Abstract
Monoclonal B cell lymphocytosis (MBL) and chronic lymphocytic leukemia (CLL) are clonal B cell disorders associated with increased risk of infections and impaired vaccination responses. We investigated the immunogenicity of recombinant zoster vaccine (RZV) in these patients. Individuals with MBL/untreated CLL and Bruton tyrosine kinase inhibitor (BTKi)-treated CLL patients were given two doses of RZV separated by two months. Responses assessed at 3-months and 12-months from the first dose of RZV by an anti-glycoprotein E ELISA antibody assay and by dual-color IFN-γ and IL-2 FLUOROSPOT assays were compared to historic controls matched by age and sex. Sixty-two patients (37 MBL/untreated CLL and 25 BTKi-treated CLL) were enrolled with a median age of 68 years at vaccination. An antibody response at 3 months was seen in 45% of participants, which was significantly lower compared to historic controls (63%, P=0.03). The antibody response did not significantly differ between MBL/untreated CLL and BTKi-treated CLL (51% vs 36%, respectively, P=0.23). The CD4+ T cell response to vaccination was significantly lower in study participants compared to controls (54% vs 96%, P<0.001), mainly due to lower responses among BTKi-treated patients compared to untreated MBL/CLL (32% vs 73%, P=0.008). Overall, only 29% of participants achieved combined antibody and cellular responses to RZV. Among participants with response assessment at 12 months (n=47), 24% had antibody titers below response threshold. Hypogammaglobulinemia and BTKi therapy were associated with reduced T cell responses in a univariate analysis. Strategies to improve vaccine response to RZV among MBL/CLL patients are needed.
Introduction
Herpes zoster, which represents reactivation of latent varicella zoster virus (VZV), manifests typically as a painful, vesicular rash in a dermatomal distribution. More than 1.1 million cases of herpes zoster occur each year in the United States, with its incidence increasing with age.1,2 Chronic lymphocytic leukemia (CLL) is one of the most common leukemias in adults in the US; the median age at diagnosis is 72 years.3 CLL is associated with significant immune dysfunction leading to an increased risk of infectious complications.4,5 The incidence of herpes zoster among CLL patients treated with chemoimmunotherapy is 7–15%.6,7 Data on the risk of herpes zoster in CLL patients treated with novel agents such as Bruton tyrosine kinase inhibitors (BTKi) and BCL-2 antagonists is limited.8,9
The cornerstone for prevention of herpes zoster in immune competent individuals is vaccination. The first herpes zoster vaccine was a live virus vaccine that is contraindicated in patients with CLL who have significant immune dysfunction.10 In the US, Canada, Australia, and some EU countries, a non-live herpes zoster vaccine (recombinant zoster vaccine [RZV] Shingrix; GlaxoSmithKline Biologicals, SA, Belgium) that is safe in immune compromised patients is available. RZV is a 2-dose subunit herpes zoster vaccine containing recombinant glycoprotein E (gE) in combination with a potent adjuvant. It is recommended for adults ≥50 years old based on two pivotal trials that demonstrated ≥92% efficacy against herpes zoster and post-herpetic neuralgia across all age groups.11,12 RZV is safe in patients with HIV, renal transplantation, and solid tumors receiving chemotherapy. In addition, its safety and efficacy has been demonstrated in autologous hematopoietic stem cell transplant recipients and patients with hematologic malignancies.13–17 In a phase 3 study of patients with hematologic malignancies, RZV was 87% effective within the 2-year follow-up period and induced humoral immune responses in 80% of study participants.18 That study excluded patients with CLL and other non-Hodgkin B cell lymphoma from the primary analyses. Two studies have evaluated the response to RZV vaccine among CLL patients treated with BTKi. Both studies demonstrated high antibody response rates to RZV, and one study demonstrated gE-specific cell-mediated immunity in ~ 80% vaccinees.19,20 The goal of our study was to further assess T cell-mediated immune response and gE-specific antibody responses after RZV administration to patients with CLL and to compare these to responses of historic controls. We also included individuals with monoclonal B cell lymphocytosis (MBL), a precursor condition to CLL,21 which is associated with immune deficits and an increased risk of infections.22–24
Methods
Study Design and Participants
Individuals with MBL and CLL cared for at Mayo Clinic, Rochester, MN who were naïve to RZV were eligible to participate in this study (patients who received prior live attenuated zoster vaccine were allowed). Two cohorts were enrolled: Cohort 1 consisted of individuals with MBL and previously untreated CLL; and Cohort 2 consisted of CLL participants who were on BTKi therapy. The pre-vaccination characteristics at the time of study entry and history of prior vaccination with the live attenuated herpes zoster vaccine were ascertained. Individuals who participated provided written consent after the study was approved by the Mayo Clinic Institutional Review Board. Since the study was conducted after Food and Drug Administration (FDA) approval of RZV, all patients received the vaccine as standard of care. Participants received two doses of RZV separated by 2 months. Blood samples were collected prior to vaccination, 1 month after the second dose of RZV (3 months from the first dose of RZV), and at 12 and 24 months after the first dose of vaccine. Blood sample collection at the 24-month time point is still ongoing and results from this interval are not included in this report.
Assessment of Immunogenicity
Antibody was measured by ELISA designed to detect anti-gE antibodies at pre-vaccination and 3 and 12 months after the first dose of RZV administration. Assays were conducted at the Centers for Disease Control and Prevention (CDC; Dr. Scott Schmid) as previously described.25 Results are reported in ELISA units/µl after interpolation of the optical density (OD) values onto a standard curve constructed using a laboratory control serum and Prism 9.1 software (Graph pad LLC). We defined a humoral response to vaccination as the proportion with anti-gE antibody concentration after vaccination that was ≥4 times the pre-vaccination concentration, as previously described.18,20,26 Antibody responses were also reported as the geometric mean titer in each group. The gE-specific T cell response was measured by dual-color interferon γ (IFN- γ) and interleukin 2 (IL-2) FLUOROSPOT (Mabtech) on peripheral blood mononuclear cells (PBMCs) depleted of ≥50% leukemic of B cells using magnetic beads coated with anti-CD19 monoclonal antibodies (STEMCELL Technologies, Catalog # 17854). Results are reported as spot-forming cells (SFC)/106 PBMC in wells stimulated with overlapping gE peptides as previously described.27 We also report the geometric mean count (GMC) of SFCs/106 PBMC at pre-vaccination and 3 months after the first dose of vaccine, and calculated the geometric mean fold rise (GMFR) in SFCs during this interval. GMFR was calculated as anti-log10 (mean [log10 SFC3 month/SFC0 month]). Cell-mediated immune response assays were conducted at the University of Colorado (Dr. Adriana Weinberg). The FLUOROSPOT well reader (MJJ) was blinded to cohort allocation of the samples. The cell-mediated immune response rate was defined as the proportion of participants who achieved a ≥2-fold increase in IFN- γ and IL-2 SFC/106 PBMC 3 months after the first dose of RZV compared with pre-vaccination levels, as previously described.18 We compared the response data to a historic cohort of age-and sex-matched healthy controls who received RZV, as previously described.27
Statistical analyses
Chi-square or Fisher’s exact tests were used to measure differences in response rates and Kruskal-Wallis tested for differences in continuous variables between two comparison groups: 1) patients with MBL/CLL versus age- and sex-matched controls; 2) untreated MBL/CLL versus BTKi-treated CLL patients. Median titer values and proportions of responders along with 95% confidence intervals (CI) were calculated. Among all participants, univariable logistic regression analysis was used to investigate the association between pre-vaccination characteristics and antibody and T cell responses. Statistical analyses were conducted using SAS version 9.0.
Results
A total of 62 participants were included in the study (37 in Cohort 1, consisting of MBL and untreated CLL, and 25 in Cohort 2, including 24 patients on ibrutinib and 1 patient on acalabrutinib therapy]. The pre-vaccination characteristics of all participants are shown in Table 1. The median age at vaccination was 68 years [range 32–85], and 40 (65%) were male. Prior receipt of live attenuated zoster vaccine was 21%, similar between cohort 1 and cohort 2. Of the BTKi-treated cohort, the median time on BTKi was 1.4 years (range 0.04–5.1).
Table 1:
Pre-vaccination Characteristics of all patients
| Characteristic at enrollment | MBL/Untreated CLL [range] or (%) | CLL patients on BTKi [range] or (%) | Total | |
|---|---|---|---|---|
| Number | 37 | 25 | 62 | |
| Median age at vaccination, years | 64 [32–85] | 71 [48–82] | 68 [32–85] | |
| Male | 23 (62) | 17 (68) | 40 (65) | |
| Time from MBL/CLL diagnosis or start of BTKi to enrollment, years | 0.9 [0–16.8] | 1.4 [0.04–5.1] | 1.2 [0–16.8] | |
| Median ALC (x109/L) | 13.2 [2–81] | 4.2 [0.3–229] | 8.9 [0.3–229] | |
| Median WBC (x109/L) | 18.7 [6.7–88.7] | 9.7 [6.4–233.7] | 16.1 [6.4–233.7] | |
| Rai Stage | 0 | 20 (54) | 3 (12) | 23 (37) |
| I | 14 (38) | 5 (20) | 19 (31) | |
| II | 1 (3) | 5 (20) | 6 (10) | |
| III | 1 (3) | 2 (8) | 3 (5) | |
| IV | 1 (3) | 10 (40) | 11 (18) | |
| Unmutated IGHV genes | 14/31 (45) | 19 (76) | 33/56 (59) | |
| FISH | None Detected | 9 (27) | 3 (13) | 12 (21) |
| Del13q | 18 (53) | 13 (54) | 31 (53) | |
| Trisomy12 | 4 (12) | 3 (13) | 7 (12) | |
| Del11q | 2 (6) | 3 (13) | 5 (9) | |
| Del17p | 1 (3) | 2 (8) | 3 (5) | |
| Missing | 3 | 1 | 4 | |
| CLL-IPI | Low | 14 (47) | 3 (13) | 17 (32) |
| Intermediate | 12 (40) | 11 (46) | 23 (43) | |
| High | 3 (10) | 8 (33) | 11 (20) | |
| Very high | 1 (3) | 2 (8) | 3 (6) | |
| Missing | 7 | 1 | 8 | |
| Prior receipt of live attenuated zoster vaccine | 8 (22) | 5 (20) | 13 (21) | |
| Time from receipt of live attenuated zoster vaccine to enrollment, years | 8.8 [0–11.1] | 7.1 [2.0–10.3] | 8.2 [0–11.1] | |
| Receipt of IVIg at any time prior to enrollment | 1 (3) | 4 (16) | 5 (8) | |
| Receipt of IVIg within 90 days of enrollment | 0 (0) | 3 (13) | 3 (5) | |
Abbreviations used: MBL, monoclonal B cell lymphocytosis; CLL, chronic lymphocytic leukemia; BTKi, Bruton tyrosine kinase inhibitor; ALC, Absolute Lymphocyte Count; WBC, White Blood cell Count; IGHV, immunoglobulin heavy chain gene variable region; IVIG: intravenous immunoglobulin; FISH, fluorescence in situ hybridization; CLL-IPI, CLL-International prognostic index
Antibody Response
The median pre-vaccination anti-gE antibody concentration was 61.6 EU/ µL (95% CI 35.7–99.7) among 62 CLL/MBL participants compared to 43.2 EU/ µL (95% CI 29.4–58.6) in the control group (P=0.07, Figure 1A). Three months following the first RZV administration, the median anti-gE antibody concentration was 237.8 EU/ µL (95% CI 210.3–269.0) among MBL/CLL participants compared to 237.0 EU/µL (95% CI 229.4–245.6) in the control group (P=0.99, Figure 1B). In the MBL/CLL group, 45% of individuals responded to RZV with a ≥4-fold increase in antibodies over pre-vaccination compared to 63% in the control group (P=0.03, Supplemental Figure 1A).
Figure 1: Comparison of anti-gE antibody concentration between MBL/CLL participants to controls.
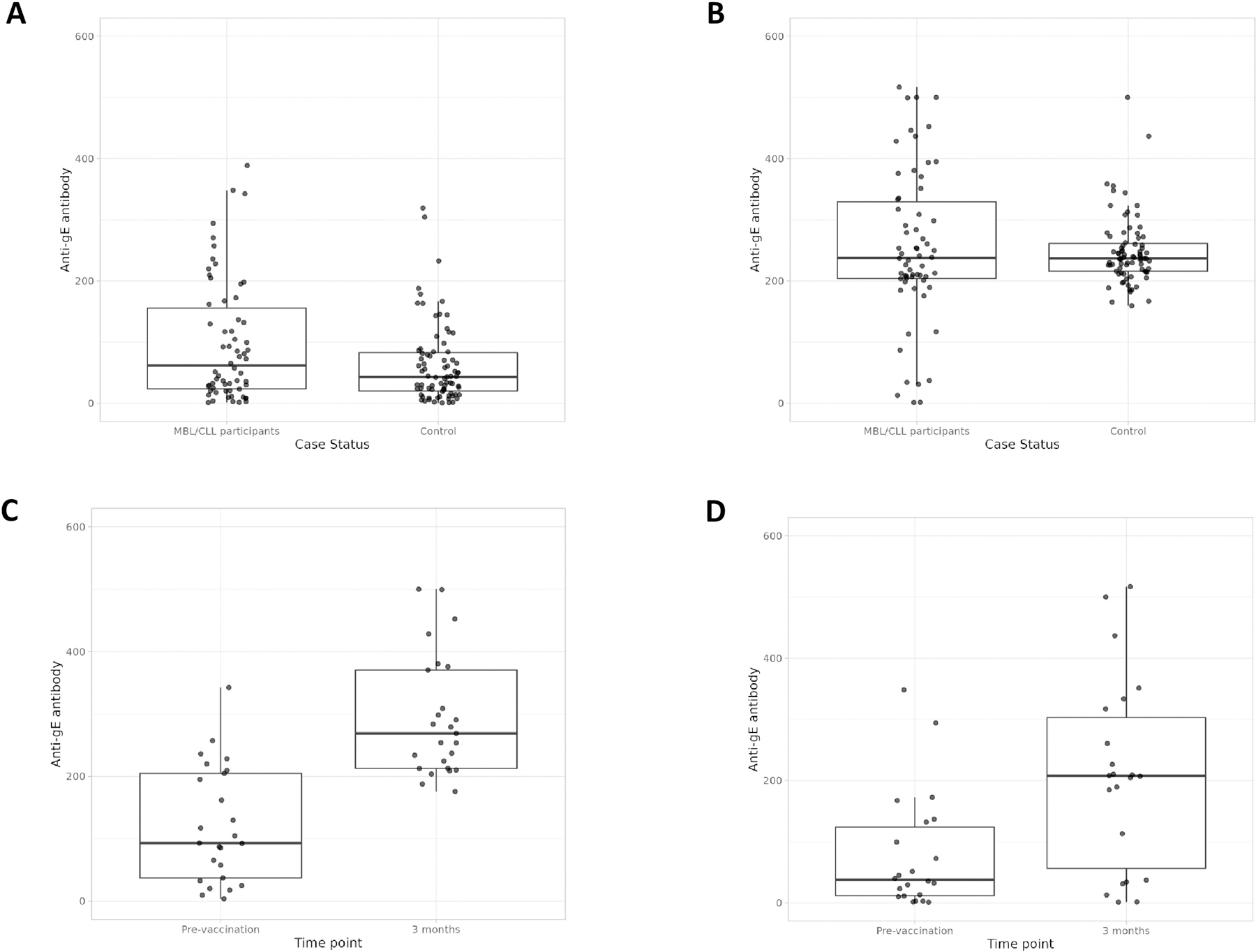
Figure 1A: Pre-vaccination anti-gE level in MBL/CLL participants (n=62) compared to age- and sex-matched controls (n=68; P=0.07); Figure 1B: Anti-gE antibody level 3 months after the first dose of recombinant zoster vaccine (RZV) in MBL/CLL participants compared to age- and sex-matched healthy controls (P=0.99); Figure 1C: Comparison of pre-vaccination anti-gE antibody level in untreated MBL/CLL (n=37) to 3 months post first dose of RZV vaccine (P<0.001); Figure 1D: Comparison of pre-vaccination anti-gE antibody level in CLL patients treated with Bruton tyrosine kinase inhibitors (BTKi) to 3 months after the first dose of RZV vaccine (P=0.004)
Among MBL/previously untreated CLL participants (Cohort 1), the median anti-gE antibody concentration increased from 93.0 EU/ µL (95% CI 57.7–195.2) at pre-vaccination to 269.0 EU/µL (95% CI 224.5–308.7) 3 months following the first dose of RZV (P<0.001, Figure 1C). Among patients with CLL on therapy with BTKi (Cohort 2), the median anti-gE antibody concentration increased from 37.9 EU/µL (95% CI 13.6–132.2) pre-vaccination to 207.7 EU/µL (95% CI 113.2–317.1) 3 months following the first dose of RZV vaccination (P=0.004, Figure 1D). The corresponding response rates were not significantly different among participants in Cohort 1 compared to Cohort 2 (51% vs. 36%, P=0.23, Supplemental Figure 1B).
Forty-seven participants had antibody data at pre-vaccination, and at 3 and 12 months following the first dose of RZV. Of these, 26 (55%) did not have a humoral response at both the 3- and 12-months after the first dose of RZV vaccination; 15 (32%) had a humoral response at 3 months that was sustained at 12 months; 5 (11%) had a response at 3 months that was not sustained at 12 months; and 1 (2%) did not have a humoral response at 3 months and had a response at 12 months. Supplemental Figure 2A shows the median anti-gE antibody concentration over time among all CLL/MBL participants compared to controls, and among those in Cohort 1 and Cohort 2 (Supplemental Figure 2B and Supplemental 2C, respectively).
T Cell Response
The cell-mediated immune response was measured in a subset of participants (n=41). The pre-vaccination characteristics of participants with cell-mediated immune responses measured are shown in Supplemental Table 1. The GMC of the SFCs/106 PMBCs was significantly higher at 3 months post-vaccination compared to pre-vaccination among both controls and MBL/CLL participants for both IFN-γ and IL-2 (Table 2). However, the magnitude of the response as measured by GMFR was significantly lower in MBL/CLL vaccinees compared to controls (2.2 vs. 19, P<0.001 for IFN-γ; and 6.5 vs. 32.6, P<0.001 for IL-2). The IFN- γ response rate (defined as ≥2-fold increase in SFC/106 PBMC) was significantly lower among MBL/CLL participants compared to controls (56% vs. 96%, P<0.001); the difference in the IL-2 response between MBL/CLL and controls reached borderline statistical significance (93% vs. 100%, P=0.051). The overall cell-mediated immune response (defined as a response to both IFN- γ and IL-2) was significantly lower in MBL/CLL participants compared to controls (54% vs. 96%, P<0.001). Figure 2 depicts a image of FLUOROSPOT result from a representative CLL patient treated with BTKi (left panel) and an untreated CLL patient (right panel).
Table 2:
Cellular immune response to recombinant zoster vaccine in age- and sex-matched healthy controls compared to MBL/CLL and among untreated MBL/CLL compared to CLL patients treated with Bruton tyrosine kinase inhibitors
| Controls (n=68) compared to MBL/CLL (n=41) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | IFN-γ | IL-2 | |||||
| Controls (n=68) | MBL/CLL (n=41) | p-value | Controls (n=68) | MBL/CLL (n=41) | p-value | ||
| Median SFCs (range) | Pre-vaccination | 11 (1–64) | 28.5 (3–96) | <0.001 | 15 (1–120) | 4.5 (0.5–75.5) | 0.02 |
| 3 months | 231 (2–890) | 78.5 (2–335.5) | <0.001 | 424 (16–1184) | 95.5 (1.5–297) | <0.001 | |
| Pre-vaccination vs. 3 months p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Median GMFR (range) | 19.0 (0.7–794) | 2.2 (0.2–29.7) | <0.001 | 32.6 (2–1116) | 6.5 (1.5–165.5) | <0.001 | |
| Response Rate | 65 [96%] | 23 [56%] | <0.001 | 68 [100%] | 38 [93%] | 0.051 | |
| IFN-γ and IL-2 | |||||||
| Overall Vaccine Response Rate* | Controls (n=68) | MBL/CLL (n=41) | p-value | ||||
| 65 (96%) | 22 (54%) | <0.001 | |||||
| MBL/untreated CLL (n=22) compared to CLL treated with BTKi (n=19) | |||||||
| Characteristic | IFN-γ | IL-2 | |||||
| MBL/untreated CLL (n=22) | CLL treated with BTKi (n=19) | p-value | MBL/untreated CLL (n=22) | CLL treated with BTKi (n=19) | p-value | ||
| Median SFCs (range) | Pre-vaccination | 21.8 (3–96) | 32 (4–75.5) | 0.46 | 4.8 (1–75.5) | 3.5 (0.5–31) | 0.67 |
| 3 months | 131 (2–335.5) | 52.5 (3–191) | 0.02 | 166.3 (5.5–297) | 34 (1.5–236.5) | 0.001 | |
| Pre-vaccination vs. 3 months p-value | <0.001 | 0.06 | <0.001 | 0.002 | |||
| Median GMFR (range) | 5.2 (0.2–29.7) | 1.9 (0.5–24.9) | 0.03 | 25 (1.6–165.5) | 4.1 (1.5–157.7) | 0.003 | |
| Response Rate | 16 [73%] | 7 [37%] | 0.02 | 21 [96%] | 17 [89%] | 0.59 | |
| IFN-γ and IL-2 | |||||||
| Overall Vaccine Response Rate * | Untreated MBL/CLL (n=22) | CLL treated with BTKi (n=19) | p-value | ||||
| 16 [73%] | 6 [32%] | 0.008 | |||||
Abbreviations: BTKi, Bruton tyrosine kinase inhibitor; CLL, Chronic lymphocytic leukemia; GMC: geometric mean count; GMFR: geometric mean fold rise; IFN-γ, Interferon- γ; IL-2, Interleuekin-2; MBL, Monoclonal B-cell lymphocytosis; RZV, Recombinant zoster vaccine; SFCs, spot-forming cells
The overall cellular vaccine response rate was defined as the proportion of participants who achieved a ≥2-fold increase in IFN- γ and IL-2 SFC/106 PBMC 3 months after the first dose of RZV compared with pre-vaccination pre-vaccination levels.
Figure 2:
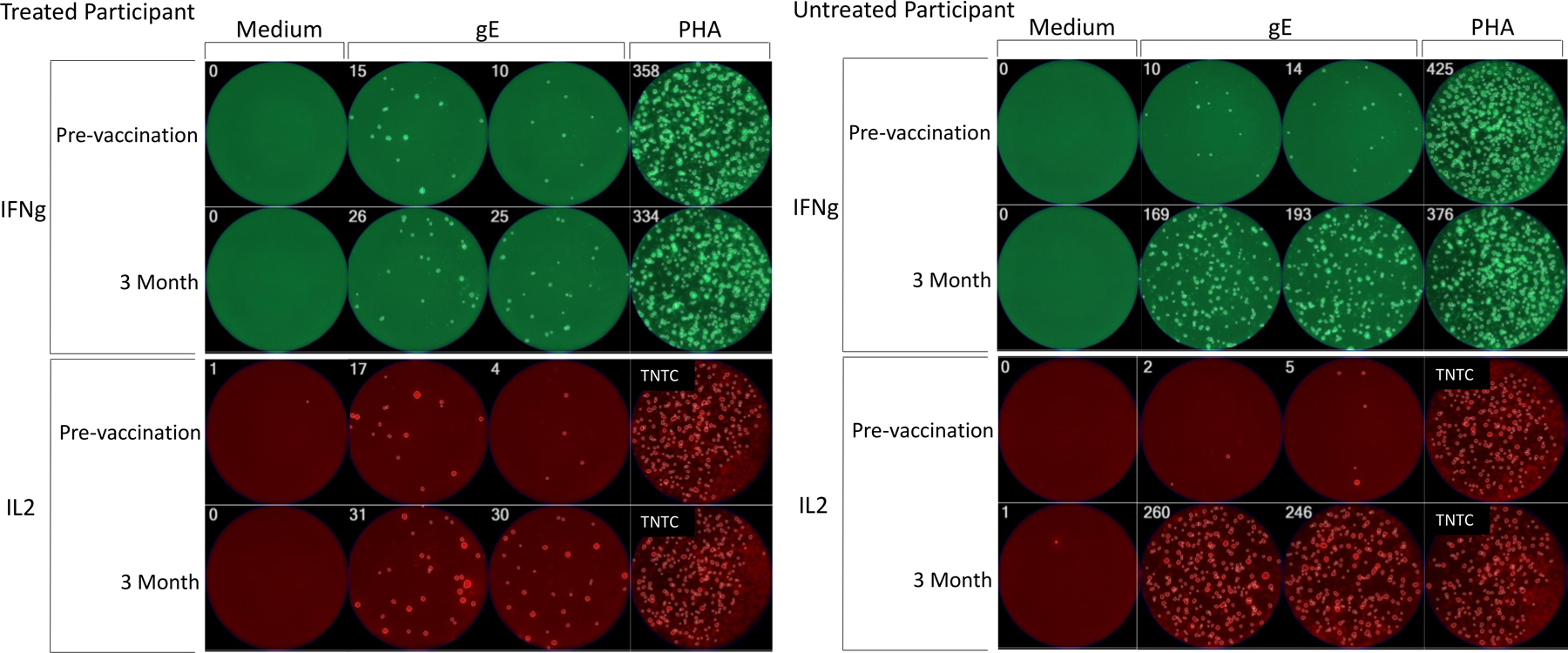
Representative images of FLUOROSPOT results in CLL patients treated with Bruton tyrosine kinase inhibitor (left panel) and untreated (right panel) CLL participants. The images show IFNg (green fluorescence) and IL2 (red fluorescence) spot-forming cells (SFC) in wells containing 250,000 PBMC/well incubated for 48 h with medium (negative control), gE overlapping peptides, and PHA mitogen (positive control). The number of spot forming cells (SFC) per well is indicated in the left upper corner of each quadrant. TNTC indicates too numerous to count. The top rows show similarly low IFNg and IL2 responses to gE peptide stimulation before vaccination in the treated and untreated CLL participants, while the bottom rows show that at 30 days after the 2nd dose of the treated CLL participant has visibly lower numbers of SFC/well compared with the untreated participant. There are no appreciable differences in the medium- and PHA-stimulated controls between the two participants.
Table 2 also shows that the GMC of SFCs/106 PBMCs was significantly higher at 3 months post- compared to pre-vaccination among MBL/ untreated CLL for both IFN- γ and IL-2, whereas CLL participants treated with BTKi had significant increases for IL-2 but not for IFN- γ. MBL/untreated CLL participants had a higher GMFR compared to CLL participants treated with BTKi (5.2 vs. 1.9, P=0.03 for IFN- γ and 25 vs. 4.1, P=0.003 for IL-2). The IFN-γ response was significantly lower among CLL participants treated with BTKi compared to untreated MBL/CLL participants (37% vs. 73%, P=0.02). There was no significant difference in the IL-2 response rate between the two groups (89% vs. 96%, P=0.59). However, the overall cell-mediated immune response (defined as a response to both IFN-γ and IL-2) was significantly lower in CLL participants treated with BTKi compared to untreated MBL/CLL participants (32% vs. 73%, P=0.008).
Combined humoral and cellular immune responses
Among the 41 patients in whom data for both humoral and cellular immune responses were available, 12 (29%) individuals met the criteria for both humoral and cell-mediated immune responses to vaccination (7 in MBL/untreated CLL and 5 in BTKi-treated CLL). Supplemental Table 2 shows the distribution of responses across both cohorts.
Factors predicting response to RZV
Univariable analysis of factors (Supplemental Table 3) showed that participants with hypogammaglobulinemia (serum IgG < 600 mg/dL, odds ratio [OR]= 0.13, 95% CI 0.03–0.72, P=0.02) and those receiving BTKi (OR: 0.17, 95% CI: 0.04–0.66, P=0.01) were associated with lower cell-mediated immune responses to vaccine. In contrast, participants who had received prior live attenuated zoster vaccine had a borderline significant higher cell-mediated immune response (OR: 4.86, 95% CI 0.9–26.7, P=0.07). Other variables including age at vaccination, sex, and other CLL-specific characteristics including total white blood cell count, Rai Stage, fluorescence in situ hybridization (FISH), and immunoglobulin heavy chain variable gene (IGHV) mutation status did not affect cell-mediated immune response to RZV. None of these factors were associated with a lower humoral vaccine response on univariable analysis.
Discussion
In this prospective study, we found that RZV administration to patients with MBL/CLL was less effective in inducing gE-specific antibody and cell-mediated immune responses than was vaccination of age and sex matched healthy controls. The finding of lower cell-mediated immune responses in MBL/CLL is particularly concerning because protection against herpes zoster is mediated by a robust cellular immune response.28 Although a correlate of protection for RZV against herpes zoster has not been identified, we previously showed that what distinguished RZV from the less effective live zoster vaccine is higher gE-specific and more persistent gE- and VZV-specific cell mediated immune response.27 Thus, the decreased cell mediated immune response of MBL/CLL to RZV may be associated with lower efficacy of the vaccine in this population, similar to what was observed in autologous hematopoietic stem cell transplant recipients.17 T cell defects, including an increase in T regulatory cells, exhausted T cells and an impaired immunological synapse formation are well described in patients with CLL and likely contribute to the decreased cell-mediated immune responses to RZV administration.29,30 This is in agreement with previous studies from our group showing that increased PD-1 expression on CD4+ T cells was associated with lower responses to the live zoster vaccine in older adults.31 The lower immune responses of individuals with MBL in our study is not surprising given that we and others have previously described that despite the small clone size in MBL, the malignant B cells directly impair T cell function.32–34
Patients receiving BTKi therapy for CLL had lower T cell responses to RZV vaccination compared to untreated CLL. BTKi therapy in CLL, in particular using ibrutinib, is known to improve CD4+ and CD8+ T cell numbers, and also decrease the expression of PD-1, CD200, and CTLA-4 expression on T cells.35 In addition, CLL patients treated with ibrutinib demonstrate a broader T cell receptor repertoire diversity, suggesting a partial restoration of the immune system.36 However, ibrutinib also inhibits interleukin-2 inducible kinase, which is important for the activity of T cells and this may explain the decreased functionality of T cells.37 The exact reasons for the lack of response to RZV in BTKi treated patients in our study remains unclear, and further mechanistic studies need to be conducted to understand this. It is unlikely that the relatively short duration of ibrutinib therapy (median 1.4 years prior to administration of RZV) failed to correct the low cell-mediated immune response, since vast majority of the improvement in the T cell profile occurs in the first year after starting ibrutinib therapy.38 We were unable to compare the differential effects of response to RZV vaccination in ibrutinib treated patients compared to those treated with a second generation BTKi such as acalabrutinib, since we only enrolled one patient who was on acalabrutinib at the time of RZV vaccination. Given the differences in the restoration of T cell profile between ibrutinib and acalabrutinib,39 future studies specifically addressing this group of patients will be important.
Humoral responses to RZV in our study were significantly lower compared to age- and sex-matched healthy controls. The 56% response rate is similar to that reported in the only randomized clinical trial of RZV administration in patients with hematologic malignancies – where the humoral response rate was 50% among participants with CLL and other B cell Non-Hodgkin lymphoma (these participants were excluded from the primary analysis in that study).18 Pleyer and colleagues reported a similar seroconversion rate (50%) in their study assessing humoral immune response to RZV vaccination in 30 participants with CLL.20 Similar to our study, there were no differences noted in the humoral immune response between previously untreated CLL and those receiving BTKi. These findings are in contrast to those reported by Zent and colleagues where a seroconversion rate of 75% was noted in 32 participants;26 although a third of the study population consisted of individuals with Waldenstrom macroglobulinemia, which may explain the discrepant results. Results from our study indicate that 1 in 4 patients who demonstrate an initial antibody response to RZV lose that response at 12 months; a finding that has not been reported previously for CLL patients. A similar decrease in antibody responses was seen among historic controls seen in our study, although this was not observed in individuals with other hematological cancers who received RZV.18 It is also worth noting that absence of humoral response did not preclude a cellular immune response. Of 41 participants in our study who had both humoral and cellular immune responses assessed, 10 (24%) exhibited a cell-mediated immune response in the absence of a humoral response.
Our findings add to the growing literature41 demonstrating lower immunogenicity of a variety of vaccinations in the CLL population including vaccines against influenza,42 pneumococcal infection, tetanus toxoid and Haemophilus influenzae and more recently mRNA-based vaccine against severe acute coronavirus 2 (SARS-CoV-2).43 Hypogammaglobulinemia and BTKi therapy have been shown to be associated with lower immunogenicity in most of these studies, indicating that the immune microenvironment at the time of vaccination plays an important role in determining response to vaccination. Efforts to improve vaccine responses in CLL patients using adjuvants such as ranitidine and granulocyte-monocyte colony stimulating factor (GM-CSF) have been unsuccessful;44,45 but a small study showed lenalidomide increased antibody levels among patients with multiple myeloma who received conjugated pneumococcal vaccination.46 A higher vaccine dose may have a role in improving vaccine responses in CLL, as was shown for influenza vaccine in older adults.47,48 More likely, booster vaccine doses will be helpful to augment immune responses, as has been recently shown in solid organ transplant recipients who were given a third dose of an mRNA based vaccine against SARS-CoV-2.49
The strengths of this study are several-fold. We provide both humoral and cellular vaccine response early after vaccination and at 12 months, providing data on durability of such responses. In addition to CLL, we included individuals with MBL, an immunocompromised population known to have an increased risk of serious infections. We compared data to historic age- and sex-matched controls tested in the same laboratory, strengthening the interpretation of the results. And lastly, patients with CLL/MBL have high circulating clonal B cells, which can be challenging to assess cellular mediated immune responses. In this study, we partially depleted the clonal B cell compartment, thus increasing the robustness of the cell-mediated immune response findings. This study, however, has several limitations. Since RZV was administered as part of routine clinical practice, all adverse events related to vaccination were not systematically collected; however, no serious adverse events were noted. Another limitation was that the rates of herpes zoster infection and post herpetic neuralgia were not assessed – in part, because a subset of CLL patients on BTKi were on prophylactic antiviral medications, which would confound any such assessment. We did not have adequate power to conduct multivariable analyses to determine factors associated with lower cell-mediated immune response. A RZV-generated threshold T cell response associated with protection against herpes zoster or post-herpetic neuralgia has not been identified. Therefore, we cannot be certain that the T cell response achieved by MBL/CLL patients who received RZV will be protective. Although the median pre-vaccination anti-gE antibody concentration was significantly higher in MBL/CLL participants compared to controls, it was likely due to the receipt of intravenous immunoglobulin (IVIG) in 5 patients. We performed sensitivity analyses after removing these 5 patients which showed no significant difference in median pre-vaccination anti-gE antibody concentration between MBL/CLL (57.7 EU/ µL; 95% CI 32.5–104.6) and controls (43.2 EU/ µL; 95% CI 29.4–58.6; P=0.11). Finally, long term immune response data from vaccination with RZV beyond the first year is not available as of current follow-up; but will be reported in the future.
In summary, our study demonstrates that RZV does not generate cell-mediated and antibody responses among MBL/CLL participants that are comparable to age- and sex-matched controls. This observation is primarily driven by the lower responses in BTKi-treated patients. Despite the lower immunogenicity, given the absence of serious adverse events in our study and at least one other prospective study,20 we recommend administering this vaccine to all individuals with MBL and CLL. In addition, continued prophylaxis with acyclovir against herpes zoster may be necessary in a subset of CLL patients with hypogammaglobulinemia and those on therapy with BTKi until additional studies show efficacy of RZV in this population.
Supplementary Material
Acknowledgments
The preliminary results of this study were presented as a poster presentation at the 2020 Annual American Society of Hematology Meeting (that was held virtually due to the COVID-19 pandemic). The conduct of this research was supported in part by a generous patient who was cared for at Mayo Clinic and the Henry J Predolin Foundation.
Footnotes
Conflicts of Interest
SAP: Research funding has been provided to the institution from Pharmacyclics, MorphoSys, Janssen, AstraZeneca, TG Therapeutics, Bristol Myers Squibb, Merck, AbbVie, and Ascentage Pharma for clinical studies in which SAP is a principal investigator. SAP has also participated in Advisory Board meetings of Pharmacyclics, AstraZeneca, Genentech, Gilead, GlaxoSmithKline, Verastem Oncology, and AbbVie (he was not personally compensated for his participation).
NEK: Research funding from: Acerta Pharm, Pharmacyclics, MEI Pharma, and Tolero. He is on a data safety Monitoring committee for: Agios Pharm, Celgene, Sunesis, Cytomx Therapeutics, Morpho-Sys, Rigel Pharm and Juno Therapeutics. He is on an advisory Board for Astra Zeneca, Cytomx Therapeutics, Pharmacyclics Dava Oncology, Acerta Pharma BV, and Juno Therapeutics.
SSK: SSK is an inventor on patents in the field of CAR immunotherapy that are licensed to Novartis (through an agreement between Mayo Clinic, University of Pennsylvania, and Novartis). RS, MJC, and SSK are inventors on patents in the field of CAR immunotherapy that are licensed to Humanigen (through Mayo Clinic). SSK and MH are inventors on patents in the field of CAR immunotherapy that are licensed to Mettaforge (through Mayo Clinic). SSK receives research funding from Kite, Gilead, Juno, Celgene, Novartis, Humanigen, MorphoSys, Tolero, Sunesis, and Lentigen.
WD: Research funding from Merck and DTRM. Advisory board: Merck and Octapharma (no personal compensation).
YW: Research funding (to the institution) from Incyte, InnoCare, Norvatis, and Genentech.
MJL: Research funding and Advisory board for GlaxoSmithKline.
AW: Research funding (to the institution) from NIH, GlaxoSmithKline, Merck and Janssen.
All other authors declare no conflict of interest.
References
- 1.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. Journal of General Internal Medicine 2005;20:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: Shingles (herpes zoster). (Accessed June 4, 2021, at https://www.cdc.gov/shingles/hcp/clinical-overview.html.)
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 4.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for diagnosis, indications for treatment, response assessment and supportive management of chronic lymphocytic leukemia. Blood 2018. [DOI] [PubMed] [Google Scholar]
- 5.Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol 2006;33:240–9. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–74. [DOI] [PubMed] [Google Scholar]
- 7.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008;112:975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giridhar KV, Shanafelt T, Tosh PK, Parikh SA, Call TG. Disseminated herpes zoster in chronic lymphocytic leukemia (CLL) patients treated with B-cell receptor pathway inhibitors. Leuk Lymphoma 2017;58:1973–6. [DOI] [PubMed] [Google Scholar]
- 9.Davids MS, Hallek M, Wierda W, et al. Comprehensive Safety Analysis of Venetoclax Monotherapy for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Clinical Cancer Research 2018;24:4371–9. [DOI] [PubMed] [Google Scholar]
- 10.Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices Centers for Disease C, Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57:1–30. [PubMed] [Google Scholar]
- 11.Cunningham AL, Heineman TC, Lal H, et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J Infect Dis 2018;217:1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep 2018;67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014;124:2921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015;211:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial. Clin Infect Dis 2020;70:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: A randomized trial. Cancer 2019;125:1301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastidas A, de la Serna J, El Idrissi M, et al. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2019;322:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 2019;19:988–1000. [DOI] [PubMed] [Google Scholar]
- 19.Zent CS, Brady MT, Delage C, et al. Short term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial BTK inhibitor therapy for CLL or lymphoplasmacytic lymphoma. Leukemia 2021;35:1788–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood 2021;137:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia 2010;24:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanafelt TD, Kay NE, Parikh SA, et al. Risk of serious infection among individuals with and without low count monoclonal B-cell lymphocytosis (MBL). Leukemia 2021;35:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira J, Rabe KG, Cerhan JR, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia 2013;27:136–41. [DOI] [PubMed] [Google Scholar]
- 24.Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood Rev 2018;32:387–99. [DOI] [PubMed] [Google Scholar]
- 25.Schmid DS, Miao C, Leung J, Johnson M, Weinberg A, Levin MJ. Comparative Antibody Responses to the Live-Attenuated and Recombinant Herpes Zoster Vaccines. J Virol 2021;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zent CS, Brady MT, Delage C, et al. Short term results of vaccination with adjuvanted recombinant varicella zoster glycoprotein E during initial BTK inhibitor therapy for CLL or lymphoplasmacytic lymphoma. Leukemia 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest 2018;128:4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MJ L Corerlates of Protection. 7th Edition ed. Philadelphia: Elseiver; 2017. [Google Scholar]
- 29.Vlachonikola E, Stamatopoulos K, Chatzidimitriou A. T Cells in Chronic Lymphocytic Leukemia: A Two-Edged Sword. Front Immunol 2020;11:612244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood 2012;120:1412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg A, Pang L, Johnson MJ, et al. The Effect of Age on the Immunogenicity of the Live Attenuated Zoster Vaccine Is Predicted by Baseline Regulatory T Cells and Varicella-Zoster Virus-Specific T Cell Immunity. J Virol 2019;93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008;118:2427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood 2013;121:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh SARA, Boysen J, Lesnick C, Ding W, Medina K, Ionnaou N, Jelinek DF, Slager SL, Kay NE, Shanafelt TD. Longitudinal Evaluation of T-cells in Clinical Monoclonal B-cell Lymphocytosis (MBL). 21st Annual Congress of European Haematology Association; 2016; Copenhagen, Denmark: Haematologica. [Google Scholar]
- 35.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. The Journal of Clinical Investigation 2017;127:3052–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin Q, Sivina M, Robins H, et al. Ibrutinib Therapy Increases T Cell Repertoire Diversity in Patients with Chronic Lymphocytic Leukemia. J Immunol 2017;198:1740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122:2539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roessner PM, Seiffert M. T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease? Leukemia 2020;34:2012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest 2017;127:3052–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med 2016;375:1019–32. [DOI] [PubMed] [Google Scholar]
- 41.Whitaker JA, Shanafelt TD, Poland GA, Kay NE. Room for improvement: immunizations for patients with monoclonal B-cell lymphocytosis or chronic lymphocytic leukemia. Clin Adv Hematol Oncol 2014;12:440–50. [PubMed] [Google Scholar]
- 42.Whitaker JA, Parikh SA, Shanafelt TD, et al. The humoral immune response to high-dose influenza vaccine in persons with monoclonal B-cell lymphocytosis (MBL) and chronic lymphocytic leukemia (CLL). Vaccine 2021;39:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jurlander J, de Nully Brown P, Skov PS, et al. Improved vaccination response during ranitidine treatment, and increased plasma histamine concentrations, in patients with B cell chronic lymphocytic leukemia. Leukemia 1995;9:1902–9. [PubMed] [Google Scholar]
- 45.Safdar A, Rodriguez GH, Rueda AM, et al. Multiple-dose granulocyte-macrophage-colony-stimulating factor plus 23-valent polysaccharide pneumococcal vaccine in patients with chronic lymphocytic leukemia: a prospective, randomized trial of safety and immunogenicity. Cancer 2008;113:383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noonan K, Rudraraju L, Ferguson A, et al. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res 2012;18:1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014;371:635–45. [DOI] [PubMed] [Google Scholar]
- 48.Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med 2017;5:738–46. [DOI] [PubMed] [Google Scholar]
- 49.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med 2021;385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


