Sensory phenotype is associated with the impact of neuropathic pain conditions on well-being, daily functionality, and quality of life, whereas the most severe impairments are observed in the sensory loss phenotype. Somatosensory phenotype should therefore be considered for overall pain management.
Keywords: PRO, Sensory phenotype, Quality of life, Functionality, Emotional well-being, Neuropathic pain, QST
Abstract
Neuropathic pain highly affects quality of life, well-being, and function. It has recently been shown based on cluster analysis studies that most patients with neuropathic pain may be categorized into 1 of 3 sensory phenotypes: sensory loss, mechanical hyperalgesia, and thermal hyperalgesia. If these phenotypes reflect underlying pathophysiological mechanisms, they may be more relevant for patient management than underlying neurological diagnosis or pain intensity. The aim of this study was thus to examine the impact of these sensory phenotypes on mental health, functionality, and quality of life. Data of 433 patients from the IMI/EuroPain network database were analyzed, and results of HADS-D/A, Pain Catastrophizing Scale, Euro Quality of Life 5D/-VAS, Brief Pain Inventory, and Graded Chronic Pain Scale between the sensory phenotypes were compared using multiple regression analysis. There was no difference in chronic pain grade, pain intensity, depression, or anxiety scores between phenotypes. Pain interference (Brief Pain Inventory) was higher (P = 0.002); self-reported health state lower (Euro Quality of Life 5D VAS, P = 0.02); and problems regarding mobility (P = 0.008), usual activities (P = 0.004), and self-care (P = 0.039) more prominent (EQ5-D) in the sensory loss compared with the thermal hyperalgesia phenotype. Patients with sensory loss also showed higher pain catastrophizing scores (P = 0.006 and 0.022, respectively) compared with the 2 other groups. Sensory phenotype is associated with the impact of neuropathic pain conditions on well-being, daily functionality, and quality of life but is less associated with pain intensity. These results suggest that the somatosensory phenotype should be considered for personalized pain management.
1. Introduction
Neuropathic pain syndromes develop as a result of an injury or disease in the somatosensory system and are accompanied by both positive and negative sensory signs and symptoms.18 It is assumed that biomarkers based on these sensory signs and symptoms may represent different underlying pathophysiological mechanisms, eg, deafferentation, peripheral and central sensitization, or dysfunction of endogenous pain modulation. Precision (personalized) management of neuropathic pain based on mechanisms revealed by sensory clinical biomarker signs is an increasingly promising approach.13
The German Research Network on Neuropathic Pain (DFNS) has developed a standardized quantitative sensory testing (QST) protocol and normative data as an instrument to robustly assess sensory signs in patients with neuropathic pain.36 This clinical test battery assesses 13 parameters including thermal and mechanical detection, pain thresholds, vibration threshold, dynamic mechanical allodynia, wind-up ratio, and pressure pain threshold for the assessment of peripheral small and large fibers or their central pathways. By comparing z-transformed patient values with a database of healthy controls, positive (gain of function) and negative (loss of function) sensory signs can be evaluated.28 Furthermore, Baron et al. have found that patients with neuropathic pain can be allocated to distinct sensory phenotypes based on their somatosensory profiles: sensory loss, mechanical hyperalgesia, and thermal hyperalgesia.44
Previous studies have shown that the prevalence of depression and anxiety is increased among patients suffering from neuropathic pain compared with healthy subjects and patients with non-neuropathic pain.2,14,20,29,37 Quality of life is negatively affected, especially by pain interference.7,11,21,22,30,33,41 In addition, subjective disability is affected by psychological variables such as coping strategies and catastrophizing.25
No study has compared the role of sensory phenotype on disability, well-being, and quality of life. All previous studies focused on pain intensity or on underlying neurological diseases.
If the sensory phenotypes, ie, sensory loss, mechanical hyperalgesia, and thermal hyperalgesia, reflect underlying pathophysiological mechanisms, they may be more relevant for future patient stratification and thereby contribute to our knowledge on future treatments than underlying neurological diagnosis or pain intensity. The aim of this study was thus to examine the impact of these sensory sign phenotypes on quality of life, functionality, and emotional well-being in patients with neuropathic pain.
2. Methods
2.1. Patients
A total of 433 patient records from the database of the European consortia Innovative Medicines Initiative EUROPAIN and NEUROPAIN with a diagnosis of a painful neuropathy of different etiologies were included in this study. The NEUROPAIN project is an investigator-initiated European multicenter study with Prof. Dr. Ralf Baron as a principle investigator and 10 coinvestigator sites. Data were collected at 13 European sites and imported monthly into the central database between 2010 and 2014. The study was performed in accordance with the Declaration of Helsinki and approved by the respective ethics committee of each participating site. Written informed consent was obtained from all patients before inclusion to the study. All centers underwent strict quality control45 and have been shown to have largely homogenous results.43
Diagnosis, inclusion, and examination of the patients were made in the different centers specialized in diagnosis and treatment of neuropathic pain (authors). Painful conditions were based on the patients' self-report with a pain intensity rating of ≥ 2 for the past 4 weeks and a current pain intensity of ≥ 2 on a numerical rating scale (NRS [0-10, 0 = no pain and 10 = worst imaginable pain]) as assessed by the Brief Pain Inventory (BPI, see below), ie, patients with current or average pain intensity of 1 on the 0 to 10 NRS scale were not included. In addition, all patients enrolled in the study had to fulfill the following criteria: age ≥18 years; sufficient language skills; no pain at another localization needing treatment with opioids, antidepressants, or anticonvulsants; no treatment with anticonvulsants or antidepressants with known efficacy on neuropathic pain for other reasons; no severe focal or systemic neurological diseases or diagnosed major cognitive or psychiatric disorders; and no spinal stenosis or peripheral vascular disease (Fontaine stage II or higher).
2.2. Quantitative sensory testing and phenotype allocation
All patients were examined in the painful body area and the corresponding contralateral side using the QST test battery according to the protocol of the German Research Network on Neuropathic pain (DFNS). This includes assessment of warm detection threshold (WDT) and cold detection threshold, paradoxical heat sensation, alternating warm and cold stimuli (TSL), heat pain threshold and cold pain threshold, mechanical detection threshold, mechanical pain sensitivity (MPS), mechanical pain threshold, pressure pain threshold, vibration detection threshold, dynamic mechanical allodynia, and wind-up ratio. Testing was performed as described previously.36 Individual patient's data were z-transformed using reference data of age-matched and sex-matched healthy controls.28,32 The allocation to 1 of 3 distinct sensory phenotypes (sensory loss, mechanical hyperalgesia, and thermal hyperalgesia) was performed using an algorithm published previously.44 Figure 1 shows a projection of the 11-dimensional cluster analysis space onto 2 dimensions: WDT and MPS. Sensory loss and mechanical hyperalgesia clusters are separated along the MPS axis by about 2.3 z, whereas a thermal hyperalgesia cluster is separated from the other 2 along the WDT axis by 1.7 to 1.9 z. With the total n = 433, these differences are highly significant.
Figure 1.
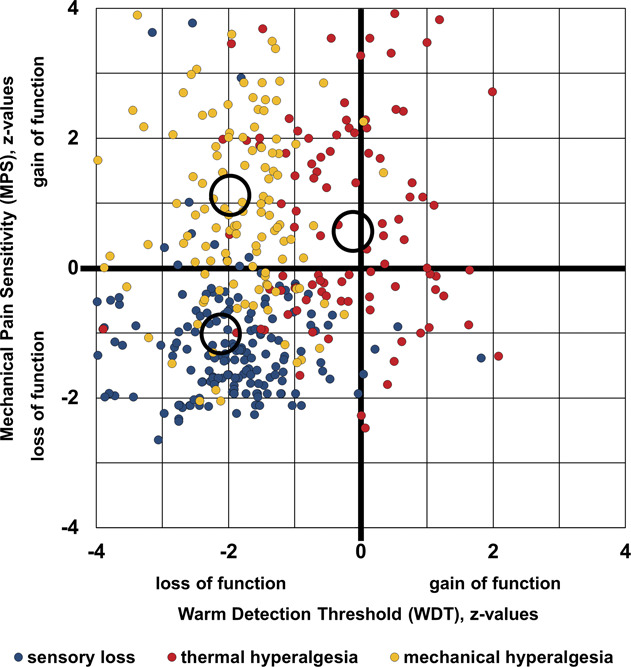
Cluster separation projected onto 2-dimensional space. Scatter plot of the 2 quantitative sensory testing (QST) parameters that gave the best cluster separation: mechanical pain sensitivity (MPS) plotted against warm detection threshold (WDT). Blue dots: cluster 1 “sensory loss” (n = 193); red dots: cluster 2 “thermal hyperalgesia” (n = 103); and yellow dots: cluster 3 “mechanical hyperalgesia” (n = 137). Circles indicate centroids of each cluster.
2.3. Questionnaires
Patients were asked to fill in several questionnaires, including BPI, Graded Chronic Pain Scale (GCPS), Hospital Anxiety and Depression Scale (HADS-A/-D), Euro Quality Of Life 5D (EQ-5D), and Pain Catastrophizing Scale (PCS).
2.3.1. Brief Pain Inventory
The BPI was initially developed to assess different aspects of pain in patients with tumor but has been shown to be equally appropriate for other pain conditions.9 It is a self-report questionnaire including 4 items to assess pain severity and 7 items to assess pain interference in 2 subscores. In assessing pain intensity, patients were asked about pain at its “worst,” “least,” “average,” and “now” (current pain) level on a 0 to 10 scale with 0 = no pain to 10 = worst imaginable pain within the past 4 weeks. A mean severity score of the 4 pain items was used to assess pain severity as recommended by the developers of the BPI.8
The pain interference score was calculated as the mean of the 7 pain interference items assessing to what extent the patient has been impaired over the past 24 hours regarding general activity, walking, work, mood, enjoyment of life, relations with others, and sleep on a 0 to 10 scale with 0 = “does not interfere” to 10 = “interferes completely.”9 The BPI has proven to be a reliable and valid instrument in other pain studies and is recommended also for the assessment of neuropathic pain.3,20
2.3.2. Graded Chronic Pain Scale
The GCPS is a tool to measure pain intensity and pain-related disability to code chronic pain severity as a 4-level categorical variable. The scale includes 3 pain intensity items and 4 disability items. Pain intensity is calculated using the mean of questions 1 to 3 (pain right now, worst pain, and average pain) on a 0 to 10 scale (ranging from 0 = no pain to 10 = worst imaginable pain) multiplied by 10.
The disability score is derived from questions 5 to 7 as the mean interference value on a 0 to 10 scale (daily activities, social activities, and work activities) multiplied by 10 and translated into 0 to 3 disability points using a provided table. The number of days in the past 6 months the patient had been kept from usual activities (question 4) is as well translated into disability points and added to the previous disability points. Chronic pain grade is then defined as 1 or 2 in patients scoring less than 3 disability points (grade 1: pain intensity <50, ie, represents low pain intensity and minor impairment and grade 2: pain intensity ≥50, ie, represents high pain intensity and minor impairment). Patients scoring 3 to 4 disability points are rated grade 3 (high pain-related impairment that is moderately limiting) while patients scoring 5 to 6 disability points are rated grade 4 regardless of pain intensity, ie, high pain-related impairment that is strongly limiting.24
2.3.3. Hospital Anxiety and Depression Scale
The HADS is a self-rating questionnaire developed for screening and assessment of depression and anxiety in patients with physical health issues. The HAD Scale contains 2 subscales with 7 items each for anxiety and depression, assessing symptoms within the past 14 days. Each item presents a statement (eg, “I feel tense or wound up”) and provides 4 possible answers specific for each statement (scored from 0 to 3 on a Likert scale). Thus, for each subscale the maximum score is 21.46 A cutoff score of 8 points is regarded as “suspected depressed or anxious mood,” giving a specificity of 0.78 and a sensitivity of 0.9 for anxiety and a specificity of 0.79 and a sensitivity of 0.83 for depression.5 Values above the cutoff are considered “abnormal.”
2.3.4. Euro Quality of Life 5D-3L and EQ-VAS
Euro Quality of Life 5D and EQ-VAS are standardized self-rating measures of health status and are 2 of the most widely used instruments for measuring health-related quality of life. The EQ-5D-3L comprises 5 dimensions, each representing different aspects of health: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. For each dimension there are 3 levels: “no problems,” “some problems,” and “extreme problems” (coded 1-3). Patients are asked to select the most appropriate level in each dimension. In this study, we only used the items for mobility, self-care, and usual activities because more appropriate instruments to measure pain or discomfort and anxiety or depression were available within other questionnaires.
The EQ-VAS is a 100-step visual analogue scale recording the patients' current self-rated overall health status (0 = worst possible health and 100 = perfect health).6,35
2.3.5. Pain Catastrophizing Scale
Pain catastrophizing is commonly defined as an exaggerated negative mental mindset during actual or anticipated painful experiences, comprising 3 dimensions: rumination, magnification, and helplessness. The PCS is a well-validated questionnaire used for assessing catastrophizing, especially in the context of different types of chronic pain.38–40 Participants are asked to indicate the degree of which they experienced given thoughts or feelings during past painful events on a 5-point scale (ranging from 0 = “not at all” to 4 = “all the time”) in 13 different items. The PCS total score is determined by summing responses to all 13 items (0-52 points), whereas subscales can be computed by summing only the corresponding items for rumination, magnification, or helplessness. A total score of 30 is considered a relevant level of catastrophizing, representing the 75th percentile of PCS scores in clinic samples of patients with chronic pain.
2.4. Statistical analysis
Statistical analysis of collected data was performed using IBM SPSS statistics for Windows (version 23.0, NY). Because age and diagnoses were not distributed equally in the different phenotypes and patients with certain diagnoses (eg, painful polyneuropathy [PNP] or spinal cord injury) or older patients might be more likely to have a lower quality of life and more severe limitations than others, these factors were taken into account for analysis. As these covariates were not distributed homogenously, multiple logistic regression analyses were performed with cluster, age, and disease as independent variables. The χ2 test was used for comparison of chronic pain grade. For normally distributed variables, as assessed by the Shapiro–Wilk test, the Pearson correlation coefficient was used to calculate correlations, whereas for not normally distributed variables, the Spearman correlation coefficient was used. P < 0.05 was considered significant.
3. Results
3.1. Patients
Patients' characteristics are presentend in Table 1. The most frequent diagnosis among patients was PNP (n = 180) including diabetic and nondiabetic polyneuropathies ([ICD-11 code]: 8C0Z), followed by peripheral nerve injury (PNI; n = 89, [ND56.4]), radiculopathy (RL; n = 61, [8B93.Y]), syringomyelia (Syr; n = 37, [8D66.Z]), central poststroke pain (CPSP; n = 25, [MG30.50]), postherpetic neuralgia (PHN; n = 24, [1E91.5]), trigeminal neuralgia (TN; n = 9, [8B82.0]), and spinal cord injury (SCI; n = 8, [ND51.2]).
Table 1.
Patients' characteristics.
| Diagnosis [n] (%) | All (n = 433) | Sensory loss (n = 193) | Thermal hyperalgesia (n = 103) | Mechanical hyperalgesia (n = 137) | P |
|---|---|---|---|---|---|
| CPSP PHN PNI PNP RL SCI Syr TN |
25 (5.77%) 24 (5.54%) 89 (20.55%) 180 (41.57%) 61 (14.09%) 8 (1.85%) 37 (8.55%) 9 (2.08%) |
10 (5.18%) 3 (1.55%) 28 (14.51%) 100 (51.81%) 29 (1.55%) 3 (9.84%) 19 (9.84%) 1 (0.52%) |
4 (3.88%) 9 (8.74%) 26 (25,.4%) 32 (31.07%) 16 (15.53%) 1 (0.97%) 10 (9.71%) 5 (4.85%) |
11 (8.03%) 12 (8.76%) 35 (25.55%) 48 (35.04%) 16 (11.68%) 4 (2.92%) 8 (5.84%) 3 (2.19%) |
P < 0.01 (sensory loss vs thermal hyperalgesia; sensory loss vs mechanical hyperalgesia) |
| Gender (f/m) [%] | 52.42%/47.58% | 48.19%/51.81% | 57.28%/42.72% | 54.74%/45.26% | n.s. |
| Age [mean ± SD] (range) | 57.23 ± 14.80 (21-90) | 58.93 ± 14.38 (21-90) | 53.54 ± 14.34 (21-87) | 57.60 ± 15.24 (22-89) | P = 0.008 (sensory loss vs thermal hyperalgesia) |
| GCPS grade [n = 400] (%) | |||||
| Grade 1 | 14.96% | 12.85% | 16.84% | 16.54% | n.s. |
| Grade 2 | 40.90% | 37.99% | 44.21% | 42.52% | |
| Grade 3 | 26.18% | 29.61% | 23.16% | 23.62% | |
| Grade 4 | 17.96% | 19.55% | 15.79% | 17.32% |
CPSP, central poststroke pain; GCPS, Graded Chronic Pain Scale where grade 1: low pain intensity and minor impairment, grade 2: high pain intensity and minor impairment, grade 3: high pain-related impairment that is moderately limiting, and grade 4: high pain-related impairment that is strongly limiting; PHN, postherpetic neuralgia; PNI, peripheral nerve injury; PNP, painful polyneuropathy; RL, radiculopathy; SCI, spinal cord injury; Syr, syringomyelia; TN, trigeminal neuralgia.
The underlying neurological diseases differed between sensory phenotypes because the sensory loss phenotype included a high percentage of patients suffering from polyneuropathy (Table 1). In addition, patients in the “sensory loss” phenotype were older compared with those in the “thermal hyperalgesia” phenotype, but not to the “mechanical hyperalgesia” phenotype (Table 1). There was no gender difference between the 3 sensory phenotypes.
Most of the patients were classified with chronic pain grade 2 (ie, high pain intensity with minor impairment): This was also the most frequent grade within each phenotype followed by grade 3 (ie, high pain-related impairment that is moderately limiting). Overall, chronic pain grades were similarly distributed across all phenotypes (Table 1).
3.2. Multiple logistic regression analyses
Multiple logistic regression analyses were performed with cluster, age, and disease as independent variables and the following dependent variables: pain, emotional well-being, quality of life, functionality, and pain catastrophizing. Sensory phenotype clusters were stronger predictors than underlying disease and age, as described below in detail.
3.3. Questionnaires
3.3.1. Pain
Pain intensity showed a trend towards lower values in the thermal hyperalgesia compared with the sensory loss phenotype, but these results were not consistent across questionnaires addressing pain intensities (GCPS, BPI, and subscores [Tables 1–3]) and can therefore be explained by the lower amount of patients with CPSP in the thermal hyperalgesia phenotype (Table 3).
Table 3.
Results of multiple logistic regression analysis.
| Regression coefficient B | Standard error | P | 95% confidence interval | |
|---|---|---|---|---|
| BPI pain severity | ||||
| Cluster 2 (thermal hyperalgesia) | −0.517 | 0.257 | 0.045 | −1.023 to 0.011 |
| Chronic poststroke pain | 1.083 | 0.444 | 0.015 | 0.211 to 1.955 |
| BPI interference score | ||||
| Cluster 2 (thermal hyperalgesia) | −0.896 | 0.294 | 0.002 | −1.475 to 0.318 |
| Life quality | ||||
| EQ5-D-VAS | ||||
| Cluster 2 (thermal hyperalgesia) | 8.850 | 3.767 | 0.02 | 1.424 to 16.276 |
| Trigeminal neuralgia | 23.877 | 8.048 | 0.003 | 8.013 to 39.740 |
| Pain Catastrophizing Scale | ||||
| Total score | ||||
| Cluster 2 (thermal hyperalgesia) | −4.410 | 1.603 | 0.006 | −7.561 to 1.259 |
| Cluster 3 (mechanical hyperalgesia) | −3.324 | 1.446 | 0.022 | −6.167 to 0.482 |
| PCS magnification | ||||
| Cluster 2 (thermal hyperalgesia) | −1.273 | 0.393 | 0.001 | −2.046 to 0.499 |
| Cluster 3 (mechanical hyperalgesia) | −0.746 | 0.355 | 0.036 | −1.444 to 0.049 |
| PCS helplessness | ||||
| Cluster 2 (thermal hyperalgesia) | −2.072 | 0.762 | 0.007 | −3.569 to 0.574 |
| Cluster 3 (mechanical hyperalgesia) | −1.703 | 0.687 | 0.014 | −3.054 to 0.352 |
Reference group is cluster 1 (sensory loss). For a better overview, only significant parameters are shown.
BPI, Brief Pain Inventory; PCD, Pain Catastrophizing Scale.
The “sensory loss” phenotype showed the highest BPI pain interference score. This score was higher than the score in the “thermal hyperalgesia” but not the “mechanical hyperalgesia” phenotype (P = 0.002, Fig. 2 and Tables 2 and 3). Both pain intensity and pain interference showed a mild negative correlation with self-reported QoL (EQ5-VAS, r = −0.3 (pain intensity); r = −0.33 (pain interference); P < 0.001). BPI pain interference showed a moderate positive correlation with depression (r = 0.53, P < 0.001) and anxiety (r = 0.47, P < 0.001) scores.
Figure 2.
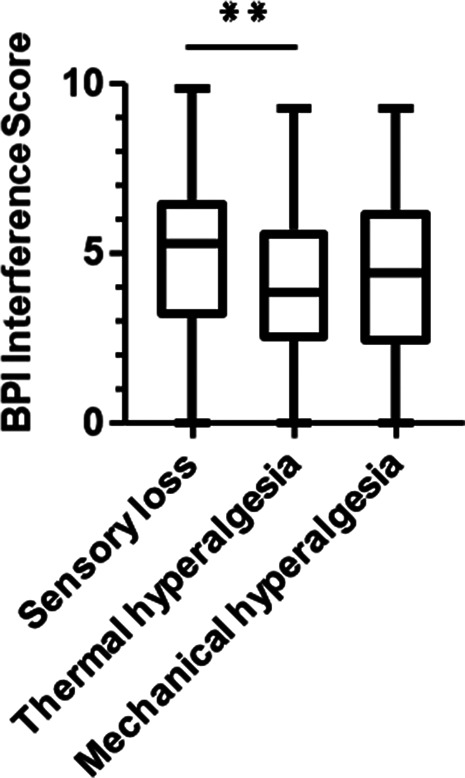
Box plot showing the BPI interference scores of all 3 phenotypes. Differences were significant between “sensory loss” and “thermal hyperalgesia.” **P = 0.002 (logistic regression analysis). BPI was completed by n = 404 participants. BPI, Brief Pain Inventory.
Table 2.
Results of questionnaires.
| All | Sensory loss | Thermal hyperalgesia | Mechanical hyperalgesia | P | |
|---|---|---|---|---|---|
| BPI pain severity, [mean ± SD] (range) | 5.37 ± 1.95 (0-10) | 5.52 ± 1.89 (0-9.75) | 4.99 ± 1.86 (1-9) | 5.43 ± 2.07 (0-10) | n.s. |
| BPI interference score [mean ± SD] (range) | 4.60 ± 2.29 (0-9.86) | 4.99 ± 2.22 (0-9.86) | 4.08 ± 2.11 (0-9.29) | 4.44 ± 2.43 (0-9.29) | 0.002 (thermal hyperalgesia vs sensory loss) |
| HADS-A score (n = 410) [mean ± SD] (range) | 7.88 ± 4.41 (0-20) | 8.09 ± 4.39 (0-19) | 7.56 ± 4.19 (0-18) | 7.83 ± 4.61 (0-20) | n.s. |
| HADS-A abnormal [n] (%) | 212 (51.8%) | 102 (56.1%) | 45 (46.9%) | 65 (49.2%) | 0.047 (thermal hyperalgesia vs sensory loss) |
| HADS-D score (n = 410) [mean ± SD] (range) | 7.43 ± 4.52 (0-21) | 7.91 ± 4.40 (0-21) | 7.24 ± 4.60 (0-18) | 6.92 ± 4.61 (0-20) | n.s. |
| HADS-D abnormal [n] (%) | 205 (50.0%) | 97 (53.3%) | 46 (47.9%) | 62 (47.0%) | n.s. |
| EQ5 mobility abnormal (n = 225) [n] (%) | 132 (58.7%) | 70 (53.3%) | 18 (13.6%) | 44 (33.3%) | 0.008 (thermal hyperalgesia vs sensory loss) |
| EQ5 usual activity abnormal (n = 225) [n] (%) | 156 (69.3%) | 75 (48.1%) | 26 (16.7%) | 55 (35.3%) | 0.004 (thermal hyperalgesia vs sensory loss) |
| EQ5 self-care (n= = 25) [n] (%) | 52 (23.1%) | 29 (28.7%) | 7 (15.2%) | 16 (20.5%) | 0.039 (thermal hyperalgesia vs sensory loss) |
| EQ5-VAS [mean ± SD] (range) | 55.6 ± 21.2 (4-96) | 52.23 ± 18.63 (8-95) | 61.61 21.97 (10-96) | 56.37 ± 23.20 (4-95) | 0.02 (thermal hyperalgesia vs sensory loss) |
| PCS total score [mean ± SD] (range) | 23.63 ± 12.30 (0-52) | 25.76 ± 11.46 (0-52) | 21.73 ± 11.78 (0-52) | 22.11 ± 13.38 (0-52) |
0.006 (thermal hyperalgesia vs sensory loss) 0.022 (mechanical hyperalgesia vs sensory loss) |
| PCS rumination [mean ± SD] (range) | 8.07 ± 4.51 (0-16) | 8.59 ± 4.25 (0-16) | 7.65 ± 4.27 (0-16) | 7.67 ± 4.96 (0-16) | n.s. |
| PCS magnification [mean ± SD] (range) | 4.55 ± 3.07 (0-12) | 5.17 ± 3.02 (0-12) | 3.89 ± 2.92 (0-12) | 4.20 ± 3.10 (0-12) |
0.001 (thermal hyperalgesia vs sensory loss) 0.036 (mechanical hyperalgesia vs sensory loss) |
| PCS helplessness [mean ± SD] (range) | 11.01 ± 5.84 (0-24) | 11.99 ± 5.46 (0-24) | 10.20 ± 5.70 (0-24) | 10.24 ± 6.27 (0-24) |
0.007 (thermal hyperalgesia vs sensory loss) 0.014 (mechanical hyperalgesia vs sensory loss) |
BPI, Brief Pain Inventory; HADS, Hospital Anxiety and depression Scale with A = Anxiety and D = depression parts; n.s., not significant.
P values refer to the results of logistic regression analysis (refer to Table 3).
3.3.2. Anxiety and depression
High scores for depression (HADS-D) and anxiety (HADS-A) being slightly below or above the suggested cutoff of ≥ 8 were found across all 3 phenotypes (Table 2). For depression scores, there were neither differences between clusters nor the number of patients with clinically relevant scores (Table 2). Although anxiety scores did not differ between phenotypes, the “sensory loss” phenotype was associated with the highest proportion of patients with “abnormal” results, which was higher compared with the thermal hyperalgesia phenotype (Table 4). However, age was lower in the thermal hyperalgesia phenotype which could additionally have influenced the result (Table 4). Both anxiety and depression scores showed a mild negative correlation with self-reported QoL (EQ5-VAS, r = −0.27 (anxiety score); r = −0.25 (depression score); P < 0.001).
Table 4.
Results of logistic regression analysis.
| ExpB (odds ratio) | P | 95% confidence interval | |
|---|---|---|---|
| HADS-A | |||
| Cluster 2 (thermal hyperalgesia) | 0.582 | 0.047 | 0.341-0.994 |
| Age | 0.984 | 0.044 | 0.969-1.0 |
| Restless leg syndrome | 2.653 | 0.002 | 1.410-4.992 |
| EQ5 mobility | |||
| Cluster 2 (thermal hyperalgesia) | 0.344 | 0.008 | 0.157-0.754 |
| Postherpetic neuralgia | 0.262 | 0.027 | 0.080-0.861 |
| EQ5 usual activity | |||
| Cluster 2 (thermal hyperalgesia) | 0.268 | 0.004 | 0.110-0.653 |
| Age | 0.955 | 0.001 | 0.931-0.980 |
| EQ5 self-care | |||
| Cluster 2 (thermal hyperalgesia) | 0.350 | 0.039 | 0.129-0.948 |
Reference group is cluster 1 (sensory loss). For a better overview, only significant parameters are shown.
3.3.3. Quality of life
The EQ-5D was only completed by n = 225 participants (59%). However, the proportion of patients who completed the EQ5 did not differ between phenotypes (sensory loss 101/193 (52.3%), thermal hyperalgesia 46/103 (44.6%), and mechanical hyperalgesia: 78/137 (56.9%); P = 0.168). When comparing EQ5 responders vs nonresponders, responders of EQ5 were younger (P = 0.004) and had lower values on HADS-D (P = 0.001) than nonresponders while no differences were observed in the number of patients with abnormal values on HADS-D. No other differences, eg, phenotype, age, sex, pain interference, and PCS total score, could be observed.
We examined 3 of the 5 dimensions of the EQ-5D questionnaire: mobility, self-care, and usual activities. Because a low number of participants (n = 29, 12.9% of the total sample) reported “extreme problems” in these dimensions, we pooled together the variables “extreme problems” and “some problems” making it a dichotomous variable (“problems” and “no problems”). The “sensory loss” phenotype was associated with the highest frequency of patients reporting problems with mobility (n = 70, 69.3%): this proportion was higher than in the “thermal hyperalgesia” phenotype (P = 0.008; Fig. 3A and Tables 2 and 4). Similarly, the “sensory loss” phenotype showed the highest percentage of patients stating problems with usual activities (n = 75, 74.3%), which was higher than that in the thermal hyperalgesia phenotype (P = 0.004, Fig. 3B and Tables 2 and 4), but patients in the sensory loss phenotype were also older (Table 4). Interestingly, most patients (n = 173, 76.9%) reported no problems regarding self-care (Fig. 3C, Table 2). Nevertheless, self-care was reduced in the sensory loss phenotype compared with the thermal hyperalgesia phenotype (P = 0.039, Fig. 3C and Tables 2 and 4).
Figure 3.
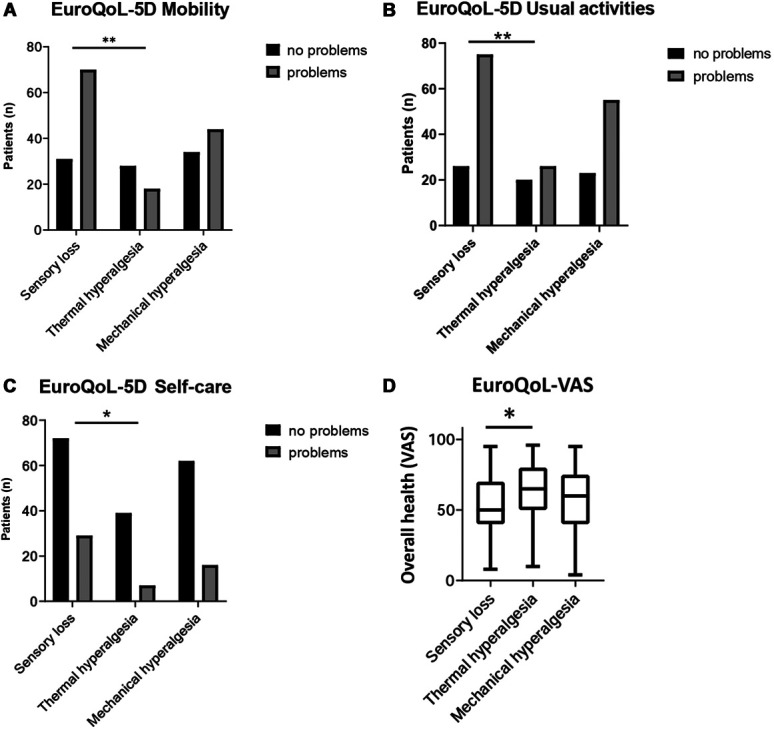
EQ-5D/-VAS was completed by n = 225 participants. Bar chart showing the distribution of patients with and without problems regarding mobility (A), usual activities (B), and self-care (C) according to EQ-5D. “Some problems” and “extreme problems” were subsumed to solely “problems.” *P < 0.05, **P < 0.01 for logistic regression analysis. (D) Box plot showing the results of the EQ-VAS (visual analogue scale) representing the overall health state. * P < 0.05. EQ-5D, Euro Quality of Life 5D.
Overall health state measured on the EQ-VAS was rated lower in the “sensory loss” phenotype than that in the “thermal hyperalgesia” phenotype but did not differ from the “mechanical hyperalgesia” phenotype (P = 0.02; Fig. 3D and Tables 2 and 3).
3.3.4. Pain catastrophizing
The mean scores of pain catastrophizing on the PCS were below the cutoff, representing a clinically relevant level of catastrophizing in all 3 phenotypes (Table 2). The frequency of values above the cutoff did not differ across phenotypes. However, PCS total scores as well as subscores for magnification and helplessness were higher in the “sensory loss” phenotype compared with the “thermal hyperalgesia” phenotype (Fig. 4, Tables 2 and 3). There was a moderately negative correlation between PCS total score and self-rated health state on EQ-VAS (r = −0.33, P < 0.005) and a positive correlation between PCS total score and anxiety score (r = 0.54, P < 0.001), depression score (r = 0.51, P < 0.001), BPI pain interference (r = 0.5, P < 0.001), and BPI pain severity (r = 0.37, P < 0.001).
Figure 4.
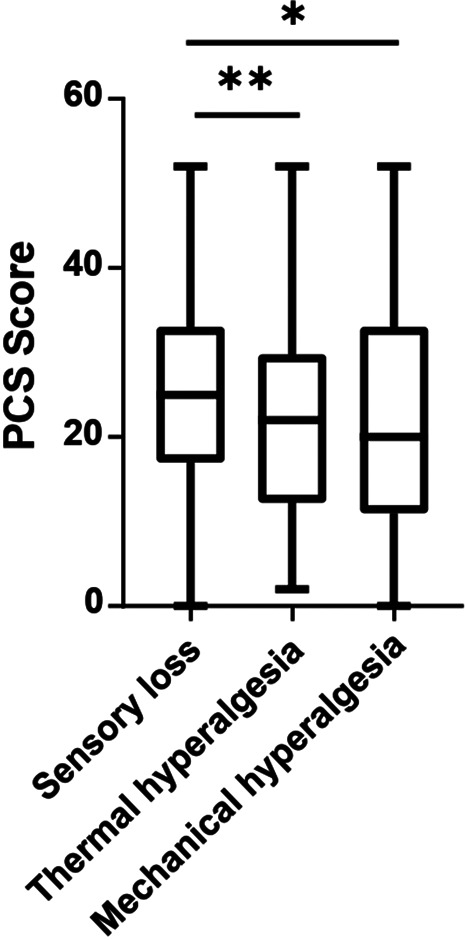
PCS was completed by n = 407 participants. Box plot showing the results of the Pain Catastrophizing Scale (PCS). The mean in all 3 phenotypes was below the cutoff of 30. *P = 0.022, **P = 0.006 for logistic regression analysis.
4. Discussion
The objective of this study was to examine different variables of mental health and functionality in association to the sensory phenotype. The results show that the sensory phenotype has a relevant impact on well-being, daily functionality, and QoL, which are mainly independent from age or underlying neurological diagnoses. Interestingly, the sensory phenotype (including tests of evoked pain) does not seem to be associated with ongoing pain intensity itself but rather other dimensions associated with chronic neuropathic pain conditions such as pain interference, impaired mobility, QoL, and catastrophizing. These aspects are important constituents of the clinical severity of chronic pain conditions and may be independent of reported ongoing pain intensity. Patients with prevailing loss of somatosensory functions, represented by the “sensory loss” phenotype, reported the highest interference score on the BPI. Consistently, “sensory loss” reveals a high proportion of patients having problems with mobility, usual activities, and self-care. Furthermore, overall health was rated the lowest in the “sensory loss” phenotype while pain catastrophizing was highest in this group. The presence of depressive and anxiety symptoms was high across all phenotypes, suggesting that these dimensions are less affected by the sensory phenotype than by pain intensity itself, which was generally similar between phenotypes. This is consistent with previous reports showing the association of painful conditions and depressive symptoms.2,23,27,31,37
The finding that the “sensory loss” phenotype was rated as the most impaired and showed the highest scores in pain catastrophizing might be a possible explanation for the subjective lower heath state and higher pain interference observed in this phenotype. Recent studies have shown that catastrophic thinking is associated with decreased QoL and high levels of pain interference.15,17 Furthermore, it has been shown that pain catastrophizing predicted pain outcomes (pain intensity and pain interference) negatively in longitudinal treatment studies.10,34 Interestingly, physical activity was found to be a mediator in the relationship between pain catastrophizing and QoL in patients with painful diabetic neuropathy.17 This is consistent with our data, showing a moderate negative correlation between overall health state (QoL) and pain catastrophizing. Similarly, it has been shown that participation in social activities is a major mediator for QoL in patients with spinal cord injuries.30 Two-thirds of patients with the “sensory loss” phenotype reported problems with mobility and usual activities, which might contribute to their reduced QoL. The impact of sensory loss on mobility and usual activities is illustrated by findings that both the loss of sensory function and proprioception are major predictors for decreased postural stability,1,12,26 thus affecting the ability to walk. Consistent with this finding, half of the patients in the “sensory loss” phenotype were suffering from PNP, which has been shown to be interrelated with impaired QoL and daily functionality, including sleep, enjoyment of life, normal work, as well as general and social activities.4,16,20
In addition, lower ratings in QoL within the “sensory loss” phenotype could possibly be related to the limited treatment options for symptoms of somatosensory loss. Although there are several therapeutic options available for the treatment of positive sensory symptoms (ie, hyperalgesia),27 there is a lack of mechanism-based treatment options for patients with negative symptoms.18 Because perceived helplessness is 1 of the 3 dimensions included in catastrophizing,38 the deficiency of options for symptom relief might also contribute to higher ratings in pain catastrophizing in the “sensory loss” phenotype. However, catastrophizing scores in our study were below the cutoff for clinically relevant symptoms, which suggests that the overall impact of catastrophizing is minor on our results.
Our findings are well in line with our previous study in patients suffering from chronic low back pain, showing that symptom intensity, impairment of QoL, and functionality are all important therapeutic outcome parameters but not necessarily reported in combination with one another; some patients might be highly impaired in QoL or functionality with low pain intensity ratings, whereas others report higher pain intensity but are less impaired in QoL and functionality.19 Similarly, our results show that not only ongoing pain intensity but also, more importantly, the presence of certain sensory symptoms, ie, loss of somatosensory function, hyperalgesia, or allodynia (ie, including evoked pain), affects QoL and functionality. Because controlling these somatosensory symptoms might be important for the success of pain management in regard to the overall well-being of a patient with pain, the somatosensory phenotype should be evaluated as part of the treatment regimen.
Some limitations of the study should be acknowledged. Not all participants filled out the complete range of patient-reported outcome measures, particularly the EQ-5D. Although EQ-5D noncompleters were older and had higher depression scores than completers, the number of patients above the cutoff values for suspected depression did not differ between groups. Because age only influenced EQ-5D usual activities, and not other dimensions, such as mobility or self-care, we believe that this had a minor impact on our results.
Another potential limitation is that we generally did not document the exact causes of PNP in patients within the sensory loss phenotype, in particular we could not differentiate diabetic from nondiabetic polyneuropathies. Hence, we cannot exclude that some results obtained in patients with polyneuropathy were related to the presence of physical comorbidities, particularly in patients with diabetes or chemotherapy-induced neuropathy. Furthermore, patients with polyneuropathy, especially, may have been inhomogeneous according to parameters such as proprioceptive or motor deficits. Thus, we cannot claim causality for the sensory profiles from data on association.
5. Conclusion
We found that the sensory phenotype has an important impact on emotional well-being and daily functionality in patients with neuropathic pain, but interestingly, it does not seem to be associated with reported pain intensity. Therefore, not only pain intensity but also quality of life, pain-related psychological factors, and daily functionality should be considered as valuable outcome parameters for the evaluation of pain management, particularly in patients presenting with negative sensory signs. According to these findings, patients with neuropathic pain and the sensory loss profile may benefit particularly from enhanced physiotherapy and education on self-care. However, this should be verified by prospective studies.
Furthermore, because pain catastrophizing was a predictor for pain outcomes, it should be addressed in patients with neuropathic pain. Further studies are needed to collect more detailed and differentiated data on specific areas of patients' daily life to identify factors responsible for the impairment of QoL and functionality in a more accurate way. Future work should also examine the impact of therapeutic success, patient's compliance, impairment due to the underlying disease, and the presence of comorbidities on QoL and daily functionality.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgements
This project has received funding from an Innovative Medicines Initiative 1 EUROPAIN under grant No [115007] and from an Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No [777500]. This joint undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. (www.imi.europe.eu; www.imi-paincare.eu).
The statements and opinions presented here reflect the authors' view and neither IMI nor the European Union, EFPIA, or any associated partners are responsible for any use that may be made of the information contained herein.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
J. Gierthmühlen and J. Böhmer contributed equally to this work.
Contributor Information
Johann Böhmer, Email: johannboehmer@icloud.com.
Nadine Attal, Email: nadine.attal@aphp.fr.
Didier Bouhassira, Email: didier.bouhassira@aphp.fr.
Rainer Freynhagen, Email: r.freynhagen@krankenhaus-tutzing.de.
Maija Haanpää, Email: maija.haanpaa@ilmarinen.fi.
Per Hansson, Email: per.hansson@ki.se.
Troels Staehelin Jensen, Email: tsjensen@clin.au.dk.
Jeffrey Kennedy, Email: jdkennedy2@gmail.com.
Christoph Maier, Email: christoph.maier@ruhr-uni-bochum.de.
Andrew S.C. Rice, Email: a.rice@imperial.ac.uk.
Juliane Sachau, Email: juliane.sachau@uksh.de.
Märta Segerdahl, Email: msst@lundbeck.com.
Sören Sindrup, Email: Soeren.Sindrup@rsyd.dk.
Thomas Tölle, Email: toelle@lrz.tu-muenchen.de.
Rolf-Detlef Treede, Email: Rolf-Detlef.Treede@medma.uni-heidelberg.de.
Lise Ventzel, Email: lise.ventzel@oncology.au.dk.
Jan Vollert, Email: j.vollert@imperial.ac.uk.
Ralf Baron, Email: r.baron@neurologie.uni-kiel.de.
References
- [1].Anson E, Bigelow RT, Swenor B, Deshpande N, Studenski S, Jeka JJ, Agrawal Y. Loss of peripheral sensory function explains much of the increase in postural sway in healthy older adults. Front Aging Neurosci 2017;9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. PAIN 2011;152:2836–43. [DOI] [PubMed] [Google Scholar]
- [3].Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain 2004;5:491–7. [DOI] [PubMed] [Google Scholar]
- [4].Benbow SJ, Wallymahmed ME, Macfarlane IA. Diabetic peripheral neuropathy and quality of life. QJM 1998;91:733–7. [DOI] [PubMed] [Google Scholar]
- [5].Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- [6].Brooks R, De Charro F. EuroQol: the current state of play. Health Policy 1996;37:53–72. [DOI] [PubMed] [Google Scholar]
- [7].Burke D, Lennon O, Fullen BM. Quality of life after spinal cord injury: the impact of pain. Eur J Pain 2018;22:1662–72. [DOI] [PubMed] [Google Scholar]
- [8].Cimino Brown D. Brief Pain Inventory User Guide. 2017. Available at: www.CanineBPI.com. [Google Scholar]
- [9].Cleeland C. The brief pain inventory user guide. Br Pain Invent 2009:3–4. Available at: http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf. [Google Scholar]
- [10].Craner JR, Sperry JA, Evans MM. The relationship between pain catastrophizing and outcomes of a 3-week comprehensive pain rehabilitation program. Pain Med 2016;17:2026–35. [DOI] [PubMed] [Google Scholar]
- [11].Cruz-Almeida Y, Alameda G, Widerström-Noga EG. Differentiation between pain-related interference and interference caused by the functional impairments of spinal cord injury. Spinal Cord 2009;47:390–6. [DOI] [PubMed] [Google Scholar]
- [12].Deshpande N, Simonsick E, Metter EJ, Ko S, Ferrucci L, Studenski S. Ankle proprioceptive acuity is associated with objective as well as self-report measures of balance, mobility, and physical function. Age (Dordr) 2016;38:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Forstenpointner J, Rehm S, Gierthmühlen J, Baron R. Stratification of neuropathic pain patients: the road to mechanism-based therapy? Curr Opin Anaesthesiol 2018;31:562–8. [DOI] [PubMed] [Google Scholar]
- [14].Freynhagen R, Parada HA, Calderon-Ospina CA, Chen J, Rakhmawati Emril D, Fernández-Villacorta FJ, Franco H, Ho KY, Lara-Solares A, Li CCF, Mimenza Alvarado A, Nimmaanrat S, Dolma Santos M, Ciampi de Andrade D. Current understanding of the mixed pain concept: a brief narrative review. Curr Med Res Opin 2019;35:1011–18. [DOI] [PubMed] [Google Scholar]
- [15].Furrer A, Michel G, Terrill AL, Jensen MP, Müller R. Modeling subjective well-being in individuals with chronic pain and a physical disability: the role of pain control and pain catastrophizing. Disabil Rehabil 2019;41:498–507. [DOI] [PubMed] [Google Scholar]
- [16].Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 2000;47:123–8. [DOI] [PubMed] [Google Scholar]
- [17].Geelen CC, Kindermans HP, van den Bergh JP, Verbunt JA. Perceived physical activity decline as a mediator in the relationship between pain catastrophizing, disability, and quality of life in patients with painful diabetic neuropathy. Pain Pract 2017;17:320–8. [DOI] [PubMed] [Google Scholar]
- [18].Gierthmühlen J, Baron R. Neuropathic pain. Semin Neurol 2016;36:462–8. [DOI] [PubMed] [Google Scholar]
- [19].Gierthmühlen J, Greinacher J, Höper J, Oberlojer V, Lankes M, Traulsen F, Hüllemann P, Borzikowsky C, Reimer M, Baron R. Sensory symptoms in low back pain—how do they matter? Curr Med Res Opin 2018;34:657–67. [DOI] [PubMed] [Google Scholar]
- [20].Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005;30:374–85. [DOI] [PubMed] [Google Scholar]
- [21].Gutierrez DD, Thompson L, Kemp B, Mulroy SJ, Winstein CJ, Gordon J, Brown DA, Knutson L, Fowler E, DeMuth S, Kulig K, Sullivan K. The relationship of shoulder pain intensity to quality of life, physical activity, and community participation in persons with paraplegia. J Spinal Cord Med 2007;30:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haroun OMO, Vollert J, Lockwood DN, Bennett DLH, Pai VV, Shetty V, Wakade AV, Khodke AS, Schilder A, Pfau D, Enax-Krumova EK, Maier C, Treede R-D, Rice ASC. Clinical characteristics of neuropathic pain in leprosy and associated somatosensory profiles. PAIN Rep 2019;4:e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, Dascal J, Marcia L, Gohar Y, Eskander L, Yadegar J, Hanna S, Sadek A, Aguilar-Hernandez L, Danovitch I, Louy C. Pain and depression: a systematic review. Harv Rev Psychiatry 2018;26:352–63. [DOI] [PubMed] [Google Scholar]
- [24].Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. PAIN 1992;50:133–49. [DOI] [PubMed] [Google Scholar]
- [25].Kröner-Herwig B, Jäkle C, Frettlöh J, Peters K, Seemann H, Franz C, Basler HD. Predicting subjective disability in chronic pain patients. Int J Behav Med 1996;3:30–41. [DOI] [PubMed] [Google Scholar]
- [26].Kwon HJ, Kim JS, Kim YJ, Kwon SJ, Yu JN. Sensory impairment and health-related quality of life. Iran J Public Health 2015;44:772–82. [PMC free article] [PubMed] [Google Scholar]
- [27].Macone A, Otis J. Neuropathic pain. Semin Neurol 2018;38:644–53. [DOI] [PubMed] [Google Scholar]
- [28].Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- [29].McCallum LM, Damms NA, Sarrigiannis PG, Zis P. Anxiety and depression in patients with suspected carpal tunnel syndrome—a case controlled study. Brain Behav 2019;9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Müller R, Landmann G, Béchir M, Hinrichs T, Arnet U, Jordan X, Brinkhof MWG. Chronic pain, depression and quality of life in individuals with spinal cord injury: mediating role of participation. J Rehabil Med 2017;49:489–96. [DOI] [PubMed] [Google Scholar]
- [31].Naranjo C, Del Reguero L, Moratalla G, Hercberg M, Valenzuela M, Failde I. Anxiety, depression and sleep disorders in patients with diabetic neuropathic pain: a systematic review. Expert Rev Neurother 2019;19:1201–9. [DOI] [PubMed] [Google Scholar]
- [32].Pfau DB, Krumova EK, Treede RD, Baron R, Toelle T, Birklein F, Eich W, Geber C, Gerhardt A, Weiss T, Magerl W, Maier C. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. PAIN 2014;155:1002–15. [DOI] [PubMed] [Google Scholar]
- [33].Phillips TJC, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, De Williams ACC, Orengo C, Bennett DLH, Bodi I, Cox S, Maier C, Krumova EK, Rice ASC. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: a cross-sectional deep profiling study. PAIN 2014;155:1846–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Racine M, Moulin DE, Nielson WR, Morley-Forster PK, Lynch M, Clark AJ, Stitt L, Gordon A, Nathan H, Smyth C, Ware MA, Jensen MP. The reciprocal associations between catastrophizing and pain outcomes in patients being treated for neuropathic pain: a cross-lagged panel analysis study. PAIN 2016;157:1946–53. [DOI] [PubMed] [Google Scholar]
- [35].Van Reenen M, Oppe M, Boye K, Herdman M, Kennedy-Martin M, Kenndy-Martin T, Slaap B. EuroQol research foundation. EQ-5D-3L user guide. EuroQol Res Found 2018:1–33. Available at: https://euroqol.org/publications/user-guides. [Google Scholar]
- [36].Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [37].Sadosky A, Schaefer C, Mann R, Bergstrom F, Baik R, Parsons B, Nalamachu S, Nieshoff E, Stacey BR, Anschel A, Tuchman M. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional survey. Diabetes Metab Syndr Obes Targets Ther 2013;6:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sullivan M, Bishop S, Pivik J. The pain catastrophizing scale: user Manual. Psychol Assess 1995;7:524–32. [Google Scholar]
- [39].Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. PAIN 2005;113:310–15. [DOI] [PubMed] [Google Scholar]
- [40].Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- [41].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice ASC, Bennett DLH. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. PAIN 2016;157:1132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. A classification of chronic pain for ICD-11. PAIN 2015;156:1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vollert J, Attal N, Baron R, Freynhagen R, Haanpää M, Hansson P, Jensen TS, Rice ASC, Segerdahl M, Serra J, Sindrup SH, Tölle TR, Treede RD, Maier C. Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. PAIN 2016;157:750–8. [DOI] [PubMed] [Google Scholar]
- [44].Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmühlen J, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Magerl W, Ramirez JD, Rice ASC, Schuh-Hofer S, Segerdahl M, Serra J, Shillo PR, Sindrup S, Tesfaye S, Themistocleous AC, Tölle TR, Treede R-D, Baron R. Stratifying patients with peripheral neuropathic pain based on sensory profiles. PAIN 2017;158:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vollert J, Mainka T, Baron R, Enax-Krumova EK, Hüllemann P, Maier C, Pfau DB, Tölle T, Treede RD. Quality assurance for Quantitative Sensory Testing laboratories: development and validation of an automated evaluation tool for the analysis of declared healthy samples. PAIN 2015;156:2423–30. [DOI] [PubMed] [Google Scholar]
- [46].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]


