Abstract
Techniques to monitor honey bee (Apis mellifera) egg production in cages allow researchers to study how different environmental factors contribute to reproduction. However, although the conditions required to facilitate queen egg production in a laboratory setting have been established, limited work has addressed the requirements for stimulating and monitoring worker egg laying. Here, we documented that drone laying workers will lay eggs in Queen Monitoring Cages (QMC), specialized cages designed to facilitate queen egg laying under controlled conditions. Egg production and worker mortality were compared between QMCs containing queens and those containing drone laying workers. High-definition images of the last abdominal segments of living first-instar larvae hatched from worker laid eggs and those putatively laid by queens were qualitatively compared to identify candidate characteristics to determine their sex.
Keywords: fecundity, haplodiploidy, drone rearing, anarchistic workers
Queen monitoring cages (QMCs) are a tool that can be used to study honey bee queen oviposition under controlled laboratory conditions. When using QMCs, it is recommended to use newly eclosed worker bees (NEWs) as attendants (Fine et al. 2021) because of the responsiveness of typically younger, more nurse-like bees to queen pheromone (Pham-Delègue et al. 1993), and because of the reduced likelihood that the workers will exhibit developed ovaries capable of producing drone eggs within the recommended 2-wk duration of a QMC experiment (Hoover et al. 2003). However, the occurrence of drone laying workers and their propensity to lay in QMCs has not been previously documented. Although there has been considerable work done in field settings and observation colonies to study worker egg laying (Holmes and Beekman 2017), the development of a system to quantitatively study the phenomenon under controlled laboratory conditions would facilitate experiments investigating the occurrence and behavior of anarchistic worker bees. Here, we demonstrate that the same conditions necessary for stimulating queen egg laying in QMCs can be used to stimulate workers to lay.
Observations of drone laying workers in QMCs made during a 39-d period in Davis, CA are described, and images of anatomical features reported to vary between first-instar male and female larvae are qualitatively compared between worker-laid and putatively queen-laid larvae.
Materials and Methods
Cage Assembly
On December 2, 2020, 6 QMCs were assembled using queens of Carniolan lineage shipped from a California queen breeder (queenright QMCs). Because of the seasonal decline in brood rearing during the winter months in California, newly eclosed bees could not be easily obtained. Approximately 50–100 workers shipped with the queens were added per QMC instead of NEWs. As part of a separate study, three additional queenright QMCs were subjected to exposure to 500 ppm pyriproxyfen in sucrose solution for 12 h. Only images of the larvae hatched from eggs produced in these cages are represented in this report. The remaining workers were divided between 3 QMCs without queens, adding 50–100 workers to each (queenless QMCs). All QMCs were provisioned with diet and placed in an incubator set at 34.5°C and 60 ± 10% RH as described by Fine et al. (2021).
Once all cages were producing eggs, egg production and mortality rates in QMCs were recorded every 24–48 h for 27 d.
Larval Imaging
Images of the ninth and tenth abdominal segments of live first-instar larvae eclosing from eggs laid in QMCs were taken during the experiment to identify conspicuous differences between the male and female larval epiproct. Egg laying plates containing eggs were taken from queenless and queenright QMCs, covered with a PCR plate seal, and permitted to hatch in a desiccator maintained at 95% RH using a saturated potassium sulfate solution in an incubator maintained at the conditions described above. Approximately 72 h after collection, photos of living larvae were taken at 200× magnification using an Olympus BX53 System Microscope and a UC90 Digital Camera (Olympus Corporation, Tokyo, Japan) and evaluated using methods described by Santomauro and Engels (2002).
Statistical Analysis
Statistical analysis was performed using JMP Pro version 15 software. Figures were created using Photoshop CC 2019 (Adobe Inc., San Jose, CA) and JMP Pro 15. Differences between queenright and queenless QMCs in the total number of eggs produced and the number of worker deaths in QMCs from days 13 and 39 were evaluated using two-sided Student’s t-tests.
Results
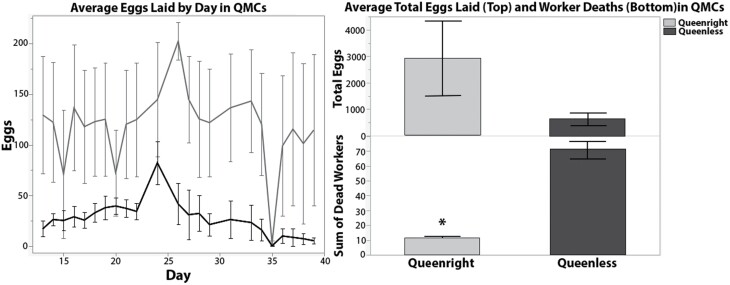
All 3 queenright QMCs began to produce eggs by 5 d after cage assembly, and 13 d after assembly, the 3 queenless QMCs also began to produce eggs. Between days 13 and 39, there was no significant difference in the total number of eggs laid in queenright and queenless QMCs (t = −1.604, n = 3, DF = 2.114, P = 0.253), but queenright QMCs had significantly lower rates of worker mortality (t = 0.004, n = 3, DF = 2.178, P = 0.004; Fig. 1).
Fig. 1.
Left: Daily average ± SE egg laying in queenright (blue) and queenless QMCs (red). Right: Average total eggs laid (top) and worker mortality recorded per QMC in queenright (blue) and queenless QMCs (red). Significant difference indicated by ‘*’.
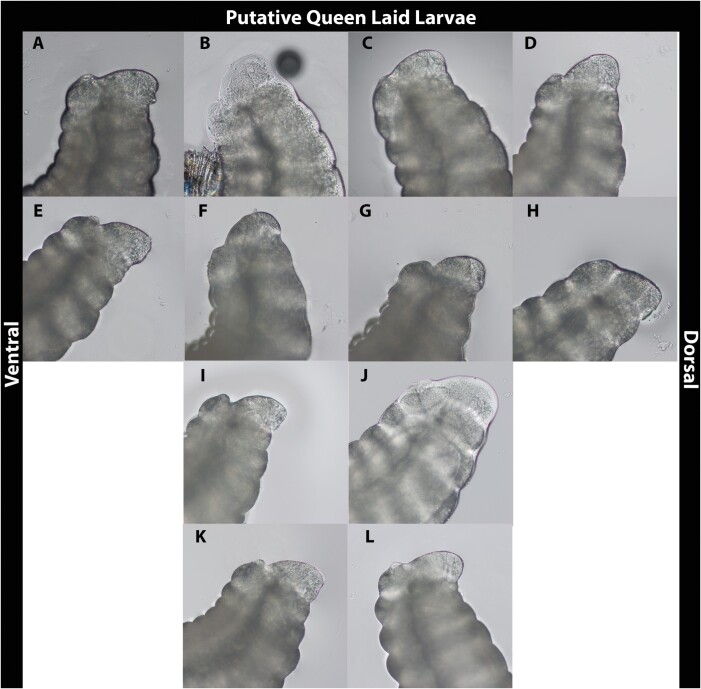
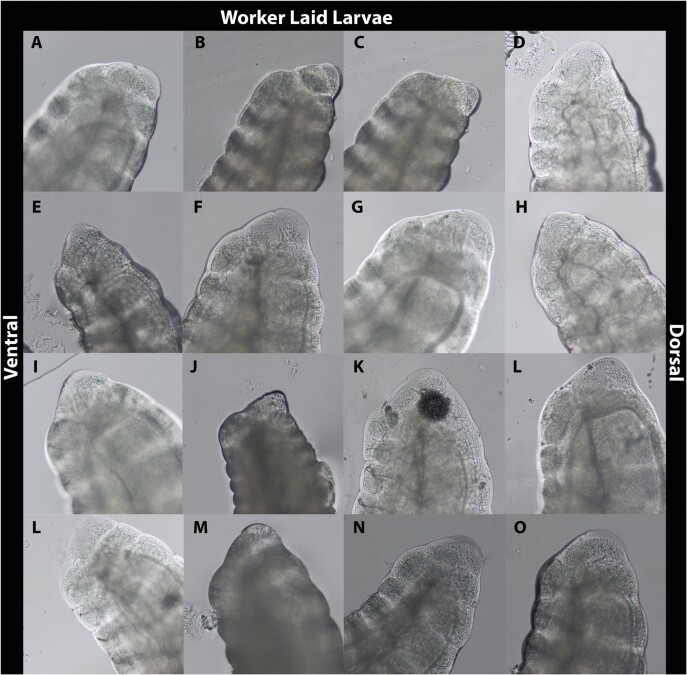
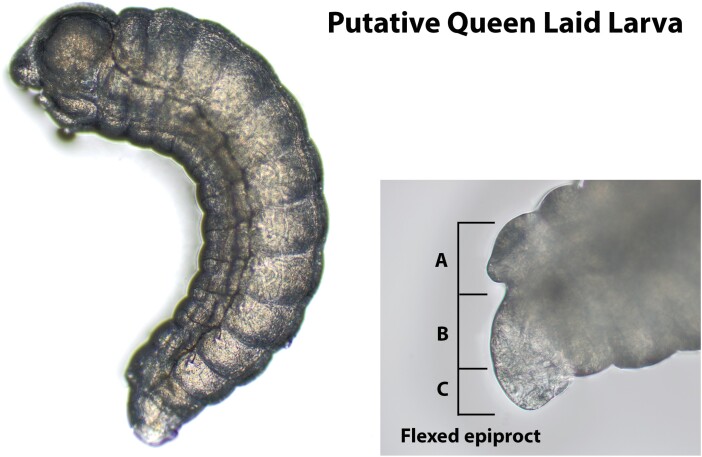
Images of putatively queen laid and worker laid larvae are shown in Figs. 2 and 3, respectively. Most images taken of larvae putatively laid by queens depict distinct features compared to those definitively laid by workers (Fig. 4), though some features reported to be useful for making this distinction are difficult to discern. Santomauro and Engels (2002) suggest that a longer length of the ventral epiproct relative to the dorsal (Figure 4B and C respectively), a smaller proportion of the ninth segment visible under the tenth (Figure 4A) relative to the ventral epiproct, and the extension of the dorsal epiproct over the ninth segment when the larva is flexed can be used to identify female larvae. These features were not universally obvious, and differences between the proportions of the dorsal and ventral portion of the epiprocts in putatively queen laid larvae versus worker laid larvae were not immediately apparent. One characteristic observed in the majority of queen laid eggs that was absent in all worker laid eggs was a mound protruding from the ninth abdominal segment below the epiproct (Fig. 4A). This feature was present in all but one of the queen-laid larvae (Fig. 2H).
Fig. 2.
The latter abdominal segments of first-instar larvae laid in queenright QMCs at 200× magnification.
Fig. 3.
The latter abdominal segments of presumed male, first-instar larvae laid in queenless QMCs.
Fig. 4.
A first-instar larva laid in a queenright QMC at 10× magnification (left) and a 200× magnified image of its flexed epiproct. (A) The ninth abdominal segment visible beneath the tenth. (B) The ventral portion of the epiproct. (C) The dorsal portion of the epiproct.
Discussion
The eggs observed in queenless QMCs indicates the presence of drone laying workers at day 13 and suggests the possibility that workers in the queenright QMCs may have also become reproductively active and started to lay drone eggs. Although the presence of a mated queen is a strong inhibitor of worker ovary activation (Hoover et al. 2003), the parentage of the eggs produced in the queenright QMCs cannot be determined with total confidence. However, the lower rate of worker mortality observed in queenright QMCs compared to queenless suggests that the queens’ pheromone blend produced a strong physiological response in workers in queenright QMCs. Queen pheromone (Pankiw et al. 1998) inhibits rising juvenile hormone titers and delays the onset of foraging, which typically occurs prior to senescence (Woyciechowski and Moroń 2009). The increased survival of the bees in queenright cages was likely due to exposure to the pheromone blend of a mated queen, which would likely inhibit worker ovary activation. The 13-d period prior to the onset of worker egg laying behaviors in queenless QMCs with workers of an unknown age indicates that workers in QMC experiments conducted over a 2-wk period with NEWs are unlikely to become reproductively active. Likewise, the progression of egg production in queenless QMCs indicates that egg laying behaviors of anarchistic worker bees can only be observed after 2 wk.
Here, we demonstrate that in the absence of a queen, workers can become reproductively active and begin laying in QMCs populated with 50 to 100 worker bees when given the same nutritional and environmental stimulus as cages containing queens. The lack of differences in the number of eggs produced by workers compared to queens was likely due to the small sample size (n = 3) and the lack of data on the number of laying workers present in each queenless QMC. In the future, parameters including the number of workers used to populate cages and nutritional and environmental inputs can be refined to optimally support reproductively active workers, increase worker egg laying, and decrease mortality.
Although the sex of the embryos produced in this work was not definitively determined, the images taken of live first-instar larvae may be useful in identifying candidate features to use when performing in vivo assessments of first instars. Within a colony, honey bee queens lay mostly fertilized eggs that develop into female larvae and a small number of unfertilized eggs that develop into haploid male drone larvae. Therefore, we anticipated that most of the larvae hatching from eggs laid in queenright QMCs would be female. Conversely, reproductively active workers can only lay unfertilized eggs that develop into male larvae. Therefore, we assumed that worker laid larvae from queenless QMCs were male.
We hypothesize that the mound protruding from the ninth abdominal segment below the epiproct of the majority of queen-laid larvae may eventually develop into the ovipositor in queens or the stinger in workers. The results presented here suggest that this characteristic may be more suitable for determining the sex of live first-instar honey bee larvae from still images.
The sex ratio of the offspring produced in laboratory assays could provide information on the queen’s reproductive status, sperm viability (Collins 2004), and the occurrence of reproductively active workers (Hoover et al. 2003). Similarly, although theletoky has never been documented in Apis mellifera subspecies besides capensis (Chapman et al. 2015), the ability to discern the sex of living larvae could prove useful in studying the phenomenon. In the future, morphometric analysis paired with a definitive identification of sex via microsatellite markers (Ratnieks and Keller 1998) will determine the utility of the traits observed here such as the putative ovipositor or stinger in distinguishing between male and female first-instar larvae.
Acknowledgments
Thank you to Bernardo Niño for his assistance with this project. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Contributor Information
Julia D Fine, Invasive Species and Pollinator Health Research Unit, USDA-ARS, 3026 Bee Biology Road, Davis, CA 95616, USA.
Eliza M Litsey, Invasive Species and Pollinator Health Research Unit, USDA-ARS, 3026 Bee Biology Road, Davis, CA 95616, USA.
Funding
This research was supported by USDA project 2030-21000-055-000-D.
Authors’ Contributions
E.M.L. and J.D.F. conceptualized of the research, E.M.L. performed the investigation, J.D.F. conducted the formal analysis, J.D.F. wrote the original manuscript, and E.M.L. reviewed and revised the final version.
References Cited
- Chapman, N. C., Beekman M., Allsopp M. H., Rinderer T. E., Lim J., Oxley P. R., and Oldroyd B. P.. . 2015. Inheritance of thelytoky in the honey bee Apis mellifera capensis. Heredity. 114: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, A. M. 2004. Functional longevity of honey bee, Apis mellifera, queens inseminated with low viability semen. J. Apic. Res 43: 167–171. [Google Scholar]
- Fine, J. D., Torres K. M., Martin J., and Robinson G. E.. . 2021. Assessing agrochemical risk to mated honey bee queens. JoVE J. Vis. Exp. e62316. [DOI] [PubMed] [Google Scholar]
- Holmes, M. J., and Beekman M.. . 2017. When does cheating pay? Worker reproductive parasitism in honeybees. Insectes Sociaux. 64: 5–17. [Google Scholar]
- Hoover, S. E. R., Keeling C. I., Winston M. L., and Slessor K. N.. . 2003. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften. 90: 477–480. [DOI] [PubMed] [Google Scholar]
- Pankiw, T., Huang Z.-Y., Winston M. L., and Robinson G. E.. . 1998. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J. Insect Physiol. 44: 685–692. [DOI] [PubMed] [Google Scholar]
- Pham-Delègue, M. H., Trouiller J., Caillaud C. M., Roger B., and Masson C.. . 1993. Effect of queen pheromone on worker bees of different ages: behavioural and electrophysiological responses. Apidologie. 24: 267–281. [Google Scholar]
- Ratnieks, F. L. W., and Keller L.. . 1998. Queen control of egg fertilization in the honey bee. Behav. Ecol. Sociobiol. 44: 57–61. [Google Scholar]
- Santomauro, G., and Engels W.. . 2002. Sexing of newly hatched live larvae of the honey bee, Apis mellifera, allows the recognition of diploid drones. Apidologie. 33: 283–288. [Google Scholar]
- Woyciechowski, M., and Moroń D.. . 2009. Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insectes Sociaux. 56: 193–201. [Google Scholar]