Supplemental Digital Content is Available in the Text. Social observational learning can augment the effect of pain medication in patients with chronic low back pain leading to a reduction in perceived pain-related disability.
Keywords: Placebo analgesia, Social learning, Chronic back pain, Placebo effect, Sham patient, Additive placebo effect
Abstract
Clinical research on social observational learning (SoL) as an underlying mechanism for inducing expectancy and eliciting analgesic placebo effects is lacking. This double-blinded randomized controlled clinical trial investigated the influence of SoL on medication-augmenting placebo effects in 44 patients with chronic low back pain. Our hypothesis was that observing positive drug effects on pain and mobility in another patient could increase pain reduction and functional capacity. To test this, we compared the effects of observing positive treatment outcomes in a sham patient (the social learning group [SoLG]) vs hearing the same sham patient report neutral effects (the control group). In the SoLG, the sham patient told peers about pain reduction due to amitriptyline and demonstrated his improved mobility by bending forwards and sideways while he told the control group only that he was taking amitriptyline. The primary outcome was a reduction in clinical low back pain self-ratings. The secondary outcome was perceptions of pain-related disability. The exploratory outcome was mood and coping statements. Data collection occurred before and after the intervention and 2 weeks later. After the intervention, pain decreased in both groups (F [1, 41] = 7.16, P < 0.05, d = 0.83), with no difference between groups. However, the SoLG showed a significantly larger decrease in perceived disability (F [1, 41] = 5, P < 0.05, d = 0.63). The direct observation of patient with chronic low back pain of positive treatment outcomes in the sham patient seems to have enhanced the treatment effects while indirect verbal reports of reduced pain did not.
1. Intr. oduction
There is an abundance of evidence of analgesic placebo effects,11,14 with research tending to focus on the underlying neurobiological pathways and molecular substrates of placebo analgesia and their interaction with psychological mechanisms.14,20,28,37 Expectations are considered to play a central role in mediating the mechanisms and their effects,8 but further research is needed, including investigations into social observational learning (SoL) as the underlying mechanism inducing expectancy and eliciting placebo effects, alongside classical conditioning and verbal instructions, and the translation to patients with chronic pain (eg, augmentation of the placebo effects of effective medication).
Although verbal suggestions and classic conditioning have been shown to elicit placebo effects,7,15,35,52 SoL has not been extensively researched. The Bandura social learning theory4,5 indicates that observational learning should also elicit clinically relevant placebo effects. Current research shows that SoL plays a central role in activating expectancies64 and thus promoting placebo analgesia.12,37,51 One study found empathy to be a moderating factor. The influence of SoL on pain perception has been previously demonstrated,2,16,19,60 its basic principles have been researched,31,59 and it has been defined as an independent type of learning.41
To deduce the clinical implications of the placebo effects, it is vital to show that such mechanisms are also found in patients with chronic pain to improve pharmacological pain management.36
Analgesic placebo effects—such as core pharmacological efficacy—are not only triggered by neutral substances (the placebo) but also by verum treatments. The effect of an active analgesic component can be enhanced by placebo effects.13 Accordingly, the effectiveness of pain therapeutics is indicated by the pharmacological and the psychological (placebo) components. The analgesic effects of placebos are mediated by expectancies of positive treatment outcomes, which are elicited by verbal instructions and classical conditioning.7,15,35,52 However, recent research has shown that conditioning also has effects that are not mediated by expectations.15,54 Positive expectancies of analgesics can activate the inherent psychological component.24 Because this placebo effect accompanies the effect of the verum medication, it is described as an augmented therapeutic placebo effect.36 To date, no studies of SoL have investigated augmented placebo effects46 on pharmacological treatments in a clinical context (eg, augmented placebo component of amitriptyline).
Our central questions were as follows: (1) is it possible to induce the augmented placebo components of a pain medication such as amitriptyline (prescribed before the beginning of the study) in clinical pain of patients with chronic low back pain (CLBP) using SoL? (2) Is it possible to achieve this augmented placebo effect on pain experience and functional capacity using SoL? We hypothesized that SoL would enhance the positive treatment effects of pain medication in patients with CLBP.
For this purpose, we examined a sample of 44 patients with CLBP in an experimental setting, with an assumed patient (the “sham patient”) talking about the positive effects of the medication that the patients were already taking and demonstrating his own progress in mobility.
2. Method.
2.1. Participants.
The participants were 44 adult patients with CLBP that had lasted longer than 6 months. They were recruited at the University Hospital Hamburg-Eppendorf (UKE), Germany. The sample comprised 18 men (9 per group) and 26 women (13 per group). Their ages ranged between 35 and 82 years, with a mean age of 62.79 years. The inclusion criteria were persistent and ongoing CLBP for more than 6 months, medication that had started at least 3 weeks before the start of the study, and treatment with amitriptyline (M – 21.31) in the outpatient clinic for chronic pain of the University Hospital Hamburg-Eppendorf. The pain specialist in charge excluded patients with acute or chronic mental disorders, as defined by the International Classification of Diseases-10,65 other than F45.41 (“chronic pain disorder with somatic and psychological factors”), F32.0 (“mild depression”), or F32.1 (“moderate depression”); insufficient language skills or reading abilities; cognitive impairment; or intake of medication affecting awareness. The pain specialist also excluded patients exhibiting “red flags” such as acute trauma, unexplained weight loss, or long-lasting fever.38 The mental health problems were diagnosed by psychotherapists. The pain specialist in charge selected the patients and invited them to participate in the study.
Each patient was randomly assigned to one of the 2 groups: either the “social learning group” (SoLG) or the “control group” (CG). In a process of simple randomization, the sham patient drew a lot to identify the role that he would play (SoLG or CG). The group assignments were not known to the examiners until the survey had been completed; thus, the examiners were completely blinded. The study was approved by the local institutional review board, followed the guidelines of the Declaration of Helsinki, and was registered to the German Clinical Trials registry (DRKS00011230).
2.2. Setting.
The study took place at the outpatient pain center in the University Hospital Hamburg-Eppendorf while the participants were attending a scheduled routine appointment. This setting was chosen to strengthen the credibility of the sham patient.
2.3. Procedure.
At the time of the study, amitriptyline was recommended in the S3 guidelines for the treatment of patients with CLBP,9 as part of an overall therapeutic concept for the treatment of CLBP, such as an interdisciplinary treatment or treatment with analgesics.23 The treatment regimen in the pain outpatient clinic (therapy-as-usual) followed these guidelines, and owing to individual indication, metamizole was also part of the drug treatment regimen. Amitriptyline is a tricyclic antidepressant and analgesic that acts as a nonselective monoamine reuptake inhibitor.21 Only 30% of the antidepressive active dose is needed for effective pain medication.9 It was recommended in a low analgesic dosage in the S3 guidelines as an adjuvant analgesic for the treatment of low back pain, as the standard medication treatment.9 The standard medication amitriptyline was used in our SoL intervention. Amitriptyline was prescribed according to the analgesic dosage range (4-50 mg), rather than the antidepressive dosage (75-300 mg53). The medication was documented before the study commenced and kept stable throughout: the patients were not allowed to change the dose or intake times of the medication during the 2-week period. The patients were also monitored at the 2-week follow-up for deviations in their medication.
2.4. Study design
This controlled clinical study used a parallel group design with repeated measures. This was a double-blind experimental trial, and the participants were randomly assigned to 2 groups (SoLG and CG) by simple randomization. The pain specialist, the participants, and the experimenter were blinded to the group allocations. The patients did not know that there were 2 groups or to which of the groups they had been assigned. A male sham patient described his positive treatment outcomes to participants in the SoLG group and did not refer to treatment outcomes with the CG.
Before a routine appointment with their familiar pain specialist, the patients were informed that options for improving the efficacy of amitriptyline were being tested. They were not informed about the specific goal of the study (the investigation of the augmented placebo effect) or that they would be divided into 2 groups.
The baseline data collection took place in the waiting room, before the appointment, with the study assistant (a medical student) available for questions.
The appointment with the pain specialist (an anesthesiologist) was a 20-minute medical consultation in which the patients were asked about the following:
The effectiveness of their pain medication.
The tolerability of their medication (amitriptyline).
Their general health condition.
The appointment was not focused on functional capacity. At the end of the patients' regular follow-up, the pain specialist left the treatment room, stating that he had to retrieve a new prescription pad. The sham patient, a male pensioner with a neat appearance and no medical or psychological training, entered the room on the pretext of looking for the pain specialist. The sham patient followed a set script for both groups (available as supplemental digital content at http://links.lww.com/PAIN/B521). In the SoLG, he said that his CLBP had considerably reduced since he started taking amitriptyline. He also said that his mobility had improved, and he demonstrated this by leaning forward and bending sideways. He commented that this movement was now painless. He gave equal time to discussing his improved mobility and pain reduction. By contrast, the sham patient asked the CG group where he should leave the questionnaire.
Immediately after the appointment (approximately 20 minutes after baseline collection), the postintervention data were collected. The participants were then contacted 2 weeks later to fill out the last questionnaire (posttest). On average, it took the patients 15 to 30 minutes to complete each questionnaire. We offered a face-to-face debriefing after posttest, with a debriefing script used to inform the participants about the deception and the true nature of the study. None of the participants objected to the study's goal or having been deceived. Figure 1 provides a detailed timeline. In addition, at postintervention, the participants were asked about their awareness of the sham patient.
Figure 1.
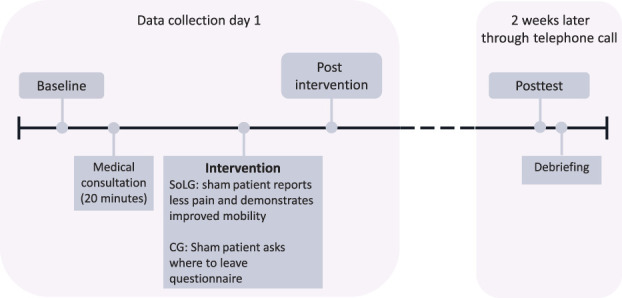
Detailed timeline of the experiment with data collection points and intervention.
2.5. Dependent variables and measurements
The primary outcome was measured using the average daily clinical back pain self-reports (see below). The participants were asked to report daily pain changes. Their everyday disability scores were also collected, and these provided data on the secondary outcome (see below).
2.5.1. Assessment of clinical back pain
The patients were asked to rate the intensity of their current back pain on an 11-point numerical rating scale (NRS), from 0 (“no pain”) to 10 (“worst pain imaginable”),55 using a questionnaire at baseline and a telephone interview at posttest. They gave ratings for the most intense pain and their average pain during the previous 6 months at baseline (questionnaire) and, at posttest, during the previous 2 weeks (telephone interview; primary outcome). At baseline, they were asked to rate their average pain over the previous 6 months (“no pain diary”) to enable an estimation of their mean clinical pain. At posttest, they were asked to rate their average pain over the previous 2 weeks to enable an estimation of their mean pain during the trial period (“no pain diary”). In a similar manner, they rated the average level of disability caused by their CLBP and affecting their daily activities, using an 11-point NRS from 0 (“no impairment”) to 11 (“unable to do any activity”), for the preceding 6 months at baseline and the previous 2 weeks at posttest. Average mood was rated on an 11-point NRS from 0 (“good, no impairment”) to 10 (“bad, severe impairment”) for the previous 6 months at baseline and 2 weeks at posttest (exploratory outcome).
For a measurement of pain severity over time, we asked the participants to report their perceived overall health status in the previous year and their perceived overall health status since the onset of their CLBP, using a 5-point scale with the following question and Likert answers:
How would you describe your health status compared with a year ago/since the onset of the CLBP? Much better than a year ago/since the onset of CLBP, somewhat better than a year ago/since the onset of CLBP, the same as one year ago/since the onset of CLBP, somewhat worse than a year ago/since the onset of CLBP, or much worse than a year ago/since the onset of CLBP. The patients also had the option of reporting the location and frequency of their pain.
2.5.2. Assessment of disability
A 12-item scoring system was used to assess the patients' functional disability. This was the “Hannover Functional Ability Questionnaire for diagnosis of functional disability in everyday life caused by back ache,”38,39 the German Funktionsfragebogen Hannover zur alltagsnahen Diagnostik der Funktionsbeeinträchtigung durch Rückenschmerzen, (FFbHR)39 (secondary outcome). This questionnaire has been shown to correlate with the Roland–Morris scale for disability in low back pain.49,50 The response options are able to perform without difficulties, able to perform with difficulties, and unable to perform or only with help. The scores for the items were calculated, divided by the total number of items, and then standardized. The results are values between 0% and 100% of functional capacity.
2.5.3. Assessment of the mental state
Depressed mood in the previous week was assessed using the 20-item scoring system “the general scale of depression” (ADS)30 and the adapted German version of the Center for Epidemiologic Studies Depression Scale46) (exploratory outcome). Four items were inverted. The item scores were used to calculate a total score in the range of 0 to 60.
2.5.4. Assessment of patients' mental pain behavior
The patients' mental behavior (exploratory outcome) was assessed using an 18-item scoring system measuring the occurrence of pain-related negative and active coping self-statements, with 6 options for responses, ranging from 1 (“never”) to 6 (“nearly always”). The tool was the Fragebogen zur Erfassung schmerzbezogener Selbstinstruktionen (FSS) or the “questionnaire for assessment of pain-related self-statements.”25) The patients were asked to rate how often they made pain-related negative and active coping self-statements. There were 9 active coping self-statements that supported constructive pain coping and 9 negative coping self-statements that hindered constructive pain coping. For active and negative coping self-statements, the item values were combined to give a total score in the range of 9 to 54.
2.5.5. Assessment of expectancy pain relief and mobility
Expected pain relief was assessed with the question, “What kind of pain relief do you expect from amitriptyline?” Expected mobility was assessed by asking, “What kind of mobility improvement do you expect from amitriptyline?” The participants responded to both questions on a 6-point scale (1 = very good, 2 = good, 3 = satisfactory, 4 = sufficient, 5 = deficient, and 6 = inadequate).
2.5.6. Assessment of perceptions of the sham patient
At the postintervention visit (2 weeks later), participants were asked whether they had noticed the sham patient and were invited to respond using a 6-point scale: There was no one else in the room, noticed him very little, noticed him a little, did not pay attention to the other person, noticed him, or noticed him very much.
The following dependent variables were collected at baseline and posttest: ratings of the most intense pain and average pain, activity and mood ratings, perceived overall health status, functional disability (FFbHR39), depressed mood (ADS30), and coping statements (FSS25). The measurements collected at postintervention were as follows: current pain intensity, mood, expected pain relief and mobility, and detection of the sham patient.
2.5.7. Assessment of empathy
Empathy was measured with the “empathic concern scale” of the Interpersonal Reactivity Index18 as used in the study by Colloca and Benedetti.12 The patients were asked to rate statements on empathy on a 5-point scale (0 = not applicable at all” and 4 = highly applicable). We used the German version.42
2.6. Statistical analysis
Generalized linear models were estimated by using primary intervention effects with repeated-measures analyses of variance (ANOVAs). We calculated a repeated-measures ANOVA and Cohen d to assess effect sizes (baseline and posttest) to determine the course for each group and to analyze average daily clinical pain, FSS,25 ADS,30 and FFbHR.39 These variables were collected only at baseline and posttest because the time interval between baseline and postintervention (approximately 45 minutes) was too short to identify changes using these questionnaires, which were designed for longer periods.
The parameter d was reported to quantify the effect size of the F-tests. The presumed alpha error was 0.05. When necessary (e.g. a significant Mauchly test), a Greenhouse–Geisser correction was used, resulting in partial degrees of freedom. Using G*Power, we estimated that a sample size of 44 was needed to detect small effect sizes (f = 0.25) in a repeated-measures ANOVA with 2 groups.22 All statistical analyses were performed using SPSS 22 (SPSS, Inc, Chicago, IL).
3. Results
3.1. Sample characteristics
A total of 342 patients with back pain persisting for at least 6 months and a prescription for amitriptyline at any point were screened for eligibility. Of this total, 47 patients were included, 3 of whom did not complete the study, leaving 44 data sets for analysis. Of the excluded patients, 201 did not meet the inclusion criteria (cessation of treatment with amitriptyline or severe depression), 68 declined to participate, and 27 could not be reached by telephone for other reasons (Fig. 2). Data collection took place between October 2016 and April 2018 and ended when the sample size goal was achieved.
Figure 2.
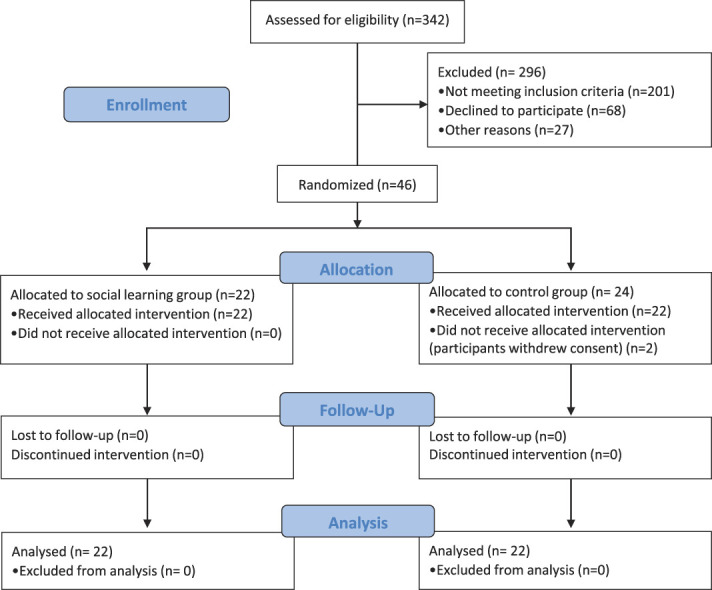
Consort flowchart.
During the study, pain severity and medical history were recorded. In addition to taking amitriptyline, 59.1% of the patients (68% of the SoLG and 50% of the CG) also took metamizole, 56.8% (72.7% SoLG and 4.9% CG) anticonvulsants, and 43.2% (43.2% SoLG and 43.2% CG) opioids. In addition, 13.6% (9.1% SoLG and 18.2% CG) received nonsteroidal anti-inflammatory drugs, 11.4% (4.5% SoLG and 18.2% CG) lidocaine, and 4.5% (0% SoLG and 9.1% CG) duloxetine. One patient in the SoLG (2.3%) was taking mirtazapine and 1 in the CG was taking triptans.
The average age of the participants was 62.79 years, and 61.36% were female (c.f. Table 1). The mean pain duration was 165.52 months (range = 6-552 months), mean perceived overall health compared with 1 year ago was 2.02 (range = 1-5), and mean perceived overall health compared with onset of CLBP was 1.89 (range = 1-5). The mean dosage of amitriptyline prescription was 92.52 (c.f. Table 1).
Table 1.
Baseline demographic and clinical characteristics.
| Social learning group (n = 22) | Control group (n = 22) | Overall | Difference (P, χ2) | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age | 61.91 (12.86) | 63.75 (12.67)1) | 62.79 | 1.84 (F = 0.22, P = 0.64) |
| Sex: Female | 13 (59.09%) | 14 (63.64%) | 61.36% | 4.55% (χ2 = 0.1, P = 0.76) |
| Clinical characteristics | ||||
| Back pain duration in total (mo) | 151 (154.59) | 180.05 (140.12) | 165.52 | 29.05 (F = 0.43, P = 0.52) |
| Back pain duration of current intensity (mo) | 57.36 (53.74) | 97.68 (84.60) | 77.52 | 40.32 (F = 3.56, P = 0.07) |
| Disability caused by back pain | 6.14 (2.71) | 6.05 (2.77) | 6.09 | 0.09 (F = 0.01, P = 0.91) |
| Perceived health compared with 1 year ago | 2 (1.23) | 2.05 (1.53) | 2.02 | 0.05 (F = 0.01, P = 0.91) |
| Perceived health compared with onset chronic pain | 1.95 (1.33) | 1.81 (1.47) | 1.89 | 0.14 (F = 0.1, P = 0.75) |
| Average pain (numerical rating scale 0-10) | 6.36 (1.94) | 6.23 (1.77) | 6.3 | 0.13 (F= 0.06, P = 0.81) |
| Back pain intensity (strongest pain in the past 6 mo) | 8.18 (1.82) | 8.27 (1.70) | 8.23 | 0.09 (F = 0.03, P = 0.86) |
| Pain-related active coping self-statement | 37.14 (8.01) | 31.45 (7.55) | 5.86 | 5.69 (F = 5.86, P = 0.02) |
| Pain-related negative coping self-statement | 26.68 (9.68) | 29.73 (11.04) | 28.20 | 3.05 (F = 0.95, P = 0.34) |
| Depressed mood (Allgemeine Depressionsskala) | 21.09 (10.38) | 22.00 (9.15) | 21.53 | 0.91 (F = 0.09, P = 0.76) |
| Functional capacity in % (FFbHR) | 50.38 (26.32) | 59.09 (21.69) | 54.73 | 8.71 (F = 1.44, P = 0.24) |
| Duration of amitriptyline intake (mo) | 19.91 (24.64) | 18.36 (24.00) | 19.14 | 1.55 (F = 0.04, P = 0.83) |
| Dosage of amitriptyline (mg) | 48.91 (90.94) | 49.95 (96.21) | 92.52 | 1.04 (F = 2.61, P = 0.11) |
Data are mean values (SD), n (%), or n. Bold indicates only pain-related active coping self-statement revealed significant differences between groups with significantly more active coping self-statements in the social learning group. 1). n = 20 in the control group because 2 participants did not fill out their age.
3.2. Baseline comparison
The SoLG and the CG did not differ significantly regarding the main outcome of average pain at baseline (F [1, 42] = 0.06, P = 0.81; c.f. Table 1). There was no significant difference between the groups at baseline in the use of pain-related negative coping self-statements (F [1, 42] = 0.95, P = 0.34; c.f. Table 1). Despite the randomization, the SoLG reported significantly more pain-related active coping self-statements than the CG did (F [1, 42] = 5.86, P = 0.02; c.f. Table 1). At baseline, there was no significant difference between the groups in depressed mood (F [1, 42] = 0.09, P = 0.76) or functional capacity (F [1, 42] = 1.44, P = 0.24; c.f. Table 1).
There were no significant differences between the groups regarding age (F [1, 41] = 0.22, P = 0.64), pain duration (F [1, 43] = 0.43, P = 0.52), perceived overall health compared with 1 year ago (F [1, 43] = 0.01, P = 0.91), perceived overall health compared with onset of CLBP (F [1, 43] = 0.1, P = 0.75), or dosage of amitriptyline prescription (F [1, 43] = 2.61, P = 0.11; c.f. Table 1). The χ2 tests were conducted to analyze the sex distribution between the 2 groups, and this revealed no significant difference (χ2 = 0.1; c.f. Table 1).
3.3. Primary outcome
It is important to note that “baseline” refers to the period before the intervention, “postintervention” to directly after the intervention, and “posttest” to 2 weeks after the intervention (c.f. Fig. 1).
3.3.1. Average pain assessment: significant improvement in both groups, but no difference between groups
The results showed a significant main effect on the variable “average pain,” and both groups reported a significant reduction in pain between baseline and posttest (F [1, 41] = 7.16, P = 0.01), resulting in a large effect size of d = 0.82 (c.f. Table 2). However, there was neither a significant group main effect (F [1, 41] = 0.47, P = 0.5) nor a significant interaction between “time course” and “group” (F [3, 44] = 0.69, P = 0.41). Hence, both groups experienced less pain, and the SoLG did not benefit significantly more in this way than the CG (c.f. Table 2).
Table 2.
Changes from baseline to posttest in average pain ratings, cognitive and emotional processing of pain, and self-ratings of functional capacity.
| Average pain | Functional capacity | Pain-related active coping self-statements | Pain-related negative self-statements | Depressed mood | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Posttest | Baseline | Posttest | Baseline | Posttest | Baseline | Posttest | Baseline | Posttest | |||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Social learning | 6.36 | 1.94 | 5.91 | 1.85 | 50.38 | 26.32 | 53.60 | 24.68 | 37.14 | 8.01 | 38.45 | 7.51 | 26.68 | 9.68 | 23.55 | 9.32 | 21.09 | 10.38 | 14.68 | 10.49 |
| Control group | 6.23 | 1.77 | 5.36 | 1.81 | 59.09 | 21.69 | 55.87 | 23.35 | 31.45 | 7.55 | 35.27 | 6.74 | 29.73 | 11.04 | 24.45 | 9.40 | 22.00 | 9.15 | 14.71 | 8.21 |
| ANOVA | F (1,41) | P | d | F (1,41) | P | d | F (1,41) | P | d | F (1,41) | P | d | F (1,41) | P | d | |||||
| Time | 7.16 | 0.01 | 0.82 | 0.00 | ≤1.0 | 0 | 5.63 | 0.02 | 0.73 | 11.41 | 0.002 | 1.04 | 40.19 | < 0.05 | 1.98 | |||||
| ANOVA | F (1,41) | P | d | F (1,41) | P | d | F (1,41) | P | d | F (1,41) | P | d | F (1,41) | P | d | |||||
| Group | 0.47 | 0.5 | 0.2 | 0.6 | 0.44 | 0.24 | 5.04 | 0.03 | 11.18 | 0.53 | 0.47 | 0.23 | 0.03 | 0.86 | 0.06 | |||||
| ANOVA | F (3,44) | P | d | F (3,44) | P | d | F (3,44) | P | d | F (3,44) | P | d | F (3,44) | P | d | |||||
| Time × group | 0.69 | 0.41 | 0.26 | 4.22 | 0.046 | 0.63 | 1.33 | 0.25 | 0.35 | 0.74 | 0.4 | 0.26 | 0.17 | 0.69 | 0.13 | |||||
Mean values (M) and SDs for both groups at baseline and at posttest. The results of a repeated-measures analysis of variance (ANOVA) for the main effect time, group, and interaction of time and group (ie, difference between both groups in baseline to posttest changes) with F values (F), probability (P), and effect size Cohen d (d). Bold indicates average pain, active, and negative pain-related self-statements and depressed mood improved significantly from baseline to posttest, but there was no difference between groups. The effect time and group interaction was only significant for functional capacity.
3.4. Secondary outcomes
3.4.1. Pain behavior—functional capacity: only the social learning group benefited significantly
The results showed a significant interaction between “time course” and “group” (F [3, 44] = 4.22, P = 0.046; c.f. Table 2 and Fig. 3). The SoLG saw a more significant increase in functional capacity than the CG did (c.f. Table 2 and Fig. 3). This effect was medium-sized (d = 0.63; c.f. Table 2 and Fig. 3). There was no significant “group main effect” (F [1, 41] = 0.6, P = 0.44). The SoLG saw an increase in functional capacity of 3.22%, whereas the CG saw a decrease of 3.22% (c.f. Table 2).
Figure 3.
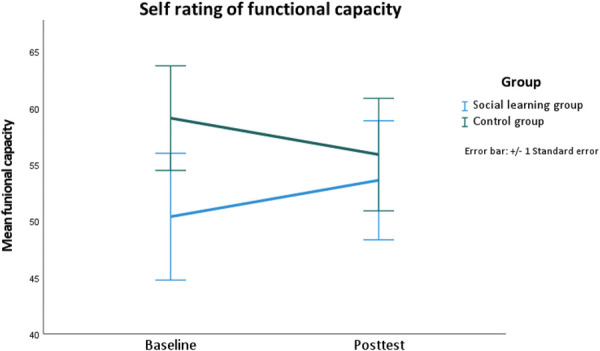
Difference between baseline and 2 weeks after the intervention for functional capacity separated by group.
3.4.2. Pain behavior—cognitive processing (exploratory outcome): there was a significant reduction in pain-related negative self-statements and a significant increase in active coping self-statements in both groups
Both groups saw a significant increase in the occurrence of pain-related active coping self-statements (F [1, 41] = 5.63, P = 0.02) and a significant decrease in pain-related negative coping self-statements (F [1, 41] = 11.41, P = 0.002; c.f. Table 2). The effect size for pain-related active coping self-statements was medium-sized (d = 0.73) but large for pain-related negative coping self-statements (d = 1.04; c.f. Table 2). No significant interaction was found between “time course” and “group” for pain-related active coping self-statements (F [3, 44] = 1.33, P = 0.25) or pain-related negative coping self-statements (F [3, 44] = 0.74, P = 0.4; c.f. Table 2). There was a significant “group main effect” for active coping self-statements (F [1, 41] = 5.04, P = 0.03), but not for negative coping self-statements (F [1, 41] = 0.53, P = 0.47).
3.4.3. Pain behavior—emotional processing: there was a significant reduction in symptoms of depression in both groups, but no difference between the groups
The results showed a significant main effect of the intervention on depressed mood (F [1, 41] = 40.19, P <0.05, d = 1.98; c.f. Table 2). There was no significant interaction between “time course” and “group” (F [2, 44] = 0.17, P = 0.69; c.f. Table 2); thus, both groups improved and did not significantly differ in their courses. There was no significant “group main effect” for depressed mood (F [1, 41] = 0.03, P = 0.86, c.f. Table 2).
3.4.4. Expected pain relief did not change from baseline to postintervention, but it did increase significantly from postintervention to posttest
There was a significant change in expected pain relief over the 3 data collection points (baseline, postintervention, and posttest; F (2, 42) = 6.28, P < 0.05, c.f. Tables 3 and 4), but there was no difference in this change between the groups (Time*group; F [2, 40] = 0.99, P = 0.38, c.f. Tables 3 and 4). Although the contrasts between baseline and postintervention were not significantly different (F [2, 40] = 3.03, P = 0.09, c.f. Tables 3 and 4), the contrasts between postintervention and posttest were (F [2, 40] = 6.6, P < 0.05, c.f. Tables 3 and 4), with an effect size of d = 0.79 (c.f. Tables 3 and 4).
Table 3.
Baseline, postintervention, and posttest scores in expected pain relief by amitriptyline.
| Expected pain relief by amitriptyline | ||||||
|---|---|---|---|---|---|---|
| Baseline | Postintervention | Posttest | ||||
| M | SD | M | SD | M | SD | |
| Social learning | 3.27 | 1.75 | 3 | 1.69 | 2.36 | 1.68 |
| Control group | 3.86 | 2.66 | 3.77 | 3.69 | 3.45 | 2.77 |
Mean values (M) and SDs for both groups at baseline, postintervention, and posttest.
Table 4.
Changes from baseline, postintervention, and posttest in expected pain relief by amitriptyline.
| Expected pain relief by amitriptyline | |||
|---|---|---|---|
| F value | Probability (P) | Effect size (d) | |
| ANOVA | F (2,42) | P | d |
| Time | 6.28 | <0.05 | 1.1 |
| ANOVA | F (2,42) | P | d |
| Group | 1.56 | 0.22 | 0.39 |
| ANOVA | F (2,40) | P | d |
| Time × group | 0.99 | 0.38 | 0.26 |
| Time | F (2,40) | P | d |
| Baseline vs postintervention | 3.03 | 0.09 | 0.54 |
| Time | F (2,40) | P | d |
| Postintervention vs posttest | 6.6 | <0.05 | 0.79 |
| Time × group | F (2,40) | P | d |
| Baseline vs postintervention | 0.76 | 0.39 | 0.27 |
| Time × group | F (2,40) | P | d |
| Postintervention vs posttest | 0.73 | 0.4 | 0.26 |
The results of a repeated-measures analysis of variance (ANOVA) for the main effect time and interaction of time and group (ie, difference between both groups in baseline, postintervention, and posttest changes) with F values (F), probability (P) and effect size Cohen d (d). In addition, contrasts of time (ie, both groups together) comparing expectation ratings at baseline vs postintervention as well as ratings at postintervention vs posttest. Bold indicates only the comparison between postintervention and posttest yielded a significant difference. The contrast time × group (ie, comparing the change from one data point to another between groups) did not result in significant differences.
3.4.5. Explicit memory of sham patient was low, and there was no difference between the groups
The responses were summarized in 2 categories: did not notice the sham patient (“there was no one else in the room” to “did not pay attention to the other person”) and noticed the sham patient (“noticed him” to “noticed him very much”). Only 6 participants in the SoLG and 4 in the CG indicated that they remembered the sham patient at all (c.f. Table 5). There was no significant difference between the groups (χ2 = 0.52, P = 0.47, c.f. Table 5).
Table 5.
Memory of the sham patient.
| Social learning group (n = 22) | Control group (n = 22) | Comparison (P, χ2) | |
|---|---|---|---|
| No memory of sham patient | 16 (72.73%) | 18 (81.82%) | 0.47(0.52) |
| Aware of sham patient | 6 (27.27%) | 4 (18.18%) |
Data are n (%) or n. χ2 for group comparison (ie, were more participants in 1 group aware of the sham patient than the other?) was not significant.
3.4.6. Age, sex, and empathy did not moderate the effects of social observational learning
Moderator analyses were used to determine whether SoL was dependent on age, sex, or empathy scores as moderators. The change in average pain and in functional capacity was used as dependent variables, and group allocation, age, sex, and empathy as well as the interaction terms were used as predictors. In the model with the predictors, group allocation (β = −0.84, P = 0.31), age (β = −0.47, P = 0.35), and the interaction term of group allocation and age (β = 1.15, P = 0.24) did not significantly predict the change in average pain. The regression model with group allocation (β = −0.04, P = 0.94), sex (β = −0.21, P = 0.67), and the interaction of group allocation and sex (β = 0.24, P = 0.74) also did not predict the change in average pain. The predictors group allocation (β = −0.58, P = 0.38), empathy (β = −0.71, P = 0.13), and the interaction group allocation and empathy (β = 1.18, P = 0.25) did not significantly predict the change in average pain.
The predictors group allocation (β = −0.57, P = 0.47), age (β = 0.007, P = 0.99), and the interaction of group allocation and age (β = 0.29, P = 0.76) did not predict the change in functional capacity. In a model with the predictors group allocation (β = −0.18, P = 0.73), sex (β = 0.18, P = 0.7), and the interaction term group allocation and sex (β = −0.18, P = 0.8), the predictors could not significantly predict change in functional capacity. A regression model with group allocation (β = −1.2, P = 0.051), empathy (β = −0.77, P = 0.09), and the interaction group allocation and empathy (β = 1.2, P = 0.12) could also not predict change in functional capacity.
4. Discussion
We investigated the influence of SoL on the effectiveness of amitriptyline in a randomized double-blind controlled clinical study with a sample of 44 patients with CLBP. Two weeks after the intervention, both groups reported lower average pain. However, the SoLG showed a significant improvement in perceived mobility while the CG reported a decline. The SoL was intended to augment the placebo component of an already established medication regimen.
4.1. An analgesic placebo effect was seen in both groups due to the patient–physician relationship
The significant pain reduction seen after 2 weeks in both groups could be attributed to the interviews with the pain specialist that were attended by both groups. To support this conclusion, a comparison with a natural history group would have been required.44 Nevertheless, the significantly different courses of the groups' functional capacities indicate that the change cannot be attributed to the mere natural course of the CLBP. All the patients had been taking the medication for at least 4 months before the study and were thus accustomed to its effects. The pain reduction in both groups could be due to the interviews refocusing their attention on the medication's pain-relieving effects and acting as a specific cue (Fig. 4), thereby eliciting the augmented analgesic placebo effect. Highlighting beneficial aspects in a positive atmosphere is an aspect of “open medication” that elicits and increases placebo effectiveness,1,6,13 and a positive physician–patient relationship strongly promotes these effects.26,27,32,33,48,58 However, we did not find a change in expectations immediately after the pain specialist interview, and thus, the mode of action seems to be the refocusing of the patients' attention on the beneficial effect of the medicine. Although the mean reduction in average pain (difference = 0.66) was statistically significant, clinical significance starts at a 2-point reduction on the 10-point NRS.56 Nevertheless, it is remarkable that a short intervention of just 20 minutes with the physician could have such a significant effect. Future studies are needed to explore whether exposure to repetitive SoL could produce a clinically relevant reduction.
Figure 4.
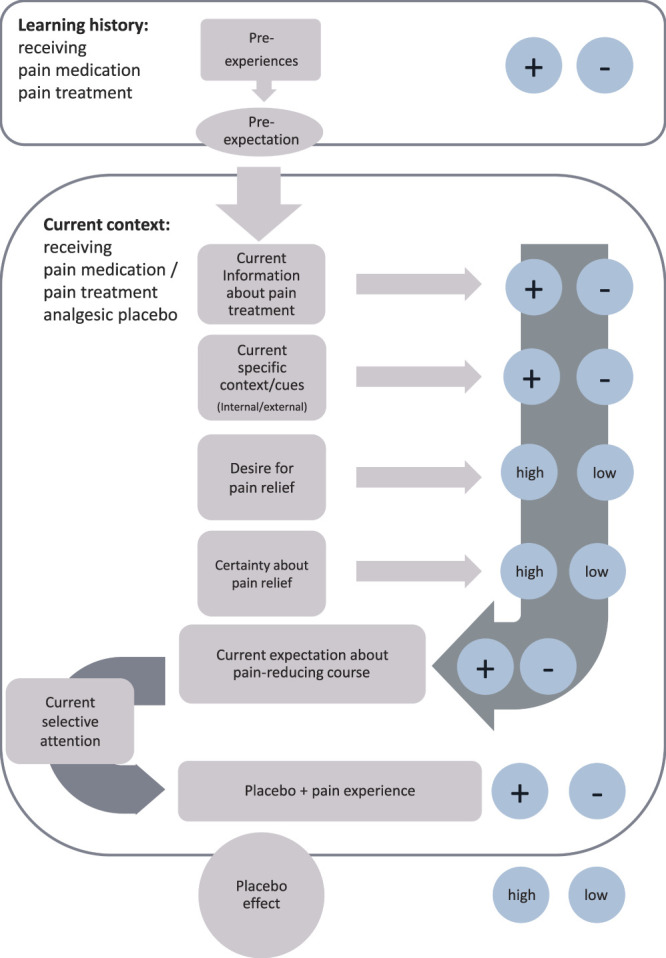
Learning model of placebo effect imbedded in learning history and contextual cues.
4.2. Social observational learning augmented the placebo effect on functional capacity, but not on pain intensity
The improvement in perceived mobility in the SoLG and decline in the CG is an indication of the power of SoL for improving functional capacity. However, this effect was not found for average pain reduction, and thus, it may be that a visual observation leaves a stronger impression than a verbal report.66 The SoLG directly observed the sham patient's improvement in mobility because he demonstrated movements that were not possible before taking amitriptyline. By contrast, they could not directly observe the decreased pain intensity through simple changes in facial expression. Research which demonstrates that SoL can induce placebo effects showed changes in facial expressions in demonstrators.12,57,64,67 We therefore assume that pain reduction through SoL requires direct observation of pain reduction through changes in facial expression or other behavior. In our study, both groups were asked about their functional capacity. This focused their attention on their perceptions of their disabilities in everyday life. The SoLG had a positive SoL experience from observing the sham patient and had improved in functional capacity, as we hypothesized. The CG, on the other hand, had focused its attention on functional capacity but had not had a positive experience and was thus dissatisfied with its level of impairment, thus decreasing its perceptions of its functional capacity.
The mean improvement in functional capacity (3.6%) was not clinically significant. However, the improvement in the placebo responders (13 of 22) is high (10.26%) and similar to that reported as clinically significant (12%, as suggested by 39). Our study is one of the first to translate mechanisms of SoL into the clinical arena with the intent to increase therapeutic responses. In future, larger studies are needed to demonstrate the effectiveness of SoL as an adjuvant intervention.
4.3. Change in expectations 3 weeks after the intervention
Our study did not find any changes in treatment expectations (expected pain intensity) directly after the intervention. We postulate that the physician interviews were a specific cue that refocused the participants' attention on the positive effects of the medication (Fig. 3).
The influence of expectations on placebo outcomes has been demonstrated (for a review, see Refs. 34 and 43). However, our results differ because, while there was no change in expectation ratings, there was nonetheless a pain reduction. The studies demonstrating the influence of expectations on placebo outcomes differ from our investigation in various aspects. First, they used expectation ratings as predictors after the placebo interventions but did not verify whether participants' expectations had changed before or after the interventions.17,29,45,61 This suggests that changing expectations through an intervention has not been as thoroughly researched as expectancy as a predictor. Second, previous studies have collected data on experimentally induced pain.45,52 This creates a strong focus on the intervention itself; while in our study, the presence of the sham patient was concealed by a cover story. In addition, predictions (ie, expectations) of pain intensity were far more important in these studies because the pain stimulus arrived in the short term. When the pain experience is immediate (ie, occurring seconds or minutes later), the attention-focusing cue is much more salient. We propose that without sufficient salience (ie, when there is no threat of immediate pain) patients do not immediately change their expectations. Linde et al.40 also rated expectations after 3 sessions of acupuncture and found no changes, which may be in line with our argument that, in a more clinical setting, intentional and explicit expectation ratings are evident only after longer periods. Expectations (ie, predictions of pain intensity) are based on experienced treatment effects. In experimental studies, participants receive immediate feedback. In a clinical setting (especially with medication), the patient must wait before they can identify whether the medication has worked. This might explain why there was no immediate change in expectations after the intervention. This is supported by the findings of Colloca et al.,15 which showed that treatment outcomes are mediated by previous therapeutic outcomes and not expectations.
4.4. No moderating effects of empathy
Although one of the first studies on SoL on placebo effects12 found empathy to be a moderator, subsequent research3,47 did not find this effect. We could not find this effect in our study either.
4.5. Limitations
After the start of this study amitriptyline was removed from the German guidelines for CLBP treatment10 limiting our possibility to explore long-term effects of the SoL on the medication. Long-term studies on SoL should be developed in clinical settings. In this study, both groups showed significant improvements in average pain, mood, and cognitive coping. While we attribute these changes to contextual factors, they could also be the result of regression to the mean. To exclude this, one would need to include a natural history group to demonstrate the natural fluctuation of CLBP. Owing to the lack of natural history group, we cannot unequivocally attribute the observed changes to the SoL behavioral intervention.
A shortcoming of this study is its sample size, which was small. Even if the sample size were underestimated for the primary and secondary outcomes, the power of the study was sufficient to find a significant effect on functional capacity.
In our study, the participants were already taking the amitriptyline medication, and our aim was to use SoL to augment the effects of an existing treatment. However, the effects may have been stronger at the beginning of the treatment than after several weeks or months of treatment experience. Our experimental procedure could have resulted in stronger effects in the case of a de novo amitriptyline treatment.
4.6. Outlook
We have demonstrated that the augmented placebo component of amitriptyline on functional capacity can be enhanced by observing another person experiencing greater mobility as a result of receiving the same treatment. This should be implemented in theoretical models. The augmentation of pain-free mobility is an important part of therapy because it reduces pain behaviors and increases quality of life. In line with the fear-avoidance model,63,62 the SoL experience could lessen fear of pain and enable participants to enjoy more active lifestyles. One clinical use could involve interactions between a patient just beginning therapy with another who has completed therapy and regained mobility.
Our results have various clinical implications. Many studies are conducted under controlled laboratory settings, and hence, the pain experience is immediate and, as argued above, changes in expectations are quickly measurable. In an everyday clinical setting, patients do not take the medication immediately after prescription, and the effects of the pain medication are often built-up and dose dependent. Over time, the physician's advice for the medication may be long forgotten and the specific cue may have lost its salience. Sharing the observations of a patient who has improved could create a tangible memory, comprising an easy-to-add adjuvant intervention. In group sessions, patients could share their positive experiences with others and describe their improvements. Videos could be shown of patients before and after a treatment, highlighting the improvements in their functional capacity.
The participants' reported lack of awareness of the sham patient was unexpected. In future studies on the implementation of SoL in clinical settings, it is important to ensure that the patients recall the encounter. This might be achieved by setting up multiple encounters. As in other studies conducted in laboratory settings,3,12 the patients could take notes on pain improvement, pain expression, functional capacity, and so on. To increase salience, a video of a patient with everyday problems due to CLBP could be shown. Augmenting placebo effects through SoL can be exploited for optimal pain management, and clinical studies should investigate how this can be of benefit for the daily lives of patients with CLBP.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B521.
Acknowledgements
Author contribution: conception: M. Schwartz, L.-M. Fischer, C. Bläute, J. Stork, L. Colloca, C. Zöllner, and R. Klinger; design: M. Schwartz, L.-M. Fischer, J. Stork, and R. Klinger; acquisition of data: L.-M. Fischer, C. Bläute, and J. Stork; data analysis: M. Schwartz, L.-M. Fischer, and R. Klinger; interpretation of data: M. Schwartz, L.-M. Fischer, and R. Klinger; writing of the manuscript: M. Schwartz, L.-M. Fischer, C. Bläute, J. Stork, L. Colloca, C. Zöllner, and R. Klinger. All authors are familiar with the content, take responsibility for the completeness and accuracy of the content, and have approved the final version of the manuscript.
This research was funded by grants from the Deutsche Forschungsgemeinschaft DFG (the German Research Foundation): FOR 1328-1 to R. Klinger (Kl 1350-3.1) and SFB/TRR 289 Project No. 422744262 to R. Klinger.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
M. Schwartz and L.-M. Fischer contributed equally to this work.
German Clinical Trials Register (No. DRKS00011230).
Contributor Information
Marie Schwartz, Email: ma.schwartz@uke.de.
Laura-Marie Fischer, Email: L-MFischer@web.de.
Corinna Bläute, Email: c.blaeute@uke.de.
Jan Stork, Email: JStork@tabea-krankenhaus.de.
Luana Colloca, Email: colloca@umaryland.edu.
Christian Zöllner, Email: c.zoellner@uke.de.
References
- [1].Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. PAIN 2001;90:205–15. [DOI] [PubMed] [Google Scholar]
- [2].Ashton-James CE, Richardson DC, Williams ACdC, Bianchi-Berthouze N, Dekker PH. Impact of pain behaviors on evaluations of warmth and competence. PAIN 2014;155:2656–61. [DOI] [PubMed] [Google Scholar]
- [3].Bajcar EA, Wiercioch-Kuzianik K, Farley D, Adamczyk WM, Buglewicz E, Bąbel P. One of us or one of them? The effects of the model's and observer's characteristics on placebo analgesia induced by observational learning. PLoS One 2020;15:e0243996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bandura A, Ross D, Ross SA. Transmission of aggression through imitation of aggressive models. J Abnormal Soc Psychol 1961;63:575. [DOI] [PubMed] [Google Scholar]
- [5].Bandura A. Social foundations of thought and action. Englewood Cliffs, New Jersey: Prentice Hall, 1986:23–8. [Google Scholar]
- [6].Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet 1995;346:1231. [DOI] [PubMed] [Google Scholar]
- [7].Bingel U. Avoiding nocebo effects to optimize treatment outcome. JAMA 2014;312:693–4. [DOI] [PubMed] [Google Scholar]
- [8].Bingel U. Placebo 2.0: the impact of expectations on analgesic treatment outcome. PAIN 2020;161:S48–56. [DOI] [PubMed] [Google Scholar]
- [9].Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), (AWMF) AdWMF. Nationale VersorgungsLeitlinie Kreuzschmerz – Langfassung. Version 1.2. 2010. 2010. Available at: http://wwwversorgungsleitliniende/themen/kreuzschmerz. Accessed January 24, 2021. [Google Scholar]
- [10].Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), (AWMF) AdWMF. Nationale VersorgungsLeitlinie Nicht-spezifischer Kreuz-schmerz – Langfassung, 2. Auflage. Version 1. 2017. Available at: http://wwwversorgungsleitliniende/themen/kreuzschmerz. Accessed January 24, 2021. [Google Scholar]
- [11].Colloca L, Barsky AJ. Placebo and nocebo effects. N Engl J Med 2020;382:554–61. [DOI] [PubMed] [Google Scholar]
- [12].Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. PAIN 2009;144:28–34. [DOI] [PubMed] [Google Scholar]
- [13].Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol 2004;3:679–84. [DOI] [PubMed] [Google Scholar]
- [14].Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: psychological and neurobiological mechanisms. PAIN 2013;154:511–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Colloca L, Akintola T, Haycock NR, Blasini M, Thomas S, Phillips J, Corsi N, Schenk LA, Wang Y. Prior therapeutic experiences, not expectation ratings, predict placebo effects: an experimental study in chronic pain and healthy participants. Psychotherapy Psychosomatics 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Colloca L. Placebo, nocebo, and learning mechanisms. In: Benedetti F, Enck P, Frisaldi E, Schedlowski M, editors. Placebo. Berlin: Springer Berlin Heidelberg, 2014. pp. 17–35. [Google Scholar]
- [17].Cormier S, Lavigne GL, Choinière M, Rainville P. Expectations predict chronic pain treatment outcomes. PAIN 2016;157:329–38. [DOI] [PubMed] [Google Scholar]
- [18].Davis MH. A multidimensional approach to individual differences in empathy, 1980. [Google Scholar]
- [19].De Ruddere L, Goubert L, Vervoort T, Kappesser J, Crombez G. Impact of being primed with social deception upon observer responses to others' pain. PAIN 2013;154:221–6. [DOI] [PubMed] [Google Scholar]
- [20].Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov 2013;12:191–204. [DOI] [PubMed] [Google Scholar]
- [21].Fangmann P, Assion HJ, Juckel G, González CÁ, López-Muñoz F. Half a century of antidepressant drugs: on the clinical introduction of monoamine oxidase inhibitors, tricyclics, and tetracyclics. Part II: tricyclics and tetracyclics. J Clin Psychopharmacol 2008;28:1–4. [DOI] [PubMed] [Google Scholar]
- [22].Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- [23].Finnerup NB. Nonnarcotic methods of pain management. N Engl J Med 2019;380:2440–8. [DOI] [PubMed] [Google Scholar]
- [24].Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet 2010;375:686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Flor H, Turk D. Pain-related cognitions, pain severity, and pain behaviors in chronic pain patients. PAIN 1987;30:S416. [Google Scholar]
- [26].Gallagher J, Waldron FL, Stack J, Barragry J. Dress and address: patient preferences regarding doctor's style of dress and patient interaction. Irish Med J 2008;101:211–13. [PubMed] [Google Scholar]
- [27].Gherardi G, Cameron J, West A, Crossley M. Are we dressed to impress? A descriptive survey assessing patients' preference of doctors' attire in the hospital setting. Clin Med 2009;9:519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gollub RL, Kirsch I, Maleki N, Wasan AD, Edwards RR, Tu Y, Kaptchuk TJ, Kong J. A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. J Pain 2018;19:515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haanstra TM, Kamper SJ, Williams CM, Spriensma AS, Lin C-WC, Maher CG, De Vet HC, Ostelo RW. Does adherence to treatment mediate the relationship between patients' treatment outcome expectancies and the outcomes of pain intensity and recovery from acute low back pain?. PAIN 2015;156:1530–6. [DOI] [PubMed] [Google Scholar]
- [30].Hautzinger M, Bailer M. Allgemeine Depressionsskala (ADS). Deutsche Form der Center for Epidemiologic Studies Depression Scale (CES-D): Beltz Text, 1993. [Google Scholar]
- [31].Hoppitt W, Laland KN. Social learning: an introduction to mechanisms, methods, and models: Princeton University Press, 2013. [Google Scholar]
- [32].Howe LC, Goyer JP, Crum AJ. Harnessing the placebo effect: exploring the influence of physician characteristics on placebo response. Health Psychol 2017;36:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008;336:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ 2020:370. [DOI] [PubMed] [Google Scholar]
- [35].Kirsch I, Kong J, Sadler P, Spaeth R, Cook A, Kaptchuk TJ, Gollub R. Expectancy and conditioning in placebo analgesia: separate or connected processes?. Psychol Conscious Theor Res Pract 2014;1:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Klinger R, Colloca L, Bingel U, Flor H. Placebo analgesia: clinical applications. PAIN 2014;155:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koban L, Jepma M, López-Solà M, Wager TD. Different brain networks mediate the effects of social and conditioned expectations on pain. Nat Commun 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Koes BW, van Tulder M, Lin C-WC, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J 2010;19:2075–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kohlmann T. Der Funktionsfragebogen Hannover zur alltagsnahen Diagnostik der Funktionsbeeintrachtigung durch Ruckenschmerzen (FFbH-R). Rehabilitation 1996;35:I–VIII. [PubMed] [Google Scholar]
- [40].Linde K, Witt CM, Streng A, Weidenhammer W, Wagenpfeil S, Brinkhaus B, Willich SN, Melchart D. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. PAIN 2007;128:264–71. [DOI] [PubMed] [Google Scholar]
- [41].Mazur JE. Lernen und Verhalten [Learning and behavior] (6th ed.). München, Germany: Pearson Studium, 2006. [Google Scholar]
- [42].Paulus C. Der Saarbrücker Persönlichkeitsfragebogen SPF (IRI) zur Messung von Empathie [The Saarbrücker personality questionnaire (IRI) measuring empathy]. 2009. Retrieved from http://psydok.sulb.uni-saarland.de/volltexte/2009/2363/. [Google Scholar]
- [43].Peerdeman KJ, van Laarhoven AI, Keij SM, Vase L, Rovers MM, Peters ML, Evers AW. Relieving patients' pain with expectation interventions: a meta-analysis. PAIN 2016;157:1179–91. [DOI] [PubMed] [Google Scholar]
- [44].Peñalver-Barrios ML, Lisón JF, Ballester-Salvador J, Schmitt J, Ezzedinne-Angulo A, Arguisuelas MD, Doménech J. A novel (targeted) kinesio taping application on chronic low back pain: Randomized clinical trial. PloS One 2021;16:e0250686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. PAIN 1999;83:147–56. [DOI] [PubMed] [Google Scholar]
- [46].Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- [47].Raghuraman N, Wang Y, Schenk LA, Furman AJ, Tricou C, Seminowicz DA, Colloca L. Neural and behavioral changes driven by observationally-induced hypoalgesia. Sci Rep 2019;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rehman SU, Nietert PJ, Cope DW, Kilpatrick AO. What to wear today? Effect of doctor's attire on the trust and confidence of patients. Am J Med 2005;118:1279–86. [DOI] [PubMed] [Google Scholar]
- [49].Roese I, Kohlmann T, Raspe H. Measuring functional capacity in backache patients in rehabilitation: a comparison of standardized questionnaires. Die Rehabil 1996;35:103–8. [PubMed] [Google Scholar]
- [50].Roland M, Morris R. A study of the natural history of back pain: Part IDevelopment of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141–4. [DOI] [PubMed] [Google Scholar]
- [51].Schenk L, Krimmel SR, Colloca L. Observe to get pain relief: current evidence and potential mechanisms of socially-learned pain modulation. PAIN 2017;158:2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schmitz J, Müller M, Stork J, Eichler I, Zöllner C, Flor H, Klinger R. Positive treatment expectancies reduce clinical pain and perceived limitations in movement ability despite increased experimental pain: a randomized controlled trial on sham opioid infusion in patients with chronic back pain. Psychotherapy Psychosomatics 2019;88:203–14. [DOI] [PubMed] [Google Scholar]
- [53].Schneider F, Härter M, Schorr S. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression. Second Ed. Berlin, Germany: Springer, 2017. https://www. leitlinien. de/ nvl/ depression/ [Google Scholar]
- [54].Sölle A, Worm M, Benedetti F, Sabine Bartholomäus T, Schwender‐Groen L, Klinger R. Targeted use of placebo effects decreases experimental itch in atopic dermatitis patients: a randomized controlled trial. Clin Pharmacol Ther 2021;110:486–97. [DOI] [PubMed] [Google Scholar]
- [55].Strong J, Ashton R, Chant D. Pain intensity measurement in chronic low back pain. Clin J Pain 1991;7:209–18. [DOI] [PubMed] [Google Scholar]
- [56].Suzuki H, Aono S, Inoue S, Imajo Y, Nishida N, Funaba M, Harada H, Mori A, Matsumoto M, Higuchi F. Clinically significant changes in pain along the Pain Intensity Numerical Rating Scale in patients with chronic low back pain. PLoS One 2020;15:e0229228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Swider K, Babel P. The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. PAIN 2013;154:1312–17. [DOI] [PubMed] [Google Scholar]
- [58].Thomas KB. General practice consultations: is there any point in being positive? Br Med J (Clin Res Ed) 1987;294:1200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Trost Z, France CR, Vervoort T, Lange JM, Goubert L. Learning about pain through observation: the role of pain-related fear. J Behav Med 2014;37:257–65. [DOI] [PubMed] [Google Scholar]
- [60].Trost Z, Van Ryckeghem D, Scott W, Guck A, Vervoort T. The effect of perceived injustice on appraisals of physical activity: an examination of the mediating role of attention bias to pain in a chronic low back pain sample. J Pain 2016;17:1207–16. [DOI] [PubMed] [Google Scholar]
- [61].Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients: an empirical investigation. PAIN 2003;105:17–25. [DOI] [PubMed] [Google Scholar]
- [62].Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. PAIN 2012;153:1144–7. [DOI] [PubMed] [Google Scholar]
- [63].Vlaeyen JW, Crombez G, Linton SJ. The fear-avoidance model of pain. PAIN 2016;157:1588–9. [DOI] [PubMed] [Google Scholar]
- [64].Vogtle E, Barke A, Kroner-Herwig B. Nocebo hyperalgesia induced by social observational learning. PAIN 2013;154:1427–33. [DOI] [PubMed] [Google Scholar]
- [65].World Health Organization: The ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. Geneva, Switzerland: World Health Organization, 1993. [Google Scholar]
- [66].Zentall TR. Cognitive and Noncognitive Aspects of Social Learning. In: Animal Creativity and Innovation. Academic Press, 2015. pp. 335–374. [Google Scholar]
- [67].Zhang H, Zhou L, Wei H, Lu X, Hu L. The sustained influence of prior experience induced by social observation on placebo and nocebo responses. J Pain Res 2017;10:2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B521.


