Abstract
The first step of amino acid degradation in lactococci is a transamination, which requires an α-keto acid as the amino group acceptor. We have previously shown that the level of available α-keto acid in semihard cheese is the first limiting factor for conversion of amino acids to aroma compounds, since aroma formation is greatly enhanced by adding α-ketoglutarate to cheese curd. In this study we introduced a heterologous catabolic glutamate dehydrogenase (GDH) gene into Lactococcus lactis so that this organism could produce α-ketoglutarate from glutamate, which is present at high levels in cheese. Then we evaluated the impact of GDH activity on amino acid conversion in in vitro tests and in a cheese model by using radiolabeled amino acids as tracers. The GDH-producing lactococcal strain degraded amino acids without added α-ketoglutarate to the same extent that the wild-type strain degraded amino acids with added α-ketoglutarate. Interestingly, the GDH-producing lactococcal strain produced a higher proportion of carboxylic acids, which are major aroma compounds. Our results demonstrated that a GDH-producing lactococcal strain could be used instead of adding α-ketoglutarate to improve aroma development in cheese.
Enzymatic degradation of amino acids plays an important role in the development of flavor cheese. In particular, branched-chain amino acids are precursors of cheesy aroma compounds, such as isovalerate (precursor, Leu) and isobutyrate (Val), which are major aroma compounds of cheese, and aromatic amino acids are precursors of floral or phenolic aroma compounds, such as phenylacetate and phenylacetaldehyde (Phe), indole (Trp), and phenol (Tyr). The first step of branched-chain and aromatic amino acid degradation by lactococci, which are widely used as starters, is a transamination (6, 36; S. Thirouin, L. Rijnen, J. C. Gripon, and M. Yvon, Club des bactéries lactiques—7ème Colloq., abstr. M4, 1995). This reaction is catalyzed by branched-chain or aromatic aminotransferases and requires an α-keto acid as the amino group acceptor (6, 21, 36, 38). We have previously shown that in semihard cheese the presence of such a keto acid is the first limiting factor for amino acid transamination, since adding α-ketoglutarate (α-KG) greatly enhances the formation of cheese flavor from amino acids (37).
As an alternative to adding α-KG, we thought of using a lactic acid bacterial strain that is capable of producing a sufficient amount of α-KG from precursors present in cheese. Large amounts of glutamate are present in cheese, and this compound is a direct precursor of α-KG, since the conversion requires only oxidative deamination by a glutamate dehydrogenase (GDH). Unfortunately, we did not detect GDH activity in several strains of Lactococcus lactis and other lactic acid bacteria and did not find a gene homologous to known gdh sequences in the L. lactis IL1403 genome (P. Renault, personal communication).
Before large-scale screening for a GDH-containing strain is begun, it is essential to verify that a strain with such activity can produce α-KG and transform amino acids to aroma compounds under cheese-ripening conditions. To do this, we expressed a heterologous gdh gene in L. lactis. The GDH enzymes have been divided into two classes on the basis of metabolic specificity and oligomeric structure (27, 31). NAD+-dependent GDHs are either tetrameric or hexameric and are mainly involved in glutamate catabolism, while NADP+-dependent GDHs are hexameric and are mainly involved in ammonia assimilation and hence in glutamate synthesis. Catabolic NAD+-dependent GDHs have been found in several microorganisms, including Saccharomyces cerevisiae (34), Candida utilis (9), Neurospora crassa (2, 8), Streptomyces fradiae (17), Peptostreptococcus asaccharolyticus (28), Halobacterium halobium (3), Thermus thermophilus (23), Clostridium symbiosum (32), and Clostridium difficile (16), but only a few of these enzymes have been genetically and biochemically characterized (the C. symbiosum, C. difficile, and P. asaccharolyticus enzymes). Of the known gdh genes, the gdh gene of P. asaccharolyticus seems to be suitable for expression in L. lactis, since it is a gene from a gram-positive coccus that is phylogenetically closely related to L. lactis. Also, its promoter region (28) is highly homologous to the consensus promoter region of L. lactis (5), and the gene has a low G+C content (36%), like L. lactis genes (36 to 38%) (15). Moreover, this gene has already been successfully cloned with its own promoter in Escherichia coli, in which it was highly expressed (28). In P. asaccharolyticus GDH acts during hydroxyglutarate fermentation of glutamate, in which its role consists of degrading glutamate (11).
In this study, we cloned the P. asaccharolyticus gdh gene in L. lactis and examined the impact of expression of this gene on conversion of amino acids to aroma compounds in in vitro tests and in a cheese model in which radiolabeled amino acids were used as tracers. Tritiated phenylalanine and leucine were used as radiolabeled substrates that represented aromatic and branched-chain amino acids, respectively, since members of these two amino acid groups are major precursors of aroma compounds.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. P. asaccharolyticus was grown anaerobically in brain heart infusion broth (Difco) (1) supplemented with 0.5% (wt/vol) yeast extract, hemin (5 mg/liter), and cysteine (0.3 g/liter) at 37°C. E. coli was grown in Luria-Bertani medium (24) at 37°C with aeration. L. lactis strains were grown either in M17 medium (33) supplemented with 0.5% (wt/vol) lactose or 0.5% (wt/vol) glucose or in a chemically defined medium (CDM) (25). When necessary, erythromycin (5 μg · ml−1 for L. lactis and 150 μg · ml−1 for E. coli) or ampicillin (50 μg · ml−1 for E. coli) was added to the culture medium. For growth experiments, L. lactis was cultivated in buffered 75 mM β-glycerophosphate or in nonbuffered milk (reconstituted with NILAC milk powder [NIZO, Ede, The Netherlands] at a concentration of 10% [wt/vol] in distilled sterilized water) at 30°C. For milk cultures, precultures were grown in NILAC milk, which contained erythromycin for modified strains, by inoculating a series of tubes at concentrations of 0.5, 1, 2, and 4% with a preculture grown on M17 medium containing lactose. The most highly developed preculture that was not coagulated after incubation for 1 night at 30°C was then used to inoculate milk at a concentration of 2%. The optical densities at 480 nm of milk cultures were determined after 10-fold dilution with 5 mM EDTA (pH 12). Viable cell counts were determined by using M17 medium containing lactose with and without erythromycin selection.
DNA techniques.
DNA restriction and modification enzymes were purchased from GIBCO-BRL and Eurogentec and were used as recommended by the suppliers. Oligonucleotides were synthesized by Eurogentec (Seraing, Belgium).
L. lactis electrocompetent cells were prepared and transformed as described by Holo and Nes (10), with minor modifications, and E. coli electrocompetent cells were prepared and transformed as described by Sambrook et al. (24). Chromosomal DNA from P. asaccharolyticus was isolated as described by Snedecor et al. (28) and was extracted by isopropanol precipitation followed by ethanol precipitation. Plasmid DNA was prepared by using a plasmid purification kit obtained from Qiagen Inc. (Chatsworth, Calif.) for E. coli and by using the O'Sullivan-Klaenhammer method for L. lactis (19). PCR amplification was carried out with a Perkin-Elmer model 2400 DNA thermal cycler by using Taq polymerase. Samples used for sequencing were prepared with a PRISM Ready Reaction Dye Deoxy terminator cycle sequencing kit (Applied Biosystems, Warrington, Great Britain) by using the PCR apparatus, and the sequences were determined with an automatic sequencer (model 370A DNA sequencer; Applied Biosystems).
Construction of the gdh-producing (gdh+) strain.
A 1.6-kb DNA fragment containing the gdh gene and its promoter was amplified by PCR from the total DNA of P. asaccharolyticus by using two oligonucleotides chosen from the DNA sequence (28) (5′AGCTGATTAGCTATGAGT and 5′AAGTTCTGCTTATTTCGC). The PCR product was cloned in the cloning site of the pGEMT-Easy vector to obtain pTIL221, which was used to transform TG1 cells, which resulted in TIL321.
Plasmid pTIL221 was cloned in the PstI site of lactococcal expression vector pILN13 at a high copy number to obtain pTIL223, which was used to transform L. lactis TIL46 cells and create TIL323. A control strain (TIL324) was constructed by introducing the same plasmid construction without the gdh gene (pTIL224) into TIL46. Final constructions were verified by plasmid extraction and plasmid DNA sequencing.
Determination of GDH activity.
The GDH activities of cell extracts were determined by measuring the glutamate-dependent reduction of NAD as described by Johnson and Westlake (12). The reaction medium contained 10 mM l-glutamate, 1 mM NAD+, 40 mM Tris-HCl buffer (pH 8.8), and cell extract. Changes in NADH concentration were monitored at room temperature by measuring the absorbance at 340 nm, and the results were expressed in micromoles of NADH produced per minute per milligram of protein. Cell extracts were prepared as previously described (21), and protein concentrations were determined by the Bradford assay (4) by using bovine serum albumin as the standard.
Amino acid catabolism in vitro.
Amino acid catabolism by the different strains (TIL46, TIL323, and TIL324) was studied as previously described (21) by incubating whole CDM-cultivated cells in various reaction media containing l-[2,6-3H]phenylalanine (60 Ci mmol−1) or l-[4,5-3H]leucine (60 Ci mmol−1) as the tracer. The reaction mixtures contained 70 mM buffer (phosphate buffer [pH 5.5 or 6.5] or Tris-HCl buffer [pH 8]), unlabeled amino acid at a concentration of 3 mM, and radiolabeled amino acid at a concentration of 0.05 μM. In some cases, glucose (0.3%), α-KG (10 mM), or glutamate (10 mM) was added. Aliquots of the reaction mixtures were analyzed at zero time and after incubation for 40 h at 37°C by reverse-phase or ion-exclusion high-performance liquid chromatography with both UV detection (214 nm) and radioactivity detection as previously described (36, 37).
Amino acid catabolism in a cheese model.
Amino acid catabolism by the gdh+ strain and the control strain (TIL324) was also studied under cheese-ripening conditions by using the cheese model (Ch-Easy) developed by NIZO (26). The trials were performed in small sterile tubes containing 2 g ± 0.05 g of cheese paste. For each strain, whole cells were harvested from a culture in buffered NILAC milk containing erythromycin at an optical density of 4.5. The cells were washed twice with 0.5 M glycerophosphate (pH 7) and resuspended in the same buffer to obtain a cellular concentration of 1010 cells · ml−1. The cells were added aseptically to the cheese paste at a concentration of 2 × 109 cells · g−1. Fifty microliters of an α-KG solution (15 mg · g of paste−1) was added in one-half of the trials carried out with the control strain, while the same volume of water was added in the other half of the trials carried out with the control strain and the trials carried out with the gdh+ strain. To monitor amino acid catabolism, radiolabeled Phe and Leu (10 μCi · g−1 in 50 μl) were also added to the paste except for the paste used for the free amino acid and α-KG analysis and for microbiological controls. The paste mixture was homogenized before incubation at 13°C, and all analyses were performed at zero time and after 4 weeks of incubation. Radiolabeled metabolites were extracted and analyzed as previously described (37). α-KG and free amino acid contents were determined as previously described, with minor modifications (37). All cheese model experiments were done in duplicate by using cells from different cultures.
RESULTS
Expression of gdh in L. lactis.
Expression of gdh from P. asaccharolyticus in E. coli was verified by determining the GDH activity in a TIL321 cellular extract. The GDH specific activity was 6.8 μmol per mg of protein per min, while no activity was found in TG1; these findings demonstrated that the level of expression of gdh in E. coli was high, as previously observed by Snedecor et al. (28). By using lactococcal expression vector pILN13 at a high copy number (45 to 65 copies/cell), gdh was also successfully expressed in L. lactis, although the enzyme activity was 8-fold lower than the enzyme activity in E. coli. Indeed, the GDH specific activity of strain TIL323 was 0.9 μmol per mg of protein per min, while no activity was detected in control strain TIL324.
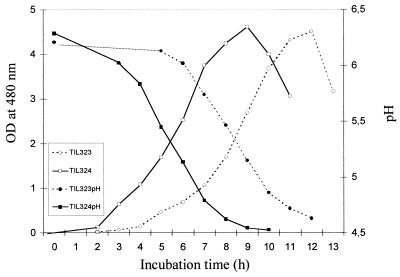
GDH activity in L. lactis affected growth in CDM and in buffered milk only very slightly (results not shown), but in nonbuffered milk the gdh+ strain grew and acidified the medium more slowly than the wild-type strain (Fig. 1).
FIG. 1.
Growth curves and acidification of the wild-type strain and the gdh+ strain in nonbuffered milk. The data are means based on two determinations. OD at 480 nm, optical density at 480 nm.
The constructions appeared to be stable in L. lactis, since the viable cell counts with and without erythromycin selection after growth in milk without erythromycin were not significantly different (results not shown).
Amino acid catabolism in vitro.
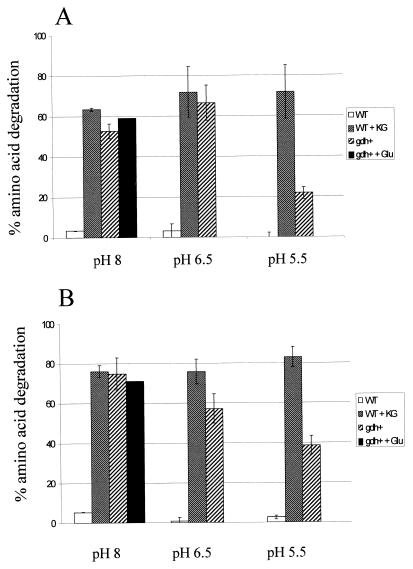
Amino acid catabolism by the gdh+ strain (TIL323) was compared to amino acid catabolism by the wild-type strain (TIL46) and the control strain (TIL324) in vitro by using radiolabeled Phe or Leu as the tracer. Amino acid degradation was determined under different reaction conditions (Fig. 2). Degradation by the wild-type strain and degradation by the control strain were similar in each medium (results not shown), and the levels of degradation were very low in each medium when α-KG was not present. When α-KG was added to the media at pH 8, the wild-type strain degraded large amounts of Leu and Phe. At the same pH when α-KG was not present, the gdh+ strain also degraded large amounts of Leu and Phe, and adding glutamate did not increase the level of degradation. In fact, α-KG accumulated in cells of the gdh+ strain during growth (approximately 2 mM), while no α-KG was found in the wild-type strain. Moreover, all of the cells also contained a large amount of glutamate which could be used by the GDH. Therefore, in this case adding glutamate did not seem to be essential for α-KG production. These results indicate that the gdh+ strain does produce α-KG, which can be used as a cosubstrate for amino acid transamination. Since the amount of α-KG present in the cells due to accumulation during growth (18 nmol) was much smaller than the amount of amino acid transformed (750 nmol), the GDH must also have been active during incubation in order to generate α-KG from glutamate. Amino acid degradation by the wild-type strain was not affected by a decrease in the pH to 5.5, indicating that transaminase activity was not actually reduced by this external pH. Conversely, amino acid degradation by the modified strain gradually decreased as the reaction pH decreased, indicating that GDH activity was inhibited by a lower pH, which limited the amount of α-KG available for transamination.
FIG. 2.
Percentages of amino acids degraded in reaction media at pH 8, 6.5, and 5.5 by the wild-type strain without added α-KG (WT) or with added α-KG (WT + KG) and by the gdh+ strain without added glutamate (gdh+) or with added glutamate (gdh+ + Glu). (A) Phenylalanine degradation. (B) Leucine degradation. Amino acid degradation was monitored by measuring the radioactivity associated with the residual amino acid introduced into the reaction mixture as the tracer after incubation for 40 h at 37°C. The error bars indicate standard deviations based on duplicate or triplicate determinations.
Microbial control and free amino acid analysis in a cheese model.
The microbial control values and free amino acid contents before incubation and after 4 weeks of incubation and the amounts of ammonia produced in 4 weeks are shown in Table 2. The amount of free amino acids in the cheese model before incubation was approximately 40 μmol per g of cheese paste, which is much greater than the amount present in semihard cheeses before ripening (approximately 4 μmol per g) (21a, 37). This is because the basis of the cheese model is a 4-week-old Gouda type of cheese (26). The lactococcal inoculation levels in the cheese model were very similar for the different strains in both trials and corresponded to the expected levels (2 × 109 cells per g). The level of survival of the gdh+ mutant cells after 4 weeks of incubation was not significantly different from the level of survival of the control strain, while adding α-KG in the cheese model seemed to increase the level of survival of the control strain. The modified strains appeared to be stable during incubation, since the viable cell counts with and without erythromycin selection were similar (results not shown). In the first experiment, the amounts of free amino acids released in the cheese model incubated with the gdh+ strain were greater than the amounts released in the cheese model incubated with the control strain; however, this finding was not confirmed in the second experiment. The GDH+ mutant produced twice as much ammonia as the control strain, probably due to the deamination activity of GDH.
To compare the free amino acid compositions in the cheese model incubated with both strains, the amount of each amino acid was expressed as a percentage of the total amount of free amino acid in the cheese model (Table 3). As previously observed in semihard cheese made with the same strain (21a, 37), the main free amino acids released during ripening in the cheese model were glutamate and leucine, as well as (to a lesser extent) phenylalanine, asparagine, lysine, ornithine (or arginine), valine, and proline. In the cheese model when the control strain was used, adding α-KG significantly increased the level of Glu and significantly decreased the levels of Leu, Ile, Val, Asp, Met, Phe, Tyr, and Trp, indicating that transamination of these amino acids occurred. Expression of the heterologous gdh gene in L. lactis also resulted in decreases in the proportions of Leu, Val, Phe, Tyr, and Trp in the cheese model compared to the proportions of the amino acids in the cheese model incubated with the control strain without α-KG, but the glutamate level did not increase simultaneously. These results indicate that GDH did utilize glutamate to generate α-KG, which allowed transamination of aromatic and branched-chain amino acids to occur. However, on the basis of these analyses it is not possible to compare the absolute extents of amino acid degradation, since the amounts of free amino acids released were not the same for both strains.
Amino acid catabolism in the cheese model.
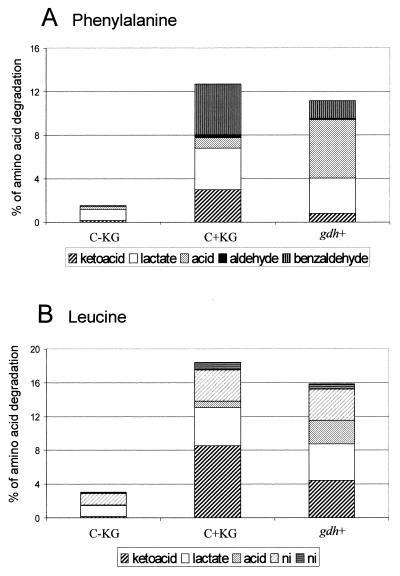
Amino acid catabolism was monitored in the cheese model by using radiolabeled Phe and Leu as tracers (Fig. 3). The results obtained with the cheese model when the control strain was used were very similar to the results obtained previously with experimental St. Paulin type cheeses made with the wild-type strain (21a, 37). Indeed, in the cheese model when the control strain was used without α-KG, the level of amino acid degradation was very low, and it increased substantially when α-KG was added. The major metabolites produced were also similar to those produced in semihard cheese and were keto acids, hydroxy acids (from Phe and Leu), and benzaldehyde (from Phe), as well as (to a lesser extent) carboxylic acids (from Phe and Leu), aldehyde (from Phe), and some unidentified compounds (from Leu). In the cheese model when the gdh+ strain was used without α-KG, the levels of Phe and Leu degradation were also much greater than the levels of degradation in the cheese model when the control strain was used without α-KG and almost reached the levels of degradation obtained with the control strain when α-KG was added. This confirmed that the gdh+ strain produced α-KG from glutamate under cheese-ripening conditions, which allowed amino acid transamination to occur. Interestingly, in the cheese model, the gdh+ strain produced much more carboxylic acid from α-keto acids than the wild-type strain produced when α-KG was added, indicating that the levels of α-keto acid dehydrogenase activities which were probably involved in the conversion were higher (22). In contrast, lower levels of benzaldehyde, which was formed by spontaneous oxidation of the keto acid of Phe (18), were produced in the cheese model when the gdh+ strain was used.
FIG. 3.
Amino acid degradation in the cheese model by the control strain without added α-KG in the cheese paste (C-KG) or with added α-KG (C+KG) and by the gdh+ strain (gdh+). (A) Metabolites produced from phenylalanine. (B) Metabolites produced from leucine. The data are means of results from two trials. The percentages of carboxylic acids, keto acids, and benzaldehyde produced by the gdh+ strain were significantly different from the percentages produced by the control strain in the presence of α-KG. ni, not identified.
DISCUSSION
In order to verify that a GDH-producing lactococcal strain is capable of producing sufficient α-KG to transform amino acids to aroma compounds under cheese-ripening conditions, we introduced the gdh gene from P. asaccharolyticus with its own promoter into L. lactis. As expected from the high levels of homology between the transcription and translation signal sequences of P. asaccharolyticus and the consensus sequences of L. lactis, the heterologous gene was successfully expressed in L. lactis. The GDH activity of the gdh+ L. lactis strain in the direction of oxidative deamination was 0.9 μmol per mg per min, which is only fivefold lower than the level of activity found in extracts of P. asaccharolyticus grown on glutamate, in which high levels of GDH are produced (12, 14).
In contrast to the wild-type strain, the gdh+ strain was able to degrade amino acids to aroma compounds in the absence of α-KG in vitro at pH 8, and the extent of degradation was similar to the extent observed with the wild-type strain in a medium containing α-KG. However, in vitro GDH activity, which is optimal at pH 8 to 8.8, was reduced when the pH was decreased to 5.5, which is the pH of cheese. This decrease in activity led to a decrease in amino acid degradation, since α-KG was limiting for transamination. However, in the cheese model, the GDH activity of the gdh+ strain was still high enough that amino acid degradation could occur, similar to the degradation obtained when α-KG is added in the cheese model with the wild-type strain. In fact, the level of amino acid degradation in the cheese model when α-KG is added is lower than the level in liquid medium since the level of uptake of exogenous α-KG in cells is probably lower in solid cheese than in liquid medium.
Surprisingly, the gdh+ strain produced much more carboxylic acid from the α-keto acids than the wild-type strain produced, and the gdh+ strain produced less benzaldehyde from phenylpyruvate. Since α-keto acid oxidative decarboxylation by α-keto acid dehydrogenase occurs mainly at pH 5.5 to 6.5 (29), requires NAD+, and is inhibited by NADH (30) and since, in contrast, chemical oxidation of phenylpyruvate to benzaldehyde mainly occurs at pH values greater than 7 and uses oxygen (18), we can assume that the intracellular pH of the gdh+ strain was lower than the intracellular pH of the wild-type strain and the ratio of NAD+ to NADH was much higher. However, this was not expected, since glutamate oxidation by GDH generates reduced coenzyme and ammonia, so additional studies are needed to explain the change in metabolism.
However, whatever the reason for the changes in metabolism, the intensification of keto acid transformation to carboxylic acids is very interesting in terms of the development of flavor in cheese. Indeed, carboxylic acids, such as isovaleric acid and isobutyric acid, are very potent aroma compounds and contribute greatly to cheese flavor (35). Ammonia produced by glutamate deamination could also generate ammoniacal flavor. However, the ammonia concentration in cheese prepared with the gdh+ strain reached only 0.2 mg/g, which is 10-fold lower than the concentration found in Camembert and Brie (13), in which ammonia really contributes to the cheese flavor. Now, experimental cheese trials have to be performed to verify the value of such a strain for cheese making. In fact, the experiments performed with the cheese model show that a GDH-producing strain has potential value for cheese ripening, but this model does not take into account development of the strain in milk, which seems to be slowed down.
In lactic acid bacteria and in other bacteria used in the food industry, the presence of GDH activity or of a gene encoding GDH has not been reported previously. However, considering the effect of gdh expression in L. lactis on the formation of aroma from amino acids, it would be interesting to perform large-scale screening of these bacteria for GDH activity.
In conclusion, we demonstrated that a GDH-producing lactococcal strain could be used instead of adding α-KG to cheese to enhance amino acid degradation to aroma compounds.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| L. lactis subsp. cremoris strains | ||
| NCDO763 | Wild-type strain | National Collection of Food Bacteria (Shinfield, Reading, England) |
| TIL46 | NCDO763 cured of its 2-kb plasmid | |
| TIL323 | TIL46 with pTIL322, GDH+ | This study |
| TIL324 | TIL46 with pTIL325, GDH− | This study |
| E. coli strains | ||
| TG1 | 7 | |
| TIL321 | TG1 with pTIL320 | This study |
| P. asaccharolyticus ATCC 14963 | Wild-type strain | American Type Culture Collection (Rockville, Md.) |
| Plasmids | ||
| pGEMT-Easy | Cloning vector (T-overhangs) | Promega Corp., Madison, Wis. |
| 3.0 kb, AmprlacZ f1ori | ||
| pILN13 | 4.8 kb, repE EryrrepF, high (45 to 65) or low (6 to 9) copy number | 20 |
| pTIL320 | pGEMT-Easy + gdh of P. asaccharolyticus | This study |
| pTIL322 | pTIL320 + pILN13 | This study |
| pTIL325 | pGEMT-Easy + pILN13 | This study |
TABLE 2.
Microbial control values and free amino acid concentrations in Ch-easy cheese model preparations containing the control strain or the gdh+ strain with or without α-KG
| Ch-easy trial | Strain | Presence of α-KG | Free amino acid concn at zero time (μmol · g−1) | Free amino acid concn at 4 weeks (μmol · g−1) | NH3 formed in 4 weeks (μmol · g−1) | No. of viable cells at zero time (CFU · g−1) | No. of viable cells at 4 weeks (CFU · g−1) |
|---|---|---|---|---|---|---|---|
| 1 | Control | + | 38.40 | 47.25 | 3.48 | 2.0 × 109 | 2.6 × 108 |
| Control | − | 49.94 | 3.27 | 2.0 × 109 | 1.6 × 106 | ||
| gdh+ | − | 68.03 | 6.60 | 2.0 × 109 | 5.7 × 106 | ||
| 2 | Control | + | 42.85 | 53.70 | 2.57 | 2.3 × 109 | 1.1 × 109 |
| Control | − | 51.88 | 2.64 | 2.6 × 109 | 1.5 × 107 | ||
| gdh+ | − | 56.12 | 4.41 | 2.2 × 109 | 1.0 × 106 |
TABLE 3.
Amino acid composition of control Ch-easy preparation containing the wild-type strain and effect of adding α-KG or effect of GDH activity
| Amino acid | % of total amino acidsa | Difference (%) forb:
|
|
|---|---|---|---|
| Wild type + α-KG | gdh+ | ||
| Asp | 2.34 | −0.33 | +0.19 |
| Thr | 2.76 | −0.03 | +0.14 |
| Ser | 3.12 | −0.11 | +0.37 |
| Asn | 8.03 | −0.20 | −0.99 |
| Glu | 18.97 | +6.74 | −0.83 |
| Gln | 3.34 | +0.01 | +1.18 |
| Pro | 6.04 | +0.38 | +0.33 |
| Gly | 1.87 | +0.08 | +0.22 |
| Ala | 3.72 | −0.08 | +0.09 |
| Cit | 0.70 | +0.05 | −0.02 |
| Val | 5.96 | −0.78 | −0.33 |
| Cys | 0.00 | +0.00 | +0.00 |
| Met | 1.71 | −0.21 | +0.02 |
| Ile | 2.22 | −0.53 | +0.26 |
| Leu | 15.87 | −3.15 | −1.64 |
| Tyr | 2.86 | −0.49 | −0.09 |
| Phe | 5.72 | −1.36 | −0.45 |
| His | 1.24 | −0.01 | +0.32 |
| Trp | 1.85 | −0.09 | −0.29 |
| Orn | 6.16 | −0.06 | −0.02 |
| Lys | 5.50 | +0.17 | +1.54 |
| Arg | 0.00 | +0.00 | +0.00 |
Means based on two determinations.
The boldface type indicates values that are greater than the variability between the two trials.
ACKNOWLEDGMENTS
This work was supported by FAIR contract CT97-3173 and TMR grant ERB 4001 GT954921 from the Commission of European Communities.
We thank A. Clara for preparing the anaerobic culture of P. asaccharolyticus, G. Smit (NIZO) for providing the Ch-easy model, and A.-M. Wall (INRA Translation Unit, Jouy-en-Josas, France) for revising the English version of the manuscript.
REFERENCES
- 1.Atlas R M, Parks L C. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1993. p. 149. [Google Scholar]
- 2.Austen B M, Haberland M E, Nyc J F, Smith E L. Nicotinamide adenine dinucleotide-specific glutamate dehydrogenase of Neurospora. J Biol Chem. 1977;252:8142–8149. [PubMed] [Google Scholar]
- 3.Bonete M J, Perez-Pomares F, Ferrer J, Camacho M L. NAD-glutamate dehydrogenase from Halobacterium halobium: inhibition and activation by TCA intermediates and amino acids. Biochim Biophys Acta. 1996;1289:14–24. doi: 10.1016/0304-4165(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.de Vos W M, Simons G F M. Gene cloning and expression systems in lactococci. In: Gasson M J, de Vos W M, editors; Gasson M J, de Vos W M, editors. Genetics and biotechnology of lactic acid bacteria. London, United Kingdom: Blackie Academic and Professional; 1994. pp. 54–105. [Google Scholar]
- 6.Gao S, Oh D H, Broadbent J R, Johnson M E, Weimer B C, Steele J L. Aromatic amino acid catabolism by lactococci. Lait. 1997;77:371–381. [Google Scholar]
- 7.Gibson T J. Studies on the Epstein Barr virus genome. Ph.D. thesis. Cambridge, England: University of Cambridge; 1984. [Google Scholar]
- 8.Haberland M, Smith E L. Nicotinamide adenine dinucleotide-specific glutamate dehydrogenase of Neurospora crassa. J Biol Chem. 1980;255:7984–7992. [PubMed] [Google Scholar]
- 9.Hemmings B A. Phosphorylation of NAD-dependent glutamate dehydrogenase from yeast. J Biol Chem. 1978;253:5255–5258. [PubMed] [Google Scholar]
- 10.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornby D P, Engel P C. Characterization of Peptostreptococcus asaccharolyticus glutamate dehydrogenase purified by dye-ligand chromatography. J Gen Microbiol. 1984;130:2385–2394. doi: 10.1099/00221287-130-9-2385. [DOI] [PubMed] [Google Scholar]
- 12.Johnson W M, Westlake W S. Purification and characterization of glutamic acid dehydrogenase and α-ketoglutaric acid reductase from Peptococcus aerogenes. Can J Microbiol. 1972;18:881–891. doi: 10.1139/m72-136. [DOI] [PubMed] [Google Scholar]
- 13.Karahadian C, Lindsay R C. Integrated roles of lactate, ammonia, and calcium in texture development of mold surface-ripened cheese. J Dairy Sci. 1987;70:909–918. [Google Scholar]
- 14.Kew O M, Woolfolk C A. Preparation of glutamate dehydrogenase from Micrococcus aerogenes. Biochem Biophys Res Commun. 1970;39:1126–1133. doi: 10.1016/0006-291x(70)90676-5. [DOI] [PubMed] [Google Scholar]
- 15.Kippler-Balz R, Fischer G, Schleiffer K H. Nucleic acid hybridization of group N and group D streptococci. Curr Microbiol. 1982;7:245–250. [Google Scholar]
- 16.Lyerly D M, Barraso L A, Wilkins T D. Identification of the latex test-reactive protein of Clostridium difficile as glutamate dehydrogenase. J Clin Microbiol. 1991;29:2639–2642. doi: 10.1128/jcm.29.11.2639-2642.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen K T, Nguyen L T, Kopeckey J, Behal V. Properties of NAD-dependent glutamate dehydrogenase from the tylosin producer Streptomyces fradiae. Can J Microbiol. 1997;43:1005–1010. [Google Scholar]
- 18.Nierop-Groot M N, de Bont J A M. Conversion of phenylalanine to benzaldehyde initiated by an aminotransferase in Lactobacillus plantarum. Appl Environ Microbiol. 1998;64:3009–3013. doi: 10.1128/aem.64.8.3009-3013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Sullivan D J, Klaenhammer T R. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol. 1993;59:2730–2733. doi: 10.1128/aem.59.8.2730-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renault P, Corthier G, Goupil N, Delorme C, Ehrlich S D. Plasmid vectors for Gram-positive bacteria switching from high to low copy number. Gene. 1996;183:175–182. doi: 10.1016/s0378-1119(96)00554-9. [DOI] [PubMed] [Google Scholar]
- 21.Rijnen L, Bonneau S, Yvon M. Genetic characterization of the lactococcal aromatic aminotransferase and its involvement in the conversion of amino acids to aroma compounds. Appl Environ Microbiol. 1999;65:4873–4880. doi: 10.1128/aem.65.11.4873-4880.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Rijnen, L., A. Delacroix-Buchet, O. Demaizieres, J. L. Le Quéré, J.-C. Gripon, and M. Yvon. Inactivation of lactococcal aromatic aminotransferase prevents the formation of floral aroma compounds from aromatic amino acids in semihard cheese. Int. J. Dairy Res., in press.
- 22.Roudot-Algaron F, Yvon M. Le catabolisme des acides aminés aromatiques et des acides aminés à chaîne ramifiée chez Lactococcus lactis. Lait. 1998;78:23–30. [Google Scholar]
- 23.Ruiz J L, Ferrer J, Camacho M, Bonete M J. NAD-specific glutamate dehydrogenase from Thermus thermophilus HB8: purification and enzymatic properties. FEMS Microbiol Lett. 1998;159:15–20. [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 25.Smid E J, Konings W N. Relationship between utilization of proline and proline-containing peptides and growth of Lactococcus lactis. J Bacteriol. 1990;172:5286–5292. doi: 10.1128/jb.172.9.5286-5292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smit G, Braber A, van Spronsen W, van de Berg G, Exterkate F A. Ch-easy model: a cheese-bases model to study cheese ripening. Bioflaveur. 1995;95:185–190. [Google Scholar]
- 27.Smith E L, Austen B M, Blumenthal K M, Nyc J F. Glutamate dehydrogenases. In: Boyer P, editor; Boyer P, editor. The enzymes. New York, N.Y: Academic Press; 1975. pp. 293–367. [Google Scholar]
- 28.Snedecor B, Chu H, Chen E. Selection, expression and nucleotide sequencing of the glutamate dehydrogenase gene of Peptostreptococcus asaccharolyticus. J Bacteriol. 1991;173:6162–6167. doi: 10.1128/jb.173.19.6162-6167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snoep J L, de Mattos M J T, Postma P W, Neijssel O M. Involvement of pyruvate dehydrogenase in product formation in pyruvate-limited anaerobic chemostat cultures of Enterococcus faecalis NCTC 775. Arch Microbiol. 1990;154:50–55. doi: 10.1007/BF00249177. [DOI] [PubMed] [Google Scholar]
- 30.Snoep J L, de Mattos M J T, Starrenburg M J C, Hugenholz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and α-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol. 1992;174:4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stillman T J, Baker P J, Britton K L, Rice D W. Conformational flexibility in glutamate dehydrogenase: role of water in substrate recognition and catalysis. J Mol Biol. 1993;234:1131–1139. doi: 10.1006/jmbi.1993.1665. [DOI] [PubMed] [Google Scholar]
- 32.Teller J K, Smith R M, McPherson M J, Engel P C, Guest J R. The glutamate dehydrogenase gene of Clostridium symbiosum. Cloning by polymerase chain reaction, sequence analysis and over-production in Escherichia coli. Eur J Biochem. 1992;15:151–159. doi: 10.1111/j.1432-1033.1992.tb16912.x. [DOI] [PubMed] [Google Scholar]
- 33.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uno I, Matsumoto K, Adachi K, Ishikawa T. Regulation of NAD-dependent glutamate dehydrogenase by protein kinases in Saccharomyces cerevisiae. J Biol Chem. 1984;25:1288–1293. [PubMed] [Google Scholar]
- 35.Urbach G. Contribution of lactic acid bacteria to flavour compound formation in dairy products. Int Dairy J. 1995;5:877–903. [Google Scholar]
- 36.Yvon M, Thirouin S, Rijnen L, Fromentier D, Gripon J C. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl Environ Microbiol. 1997;63:414–419. doi: 10.1128/aem.63.2.414-419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yvon M, Berthelot S, Gripon J C. Adding α-ketoglutarate to semi-hard cheese curd highly enhances the conversion of amino acids to aroma compounds. Int Dairy J. 1999;8:889–898. [Google Scholar]
- 38.Yvon M, Chambellon E, Bolotin A, Roudot-Algaron F. Characterization and role of the branched-chain aminotransferase (BcaT) isolated from Lactococcus lactis subsp. cremoris NCDO 763. Appl Environ Microbiol. 2000;66:571–577. doi: 10.1128/aem.66.2.571-577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]