Abstract
PURPOSE
Acute care use is high among individuals with chronic kidney disease (CKD). It is unclear how relational continuity of primary care influences downstream acute care use. We aimed to determine if poor continuity of care is associated with greater rates of acute care use and decreased prescriptions for guideline-recommended drugs.
METHODS
We conducted a population-based retrospective cohort study of adults with stage 3-4 CKD and ≥3 visits to a primary care clinician during the period April 1, 2011 to March 31, 2014 in Alberta, Canada. Continuity was calculated using the Usual Provider Continuity index. Descriptive statistics were used to summarize patient and acute care encounter characteristics. Adjusted rates and incidence rate ratios for all-cause and CKD-related ambulatory care-sensitive condition (ACSC) hospitalizations and emergency department (ED) visits were estimated using negative binomial regression. Adjusted odds ratios for prescription use were estimated by multivariable logistic regression.
RESULTS
Among 86,475 patients with CKD, 51.3%, 30.0%, and 18.7% had high, moderate, and poor continuity of care, respectively. There were 77,988 all-cause hospitalizations, 6,489 ACSC-related hospitalizations, 204,615 all-cause ED visits, and 8,461 ACSC-related ED visits during a median follow-up of 2.3 years. Rates of all-cause and ACSC hospitalization and ED use increased with poorer continuity of care in a stepwise fashion across CKD stages. Patients with poor continuity were less likely to be prescribed a statin.
CONCLUSIONS
Poor continuity of care is associated with increased acute care use among patients with CKD. Targeted strategies that strengthen patient-physician relationships and guide physicians regarding guideline-recommended prescribing are needed.
Key words: ambulatory care, chronic kidney disease, continuity of primary care, hospitalization, relational continuity, primary health care
INTRODUCTION
In Canada, the estimated prevalence of chronic kidney disease (CKD) is approximately 3% among individuals aged ≥18 years.1 Chronic kidney disease is a complex chronic condition that often involves the management of several comorbidities such as hypertension, diabetes, and cardiovascular disease.2 International guidelines3 recommend referral to a nephrologist for patients with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2. The majority of patients with less-advanced CKD are managed exclusively in primary care settings.4
Relational continuity of care is a measure of an ongoing therapeutic relationship between a patient and their health care clinicians.5 Within primary care, relational continuity is particularly beneficial for patients with chronic conditions and who require ongoing medical care in an outpatient setting.6,7 Among such individuals, high continuity of care has been associated with decreased emergency department (ED) visits, hospitalizations, and health care costs and improved patient-relevant outcomes such as satisfaction, quality of life, and treatment adherence.8 Because acute care utilization is notably high among people with CKD (ie, 3-8 times greater than in the general population), effective primary care continuity might address health system burden and improve care experiences among this medically complex population.9,10
Prior work has shown that approximately 10% of CKD-related acute care encounters are potentially preventable, given that they are related to conditions (ie, volume overload, hyperkalemia, malignant hypertension, heart failure) associated with CKD, and timely and effective care might avoid their onset or progression.11,12 However, associations between relational continuity of care in the primary care setting and all-cause and potentially preventable acute care use among patients with CKD have not been examined. Potential relations between continuity of care and other quality-of-care indicators that might influence acute care use, such as the prescription of indicated drugs (ie, renin-angiotensin-aldosterone system [RAAS] inhibitors and statins), are also unclear. The objective of the present study was to determine if poor continuity of care is associated with greater rates of all-cause and potentially preventable acute care use and decreased prescriptions for guideline-recommended drugs.
METHODS
Data Source, Setting, and Study Population
We used an established computerized repository of provincial administrative and laboratory data from across Alberta, the Alberta Kidney Disease Network (AKDN).13 A unique patient identifier (provincial health care number) was used to link patients to various administrative data sources to capture detailed sociodemographic data, clinical information, drug dispensations, and encounters with acute and primary care services. We created a cohort of adults (aged ≥18 years) with ≥2 outpatient serum creatinine measurements during the period April 1, 2011 to March 31, 2014 in Alberta, Canada. Using the Chronic Kidney Disease Epidemiology Collaboration equation,14 CKD diagnosis was defined by a series of ≥2 serum creatinine measurements that equated to an eGFR <60 mL/min/1.73 m2 taken a minimum of 90 days and a maximum of 18 months apart, with no recovering kidney function. The index date for CKD diagnosis and CKD stage was defined by the first eGFR measurement <60 mL/min/1.73 m2. Those who were dialysis dependent, received a kidney transplant, or had kidney failure (ie, eGFR <15 mL/min/1.73 m2) before the index date were excluded because those individuals are generally managed by nephrologists. Individuals with CKD and ≥3 outpatient visits to a single primary care clinician during a 2-year period before the index date were included in our final cohort.
Relational Continuity of Primary Care
Relational continuity of primary care was defined as the proportion of outpatient visits to a primary care clinician (ie, family physician) for patients with ≥3 outpatient visits during a 2-year period before the index date. Outpatient primary care visits and visits to a usual clinician (defined as the primary care physician that a patient visited the most during a 2-year period before the index date) were identified from the Alberta Health Practitioner Claims database. The level of continuity of primary care was calculated using the Usual Provider Continuity index and categorized as poor (<0.50), moderate (0.50-0.74), or high (0.75-1.00).15 We assumed that the continuity level defined in a 2-year period before the index date was constant from the index date to the time of censoring/end of the study.
Identification of Health Care Utilization Outcomes—All-Cause and Potentially Preventable Acute Care Use
We evaluated acute care use among individuals with CKD by using the following 4 outcome measures: (1) all-cause hospitalizations, (2) all-cause ED visits, (3) CKD-related potentially preventable hospitalizations, and (4) CKD-related potentially preventable ED visits. All patients were followed from the day they entered the study (CKD diagnosis date) until death, outmigration, dialysis start, or end of study (March 31, 2014). The number of hospitalizations and ED visits during this period were recorded and used to determine the rate of hospitalization and/or ED visits for each patient (number of events/1,000 person-years).
Potentially preventable acute care use was defined as hospitalizations and ED visits for CKD-related ambulatory care-sensitive conditions (ACSCs) (ie, volume overload, hyperkalemia, malignant hypertension, heart failure).16 These were captured using the most responsible diagnosis code on the basis of International Statistical Classification of Diseases, 10th Revision (ICD-10) diagnostic coding.
Identification of Process of Care Outcomes—Prescription of RAAS Inhibitors and Statins
The Alberta Pharmaceutical Information Network contains data on nearly all outpatient pharmaceutical dispensations across the province. These data were used to ascertain individuals’ prescribed drugs. Prescription of guideline-recommended drugs for patients with CKD were defined using Anatomical Therapeutic Chemical Classification System. We determined the proportions of patients with ≥1 prescription for an RAAS inhibitor and/or a statin in the year before cohort entry.
Modifying and/or Confounding Variables
We identified cohort demographic and clinical variables from Alberta Health administrative data; these included age, sex, household location (urban vs rural), and neighborhood-level median household income quintile. Albuminuria was categorized as normal (A1), moderate (A2), or severe (A3) on the basis of prespecified cutpoints within the AKDN provincial laboratory repository.17 The presence of 30 chronic comorbidities was identified using validated International Classification of Diseases, 9th Revision (ICD-9) and ICD-10 coding algorithms in the 2 years before entering the study.18 The proportion of patients with ≥1 visit to a specialist in the 2 years before the index date was also measured.
Statistical Analysis
We summarized patient demographic and clinical characteristics using descriptive statistics (mean [SD], proportion, median [interquartile range (IQR)], and 95% CI) for the overall cohort, stratified by level of continuity of primary care. Unadjusted rates of hospitalizations and ED visits/1,000 person-years were initially calculated using Poisson regression models. Because we found evidence of overdispersion in the data, we used negative binomial regression models to estimate incident rate ratios (IRRs) for hospitalizations and ED visits. We found that CKD stage modified the relation between continuity of care and acute care use (P <.05) and thus reported IRRs stratified by CKD stage. We also evaluated whether there was an interaction by diabetes status in our models; this interaction term was consistently found to be nonsignificant, suggesting that diabetes status did not modify the association between continuity of care and the IRRs for all outcomes of interest. For all statistical modeling, we included confounding variables using a forward stepwise regression process. We set the high-continuity group as the reference category and reported unadjusted and adjusted CKD stage-stratified IRRs for all-cause hospitalizations and ED visits. These IRRs were adjusted for age, sex, household location, median household income quintile, CKD-related comorbidities, and albuminuria severity. The analysis was repeated to determine the association between continuity of primary care and rates of CKD-related ACSC hospitalizations and ED visits. Multivariable logistic regression modeling was used to estimate odds ratios (ORs) of being prescribed an RAAS inhibitor and/or a statin in the year prior for each level of relational continuity of care. We used Stata version 16 (StataCorp LLC) for all analyses and followed recommended reporting guidelines for observational studies.19,20
RESULTS
Baseline Characteristics
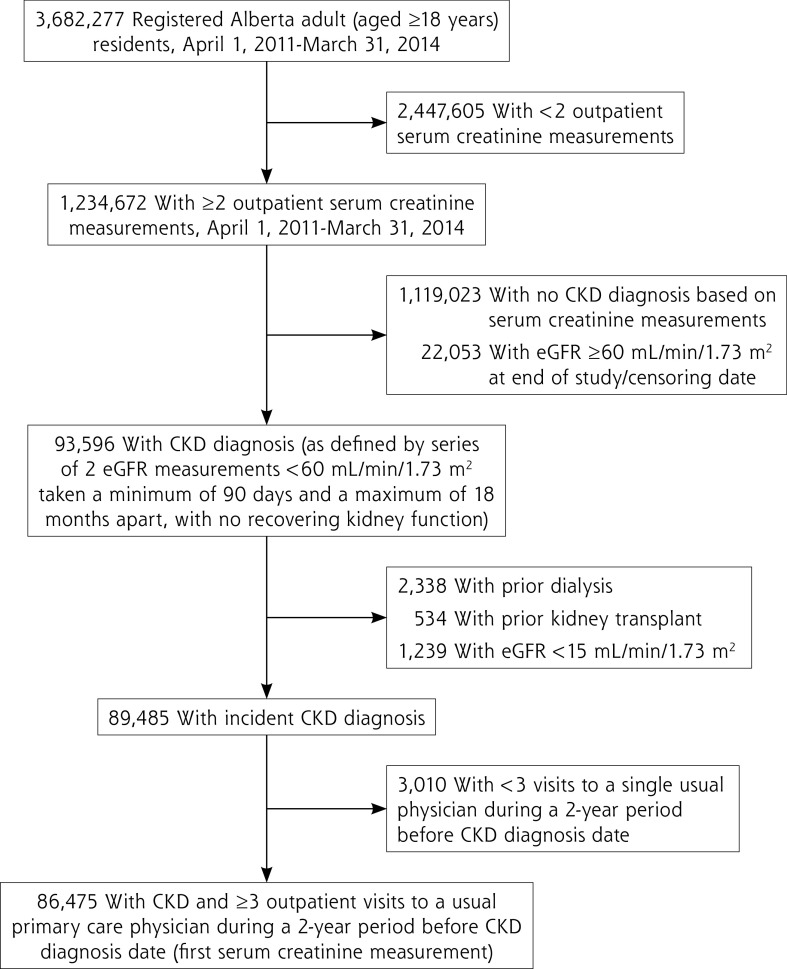
There were 1,234,672 registered adults with ≥2 outpatient serum creatinine measurements during the period April 1, 2011 to March 31, 2014 in Alberta. After exclusion of those with no CKD diagnosis, kidney failure, or <3 outpatient visits with a single primary care clinician during the 2 years before CKD diagnosis, our final cohort consisted of 86,475 patients (Figure 1).
Figure 1.
Criteria to determine final study cohort.
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
The mean age of the overall cohort was 76.0 (11.2) years, and 56.6% of patients were female (Table 1). A total of 51.3% of patients had high continuity, 30.0% had moderate continuity, and 18.7% had poor continuity. When stratified by CKD stage, the majority (61.5%) of patients with CKD stage 3a had high primary care continuity, whereas the proportion with poor continuity increased with later stages of CKD (Table 1). Patients with poor continuity were more likely to have severe albuminuria, more comorbidities, and rural residence compared with those with moderate or high continuity (Table 1).
Table 1.
Patient Demographic and Clinical Characteristics by Continuity of Primary Care
| Patient Characteristic | Level of Relational Continuity of Primary Care | |||
|---|---|---|---|---|
| Poor | Moderate | High | Overall | |
| Patients, No. (%) | 16,143 (18.7) | 25,948 (30.0) | 44,384 (51.3) | 86,475 (100.0) |
| CKD stage, No. (%)a | ||||
| 3a | 9,153 (56.7) | 15,489 (59.7) | 27,274 (61.5) | 51,916 (60.0) |
| 3b | 5,145 (31.9) | 7,960 (30.7) | 13,313 (30.0) | 26,418 (30.5) |
| 4 | 1,845 (11.4) | 2,499 (9.6) | 3,797 (8.6) | 8,141 (9.4) |
| Age, y, mean (SD) | 76.1 (12.6) | 75.7 (11.4) | 76.2 (10.5) | 76.0 (11.2) |
| Age category, y, No. (%) | ||||
| 18-44 | 360 (2.2) | 357 (1.4) | 364 (0.8) | 1,081 (1.3) |
| 45-64 | 2,469 (15.3) | 3,930 (15.1) | 5,880 (13.2) | 12,279 (14.2) |
| 65-74 | 3,611 (22.4) | 6,697 (25.8) | 11,926 (26.9) | 22,234 (25.7) |
| 75-84 | 5,571 (34.5) | 9,492 (36.6) | 17,326 (39.0) | 32,389 (37.5) |
| ≥85 | 4,132 (25.6) | 5,472 (21.1) | 8,888 (20.0) | 18,492 (21.4) |
| Female, No. (%) | 9,571 (59.3) | 14,902 (57.4) | 24,483 (55.2) | 48,956 (56.6) |
| Location of residence, | No. (%) | |||
| Urban | 13,221 (82.0) | 21,978 (84.7) | 40,339 (90.9) | 75,538 (87.4) |
| Rural | 2,909 (18.0) | 3,932 (15.2) | 3,988 (9.0) | 10,829 (12.5) |
| Albuminuria, No. (%) | ||||
| Normal/mild (A1) | 7,601 (47.1) | 13,458 (51.9) | 23,991 (54.1) | 45,050 (52.1) |
| Moderate (A2) | 2,021 (12.5) | 3,221 (12.4) | 5,660 (12.8) | 10,902 (12.6) |
| Severe (A3) | 1,440 (8.9) | 2,007 (7.7) | 2,979 (6.7) | 6,426 (7.4) |
| Unmeasured | 5,081 (31.5) | 7,262 (28.0) | 11,754 (26.5) | 24,097 (27.9) |
| Median household income quintile, No. (%) | ||||
| 1 (lowest) | 4,627 (28.7) | 6,944 (26.8) | 11,130 (25.1) | 22,701 (26.3) |
| 2 | 3,673 (22.8) | 5,893 (22.7) | 10,036 (22.6) | 19,602 (22.7) |
| 3 | 2,958 (18.3) | 5,012 (19.3) | 8,735 (19.7) | 16,705 (19.3) |
| 4 | 2,246 (13.9) | 3,687 (14.2) | 6,576 (14.8) | 12,509 (14.5) |
| 5 (highest) | 2,062 (12.8) | 3,686 (14.2) | 6,986 (15.7) | 12,734 (14.7) |
| 6 (unknown) | 577 (3.6) | 726 (2.8) | 921 (2.1) | 2,224 (2.6) |
| Comorbidities, No. (%) | ||||
| Asthma | 1,106 (6.9) | 1,385 (5.3) | 1,699 (3.8) | 4,190 (4.8) |
| Atrial fibrillation | 3,472 (21.5) | 4,741 (18.3) | 7,241 (16.3) | 15,455 (17.9) |
| Cancer | 1,723 (10.7) | 2,573 (9.9) | 3,785 (8.5) | 8,081 (9.3) |
| Congestive heart failure | 4,635 (28.7) | 5,785 (22.3) | 8,068 (18.2) | 18,488 (21.4) |
| Chronic obstructive pulmonary disease | 5,379 (33.3) | 7,239 (27.9) | 10,000 (22.5) | 22,618 (26.2) |
| Cirrhosis | 168 (1.0) | 142 (0.5) | 186 (0.4) | 496 (0.6) |
| Diabetes | 5,871 (36.4) | 8,848 (34.1) | 15,497 (34.9) | 30,216 (34.9) |
| Hypertension | 14,008 (86.8) | 22,293 (85.9) | 38,475 (86.7) | 74,776 (86.5) |
| Peripheral vascular disease | 1,084 (6.7) | 1,413 (5.4) | 2,052 (4.6) | 4,549 (5.3) |
| Comorbidities, mean (SD) | 3.9 (2.3) | 3.3 (2.0) | 3.0 (1.8) | 3.2 (2.0) |
| ≥1 Visit to a specialist in a 2-year period before index date, No. (%) | ||||
| Nephrology | 2,350 (14.6) | 3,228 (12.4) | 4,788 (10.8) | 10,366 (12.0) |
| Endocrinology | 187 (1.2) | 228 (0.9) | 310 (0.7) | 725 (0.8) |
| Oncology | 49 (0.3) | 39 (0.2) | 34 (0.1) | 122 (0.1) |
| Psychiatry | 1,756 (10.9) | 1,591 (6.1) | 1,591 (3.6) | 4,938 (5.7) |
| Cardiology | 4,332 (26.8) | 6,344 (24.4) | 10,001 (22.5) | 20,677 (23.9) |
| Respiratory | 1,600 (9.9) | 2,198 (8.5) | 2,797 (6.3) | 6,595 (7.6) |
| Internal medicine | 9,095 (56.3) | 13,088 (50.4) | 20,713 (46.7) | 42,896 (49.6) |
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
CKD stages were defined as stage 3a (eGFR 45-59 mL/min/1.73 m2), stage 3b (eGFR 30-44 mL/min/1.73 m2), and stage 4 (eGFR 15-29 mL/min/1.73 m2).
All-Cause Acute Care Use
Overall, there were 77,988 all-cause hospitalizations among 34,810 patients with CKD, with a median follow-up time of 2.3 years (IQR 1.5-2.8 years) (Table 2). The number of all-cause ED visits was 204,615 among 51,152 patients with CKD. Patients with poor or moderate continuity of primary care accounted for almost 55% of all-cause hospitalizations and ED visits.
Table 2.
All-Cause and Ambulatory Care-Sensitive Condition–Related Hospitalizations and Emergency Department Visit Characteristics by Continuity of Primary Care
| Variable | Level of Relational Continuity of Primary Care | Overall (n = 86,475) |
|||
|---|---|---|---|---|---|
| Poor (n = 16,143) |
Moderate (n = 25,948) |
High (n = 44,384) |
|||
| All-cause hospitalizations | Patients, No. | 7,938 | 11,020 | 15,852 | 34,810 |
| Hospitalizations, No. (%) | 19,835 (25.4) | 25,551 (32.8) | 32,602 (41.8) | 77,988 (100.0) | |
| Hospitalizations, median (IQR) | 0 (0-2) | 0 (0-1) | 0 (0-1) | 0 (0-1) | |
| Person-time, y (%) | 32,328.8 (17.9) | 53,698.6 (29.7) | 94,520.5 (52.4) | 180,547.9 (100.0) | |
| Length of hospital stay, d, mean (SD) | 13.7 (21.1) | 12.8 (19.2) | 12.7 (20.0) | 12.9 (20.0) | |
| Length of hospital stay, d, median (IQR) | 0 (0-7) | 0 (0-5) | 0 (0-4) | 0 (0-5) | |
| Cumulative length of hospital stay, d, median (IQR) | 15 (6-42) | 13 (5-39) | 11 (4-33) | 13 (5-37) | |
| Unadjusted hospitalization rate/1,000 person-years (95% CI) | 613.5 | 475.8 | 344.9 | 432.0 | |
| (605.6-622.7) | (470.2-481.9) | (341.9-349.4) | (429.5-435.6) | ||
| Patients, No. | 1,095 | 1,369 | 1,842 | 4,306 | |
| ACSC-related hospitalizations | ACSC hospitalizations, No. (%) | 1,714 (26.4) | 2,045 (31.5) | 2,730 (42.1) | 6,489 (100.0) |
| ACSC hospitalizations, median (IQR) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Person-time, y (%) | 32,328.8 (17.9) | 53,698.6 (29.7) | 94,520.5 (52.4) | 180,547.9 (100.0) | |
| Length of hospital stay, d, mean (SD) | 1.6 (6.7) | 1.4 (5.7) | 1.4 (6.0) | 1.4 (6.1) | |
| Length of hospital stay, d, median (IQR) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Cumulative length of hospital stay, d, median (IQR) | 0 (0-7) | 0 (0-5) | 0 (0-4) | 0 (0-5) | |
| Unadjusted ACSC hospitalization rate/1,000 person-years (95% CI) | 53.1 | 38.1 | 28.9 | 36.0 | |
| (50.6-55.6) | (36.5-39.8) | (27.9-30.1) | (35.1-36.9) | ||
| All-cause ED visits | Patients, No. | 11,234 | 16,237 | 23,681 | 51,152 |
| ED visits, No. (%) | 56,809 (27.8) | 70,147 (34.3) | 77,659 (38.0) | 204,615 (100.0) | |
| ED visits, median (IQR) | 2 (0-4) | 1 (0-3) | 1 (0-2) | 1 (0-3) | |
| Person-time, y (%) | 32,328.8 (17.9) | 53,698.6 (29.7) | 94,520.5 (52.4) | 180,547.9 (100.0) | |
| Unadjusted ED visit rate/1,000 person-years (95% CI) | 1,757.2 | 1,306.3 | 823.4 | 1,133.3 | |
| (1,744.3-1,773.3) | (1,297.2-1,316.5) | (817.6-829.2) | (1,129.9-1,139.8) | ||
| ACSC-related ED visits | Patients, No. | 1,414 | 1,731 | 2,272 | 5,417 |
| ACSC ED visits, No. (%) | 2,294 (27.1) | 2,726 (32.2) | 3,441 (40.7) | 8,461 (100.0) | |
| ACSC ED visits, median (IQR) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Person-time, y (%) | 32,328.8 (17.9) | 53,698.6 (29.7) | 94,520.5 (52.4) | 180,547.9 (100.0) | |
| Unadjusted ACSC ED visits rate/1,000 person-years (95% CI) | 71.0 | 50.8 | 36.5 | 46.9 | |
| (68.2-74.0) | (48.9-62.7) | (35.3-37.7) | (45.9-47.9) | ||
ACSC = ambulatory care-sensitive condition; ED = emergency department; IQR = interquartile range.
Among patients with CKD, those in the lowest-continuity group had unadjusted hospitalization and ED visit rates that were approximately double the rates for those in the high-continuity group (613.5 vs 344.9 hospitalizations/1,000 person-years; 1,757.2 vs 823.4 ED visits/1,000 person-years). After adjusting for relevant confounders, patients with poor continuity of care were 1.52 (95% CI, 1.47-1.57) and 1.78 (95% CI, 1.73-1.83) times more likely to experience an all-cause hospitalization and ED visit, respectively (Supplemental Figure 1). Similar trends were observed in a stepwise fashion across CKD stages, with poor continuity of care being associated with greater rate ratios for all-cause hospitalizations and ED visits (Supplemental Figure 2, Supplemental Table 2, and Supplemental Table 3).
Potentially Preventable Acute Care Use
Our cohort had 6,489 (8.3% of all hospitalizations) CKD-related ACSC hospitalizations and 8,461 (4.1% of all ED visits) CKD-related ACSC ED visits (Supplemental Table 4). More than one-half of these ACSC hospitalizations and ED visits were among individuals with poor or moderate continuity. A total of 96.9% and 88.7% of CKD-related ACSC hospitalizations and ED visits, respectively, were attributable to heart failure.
In our adjusted negative binomial models, poor continuity of care was associated with significantly greater ACSC hospitalization (IRR, 1.58; 95% CI, 1.44-1.74) and ED (IRR, 1.68; 95% CI, 1.54-1.82) rates. Similar but attenuated trends were observed for patients with moderate continuity (IRR for hospitalization, 1.23; 95% CI, 1.13-1.34 and IRR for ED visits, 1.28; 95% CI, 1.19-1.38).
When stratified by CKD stage, rate ratios for ACSC acute care use were significantly greater among patients with poor continuity in earlier stages of CKD (stages 3a and 3b) (Supplemental Figure 3, Supplemental Table 5, and Supplemental Table 6). No significant differences were observed in rate ratios for ACSC hospitalizations and ED visits across continuity of primary care among patients with stage 4 CKD.
Guideline-Recommended Drug Prescriptions
A total of 48,648 (56.9%) and 5,753 (6.7%) patients were prescribed an RAAS inhibitor or statin, respectively, at least once in the year before their CKD diagnosis date (Supplemental Table 7). There were no significant differences in the proportion and adjusted ORs of patients prescribed an RAAS inhibitor in the year before the index date across continuity of primary care (Table 3). However, patients with poor and moderate continuity of care were 0.8 (95% CI, 0.74-0.86) and 0.89 (95% CI, 0.84-0.95) times less likely to be prescribed a statin compared with patients with high continuity of care.
Table 3.
Unadjusted and Adjusted Incident Rate Ratios for Health Care Utilization (All-Cause and Ambulatory Care-Sensitive Condition-Related Hospitalizations and Emergency Department Visits) and Process of Care Outcomes by Continuity of Primary Care
| Variable | Level of Relational Continuity of Pimary Care | Overall (n = 86,475) |
||||
|---|---|---|---|---|---|---|
| Poor (n = 16,143) |
Moderate (n = 25,948) |
High (n = 44,384) |
||||
| Health care utilization outcomes | All-cause hospitalizations | Patients, No. | 7,938 | 11,020 | 15,852 | 34,810 |
| All-cause hospitalizations, No. | 19,835 | 25,551 | 32,602 | 77,988 | ||
| Unadjusted incident rate ratio of all-cause hospitalizations (95% CI) | 1.85 (1.79-1.91) |
1.41 (1.37-1.45) |
(reference) | |||
| Adjusted incident rate ratio of all-cause hospitalizations (95% CI)a | 1.52 (1.47-1.57) |
1.28 (1.25-1.32) |
(reference) | |||
| ACSC-related hospitalizations | Patients, No. | 1,095 | 1,369 | 1,842 | 4,306 | |
| ACSC-related hospitalizations, No. | 1,714 | 2,045 | 2,730 | 6,489 | ||
| Unadjusted incident rate ratio of ACSC-related hospitalizations (95% CI) | 1.96 (1.77-2.16) |
1.35 (1.24-1.48) |
(reference) | |||
| Adjusted incident rate ratio of ACSC-related hospitalizations (95% CI)b | 1.58 (1.44-1.74) |
1.23 (1.13-1.34) |
(reference) | |||
| All-cause ED visits | Patients, No. | 11,234 | 16,237 | 23,681 | 51,152 | |
| All-cause ED visits, No. | 56,809 | 70,147 | 77,659 | 204,615 | ||
| Unadjusted incident rate ratio of all-cause ED visits (95% CI) | 2.18 (2.12-2.24) |
1.6 (1.56-1.64) |
(reference) | |||
| Adjusted incident rate ratio of all-cause ED visits (95% CI)a | 1.78 (1.73-1.83) |
1.42 (1.39-1.46) |
(reference) | |||
| ACSC-related ED visits | Patients, No. | 1,414 | 1,731 | 2,272 | 5,417 | |
| ACSC-related ED visits, No. | 2,294 | 2,726 | 3,441 | 8,461 | ||
| Unadjusted incident rate ratio of ACSC-related ED visits (95% CI) | 2.1 (1.92-2.29) |
1.44 (1.33-1.55) |
(reference) | |||
| Adjusted incident rate ratio of ACSC-related ED visits (95% CI)b | 1.68 (1.54-1.82) |
1.28 (1.19-1.38) |
(reference) | |||
| RAAS inhibitor | Patients, No. | 9,387 | 14,500 | 24,761 | 48,648 | |
| Process of care outcomes | Unadjusted odds ratio (95% CI) | 1.09 (1.05-1.14) |
1.0 (0.97-1.03) |
(reference) | ||
| Adjusted odds ratio (95% CI) | 1.03 (0.98-1.07) |
0.99 (0.96-1.02) |
(reference) | |||
| Patients, No. | 7,318 | 12,147 | 21,831 | 41,296 | ||
| Statin | Unadjusted odds ratio (95% CI) | 0.75 (0.69-0.8) |
0.86 (0.81-0.92) |
(reference) | ||
| Adjusted odds ratio (95% CI) | 0.8 (0.74-0.86) |
0.89 (0.84-0.95) |
(reference) | |||
ACSC = ambulatory care-sensitive condition; ED = emergency department; IQR = interquartile range; RAAS = renin-angiotensin-aldosterone system.
Adjusted by age, sex, household location, median household income quintile, cirrhosis, chronic heart failure, peripheral vascular disease, atrial fibrillation, asthma, chronic obstructive pulmonary disease, cancer, diabetes, hypertension, and albuminuria severity.
Adjusted by age, sex, household location, median household income quintile, cirrhosis, peripheral vascular disease, atrial fibrillation, asthma, chronic obstructive pulmonary disease, cancer, diabetes, and albuminuria severity.
DISCUSSION
In this population-based cohort study, approximately 1 in 5 patients with CKD had poor continuity of primary care in Alberta. Rates of all-cause hospitalization and ED use increased with poorer continuity of care and followed in a stepwise fashion across CKD stages. Poor continuity of care was also associated with greater rates of CKD-related ACSC hospitalization and ED visits among patients with earlier stages of CKD and lower rates of guideline-recommended drug prescriptions. These findings highlight the importance of relational continuity of care among adults with earlier stages of CKD and the need for strategies to improve care coordination in this high-risk population.
In the general population, up to one-third of individuals have poor continuity of primary care.21,22 Similar findings among populations with chronic disease suggest that poor relational continuity of primary care is common and is associated with greater odds of all-cause and ACSC acute care use.23-26 This is the first Canadian study to show the association between poor relational continuity of primary care and potentially preventable hospitalizations and ED visits among those with earlier stages of CKD. Given that primary care plays an essential role in the identification and management of less-advanced CKD and associated comorbidities, strong relational continuity of care has the potential to delay irreversible progression of kidney disease and downstream complications that contribute to acute care utilization.27,28 Understanding how relational continuity can be enhanced in this complex population is essential to inform targeted health promotion strategies in the outpatient setting.
In primary care practices in Canada, CKD care evidence gaps exist in prescribing patterns of guideline-recommended preventive drugs and routine albuminuria testing.29,30 This was confirmed by our present analysis; patients with poor continuity of care were less likely to be prescribed a statin compared with individuals with high continuity of care. Gaps in guideline-recommended drug prescribing that are related to poor continuity might have contributed to high rates of potentially preventable health care utilization observed in our cohort. Thus, strategies are needed to improve primary care continuity via health care models (ie, patient enrollment primary care models) and policies (ie, booking patients with their usual physician >80% of the time, improving access to appointments, etc) before nephrology referral.31-34 High continuity of care is patient centered and should be promoted in the CKD population across all levels of health care, given that it appears to mitigate acute care needs for potentially preventable conditions.
We found that the most common CKD-related hospitalizations and ED visits were for heart failure. Individuals with kidney failure requiring dialysis are at increased risk of developing heart failure35; however, our findings suggested that potentially preventable acute care use for heart failure occurred even among those with less advanced CKD. Numerous pharmacologic and nonpharmacologic strategies for preventing heart failure, such as RAAS inhibitors, are used among non–dialysis-dependent patients.36-39 Given that transitions into nephrology care typically occur in stage 4 CKD, multidisciplinary heart failure strategies across primary care, cardiology, and nephrology care could be a practical and effective way to prevent acute decompensation in this complex population.40,41 Future research engaging primary care physicians in health promotion strategies that target common underlying comorbidities, such as heart failure, in patients with CKD is warranted.
The present study has limitations that should be considered. First, we were only able to include those patients with a diagnosis of CKD who used laboratory services. The number of patients not captured with this approach is expected to be relatively small and unlikely to invalidate our findings, given that we used population-level laboratory data from the AKDN repository and classified CKD diagnosis using ≥2 eGFR measurements taken at least 90 days apart to minimize misclassification bias of individuals with acute kidney injury. Second, we used CKD-related ACSCs to infer potentially preventable encounters that are likely influenced by a number of social determinants of health (eg, level of family support, education, etc). Those factors were not captured by our administrative data sources; however, we were able to adjust for important patient-level factors (eg, residential location, neighborhood income, etc) related to increased acute care use in our adjusted analysis. Third, we were unable to explore prescribing of sodium-glucose cotransporter 2 (SGLT2) inhibitors across levels of continuity because they were not available in Alberta during the study time frame. Given that SGLT2 inhibitors have since become part of the standard guidelines for CKD and heart failure treatments, future studies should seek to explore how primary care continuity affects SGLT2 inhibitor prescribing rates in CKD populations and whether this influences downstream acute care utilization. Finally, a fundamental limitation inherent to all observational study designs is the inability to infer causation. Thus, our associations between continuity and health utilization/process of care outcomes should be interpreted with caution.
CONCLUSIONS
In summary, approximately 1 in 5 patients with CKD had poor continuity of primary care, and the rate of all-cause and potentially preventable acute care use significantly increased with poor continuity of care. The present study suggests that if the association between continuity and CKD acute care use is causal, decreases in potentially preventable CKD-related acute care encounters might be realized via health care models and policies that strengthen patient-physician relationships and improve prescribing of guideline-recommended drugs for patients with less advanced stages of CKD. Targeted interventions aimed at those with specific comorbidities (eg, heart failure) or that enhance continuity of primary care might also lead to decreased reliance on acute care use and to improved care experiences and health outcomes for patients with CKD.
Supplementary Material
Footnotes
Conflicts of interest: authors report none.
Funding support: C.C. is supported by the Alberta Strategy for Patient Oriented Research SUPPORT Unit (AbSPORU), the Canadian Institutes of Health Research (CIHR), and the University of Calgary Cumming School of Medicine. P.R. is supported by an operating grant from the CIHR.
Authors’ contributions: C.C. and P.R. were involved in the conception and design of the study. C.C. and P.R. were responsible for drafting the manuscript and interpreting the data. C.C. contributed to the study design and conducted the analysis. All authors contributed to the conception and interpretation of study findings. All authors were responsible for revising the manuscript for important intellectual content, approved the final version, and agree to be accountable for all aspects of the work.
Ethics approval: Ethics approval for the study was obtained from the Conjoint Health Ethics Review Board at the University of Calgary. This study used secondary data; therefore, individual patient consent was not required.
Disclaimer: This study was based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study.
Data sharing: C.C. and P.R. had full access to all of the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. We are not able to make our data set available to other researchers, owing to our contractual arrangements with the provincial health ministry (Alberta Health), who is the data custodian.
References
- 1.Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013; 185(9): E417-E423. 10.1503/cmaj.120833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prichard SS. Comorbidities and their impact on outcome in patients with end-stage renal disease. Kidney Int. 2000; 57(Suppl 74): S100-S104. 10.1046/j.1523-1755.2000.07417.x [DOI] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3(1): 1-50. [Google Scholar]
- 4.Grill AK, Brimble S.. Approach to the detection and management of chronic kidney disease: what primary care providers need to know. Can Fam Physician. 2018; 64(10): 728-735. [PMC free article] [PubMed] [Google Scholar]
- 5.Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R.. Continuity of care: a multidisciplinary review. BMJ. 2003; 327(7425): 1219-1221. 10.1136/bmj.327.7425.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McWhinney IR. Primary care: core values. Core values in a changing world. BMJ. 1998; 316(7147): 1807-1809. 10.1136/bmj.316.7147.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker R, Freeman G, Boulton M, et al. Continuity of care: patients’ and carers’ views and choices in their use of primary care services. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R & D. Published Nov 2005. Accessed Apr 20, 2022. https://njl-admin.nihr.ac.uk/document/download/2027402 [Google Scholar]
- 8.van Servellen G, Fongwa M, Mockus D’Errico E.. Continuity of care and quality care outcomes for people experiencing chronic conditions: a literature review. Nurs Health Sci. 2006; 8(3): 185-195. 10.1111/j.1442-2018.2006.00278.x [DOI] [PubMed] [Google Scholar]
- 9.Ronksley PE, Tonelli M, Manns BJ, et al. Emergency department use among patients with CKD: a population-based analysis. Clin J Am Soc Nephrol. 2017; 12(2): 304-314. 10.2215/cjn.06280616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komenda P, Tangri N, Klajncar E, et al. Patterns of emergency department utilization by patients on chronic dialysis: a population-based study. PLoS One. 2018; 13(4): e0195323. 10.1371/journal.pone.0195323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronksley PE, Hemmelgarn BR, Manns BJ, et al. Potentially preventable hospitalization among patients with CKD and high inpatient use. Clin J Am Soc Nephrol. 2016; 11(11): 2022-2031. 10.2215/CJN.04690416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiebe N, Klarenbach SW, Allan GM, et al. ; Alberta Kidney Disease Network . Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014; 64(2): 230-238. 10.1053/j.ajkd.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009; 10: 30. 10.1186/1471-2369-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150(9): 604-612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breslau N, Reeb KG.. Continuity of care in a university-based practice. J Med Educ. 1975; 50(10): 965-969. 10.1097/00001888-197510000-00006 [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Manns BJ, Culleton BF, et al. ; Alberta Kidney Disease Network . Access to health care among status Aboriginal people with chronic kidney disease. CMAJ. 2008; 179(10): 1007-1012. 10.1503/cmaj.080063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmelgarn BR, Manns BJ, Lloyd A, et al. ; Alberta Kidney Disease Network . Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010; 303(5): 423-429. 10.1001/jama.2010.39 [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M, Wiebe N, Fortin M, et al. ; Alberta Kidney Disease Network . Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015; 15: 31. 10.1186/s12911-015-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147(8): 573-577. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 20.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015; 12(10): e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menec VH, Sirski M, Attawar D, Katz A.. Does continuity of care with a family physician reduce hospitalizations among older adults? J Health Serv Res Policy. 2006; 11(4): 196-201. 10.1258/135581906778476562 [DOI] [PubMed] [Google Scholar]
- 22.Menec VH, Sirski M, Attawar D.. Does continuity of care matter in a universally insured population? Health Serv Res. 2005; 40(2): 389-400. 10.1111/j.1475-6773.2005.00363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worrall G, Knight J.. Continuity of care is good for elderly people with diabetes: retrospective cohort study of mortality and hospitalization. Can Fam Physician. 2011; 57(1): e16-e20. [PMC free article] [PubMed] [Google Scholar]
- 24.Kao YH, Tseng TS, Ng YY, Wu SC.. Association between continuity of care and emergency department visits and hospitalization in senior adults with asthma-COPD overlap. Health Policy. 2019; 123(2): 222-228. 10.1016/j.healthpol.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blozik E, Bähler C, Näpflin M, Scherer M.. Continuity of care in Swiss cancer patients using claims data. Patient Prefer Adherence. 2020; 14: 2253-2262. 10.2147/PPA.S266381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CC, Chen SH.. Better continuity of care reduces costs for diabetic patients. Am J Manag Care. 2011; 17(6): 420-427. [PubMed] [Google Scholar]
- 27.Allen AS, Forman JP, Orav EJ, Bates DW, Denker BM, Sequist TD.. Primary care management of chronic kidney disease. J Gen Intern Med. 2011; 26(4): 386-392. 10.1007/s11606-010-1523-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady M, O’Donoghue D.. The role of primary care in managing chronic kidney disease. Br J Gen Pract. 2010; 60(575): 396-397. 10.3399/bjgp10X502065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manns L, Scott-Douglas N, Tonelli M, et al. A population-based analysis of quality indicators in CKD. Clin J Am Soc Nephrol. 2017; 12(5): 727-733. 10.2215/cjn.08720816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bello AK, Ronksley PE, Tangri N, et al. Quality of chronic kidney disease management in Canadian primary care. JAMA Netw Open. 2019; 2(9): e1910704. 10.1001/jamanetworkopen.2019.10704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiagi R, Chechulin Y.. The effect of rostering with a patient enrolment model on emergency department utilization. Healthc Policy. 2014; 9(4): 105-121. [PMC free article] [PubMed] [Google Scholar]
- 32.Muggah E, Hogg W, Dahrouge S, et al. Patient-reported access to primary care in Ontario: effect of organizational characteristics. Can Fam Physician. 2014; 60(1): e24-e31. [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy A, Pollack CE, Asch DA, Canamucio A, Werner RM.. The effect of primary care provider turnover on patient experience of care and ambulatory quality of care. JAMA Intern Med. 2015; 175(7): 1157-1162. 10.1001/jamainternmed.2015.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberta Medical Association . Relational continuity. Published Jun 19, 2019. Accessed Mar 8, 2022. https://actt.albertadoctors.org/CPGs/Pages/Relational-Continuity.aspx
- 35.Wang AYM, Wang M, Lam CWK, Chan IHS, Lui SF, Sanderson JE.. Heart failure in long-term peritoneal dialysis patients: a 4-year prospective analysis. Clin J Am Soc Nephrol. 2011; 6(4): 805-812. 10.2215/CJN.07130810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee D, Wang AYM.. Personalizing heart failure management in chronic kidney disease patients. Nephrol Dial Transplant. 2021; 1-8. 10.1093/ndt/gfab026 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen M, Rumjaun S, Lowe-Jones R, et al. Management and outcomes of heart failure patients with CKD: experience from an inter-disciplinary clinic. ESC Heart Fail. 2020; 7(5): 3225-3230. 10.1002/ehf2.12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee D, Rosano G, Herzog CA.. Management of heart failure patient with CKD. Clin J Am Soc Nephrol. 2021; 16(7): 1131-1139. 10.2215/CJN.14180920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed A, Rich MW, Zile M, et al. Renin-angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med. 2013; 126(2): 150-161. 10.1016/j.amjmed.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmelgarn BR, Manns BJ, Zhang J, et al. Association between multidisciplinary care and survival for elderly patients with chronic kidney disease. J Am Soc Nephrol. 2007; 18(3): 993-999. 10.1681/asn.2006080860 [DOI] [PubMed] [Google Scholar]
- 41.Calvert MJ, Shankar A, McManus RJ, Ryan R, Freemantle N.. Evaluation of the management of heart failure in primary care. Fam Pract. 2009; 26(2): 145-153. 10.1093/fampra/cmn105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.