Abstract
PURPOSE
We aimed to evaluate the effectiveness of a virtual community of practice (vCoP) in improving primary health care professionals’ (HCPs’) attitudes toward empowering patients with chronic disease.
METHODS
We conducted a cluster randomized controlled trial. Practices were units of randomization, and primary HCPs and patients were units of analysis. Sixty-three practices in Madrid, Catalonia, and the Canary Islands were randomly allocated to the intervention or control groups. Randominzation of practices was performed after HCP and patient recruitment. The patients and statistician were anonymized to group allocation; it was not possible to anonymize HCPs. The intervention was a 12-month multicomponent tailored vCoP built on the Web 2.0 concept and focused on skills toward patient empowerment. The primary outcome was Patient-Provider Orientation Scale (PPOS) score at baseline and at 12 months. The secondary outcome was the Patient Activation Measure (PAM) score.
RESULTS
A total of 321 HCPs and 1,921 patients were assessed. The intervention had a positive effect on PPOS total score (0.14 points higher in the vCoP arm; 95% CI, 0.03-0.25; P = .011) and the PPOS Sharing subscale (0.3 points higher in the vCoP arm; 95% CI, 0.15-0.44; P < .001). No effect was found for the PPOS Caring subscale, and no significant differences were found for PAM scores.
CONCLUSIONS
A vCoP led to a minor increase in the PPOS Sharing component and the total score but not in the Caring component. However, considerable uncertainty remains, given the observed attrition and other limitations of the study. Further research is needed on the effectiveness of the vCoP model and on how to improve HCP engagement.
Key words: patient empowerment, chronic diseases, patient-centered care, virtual community of practice
INTRODUCTION
The high burden for health care systems that is associated with chronic care could be decreased by boosting patient empowerment.1 Empowered patients take action to improve their quality of life and have the necessary knowledge, skills, and attitudes to adjust their behavior and work to achieve optimal well-being.2 Support interventions for patient empowerment are thus crucial for patients to participate in their care to the degree they desire.2 To provide this support, well-trained and motivated health care professionals (HCPs) are needed, particularly in primary care. Indeed, HCP attitudes and perceptions are one of the main barriers to patient empowerment.3,4
Communities of practice (CoPs) built on relationships of mutual engagement and collective learning5 might help change HCP attitudes toward patient empowerment. A CoP is “a group of people who share an interest, a set of problems, or a passion about a subject, and who deepen their knowledge and experience in the area through continuous interaction.”6 They have been shown to effectively improve both learning and quality of care.7 Although CoPs have the potential to improve quality of care,8,9 their effect has not been systematically evaluated on a large scale. To our knowledge, no randomized clinical trials have studied the effectiveness of virtual communities of practice (vCoPs) targeting HCPs. We hypothesized that a vCoP might improve HCP attitudes toward patient empowerment and shared decision making and equip them with necessary skills by using a collective learning process. We designed a cluster randomized trial, e-MPODERA, to compare the effect of usual training of HCPs working in primary care and a vCoP aimed at improving attitudes toward the empowerment of patients with chronic diseases.
METHODS
Trial Design
The e-MPODERA trial was a pragmatic, parallel, cluster randomized controlled trial in which 63 primary care practices were randomly assigned to 1 of 2 arms (allocation ratio 1:1). Full details of the methodology can be found in the study protocol.10 A cluster design was selected to avoid contamination between HCPs from the same practice because they were intervention recipients. The units of analysis were HCPs and patients.
Participants
Setting
The trial was conducted in the following 3 regions of Spain: Madrid, Catalonia, and the Canary Islands, which are independent in terms of health care management and provision. There were 262 practices in Madrid, 329 in Catalonia, and 102 in the Canary Islands. We considered the following 2 variables for stratification: area of practice (metropolitan vs north vs south, within each region) and geographic region (Madrid, Catalonia, Canary Islands).
Primary Care Practices
Primary care practices were randomly selected from region-specific practice databases. Inclusion criteria included adequate Internet connectivity and a letter of acceptance signed by the practice director.
Participants Within Clusters
Health Care Professionals
Health care professionals were general practitioners and practice nurses at the selected practices who voluntarily agreed to participate and provided signed informed consent. The inclusion criterion was not having the intention of moving from their practice during the study period.
Patients
Inclusion criteria were (1) age ≥18 years, (2) an active diagnosis in their medical record, made ≥1 year before study inclusion, of any of the following chronic diseases: hypertension, diabetes, hypercholesterolemia, obesity, heart failure, ischemic heart disease, cerebrovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or asthma, (3) having consulted their physician or nurse about ≥1 of those diseases ≥2 times in the prior year, and (4) providing signed informed consent. We excluded patients (1) temporarily residing in the area, (2) institutionalized, (3) with a terminal illness, (4) with a physical or mental disability that would have prevented them from responding to the questionnaires, and (5) without a telephone or e-mail address in the practice database (for contact with their HCP).
Intervention
e-MPODERA Virtual Community of Practice
Health care professionals allocated to the intervention received an e-mail with a Web link inviting them to register for the e-MPODERA vCoP, a virtual knowledge-sharing community of practice built on the Web 2.0 concept. To design the activities, we developed a competence framework to cover 12 core competencies and the following 3 thematic areas related to patient empowerment: health literacy, self-management support, and shared decision making.11 After a pilot study,11 the platform was prepared via a tailored training pathway. We structured the pathway into the following 3 periods: 1 month focusing on coping activities (influence of the clinician-patient relationship on empowerment) followed by 2 months of reflection activities (exercising empathy and identifying barriers for patient empowerment) and 3 cycles of 3 months, each focusing on behavioral change, goals, implementation of actions, and evaluation in clinical practice. Participants were free to start new discussion topics or ask questions. To encourage engagement, a gamification system that included individual challenges was devised. A general practitioner acted as a community manager. Participants were encouraged to access the platform weekly; however, a minimum number of visits or level of interaction was not required.
Quarterly newsletters and regular e-mail reminders were used to enhance participation. In addition, HCPs were offered a participation certificate for recruiting patients and completing the Patient-Practitioner Orientation Scale (PPOS)12,13 at baseline and at 12 months as well as a training certificate after completing ≥20 challenges and obtaining a score of 1,000 points. Access to the platform was allowed only to HCPs, not to their patients.
Control Group (Usual Training)
Health care professionals assigned to the control group did not receive any specific intervention. Training on patient empowerment is not part of continuous professional development for HCPs in Spain, although some might have received specific training (eg, in the context of scientific conferences).
Outcomes
The primary outcome was HCP attitudes toward person-centered care measured using the PPOS, a self-report instrument containing two 9-item subscales; the Sharing subscale assesses attitudes to sharing information, decision making, and power with patients, and the Caring subscale measures the provision of warmth, emotional support, and treatment of the patient as a whole person. Items range from 1 (strongly disagree) to 6 (strongly agree). The total score on the scale and subscales is divided by the corresponding number of items, thus ranging from 1 to 6. Higher scores indicate a greater person-centered orientation.
The secondary outcome was the 13-item version of the Patient Activation Measure (PAM),14 which assesses self-perceived skills, knowledge, and confidence in making health decisions and managing self-care. Items range from 1 (strongly disagree) to 4 (strongly agree), and the total score is transformed to a 0-100 scale. Higher scores indicate greater patient activation.
The PPOS was completed using a link provided by e-mail, and the PAM was completed at a visit to the practice or by telephone. Outcome variables for HCPs (PPOS) and patients (PAM) were measured at baseline and at 12 months (postintervention). No changes in inclusion criteria or outcomes were made after trial commencement. All outcomes prespecified in the trial protocol are reported in this manuscript. No harm was expected from participation in the trial.
Adherence to the Virtual Community of Practice
The number of logins to the vCoP platform by HCPs was registered.
Sample size
The sample size was calculated assuming 95% confidence, a power of 80%, and a 20% attrition rate and adjusting for clustering to detect a between-group difference of 0.2 points in PPOS score, which represents a small to moderate effect size (Cohen d = 0.4), assuming an SD of 0.50.13,15 The required size was 270 HCPs (135 per group) to be recruited from 54 practices (270/5); HCPs were expected to recruit 1,382 consecutive patients (Supplemental Appendix 1).
Randomization
After completion of baseline measures, practices were centrally randomized in blocks of 2 (an intervention and a control practice allocated simultaneously) by a statistician and stratified by region and area of practice using a random number table.
Anonymization
The patients and study statistician, but not HCPs, were anonymized to group allocation.
Statistical Methods
Descriptive statistics were calculated for the outcome and control variables. Analyses were performed on an intention-to-treat basis. Missing values were imputed by multiple imputation (Markov chain Monte Carlo method, 10 imputations per variable). For the PPOS, because data did not appear to have been missed at random, we also performed a baseline observation carried forward (BOCF) analysis. Multilevel linear or logistic regression was used, with practice as a random effect for the HCP models and practice and HCP for the patient models. The intervention effect on the dependent variables was analyzed by multilevel linear regression, adjusting for baseline PPOS and PAM scores, respectively, and for sociodemographic and clinical variables with significant differences at baseline. For the PPOS, we carried out exploratory moderator analyses, introducing to the model the interaction term of the intervention with each of the clinician sociodemographic and profession-related variables, as well as region and area of practice.
We performed 2 post hoc sensitivity analyses, excluding HCPs who had not accessed the vCoP platform (and their patients) and including them in the control group, respectively. We also explored predictors of the number of vCoP logins for the intervention group from both a continuous and categorical (≥1 vs 0) perspective. Finally, we analyzed the association between the number of logins and PPOS postintervention scores, adjusting for baseline values. This study is reported in accordance with the Consolidated Standards of Reporting Trials guidelines.16-19
RESULTS
Participants
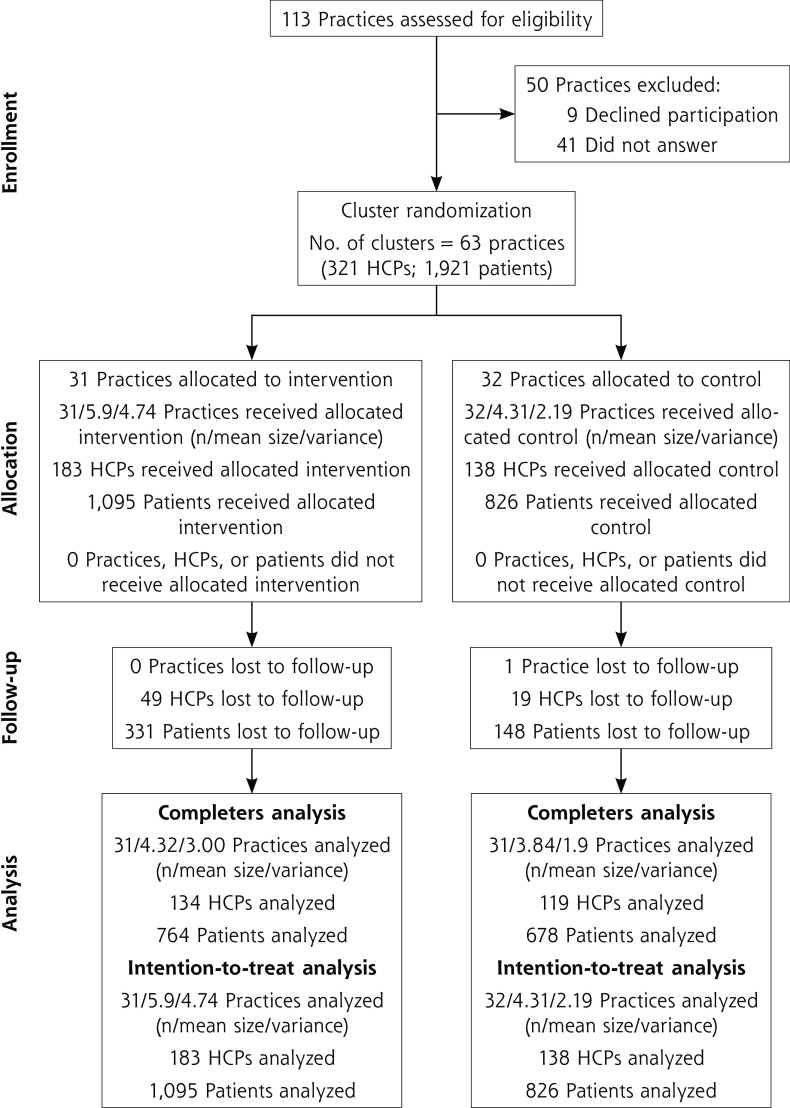
A total of 63 practices participated (31 intervention, 32 control) (Figure 1). A total of 321 HCPs (183 intervention, 138 control) recruited 1,921 patients (1,095 intervention, 826 control). Recruitment took place from October 2016 to February 2017.
Figure 1.
CONSORT flow diagram.
CONSORT = Consolidated Standards of Reporting Trials; HCP = health care professional.
Baseline Data
At baseline, the control HCP group showed a larger caseload (P = .041) and fewer nurses (P = .056) (Table 1), and the control patient group showed more obese patients (P = .008) (Table 2). These variables were included as covariates in the corresponding effectiveness analyses.
Table 1.
Baseline Characteristics of Health Care Professionals
| Total (N = 321) |
Intervention (n = 183) |
Control (n = 138) |
|
|---|---|---|---|
| Nurses, No. (%) | 131 (40.8) | 83 (45.4) | 48 (34.8) |
| Age, y, mean (SD) | 47.7 (8.79) | 47.1 (8.78) | 48.5 (8.77) |
| Female, No. (%) | 243 (75.7) | 141 (77.0) | 102 (73.9) |
| Years of experience, mean (SD) | 22.0 (8.84) | 21.9 (8.52) | 22.1 (9.29) |
| Years in primary care, mean (SD) | 17.7 (8.92) | 17.6 (8.67) | 17.8 (9.29) |
| Daily patient caseload number, mean (SD) | 29.4 (11.3) | 27.8 (10.7) | 31.4 (11.8) |
Table 2.
Baseline Characteristics of Patients
| Total (N = 1,921) |
Intervention (n = 1,095) |
Control (n = 826) |
|
|---|---|---|---|
| Age, y, mean (SD) | 64.7 (12.4) | 64.7 (12.4) | 64.6 (12.3) |
| Female, n (%) | 975 (50.8) | 524 (47.9) | 451 (54.6) |
| Educational level, No. (%) | |||
| None | 38 (2.0) | 25 (2.3) | 13 (1.6) |
| Primary | 1,014 (52.8) | 551 (50.3) | 463 (56.1) |
| Secondary | 558 (29.0) | 337 (30.8) | 221 (26.8) |
| University | 311 (16.2) | 182 (16.6) | 129 (15.6) |
| Marital status, No. (%) | |||
| Single | 144 (7.5) | 78 (7.1) | 66 (8.0) |
| Married/partner | 1,362 (70.9) | 797 (72.8) | 565 (68.4) |
| Separated | 56 (2.9) | 26 (2.4) | 30 (3.6) |
| Divorced | 129 (6.7) | 69 (6.3) | 60 (7.3) |
| Widowed | 230 (12.0) | 125 (11.4) | 105 (12.7) |
| Living alone, No. (%) | 304 (15.8) | 165 (15.1) | 139 (16.8) |
| Born outside Spain, No. (%) | 171 (8.9) | 111 (10.1) | 60 (7.3) |
| Chronic conditions, No. (%) | |||
| Hypertension | 1,406 (73.2) | 799 (73.0) | 607 (73.5) |
| Diabetes | 707 (36.8) | 425 (38.8) | 282 (34.1) |
| Hypercholesterolemia | 1,122 (58.4) | 634 (57.9) | 488 (59.1) |
| Ischemic heart disease | 155 (8.1) | 88 (8.0) | 67 (8.1) |
| Heart failure | 220 (11.5) | 127 (11.6) | 93 (11.3) |
| Cerebrovascular disease | 95 (4.9) | 54 (4.9) | 41 (5.0) |
| Chronic obstructive pulmonary disease | 174 (9.1) | 111 (10.1) | 63 (7.6) |
| Asthma | 168 (8.7) | 88 (8.0) | 80 (9.7) |
| Chronic renal disease | 168 (8.7) | 93 (8.5) | 75 (9.1) |
| Obesity | 667 (34.7) | 348 (31.8) | 319 (38.6) |
| Duration of main disease, y, mean (SD) | 11.7 (7.4) | 11.5 (7.3) | 11.9 (7.5) |
Analysis of Dropouts
A total of 68 HCPs (21.2%) and 479 patients (24.9%) dropped out. Supplemental Table 1 shows the predictors of dropping out. The HCPs lost to follow-up were significantly more likely to be in the intervention group and scored lower than completers on the PPOS Sharing and Caring subscales. In the intervention group, accessing the vCoP ≥1 time and a greater number of logins significantly related to fewer losses. Among patients, those lost to follow-up were slightly older than completers.
Outcomes and Estimation
Effectiveness of Intervention
Multiple imputations and completers analyses showed similar results (Table 3). At 12 months, HCPs in the intervention group showed a significantly greater PPOS total score compared to the control group (β = 0.14; 95% CI, 0.03-0.25; P = .011) and PPOS Sharing subscale score (β = 0.3; 95% CI, 0.15-0.44; P <.001). For the BOCF analysis, results were not significant for the total score (β = 0.09; 95% CI, 0-0.18; P = .063), and the effect was weaker than that obtained with multiple imputation for the Sharing subscale score (β = 0.18; 95% CI, 0.06-0.31; P = .004). The difference in the Caring subscale score was not statistically significant.
Table 3.
Effectiveness of Intervention
| Health Care Professionals | Completers Analysis (n = 253, k= 62) | MI Analysis (n = 321, k = 63) | BOCF Analysis (n = 321, k = 63) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention (n = 134, k = 31) |
Control (n = 119, k = 31) |
β (95% CI) P Valuea |
Intervention (n = 183, k = 31) |
Control (n = 138, k = 32) |
β (95% Cl) P Valuea |
Intervention (n = 183, k= 31) | Control (n = 138, k = 32) | β (95% CI) P Valuea | |
| Total PPOS score | 0.15 | 0.14 | 0.09 | ||||||
| Baseline | 4.43 (0.55) | 4.44 (0.54) | (0.04 to 0.27) | 4.37 (0.55) | 4.44 (0.53) | (0.03 to 0.25) | 4.37 (0.55) | 4.44 (0.53) | (0 to 0.18) |
| 12 months | 4.65 (0.56) | 4.50 (0.54) | P = .007 | 4.60 (0.66) | 4.49 (0.57) | P = .011 | 4.53 (0.58) | 4.49 (0.53) | P = .063 |
| PPOS Sharing subscale score | 0.29 | 0.3 | 0.18 | ||||||
| Baseline | 3.98 (0.71) | 3.95 (0.73) | (0.14 to 0.44) | 3.91 (0.73) | 3.94 (0.73) | (0.15 to 0.44) | 3.91 (0.73) | 3.94 (0.73) | (0.06 to 0.31) |
| 12 months | 4.32 (0.71) | 4.00 (0.74) | P <.001 | 4.28 (0.76) | 3.99 (0.8) | P <.001 | 4.16 (0.77) | 3.99 (0.73) | P = .004 |
| PPOS Caring subscale score | 0.01 | –0.02 | –0.01 | ||||||
| Baseline | 4.88 (0.51) | 4.94 (0.55) | (–0.11 to 0.13) | 4.83 (0.54) | 4.93 (0.53) | (–0.15 to 0.11) | 4.83 (0.54) | 4.93 (0.53) | (–0.11 to 0.09) |
| 12 months | 4.99 (0.58) | 5.00 (0.51) | P = .869 | 4.93 (0.77) | 4.99 (0.53) | P = .769 | 4.91 (0.6) | 4.98 (0.5) | P = .82 |
| Patients | Completers Analysis (n = 1,442, k = 61) | Ml Analysis (n = 1,921, k = 63) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention (n = 764, k= 30) |
Control (n = 678, k= 31) |
β (95% CI) P Valueb |
lntervention (n = 1,095, k = 31) |
Control (n = 826, k= 32) |
β (95% Cl) P Valueb |
||||
| PAM | 1.5 | 0.24 | |||||||
| Baseline | 65.6 (15.6) | 65.2 (16.2) ( | –1.53 to 4.53) | 65.6 (15.5) | 65.3 (16.4) | (–2.28 to 2.76) | |||
| 12 months | 65.7 (15.4) | 64.8 (15.2) | P = .331 | 65.6 (17.5) | 65.0 (16.1) | P = .852 | |||
BOCF = baseline observation carried forward; k = No. of primary care practices; MI = multiple imputation; PAM = Patient Activation Measure; PPOS = Patient-Practitioner Orientation Scale.
Note: Data for the intervention and control groups are presented as mean (SD).
Unstandardized coefficients (95% CI) and P value from multilevel linear regression models, with PPOS scores at 12 months as the dependent variable, fixed effects for health care professionals, and random effects for primary care practice (k), with adjustment for the profession (nurse or physician), patients attended per day, and baseline PPOS scores.
Unstandardized coefficients (95% CI) and P value from multilevel linear regression models, with PAM scores at 12 months as the dependent variable, fixed effects for patients, and random effects for professional and primary care practice (k), with adjustment for the rate of obesity and baseline PAM scores.
None of the HCP characteristics significantly moderated the effect of the intervention. For Sharing, sex showed a P value of .061. Separate analyses showed that the effect was significant for women (β = 0.38; 95% CI, 0.2-0.55; P < .001) but not for men (β = 0.06; 95% CI, −0.22 to 0.33; P = .688). There were no significant interactions with region or area.
The intervention had no effect on patient activation (β = 0.24; 95% CI, −2.28 to 2.76; P = .852) (Table 3). Post hoc analyses excluding HCPs who did not access the platform (together with their patients) or including them in the control group yielded significant results for the total score in the BOCF analyses (β = 0.11; 95% CI, 0.01-0.21; P = .023 and β = 0.12; 95% CI, 0.03-0.22; P = .009, respectively) and did not change the results for the PAM score.
Health Care Professional Logins to Virtual Community of Practice
A total of 18 HCPs (9.8%) never accessed the platform, and 57 (31.1%) accessed it 1 to 5 times. The median number of logins for the overall group was 8 (interquartile range [IQR] 2-29) and for those who accessed ≥1 time was 10 (IQR 3-34.5). Supplemental Table 2 shows the predictors of vCoP activity. Greater baseline Caring subscale score significantly related to accessing ≥1 time (β = 1.06; 95% CI, 0.09-2.03; P = .032). There were no significant predictors of the number of logins. The number of logins significantly predicted 12-month scores on the Sharing subscale (β = 0.004; 95% CI, 0.001-0.007; P = .02) but not on the Caring subscale (β = 0.002; 95% CI, −0.001 to 0.005; P = .254).
DISCUSSION
Our results show that at 12 months of follow-up, a vCoP produced a minor positive effect on the PPOS Sharing subscale, which translates to the PPOS total score. No effect was found for the PPOS Caring subscale. The Sharing subscale is directly related to the ultimate objective of the vCoP, to promote patient empowerment and self-care. Nonetheless, the clinical significance of the observed differences is unclear. Other studies aimed at improving HCP attitudes also found significant results only for the Sharing subscale, with similar effect sizes.20-22 In the field of mental health, Drivenes et al23 found a strong and significant cross-sectional association (odds ratio = 1.97) between Sharing score and perceiving a high level of shared decision making in consultation from the patient’s perspective, but that cannot be generalized to longitudinal data as in the present study. A ceiling effect cannot be ruled out, especially for the Caring subscale, which also showed a low internal consistency (Cronbach α = 0.48). A psychometric analysis of baseline data showed an unsatisfactory functioning of several items,24 as observed in other studies.25,26
Post hoc analysis showed a greater effect for female HCPs (P value of the interaction = .061). We do not know of published interventions showing a differential effect by gender; however, among medical students, women consistently show more patient-centered attitudes,27-29 and there is evidence that their attitudes improve more (or deteriorate less) throughout their education compared to men.30,31 Future studies should explore gender in relation to issues regarding attitudes to training in patient-centered care.
The dropout rate (21.2%) and moderate adherence to the vCoP support the need to evaluate access barriers and design motivational strategies to engage HCPs who are less patient oriented. Accessing the vCoP ≥1 time was associated with fewer dropouts, and the number of logins positively related to better PPOS postintervention scores, independent of baseline attitudes. Therefore, low-cost strategies to promote access and continuous participation in the vCoP might help to improve retention.
The intervention did not improve patient activation, suggesting that HCP training should be complemented with more comprehensive approaches including support from senior leadership, integration into organizational functioning,32 and self-management support. Changing patients’ knowledge, skills, and self-efficacy are long-term goals that require an iterative, self-correcting process.
Study Limitations
The present study has several limitations, some already mentioned, such as the uncertainty regarding the psychometric properties of the PPOS, the clinical significance of the observed differences, and participant attrition. From a statistical perspective, the apparently nonrandom attrition of HCPs could have biased the multiple imputation analysis. For this reason, we also performed a BOCF analysis. Assuming no detrimental effect of the intervention, that analysis is conservative because there were twice as many lost participants in the intervention group. Moderator analyses were post hoc and underpowered. Another important limitation is that given the characteristic of the intervention, anonymizing participants was possible for patients but not for HCPs. In addition, we do not know whether HCPs received other training on patient empowerment during the study. Finally, we did not check that all eligible patients were invited to participate, but intervention and control groups were similar in terms of baseline characteristics, and selection bias does not appear likely.
Implications for Practice and Future Research
The present study highlights the need to investigate the relation between the intensity of a virtual intervention and learning goals. Adapting training programs to the needs and availability of individual HCPs is attractive, but in light of our present results, it would appear that focusing on acquiring a set of standard competencies might add value. The learning objectives and competencies developed in the present study might be a good starting point. Future research should also assess health outcomes because they are the ultimate objective of health care.
CONCLUSIONS
A vCoP for primary HCPs produced a significant improvement in participants’ attitudes toward sharing information and decisions with patients, but the clinical significance of the observed difference is unclear. There were no changes in the PPOS Caring subscale dimension or in patient activation. Health care professionals in the intervention group and those with a less patient-centered orientation at baseline were more likely to drop out of the study. Design limitations preclude drawing definitive conclusions. Further research is needed on the effectiveness of the vCoP model and on how to improve HCP motivation and engagement.
Supplementary Material
Acknowledgments
English language support was provided by Anne Murray, Helena Vall, and Mariana Aparicio.
Footnotes
Conflicts of interest: authors report none.
Funding support: This study was financed by Instituto de Salud Carlos III and Cofinanced by Fondo Europeo de Desarrollo Regional, Ministerio de Economía y Competitividad, Gobierno de España (PI15/00164, PI15/00586, PI15/00566). This is a public research funding source and played no role in study design, data collection, analysis or interpretation, or the manuscript’s writing. The research retains complete independence in the conduct of the study. The Fondo de Investigación Sanitaria evaluation committee will annually inspect study progress and adherence to the study protocol.
Previous presentations: Presented in part at the Cochrane Colloquium, October 22, 2019, Santiago, Chile; the International Conference on Communication in Healthcare, October 27-30, 2019, San Diego, California; the 10th International Shared Decision Making Conference, July 2019, Quebec City, Canada; and the 19th International Conference on Integrated Care, April 1-3, 2019, San Sebastian, Basque Country, Spain.
Trial registration: ClinicalTrials.gov: NCT02757781. Registered April 25, 2016. Registration was performed before the inclusion of the first health care professional and patient.
Protocol: The full protocol of this cluster randomized control trial is available in open access.10
References
- 1.Bodenheimer T, Lorig K, Holman H, Grumbach K.. Patient self-management of chronic disease in primary care. JAMA. 2002; 288(19): 2469-2475. 10.1001/jama.288.19.2469 [DOI] [PubMed] [Google Scholar]
- 2.EU Health Programme . EMPATHiE: empowering patients in the management of chronic diseases final summary report. Published Sep 30, 2014. Accessed Mar 15, 2022. https://www.eu-patient.eu/contentassets/290c301ddd174840a75ebf0a07d7c037/final-report-20-page-summary-.pdf
- 3.European Commission . Eurobarometer qualitative study: patient involvement. Published May 21, 2012. Accessed Mar 15, 2022. https://www.digitalhealthnews.eu/download/publications/3072-eurobarometer-qualitative-study-patient-involvement
- 4.Jerant AF, von Friederichs-Fitzwater MM, Moore M.. Patients’ perceived barriers to active self-management of chronic conditions. Patient Educ Couns. 2005; 57(3): 300-307. 10.1016/j.pec.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 5.Wenger E. Communities of practice: learning as a social system. Syst Thinker. 1998;9(5). https://thesystemsthinker.com/communities-of-practice-learning-as-a-social-system/ [Google Scholar]
- 6.Li LC, Grimshaw JM, Nielsen C, Judd M, Coyte PC, Graham ID.. Evolution of Wenger’s concept of community of practice. Implement Sci. 2009; 4: 11. 10.1186/1748-5908-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazmanian PE, Davis DA.. Continuing medical education and the physician as a learner: guide to the evidence. JAMA. 2002; 288(9): 1057-1060. 10.1001/jama.288.9.1057 [DOI] [PubMed] [Google Scholar]
- 8.Ranmuthugala G, Cunningham FC, Plumb JJ, et al. A realist evaluation of the role of communities of practice in changing healthcare practice. Implement Sci. 2011; 6: 49. 10.1186/1748-5908-6-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braithwaite J, Westbrook JI, Ranmuthugala G, et al. The development, design, testing, refinement, simulation and application of an evaluation framework for communities of practice and social-professional networks. BMC Health Serv Res. 2009; 9: 162. 10.1186/1472-6963-9-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González-González A, Orrego C, Perestelo-Perez L, et al. Effectiveness of a virtual intervention for primary healthcare professionals aimed at improving attitudes towards the empowerment of patients with chronic diseases: study protocol for a cluster randomized controlled trial (e-MPODERA project). Trials. 2017; 18(1): 505. 10.1186/s13063-017-2232-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bermejo-Caja CJ, Koatz D, Orrego C, et al. ; e-MPODERA group . Acceptability and feasibility of a virtual community of practice to primary care professionals regarding patient empowerment: a qualitative pilot study. BMC Health Serv Res. 2019; 19(1): 403. 10.1186/s12913-019-4185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krupat E, Hiam CM, Fleming MZ, Freeman P.. Patient-centeredness and its correlates among first year medical students. Int J Psychiatry Med. 1999; 29(3): 347-356. 10.2190/DVCQ-4LC8-NT7H-KE0L [DOI] [PubMed] [Google Scholar]
- 13.Shaw WS, Woiszwillo MJ, Krupat E.. Further validation of the Patient-Practitioner Orientation Scale (PPOS) from recorded visits for back pain. Patient Educ Couns. 2012; 89(2): 288-291. 10.1016/j.pec.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 14.Hibbard JH, Stockard J, Mahoney ER, Tusler M.. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004; 39(4 Pt 1): 1005-1026. 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell MK, Grimshaw JM, Elbourne DR.. Intracluster correlation coefficients in cluster randomized trials: empirical insights into how should they be reported. BMC Med Res Methodol. 2004; 4: 9. 10.1186/1471-2288-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Hopewell S, Schulz KF, et al. ; CONSORT . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012; 10(1): 28-55. 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 17.Hopewell S, Clarke M, Moher D, et al. ; CONSORT Group . CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008; 5(1): e20. 10.1371/journal.pmed.0050020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008; 148(4): 295-309. 10.7326/0003-4819-148-4-200802190-00008 [DOI] [PubMed] [Google Scholar]
- 19.Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012; 345: e5661. 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann TC, Bennett S, Tomsett C, Del Mar C.. Brief training of student clinicians in shared decision making: a single-blind randomized controlled trial. J Gen Intern Med. 2014; 29(6): 844-849. 10.1007/s11606-014-2765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross EF, Haidet P.. Attitudes of physical therapy students toward patient-centered care, before and after a course in psychosocial aspects of care. Patient Educ Couns. 2011; 85(3): 529-532. 10.1016/j.pec.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 22.Sweeney K, Baker P.. Promoting empathy using video-based teaching. Clin Teach. 2018; 15(4): 336-340. 10.1111/tct.12693 [DOI] [PubMed] [Google Scholar]
- 23.Drivenes K, Haaland VØ, Mesel T, Tanum L.. Practitioners’ positive attitudes promote shared decision-making in mental health care. J Eval Clin Pract. 2019; 25(6): 1041-1049. 10.1111/jep.13275 [DOI] [PubMed] [Google Scholar]
- 24.Perestelo-Pérez L, Rivero-Santana A, González-González AI, et al. Cross-cultural validation of the patient-practitioner orientation scale among primary care professionals in Spain. Health Expect. 2021; 24(1): 33-41. 10.1111/hex.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Zou R, Fu H, Qian H, Yan Y, Wang F.. Measuring the preference towards patient-centred communication with the Chinese-revised Patient-Practitioner Orientation Scale: a cross-sectional study among physicians and patients in clinical settings in Shanghai, China. BMJ Open. 2017; 7(9): e016902. 10.1136/bmjopen-2017-016902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira CM, Amaral CF, Ribeiro MM, et al. Cross-cultural validation of the Patient-Practitioner Orientation Scale (PPOS). Patient Educ Couns. 2013; 91(1): 37-43. 10.1016/j.pec.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 27.Haidet P, Dains JE, Paterniti DA, et al. Medical student attitudes toward the doctor-patient relationship. Med Educ. 2002; 36(6): 568-574. 10.1046/j.1365-2923.2002.01233.x [DOI] [PubMed] [Google Scholar]
- 28.Tsimtsiou Z, Kerasidou O, Efstathiou N, Papaharitou S, Hatzimouratidis K, Hatzichristou D.. Medical students’ attitudes toward patient-centred care: a longitudinal survey. Med Educ. 2007; 41(2): 146-153. 10.1111/j.1365-2929.2006.02668.x [DOI] [PubMed] [Google Scholar]
- 29.Michael K, Dror MG, Karnieli-Miller O.. Students’ patient-centered-care attitudes: the contribution of self-efficacy, communication, and empathy. Patient Educ Couns. 2019; 102(11): 2031-2037. 10.1016/j.pec.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa H, Son D, Eto M, Kitamura K, Kiuchi T.. Changes in patient-centered attitude and confidence in communicating with patients: a longitudinal study of resident physicians. BMC Med Educ. 2018; 18(1): 20. 10.1186/s12909-018-1129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahlqvist M, Gunnarsson RK, Dahlgren G, Nordgren S.. Patient-centred attitudes among medical students: gender and work experience in health care make a difference. Med Teach. 2010; 32(4): e191-e198. 10.3109/01421591003657451 [DOI] [PubMed] [Google Scholar]
- 32.The Health Foundation . Sustaining and Spreading Self-Management Support - Lessons from Co-Creating Health Phase 2. The Health Foundation; 2013. https://www.health.org.uk/publications/sustaining-and-spreading-self-management-support [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.