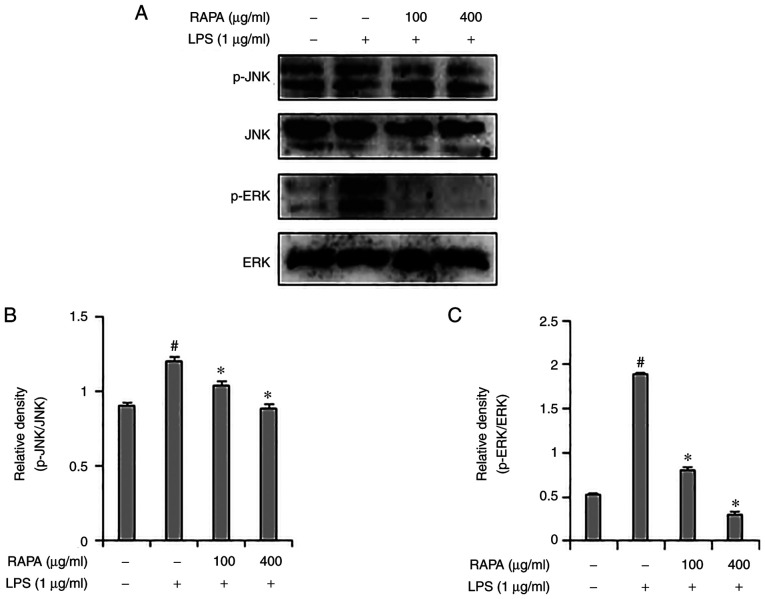
Representative members of the MAPK signaling pathway include JNK, p38 and ERK1/2, which are important signal transmitters in the inflammatory response. MAPKs are associated with COX-2 expression in iNOS and play an important role in NF-κB activation (32,33). Western blotting was used to assess whether the anti-inflammatory effects of RAPA were mediated through the MAPK signaling pathway. Increased ERK1/2 and JNK phosphorylation were observed in the LPS-treated group (Fig. 4), and RAPA pretreatment inhibited ERK1/2 and JNK phosphorylation. Of note, p38 activation was not affected (data not shown). These results suggest that RAPA is involved in anti-inflammatory activity through inhibition of the ERK1/2 and JNK pathways.
Figure 4.
Effect of RAPA on JNK and ERK phosphorylation in LPS-treated RAW 264.7 cells. Cells were pretreated with the indicated concentrations of RAPA for 1 h and subsequently stimulated with 1 µg/ml LPS for 30 min. JNK and ERK expression were measured by western blot analysis (A) and analyzed using ImageJ software (B) and (C). Data are presented as the mean ± SD. #P<0.05 vs. untreated cells; *P<0.05 vs. LPS treated cells. RAPA, red ginseng marc, Artemisia scoparia Waldst.et Kit, Paeonia japonica Miyabe & Takeda, and Angelica gigas Nakai extract mixture.
Effect of RAPA on the activity of NF-κB signaling pathway in LPS-treated RAW 264.7 cells
To investigate the effects of RAPA on the NF-κB signaling pathway, western blotting and immunofluorescence staining were performed. Western blot results demonstrated that RAPA treatment effectively inhibited the activity of NF-κB and IκB in LPS-treated RAW 264.7 cells (Fig. 5A). Immunofluorescence staining results also showed that RAPA treatment inhibited the translocation of NF-κB to the nucleus (Fig. 5B). The NF-κB signaling pathway plays a key role in LPS-induced inflammation models. In the resting state, NF-κB is present in the cytoplasm and is bound to the repressor protein IκB, which has no transcriptional activity. Stimulation by LPS liberates and activates NF-κB from IκB, and it then translocates to the nucleus to induce transcription of inflammatory genes (34,35). In this study, the activation of NF-κB and IκB was significantly increased in the LPS-treated RAW 264.7 cells, and this was decreased by RAPA pretreatment. Therefore, in the LPS inflammation model, it was hypothesized that RAPA may suppress excessive inflammatory responses by inhibiting the NF-κB signaling pathway. Medicinal plants with anti-inflammatory activity contain substances such as alkaloids, flavonoids, saponins, condensed tannins, terpenoids, phenylpropanoids and hydrolysable tannins (36). Whilst RAPA was shown to exhibit an anti-inflammatory effect, it is not known which specific component(s) exerted the anti-inflammatory effects.
Figure 5.
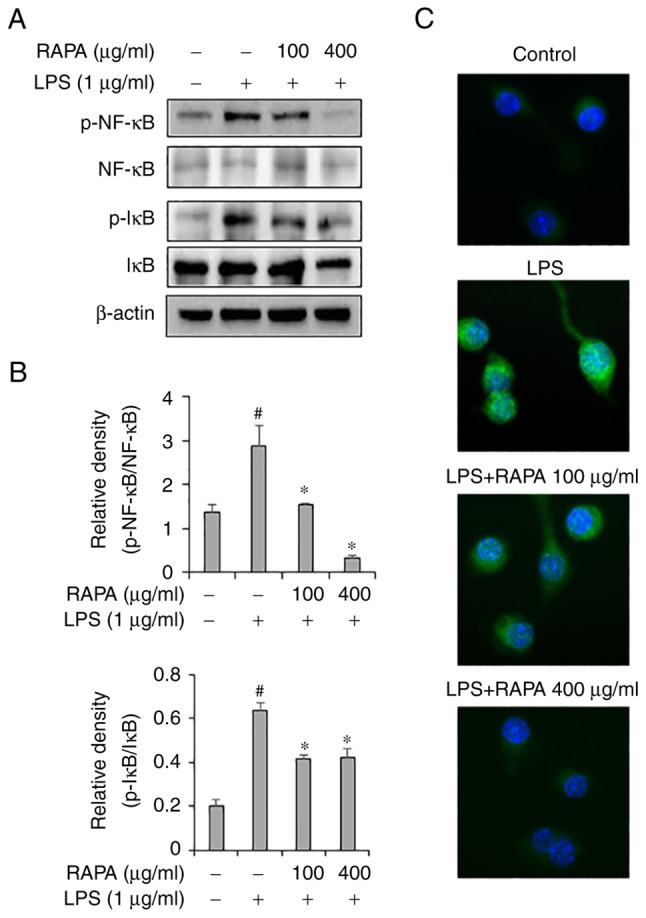
Effect of RAPA on NF-kB signaling pathway activity in LPS-treated RAW 264.7 cells. Cells were pretreated with the indicated concentrations of RAPA for 1 h and subsequently stimulated with 1 µg/ml LPS for 30 min. Phosphorylation of NF-κB and IκB were measured by western blot analysis (A)and analyzed using ImageJ software (B) and (C). Translocation of NF-κB was determined by immunofluorescence analysis (C). Scale bar, 40 µm. NF-κB was stained green, the nucleus was stained with DAPI (blue). Each bar represents the mean ± SD. #P<0.05 vs. untreated cells; *P<0.05 vs. LPS treated cells. RAPA, red ginseng marc, Artemisia scoparia Waldst.et Kit, Paeonia japonica Miyabe & Takeda, and Angelica gigas Nakai extract mixture.
Effect of RAPA on ROS generation in LPS-treated RAW 264.7 cells
Flow cytometry was performed to investigate the effects of RAPA on ROS generation in LPS-treated RAW 264.7 cells. The results showed that LPS treatment significantly increased ROS production in cells (Fig. 6). However, pretreatment with RAPA inhibited the production of LPS-induced ROS. LPS-induced ROS participates in the regulation of NF-κB activation, and activated NF-κB is involved in the production of COX-2, iNOS, and cytokines in macrophages (37). Thus, it is hypothesized that the inhibition of NF-κB activity in RAPA pretreated LPS-treated cells may have contributed to the inhibition of ROS production.
Figure 6.
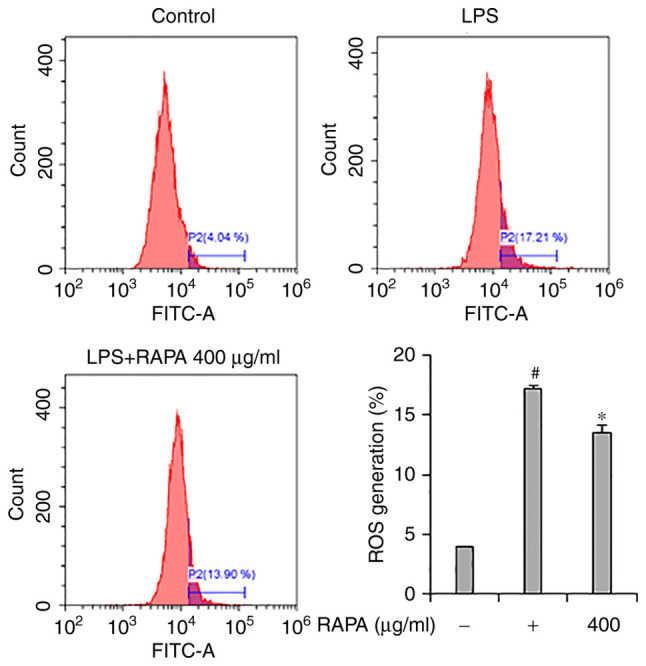
Effects of RAPA on ROS production in LPS-treated RAW 264 7 cell. ROS scavenging was determined using carboxy-H2DCFDA. Data are presented as the mean ± SD. #P<0.05 vs. untreated cells; *P<0.05 vs. LPS treated cells. RAPA, red ginseng marc, Artemisia scoparia Waldst.et Kit, Paeonia japonica Miyabe & Takeda, and Angelica gigas Nakai extract mixture.
In conclusion, the anti-inflammatory effects of RAPA in LPS-treated cells were demonstrated. It is hypothesized that the anti-inflammatory effects of RAPA are achieved though the induction of production of inflammatory mediators such as IL-6, COX-2, iNOS, and TNF-α via inhibition of the MAPK and NF-κB signaling pathway. These results suggest that RAPA may be used as an anti-inflammatory agent derived from natural products.
Acknowledgements
Not applicable.
Funding Statement
Funding: This research was financially supported by the Ministry of Small and Medium-sized Enterprises and Startups, under the ‘Regional Specialized Industry Development Plus Program (R&D, Project no. S3088018)’ supervised by the Korea Technology and Information Promotion Agency.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JYS and BOC conceived and designed the experiments. JYS and ESK participated in the design of the study and drafting of the manuscript. JYS and ESK performed the majority of the experiments. JHP participated in the cell culture and western blotting. JYS and SIJ confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. The data included in this study were previously published in the ‘Korean Society and Food Science Nutrition 2021 International Symposium and Annual meeting’.
References
- 1.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 2.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–226. 229; discussion 230-2. [PubMed] [Google Scholar]
- 3.Pahwa R, Goyal A, Bansal P, Jialal I. Chronic inflammation. 2018. [PubMed] [Google Scholar]
- 4.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 5.Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107:391–397. doi: 10.1111/cas.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldsby R, Kindt TJ, Osborne BA, Kuby J. In: Immunology (5: e uppl.). 5th Edition. W. H. Freeman and Company, New York, 2003. [Google Scholar]
- 7.Hume DA, Wells CA, Ravasi T. Transcriptional regulatory networks in macrophages. Novartis Found Symp. 2007;281:2–18. doi: 10.1002/9780470062128.ch2. [DOI] [PubMed] [Google Scholar]
- 8.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 9.Zelová H, Hošek J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 10.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 11.Farinelli I, Martelletti P. Aspirin and tension-type headache. J Headache Pain. 2007;8:49–55. doi: 10.1007/s10194-006-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonabello A, Galmozzi MR, Canaparo R, Isaia GC, Serpe L, Muntoni E, Zara GP. Dexibuprofen (S+-isomer ibuprofen) reduces gastric damage and improves analgesic and antiinflammatory effects in rodents. Anesth Analg. 2003;97:402–408. doi: 10.1213/01.ANE.0000073349.04610.42. [DOI] [PubMed] [Google Scholar]
- 13.Wiechert R. Modern steroid problems. Angew Chem Int Ed Engl. 1970;9:321–332. doi: 10.1002/anie.197003211. [DOI] [PubMed] [Google Scholar]
- 14.Grennan D, Wang S. Steroid side effects. JAMA. 2019;322(282) doi: 10.1001/jama.2019.8506. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Bajwa BS, Kuldeep S, Kalia AN. Anti-inflammatory activity of herbal plants: A review. Int J Adv Pharm Biol Chem. 2013;2:272–281. [Google Scholar]
- 16.Baeg IH, So SH. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Yang WS, Yu T, Sung GH, Park KW, Yoon K, Son YJ, Hwang H, Kwak YS, Lee CM, et al. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Bak MJ, Hong SG, Lee JW, Jeong WS. Red ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SB, Lee JS, Moon SO, Lee HD, Yoon YS, Son CG. A standardized herbal combination of Astragalus membranaceus and Paeonia japonica, protects against muscle atrophy in a C26 colon cancer cachexia mouse model. J Ethnopharmacol. 2021;267(113470) doi: 10.1016/j.jep.2020.113470. [DOI] [PubMed] [Google Scholar]
- 20.Lim SY, Jang JH, Lee HJ, Park SS, Kim SR, Lee KM, Kim JK, Park H, Jung HK. Characteristics and phylogenetic analysis of the complete chloroplast genome of Paeonia japonica (Paeoniaceae) Mitochondrial DNA B Resour. 2021;6:734–735. doi: 10.1080/23802359.2020.1860718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowndhararajan K, Kim S. Neuroprotective and cognitive enhancement potentials of Angelica gigas Nakai Root: A Review. Sci Pharm. 2017;85(21) doi: 10.3390/scipharm85020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, Wang L, He C, Zhao J, Si L, Huang H. Artemisia scoparia: Traditional uses, active constituents and pharmacological effects. J Ethnopharmacol. 2021;273(113960) doi: 10.1016/j.jep.2021.113960. [DOI] [PubMed] [Google Scholar]
- 23.Boudreau A, Burke SJ, Collier JJ, Richard AJ, Ribnicky DM, Stephens JM. Mechanisms of Artemisia scoparia's anti-inflammatory activity in cultured adipocytes, macrophages, and pancreatic β-cells. Obesity (Silver Spring) 2020;28:1726–1735. doi: 10.1002/oby.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn JH, Park YL, Song AY, Kim WG, Je CY, Jung DH, Kim YJ, Oh J, Cho JY, Kim DJ, Park JH. Water extract of Artemisia scoparia Waldst. & Kitam suppresses LPS-induced cytokine production and NLRP3 inflammasome activation in macrophages and alleviates carrageenan-induced acute inflammation in mice. J Ethnopharmacol. 2021;268(113606) doi: 10.1016/j.jep.2020.113606. [DOI] [PubMed] [Google Scholar]
- 25.Cho BO, Shin JY, Kang HJ, Park JH, Hao S, Wang F, Jang SI. Anti-inflammatory effect of Chrysanthemum zawadskii, peppermint, Glycyrrhiza glabra herbal mixture in lipopolysaccharide-stimulated RAW264. 7 macrophages. Mol Med Rep. 2021;24(532) doi: 10.3892/mmr.2021.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nayak SS, Ghosh AK, Debnath B, Vishnoi SP, Jha T. Synergistic effect of methanol extract of Abies webbiana leaves on sleeping time induced by standard sedatives in mice and anti-inflammatory activity of extracts in rats. J Ethnopharmacol. 2004;93:397–402. doi: 10.1016/j.jep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Stoclet J, Muller B, Andriantsitohaina R, Kleschyov A. Overproduction of nitric oxide in pathophysiology of blood vessels. Biochemistry (Mosc) 1998;63:826–832. [PubMed] [Google Scholar]
- 28.Shin JY, Park JH, Che DN, Kang HJ, Cho BO, Lim YT, Jang SI. Protective effects of halophyte complex extract against UVB-induced damage in human keratinocytes and the skin of hairless mice. Exp Ther Med. 2021;22(682) doi: 10.3892/etm.2021.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Escobar R, Moreno R, Garcıa-Creus M. Seasonal changes of mineral nutrients in olive leaves during the alternate-bearing cycle. Scientia Horticulturae. 1999;82:25–45. [Google Scholar]
- 30.Bastidas-Coral AP, Bakker AD, Zandieh-Doulabi B, Kleverlaan CJ, Bravenboer N, Forouzanfar T, Klein-Nulend J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 differentially affect osteogenic differentiation of human adipose stem cells. Stem Cells Int. 2016;2016(1318256) doi: 10.1155/2016/1318256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi L, Zhou Z, Zheng Y, Chang M, Huang X, Guo F, Zhao Q, Huan J. Suppressive effects of GSS on lipopolysaccharide-induced endothelial cell injury and ALI via TNF-α and IL-6. Mediators Inflamm. 2019;2019(4251394) doi: 10.1155/2019/4251394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiura R, Satoh R, Ishiwata S, Umeda N, Kita A. Role of RNA-Binding Proteins in MAPK signal transduction pathway. J Signal Transduct. 2011;2011(109746) doi: 10.1155/2011/109746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du W, Hu H, Zhang J, Bao G, Chen R, Quan R. The mechanism of MAPK signal transduction pathway involved with electroacupuncture treatment for different diseases. Evid Based Complement Alternat Med. 2019;2019(8138017) doi: 10.1155/2019/8138017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia YF, Liu LP, Zhong CP, Geng JG. NF-kappaB activation for constitutive expression of VCAM-1 and ICAM-1 on B lymphocytes and plasma cells. Biochem Biophys Res Commun. 2001;289:851–856. doi: 10.1006/bbrc.2001.6067. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Chen J, Lin L, Pan G, Zhang S, Chen H, Zhang M, Xuan Y, Wang Y, You Z. Quzhou Fructus Aurantii Extract suppresses inflammation via regulation of MAPK, NF-κB, and AMPK signaling pathway. Sci Rep. 2020;10(1593) doi: 10.1038/s41598-020-58566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunes CDR, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, Vieira IJC, Barros de Oliveira D. Plants as sources of anti-inflammatory agents. Molecules. 2020;25(3726) doi: 10.3390/molecules25163726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.