Abstract
Background
Healthy sleep is an important component of childhood development. Changes in sleep architecture, including sleep stage composition, quantity, and quality from infancy to adolescence are a reflection of neurologic maturation. Hospital admission for acute illness introduces modifiable risk factors for sleep disruption that may negatively affect active brain development during a period of illness and recovery. Thus, it is important to examine non‐pharmacologic interventions for sleep promotion in the pediatric inpatient setting.
Objectives
To evaluate the effect of non‐pharmacological sleep promotion interventions in hospitalized children and adolescents on sleep quality and sleep duration, child or parent satisfaction, cost‐effectiveness, delirium incidence, length of mechanical ventilation, length of stay, and mortality.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, three other databases, and three trials registers to December 2021. We searched Google Scholar, and two websites, handsearched conference abstracts, and checked reference lists of included studies.
Selection criteria
Randomized controlled trials (RCTs) or quasi‐RCTs, including cross‐over trials, investigating the effects of any non‐pharmacological sleep promotion intervention on the sleep quality or sleep duration (or both) of children aged 1 month to 18 years in the pediatric inpatient setting (intensive care unit [ICU] or general ward setting).
Data collection and analysis
Two review authors independently assessed trial eligibility, evaluated risk of bias, extracted and synthesized data, and used the GRADE approach to assess certainty of evidence. The primary outcomes were changes in both objective and subjective validated measures of sleep in children; secondary outcomes were child and parent satisfaction, cost‐effectiveness ratios, delirium incidence or delirium‐free days at time of hospital discharge, duration of mechanical ventilation, length of hospital stay, and mortality.
Main results
We included 10 trials (528 participants; aged 3 to 22 years) in inpatient pediatric settings. Seven studies were conducted in the USA, two in Canada, and one in Brazil. Eight studies were funded by government, charity, or foundation grants. Two provided no information on funding.
Eight studies investigated behavioral interventions (massage, touch therapy, and bedtime stories); two investigated physical activity interventions. Duration and timing of interventions varied widely. All studies were at high risk of performance bias due to the nature of the intervention, as participants, parents, and staff could not be masked to group assignment.
We were unable to perform a quantitative synthesis due to substantial clinical heterogeneity.
Behavioral interventions versus usual care
Five studies (145 participants) provided low‐certainty evidence of no clear difference between multicomponent relaxation interventions and usual care on objective sleep measures. Overall, evidence from single studies found no clear differences in daytime or nighttime sleep measures (33 participants); any sleep parameter (48 participants); or daytime or nighttime sleep or nighttime arousals (20 participants). One study (34 participants) reported no effect of massage on nighttime sleep, sleep efficiency (SE), wake after sleep onset (WASO), or total sleep time (TST) in adolescents with cancer. Evidence from a cross‐over study in 10 children with burns suggested touch therapy may increase TST (391 minutes, interquartile range [IQR] 251 to 467 versus 331 minutes, IQR 268 to 373; P = 0.02); SE (76, IQR 53 to 90 versus 66, IQR 55 to 78; P = 0.04); and the number of rapid eye movement (REM) periods (4.5, IQR 2 to 5 versus 3.5, IQR 2 to 4; P = 0.03); but not WASO, sleep latency (SL), total duration of REM, or per cent of slow wave sleep.
Four studies (232 participants) provided very low‐certainty evidence on subjective measures of sleep. Evidence from single studies found that sleep efficiency may increase, and the percentage of nighttime wakefulness may decrease more over a five‐day period following a massage than usual care (72 participants). One study (48 participants) reported an improvement in Children's Sleep Habits Questionnaire scores after discharge in children who received a multicomponent relaxation intervention compared to usual care. In another study, mean sleep duration per sleep episode was longer (23 minutes versus 15 minutes), and time to fall asleep was shorter (22 minutes versus 27 minutes) following a bedtime story versus no story (18 participants); and children listening to a parent‐recorded story had longer SL than when a parent was present (mean 57.5 versus 43.5 minutes); both groups reported longer SL than groups who had a stranger‐recorded story, and those who had no story and absent parents (94 participants; P < 0.001).
In one study (34 participants), 87% (13/15) of participants felt they slept better following massage, with most parents (92%; 11/12) reporting they wanted their child to receive a massage again. Another study (20 participants) reported that parents thought the music, touch, and reading components of the intervention were acceptable, feasible, and had positive effects on their children (very low‐certainty evidence).
Physical activity interventions versus usual care
One study (29 participants) found that an enhanced physical activity intervention may result in little or no improvement in TST or SE compared to usual care (low‐certainty evidence). Another study (139 participants), comparing play versus no play found inconsistent results on subjective measures of sleep across different ages (TST was 49% higher for the no play groups in 4‐ to 7‐year olds, 10% higher in 7‐ to 11‐year olds, and 22% higher in 11‐ to 14‐year olds). This study also found inconsistent results between boys and girls (girls in the first two age groups in the play group slept more than the no play group).
No study evaluated child or parent satisfaction for behavioral interventions, or cost‐effectiveness, delirium incidence or delirium‐free days at hospital discharge, length of mechanical ventilation, length of hospital stay, or mortality for either behavioral or physical activity intervention.
Authors' conclusions
The included studies were heterogeneous, so we could not quantitatively synthesize the results. Our narrative summary found inconsistent, low to very low‐certainty evidence. Therefore, we are unable to determine how non‐pharmacologic sleep promotion interventions affect sleep quality or sleep duration compared with usual care or other interventions.
The evidence base should be strengthened through design and conduct of randomized trials, which use validated and highly reliable sleep assessment tools, including objective measures, such as polysomnography and actigraphy.
Keywords: Adolescent; Child; Female; Humans; Male; Child, Hospitalized; Delirium; Delirium/prevention & control; Intensive Care Units; Randomized Controlled Trials as Topic; Respiration, Artificial; Sleep
Plain language summary
Methods for promoting sleep in children and young people in hospital without using medicines (non‐medicinal)
Key messages
We are uncertain how effective non‐medicinal sleep promotion methods are for children and young people in hospital.
Studies that use established, reliable research methods are needed to investigate non‐medicinal sleep promotion in hospitalized children and young people, to identify methods that work.
Why is sleep important for children in hospital?
Sleep is an important part of healthy childhood development and helps to keep children healthy. Sleep patterns change throughout childhood, as children’s brains develop. When children are ill and stay in hospital, their sleep may be of poor quality, or disrupted by the constant noise and light, medical treatments, monitoring by nurses, stress, and pain.
While medicines can be used to try to improve sleep in hospitalized children, studies show they do not work particularly well, and can make the quality of sleep worse. Non‐medicinal ways of promoting sleep can be used instead. These may include changes to the hospital environment, music, massage, or other methods.
What did we want to find out?
We wanted to find out whether non‐medicinal ways of trying to improve sleep work better than usual care or other methods in hospitalized children.
We wanted to look at how well these different methods worked on:
‐ the quality and quantity of sleep in the children;
‐ child and parent satisfaction;
‐ length of time for which breathing was supported by a ventilator (ventilator support);
‐ delirium;
‐ cost‐effectiveness;
‐ length of hospital stay; and
‐ mortality.
We also wanted to find out if non‐medicinal methods were associated with any unwanted effects.
What did we do?
We looked for studies that compared non‐medicinal methods for improving sleep with usual care, or other methods in children in hospital.
We compared and summarized the results of the studies, and rated our confidence in the evidence, based on factors, such as study methods and sizes.
What did we find?
We found 10 trials from three countries, which involved 528 children and young people (aged from 4 to 22 years). Eight studies were funded by non‐profit organizations or government sources.
All the children and young people were staying in hospital for more than 48 hours on regular wards, or in children’s intensive care units. The methods used to try to improve sleep included behavioral interventions (relaxation, including music, reading, and quiet time; touch therapy, and massage), and physical activity interventions.
There were many differences between studies in the participants, the methods used to measure the quantity and quality of sleep, and the way the results were analyzed. As a result, we could not combine the findings of trials that investigated similar methods; instead, we provided a descriptive summary.
Behavioral interventions
Studies that combined methods of relaxation found that these may make little or no difference on the amount or quality of sleep compared to usual care.
Touch therapy may improve total sleep time and sleep quality in children with burns. Massage and bedtime stories may also improve sleep. However, we have little confidence in these results, because of differences in the study populations and the measurement methods they used.
Children and parents may be satisfied with both massage and multicomponent relaxation methods for improving sleep. However, we are not confident about this result, as the people in the studies knew which sleep‐improving method they received, and there were not enough studies for us to be certain about their results.
We did not identify any studies that reported length of ventilator support, delirium, cost‐effectiveness, length of hospital stay, or mortality.
Physical activity interventions
One study showed that using a stationary (exercise) bicycle to improve sleep may not improve total sleep time or quality of sleep compared to usual care. Another study investigated whether organized play improved sleep; it found inconsistent results for boys and girls, and for children in different age groups. No study evaluated child or parent satisfaction, cost‐effectiveness, delirium, length of ventilator support, length of hospital stay, or mortality.
What are the limitations of the evidence?
We did not identify any studies that reported the effects of complementary interventions, such as aromatherapy, acupressure, or acupuncture.
We did not identify any studies for any intervention that reported length of ventilator support, delirium, cost‐effectiveness, length of hospital stay, or mortality.
We are not confident in any of these results; it is possible that these results would change if we had more evidence.
How up to date is this review?
This evidence is current to December 2021.
Summary of findings
Summary of findings 1. Behavioral interventions versus usual care for sleep promotion in pediatric inpatients.
|
Patient or population: children between the ages of 1 month and 18 years who were admitted to a pediatric inpatient setting from another location Settings: pediatric inpatient ward or intensive care unit Interventions: multicomponent relaxation interventions, massage, healing touch, bedtime stories Comparison: usual care | ||||
| Outcomes | Impact |
Number of participants (studies)* |
Certainty of the evidence (GRADE) | Comments |
|
Objective measures of sleep Measured with: TST (min), SE (%), SL (min), WASO (min) Timing of assessment: during hospitalization |
|
145 (5 studies) |
⊕⊕⊝⊝ Lowa | Mean age 10.1 years (range 4 to 22 years) |
|
Subjective sleep quality or quantity Measured with: video, questionnaire, or observation scale Timing of assessment: during hospitalization (3 studies) and after hospital discharge (1 study) |
|
232 (4 studies) |
⊕⊝⊝⊝
Very lowb |
Mean age not provided for all studies (range 3 to 18 years) |
|
Participant or parent satisfaction Measured with: surveys Timing of assessment: during hospitalization |
|
54 (2 studies) |
⊕⊝⊝⊝ Very lowc | Mean age 12.6 years (range not available) |
| Cost‐effectiveness | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Delirium incidence or delirium‐free days at hospital discharge | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Length of mechanical ventilation | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Length of hospital stay | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| CSHQ: Children's Sleep Habits Questionnaire; ICU: intensive care unit; min: minutes; PICU: pediatric ICU; REM: rapid eye movement; SE: sleep efficiency; SL: sleep latency; TST: total sleep time; vs: versus; WASO: wake after sleep onset | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||
aDowngraded two levels due inconsistency (differences in population and differences in interventions) bDowngraded two levels due to risk of bias (high risk of bias from inability to blind outcome assessors for this outcome), and one level due to inconsistency (differences in intervention and population) cDowngraded two levels for imprecision (small sample size and inadequate data [small number of included studies; only two trials identified for comparison]); and one level for risk of bias (lack of blinding)
Summary of findings 2. Physical activity interventions versus usual care for sleep promotion in pediatric inpatients.
|
Patient or population: children between the ages of 1 month and 18 years who were admitted to a pediatric inpatient setting from another location Settings: pediatric inpatient ward or intensive care unit Intervention: early physical activity intervention (stationary bicycle‐style exerciser) and play Comparison: usual care | ||||
| Outcomes | Impact | Number of participants (studies)* | Certainty of the evidence (GRADE) | Comments |
|
Objective measures of sleep Measured with: TST (min), SE (%) Timing of assessment: days 0 to 3 |
One study compared the effect of an enhanced physical activity intervention, using a stationary bicycle‐style exerciser vs standard care using actigraphy. There was no evidence of a difference in SE or TST between the groups. | 29 (1 study) |
⊕⊕⊝⊝ Lowa | Mean age 12.5 years (range 7.4 to 18.2) |
|
Subjective sleep quality or quantity Measured with: sleep logs (observation) Timing of assessment: during hospitalization |
One study compared the effect of play activities vs no play on sleep in children hospitalized for respiratory disease, using sleep logs. TST was 49% higher for the non‐play groups in 4 to 7 year olds compared to play groups, 10% higher in 7 to 11 year olds and 22% higher in 11 to 14 year olds. Girls in the first two age groups slept more in the play group. | 139 (1 study) |
⊕⊝⊝⊝
Very Lowb |
Age range 4 to 14 years |
| Participant or parent satisfaction | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Cost‐effectiveness | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Delirium incidence or delirium‐free days at hospital discharge | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Length of mechanical ventilation | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Length of hospital stay | None of the included studies provided data on this outcome. | ‐ | ‐ | ‐ |
| Min: minutes; SE: sleep efficiency; TST: total sleep time; vs: versus | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||
aDowngraded two levels due to imprecision (small sample size) bDowngraded two levels for high risk of bias (6/7 domains unclear or high risk of bias), and one level for imprecision (inadequate data due to small number of included studies)
Background
Description of the condition
Sleep is a basic human need. The process of rest and restoration occurs during the natural suspension of consciousness that occurs during sleep (Kryger 2005; Kudchadkar 2009; Kudchadkar 2014a; Kudchadkar 2014b; Murali 2003). Sleep is not a passive process, but one that is characterized by dynamic physiological changes. While the function of sleep remains elusive, the physiological effects and consequences of altered sleep can impact multiple systems throughout the body (Kudchadkar 2014a).
It is well known that sleep needs are in a constant state of change as a child matures from the neonatal to adolescent period, reflecting brain maturation (Feinberg 2013). During recovery from illness, children admitted to the hospital are exposed to a multitude of risk factors for sleep disruption, including noise, pain, anxiety, medications, interruptions for nursing care, and invasive medical interventions. Studies that used both objective and subjective assessment tools have demonstrated severe sleep disruption in hospitalized children (Cook 2020; Cureton‐Lane 1997a; Erondu 2019; Hinds 2007; Kudchadkar 2014a; Kudchadkar 2016c; Meltzer 2012). Sleep disruption occurs at a time when recovery and healing are the goal, potentially interferes with fundamental physiological processes, and can lead to increased energy release, impaired immunity, and delirium (Barnes 2016; Kamdar 2015). In addition, sleep disturbances from infancy through adolescence are associated with changes in brain morphology, and worsened short‐ and long‐term neurocognitive outcomes (Cheung 2017; Kocevska 2017; Saré 2016).
Although there are proven, inexpensive, and non‐invasive modalities to promote sleep, such methods are rarely used for children in the hospital setting (Kudchadkar 2014b). A recent systematic review of the literature surrounding sleep in the pediatric intensive care unit (ICU) found only nine, mostly observational, studies that investigated the quality of sleep in critically ill children (Kudchadkar 2014a). All the studies demonstrated significant sleep fragmentation and decreases in slow‐wave sleep. Slow‐wave sleep is the most restorative aspect of sleep, and an integral component of cognitive maturation during childhood and adolescence (Kudchadkar 2014a). In the hospital floor setting, sleep disturbance due to noise, pain, vital sign checks, and anxiety is associated with increased night awakenings and longer sleep latency (time to sleep onset [Meltzer 2012]). Among children recovering from major surgery, approximately 40% demonstrate no identifiable difference between nighttime and daytime activity levels, measured by actigraphy, suggesting significant circadian rhythm (sleep‐wake cycle) disturbance (Kudchadkar 2016b). Sleep promotion is not a priority in hospital culture, despite increasing evidence in the literature that sleep loss and fragmentation in critically‐ill adults increase the risk of delirium, which is an important risk factor for increased morbidity and mortality (Pandharipande 2006; Smith 2011). A recent Cochrane Review summarized the available evidence for non‐pharmacological interventions to promote sleep in critically ill adults admitted to the ICU (Hu 2015). Another systematic review summarized evidence on the effectiveness of non‐pharmacologic interventions for preterm infants in the neonatal ICU (Liao 2018a). However, the role of non‐pharmacological interventions for children and adolescents in other pediatric inpatient settings has not been synthesized.
Hospitals across the country are noisy, brightly lit environments, where care providers undertake multiple interventions throughout the day and night to assist children’s recovery. Few hospitals use noise reduction strategies to target World Health Organization (WHO) recommended levels (less than 30 A‐weighted decibels [dBA] for day and nighttime), and the current literature demonstrates that ICU noise levels are often greater than 50 dBA, regardless of the time of day, with several intermittent peaks that exceed 80 dBA (Busch‐Vishniac 2005; Kudchadkar 2014b; Liu 2005). In addition, a lack of natural sunlight and abolished patterns of normal light‐dark exposure is common in the hospital setting, with adverse effects on sleep architecture (the basic structural organization of normal sleep) and circadian rhythms (Glotzbach 1993; Kudchadkar 2016a). A 2007 study showed that 6% of all hospitalized children are prescribed medications to promote sleep, which can include opioids, benzodiazepines, and diphenhydramine (Meltzer 2007). Although these pharmacologic agents may decrease the time to sleep onset, they can significantly impact sleep architecture and lead to increased sleep fragmentation (Kudchadkar 2009).
Description of the intervention
Given the adverse effects of sedatives and analgesics on sleep physiology, the use of non‐pharmacological interventions has been explored in hospitalized adults, primarily in the ICU environment (Hu 2015; Kamdar 2014), and infants in the neonatal ICU (NICU [Liao 2018a]). These interventions can be categorized as: (1) environmental (noise and lighting modification; (2) behavioral (massage, music therapy, guided imagery); and (3) physical therapy interventions (mobility and exercise during the day to improve sleep at night [Kamdar 2016; Saliski 2015]). Possible environmental interventions include: earplugs, alarm modifications, headphones, white noise, or unit‐based 'quiet hours' (Foreman 2015; Freedman 2001; Hu 2015; Walder 2000), cycled lighting, and bright light therapy (Simons 2016; Taguchi 2007). Behavioral and complementary interventions that have been investigated in hospitalized adults include massage, aromatherapy, acupressure, and relaxation interventions, including music therapy and guided imagery (Richards 1998; Richardson 2003). All of these modalities have potential applications for the pediatric population, with the addition of kangaroo care (method of holding an infant that involves skin‐to‐skin contact) in the NICU setting (Baley 2015). Although potential pharmacological therapies, such as melatonin and atypical antipsychotic medications, are increasingly being used in the pediatric hospital setting with a goal of sleep promotion or delirium prevention (or both), the short‐ and long‐term effects of these agents are not well studied in the developing brain. Therefore, a focus on non‐invasive, low‐risk interventions is needed to promote sleep in hospitalized children undergoing active neurocognitive development.
How the intervention might work
There is a growing body of literature supporting the effectiveness of non‐pharmacological interventions in promoting sleep in hospitalized adults (Kamdar 2014; Koo 2008; Richards 2003; Richardson 2007; Scotto 2009), and neonates (Abdeyazdan 2016; Almadhoob 2020; Liao 2018a). Hospitalized children and adolescents also encounter disrupted sleep, and are just as likely to experience side effects with pharmacological interventions that promote sleep. Environmental, behavioral, and physical activity interventions that promote sleep in the home environment should have similar benefits when translated to the hospital environment, with augmented effects when used in combination (Berger 2021).
Environmental interventions, including noise reduction and lighting optimization, are safe and potentially effective strategies to promote healthy sleep in children. Sudden peaks in noise levels that occur frequently in the adult ICU increase arousals from sleep, demonstrated by polysomnography, the gold‐standard test for sleep measurement (Aaron 1996). In the pediatric ICU (PICU), noise is a strong predictor of sleep state, with louder noises correlating to increased awakenings from sleep (Cureton‐Lane 1997a). Reducing the background level, as well as peak levels of noise, with noise reduction interventions decreases the likelihood of arousing a child from sleep in the hospital setting, improving sleep continuity and quality. Continuous exposure to artificial light can abolish the normal circadian rhythm and diminish the normal peak in melatonin secretion at night. Day‐night cycling of light could help normalize the circadian rhythm of hospitalized children and decrease time to sleep onset.
Behavioral interventions, such as massage and touch therapy, provide modes of relaxation to decrease stress and anxiety that is common for hospitalized infants and children, with potential to decrease time to sleep onset and improve sleep continuity and quality (Mindell 2018/08).
Physical activity interventions engage the hospitalized child in activities and exercise during the day to promote rest at night (Hopkins 2015; Wieczorek 2016).
Finally, complementary and alternative therapies, such as aromatherapy or acupuncture, may help decrease anxiety and improve pain, with improved time to sleep onset, sleep quality, and sleep continuity (Cao 2009).
Most importantly, all these interventions must be easy for caregivers to implement, and ideally, should have high compliance amongst children and adolescents to optimize efficacy, and effectively change the culture for sleep promotion (Kudchadkar 2014b).
Why it is important to do this review
Despite evidence that sleep disruption in hospitalized adults has a negative impact on outcomes, and inexpensive sleep promotion interventions can decrease morbidity for both adults and neonates, there is a lack of awareness in the pediatric community about modifiable risk factors for sleep disruption in hospitalized children and adolescents outside of the NICU environment. There is a critical need for a synthesis of the current evidence in children older than one month, to understand the potential benefits of specific sleep promotion interventions in a population undergoing active neurocognitive development. There are several risk factors for sleep fragmentation in acutely ill children, and several medications used to improve sleep (i.e. benzodiazepines, diphenhydramine) that may, in fact, have a negative effect on sleep quality. Therefore, it is important to evaluate the effect of non‐pharmacologic interventions to promote sleep in this vulnerable population.
Objectives
To evaluate the effect of non‐pharmacological sleep promotion interventions in hospitalized children and adolescents on:
sleep quality and sleep duration; and
child or parent satisfaction, cost‐effectiveness, delirium incidence, length of mechanical ventilation, length of stay, and mortality
Methods
Criteria for considering studies for this review
Types of studies
In order to ensure recommendations based on highest level evidence, we only included randomized controlled trials (RCTs), randomized cross‐over trials, and quasi‐RCTs (participant allocation not strictly random, i.e. date of birth or alternation).
Types of participants
Infants, children, and adolescents aged between 1 month and 18 years, admitted to the hospital for more than 48 hours. Infants and children could have been in for surgical or medical reasons, or needed mechanical ventilation. We also considered studies with participants both above and below the age of 18 years, if they were in an inpatient pediatric hospital setting, and the study also included children and adolescents between 1 month and 18 years.
We excluded studies that focused on infants < 1 month of age (44 weeks' postmenstrual age), or studies with infants > 1 month who had never left the hospital after birth, due to differences in sleep architecture in newborns, and in particular preterm infants. We also excluded studies focused solely on children with central or obstructive sleep apnea, because non‐pharmacologic interventions are unlikely to impact the physiologic and anatomic aspects of these conditions.
Types of interventions
We included studies investigating the effects of any of the following non‐pharmacological sleep promotion interventions on the sleep quality or sleep duration (or both) of children and adolescents aged 1 month to 18 years, in the pediatric inpatient setting.
Environmental interventions, including, but not limited to: earplugs, headphones, alarm modifications, white noise, or unit‐based 'quiet hours', lighting control or cycling, eye masks, and bright light therapy, or a combination
Behavioral Interventions, including, but not limited to: massage, music therapy, guided imagery, and kangaroo care (skin‐to‐skin contact)
Physical activity interventions, such as mobility or exercise during the day
Complementary and alternative therapies, such as aromatherapy and acupressure or acupuncture
Any other non‐pharmacological intervention intended to promote the sleep of children in the hospital
Studies could include one intervention, or a combination of interventions in a treatment arm, and compare them to usual care or an alternative intervention.
Types of outcome measures
Primary outcomes
Changes in objective and validated measures of sleep, assessed using a polysomnography or actigraphy (or both); specifically, total sleep time (TST, based on age‐based normative data), percentage rapid eye movement (REM) sleep, minutes of wake, percentage of slow‐wave sleep, sleep efficiency (Laffan 2010), and Sleep Fragmentation Index (SFI), during the period of the intervention (Haba‐Rubio 2004)
-
Subjective measures of sleep, as measured by:
parent and nurse surveys; and
subjective, validated sleep assessment tools, such as the Patient Sleep Behavior Observation Tool (Corser 1996; Cureton‐Lane 1997a), or the Children's Sleep Habits Questionnaire (Owens 2000).
We excluded studies if they did not report any outcomes relevant to sleep (objective or subjective measures, outlined above), given the focus of the review and the fact that interventions, like physical activity, massage, etc. are used for a multitude of purposes.
Secondary outcomes
Participant or parent satisfaction, as described by the study authors
Cost‐effectiveness ratios
Delirium incidence or delirium‐free days at time of hospital discharge
Length of mechanical ventilation (days)
Length of hospital stay (days)
Mortality
Search methods for identification of studies
We ran the first searches for this review in April 2018 and updated them in March 2019. We ran further updates between October 2020 and December 2021, except for Grey Literature Reports, which was not maintained after 2017.
Electronic searches
We searched the following databases and trials registers.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 12) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 12 December 2021)
PubMed US National Library of Medicine (www.ncbi.nlm.nih.gov/pubmed; searched 12 December 2021)
Embase Elsevier (1980 to 12 December 2021)
CINAHLPlus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1950 to 12 December 2021)
Cochrane Database of Systematic Reviews (CDSR; 2021, Issue 12) in the Cochrane Library (searched 12 December 2021)
Epistemonikos (www.epistemonikos.org/en; searched March 2019 and 10 December 2021)
ProQuest Digital Dissertations and Theses (searched March 2019 and 10 December 2021)
ClinicalTrials.gov (clinicaltrials.gov; searched 12 December 2021)
ISRCTN Registry (www.isrctn.com; searched 9 December 2021)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; trialsearch.who.int/AdvSearch.aspx; searched 12 December 2021)
We also searched the grey literature, using the following websites.
OpenGrey (www.opengrey.eu; searched 17 April 2018, archived Summer 2021, and updated 10 December 2021)
Grey Literature Reports at the New York Academy of Medicine Library (greylit.org; searched 17 April 2018, not updated after 2017, thus, search not updated).
We did not limit our search publication date or language, or exclude studies based on the year the study was performed, if all other inclusion criteria were met (Criteria for considering studies for this review). The exact search strategies for each source are reported in Appendix 1.
Searching other resources
We searched reference lists of included studies and relevant review articles, as well as Google Scholar (scholar.google.com; 1980 to present, searched 10 December 2021), using the terms 'sleep' AND 'hospital' AND 'child'. We also handsearched relevant conference proceedings on 31 March 2020, including the Associated Professional Sleep Societies and the American Thoracic Society Conferences, and key sleep and pediatric journals, including the following:
Sleep (1990 to 1 December 2021);
Journal of Clinical Sleep Medicine (2005 to 1 December 2021);
American Journal of Respiratory and Critical Care Medicine (1995 to 1 December 2021);
Sleep Medicine Reviews (1998 to 1 December 2021);
Pediatrics (1990 to 1 December 2021);
Pediatric Critical Care Medicine (2000 to 1 December 2021);
Hospital Pediatrics (2011 to 1 December 2021); and
JAMA Pediatrics (1990 to 1 December 2021).
Data collection and analysis
We only reported the methods used for this review. For methods that will be used in future updates of this review, please see Kudchadkar 2017 and Appendix 2.
Selection of studies
After merging the results of the above literature search strategy in Endnote (Endnote 2016), we removed duplicates and exported titles and abstracts into Excel (Microsoft 2016). We randomly assigned each study a number, and rearranged them into numeric order. Two pairs of review authors (JB, SB, RP, TW) screened an equal number of reports. The two review authors in each pair independently reviewed the titles and abstracts based on the inclusion criteria, defined under Criteria for considering studies for this review. Each review author classified abstracts as yes – include, or no – exclude. Each pair of review authors reviewed and discussed disagreements to reach consensus. If there was disagreement after discussion, they included the study for full‐text review. If there was any doubt of eligibility, we obtained the full text of the paper for review. The same pair of authors reviewed the full texts of the 'include' group of titles and abstracts. They discussed disagreements until a consensus was reached. A study was excluded from data extraction if both review authors agreed it should be excluded after they reviewed the full text. The final list of studies chosen by both pairs of review authors for data extraction were merged again and renumbered for random assignment to two independent review authors, as detailed in Data extraction and management. Results of the selection process are presented in Figure 1 (Moher 2009).
1.
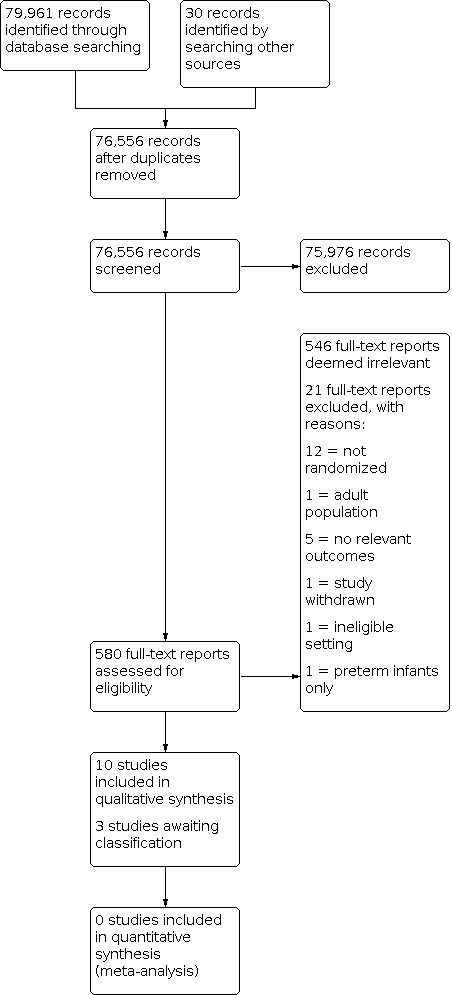
PRISMA flow diagram for included studies
Data extraction and management
Two review authors independently extracted data using a standardized form in Covidence, developed collectively by the review authors (Covidence). One review author entered data onto the form and the other member of the pair re‐entered the data using a double‐data strategy. All disagreements were resolved by group consensus and third party consultation, as needed. We extracted the following data.
-
Study characteristics
country of study
year of study
study design
method of randomization (if applicable)
unit of analysis
setting (inpatient general floor, subspecialty floor [i.e. oncology, surgery, etc.], PICU, or NICU)
outcome measures
conflict of interest and declaration of conflict of interest
-
Participant characteristics
age
sex
race
inclusion/exclusion criteria
comorbidity (prematurity, developmental delay, traumatic brain injury, surgery)
admission diagnosis
hospital length of stay
-
Intervention
type of intervention (earplugs, earmuffs, headphones, music therapy, white noise, unit‐based 'quiet‐time' protocol, alarm modifications, etc.)
timing of intervention
duration of intervention
frequency of intervention
any other associated interventions (i.e. sleep promotion 'bundles' using multiple interventions to promote sleep)
Assessment of risk of bias in included studies
Two review authors (JS, RM) independently assessed the included studies for sources of systematic bias. We assessed studies using the Cochrane RoB 1 tool, recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For each included study, we classified risk of bias as low, high, or unclear for each of the following domains: random sequence generation and allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias (other bias); see Appendix 3). The two review authors discussed and resolved disagreements, or collaborated with a third or fourth review author, if needed.
Measures of treatment effect
Continuous data
For individual studies, after verifying a normal distribution of continuous outcomes, we calculated mean values for the primary outcomes of interest (TST, SFI, sleep efficiency), and presented these with 95% confidence intervals (CI). Specifically, we calculated the mean difference (MD) when studies assessed the same outcome using the same assessment measure, and planned to present the standardized mean difference (SMD) when studies used different assessment measures for the same outcome.
Unit of analysis issues
Due to the nature of the intervention, we thought it was possible that we would encounter studies using cluster‐randomization, and cross‐over trial designs, and studies with more than two intervention groups. For these studies, we conducted the analysis as recommended in the Cochrane Handbook, and outlined below (Higgins 2021).
Cross‐over trials
We analyzed continuous data from a two‐period, two‐intervention, cross‐over trial using a paired t‐test. A paired analysis was possible if any of the following were available:
individual participant data;
means and standard deviations or errors of participant‐specific differences between measurements from the experimental and control groups;
MD and either a t statistic from paired t‐test or CI from a paired analysis; or
a graph of measurements on interventions and controls where individual matched data values could be identified and extracted.
Dealing with missing data
When possible, we contacted the original investigators of the included studies to provide missing participant data, including reasons for dropping out, and missing information regarding study design, to assist with risk of bias assessments.
Assessment of heterogeneity
By assessing variations in participant, intervention, and outcome characteristics, we examined clinical as well as methodological heterogeneity. These included the following.
Participant characteristics: age, sex, comorbidities, inclusion/exclusion criteria
Intervention characteristics: type of intervention, timing, duration
Outcome characteristics: method of measurement, timing
When clinical heterogeneity was too great, we planned to provide a narrative summary.
Assessment of reporting biases
We sought both published and unpublished literature to address publication bias. However, there were not enough studies to create a funnel plot to investigate reporting bias.
Data synthesis
We did not perform a meta‐analysis due to substantial clinical and methodological heterogeneity (see Assessment of heterogeneity). Instead, we undertook a narrative synthesis of included studies, presenting information in relevant tables (study characteristics and results, including comparisons between interventions, i.e. behavioral interventions versus usual care, physical activity interventions versus usual care). We also presented a comprehensive discussion of each study’s methodology or risk of bias (or both) that may have affected the quantitative result obtained. We reported the results without meta‐analysis, using the 2020 Synthesis without meta‐analysis (SWiM) in systematic reviews reporting guideline (Campbell 2020). We used the SWiM reporting guideline to ensure transparent reporting of our narrative synthesis, including how we grouped studies, metrics, and methods used for synthesis, data presentation, and presentation of limitations.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses, using the statistical test for planned subgroup differences, however, we had too few trials, small sample sizes, and insufficient data available to do so. See Appendix 2 for subgroup details.
Sensitivity analysis
We planned to conduct sensitivity analyses. However, the number of trials, sample sizes, and data available were insufficient to do so. See Appendix 2 for details.
Summary of findings and assessment of the certainty of the evidence
We summarized the results of each comparison (behavioral interventions versus usual care; physical activity interventions versus usual care), for the primary outcomes (objective and subjective measures of sleep), and the secondary outcomes (participant or parent satisfaction) in Table 1 and Table 2. We created the tables using GRADEpro GDT and Review Manager 5 software (GRADEpro GDT; Review Manager 2020). Using the GRADE approach, two review authors independently assessed the certainty of the body of evidence for each outcome as high, moderate, low, or very low, according to the presence of the following criteria:
limitations in design and implementation;
indirectness of evidence;
unexplained heterogeneity or inconsistent results;
imprecision of results; and
high risk of publication bias.
We provided a narrative description of important, clinically‐relevant findings.
Results
Description of studies
Results of the search
We ran the searches in May 2018, August 2019, October 2020, and December 2021. We retrieved a total of 79,991 records and imported them into Covidence for screening; we identified and discarded 3435 duplicates (Covidence). We screened the titles and abstracts of 76,556 records, and excluded 75,976 irrelevant records. We assessed 580 full‐text reports and excluded 567 that did not meet the inclusion criteria. We identified 10 included studies, and three studies that are awaiting classification (Beardslee 1974; Ctri 2020; NCT03453814). The flow of studies for this review is shown in Figure 1.
Included studies
We included 10 studies in this review (Cone 2014; Field 1992; Hinds 2007a; Jacobs 2016; Papaconstantinou 2018; Potasz 2010; Rennick 2018; Rogers 2019; White 1983; White 1990). One study was only available as a conference abstract (Potasz 2010). The included studies were published between 1983 and 2019.
Study types
Eight of the included studies were parallel trials, randomized (RCT) at the individual level (Field 1992; Hinds 2007a; Jacobs 2016; Papaconstantinou 2018; Potasz 2010; Rennick 2018; White 1983; White 1990). One study was a cross‐over trial (Cone 2014), and one was a cluster‐randomized trial (Rogers 2019).
Participants and settings
In total, there were 528 participants in the included studies. Sample sizes ranged from 6 (Cone 2014), to 139 (Potasz 2010).
Seven studies were conducted in the United States (Cone 2014; Field 1992; Hinds 2007a; Jacobs 2016; Rogers 2019; White 1983; White 1990), two in Canada (Papaconstantinou 2018; Rennick 2018), and one in Brazil (Potasz 2010).
The hospital setting varied between studies, and included an inpatient burn unit (Cone 2014), inpatient psychiatric unit (Field 1992), inpatient oncology unit (Hinds 2007a; Jacobs 2016; Rogers 2019), inpatient general pediatric unit (Papaconstantinou 2018; Potasz 2010; White 1983; White 1990), and a pediatric ICU (Rennick 2018). These settings reflected the populations of focus: children and adolescents with burns; with psychiatric disorders; those who were critically ill; those who were on oncology units, including those with central nervous system tumors; on general pediatric units; and those admitted for surgery.
Given the mix of included inpatient settings, the age of participants across studies ranged from 4 years to 22 years. Studies including participants 18 years and over, and children under 18; all were conducted in a pediatric inpatient setting.
Interventions
The most commonly studied category of non‐pharmacologic intervention (8 studies) was behavioral interventions (Cone 2014; Field 1992; Jacobs 2016; Papaconstantinou 2018; Rennick 2018; Rogers 2019; White 1983; White 1990). Two studies investigated physical activity interventions (Hinds 2007a; Potasz 2010).
No studies reported a focused environmental intervention, however, the multicomponent relaxation interventions did incorporate elements that included quiet time at night (Papaconstantinou 2018; Rennick 2018; Rogers 2019). There were no studies of complementary therapies, including acupuncture or acupressure.
The non‐pharmacologic sleep promotion interventions investigated in the included studies were heterogenous in the methods, timing, frequency, duration, and components; and ranged from short, one‐time interventions (touch therapy [Cone 2014]) to the entire hospital admission (Rennick 2018). The cross‐over trial had no specified washout period between intervention periods (Cone 2014).
1. Behavioral interventions
Multicomponent relaxation intervention
Three studies used a combination of behavioral approaches to develop a multicomponent relaxation intervention (Papaconstantinou 2018; Rennick 2018; Rogers 2019).
Papaconstantinou 2018 examined the effect of a Relax to Sleep intervention on sleep in general pediatric, surgery, or cardiology units. The Relax to Sleep intervention is comprised of one‐on‐one education for parents, with information about sleep, age, and developmentally appropriate instructions to train children in the use of diaphragmatic breathing exercises. Younger children were given a storybook to facilitate breathing exercises, and older children, a music file with a relaxation script; the exercises were practiced with the researcher. Guidance for parents included the importance of exposing their child to bright light each day.
Rennick 2018 investigated the effect of a pediatric intensive care unit (PICU) soothing intervention, consisting of parental comforting (touch and reading), followed by a quiet period of music. A fleece headband was also offered to deliver soft music, or just reduce external noise after the reading session, for up to one hour. The intervention continued throughout the PICU and hospital ward stay.
In Rogers 2019, the multicomponent intervention aimed at relaxation included age‐appropriate sleep education and relaxation training on Day 0, followed by a parental‐implemented relaxation technique (book, massage). Stimulus control measures included measures to decrease nighttime disrupters, including 90‐minute protected periods for sleep with bundled care.
Massage
Two studies investigated the effect of massage on sleep outcomes.
In Field 1992, massage was administered for 30 minutes a day, for a duration of 5 days.
Jacobs 2016 administered massage 20 to 30 minutes a day, for two to three nights, after a baseline phase of two nights.
Parent‐recorded bedtime stories
Two studies investigated the effect of parent‐recorded bedtime stories on sleep (White 1983; White 1990).
In White 1983, parents recorded a 10‐minute bedtime story, which was played for the children in the intervention group on nights two and three, after one night of baseline.
The interventions in White 1990 consisted of (1) parent‐recorded story with parent absent; (2) stranger‐recorded story with parent absent; (3) no story and parent absent; (4) no story and parent present (convenience sample). Stories were only played on night two. For both studies, parents had the option to add individualized comments, or modify the story as they deemed fit.
Touch therapies
One cross‐over study examined the use of healing touch, which consisted of non‐invasive and gentle use of the practitioner’s hands, either directly on the clothed body or slightly above the child's body (Cone 2014). The touch therapy procedure was comprised of three techniques: (1) magnetic clearing (15 to 30 full length, smooth, continuous passes with both hands, one to six inches from the body); (2) mind clearing, with hands placed lightly on, or above, several sites around face, neck, and forehead; (3) chakra connection involved the practitioner placing their hands on the major and minor chakras of the body. Participants received both the touch therapy and usual care on successive nights; the order of intervention was randomized.
2. Physical activity interventions
Two studies investigated the effect of physical activity on sleep (Hinds 2007a; Potasz 2010)
Enhanced Physical Activity
Hinds 2007a explored an enhanced physical activity intervention with a stationary bicycle‐style exerciser, which was used for 30 minutes twice daily, for two to four days of hospitalization.
Play
In a conference abstract, Potasz 2010 reported the effect of play versus no play, where play included a visit to a toy library twice a day for one hour at a time, and taking toys back to the room, where trained professional facilitated activities.
Comparators
All studies compared the intervention to usual care (standard clinical care), with the exception of White 1990, in which the control group was parent present, and no story.
Outcomes
Included studies investigated the effect of non‐pharmacologic sleep promotion interventions on objective or subjective sleep outcomes, or both. None of the trials measured all outcomes relevant to this review.
Objective sleep assessment
One study used polysomnography to measure total sleep time, sleep efficiency, sleep latency, wake after sleep onset, % time in non‐rapid eye movement sleep stage 1 (N1), N2, N3, rapid eye movement (REM), and total minutes of N1, N2, N3, and REM (Cone 2014).
Five studies used actigraphy (Hinds 2007a; Jacobs 2016; Papaconstantinou 2018; Rennick 2018; Rogers 2019). The actigraphic variables reported by these studies varied. Hinds 2007a reported 24‐hour sleep efficiency and total sleep time; while Jacobs 2016 explored those metrics in addition to nighttime‐specific total sleep time, sleep efficiency, wake after sleep onset, and long sleep episode. In Papaconstantinou 2018, sleep metrics were measured by daytime and nighttime periods (total sleep time, longest sleep period), in addition to nighttime awakenings, and wake after sleep onset. Parameters measured in Rennick 2018 included both daytime and nighttime sleep minutes, and nighttime arousals on the ward and at home; actigraphy was not used in the PICU due to concerns that sedatives and immobility might affect measures. Rogers 2019 reported daytime and nighttime percent sleep, mean duration of sleep episodes, longest sleep episode, mean duration of wake episodes, wake episodes, and longest wake episode.
Subjective sleep assessment
Five studies reported use of subjective sleep assessment (Field 1992; Papaconstantinou 2018; Potasz 2010; White 1983; White 1990). Field 1992 observed videos recorded during nighttime sleep, and coded them for active sleep versus awake, and lighting quietly, awake, and active. Papaconstantinou 2018 reported the effect of their intervention on the Children's Sleep Habits Questionnaire (Owens 2000), which was completed at baseline and five to seven days after hospital discharge. The conference abstract by Potasz 2010 reported use of sleep logs, without additional information about validation or reliability. Finally, two studies used the Sleep Onset Latency Behavior Catalog and Falling Asleep Behavioral Inventory, both of which comprised 54 different falling asleep behaviors to characterize participants' sleep behavior, for 45 minutes on each night of observation (White 1983; White 1990).
Participant/parent satisfaction
Two studies reported participant, or parental satisfaction, or both, with the non‐pharmacologic intervention used. Jacobs 2016 used an end‐of‐study questionnaire, using Likert‐scale responses on the perception of massage, and how the participant slept and felt after massage, along with open‐ended questions. In Rennick 2018, parental perception about the intervention was measured by research assistant observation, and recoding of child and parent responses.
Funding of studies
Eight studies were funded by grants (Cone 2014; Field 1992; Hinds 2007a; Papaconstantinou 2018; Rennick 2018; Rogers 2019; White 1983; White 1990); two provided no information on funding (Jacobs 2016; Potasz 2010). No sources of corporate funding were reported.
Excluded studies
We excluded 518 reports following full‐text review of 529 reports; we removed 17 because they were duplicates. We describe the reasons for excluding 21 studies in the Characteristics of excluded studies table. The most common reasons for excluding studies were observational study design (N = 8), and no relevant outcomes for this review (N = 5).
Risk of bias in included studies
We assessed risk of bias using the Cochrane RoB 1 tool. Please see the Characteristics of included studies tables for further details. Figure 2 and Figure 3 display the risk of bias summary figures.
2.

Risk of bias graph summarizing the risk of bias across included studies
3.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study
Green circle: low risk of bias Yellow: unclear risk of bias Red circle: high risk of bias
Allocation
Random sequence generation
Out of 10 studies, we assessed seven at low risk of bias for random sequence generation (Cone 2014; Field 1992; Hinds 2007a; Papaconstantinou 2018; Rennick 2018; Rogers 2019; White 1983). These studies all had clear descriptions of the randomization procedures. Two studies did not provide specifics on their randomization strategy and we classed them at unclear risk of bias (Jacobs 2016; Potasz 2010). We assessed one study at high risk of bias, because only children whose parents agreed not to stay at the bedside were randomized; if parents wanted to be present, they were allocated to the 'parent present' group (White 1990).
Allocation concealment
We rated studies at low risk of bias for allocation concealment if there were sufficient details for randomization of the sequence. We assessed six studies at low risk of bias (Cone 2014; Field 1992; Hinds 2007a; Papaconstantinou 2018; Rennick 2018; Rogers 2019; White 1983). Allocation concealment was unclear in two studies, due to insufficient information (Jacobs 2016; Potasz 2010). We assessed one study at high risk of bias as allocation could be dependent on parental presence at the bedside (White 1990).
Blinding
Performance bias
We assessed all 10 included studies at high risk of performance bias because of the inability and challenge of blinding participants and personnel to the intervention in all cases (Cone 2014; Field 1992; Hinds 2007a; Jacobs 2016; Papaconstantinou 2018; Potasz 2010; Rennick 2018; Rogers 2019; White 1983; White 1990).
Detection bias
We assessed only two studies at low risk of bias for detection because the outcomes assessors were adequately blinded (Jacobs 2016; Papaconstantinou 2018). These studies relied on objective methods of assessment that would not be affected by knowledge of the intervention assignment. We assessed three studies at unclear risk of bias, due to inadequate information (Cone 2014; Field 1992; Potasz 2010). We assessed four studies at high risk of detection bias, because all of them used subjective assessment of sleep outcomes, and there was potential knowledge of the intervention assignment (Hinds 2007a; Rennick 2018; Rogers 2019; White 1983; White 1990).
Incomplete outcome data
We assessed five studies at low risk of attrition bias, because they reported no or minimal loss to follow‐up after enrollment (Cone 2014; Hinds 2007a; Papaconstantinou 2018; Rennick 2018; Rogers 2019). There was insufficient information to make a determination of bias for three studies (Jacobs 2016; Potasz 2010; White 1990). We assessed two studies at high risk of bias (Field 1992; White 1983). White 1983 reported 13 dropouts due to early discharge or parental decision to room‐in; Field 1992 reported that 32% of sleep videos were unavailable, due to compliance or technical difficulties.
Selective reporting
We did not assesses any studies at high risk of selective reporting bias. We assesses five studies at unclear risk of bias due to insufficient information in the text (Field 1992; Hinds 2007a; Potasz 2010; Rennick 2018; White 1990); and five studies at low risk of bias, because they fully and completely reported all prespecified outcomes (Cone 2014; Jacobs 2016; Papaconstantinou 2018; Rogers 2019; White 1983).
Other potential sources of bias
We assessed four studies at unclear risk of bias from other potential sources; three due to insufficient information (Potasz 2010; White 1983; White 1990), and one because the order of touch therapy in the cross‐over study may have affected overall sleep time, due to insufficient washout time (Cone 2014).
Effects of interventions
The summary of findings for both behavioral interventions and physical activity interventions are presented in Table 1 and Table 2. We identified considerable between‐study clinical heterogeneity of participant populations, measurement tools and metrics used, study duration, and timing of outcome assessment. Thus, we were unable to pool the results in a meta‐analysis for any of the outcomes. When possible, we calculated between‐group differences and 95% CIs for individual studies. When data were insufficient to provide this information, we presented the data as described in Data synthesis, using Synthesis without meta‐analysis (SWiM) reporting guidelines (Campbell 2020), and the Cochrane Handbook (McKenzie 2021). We found inconsistent effects for all non‐pharmacologic sleep promotion interventions on objective and subjective measures of sleep.
Behavioral interventions versus usual care
Primary outcomes
1. Changes in objective and validated measures of sleep (polysomnography (PSG) or actigraphy)
We rated the certainty of evidence for all of the outcome changes in objective and validated measures of sleep as low.
Multicomponent relaxation intervention
Table 3 presents results for the three included studies with sufficient data that examined multicomponent relaxation interventions on an objective measure of sleep, using actigraphy (Papaconstantinou 2018; Rennick 2018; Rogers 2019). We did not synthesize these results because there were inconsistencies in the reporting of actigraphy metrics across studies, and small sample sizes, which did not allow us to translate medians (interquartile range [IQR]) to means (standard deviation [SD]) with confidence.
1. Multicomponent relaxation interventions versus usual care: objective sleep outcomes assessed using actigraphy.
| Study | Sleep outcomes | Mean (95% CI) | P value | ||
| Relax to sleep (N = 20) | Usual care (N = 23) | Group difference | |||
| Papaconstantinou 2018 | Total daytime sleep (in minutes) | 76.9 (50 to 106.9) | 113.6 (78.1 to 149.2) | −36.7 (−83.5 to 10.10) | 0.121 |
| Total nighttime sleep (in minutes) | 419.3 (385.7 to 453) | 369.7 (323.7 to 415.7) | 49.64 (‐7.19 to 106.5) | 0.085 | |
| Nocturnal awakenings (number) | 14.67 (11.94 to 17.40) | 14.69 (12.64 to 16.73) | ‐0.02 (‐3.27 to 3.23) | 0.989 | |
| Longest nocturnal sleep period (in minutes) | 116.6 (85.28 to 147.9) | 100.9 (81.02 to 120.8) | 15.65 (‐19.33 to 50.62) | 0.372 | |
| ‐ | Mean (SD) | Mean (95% CI) | P value | ||
| Wake after sleep onset (in minutes) | 163.9 (93.63) | 209.2 (121.2) | ‐45.34 (‐112.8 to 22.15) | 0.182 | |
| Rennick 2018 | Median (IQR) | ||||
| PICU soothing (N = 9) | Usual care (N = 6) | ||||
| Total daytime sleep (in minutes) | 300.6 (163.9 to 365.7) | 239.1 (169.63 to 374.6) | |||
| Total nighttime sleep (in minutes) | 510 (434 to 550.8) | 526 (413.4 to 596.1) | |||
| Arousals (number) | 41.33 (30.3 to 51.2) | 31 (22.1 to 34.4) | |||
| Rogers 2019 | Mean (± SD) | Cohen d | |||
| Intervention (N = 17) | Usual care (N = 16) | ||||
| Daytime percentage of sleep (%) | 17.3 (± 10.3) | 56.6 (± 10.5) | 0.114 | ||
| Daytime mean duration of sleep episodes (in minutes) | 16.1 (± 5.9) | 16.9 (± 6.2) | 0.132 | ||
| Daytime sleep episodes (number) | 4.6 (± 2.7) | 5.5 (± 3.8) | 0.273 | ||
| Daytime longest sleep episode (in minutes) | 41.8 (± 14.8) | 45.5 (± 16.3) | 0.238 | ||
| Daytime mean duration wake episodes (in minutes) | 167.2 (± 115.1) | 159.2 (± 126.8) | 0.066 | ||
| Daytime wake episodes (number) | 5.8 (± 2.4) | 7.2 (± 3.9) | 0.432 | ||
| Daytime longest wake episode (in minutes) | 361.1 (± 124.6) | 342.1 (± 139.6) | 0.144 | ||
| Nighttime percentage of sleep (%) | 56.6 (± 10.5) | 58.3 (± 12.4) | 0.157 | ||
| Nighttime mean duration sleep episodes (in minutes) | 23.1 (± 8.5) | 26.3 (± 14.5) | 0.269 | ||
| Nighttime sleep episodes (number) | 12.3 (± 2.2) | 12.5 (± 3.0) | 0.076 | ||
| Nighttime longest sleep episode (in minutes) | 87.6 (± 32.0) | 92.9 (± 26.0) | 0.182 | ||
| Nighttime mean duration wake episodes (in minutes) | 17.1 (± 5.4) | 17.9 (± 9.4) | 0.104 | ||
| Nighttime wake episodes (number) | 10.7 (± 2.4) | 10.9 (± 2.6) | 0.080 | ||
| Nighttime longest wake episode (in minutes) | 104.8 (± 26.8) | 112.0 (± 60.0) | 0.155 | ||
CI: confidence intervals; N: number; SD: standard deviation.
Papaconstantinou 2018 found no clear difference in total nighttime sleep between the Relax to Sleep Intervention (mean difference [MD] 50 minutes, 95% confidence interval [CI] ‐7.19 to 106.5; 1 study; 48 participants) and usual care (mean total sleep duration 370 minutes); and no clear difference in the longest continuous nighttime sleep period (MD 16 minutes, 95% CI ‐19.33 to 50.62). They also suggested there may be a decrease in wake after sleep onset (WASO) with the intervention (MD ‐45 minutes, 95% CI ‐112.8 to 22.15).
A second study, Rennick 2018, with 20 participants, suggested PICU soothing may slightly increase median sleep duration during daytime hours over usual care (300.62 minutes, IQR 163.9 to 365.7 versus 240 minutes, IQR 170 to 375). There may be no clear difference between PICU soothing and usual care on median sleep duration at night (510 minutes, IQR 434 to 550.8 versus 526 minutes, IQR 413.44 to 596.06).
Rogers 2019 (33 participants) suggested that there may be no clear difference across the study period for any daytime or nighttime sleep variables in children receiving the multicomponent intervention compared to controls, including percentage of sleep, number of sleep episodes, mean duration of sleep episodes, longest sleep episode, mean duration of wake episodes, number of wake episodes, or longest wake episodes. See Table 3 for breakdown of results.
Touch Therapy
One cross‐over RCT with 10 participants investigated the effect of healing touch compared to usual care on sleep, using polysomnography (Table 4). The study authors reported that healing touch may be more effective than usual care for total sleep time (391 minutes, IQR 251 to 467 versus 331 minutes, IQR 268 to 373; P = 0.02). There could also be an increase in sleep efficiency with healing touch (76%, IQR 53 to 90 versus 66%, IQR 55 to 78; P = 0.04); more REM periods (4.5, IQR 2 to 5 versus 3.5, IQR 2 to 4; P = 0.03), and more N2 minutes (213 minutes, IQR 136 to 236 versus 160, IQR 107 to 204; P = 0.03). However, there may be no difference in sleep latency, number of awakenings, WASO, N3 minutes, or the proportion of time spent in each sleep stage (Cone 2014).
2. Healing touch versus usual care: objective sleep outcomes assessed using polysomnography.
| Study | Objective sleep outcome | Sleep outcome: median (interquartile range) | P value | |
| Healing touch (N = 10) | Usual care (N = 10) | |||
| Cone 2014 | Total sleep time (in minutes) | 391 (251 to 467) | 331 (268 to 373) | 0.02 |
| Sleep efficiency (%) | 76 (53 to 90) | 66 (55 to 78) | 0.04 | |
| Sleep latency (in minutes) | 8.4 (2.8 to 34.9) | 17.2 (13.5 to 48) | 0.20 | |
| Awakenings (number) | 32 (25 to 38) | 30 (23 to 32) | 0.42 | |
| Wake after sleep onset | 99 (46 to 210) | 107 (53 to 194) | 0.77 | |
| REM periods (number) | 4.5 (2.0 to 5.0) | 3.5 (2.0 to 4.0) | 0.03 | |
| N1 stage (in minutes) | 26 (21 to 32) | 28 (18 to 30) | 1.0 | |
| N2 stage (in minutes) | 213 (136 to 236) | 160 (107 to 204) | 0.03 | |
| N3 stage (in minutes) | 63 (29 to 93) | 34 (8 to 120) | 1.0 | |
| REM stage (in minutes) | 83 (62 to 91) | 60 (40 to 86) | 0.20 | |
| N1 (% of normal) | 212 (174 to 241) | 304 (197 to 531) | 0.11 | |
| N2 (% of normal) | 110 (103 to 136) | 123 (85 to 132) | 0.23 | |
| N3 (% of normal) | 67 (44 to 110) | 49 (31 to 125) | 0.43 | |
| REM (% of normal) | 95 (82 to 101) | 86 (47 to 116) | 0.04 | |
N1, N2, N3: non‐rapid eye movement sleep stage 1, 2, 3; REM: rapid eye movement
Massage
Table 5 presents the results of Jacobs 2016 (34 participants), which investigated the effect of massage compared to standard care on actigraph sleep parameters. There was insufficient information to report univariate statistics, and SDs or CIs, thus we presented the results of the mixed‐effect model. The mixed‐effect model showed that total sleep time (TST) may be similar for the massage group (391 minutes at baseline plus 53 minutes at end) compared to standard care (431 minutes at baseline minus 30 minutes at end), as was sleep efficiency.
3. Massage versus usual care: objective sleep outcomes assessed using actigraphy.
| Study | Objective sleep outcome | Main effect of intervention (beta) | P value |
| Jacobs 2016 | Total sleep time (in minutes) | ‐60.6 | 0.22 |
| Total nighttime sleep (in minutes) | ‐26.6 | 0.51 | |
| Sleep efficiency (%) | ‐8.0 | 0.12 | |
| Nighttime wake episodes (number) | ‐1.3 | 0.59 | |
| Wake after sleep onset nighttime (in minutes) | 39.6 | 0.35 | |
| Nighttime long sleep episode (in minutes) | ‐1.1 | 0.35 |
Mixed‐effects model controlling for age, gender, and reason of admission. Measures of variation and comparisons between interventions were not provided for the univariate analysis.
2. Changes in subjective measures of sleep (parent/participant surveys and validated sleep assessment tools)
We rated the certainty of the evidence contributing to this outcome as very low, meaning we are very uncertain about the findings.
Multicomponent relaxation intervention
One study reported the effect of a multicomponent relaxation intervention (Relax to Sleep) compared to usual care on Children's Sleep Habits Questionnaire (CSHQ) scores, five to seven days after discharge from the hospital (Papaconstantinou 2018). The Relax to Sleep program may reduce scores on the CSHQ more than usual care (MD ‐3.2, 95% CI ‐6.21 to ‐0.19; P = 0.037; 1 study, 48 participants), but the evidence is very uncertain.
Massage
One study, Field 1992, reported the effect of massage versus usual care on sleep, using video observation (72 participants). Videos were coded for (1) quiet sleep (no body movements), (2) active sleep (body movement), (3) awake and lying quietly, and (4) active and awake. Only 68% of tapes were available, due to noncompliance and technical issues. The findings suggested that massage may increase the per cent of time asleep over five days (MD 11.6 minutes; P = 0.01) more than the control group (MD 2.4; P = ns), and may decrease nighttime wakefulness (% time awake) over the same period (MD ‐11.2; P = 0.05) over usual care (MD 2.3). However we are very uncertain about the evidence.
Bedtime stories
Two studies (112 participants) from the same author group observed and reported the effect of bedtime stories on sleep behaviors, with the Sleep Onset Behavior Catalog (White 1983; White 1990).
In White 1983, the authors compared parent‐recorded bedtime story versus no bedtime story. They found that parent‐recorded bedtime stories may lead to longer mean duration of sleep (measured on the third night) compared to usual care (23 minutes versus 15 minutes; 18 participants). Time to fall asleep might also be shorter following parent‐recorded bedtime stories (22 minutes versus 27 minutes) versus no story. The authors neither reported the P values for these results nor stated if these differences between the groups were statistically significant.
White 1990 (94 participants) evaluated the effect of recorded bedtime stories on sleep latency in four groups, using the Sleep Onset Behavior Catalog: parent‐recorded/parents absent versus stranger‐recorded/parents absent; versus no story/parents absent; versus no story/parents present. Children listening to a parent‐recorded story may have longer sleep latencies (mean 57.5 minutes) compared to when a parent is present (43.5 minutes); both groups had longer sleep latency than those who had the stranger‐recorded story, and no story/parent absent (P < 0.001). However the evidence is very uncertain.
Secondary outcome
Participant or parent satisfaction, or both
The certainty of evidence for participant or parent satisfaction, or both, was rated as very low.
One study investigating the effects of massage versus usual care suggested that satisfaction may be increased in the massage group compared to usual care (Jacobs 2016; 34 participants). They reported that 87% (13/15) of participants felt they slept better, and most parents in the study (92%; 11/12) reported that they wanted their child to receive massage in the future.
Another study of multicomponent relaxation intervention versus usual care in children in pediatric ICU reported that parents thought the music, touch, and reading components of the intervention were acceptable and feasible, with positive effects on their children (Rennick 2018; 20 participants).
None of the studies of behavioral interventions evaluated cost‐effectiveness, delirium incidence or delirium‐free days at hospital discharge, length of mechanical ventilation, length of hospital stay, or mortality.
Physical activity interventions versus usual care
Primary outcome
Changes in objective and validated measures of sleep (PSG or actigraphy)
Enhanced physical activity
One study investigated enhanced physical activity compared to usual care (Hinds 2007a; 29 participants). The results suggested that there may be no clear difference between the groups in either total sleep time or sleep efficiency, shown in Table 6. We rated the certainty of this evidence as low.
4. Enhanced physical activity versus usual care: objective sleep outcomes measured using actigraphy.
| Study | Objective sleep outcome | Mean (SD) | P value | |
| Enhanced physical activity (N = 12) | Usual care (N = 15) | |||
| Hinds 2007a | Total sleep time (in minutes) | 606.3 (144.6) | 562.3 (122.4) | 0.47 |
| Sleep efficiency (%) | 73 (15.6) | 71 (16.7) | 0.85 | |
N: number; SD: standard deviation.
Changes in subjective measures of sleep (parent/participant surveys and validated sleep assessment tools)
Play activities
One study investigated play versus a no‐play group to promote sleep, using sleep logs (Potasz 2010; 139 participants). For this study, we only have a conference abstract and as such, there is insufficient information to determine effect estimates between groups. The findings provided in the abstract suggest that there may be inconsistent findings across age groups and gender. The authors reported that TST in the no‐play group was 49% higher in four‐ to seven‐year‐old boys, 9.5% higher in 7.1‐ to 11‐year‐old boys, and 22% higher in 11.1‐ to 14‐year‐old boys. However, girls from the two youngest age groups may have more sleep in the play group (4% higher for 4‐ to 7‐year‐old girls, and 14% higher for 7.1‐ to 11‐year‐old girls); girls in the oldest age group had 46.1% more sleep in the no‐play group group. Boys in the usual care group had more daytime sleep across all age groups (4 to 7 years: 26%; 7.1 to 11 years: 46%; 11.1 to 14 years: 36%), similar to girls in the oldest two age groups of the no‐play group (7.1 to 11 years: 23%; 11.1 to 14 years: 59%). In contrast, the youngest girls had more sleep during the day in the play group (13%). We rated the certainty of this evidence as very low.
Secondary outcomes
None of the studies of physical interventions evaluated participant or parent satisfaction, cost‐effectiveness, delirium incidence or delirium‐free days at hospital discharge, length of mechanical ventilation, length of hospital stay, or mortality.
Discussion
Summary of main results
Our review sought to assess the effects of non‐pharmacologic sleep promotion interventions for hospitalized children and adolescents. All included studies took place in inpatient pediatric settings, including a pediatric intensive care unit (ICU), and burn, oncology, general pediatric, surgical, cardiology, and psychiatric units. The types of interventions varied widely, including behavioral interventions, such as multicomponent relaxation interventions, massage, and touch therapy; and physical activity interventions, such as exercise bicycle and age‐appropriate play. The timing, duration, and frequency varied across interventions, anywhere from one‐time interventions to the entire duration of hospital admission.
We included 10 studies, with a total of 528 participants, who ranged in age from 3 years to 22 years. Compared with usual care or controls, behavioral and physical activity interventions showed inconsistent effects across objective and subjective metrics of sleep quality and quantity. A study of touch therapy found it may improve total sleep time, sleep efficiency, and number of REM periods, with low‐certainty evidence. Other studies, including multicomponent relaxation interventions, bedtime stories, massage, physical activity, and play interventions demonstrated variable effects on sleep quality and duration, with low‐ or very low‐certainty evidence.
Overall completeness and applicability of evidence
It is remarkable that none of the included studies enrolled children under three years of age, who have a high burden of hospitalization and are undergoing neurodevelopment most rapidly. For example, recent point prevalence studies in the USA, Canada, and Europe found that two‐thirds of all children admitted to pediatric ICUs for more than 72 hours are under the age of three years (Choong 2021; Ista 2020; Kudchadkar 2020). The majority of studies in this review evaluated the effect of non‐pharmacologic sleep promotion interventions in school‐age children and adolescents, identifying major knowledge gaps for a vulnerable infant and toddler inpatient population. Thus, the omission of children in younger age groups limits generalizability of the findings. Also, none of the studies specifically addressed children with functional impairments, for whom specific interventions may not be applicable (i.e. lighting interventions in blind children, noise interventions for deaf children, physical activity interventions for children with limited mobility). However, the included studies did represent a wide spectrum of pediatric inpatient settings, which is a strength of the evidence.
None of the studies focused on specific environmental interventions, such as noise reduction and lighting optimization, which may be more feasible and generalizable across settings than multicomponent interventions. None of the studies investigated important patient‐centered outcomes, including delirium, duration of mechanical ventilation, cost, and mortality.
Finally, most of the included studies had a small number of participants, and the largest study was only retrievable as a conference abstract, limiting our ability to evaluate those data.
Quality of the evidence
We used the GRADE process to judge the certainty of the evidence. We judged the evidence to be of low‐ and very low‐certainty, due to limitations in study design (high risk of performance bias), low numbers of included trials, and small sample size limitations. There was variable reporting among trials for random sequence generation and concealment of allocation. Both objective and subjective assessments of sleep quality and quantity were affected by substantial variability in the types of outcomes measured. There were also substantial differences in populations and interventions, which decreased our certainty of the evidence for behavioral interventions. The 10 included studies largely focused on different populations, including children admitted to inpatient psychiatry; children with oncologic diagnoses, including central nervous system tumors; critically ill children; and children in general pediatric and surgical units, which may limit generalizability of the interventions across pediatric inpatients (inconsistency). Finally, all studies were single‐center, which limits generalizability across different hospital settings.
Potential biases in the review process
We are confident that this review comprehensively summarizes the currently available evidence from randomized controlled trials of non‐pharmacologic sleep promotion interventions for pediatric inpatients. Two review authors independently screened titles and abstracts and full‐text reports, extracted data, and assessed risk of bias and GRADE levels. We followed up on all ongoing trials to delineate availability of results and publication. We did not exclude any potentially relevant trials, and took all measures to minimize the introduction of additional bias. However, there remains a possibility that we failed to identify relevant unpublished trials contributing positive or negative results, as well as studies not indexed in English language databases. There is also the potential of non‐financial, unconscious bias, due to author interest in this research and clinical topic, as evidenced by previous publications relevant to the topic (Kudchadkar 2009; Kudchadkar 2014a; Kudchadkar 2014b; Kudchadkar 2016a).
Agreements and disagreements with other studies or reviews
We did not identify other reviews by other authors for this research question. One systematic review focused on sleep in critically ill children (Kudchadkar 2014a). It was limited to the pediatric ICU setting and was broader in scope, including all descriptive studies, interventions, and outcomes relevant to sleep in the pediatric ICU. Another systematic review focused specifically on non‐pharmacologic sleep promotion interventions in preterm infants in neonatal ICUs, a population that was specifically excluded in this review (Liao 2018a). None of the trials included in the current Cochrane Review were included in Kudchadkar 2014a or Liao 2018a.
Authors' conclusions
Implications for practice.
The randomized controlled trials included in this Cochrane Review provided insufficient information for review authors to determine the most effective non‐pharmacologic interventions for sleep promotion in hospitalized children. The available evidence is limited, and of low‐ or very low‐certainty; however, it provides a foundation for the development of rigorous studies in vulnerable patient populations. Importantly, a critically important age group and patient population, infants and toddlers admitted to any inpatient setting were not represented in any of the trials, since we did not consider infants in neonatal ICUs for this review. Furthermore, none of the trials evaluated important participant‐centered outcomes, including delirium, duration of mechanical ventilation, and mortality.
Implications for research.
Future clinical trials investigating interventions for non‐pharmacologic sleep promotion in the pediatric inpatient setting must prioritize the inclusion of hospitalized infants and young children (ward and ICU) in addition to other age groups, to enable analysis of efficacy according to age. A focus on these young patients will help identify best practices for sleep promotion in a vulnerable population undergoing rapid neurocognitive and physical development.
While the study of multifaceted interventions is pragmatic, feasibility and sustainability of interventions is key. Thus, it would also be beneficial to investigate single interventions, such as noise reduction and lighting optimization versus usual care to determine the most effective combinations for multifaceted interventions. Future trials should also prioritize use of objective assessment tools and measures. Polysomnography and actigraphy should be considered the first line for sleep measurement, with consistent reporting of validated measures for both sleep quality and quantity. However, the challenges of using and interpreting actigraphy and polysomnography in acutely ill children must also be considered, with a focus on efficient methods for sleep measurement that do not interfere with care or a child's natural sleep pattern. Only validated subjective tools for sleep measurement should be used, when possible. Development of surveys to address gaps in infant and toddler sleep assessment is needed. Research must also evaluate the evolution and recovery of sleep patterns after transfer or discharge from the ICU. Finally, research focused on non‐pharmacologic sleep promotion interventions should include core child‐centered outcomes, such as delirium, duration of mechanical ventilation, cost, and mortality. While non‐pharmacologic sleep promotion may seem like a common sense approach to care, rigorous interprofessional research is needed to affect culture change in the pediatric inpatient setting to prioritize sleep hygiene.
History
Protocol first published: Issue 12, 2017
Acknowledgements
The preparation of the manuscript was supported by Cochrane Developmental, Psychosocial and Learning Problems; the Johns Hopkins University School of Medicine; the Johns Hopkins University Department of Anesthesiology and Critical Care Medicine; and the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded, in part, by grant number UL1 TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The content is solely the responsibility of the authors, and does not necessarily represent the official views of John Hopkins ICTR, NCATS, or the National Institutes of Health.
The Editorial Team is grateful to the following reviewers for their time and comments: Lauri A Linder, PhD, APRN, CPON, FAAN, University of Utah, College of Nursing, USA; Elizabeth Royle; and Renée A Shellhaas, MD, MS, University of Michigan, Department of Pediatrics (Division of Pediatric Neurology), USA.
The CRG Editorial Team is grateful to Victoria Pennick for copyediting this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials, in the Cochrane Library
Searched 11 April 2018, 27 March 2019, 30 October 2020, 12 December 2021
#1 MeSH descriptor: [Sleep] explode all trees
#2 MeSH descriptor: [Sleep Stages] explode all trees
#3 MeSH descriptor: [Sleep, REM] explode all trees
#4 MeSH descriptor: [Sleep Deprivation] explode all trees
#5 (sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence):ti,ab
#6 MeSH descriptor: [Circadian Rhythm] explode all trees
#7 "circadian"[tiab]
#8 (rest OR resting OR nap OR naps OR napping):ti,ab
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 MeSH descriptor: [Noise] explode all trees
#11 ("noise" OR "noises" OR "noisy"):ti,ab
#12 MeSH descriptor: [Sound] this term only
#13 ("sound" OR "sounds" OR "sound masking"):ti,ab
#14 MeSH descriptor: [Ear Protective Devices] explode all trees
#15 ("ear protective device" OR "ear protective devices" OR "earplug" OR "earplugs" OR "ear plug" OR "ear plugs" OR "earmuff" OR "earmuffs" OR "ear muff" OR "ear muffs" OR "headphone" OR "headphones" OR "head phone" OR "head phones" OR "hat" OR "hats" OR "music"):ti,ab
#16 MeSH descriptor: [Light] explode all trees
#17 (light*):ti,ab
#18 MeSH descriptor: [Eye Protective Devices] explode all trees
#19 ("eye protective device" OR "eye protective devices" OR "eye masks" OR "eye mask"):ti,ab
#20 MeSH descriptor: [Polysomnography] explode all trees
#21 ("polysomnography" OR "polysomnographic" OR "polysomnogram" OR "polysomnograms"):ti,ab
#22 MeSH descriptor: [Actigraphy] explode all trees
#23 ("actigraphy" OR "actigraphic" OR "actigram" OR “actigrams"):ti,ab
#24 MeSH descriptor: [Music Therapy] explode all trees
#25 ("musical" OR musicotherap* OR "song" OR "songs" OR lullab* OR sing OR sings OR "singing" OR "sang" OR sung):ti,ab
#26 MeSH descriptor: [Complementary Therapies] this term only
#27 MeSH descriptor: [Mind‐Body Therapies] this term only
#28 MeSH descriptor: [Aromatherapy] explode all trees
#29 MeSH descriptor: [Relaxation Therapy] explode all trees
#30 MeSH descriptor: [Biofeedback, Psychology] explode all trees
#31 MeSH descriptor: [Imagery (Psychotherapy)] explode all trees
#32 MeSH descriptor: [Therapeutic Touch] explode all trees
#33 MeSH descriptor: [Breathing Exercises] explode all trees
#34 MeSH descriptor: [Massage] explode all trees
#35 MeSH descriptor: [Acupressure] explode all trees
#36 MeSH descriptor: [Acupuncture] explode all trees "
#37 ("non‐pharmacological" OR massag* OR "acupressure" OR "acupuncture" OR aromatherapy OR complementary therap* OR alternative therap* OR mind‐body therap* OR biofeedback OR breathing exercise* OR imagery OR muscle relaxation OR relaxation therap* OR therapeutic touch):ti,ab
#38 (environmental intervention* OR "quiet time" OR "alarm modifications" OR "alarm modification"):ti,ab
#39 MeSH descriptor: [Social Support] explode all trees
#40 (social support):ti,ab
#41 MeSH descriptor: [Physical Therapy Modalities] explode all trees
#42 ("physical therapy" OR "physical therapies" OR "rehabilitation" OR "rehab"):ti,ab
#43 "Kangaroo Care" OR "Kangaroo Mother Care"
#44 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43
#45 #9 OR #44
#46 MeSH descriptor: [Intensive Care Units] explode all trees
#47 ("ICU" OR ICUs):ti,ab
#48 MeSH descriptor: [Intensive Care Units, Pediatric] explode all trees
#49 ("PICU" OR PICUs OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units"):ti,ab
#50 MeSH descriptor: [Intensive Care Units, Neonatal] explode all trees
#51 ("NICU" OR NICUs OR "neonatal unit" OR "neonatal units")ti,ab
#52 ("intensive care" OR "intensive therapy" OR "high dependency"):ti,ab
#53 MeSH descriptor: [Critical Care] explode all trees
#54 ("critical care"):ti,ab
#55 MeSH descriptor: [Burn Units] explode all trees
#56 ("burn units" OR "burn unit"):ti,ab
#57 MeSH descriptor: [Recovery Room] explode all trees
#58 ("recovery room" OR "recovery rooms"):ti,ab
#59 MeSH descriptor: [Coronary Care Units] explode all trees
#60 ("coronary care unit" OR "coronary care units" OR "cardiac care unit" OR "cardiac care units" OR "CCU"):ti,ab
#61 MeSH descriptor: [Hemodialysis Units, Hospital] explode all trees "Respiratory Care Units"[MeSH] OR
#62 ("respiratory care unit" OR "respiratory care units"):ti,ab
#63 MeSH descriptor: [Inpatients] explode all trees
#64 (hospitali* OR "inpatients" OR "inpatient" OR "in hospital" OR "hospital ward" OR "hospital wards"):ti,ab
#65 ("pediatric wards" OR "pediatric ward" OR "paediatric wards" OR "paediatric ward" OR "patient rooms" OR "patient room"):ti,ab
#66 MeSH descriptor: [Critical Illness] explode all trees
#67 ("critically ill" OR "critical illness" OR "critical illnesses" ):ti,ab
#68 MeSH descriptor: [respiration, artificial] explode all trees
#69 ("artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses" OR "acute care"):ti,ab
#70 MeSH descriptor: [Hemodialysis Units, Hospital] explode all trees
#71 ("hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit"):ti,ab
#72 ("post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit" OR "post anaesthesia care units" OR "PACU" OR "PACUs"):ti,ab
#73 #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72
#74 #45 AND #7
PubMed, US National Library of Medicine
Searched 11 April 2018, 27 March 2019, 30 October 2020, 12 December 2021
1. "Sleep"[Mesh] 2. "Sleep Stages"[Mesh] 3. "Sleep, REM"[Mesh] 4. "Sleep Deprivation"[Mesh] 5. (sleep[tiab] OR sleeping[tiab] OR slept[tiab] OR sleeps[tiab] OR sleepless[tiab] OR sleeplessness[tiab] OR sleepy[tiab] OR asleep[tiab] OR insomnia[tiab] OR insomniac[tiab] OR somnolent[tiab] OR somnolence[tiab])) 6. "Circadian Rhythm"[Mesh] OR "circadian"[tiab] 7. rest[tiab] OR resting[tiab] OR nap[tiab] OR naps[tiab] OR napping[tiab] 8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 9. "Noise"[Mesh] OR "noise"[tiab] OR "noises"[tiab] OR "noisy"[tiab] 10. "Sound"[Mesh:noexp] OR "sound"[tiab] OR "sounds"[tiab] OR "sound masking"[tiab] 11. "Ear Protective Devices"[Mesh] OR "ear protective device"[tiab] OR "ear protective devices"[tiab] OR "earplug"[tiab] OR "earplugs"[tiab] OR "ear plug"[tiab] OR "ear plugs"[tiab] OR "earmuff"[tiab] OR "earmuffs"[tiab] OR "ear muff"[tiab] OR "ear muffs"[tiab] OR "headphone"[tiab] OR "headphones"[tiab] OR "head phone"[tiab] OR "head phones"[tiab] OR "hat"[tiab] OR "hats"[tiab] OR "music"[tiab] 12. "Light"[Mesh] OR light*[tiab] OR "Eye Protective Devices"[Mesh] OR "eye protective device"[tiab] OR "eye protective devices"[tiab] OR "eye masks"[tiab] OR "eye mask"[tiab] 13. "Polysomnography"[Mesh] OR "polysomnography"[tiab] OR "polysomnographic"[tiab] OR "polysomnogram"[tiab] OR "polysomnograms"[tiab] OR "Actigraphy"[Mesh] OR "actigraphy"[tiab] OR "actigraphic"[tiab] OR "actigram"[tiab] OR “actigrams"[tiab] 14. "Music Therapy"[MeSH Terms] OR "musical"[tiab] OR musicotherap*[tiab] OR "song"[tiab] OR "songs"[tiab] OR lullab*[tiab] OR sing[tiab] OR sings[tiab] OR "singing"[tiab] OR "sang"[tiab] OR sung[tiab] 15. "Complementary Therapies"[Mesh:noexp] OR "Mind‐Body Therapies"[Mesh:noexp] OR "Aromatherapy"[Mesh] OR "Relaxation Therapy"[Mesh] OR "Biofeedback, Psychology"[Mesh] OR "Imagery (Psychotherapy)"[Mesh] OR "Therapeutic Touch"[Mesh] OR "Breathing Exercises"[Mesh] OR "Massage"[Mesh] OR "Acupressure"[Mesh] 16. "non‐pharmacological"[tiab] OR massag*[tiab] OR "acupressure"[tiab] OR aromatherapy[tiab] OR complementary therap*[tiab] OR alternative therap*[tiab] OR mind‐body therap*[tiab] OR biofeedback[tiab] OR breathing exercise*[tiab] OR imagery[tiab] OR muscle relaxation[tiab] OR relaxation therap*[tiab] OR therapeutic touch[tiab] 17. environmental intervention*[tiab] OR "quiet time"[tiab] OR "alarm modifications"[tiab] OR "alarm modification"[tiab] 18. "Social Support"[Mesh] OR social support[tiab] 19. "Physical Therapy Modalities"[Mesh] OR "physical therapy"[tiab] OR "physical therapies"[tiab] OR "rehabilitation"[tiab] OR "rehab"[tiab] 20. "Kangaroo Care"[All Fields] OR "Kangaroo Mother Care"[All Fields] 21. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 22. #8 OR #21 23. "Intensive Care Units"[Mesh] OR "ICU"[tiab] 24. "Intensive Care Units, Pediatric"[MeSH] OR "PICU"[tiab] OR "pediatric unit"[tiab] OR "pediatric units"[tiab] OR "paediatric unit"[tiab] OR "paediatric units"[tiab] 25. "Intensive Care Units, Neonatal"[MeSH] OR "NICU"[tiab] OR "neonatal unit"[tiab] OR "neonatal units"[tiab] 26. "intensive care"[tiab] OR "intensive therapy"[tiab] OR "high dependency"[tiab] OR "Critical Care"[Mesh] OR "critical care"[tiab] 27. "Burn Units"[Mesh] OR "burn units"[tiab] OR "burn unit"[tiab] 28. "Recovery Room"[MeSH] OR "recovery room"[tiab] OR "recovery rooms"[tiab] 29. "Coronary Care Units"[MeSH] OR "coronary care unit"[tiab] OR "coronary care units"[tiab] OR "cardiac care unit"[tiab] OR "cardiac care units"[tiab] OR "CCU"[tiab] 30. "Respiratory Care Units"[MeSH] OR "respiratory care unit"[tiab] OR "respiratory care units"[tiab] 31. hospitali*[tiab] OR "Inpatients"[Mesh] OR "inpatients"[tiab] OR "inpatient"[tiab] OR "in hospital"[tiab] OR "hospital ward"[tiab] OR "hospital wards"[tiab] 32. "pediatric wards"[tiab] OR "pediatric ward"[tiab] OR "paediatric wards"[tiab] OR "paediatric ward"[tiab] OR "patient rooms"[tiab] OR "patient room"[tiab] 33. "critically ill"[tiab] OR "Critical Illness"[Mesh] OR "critical illness"[tiab] OR "critical illnesses"[tiab] OR "acute illness"[tiab] OR "acute illnesses"[tiab] OR "acute care"[tiab] 34. "Hemodialysis Units, Hospital"[Mesh] OR "hemodialysis units"[tiab] OR "hemodialysis unit"[tiab] OR "haemodialysis units"[tiab] OR "haemodialysis unit"[tiab] 35. "post acute care unit"[tiab] OR "post anesthesia care unit"[tiab] OR "post anesthesia care units"[tiab] OR "post anaesthesia care unit"[tiab] OR "post anaesthesia care units" OR “PACU"[tiab] 36. #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 #34 OR #35 37. #22 AND #36 38. randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab] NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]) 39. #37 AND #38
Embase.com, Elsevier
Searched 11 April 2018, 27 March 2019 and 12 December 2021
1 'sleep'/de
2 'sleep stage'/de
3 'rem sleep'/de
4 'sleep deprivation'/de
5 'sleep':ab,ti OR 'sleeping':ab,ti OR 'slept':ab,ti OR 'sleeps':ab,ti OR 'sleepless':ab,ti OR 'sleeplessness':ab,ti OR 'sleepy':ab,ti OR 'asleep':ab,ti OR 'insomnia':ab,ti OR 'insomniac':ab,ti OR 'somnolent':ab,ti OR 'somnolence':ab,ti
6 'circadian rhythm'/de OR 'circadian rhythm':ab,ti
7 'rest':ab,ti OR 'resting':ab,ti OR 'nap':ab,ti OR 'naps':ab,ti OR 'napping':ab,ti
8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
9 'noise'/de OR 'noise':ab,ti OR 'noises':ab,ti OR 'noisy':ab,ti
10 'sound'/de OR 'sound':ab,ti OR 'sounds':ab,ti OR 'sound masking':ab,ti
11 'ear protective device'/de OR 'ear protective device':ab,ti OR 'ear protective devices':ab,ti OR 'earplug':ab,ti OR 'earplugs':ab,ti OR 'ear plug':ab,ti OR 'ear plugs':ab,ti OR 'earmuff':ab,ti OR 'earmuffs':ab,ti OR 'ear muff':ab,ti OR 'ear muffs':ab,ti OR 'headphone':ab,ti OR 'headphones':ab,ti OR 'head phone':ab,ti OR 'head phones':ab,ti OR 'hat':ab,ti OR 'hats':ab,ti OR 'music':ab,ti
12 'light'/de OR light*:ab,ti OR 'eye protective device'/de OR 'eye protective device':ab,ti OR 'eye protective devices':ab,ti OR 'eye masks':ab,ti OR 'eye mask':ab,ti
13 'polysomnography'/de OR 'polysomnography':ab,ti OR 'polysomnographic':ab,ti OR 'polysomnogram':ab,ti OR 'polysomnograms':ab,ti OR 'actimetry'/de OR 'actigraphy':ab,ti OR 'actigraphic':ab,ti OR 'actigram':ab,ti OR 'actigrams':ab,ti
14 'music therapy'/de OR 'musical':ab,ti OR musicotherap*:ab,ti OR 'song':ab,ti OR 'songs':ab,ti OR lullab*:ab,ti OR 'sing':ab,ti OR 'sings':ab,ti OR 'singing':ab,ti OR 'sang':ab,ti OR 'sung':ab,ti
15 'alternative medicine'/de OR 'aromatherapy'/de OR 'relaxation training'/de OR 'biofeedback'/de OR 'guided imagery'/de OR 'breathing exercise'/de OR 'massage'/de OR 'acupressure'/de OR 'acupuncture'/de
16 'non‐pharmacological':ab,ti OR massag*:ab,ti OR 'acupressure':ab,ti OR 'acupuncture':ab,ti OR 'aromatherapy':ab,ti OR 'complementary therap*':ab,ti OR 'alternative therap*':ab,ti OR 'mind body therap*':ab,ti OR 'biofeedback':ab,ti OR 'breathing exercise*':ab,ti OR 'imagery':ab,ti OR 'muscle relaxation':ab,ti OR 'relaxation therap*':ab,ti OR 'therapeutic touch':ab,ti
17 'environmental intervention*':ab,ti OR 'quiet time':ab,ti OR 'alarm modifications':ab,ti OR 'alarm modification':ab,ti
18 'social support'/de OR 'social support':ab,ti
19 'physiotherapy'/de OR 'physical therapy':ab,ti OR 'physical therapies':ab,ti OR 'rehabilitation':ab,ti OR 'rehab':ab,ti
20 'kangaroo care' OR 'kangaroo mother care'
21 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20
22 #8 OR #21
23 'intensive care unit'/de OR 'icu':ab,ti OR 'icus':ab,ti
24 'pediatric intensive care unit'/de OR 'picu':ab,ti OR 'picus':ab,ti OR 'pediatric unit':ab,ti OR 'pediatric units':ab,ti OR 'paediatric unit':ab,ti OR 'paediatric units':ab,ti
25 'neonatal intensive care unit'/de OR 'nicu':ab,ti OR 'nicus':ab,ti OR 'neonatal unit':ab,ti OR 'neonatal units':ab,ti
26 'intensive care':ab,ti OR 'intensive therapy':ab,ti OR 'high dependency':ab,ti OR 'intensive care'/de OR 'critical care':ab,ti
27 'burn unit'/de OR 'burn units':ab,ti OR 'burn unit':ab,ti
28 'recovery room'/de OR 'recovery room':ab,ti OR 'recovery rooms':ab,ti
29 'coronary care unit'/de OR 'coronary care unit':ab,ti OR 'coronary care units':ab,ti OR 'cardiac care unit':ab,ti OR 'cardiac care units':ab,ti OR 'ccu':ab,ti
30 'respiratory care unit':ab,ti OR 'respiratory care units':ab,ti
31 'hospitali*':ab,ti OR 'hospital patient'/de OR 'hospital patient':ab,ti OR 'hospital patients':ab,ti OR 'inpatients':ab,ti OR 'inpatient':ab,ti OR 'in hospital':ab,ti OR 'hospital ward':ab,ti OR 'hospital wards':ab,ti
32 'pediatric wards':ab,ti OR 'pediatric ward':ab,ti OR 'paediatric wards':ab,ti OR 'paediatric ward':ab,ti OR 'patient rooms':ab,ti OR 'patient room':ab,ti
33 'critically ill':ab,ti OR 'critical illness'/de OR 'critical illness':ab,ti OR 'critical illnesses':ab,ti OR 'artificial ventilation'/de OR 'artificial ventilation':ab,ti OR 'artificial respiration':ab,ti OR 'mechanical ventilation':ab,ti OR 'mechanically ventilated':ab,ti OR 'acute illness':ab,ti OR 'acute illnesses':ab,ti OR 'acute care':ab,ti
34 'hemodialysis units':ab,ti OR 'hemodialysis unit':ab,ti OR 'haemodialysis units':ab,ti OR 'haemodialysis unit':ab,ti
35 'post acute care unit':ab,ti OR 'post anesthesia care unit':ab,ti OR 'post anesthesia care units':ab,ti OR 'post anaesthesia care unit':ab,ti OR 'post anaesthesia care units':ab,ti OR 'pacu':ab,ti OR 'pacus':ab,ti
36 #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35
37 #22 AND #36
38 'randomized controlled trial'/exp
39 'randomization'/exp
40 'double blind procedure'/exp
41 'single blind procedure'/exp
42 random*:ab,ti
43 #38 OR #39 OR #40 OR #41 OR #42
44 'animal'/exp OR 'animal experiment'/exp
45 'human'/exp
46 #44 AND #45
47 #44 NOT #46
48 #43 NOT #47
49 'clinical trial'/exp
50 (clin* NEAR/3 trial*):ab,ti
51 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti
52 'placebo'/exp
53 placebo*:ab,ti
54 random*:ab,ti
55 'experimental design'/exp
56 'crossover procedure'/exp
57 'control group'/exp
58 'latin square design'/exp
59 #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58
60 #59 NOT #47
61 #60 NOT #48
62 'comparative study'/exp
63 'evaluation'/exp
64 'prospective study'/exp
65 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti
66 25 OR #63 OR #64 OR #65
67 #66 NOT #47
68 #67 NOT (#48 OR #60)
69 #48 OR #61 OR #68
70 #37 AND #69
71 #37 AND #69 AND [01‐10‐2020]/sd NOT [10‐12‐2021]/sd
CINAHLPlus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
Searched 11 April 2018, 27 March 2019, 30 October 2020, 12 December 2021
1. (MH "Sleep+")
2. (MH "Sleep Stages+")
3. (MH "Sleep, REM")
4. (MH "Sleep Deprivation")
5. (TI (sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence)) OR (AB (sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence))
6. (MH "Circadian Rhythm") OR (TI (circadian) OR (AB (circadian))
7. (TI (rest OR resting OR nap OR naps OR napping)) OR (AB (rest OR resting OR nap OR naps OR napping))
8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
9. (MH "Noise") OR (TI (noise OR noises OR noisy)) OR (AB (noise OR noises OR noisy))
10. (MH "Sound") OR (TI (sound OR sounds OR "sound masking")) OR (AB (sound OR sounds OR "sound masking"))
11. (MH "Ear Protective Devices") OR (TI ("ear protective device" OR "ear protective devices" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff OR earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music)) OR (AB ("ear protective device" OR "ear protective devices" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff OR earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music))
12. (MH "Light+") OR (MH "Eye Protective Devices") OR (TI (light* OR "eye protective device" OR "eye protective devices" OR "eye masks" OR "eye mask")) OR (AB (light* OR "eye protective device" OR "eye protective devices" OR "eye masks" OR "eye mask"))
13. (MH "Polysomnography") OR (MH "Actigraphy") OR (TI (polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR actigraphy OR actigraphic OR actigram OR actigrams)) OR (AB (polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR actigraphy OR actigraphic OR actigram OR actigrams))
14. (MH "Music Therapy") OR (TI (musical OR musicotherap* OR song OR songs OR lullab* OR sing OR sings OR singing OR sang OR sung)) OR (AB (musical OR musicotherap* OR song OR songs OR lullab* OR sing OR sings OR singing OR sang OR sung))
15. (MH "Alternative Therapies") OR (MH "Mind Body Techniques") OR (MH "Aromatherapy") OR (MH "Relaxation Techniques+") OR (MH "Biofeedback") OR (MH "Guided Imagery") OR (MH "Therapeutic Touch") OR (MH "Breathing Exercises+") OR (MH "Massage+") OR (MH "Acupressure+") OR (MH "Acupuncture+")
16. (TI (non‐pharmacological OR massag* OR acupressure OR acupuncture OR aromatherapy OR "complementary therap*" OR "alternative therap*" OR "mind‐body therap*" OR biofeedback OR "breathing exercise*" OR imagery OR "muscle relaxation" OR "relaxation therap*" OR "therapeutic touch")) OR (AB (non‐pharmacological OR massag* OR acupressure OR acupuncture OR aromatherapy OR "complementary therap*" OR "alternative therap*" OR "mind‐body therap*" OR biofeedback OR "breathing exercise*" OR imagery OR "muscle relaxation" OR "relaxation therap*" OR "therapeutic touch"))
17. (TI ("environmental intervention*" OR "quiet time" OR "alarm modifications" OR "alarm modification")) OR (AB ("environmental intervention*" OR "quiet time" OR "alarm modifications" OR "alarm modification"))
18. (MH "Support, Psychosocial+") OR (TI ("social support")) OR (AB ("social support"))
19. (MH "Physical Therapy+") OR (TI ("physical therapy" OR "physical therapies" OR rehabilitation" OR rehab)) OR (AB ("physical therapy" OR "physical therapies" OR rehabilitation" OR rehab))
20. (TI ("Kangaroo Care" OR "Kangaroo Mother Care")) OR (AB ("Kangaroo Care" OR "Kangaroo Mother Care"))
21. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20
22. #8 OR #21
23. (MH "Intensive Care Units+") OR (TI ("ICU" OR "ICUs")) OR (AB ("ICU" OR "ICUs"))
24. (MH "Intensive Care Units, Pediatric") OR (TI ("PICU"[tiab] OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units")) OR (AB ("PICU"OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units"))
25. (MH "Intensive Care Units, Neonatal") OR (TI ("NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units")) OR (AB ("NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units"))
26. (MH "Critical Care+") OR (TI ("intensive care" OR "intensive therapy" OR "high dependency" OR "critical care")) OR (AB ("intensive care" OR "intensive therapy" OR "high dependency" OR "critical care"))
27. (MH "Burn Units") OR (TI ("burn units" OR "burn unit")) OR (AB ("burn units" OR "burn unit"))
28. (TI ("recovery room" OR "recovery rooms")) OR (AB ("recovery room" OR "recovery rooms"))
29. (MH "Coronary Care Units") OR (TI ("coronary care unit" OR "coronary care units" OR "cardiac care unit" OR "cardiac care units" OR "CCU")) OR (AB ("coronary care unit" OR "coronary care units" OR "cardiac care unit" OR "cardiac care units" OR "CCU"))
30. (MH "Respiratory Care Units") OR (TI ("respiratory care unit" OR "respiratory care units")) OR (AB ("respiratory care unit" OR "respiratory care units"))
31. (MH "Inpatients") OR (TI (hospitali* OR inpatients OR inpatient OR "in hospital" OR "hospital ward" OR "hospital wards")) OR (AB (hospitali* OR inpatients OR inpatient OR "in hospital" OR "hospital ward" OR "hospital wards"))
32. (TI ("pediatric wards" OR "pediatric ward" OR "paediatric wards" OR "paediatric ward" OR "patient rooms" OR "patient room")) OR (AB ("pediatric wards" OR "pediatric ward" OR "paediatric wards" OR "paediatric ward" OR "patient rooms" OR "patient room"))
33. (MH "Critical Illness") OR (MH "Respiration, Artificial+") OR (TI ("critically ill" OR "critical illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses" OR "acute care")) OR (AB ("critically ill" OR "critical illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses" OR "acute care"))
34. (TI ("hemodialysis unit" OR "hemodialysis units" OR "haemodialysis units" OR "haemodialysis unit")) OR (AB ("hemodialysis unit" OR "hemodialysis units" OR "haemodialysis units" OR "haemodialysis unit"))
35. (MH "Post Anesthesia Care Units") OR (TI ("post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit" OR "post anaesthesia care units" OR "PACU" OR "PACUs")) OR (AB ("post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit" OR "post anaesthesia care units" OR "PACU" OR "PACUs"))
36. #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 #34 OR #35
37. #22 AND #36
38. (MH "randomized controlled trials" OR MH "clinical trials" OR "random" OR "randomly" OR "randomized" OR "randomised" OR "randomization" OR "randomisation" OR "placebo" OR "placebos" OR TI trial* OR MH "comparative studies" OR MH "control group" OR MH "double‐blind studies" OR MH "single‐blind studies" OR MH "multicenter studies" OR MH "empirical research" OR MH "random assignment" OR MH "validation studies" OR "clinical trial" OR "clinical study" OR "controlled trial" OR "controlled study" OR "comparative" OR "comparison" OR "comparisons" OR "control group" OR "control groups" OR "cross section" OR "cross sectional" OR "cross sections" OR "crosssection" OR "crosssectional" OR "crosssections" OR "double blind" OR "double blinded" OR "double mask" OR "double masked" OR "multicenter" OR "multi center" OR "multicentre" OR "multi centre" OR "phase 1" OR "phase 2" OR "phase 3" OR "phase 4" OR "phase four" OR "phase I" OR "phase II" OR "phase III" OR "phase IV" OR "phase one" OR "phase three" OR "phase two" OR "post test" OR "posttest" OR "pre test" OR "pretest" OR "RCT" OR "RCTs" OR "single blind" OR "single blinded" OR "single mask" OR "single masked" OR TI "studies" OR TI "study" OR "triple blind" OR "triple blinded")
39. #37 AND #38
Cochrane Database of Systematic Reviews, in the Cochrane Library
Searched 11 April 2018, 27 March 2019, and 30 October 2020, 12 December 2021
#1 MeSH descriptor: [Sleep] explode all trees
#2 MeSH descriptor: [Sleep Stages] explode all trees
#3 MeSH descriptor: [Sleep, REM] explode all trees
#4 MeSH descriptor: [Sleep Deprivation] explode all trees
#5 (sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence):ti,ab
#6 MeSH descriptor: [Circadian Rhythm] explode all trees
#7 "circadian"[tiab]
#8 (rest OR resting OR nap OR naps OR napping):ti,ab
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 MeSH descriptor: [Noise] explode all trees
#11 ("noise" OR "noises" OR "noisy"):ti,ab
#12 MeSH descriptor: [Sound] this term only
#13 ("sound" OR "sounds" OR "sound masking"):ti,ab
#14 MeSH descriptor: [Ear Protective Devices] explode all trees
#15 ("ear protective device" OR "ear protective devices" OR "earplug" OR "earplugs" OR "ear plug" OR "ear plugs" OR "earmuff" OR "earmuffs" OR "ear muff" OR "ear muffs" OR "headphone" OR "headphones" OR "head phone" OR "head phones" OR "hat" OR "hats" OR "music"):ti,ab
#16 MeSH descriptor: [Light] explode all trees
#17 (light*):ti,ab
#18 MeSH descriptor: [Eye Protective Devices] explode all trees
#19 ("eye protective device" OR "eye protective devices" OR "eye masks" OR "eye mask"):ti,ab
#20 MeSH descriptor: [Polysomnography] explode all trees
#21 ("polysomnography" OR "polysomnographic" OR "polysomnogram" OR "polysomnograms"):ti,ab
#22 MeSH descriptor: [Actigraphy] explode all trees
#23 ("actigraphy" OR "actigraphic" OR "actigram" OR “actigrams"):ti,ab
#24 MeSH descriptor: [Music Therapy] explode all trees
#25 ("musical" OR musicotherap* OR "song" OR "songs" OR lullab* OR sing OR sings OR "singing" OR "sang" OR sung):ti,ab
#26 MeSH descriptor: [Complementary Therapies] this term only
#27 MeSH descriptor: [Mind‐Body Therapies] this term only
#28 MeSH descriptor: [Aromatherapy] explode all trees
#29 MeSH descriptor: [Relaxation Therapy] explode all trees
#30 MeSH descriptor: [Biofeedback, Psychology] explode all trees
#31 MeSH descriptor: [Imagery (Psychotherapy)] explode all trees
#32 MeSH descriptor: [Therapeutic Touch] explode all trees
#33 MeSH descriptor: [Breathing Exercises] explode all trees
#34 MeSH descriptor: [Massage] explode all trees
#35 MeSH descriptor: [Acupressure] explode all trees
#36 MeSH descriptor: [Acupuncture] explode all trees "
#37 ("non‐pharmacological" OR massag* OR "acupressure" OR "acupuncture" OR aromatherapy OR complementary therap* OR alternative therap* OR mind‐body therap* OR biofeedback OR breathing exercise* OR imagery OR muscle relaxation OR relaxation therap* OR therapeutic touch):ti,ab
#38 (environmental intervention* OR "quiet time" OR "alarm modifications" OR "alarm modification"):ti,ab
#39 MeSH descriptor: [Social Support] explode all trees
#40 (social support):ti,ab
#41 MeSH descriptor: [Physical Therapy Modalities] explode all trees
#42 ("physical therapy" OR "physical therapies" OR "rehabilitation" OR "rehab"):ti,ab
#43 "Kangaroo Care" OR "Kangaroo Mother Care"
#44 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43
#45 #9 OR #44
#46 MeSH descriptor: [Intensive Care Units] explode all trees
#47 ("ICU" OR ICUs):ti,ab
#48 MeSH descriptor: [Intensive Care Units, Pediatric] explode all trees
#49 ("PICU" OR PICUs OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units"):ti,ab
#50 MeSH descriptor: [Intensive Care Units, Neonatal] explode all trees
#51 ("NICU" OR NICUs OR "neonatal unit" OR "neonatal units")ti,ab
#52 ("intensive care" OR "intensive therapy" OR "high dependency"):ti,ab
#53 MeSH descriptor: [Critical Care] explode all trees
#54 ("critical care"):ti,ab
#55 MeSH descriptor: [Burn Units] explode all trees
#56 ("burn units" OR "burn unit"):ti,ab
#57 MeSH descriptor: [Recovery Room] explode all trees
#58 ("recovery room" OR "recovery rooms"):ti,ab
#59 MeSH descriptor: [Coronary Care Units] explode all trees
#60 ("coronary care unit" OR "coronary care units" OR "cardiac care unit" OR "cardiac care units" OR "CCU"):ti,ab
#61 MeSH descriptor: [Hemodialysis Units, Hospital] explode all trees "Respiratory Care Units"[MeSH] OR
#62 ("respiratory care unit" OR "respiratory care units"):ti,ab
#63 MeSH descriptor: [Inpatients] explode all trees
#64 (hospitali* OR "inpatients" OR "inpatient" OR "in hospital" OR "hospital ward" OR "hospital wards"):ti,ab
#65 ("pediatric wards" OR "pediatric ward" OR "paediatric wards" OR "paediatric ward" OR "patient rooms" OR "patient room"):ti,ab
#66 MeSH descriptor: [Critical Illness] explode all trees
#67 ("critically ill" OR "critical illness" OR "critical illnesses" ):ti,ab
#68 MeSH descriptor: [respiration, artificial] explode all trees
#69 ("artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses" OR "acute care"):ti,ab
#70 MeSH descriptor: [Hemodialysis Units, Hospital] explode all trees
#71 ("hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit"):ti,ab
#72 ("post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit" OR "post anaesthesia care units" OR "PACU" OR "PACUs"):ti,ab
#73 #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72
#74 #45 AND #7
Epistemonikos
Searched 18 April 2018, 27 March 2019, 10 December 2021
(#1 OR #2) AND #3
#1
Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR "circadian" OR rest OR resting OR nap OR naps OR napping
#2
"noise" OR "noises" OR "noisy" OR "sound" OR "sounds" OR "Ear Protective Devices" OR "ear protective device" OR "earplug" OR "earplugs" OR "ear plug" OR "ear plugs" OR "earmuff" OR "earmuffs" OR "ear muff" OR "ear muffs" OR "headphone" OR "headphones" OR "head phone" OR "head phones" OR "hat" OR "hats" OR "music" OR light* OR "eye protective device" OR "eye protective devices" OR "eye masks" OR "eye mask" OR "polysomnography" OR "polysomnographic" OR "polysomnogram" OR "polysomnograms" OR "Actigraphy" OR "actigraphy" OR "actigraphic" OR "actigram" OR "actigrams" “music therapy” OR "musical" OR musicotherap* OR "song" OR "songs" OR lullab* OR sing OR sings OR "singing" OR "sang" OR sung OR "Aromatherapy" OR "Biofeedback " OR "Imagery" OR "Acupressure" OR "Acupuncture" OR "non‐pharmacological" OR massag* OR aromatherapy OR “complementary therapy” OR “complementary therapies” OR “alternative therapy” OR “alternative therapies” OR “mind‐body therapy” OR “mind‐body therapies” OR “breathing exercise” OR “breathing exercises” OR imagery OR “muscle relaxation” OR “relaxation therapy” OR "Relaxation Therapies" OR “therapeutic touch” OR “environmental intervention” OR “environmental interventions” OR "quiet time" OR "alarm modifications" OR "alarm modification" OR social support OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "rehab" OR "Kangaroo Care" OR "Kangaroo Mother Care"
#3:
"Intensive Care Units" OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units" OR "intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "critical care" OR "Burn Units" OR "burn units" OR "recovery room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units" OR "CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitali* OR "inpatients" OR "inpatient" OR "in hospital" OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards" OR "paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "critical illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses" OR "acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit" OR "post anaesthesia care units" OR “PACU" OR "PACUs"
ProQuest Digital Dissertations and Theses
Searched 18 April 2018 and 27 March 2019, 10 December 2021
| Set # | Searched for | Databases |
| S1 | noft(Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping) | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
| S2 | noft(Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff ) OR noft(earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask" ) OR noft(Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies ) OR noft(sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages ) OR noft("alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support" ) OR noft("Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care") | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
| S3 | noft("Intensive Care Units" OR “intensive care unit” OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units" ) OR noft("intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units" ) OR noft("CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards" ) OR noft("paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses" ) OR noft("acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit" ) OR noft("post anaesthesia care units" OR "PACU" OR "PACUs") | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
| S4 | noft(Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping) AND ((noft(Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff) OR noft(earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask") OR noft(Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies) OR noft(sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages) OR noft("alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support") OR noft("Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care")) OR (noft("Intensive Care Units" OR "intensive care unit" OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units") OR noft("intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units") OR noft("CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards") OR noft("paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses") OR noft("acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit") OR noft("post anaesthesia care units" OR "PACU" OR "PACUs"))) | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
| S5 | noft(Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping) AND (noft(Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff) OR noft(earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask") OR noft(Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies) OR noft(sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages) OR noft("alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support") OR noft("Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care")) | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
| S6 | noft(Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping) AND (noft("Intensive Care Units" OR "intensive care unit" OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units") OR noft("intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units") OR noft("CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards") OR noft("paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses") OR noft("acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit") OR noft("post anaesthesia care units" OR "PACU" OR "PACUs")) | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
| S7 | (noft(Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping) AND (noft(Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff) OR noft(earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask") OR noft(Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies) OR noft(sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages) OR noft("alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support") OR noft("Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care"))) AND (noft("Intensive Care Units" OR "intensive care unit" OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units") OR noft("intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units") OR noft("CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards") OR noft("paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses") OR noft("acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit") OR noft("post anaesthesia care units" OR "PACU" OR "PACUs")) | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
| S8 | (noft(Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping) OR (noft(Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff) OR noft(earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask") OR noft(Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies) OR noft(sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages) OR noft("alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support") OR noft("Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care"))) AND (noft("Intensive Care Units" OR "intensive care unit" OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units") OR noft("intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units") OR noft("CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards") OR noft("paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses") OR noft("acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit") OR noft("post anaesthesia care units" OR "PACU" OR "PACUs")) | Dissertations & Theses @ Johns Hopkins University, ProQuest Dissertations & Theses Global |
ClinicalTrials.gov
Searched 7 April 2018 and 9 December 2021
Terms tested and separated into shorter strings to adhere to character limits in database; omitted terms that were redundant (MeSH, root variations, etc. and false hits, e.g. “in hospital”)
Ran each set #1‐7 separately with set #8, #9, #10, #11, #12 and #13.
1. Sleep OR "REM Sleep" OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR "Circadian Rhythm" OR circadian OR rest OR resting OR nap OR naps OR napping
___________________________________________________________________________
2. Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff
3. earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask"
4. Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies
5. sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR acupuncture OR "non‐pharmacological" OR massages
6. "alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support"
7. "Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care"
____________________________________________________________________________
8. "Intensive Care Units" OR “intensive care unit” OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units"
9. "intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units"
10. "CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards"
11. "paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses"
12. "acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit"
13. "post anaesthesia care units" OR "PACU" OR "PACUs"
ISRCTN Registry
Searched 5 April 2018, 20 August 2021, 9 December 2021
Note: Ran “sleep” terms against all “environment” terms and one set of “intervention” terms. No relevant hits.
1. Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping
___________________________________________________________________________
2. Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff
3. earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask"
4. Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies
5. sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages
6. "alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support"
7. "Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care"
____________________________________________________________________________
8. "Intensive Care Units" OR “intensive care unit” OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units"
9. "intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units"
10. "CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards"
11. "paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses"
12. "acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit"
13. "post anaesthesia care units" OR "PACU" OR "PACUs"
World Health Organization International Clinical Trials Registry Platform (ICTRP)
Searched 5 April 2018 and 10 December 2021
(Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping) Pulls 1125 records for 1093 trials
Built in children filter only used once. Checking the results for the same search without it turned up at least one potential hit.
Platform does not recognize parentheses and processes “AND” before “OR”. Searched using single term combinations was done to adapt to this.
1. Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping
___________________________________________________________________________
2. Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff
3. earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask"
4. Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies
5. sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages
6. "alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support"
7. "Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care"
____________________________________________________________________________
8. "Intensive Care Units" OR “intensive care unit” OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units"
9. "intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units"
10. "CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards"
11. "paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses"
12. "acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit"
13. "post anaesthesia care units" OR "PACU" OR "PACUs"
Grey Literature Reports
Searched 17 April 2018
Sleep OR ICU OR Intensive care unit, OR hospital child critical OR hospital child intensive OR infant hospital
Does not support Boolean searching; all words “anded” together. Automatic stemming is applied; rules unclear. No relevant hits.
Open Grey
Searched 17 April 2018 and 12 December 2021
Sleep OR sleeping OR slept OR sleeps OR sleepless OR sleeplessness OR sleepy OR asleep OR insomnia OR insomniac OR somnolent OR somnolence OR circadian OR rest OR resting OR nap OR naps OR napping
3,073 total hits
AND
"Intensive Care Units" OR “intensive care unit” OR "ICU" OR "ICUs" OR "PICU" OR "PICUs" OR "pediatric unit" OR "pediatric units" OR "paediatric unit" OR "paediatric units" OR "NICU" OR "NICUs" OR "neonatal unit" OR "neonatal units"
5 results; none relevant
AND
"intensive care" OR "intensive therapy" OR "high dependency" OR "Critical Care" OR "Burn Units" OR "burn unit" OR "Recovery Room" OR "recovery rooms" OR "Coronary Care Units" OR "coronary care unit" OR "cardiac care unit" OR "cardiac care units"
8 results; none relevant
AND
"CCU" OR "Respiratory Care Units" OR "respiratory care unit" OR hospitalized OR hospitalised OR "hospital ward" OR "hospital wards" OR "pediatric wards" OR "pediatric ward" OR "paediatric wards"
8 results; none relevant
AND
"paediatric ward" OR "patient rooms" OR "patient room" OR "critically ill" OR "Critical Illness" OR "critical illnesses" OR "artificial respiration" OR "mechanical ventilation" OR "mechanically ventilated" OR "acute illness" OR "acute illnesses"
6 results; none relevant
AND
"acute care" OR "hemodialysis units" OR "hemodialysis unit" OR "haemodialysis units" OR "haemodialysis unit" OR "post acute care unit" OR "post anesthesia care unit" OR "post anesthesia care units" OR "post anaesthesia care unit"
1 result; not relevant
AND
"post anaesthesia care units" OR "PACU" OR "PACUs"
No results
AND
Noise OR noises OR noisy OR sound OR sounds OR "sound masking" OR "Ear Protective Devices" OR "ear protective device" OR earplug OR earplugs OR "ear plug" OR "ear plugs" OR earmuff
66 results; none relevant
AND
earmuffs OR "ear muff" OR "ear muffs" OR headphone OR headphones OR "head phone" OR "head phones" OR hat OR hats OR music OR light OR "Eye Protective Devices" OR "eye protective device" OR "eye masks" OR "eye mask"
249 results; 34 medicine reviewed, none relevant; 22 physiology reviewed, none relevant;
14 biological and medical sciences, general reviewed: only 1 observational study:
Influence of variations in the light environment on sleep in preterm newborns: an observational study
http://hdl.handle.net/10068/1022087
AND
Polysomnography OR polysomnographic OR polysomnogram OR polysomnograms OR Actigraphy OR actigraphic OR actigram OR actigrams OR "Music Therapy" OR musical OR song OR songs OR lullaby OR lullabies
34 results, none relevant
AND
sing OR "Complementary Therapies" OR "Mind‐Body Therapies" OR Aromatherapy OR "Relaxation Therapy" OR "Therapeutic Touch" OR "Breathing Exercises" OR Massage OR Acupressure OR "non‐pharmacological" OR massages
8 results, none relevant
AND
"alternative therapy" OR "mind‐body therapy" OR biofeedback OR "breathing exercise" OR imagery OR "muscle relaxation" OR "environmental intervention" OR "quiet time" OR "alarm modifications" OR "alarm modification" OR "Social Support"
16 results, none relevant
AND
"Physical Therapy Modalities" OR "physical therapy" OR "physical therapies" OR "rehabilitation" OR "Kangaroo Care" OR "Kangaroo Mother Care"
23 results, none relevant
Appendix 2. Unused methods
| Section of review | Unused methods | Justification |
|
Measures of treatment effect |
Dichotomous data We had planned to report dichotomous outcomes as risk ratios (RR), and present these with 95% confidence intervals (CI) and P values. |
The included studies did not report relevant data for this method. |
|
Multiple outcome data Had there been multiple time points included in a study, we would have made comparisons at the following time points, when feasible: short‐term (within one week postintervention), medium‐term (within two months postintervention), and long‐term (any time after two months postintervention). |
We did not encounter data at multiple time points. | |
|
Time‐to‐event data We had planned to analyze time‐to‐event (survival) outcomes (i.e. incidence of delirium, death) using hazard ratios (HR), and to present these with 95% CIs. |
These outcomes were not reported in any of the included studies. | |
|
Unit of analysis issues
|
Cross‐over trials We intended to report cross‐over trials with problematic periods or carry‐over effects in the risk of bias assessment. |
We did not encounter any studies that met this criteria.
|
|
Multiple groups We planned to manage studies including multiple, correlated comparisons by combining all relevant experimental groups of the study into one group and combining all relevant control groups into one group, to create a single pair‐wise comparison, when possible. Had it not been possible to combine all relevant experimental groups of the study into one group, we would have split the control group to ensure participants were not double counted. | ||
| Dealing with missing data | Had we not been able to obtain missing data from study authors, we planned to attempt to calculate missing statistics (e.g. correlation coefficients or standard deviations) from other available statistics (e.g. standard error or CI). We had planned to impute data using all available information, and for missing participant data, we had planned to impute the data using the last observation carried forward (LOCF [Higgins 2021]). Had we made assumptions during imputation, we would have documented this on the data extraction sheet and reported it in the risk of bias tables. Had we imputed data, we would have conducted a sensitivity analysis to assess the impact on the results. In summarizing aggregate data where LOCF was not possible, we had planned to address the potential impact of the missing data on the findings of the review in the Discussion section. | We were unable to impute data or calculate missing statistics with limited data provided (i.e. SE and CI not available). |
| Assessment of heterogeneity | We had planned to use the I² statistic (%) to determine the proportion of variation between included studies that would have been due to heterogeneity; a value above 50% would have defined substantial statistical heterogeneity (Deeks 2021). We had also planned to examine the result of the Chi² test (P < 0.10 = significant heterogeneity) and CI overlap of included studies, with visual inspection of the forest plot, where poor overlap would have been suggestive of heterogeneity. As we anticipated the likelihood of high variability in participants, interventions, and outcomes between studies would be great, we planned to report Tau² — an estimate of between‐study variance — with use of a random‐effects model. | Due to clinical and methodological heterogeneity, we could not pool data in a meta‐analyses. Therefore, we could not assess statistical heterogeneity. |
| Assessment of reporting bias | If the meta‐analysis had included more than 10 studies, we planned to draw a funnel plot and inspect it visually for asymmetry, which may have been caused by publication bias, or other small study effects attributable to, for example, poor methodological quality, true heterogeneity, or chance. | There were too few studies to create a funnel plot to investigate reporting bias. |
| Data Synthesis | We had planned to conduct a meta‐analysis using Review Manager 5 (RevMan 5 [Review Manager 2020]). We had planned to make separate comparisons between each category of intervention (i.e. environmental interventions, behavioral interventions, physical therapy, complementary and alternative therapies, and any other non‐pharmacological intervention) and usual care or alternative interventions in each of the following three settings: NICU, PICU, and inpatient floor. We had planned to use a random‐effects model to combine the results of trials included in the meta‐analysis, as there would likely have been clinical and methodological heterogeneity. Using RevMan 5, we had planned to calculate the RR for dichotomous outcomes using the Mantel‐Haenszel method. Had continuous outcomes been measured in the same way across studies, we would have summarized across‐study MD estimates using the inverse variance method, with 95% CI and P values; we would have used the SMD when studies used different methods to measure the same outcome. If variance data had not been available, we would not have estimated the mean between‐group difference with 95% CI and P values. |
We identified considerable clinical heterogeneity across the included studies in participant populations, measurement tools and metrics used, study duration, and timing of outcome assessment. Thus, we were unable to pool the results in a meta‐analysis for any of the outcomes. |
| Subgroup analysis and investigation of heterogeneity | We had planned to conduct the subgroup analyses listed below by using the statistical test for subgroup differences.
|
We were unable to undertake any subgroup analyses due to insufficient details, number of studies. |
| Sensitivity analysis | We planned to perform sensitivity analyses to determine the impact of the following issues, if they were present among the included studies.
|
We did not conduct a meta‐analysis. |
Foototes
CI: confidence interval; MD: mean difference; NICU: neonatal intensive care unit; PICU: pediatric intensive care unit; RCT: randomized controlled trial; SE: standard error; SMD: standardized mean difference.
Appendix 3. Criteria for judging risk of bias in RCTs
Random sequence generation (selection bias)
Low risk of bias: sufficient use of a random component for adequate sequence generation (i.e. computer random number generator or random number tables)
High risk of bias: insufficient component of randomization (i.e. participants were assigned by date of birth or date of presentation)
Unclear risk of bias: authors did not sufficiently explain the randomization and how it was performed in order to permit a judgment of low or high risk of bias
Allocation concealment (selection bias)
Low risk of bias: adequate concealment of the allocation (i.e. use of consecutively numbered, sealed, opaque envelopes or telephone randomization)
High risk of bias: allocation was definitely not adequately concealed (i.e. open random number lists, odd or even date of birth)
Unclear risk of bias: uncertainty about whether the allocation was adequately concealed (i.e. concealment method not known)
Blinding of participants and personnel (performance bias)
We did not assess blinding of participants and personnel, as study participants, families, and personnel would have been aware of the intervention they were receiving or implementing.
Blinding of outcome assessment (detection bias)
Low risk of bias: (a) no blinding of outcome assessment, but we judged that the outcome measurement was not likely to have been influenced by the lack of blinding; or (b) blinding of outcome assessment was ensured
High risk of bias: there was no blinding, and the outcome measurement was likely to have been influenced by the lack of blinding
Unclear risk of bias: blinding of outcome assessor was not reported, and could not be confirmed through contact with study authors
Incomplete outcome data (attrition bias)
Low risk of bias: (a) no missing outcome data; (b) reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring was unlikely to introduce bias); (c) missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups; (d) for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have had a clinically relevant impact on the intervention effect estimate; (e) for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes was not enough to have had a clinically relevant impact on observed effect size; or (f) missing data were imputed using appropriate methods
High risk of bias: missing data were not accounted for, not imputed using appropriate methods, or not evenly distributed between groups
Unclear risk of bias: missing data or losses to follow‐up, or both, were not reported
Selective reporting (reporting bias)
Low risk of bias: (a) the study protocol was available, and all of the study’s prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way; or (b) the study protocol was not available, but it was clear that the published reports had included all expected outcomes, including those that were prespecified
High risk of bias: not all prespecified primary outcomes were reported
Unclear risk of bias: not enough information to determine if the study was at low or high risk of reporting bias
Other potential sources of bias
Low risk of bias: study was free from other apparent sources of bias
High risk of bias: there was a potential, additional source(s) of bias that was not captured by the other domains (i.e. bias related to study design)
Unclear risk of bias: not enough information to determine if the study was at low or high risk of other bias
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cone 2014.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: cross‐over Country: USA Funding: Shriners Hospital for Children Grant (71005) Study period: not specified Outcomes relevant to this review: validated objective measures of sleep (polysomnography) Overall objective of study: the primary aim of this investigation was to determine if healing touch mediates polysomnographic (PSG) changes during nocturnal sleep in pediatric burn patients. |
|
| Participants |
Setting: burn unit Sample size: 10 Dropouts: none specified Mean age: 15.5 years (range 13 to 20) Sex: not specified Inclusion criteria: children hospitalized for burns Exclusion criteria: not described |
|
| Interventions |
Intervention (N = 10): healing touch (HT); performed by 2 certified practitioners who followed standardized HT procedures, whereby non‐invasive and gentle use of the practitioner’s hands, either directly on the clothed body or slightly above the participant’s body, was performed. PSG began at onset of HT and continued for 8 hours (2200 to 0600). Soft music was played for both study nights for 45 minutes after the initiation of PSG recordings. Control group (N = 10 ): no healing touch; standard of care, which included soft music playing during sleep |
|
| Outcomes |
Primary outcomes: objective measures of sleep, including sleep stage (%), total sleep time (TST) in minutes, and number of awakenings, by polysomnography Secondary outcomes: not specified Timing of outcome assessment/time points: nighttime PSG at onset of healing touch/no healing touch for 8 hours |
|
| Notes | Conflict of Interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomization scheme generated by study statistician |
| Allocation concealment (selection bias) | Low risk | Comment: cross‐over study with randomization scheme generated by study statistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all outcome data reported for all participants enrolled |
| Selective reporting (reporting bias) | Low risk | Comment: methods section compared to results ‐ no issues noted |
| Other bias | Unclear risk | Comment: order of therapy (first versus not) may affect overall sleep time (i.e. if HT first would lead to increased sleep times in control night) |
Field 1992.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: parallel group Country: USA Funding: National Institute of Mental Health (NIMH) Research Scientist Award (MH00331), and NIMH research grant (MH40779) Study period: not specified Outcomes relevant to this review: validated subjective measures of sleep Overall objective of study: to examine the independent effects of the massage component on the behaviors and physiology of children and adolescents who were hospitalized for depression or adjustment disorder |
|
| Participants |
Setting: inpatient psychiatric unit Sample size: 72 Dropouts: not specified Mean age: 13 years (range 7 to 18) Sex: 40 boys, 32 girls Inclusion criteria: hospitalized children and adolescents with a diagnosis of depression or adjustment disorder according to DSM‐III‐R criteria, assessed by a physician Exclusion criteria: combination of conditions or complex medication timing schedule, or both |
|
| Interventions |
Intervention (N = 52; 26 = depressed, and 26 = adjustment disorder): the massage group received 30 minutes of back massage a day for 5 days, with pressure and smooth movements. The massage consisted of carefully timed stroking movement for 5‐minute periods in each of the 3 regions: up and down the neck, from the neck across the shoulders to the back of the neck, and from the neck to the waist and back of the neck along the vertebral column. The 15‐minute sequence was composed of 30 back and forth strokes per region at 10 seconds each. The same 15‐minute sequence was repeated in its entirety for a 30‐minute massage. The massages were given mid‐afternoon. Control group (N = 20; 10 = adjustment disorder, 10 = depression): The participant simply sat and watched a relaxing videotape (of pleasant sounds and visual images) with the student for 30 minutes. The participant was asked to remain still and quiet for the 30‐minute session. |
|
| Outcomes |
Primary outcomes: anxiety, measured by State Anxiety Inventory for Children (STAIC), Profile Of Mood States (POMS), and behavior observation ratings (7‐item behavior rating scale); activity level (actometer); physiologic and biochemical measures (pulse, salivary cortisol, urinary norepinephrine and epinephrine); and nighttime sleep (video observation) Secondary outcomes: none specified Timing of outcome assessment/time points: first and last treatment days for nighttime sleep and urine cortisol/catecholamines; for all other outcomes: 1) 30 minutes before massage or video session, or both (baseline); 2) immediately before (pre‐session); 3) immediately after (post‐session); and 4) 30 minutes after session (follow‐up) |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After group assignment by random stratification procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "After group assignment by random stratification procedure (stratified for sex, age and medication)...no significant differences were noted between massage and video groups on sex distribution, age, intelligence quotient, number of previous admissions, duration of hospital stay" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants not blinded; nurse observers blinded unless participant revealed their group assignment, which was discouraged but not prohibited |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: on self‐reported measures, not blinded; unclear if recorders of other outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 32% of sleep observation videos unavailable for coding due to non‐compliance and technical difficulties |
| Selective reporting (reporting bias) | Unclear risk | Comment: all prespecified outcome measures reported for all participants where data were available |
| Other bias | Low risk | Comment: no issues noted |
Hinds 2007a.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: parallel group Country: USA Funding: Oncology Nursing Foundation, by Cancer Center Support Grant (CA‐21765) from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities Study period: not specified Outcomes relevant to this review: sleep duration and sleep efficiency by actigraphy Overall objective of study: to assess the feasibility of an enhanced physical activity (EPA) intervention in hospitalized children and adolescents receiving treatment for a solid tumor or for acute myeloid leukemia (AML), and to assess different statistical techniques to detect the intervention’s sleep and fatigue outcomes |
|
| Participants |
Setting: inpatient floor of 2 pediatric cancer centers Sample size: 29 Dropouts: not specified Mean age: 12.5 years (range 7.4 to 18.2) Sex: 12 boys, 17 girls Inclusion criteria: 1) aged 7 to 18 years (fatigue measures validated for this age group only); 2) admitted for a 2‐ to 4‐day inpatient stay for scheduled chemotherapy, surgery, or other treatments for cancer; 3) able to understand English (study measures were in English); and 4) able to give assent according to institutional guidelines Exclusion criteria: children being treated for recurrent disease, scheduled for rehabilitation therapy after amputation or limb sparing, or experiencing inadequately controlled pain (defined as a score of 3 on a 5‐point scale for 2 consecutive measurement points), and children with CNS tumors |
|
| Interventions |
Intervention (N = 14): pedaling a stationary, bicycle‐style exerciser for 30 minutes twice daily, for 2 to 4 days of hospitalization Control group (N = 15 ): standard care |
|
| Outcomes |
Primary outcomes: sleep duration (minutes) and sleep efficiency (%) by actigraphy Secondary outcomes: fatigue (Fatigue Scale for 7 to 12 year olds, assessed by participant, parent, staff), sleep diary by parent, hemoglobin and hematocrit concentration Timing of outcome assessment/time points: actigraphy from day 0 to 3, and diary completed by parent daily. Fatigue scale completed on day of admission (Day 0), and Days 1 to 3. Hemoglobin and hematocrit daily. |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The randomization procedure established by the study biostatistician was based on Zelen’s methodology. Stratification within each diagnostic group was balanced according to center and study arm assignment. |
| Allocation concealment (selection bias) | Low risk | Comment: intervention allocations likely could not have been foreseen before or during enrollment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants were told of their assignment to the control or intervention group on the morning of Day 1 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: detection bias possible due to knowledge of allocated interventions by outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no issues noted |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Other bias | Low risk | Comment: no other bias detected |
Jacobs 2016.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: parallel group Country: USA Funding: not specified Study period: 2010 to 2012 Outcomes relevant to this review: objective measures of sleep (actigraphy), validated subjective measures of sleep, and participant satisfaction Overall objective of study: to establish the feasibility of conducting a massage intervention, and to measure preliminary effect on standard measures of sleep continuity, sleep quality, and fatigue in hospitalized adolescents with cancer |
|
| Participants |
Setting: inpatient oncology floor Sample size: 34 Dropouts: 1 participant too ill to participate after signing consent Mean age: 15.7 years (range 12 to 22) Sex: 24 boys, 10 girls Inclusion criteria: adolescents aged 12 to 21 years with any diagnosis or stage of cancer who were expected to be hospitalized for at least 4 consecutive nights Exclusion criteria: non‐English speaking, platelet count of < 20,000 |
|
| Interventions |
Intervention (N = 18): massage sessions were between 20 and 30 minutes in length and consisted primarily of Swedish massage techniques. Each session was tailored to the participant and may have addressed feet, legs, hands, arms, back, neck, shoulders, face, or head (or combination). Control group (N = 16): no massage |
|
| Outcomes |
Primary outcomes: sleep by actigraphy (sleep minutes, sleep efficiency (%), wake minutes after sleep onset, wake episodes during sleep, number of long sleep episodes, and longest sleep episode), parent perceptions of child's sleep, fatigue (Fatigue Scale Adolescent), anxiety (State Trait Anxiety Scale, State Portion), and mood (Behavioral, Affective and Somatic Experiences Scale Revised, Parent‐Report, and Child‐Report) Secondary outcomes: none Timing of outcome assessment/time points: actigraphy for 4 or 5 days, sleep diary by parent on nights that parent at bedside. Questionnaires completed at baseline (before admission or on admission, Day 3, and last day of the study). |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomization not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not explicitly described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: could not be blinded to massage |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: sleep measurement using actigraphy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Actigraph data were reliable and complete in all but two patients whose data were not used because they did not wear the actigraph for a sufficient period of time." |
| Selective reporting (reporting bias) | Low risk | Comment: no issues noted |
| Other bias | Low risk | Comment: no issues noted |
Papaconstantinou 2018.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: parallel group Country: Canada Funding: SickKids Foundation Fellowship in Complementary and Alternative Health Care and Pediatrics, and Canadian Institutes of Health Research Sleep and Biological Rhythms Team Grant Study period: not specified Outcomes relevant to this review: objective measures of sleep (actigraphy), validated subjective measures of sleep, and satisfaction Overall objective of study: to evaluate the Relax to Sleep program; a sleep intervention combining sleep health education with diaphragmatic breathing in hospitalized children |
|
| Participants |
Setting: inpatient hospital ward (pediatric, general surgery, or cardiology) Sample size: 48 Dropouts: 2 from the Relax to Sleep group lost to follow‐up because unreachable by telephone Mean age: 6.5 years (range 4 to 10) Sex: 26 boys, 22 girls Inclusion criteria: patients from the general pediatric unit, the general surgery unit, or the cardiology unit if they were between the ages of 4 and 10, in a single private room, accompanied by a caregiver staying overnight with them, and if it was anticipated the child would stay in hospital for 3 nights Exclusion criteria: patients receiving palliative care, diagnosed with a sleep or anxiety disorder, limited or abnormal movements of upper and lower extremities, too acutely ill to participate in the study, had cognitive deficits that could affect their ability to understand and carry out the intervention, or if they were receiving benzodiazepines or chloral hydrate |
|
| Interventions |
Intervention (N = 24): one‐on‐one educational session for the parent, who was guided by a standardized booklet containing information on sleep, and instructions for training the child in the use of a diaphragmatic breathing exercise Control group (N = 24): no information pertaining to sleep, sleep hygiene, or the use of diaphragmatic breathing to promote sleep |
|
| Outcomes |
Primary outcomes: sleep outcomes by actigraphy (nocturnal sleep time, daytime sleep time, longest sleep period, number of nighttime wakings, wake after sleep onset) Secondary outcomes: pain (sleep diary with Faces Pain Scale‐revised), sleep habits (Children's Sleep Habits Questionnaire), anxiety (Spence Children's Anxiety Scale and Spence Preschool Anxiety Scale), post hospital behaviors (Post‐Hospital Behavior Questionnaire), treatment adherence, feasibility, and treatment acceptance Timing of outcome assessment/time points: actigraphy over 3 days and 3 nights during the in‐hospital study period, and sleep diary data nightly. Anxiety and sleep questionnaires completed on admission and 5 to 7 days after discharge. Post‐hospital behavior questionnaire completed 5 to 7 days after discharge. |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sealed opaque envelopes Quote: "centrally controlled random allocation" |
| Allocation concealment (selection bias) | Low risk | Quote: "concealed‐group allocation" Quote: "A list of group assignments was computer‐generated by a research coordinator outside of the immediate research team and were placed in sealed opaque envelopes, which were centrally controlled." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to blind study participants or the individual who was delivering the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: research assistants performing actigraphy analysis and completing the data entry were blinded to the entire protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: minimal loss to follow‐up was experienced, with only two participants from the Relax‐to‐Sleep group lost to follow‐up because they were unreachable by telephone |
| Selective reporting (reporting bias) | Low risk | Comment: no selective outcomes reported |
| Other bias | Low risk | Comment: no issues noted |
Potasz 2010.
| Study characteristics | ||
| Methods |
Type of publication: abstract Study design: randomized controlled trial Study grouping: parallel group Country: Brazil Funding: not specified Study period: not specified Outcomes relevant to this review: validated subjective measures of sleep (sleep logs) Overall objective of study: to verify how the maintenance of play would influence sleep patterns |
|
| Participants |
Setting: general inpatient floor Sample size: 139 Dropouts: not specified Mean age: not specified (range 4 to 14 years) Sex: not specified Inclusion criteria: aged 4 to 14 years and hospitalized for respiratory illness Exclusion criteria: not specified |
|
| Interventions |
Intervention (N = 81): playing group Control group (N = 58): no playing group |
|
| Outcomes |
Primary outcomes: total sleep time (sleep log) Secondary outcomes: none noted Timing of outcome assessment/time points: during hospitalization |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information due to conference abstract |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information due to conference abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: could not blind to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information due to conference abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information due to conference abstract |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information due to conference abstract |
| Other bias | Unclear risk | Comment: insufficient information due to conference abstract |
Rennick 2018.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: parallel grouping Country: Canada Funding: Quebec Interuniversity Nursing Intervention Research Group (Groupe de Recherche Interuniversitaire en Interventions en Sciences Infirmières du Québec; GRIISIQ), now the Quebec Nursing Intervention Research Network (Réseau de Recherche en Interventions en Sciences Infirmières du Québec; RRISIQ), and the Canadian Nurses Foundation Study period: not specified Outcomes relevant to this review: participant or parent satisfaction, and sleep outcomes Overall objective of study: to examine the feasibility and acceptability of a PICU soothing intervention using touch, reading, and music |
|
| Participants |
Setting: PICU Sample size: 20 Dropouts: none specified Mean age: 7.3 years (range not specified) Sex: 13 boys, 7 girls Inclusion criteria: aged 2 to 17 years old, spoke English or French, and had a parent who spoke and read English or French, and who had agreed to be present during the intervention Exclusion criteria: diagnosed sleep, seizure, or hearing disorder; cognitive delay; previous PICU admission; or not expected to survive |
|
| Interventions |
Intervention (N = 10): PICU soothing, which consisted of a period of parental comforting (touch and reading) followed by a quiet period with music. Parents were instructed to hold their child’s hand or stroke their child softly while reassuring them they were present and to read to their child for 20 to 30 minutes, once daily. Touch and reading took place during the same time period and did not occur independently of one another. Parents selected an age‐appropriate book from a collection assembled by a Child Life Specialist. To minimize negative environmental auditory stimulation, parents were offered a choice of a fleece headband worn over the child's ears to: 1) solely reduce external noise or 2) deliver soft music. All parents chose music, and SleepPhones headbands (AcousticSheep LLC, Erie, PA) with pillow speakers were worn by PICU soothing group children after parents finished reading. The headbands remained on for up to one hour. Headbands were connected to iPods programmed with age‐appropriate playlists, developed by a Music Therapist and verified with parents. Control group (N = 10): usual care |
|
| Outcomes |
Primary outcomes: acceptability and feasibility (survey and semi‐structured interviews) Secondary outcomes: psychological well‐being, including distress (COMFORT Scale and Children's Critical Illness Impact Scale), anxiety (Revised Children's Manifest Anxiety Scale and Post Hospital Behavior Questionnaire), and sleep outcomes (sleep diaries and actigraphy) Timing of outcome assessment/time points: continuous actigraphy during hospitalization and at 3 months post‐discharge for 5 nights. Questionnaires were completed on the ward and at home. |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: group allocation was determined by parents randomly choosing a sealed, opaque envelope from the research assistant |
| Allocation concealment (selection bias) | Low risk |
Quote: "Group allocation was determined by parents randomly choosing a sealed, opaque envelope from the research assistant (RA)." Comment: intervention allocations likely could not have been foreseen before or during enrollment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "Participants, clinicians, and research staff could not be blinded to group assignment due to the nature of the intervention" Comment: allocated interventions were known by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: allocated interventions known by participants and personnel during the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome data are complete |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Other bias | Low risk | Comment: no issues noted |
Rogers 2019.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: group randomization (by month of year) Country: USA Funding: Alex’s Lemonade Stand Foundation for Childhood Cancer, and St Jude Children’s Research Hospital Cancer Center Grant/Award (CA21765) Study period: 2008 to 2011 Outcomes relevant to this review: validated objective measures of sleep Overall objective of study: to determine whether a sleep intervention compared with standard care was successful in preserving nighttime sleep in children with central nervous system cancers, hospitalized for high‐dose chemotherapy and autologous stem cell rescue, and to explore associations between sleep and fatigue during treatment |
|
| Participants |
Setting: inpatient oncology floor Sample size: 33 Dropouts: 4 children excluded for inadequate actigraphy data Mean age: 9.5 years (range 4 to 19) Sex: 20 boys, 13 girls Inclusion criteria: children with medulloblastoma or histologically similar CNS tumors, aged 4 to 19 years, able to self‐report symptoms, and English‐speaking parent and child Exclusion criteria: not specified |
|
| Interventions |
Intervention (N = 17): multicomponent cognitive and behavioral interventions delivered by one of three trained research staff, including verbal and written age‐appropriate sleep education, and relaxation training delivered on day 0. Following training, parents implemented a relaxation technique (e.g. storytelling, book reading, or massage) selected by the participant nightly, before lights out. Stimulus control measures, delivered daily throughout the study, were aimed at decreasing nighttime environmental sleep disrupters. They included establishing a lights‐out time (including turning off electronic equipment) and morning lights‐on time; choice of white noise program; thick black fabric placed over room windows to minimize light entry; and 90‐minute protected sleep periods, with bundled care between periods, instituted by nursing staff from lights out to lights on. Control group (N = 16): standard nursing care with addition of personal time spent each evening with research staff as an attention‐control measure |
|
| Outcomes |
Primary outcomes: sleep outcomes by actigraphy: total sleep time (minutes), per cent sleep (%), mean duration of sleep episodes (minutes), sleep episodes (number), longest sleep episode (minutes), wake episodes (number), longest wake episode (number), and activity score (mean number of activity counts per epoch) Secondary outcomes: fatigue (Intensity on 5‐point scale and Fatigue Scale‐Child or Fatige‐Scale‐Adolescent) Timing of outcome assessment/time points: actigraphy continuously through hospitalization and daily sleep diary completed by parents. Fatigue scales completed on Day 0, 1 to 4, and Day 5 |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study arms included the sleep intervention (INT) and standard‐of‐care (SOC) control groups. A group randomized design, randomizing by month of the year, was used to minimize condition contamination." |
| Allocation concealment (selection bias) | Low risk | Comment: no specific concerns about allocation concealment, since the study used a group randomized design where groups of participants underwent the assignment at the same time |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: research staff and clinical staff all aware of the assignment, given group randomization by month |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: group randomization by month, thus research staff and clinical staff all aware of the assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: six children were later excluded due to increased acuity |
| Selective reporting (reporting bias) | Low risk | Comment: no issues noted |
| Other bias | Low risk | Comment: no issues noted |
White 1983.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: parallel group Country: USA Funding: Department of Health, Education and Welfare (DHEW) Public Health Service (PHS) Health Research Alliance (HRA) Division of Nursing, grant number 5 R01 NU00531‐03 and grant number 5‐23 NU00100‐03 ; and DHEW PHS Bureau of Community Health Maternal Child Services grant number MCTOOOl34‐16‐0 Study period: not specified Outcomes relevant to this review: validated subjective measures of sleep Overall objective of study: to study the bedtime falling asleep behaviors of young hospitalized children, using a computer‐compatible method of observation |
|
| Participants |
Setting: general inpatient floor Sample size: 18 Dropouts: loss of 13 participants due to unanticipated early discharge and parents' decision to room‐in (i.e. sleep in child's hospital room) Mean age: 5 years and 10 months (range 3 to 8 years) Sex: not specified Inclusion criteria: aged 3 to 8 years, 3+ days of hospitalization, and parent > 18 years Exclusion criteria: controlled medications within 24 hours, deaf or aphasic, mental retardation, non‐English speaking, or critically ill or dying |
|
| Interventions |
Intervention (N = 7): 10‐minute bedtime story tape recorded by parents and individualized as desired, played to child on nights 2 and 3 of the study Control group (N = 11): no story for 3 nights of study |
|
| Outcomes |
Primary outcomes: frequency and duration of falling asleep behaviors (Falling Asleep Behavioral Inventory) Secondary outcomes: none specified Timing of outcome assessment/time points: observation for 45 minutes during bedtime on 3 consecutive nights |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Comment: randomization procedure fully described and adequate |
| Allocation concealment (selection bias) | Low risk | Comment: drawing numbers from hat |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: unable to blind to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcome assessors observing during unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 13 dropouts due to early discharge or parent decision to room‐in |
| Selective reporting (reporting bias) | Low risk | Comment: no issues noted |
| Other bias | Unclear risk | Comment: insufficient information to be able to make a judgement |
White 1990.
| Study characteristics | ||
| Methods |
Type of publication: manuscript Study design: randomized controlled trial Study grouping: parallel group Country: USA Funding: National Center for Nursing Research, National Institutes of Health, Division of Nursing bureau of Health Professions, Health Resources and Services Administration, US Public Health Service Study period: not specified Outcomes relevant to this review: validated objective measures of sleep (actigraphy), and validated subjective measures of sleep (Sleep Onset Latency Behavior Catalog) Overall objective of study: to investigate the bedtime going‐to‐sleep period or sleep onset latency of children, by testing the effects of a bedtime story on the incidence of distress, frequency and duration of self‐soothing behaviors, and length of sleep onset in young hospitalized children |
|
| Participants |
Setting: inpatient pediatric floor Sample size: 94 Dropouts: not specified Mean age: not specified (range 3 years to 8 years and 7 months) Sex: 51 boys, 43 girls Inclusion criteria: 3 to 8 years and 7 months of age, and hospitalized for at least 2 consecutive nights Exclusion criteria: medications in previous 24 hours that would have major effects on sleep |
|
| Interventions |
Intervention 1 (N = 16): parent‐recorded story, parents absent Intervention 2 (N = 14): stranger‐recorded story, parents absent Intervention 3 (N = 17): no recorded story, parents absent Intervention 4 (N = 47): no recorded story, parents present |
|
| Outcomes |
Primary outcomes: length of sleep onset, incidence of distress, and self‐soothing behaviors (observation, Sleep Onset Latency Behavior Catalog) Secondary outcomes: none specified Timing of outcome assessment/time points: variable, average of 6th hospital day for recruitment; and observation on nights 1 and 2 of study |
|
| Notes | Conflict of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: randomization procedure not fully described. Randomization only performed for 3 of 4 groups. Convenience sample used for parent‐present group based on parental preference |
| Allocation concealment (selection bias) | High risk | Comment: convenience sample used for parent‐present group, suggesting allocation may have been known |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: unable to blind for intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: observers for outcome were aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information to be able to make a judgement |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to be able to make a judgement |
| Other bias | Unclear risk | Comment: insufficient information to be able to make a judgement |
CNS: central nervous system; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; PICU: pediatric intensive care unit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd‐Elshafy 2015 | No relevant outcomes |
| Al‐Samsam 2005 | Observational study |
| Anggerainy 2019 | Not randomized |
| Carno 2004 | Observational study (not randomized) |
| Corser 1994 | Observational study (not randomized) |
| Cureton‐Lane 1997b | Observational (not randomized) |
| Dandoy 2015 | Observational study (not randomized) |
| Gamus 2016 | Not randomized |
| Hinds 2007b | Observational study |
| Hu 2015a | Systematic review in adults only |
| Kol 2015 | No relevant outcomes (no sleep outcomes, only noise levels) |
| Lassetter 2006 | Review article |
| Leventhal 1994 | No relevant outcomes |
| Liao 2018b | Systematic review focused on preterm neonates |
| Linder 2012 | Observational study |
| Liu 2020 | No relevant outcomes |
| McKissick 1990 | Observational study |
| Meltzer 2012a | Observational study |
| NCT00178321 | Withdrawn from clinicaltrials.gov; no results available |
| NCT01848158 | No relevant outcomes; no sleep outcomes reported on clinicaltrials.gov |
| Nelson 2018 | Outpatient setting only |
Characteristics of studies awaiting classification [ordered by study ID]
Beardslee 1974.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Attempted to obtain via interlibrary loan, but without success |
Ctri 2020.
| Methods | Randomized controlled trial |
| Participants | Hospitalized children |
| Interventions | Warm foot bath |
| Outcomes | Sleeping patterns |
| Notes |
NCT03453814.
| Methods |
Study design: prospective, randomized controlled study Study grouping: parallel group Study period: 1 November 2018 to 1 March 2020 Overall objective of study: to administer music therapy to participants in the PICU to observe how music affects their agitation, vital signs, and overall recovery in the unit |
| Participants |
Country: USA Setting: PICU, Oishei Children's Hospital, Buffalo, New York Sample size: 26 Dropouts: none specified Mean age: not specified but eligible age range 5 to 17 years Sex: not specified but both boys and girls were eligible to take part Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention (N = not stated): music therapy Control (N = not stated): no music therapy Administration: participants received a headset with music or dead air, once in the morning and once in the evening, for a total of 2 hours, for three days; therapy times were selected so that there were minimal disruptions. Treatments for sessions 1 and 2 of the day were the same (i.e. music/music, no music/no music). |
| Outcomes |
Primary outcome(s): agitation or sedation level of participant, measured by RASS (scored from + 4 to ‐5; positive scores equal to various levels of aggression, whereas negative score equal to various levels of sedation; a score of 0 = an alert and calm participant) Secondary outcome(s): alertness, measured by the Bispectral Index (BIS) monitor (scored from 0 to 100, where a score of 100 indicates the participant is fully awake, and a score of less than 40 is suggestive of a deep hypnotic effect) Other outcome(s): other relevant sleep outcomes not specified Timining of outcome assessment/time points: RASS and BIS scored once for each observed time point over three days |
| Notes |
Principal Investigator: David Rothstein Conflict of interest: not stated Funding: not stated but the State University of New York at Buffalo is listed as the sponsor |
PICU: pediatric intensive care unit; RASS: Richmond Agitation‐Sedation Scale
Differences between protocol and review
This review differs from the original protocol as outlined below (Kudchadkar 2017):
1. It focuses on children and adolescents in the inpatient setting, excluding infants in the neonatal ICU, for whom a recent review has been published (Liao 2018a), and due to substantial differences in sleep architecture between preterm and full term neonates, and older infants and children.
2. In the protocol, we stated we would include studies with participants above or below the stated age range if separate data could be obtained, however, we did not identify studies that met these criteria as well as the other inclusion criteria.
3. We excluded studies if they did not report any outcomes relevant to sleep (objective or subjective measures, outlined in Primary outcomes), given the focus of the review, and the fact that interventions, like physical activity, massage, etc. are used for a multitude of purposes.
4. The protocol stated we would include validated parent and nurse surveys; however, we did not identify any validated surveys. Thus, we removed this inclusion criteria to facilitate a more inclusive review, to guide future research, and highlight the need for validated tools in this domain.
5. We identified considerable clinical heterogeneity across the included studies in participant populations, measurement tools and metrics used, study duration, and timing of outcome assessment. Thus, we were unable to pool the results in a meta‐analysis for any of the outcomes, and presented mostly narrative measures of treatment effect. When possible, we calculated between‐group differences and 95% confidence intervals for individual studies. When data were insufficient to provide this information, we presented them as described in Data synthesis, and used SWiM reporting guidelines and the Cochrane Handbook (Campbell 2020; McKenzie 2021) .
Contributions of authors
Conception of the review: Sapna R Kudchadkar
Design of the review: Sapna R Kudchadkar and Blair Anton
Co‐ordination of the review: Sapna R Kudchadkar
Search and selection of studies for inclusion in the review: Blair Anton, Claire Twose, and Sapna Kudchadkar
Collection of data for the review: Jessica Berger, Tracie Walker, Ruchit Patel, Sean Barnes, Riley Mitchell, and Janey Song
Assessment of the risk of bias in the included studies: Riley Mitchell and Janey Song
Analysis of data: Sapna R Kudchadkar, Riley Mitchell, and Janey Song
Assessment of the certainty in the body of evidence: Sapna R Kudchadkar
Interpretation of data: Sapna R Kudchadkar and Jessica Berger
Writing of the review: Sapna R Kudchadkar, Jessica Berger, Tracie Walker, Ruchit Patel, Sean Barnes, Riley Mitchell, Blair Anton, Claire Twose, Janey Song, Naresh Punjabi
Sources of support
Internal sources
-
The Johns Hopkins University School of Medicine, Department of Anesthesiology and Critical Care Medicine, Baltimore, USA
Provided salary support for Dr Sapna R Kudchadkar
-
Johns Hopkins Clinical and Translational Science Award (CTSA) Number 5KL2RR025006, from the Johns Hopkins Institute for Clinical and Translational Research (ICTR), Baltimore, USA
Provided funding for Dr Sapna R Kudchadkar
-
Welch Medical Library, Johns Hopkins University, School of Medicine, USA
Provided salary support for Blair Anton and Claire Twose
External sources
No sources of support provided
Declarations of interest
SK declares a grant from the National Institutes of Health for research focused on optimizing outcomes for critically ill children, paid to Johns Hopkins University School of Medicine, Baltimore, MD, USA. SK reports publishing opinions relevant to the intervention, as her research focuses on interventions to optimize functional outcomes in critically ill children (~ 20 publications in 5 journals). SK works as a pediatric ICU physician and pediatric anesthesiologist at Johns Hopkins University School of Medicine, USA.
BA declares that she has no conflicts of interest.
NP declares that he has no conflicts of interest.
SB reports declaring opinions on topics related to this area, notably regarding delirium (three publications in two journals). SB works as a pediatric ICU physician and pediatric anesthesiologist at Johns Hopkins University School of Medicine, USA.
JB declares that she has no conflicts of interest.
RM declares that she has no conflicts of interest.
RP declares that he is a medical student at Harvard Medical School, USA.
JS declares that he has no conflicts of interest.
CT declares that she has no conflicts of interests.
TW declares that she works as a pediatric ICU physician at the University of North Carolina, USA.
New
References
References to studies included in this review
Cone 2014 {published data only}
- Cone L, Gottschlich MM, Khoury J, Simakajornboon N, Kagan RJ. The effect of healing touch on sleep patterns of pediatric burn patients: a prospective pilot study. Journal of Sleep Disorders: Treatment and Care 2014;3:2. [DOI: 10.4172/2325-9639.1000136] [DOI] [Google Scholar]
Field 1992 {published data only}
- Field T, Morrow C, Valdeon C, Larson S, Kuhn C, Schanberg S. Massage reduces anxiety in child and adolescent psychiatric patients. Journal of the American Academy of Child & Adolescent Psychiatry 1992;31(1):125-31. [DOI: 10.1097/00004583-199201000-00019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hinds 2007a {published data only}
- Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, Cremer L, et al. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. Journal of Pain and Symptom Management 2007;33(6):686-97. [DOI: 10.1016/j.jpainsymman.2006.09.025] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2016 {published data only}
- Jacobs S, Mowbray C, Cates LM, Baylor A, Gable C, Skora E, et al. Pilot study of massage to improve sleep and fatigue in hospitalized adolescents with cancer. Paediatric Blood & Cancer 2016;63(5):880-6. [DOI: 10.1002/pbc.25902] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papaconstantinou 2018 {published data only}
- Papaconstantinou EA, Hodnett E, Stremler R. A behavioral-educational intervention to promote pediatric sleep during hospitalization: a pilot randomized controlled trial. Behavioral Sleep Medicine 2018;16(4):356-70. [DOI: 10.1080/15402002.2016.1228639] [PMID: ] [DOI] [PubMed] [Google Scholar]
Potasz 2010 {published data only}
- Potasz C, Sella VG, Varela MV, Carvalho LB, Prado LF, Prado GF. Effects of play activities on sleep of hospitalized children. Sleep 2010;33:A338. [ABSTRACT #: 1013] [WEB PAGE: tinyurl.com/y453gouz] [Google Scholar]
Rennick 2018 {published data only}
- Rennick JE, Stremler R, Horwood L, Aita M, Lavoie T, Majnemer A, et al. A pilot randomized controlled trial of an intervention to promote psychological well-being in critically ill children: soothing through touch, reading, and music. Pediatric Critical Care Medicine 2018;19(7):e358-66. [DOI: 10.1097/PCC.0000000000001556] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rogers 2019 {published data only}
- Rogers VE, Zhu S, Ancoli-Israel S, Liu L, Mandrell BN, Hinds PS. A pilot randomized controlled trial to improve sleep and fatigue in children with central nervous system tumors hospitalized for high-dose chemotherapy. Pediatric Blood & Cancer 2019;66(8):e27814. [DOI: 10.1002/pbc.27814] [PMCID: PMC7416343] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1983 {published data only}
- White MA, Wear E, Stephenson G. A computer-compatible method for observing falling asleep behavior of hospitalized children. Research in Nursing & Health 1983;6(4):191-8. [DOI: 10.1002/nur.4770060407] [PMID: ] [DOI] [PubMed] [Google Scholar]
White 1990 {published data only}
- White MA, Williams PD, Alexander DJ, Powell-Cope GM, Conlon M. Sleep onset latency and distress in hospitalized children. Nursing Research 1990;39(3):134-9. [PMID: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd‐Elshafy 2015 {published data only}
- Abd-Elshafy SK, Khalaf GS, Abo-Kerisha MZ, Ahmed NT, El-Aziz MA, Mohamed MA. Not all sounds have negative effects on children undergoing cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2015;29(5):1277-84. [DOI: 10.1053/j.jvca.2015.01.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Samsam 2005 {published data only}
- Al-Samsam RH, Cullen P. Sleep and adverse environmental factors in sedated mechanically ventilated pediatric intensive care patients. Pediatric Critical Care Medicine 2005;6(5):562-7. [DOI: 10.1097/01.pcc.0000165561.40986.a6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anggerainy 2019 {published data only}
- Anggerainy SW, Wanda D, Nurhaeni N. Music therapy and story telling: nursing interventions to improve sleep in hospitalized children. Comprehensive Child and Adolescent Nursing 2019;42(Suppl 1):82-9. [DOI: 10.1080/24694193.2019.1578299] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carno 2004 {published data only}
- Carno M-A, Hoffman LA, Henker R, Carcillo J, Sanders MH. Sleep monitoring in children during neuromuscular blockade in the pediatric intensive care unit: a pilot study. Pediatric Critical Care Medicine 2004;5(3):224-9. [DOI: 10.1097/01.pcc.0000124024.92280.f9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Corser 1994 {published data only}
- Corser NC. Sleep of One- and Two-Year-Old Children in Intensive Care [Doctoral thesis]. Birmingham (AL): University of Alabama at Birmingham, 1994. [Google Scholar]
Cureton‐Lane 1997b {published data only}
- Cureton-Lane RA, Fontaine DK. Sleep in the pediatric ICU: an empirical investigation. American Journal of Critical Care 1997;6(1):56-63. [PMID: ] [PubMed] [Google Scholar]
Dandoy 2015 {published data only}
- Dandoy C, Coleman K, Petiniot L, Flesch L, Byars KC, Pate A, et al. Prospective pilot study evaluating sleep disruption in children and young adults undergoing stem cell transplantation. Pediatric Blood & Cancer 2015;62(Suppl 2):S32. [DOI: 10.1002/pbc] [POSTER: #511] [DOI] [Google Scholar]
Gamus 2016 {published data only}
- Gamus D. Nightly massage for improvement of sleep in hospitalised adolescent children with cancer – a feasibility study. Focus on Alternative and Complementary Therapies 2016;21(3-4):176-7. [DOI: 10.1111/fct.12266] [DOI] [Google Scholar]
Hinds 2007b {published data only}
- Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, McCarthy K, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncology Nursing Forum 2007;34(2):393-402. [DOI: 10.1188/07.ONF.393-402] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hu 2015a {published data only}
- Hu R-F, Jiang X-Y, Chen J, Zeng Z, Chen XY, Li Y, et al. Non-pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD008808. [DOI: 10.1002/14651858.CD008808.pub2] [PMCID: PMC6517220] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kol 2015 {published data only}
- Kol E, Aydın P, Dursun O. The effectiveness of environmental strategies on noise reduction in a pediatric intensive care unit: creation of single-patient bedrooms and reducing noise sources. Journal for Specialists in Pediatric Nursing 2015;20(3):210-7. [DOI: 10.1111/jspn.12116] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lassetter 2006 {published data only}
- Lassetter JH. The effectiveness of complementary therapies on the pain experience of hospitalized children. Journal of Holistic Nursing 2006;24(3):196-211. [DOI: 10.1177/0898010106289838] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leventhal 1994 {published data only}
- Leventhal BA. Effect of Imagery and Progressive Muscle Relaxation Training on State/Trait Anxiety in Hospitalized Adolescents with Chronic Illnesses [Doctoral thesis]. St Louis (MO): University of Missouri – St Louis, 1994. [Google Scholar]
Liao 2018b {published data only}
- Liao J-H, Hu R-F, Su L-J, Wang S, Xu Q, Qian X-F, et al. Nonpharmacological interventions for sleep promotion on preterm infants in neonatal intensive care unit: a systematic review. Worldviews on Evidenced-Based Nursing 2018;15(5):386-93. [DOI: 10.1111/wvn.12315] [PMID: ] [DOI] [PubMed] [Google Scholar]
Linder 2012 {published data only}
- Linder LA, Christian BJ. Nighttime sleep disruptions, the hospital care environment, and symptoms in elementary school-age children with cancer. Oncology Nursing Forum 2012;39(6):553-61. [DOI: 10.1188/12.ONF.553-561] [PMCID: PMC3638990] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2020 {published data only}
- Liu M-H, Zhu L-H, Peng J-X, Zhang X-P, Xiao Z-H, Liu Q-J, et al. Effect of personalized music intervention in mechanically ventilated children in the PICU: a pilot study. Pediatric Critical Care Medicine 2020;21(1):e8-14. [DOI] [PubMed] [Google Scholar]
McKissick 1990 {published data only}
- McKissick MY. Sleep and Sleep Disruption of School-Age Children in the Pediatric Intensive Care Unit: Isolation Rooms and the Open Unit [Masters thesis]. Columbus (OH): Ohio State University, College of Nursing, 1990. [Google Scholar]
Meltzer 2012a {published data only}
- Meltzer LJ, Davis KF, Mindell JA. Patient and parent sleep In a children's hospital. Pediatric Nursing 2012;38(2):64-71. [PMID: ] [PubMed] [Google Scholar]
NCT00178321 {published data only}
- NCT00178321. Improving sleep in the pediatric intensive care unit [Improving sleep and outcomes in critically ill children]. clinicaltrials.gov/ct2/show/NCT00178321 (first received 12 September 2005).
NCT01848158 {published data only}
- NCT01848158. Acupuncture to improve comfort of children on a ventilator in the intensive care unit [Safety, feasibility & effectiveness of acupuncture as an adjunct to pharmacologic treatment for sedation and analgesia in mechanically ventilated PICU patients - a pilot study]. clinicaltrials.gov/ct2/show/NCT01848158 (first received 2 May 2013).
Nelson 2018 {published data only}
- Nelson JK, James LE, Cone LC, Gottschlich MM. 320. Effectiveness of healing touch on sleep, pain, anxiety, anesthesia emergence and satisfaction. Journal of Burn Care & Research 2018;39(Suppl 1):S130. [DOI: 10.1093/jbcr/iry006.242] [DOI] [Google Scholar]
References to studies awaiting assessment
Beardslee 1974 {published data only}
- Beardslee CI. Factors which influence the sleep-wakefulness pattern of young, hospitalized children at nap time. Ann Arbor (MI): University of Pittsburgh, 1974. [Google Scholar]
Ctri 2020 {unpublished data only}
- CTRI/2020/10/028647. Efficacy of warm foot bath in promotion of early sleep onset among hospitalized children. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45782 (first received 26 October 2020).
NCT03453814 {published data only}
- NCT03453814. Music intervention for agitation reduction in the pediatric intensive care unit. clinicaltrials.gov/ct2/show/NCT03453814 (first received 27 Feburary 2018).
Additional references
Aaron 1996
- Aaron JN, Carlisle CC, Carskadon MA, Meyer TJ, Hill NS, Millman RP. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep 1996;19(9):707-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Abdeyazdan 2016
- Abdeyazdan Z, Mohammadian-Ghahfarokhi M, Ghazavi Z, Mohammadizadeh M. Effects of nesting and swaddling on the sleep duration of premature infants hospitalized in neonatal intensive care units. Iranian Journal of Nursing and Midwifery Research 2016;21(5):552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Almadhoob 2020
- Almadhoob A, Ohlsson A. Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD010333. [DOI: 10.1002/14651858.CD010333.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baley 2015
- Baley J. Skin-to-skin care for term and preterm infants in the neonatal ICU. Pediatrics 2015;136(3):596-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barnes 2016
- Barnes SS, Kudchadkar SR. Sedative choice and ventilator-associated patient outcomes: don't sleep on delirium. Annals of Translational Medicine 2016;4(2):34. [DOI: 10.3978/j.issn.2305-5839.2015.12.40] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2021
Busch‐Vishniac 2005
- Busch-Vishniac IJ, West JE, Barnhill C, Hunter T, Orellana D, Chivukula R. Noise levels in Johns Hopkins Hospital. Journal of the Acoustic Society of America 2005;118(6):3629-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI: 10.1136/bmj.l6890] [PMCID: PMC7190266] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2009
- Cao H, Pan X, Li H, Liu J. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2009 Nov;15(11):1171-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2017
- Cheung YT, Brinkman TM, Mulrooney DA, Mzayek Y, Liu W, Banerjee P, et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2017;123(17):3410-9. [DOI: 10.1002/cncr.30742] [PMCID: PMC5570612] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choong 2021
- Choong K, Zorko DJ, Awojoodu R, Ducharme-Crevier L, Fontela PS, Lee LA, et al. Prevalence of acute rehabilitation for kids in the PICU: a Canadian multicenter point prevalence study. Pediatric Critical Care Medicine 2021;22(2):181-93. [DOI: 10.1097/pcc.0000000000002601] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cook 2020
- Cook DJ, Arora VM, Chamberlain M, Anderson S, Peirce L, Erondu A, et al. Improving hospitalized children's sleep by reducing excessive overnight blood pressure monitoring. Pediatrics 2020;146(3):e20192217. [DOI: 10.1542/peds.2019-2217] [PMCID: PMC7461242 (available on 2021-09-01)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corser 1996
- Corser NC. Sleep of 1- and 2-year-old children in intensive care. Issues in Comprehensive Pediatric Nursing 1996;19(1):17-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version Accessed December 31, 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Cureton‐Lane 1997a
- Cureton-Lane RA, Fontaine DK. Sleep in the pediatric ICU: an empirical investigation. American Journal of Critical Care 1997;6(1):56-63. [PMID: ] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Endnote 2016 [Computer program]
- Endnote. Version Endnote X7. Amsterdam (NL): Thomsen Reuters, 2016. Available from endnote.com/.
Erondu 2019
- Erondu AI, Orlov NM, Peirce LB, Anderson SL, Chamberlain M, Hopkins K, et al. Characterizing pediatric inpatient sleep duration and disruptions. Sleep Medicine 2019;57:87-91. [DOI: 10.1016/j.sleep.2019.01.030] [PMCID: PMC6760863] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feinberg 2013
- Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. American Journal of Physiology 2013;304(4):R296-303. [DOI: 10.1152/ajpregu.00422.2012] [PMC3567357] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foreman 2015
- Foreman B, Westwood AJ, Claassen J, Bazil CW. Sleep in the neurological intensive care unit: feasibility of quantifying sleep after melatonin supplementation with environmental light and noise reduction. Journal of Clinical Neurophysiology 2015;32(1):66-74. [DOI: 10.1097/WNP.0000000000000110] [PMID: ] [DOI] [PubMed] [Google Scholar]
Freedman 2001
- Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. American Journal of Respiratory and Critical Care Medicine 2001;163(2):451-7. [DOI: 10.1164/ajrccm.163.2.9912128] [PMID: ] [DOI] [PubMed] [Google Scholar]
Glotzbach 1993
- Glotzbach SF, Rowlett EA, Edgar DM, Moffat RJ, Ariagno RL. Light variability in the modern neonatal nursery: chronobiologic issues. Medical Hypotheses 1993;41(3):217-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 31 December 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Haba‐Rubio 2004
- Haba-Rubio J, Ibanez V, Sforza E. An alternative measure of sleep fragmentation in clinical practice: the sleep fragmentation index. Sleep Medicine 2004;5(6):577-81. [DOI: 10.1016/j.sleep.2004.06.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hinds 2007
- Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, McCarthy K, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncology Nursing Forum 2007;34(2):393-402. [DOI: 10.1188/07.ONF.393-402] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hopkins 2015
- Hopkins RO, Choong K, Zebuhr CA, Kudchadkar SR. Transforming PICU culture to facilitate early rehabilitation. Journal of Pediatric Intensive Care 2015;4(4):204-11. [NIHMS774205] [PMC4849412] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2015
- Hu RF, Jiang XY, Chen J, Zeng Z, Chen XY, Li Y, et al. Non-pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD008808. [DOI: 10.1002/14651858.CD008808.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ista 2020
- Ista E, Scholefield BR, Manning JC, Harth I, Gawronski O, Bartkowska-Śniatkowska A, et al. Mobilization practices in critically ill children: a European point prevalence study (EU PARK-PICU). Critical Care 2020;24(1):1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamdar 2014
Kamdar 2015
- Kamdar BB, Niessen T, Colantuoni E, King LM, Neufeld KJ, Bienvenu OJ, et al. Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Critical Care Medicine 2015;43(1):135-41. [DOI: 10.1097/CCM.0000000000000610] [PMC4269569] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamdar 2016
- Kamdar BB, Combs MP, Colantuoni E, King LM, Niessen T, Neufeld KJ, et al. The association of sleep quality, delirium, and sedation status with daily participation in physical therapy in the ICU. Critical Care 2016;20:261. [DOI: 10.1186/s13054-016-1433-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocevska 2017
- Kocevska D, Muetzel RL, Luik AI, Luijk MP, Jaddoe VW, Verhulst FC, et al. The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: the Generation R Study. Sleep 2017;40(1):zsw022. [DOI: 10.1093/sleep/zsw022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Koo 2008
- Koo YJ, Koh HJ. Effects of eye protective device and ear protective device application on sleep disorder with coronary disease patients in CCU. Journal of Korean Academy of Nursing 2008;38(4):582-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kryger 2005
- Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. Philadelphia (PA): Elsevier Saunders, 2005. [Google Scholar]
Kudchadkar 2009
- Kudchadkar SR, Sterni L, Yaster M, Easley RB. Sleep in the intensive care unit. Contemporary Critical Care 2009;7(1):1-13. [http://bit.ly/1MiYc0n] [Google Scholar]
Kudchadkar 2014a
- Kudchadkar SR, Aljohani OA, Punjabi NM. Sleep of critically ill children in the pediatric intensive care unit: a systematic review. Sleep Medicine Reviews 2014;18(2):103-10. [DOI: 10.1016/j.smrv.2013.02.002] [PMC3883975] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kudchadkar 2014b
- Kudchadkar SR, Yaster M, Punjabi NM. Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: a wake-up call for the pediatric critical care community. Critical Care Medicine 2014;42(7):1592-600. [DOI: 10.1097/CCM.0000000000000326] [PMC4061156] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kudchadkar 2016a
- Kudchadkar SR, Beers MC, Ascenzi JA, Jastaniah E, Punjabi NM. Nurses' perceptions of pediatric intensive care unit environment and work experience after transition to single-patient rooms. American Journal of Critical Care 2016;25(5):e98-107. [DOI: 10.4037/ajcc2016463] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kudchadkar 2016b
- Kudchadkar SR, Shata N, Aljohani OA, AlHarbi A, Jastaniah E, Nadkarni A, et al. Day-night activity rhythms are disrupted In children admitted to the pediatric ICU after major surgery. American Journal of Respiratory and Critical Care Medicine 2016;193:A3096. [www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A3096] [Google Scholar]
Kudchadkar 2016c
- Kudchadkar SR, Easley RB, Brady KM, Yaster M. Pain and sedation management. In: Nichols DG, Shaffner DH, editors(s). Rogers’ Textbook Of Pediatric Intensive Care. 5th edition. Hong Kong (China): Wolters Kluwer, 2016:132-63. [Google Scholar]
Kudchadkar 2020
- Kudchadkar SR, Nelliot A, Awojoodu R, Vaidya D, Traube C, Walker T, et al, Prevalence of Acute Rehabilitation for Kids in the PICU (PARK-PICU) Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Physical rehabilitation in critically ill children: a multicenter point prevalence study in the United States. Critical Care Medicine 2020;48(5):634-44. [DOI: 10.1097/CCM.0000000000004291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laffan 2010
- Laffan A, Caffo B, Swihart BJ, Punjabi NM. Utility of sleep stage transitions in assessing sleep continuity. Sleep 2010;33(12):1681-6. [PMC2982738] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2018a
- Liao J-H, Hu R-F, Su L-J, Wang S, Xu Q, Qian X-F, et al. Nonpharmacological interventions for sleep promotion on preterm infants in neonatal intensive care unit: a systematic review. Worldviews on Evidence-Based Nursing 2018;15(5):386-93. [DOI: 10.1111/wvn.12315] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2005
- Liu YC, Chen CH, Wang TM, Chi CS. Noise distribution in closed and open spaces in the neonatal intensive care unit. Clinical Neonatology 2005;12(1):26–9. [http://bit.ly/1T3X7Li] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available fromtraining.cochrane.org/handbook/archive/v6.2.
Meltzer 2007
- Meltzer LJ, Mindell JA, Owens JA, Byars KC. Use of sleep medications in hospitalized pediatric patients. Pediatrics 2007;119(6):1047-55. [DOI: 10.1542/peds.2006-2773] [PMID: ] [DOI] [PubMed] [Google Scholar]
Meltzer 2012
- Meltzer LJ, Davis KF, Mindell JA. Patient and parent sleep in a children's hospital. Pediatric Nursing 2012;38(2):64-71. [PMID: ] [PubMed] [Google Scholar]
Microsoft 2016 [Computer program]
- Microsoft Excel. Version 15.0. Microsoft Corporation, 2016. Available from office.microsoft.com/excel.
Mindell 2018/08
- Mindell JA, Williamson AA. Benefits of a bedtime routine in young children: sleep, development, and beyond. Sleep Medicine Reviews 2018;40:93-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murali 2003
- Murali NS, Svatikova A, Somers VK. Cardiovascular physiology and sleep. Frontiers in Bioscience 2003;8:s636-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Owens 2000
- Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 2000;23(8):1043-51. [PMID: ] [PubMed] [Google Scholar]
Pandharipande 2006
- Pandharipande P, Ely EW. Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill. Critical Care Clinics 2006;22(2):313-27. [DOI: 10.1016/j.ccc.2006.02.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4.1. The Cochrane Collaboration, 2020.
Richards 1998
- Richards KC. Effect of a back massage and relaxation intervention on sleep in critically ill patients. American Journal of Critical Care 1998;7(4):288-99. [PMID: ] [PubMed] [Google Scholar]
Richards 2003
- Richards K, Nagel C, Markie M, Elwell J, Barone C. Use of complementary and alternative therapies to promote sleep in critically ill patients. Critical Care Nursing Clinics of North America 2003;15(3):329-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Richardson 2003
- Richardson S. Effects of relaxation and imagery on the sleep of critically ill adults. Dimensions of Critical Care Nursing 2003;22(4):182-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Richardson 2007
- Richardson A, Allsop M, Coghill E, Turnock C. Earplugs and eye masks: do they improve critical care patients' sleep? Nursing in Critical Care 2007;12(6):278-86. [DOI: 10.1111/j.1478-5153.2007.00243.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saliski 2015
- Saliski M, Kudchadkar SR. Optimizing sedation management to promote early mobilization for critically ill children. Journal of Pediatric Intensive Care 2015;4(4):188-93. [DOI: 10.1055/s-0035-1563543] [PMC4686268] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saré 2016
- Saré RM, Levine M, Hildreth C, Picchioni D, Smith CB. Chronic sleep restriction during development can lead to long-lasting behavioral effects. Physiology & Behavior 2016;155:208-17. [NIHMS750670] [PMC4720986] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scotto 2009
- Scotto CJ, McClusky C, Spillan S, Kimmel J. Earplugs improve patients' subjective experience of sleep in critical care. Nursing in Critical Care 2009;14(4):180-4. [DOI: 10.1111/j.1478-5153.2009.00344.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Simons 2016
- Simons KS, Laheij RJ, den Boogaard M, Moviat MA, Paling AJ, Polderman FN, et al. Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: a randomised controlled trial. Lancet 2016;4(3):194-202. [DOI: 10.1016/S2213-2600(16)00025-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, et al. Diagnosing delirium in critically ill children: validity and reliability of the Pediatric Confusion assessment method for the intensive care unit. Critical Care Medicine 2011;39(1):150-7. [DOI: 10.1097/CCM.0b013e3181feb489] [PMC3776416] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taguchi 2007
- Taguchi T, Yano M, Kido Y. Influence of bright light therapy on postoperative patients: a pilot study. Intensive & Critical Care Nursing 2007;23(5):289-97. [DOI: 10.1016/j.iccn.2007.04.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walder 2000
- Walder B, Francioli D, Meyer JJ, Lançon M, Romand JA. Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels. Critical Care Medicine 2000;28(7):2242-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wieczorek 2016
- Wieczorek B, Ascenzi J, Kim Y, Lenker H, Potter C, Shata NJ, et al. PICU up!: impact of a quality improvement intervention to promote early mobilization in critically ill children. Pediatric Critical Care Medicine 2016;17(12):e559-66. [DOI: 10.1097/PCC.0000000000000983] [PMC5138131] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kudchadkar 2017
- Kudchadkar SR, Barnes S, Anton B, Gergen DJ, Punjabi NM. Non-pharmacological interventions for sleep promotion in hospitalized children. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD012908. [DOI: 10.1002/14651858.CD012908] [DOI] [PMC free article] [PubMed] [Google Scholar]


