Abstract
The development of effective cognitive training (CT) interventions is critical for improving the daily lives of people with schizophrenia. At this point, it is unclear whether a so-called “bottom-up” or “top-down” CT approach is more beneficial for inducing cognitive gains and generalization in this population. The aims of this randomized controlled trial were to: 1) Compare the effects of these two types of training approaches on performance-based (MATRICS Consensus Cognitive Battery, MCCB) and neurophysiological (mismatch negativity, MMN) measures of cognition, and 2) Evaluate MMN as a potential predictor of treatment response. Ninety-nine patients with persistent schizophrenia (mean age of 51 and illness duration of 30 years) were randomly assigned in a 2:2:1 ratio to a “bottom-up” intervention that selectively targets basic auditory processing and verbal learning (Brain Fitness), a “top-down” intervention that targets a broad range of higher-order cognitive functions (COGPACK), or a control condition consisting of commercial computer games (Sporcle). Participants completed an average of 30 hours of training over 12 weeks. Despite demonstrated improvement on training tasks, we found no significant treatment effects on measures of neurocognition (MCCB), MMN, or functional capacity from either intervention. Interestingly, there was an association between an enhanced MMN response at 6 weeks and improved reasoning/problem solving at 12 weeks in the COGPACK group. Although this study had several methodological strengths, the results were mainly negative. It suggests that CT trials in schizophrenia should try to better understand mediators and moderators of treatment response to develop more personalized interventions.
Keywords: schizophrenia, cognition, randomized controlled trial, cognitive remediation, EEG, plasticity
1. Introduction
Cognitive dysfunction is a major contributor to the poor community outcomes and high levels of functional disability in people with schizophrenia (Bowie et al., 2008; Green et al., 2000). Therefore, effectively treating the cognitive deficits associated with this illness is important for functional outcomes. Cognitive training (CT) is considered an effective method for ameliorating cognitive impairment in schizophrenia. Meta-analytic studies (McGurk et al., 2007; Wykes et al., 2011) have reported a moderate effect-size improvement in cognition (0.45) and a significant, but lower, impact on daily functioning (0.36).
Traditionally, drill-and-practice CT interventions for schizophrenia have targeted higher-order neurocognitive operations (e.g., strategy learning, problem-solving, working memory) (Medalia and Choi, 2009). These “top-down” methods rest on the premise that by training more molar, complex abilities, component processes such as attention and processing speed will be engaged and trained simultaneously. CT that focuses exclusively on higher-order executive functions can induce structural and functional brain changes in people with schizophrenia, such as increased activation in prefrontal cortex and regions subserving attention and working memory (Bor et al., 2011; Haut et al., 2010; Wykes et al., 2002) and increased white matter integrity (Penades et al., 2013).
Recently, “neuroplasticity-based” interventions have been developed to train basic perceptual processes in schizophrenia, while also engaging attentional and working memory operations (Vinogradov et al., 2012). These “bottom-up” approaches are explicitly designed to drive adaptive plastic changes throughout distributed prefrontal-temporo-parietal systems (Keshavan et al., 2014). Intensive auditory processing training in schizophrenia has been shown to improve auditory perceptual abilities (Fisher et al., 2009), as well as auditory neural responses (M100) (Adcock et al., 2009; Dale et al., 2010) and gating (M50) (Popov et al., 2011), assessed with magnetoencephalography. This neuroscience-informed approach to training can also generate meaningful restoration of prefrontal functions and higher-order cognition (Biagianti et al., 2016; Dale et al., 2016). For instance, intensive training of auditory, visual, and social cognitive processes in schizophrenia patients improved working memory and normalized patterns of neural activation (Subramaniam et al., 2014).
Therefore, both “top-down” and “bottom-up” CT approaches lead to improvements in targeted cognitive outcomes in published studies, as well as detectable changes in brain functioning. Nonetheless, the literature is unclear regarding the relative efficacy and mechanistic specificity of these two approaches, as well as the critical drivers of behavioral change. Only one study (Popov et al., 2011) compared the two methods on a few outcome measures and found that intensive auditory training was superior to broad-spectrum cognitive training for sensory gating and verbal learning and memory. While these results are intriguing, the study had a relatively small sample of 39 patients total.
Regardless of which approach is used, there is a great deal of variability in individual responses to CT (Corbera et al., 2017; Murthy et al., 2012) and a high percentage of patients exhibit little or no benefit even after long hours of training (Wykes et al., 2011). Because CT relies on neuroplasticity or the brain’s capacity to alter its structure and function in response to new learning experiences, patients who do not respond well to CT may differ in their synaptic plasticity. Thus, measures of neuroplastic capacity may predict therapeutic response. One such index is mismatch negativity (MMN). MMN is an event-related potential that is elicited in response to infrequent, physically deviant tones interspersed in the repeated presentation of a standard tone (Naatanen et al., 1978). It is thought to index preattentive auditory discrimination and NMDA-dependent short-term plasticity (Stephan et al., 2006). Given the robust MMN abnormality in schizophrenia (Umbricht and Krljes, 2005) and its relationship to higher-order cognition (Wynn et al., 2010) and real-world functioning (Light and Braff, 2005), MMN may be a useful biomarker of treatment response. In fact, some studies find that MMN predicts cognitive gains following CT (Biagianti et al., 2017) and is malleable after only an hour of auditory training (Perez et al., 2017).
The goal of this randomized controlled trial was to compare the effects of a “bottom-up” versus “top-down” CT intervention relative to a placebo control on neurocognition (primary outcome), functional capacity (secondary outcome) and a neural measure of plasticity (proximal outcome) in schizophrenia. We hypothesized that the bottom-up training would lead to superior effects on the primary and secondary outcomes relative to the top-down training. As we expected a large degree of individual variability, we also hypothesized that patients who have a larger MMN amplitude at baseline would benefit more from treatment. Similarly, we predicted that those who exhibit malleability (i.e., amplitude changes) in MMN measured halfway through the training would have more substantial cognitive gains at the end of training.
2. Methods
2.1. Study Design and Procedures
This was a 12-week parallel, randomized controlled 3-group study conducted at the Veterans Affairs (VA) Greater Los Angeles Healthcare System (GLA) from November 2013 to April 2018. The trial protocol was approved by the VA Institutional Review Board; all participants had the capacity to voluntarily consent to the procedures. The study was registered under ClinicalTrials.gov (NCT01891721).
A block randomization system was used to assign participants to the three treatment arms. The allocation sequence and subject’s group membership were concealed from the staff members who recruited, consented, and assessed participants. After baseline assessments, subjects participated in 3 training sessions a week over a period of 12 weeks (36 sessions total). Training was delivered in cohorts of 5 to 8 subjects who worked on personal computer stations located at GLA. The study coordinator gave subjects individualized instruction in the use of the computerized training program and assisted subjects during their training sessions. The cognitive battery was administered at baseline and 12 weeks; electroencephalogram (EEG) assessment was administered at baseline, mid-training (6 weeks), and end of training (12 weeks). A urine toxicology screen was conducted at each assessment visit. In the rare instance when a subject tested positive, he/she was rescheduled to complete the assessments on another day.
2.2. Participants
Patients were recruited from VA outpatient treatment clinics and board-and-care residences in the community. They had a mean age of 51 years and mean duration of illness of 30 years. Of the 105 patients enrolled into the study, 6 were excluded because they failed to meet inclusion criteria or declined to participate. The final sample for this report consisted of 99 patients (86 schizophrenia, 13 schizoaffective disorder). Participants were considered clinically stable based on: no medication changes in the past six weeks, no psychiatric hospitalization in the past three months, and no changes in housing in the past two months. Exclusion criteria included having an estimated premorbid IQ below 70 based on reading ability, having an identifiable neurological disorder, seizures, or history of serious head injury with loss of consciousness longer than 15 min, meeting criteria for substance dependence in the past 6 months or abuse in the past month, or being insufficiently fluent in English as determined by the participant’s ability to understand the consent form. During the study, 77 of the 99 participants were receiving atypical antipsychotic medications, 9 typical antipsychotic medications, 5 both types of antipsychotics, 5 not taking antipsychotic medication, and 3 were missing medication information.
All patients received a diagnostic interview with the Structured Clinical Interview for DSM-IV (SCID-I; First et al., 1997). Interviewers were trained to reliability through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) (Ventura et al., 1998; Ventura et al., 1993). Clinical symptoms were evaluated using the expanded 24-item UCLA version of the Brief Psychiatric Rating Scale (BPRS; Ventura et al., 1993) and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984), respectively. The Role Functioning Scale (RFS; Goodman et al., 1993) was used to assess patients’ functioning in the past month. We also administered the Intrinsic Motivation Inventory for Schizophrenia Research (IMI-SR; Choi et al., 2010) at 6 weeks of training.
2.3. Computerized Training
2.3.1. Brain Fitness (Bottom-Up Program)
Brain Fitness (Posit Science, San Francisco; www.brainhq.com) is designed to target bottom-up operations in the auditory system through an intensive, repetitive, and adaptive training of well-defined auditory skills (Adcock et al., 2009; Fisher et al., 2009). It consists of 6 exercises ranging from simple acoustic tasks (e.g., time order judgments of rapidly successive frequency-modulated sweeps) to complex manipulations of continuous speech (e.g., narrative memory). Participants train on stimulus recognition, discrimination, and sequencing tasks under conditions of increasing working memory load, requiring them to incorporate their improvements in basic auditory perception into higher-level cognitive skills, such as verbal learning and memory (Fisher et al., 2016; Loewy et al., 2016; Tarasenko et al., 2016). To drive learning and ensure a dense reward schedule, difficulty level is continuously adjusted to maintain approximately 85% correct individual performance. In each session, participants worked with 4 of the 6 exercises (15 min per exercise).
2.3.2. COGPACK (Top-Down Program)
COGPACK (Marker Software, Ladenburg, Germany; www.COGPACK.de) is designed to provide a less-targeted training across a broad range of higher-order cognitive functions (Bender et al., 2004; Lindenmayer et al., 2013; Vita et al., 2011). The exercises cover several domains including attention, verbal and visual memory, reasoning and executive functioning, language, knowledge, and everyday skills. COGPACK also trains a variety of basic cognitive processes (i.e., scanning, hand-eye coordination, and psychomotor speed). However, these low-level cognitive exercises were not included in the protocol. There was a total of 34 exercises as well as variants of the same exercises with different levels of complexity. In each session, participants worked on 4 to 6 exercises. Similar to Brain Fitness, the program provides regular individualized feedback and adjusts the level of difficulty based on the subject’s performance during the session.
2.3.3. Sporcle (Computer Game Control)
Sporcle (www.sporcle.com) was used as a “placebo” treatment to control for the effects of computer exposure, contact with research personnel, time spent being cognitively active, and financial compensation for participation. These commercially available computer games cover trivia-type questions about geography, entertainment, science, history, literature, sports, movies, and similar topics. In each session, participants played between 8 and 16 games (1 to 15 min per game). Subjects in this condition completed 3 hours of “training” per week over 12 weeks and received the same attention from staff members and monetary reinforcements as subjects in the experimental treatment groups.
2.4. Outcome measures
2.4.1. Neurocognition
We assessed neurocognitive functioning with the MATRICS Consensus Cognitive Battery (MCCB; Kern et al., 2008; Nuechterlein et al., 2008). The MCCB includes 10 tests measuring 6 cognitive domains (speed of processing, attention, working memory, verbal memory, visual memory, reasoning and problem solving) and a social cognitive domain. A Neurocognition Composite score was based on the T-scores from the 6 cognitive domains.
2.4.2. Functional Capacity
The UCSD Performance-based Skills Assessment (UPSA; Patterson et al., 2001) was administered to evaluate 5 skill areas that are essential to functioning in the community (general organization, finance, social/communications, transportation, household chores). The UPSA involves role-play tasks with props that are performed in the laboratory as simulations of situations that the person is likely to encounter in the community.
2.4.3. Mismatch Negativity (MMN)
MMN was measured using a passive attention auditory oddball paradigm. Subjects were presented with binaural tones (1 kHz 85 dB sound pressure level, with 10 ms rise/fall) with a fixed stimulus onset asynchrony of 500 ms, using E-Prime 2.0. Standard (90% probability; 50 ms duration) and duration-deviant (10% probability; 100 ms duration) tones were presented in a fixed, pseudorandom order using foam insert earphones. Two-thousand total trials were administered. During the 20-minute EEG recording, subjects were instructed to watch a silent movie to divert attention from the stimuli. Details on EEG recording and processing can be found in the Supplement and our previous papers (e.g., Jahshan et al., 2013). MMN amplitude was measured as the mean voltage in the 145–200 ms latency range at pooled electrodes Fz, F1, F2, FCz, FC1, and FC2.
2.5. Statistical Analyses
ANOVAs and chi-square tests were used to test for group differences in demographic characteristics, baseline behavioral and EEG performance, as well as attrition rates. We identified factors associated with dropout by comparing baseline characteristics of those who discontinued the study with those who had complete outcome data.
For our primary analyses, we used the general linear mixed model (GLMM), which allows us to include all available data from all subjects in the analyses, regardless of the number of sessions completed, consistent with the intent to treat framework. For each of the primary (MCCB) and secondary (MMN and UPSA) outcome measures, our core model included group as the between subject factor, time as the within subject factor, and a group by time interaction. We were primarily interested in the group by time interactions comparing the outcome trajectories for the two active treatments, which were obtained as post-hoc contrasts. Such contrasts were also used to compare each of the active treatments to the control condition, to test for within group change and examine the magnitude of group differences at the end of treatment. Pearson’s correlations were conducted between baseline and outcome variables to identify potential covariates to be included in the GLMM.
To assess whether changes in cognition tracked with early changes in neuroplasticity, we examined correlations between MMN change scores (subtracting baseline from 6 weeks) and MCCB change scores (subtracting baseline from 12 weeks).
3. Results
3.1. Demographic and Clinical Characteristics
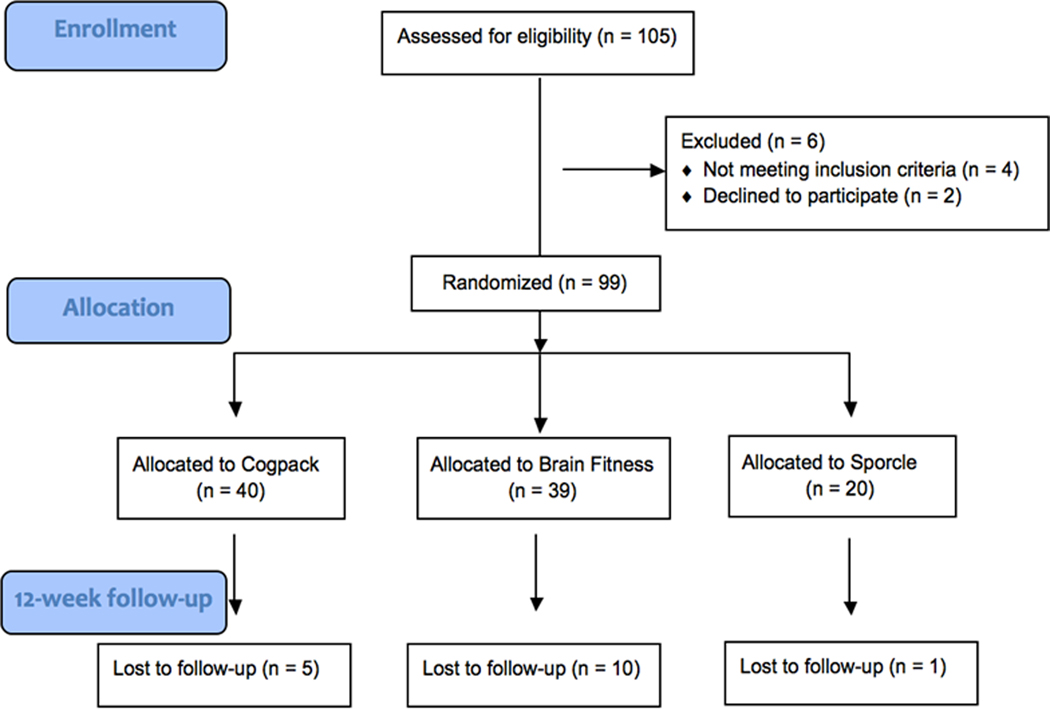
Ninety-nine patients were randomly assigned to the three treatment arms. To optimize power for the primary comparison between the active treatments, we used a 2:2:1 asymmetrical randomization procedure resulting in a total of 39 subjects in the bottom-up Brain Fitness training group, 40 in the top-down COGPACK training group, and 20 in the control Sporcle computer games group. Five subjects from COGPACK, 10 from Brain Fitness, and 1 from Sporcle dropped out before their 12-week assessment visit. Although participant attrition was higher in Brain Fitness, this difference did not reach significance, X2 (2) = 2.33, p = .31. The consort diagram is depicted in Figure 1.
Figure 1.
Consort diagram
There were no significant group differences in baseline measures of cognition, functional capacity/role functioning, or EEG. Moreover, there were no significant group differences in age, education, parental education, gender, symptom severity, duration of illness, or number of hospitalizations. All participants received an average of 30.5 (SD = 9.8) hours of training over 12 weeks in the laboratory with no group differences in the total number of sessions completed or self-reported level of intrinsic motivation. Demographics, clinical characteristics, and baseline behavioral/EEG performance are shown in Table 1.
Table 1.
Baseline characteristics
| Brain Fitness | COGPACK | Sporcle | Group differences | |
|---|---|---|---|---|
| (n = 39) | (n = 40) | (n = 20) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| ||||
| Age | 51.59 (9.44) | 51.05 (9.42) | 51.10 (9.54) | F(2,96)=.04, p=.96 |
| Education | 12.77 (2.33) | 12.75 (1.78) | 12.80 (1.20) | F(2,96)=.004, p=1.0 |
| Parental Education | 12.74 (3.19) | 12.28 (2.86) | 13.10 (3.14) | F(2,90)=.49, p=.61 |
| Gender (% Male) | 82% | 78% | 70% | X2 (2)=1.11, p=.57 |
| Race (% White) | 34% | 59% | 45% | X2 (6)=8.53, p=.20 |
| Illness Duration | 32.60 (10.77) | 26.95 (10.86) | 29.44 (13.66) | F(2,87)=2.20, p=.12 |
| Total hospitalizations | 7.56 (9.73) | 7.09 (7.31) | 7.00 (5.83) | F(2,84)=.04, p=.96 |
| BPRS total | 39.61 (8.85) | 39.80 (9.71) | 41.50 (9.02) | F(2,96)=.30, p=.74 |
| SANS total | 27.09 (15.39) | 32.09 (13.91) | 34.90 (15.52) | F(2,95)=2.06, p=.13 |
| RFS total | 18.31 (3.97) | 17.02 (4.11) | 17.65 (4.31) | F(2,96)=.97, p=.38 |
| IMI-SR total | 121.06 (15.17) | 117.50 (21.06) | 118.67 (20.37) | F(2,83)=.30, p=.74 |
| Sessions completed | 28.38 (11.37) | 31.47 (9.31) | 32.55 (6.88) | F(2,96)=1.55, p=.22 |
| MCCB composite | 42.52 (8.75) | 41.57 (8.41) | 41.18 (8.70) | F(2,96)=0.20, p=.82 |
| UPSA total | 74.85 (10.73) | 74.27 (11.72) | 77.25 (13.99) | F(2,96)=.44, p=.65 |
| MMN amplitude | −2.21 (2.12) | −2.25 (1.69) | −2.18 (2.18) | F(2,94)=.01, p=.99 |
Subjects who completed the study (n = 83) versus subjects who missed the 6-week and/or 12-week assessment visits (n = 16) did not significantly differ in age, parental education, gender, duration of illness, number of hospitalizations, motivation level, or baseline MCCB, UPSA, MMN, BPRS, SANS, or RFS. However, only in Brain Fitness, completers had significantly (p = .008) more years of education (M = 13.0, SD = 1.80) than non-completers (M = 11.6, SD = 2.12).
3.2. Within-group improvement on training programs
3.2.1. Brain Fitness
To determine if participants in the Brain Fitness group improved on the training tasks, we measured Auditory Processing Speed (APS), an index of auditory psychophysical efficiency (Biagianti et al., 2016). We derived APS from the training data on the most basic Brain Fitness exercise, a time-order judgment task of a sequence of two frequency-modulated sound sweeps. The Sound Sweeps score is the number of milliseconds at which the subject can process the interstimulus interval/tone duration ratio and maintain 80% accuracy. APS was calculated by subtracting the best Sound Sweeps score (lowest threshold achieved for each level across the subject’s total training duration) from the baseline score (threshold achieved the first time the level was played), so that a higher APS indicates better performance. All participants showed improvement on APS (M = 106.35, SD = 106.90) except for 2 participants who had an APS of 0 because they only trained for 1 or 2 hours. We also examined the percentile scores generated by BrainHQ and these data are presented in Supplemental Material.
3.2.2. COGPACK
To assess the magnitude of improvement in performance over the duration of the training, 3 of the COGPACK exercises were administered 4 times (in sessions 1, 12, 24, and 36). These exercises included 1) “Memory”, a verbal memory task that requires memorizing items on a shopping list, 2) “Comparisons”, a visual attention task that involves judging whether two words or shapes are similar or different, and 3) “Connect”, a cognitive flexibility/executive functioning task that involves connecting dots while switching between numbers and letters. Supplemental Table 1 shows the mean performance of patients on those exercises in each session. Although subjects performed better on the 3 training tasks in Session 36 compared to Session 1, repeated measures ANOVAs revealed a significant time effect only for Connect: F (3, 60) = 2.94, p = .04.
3.3. Between-group treatment effects
There were no significant main effects of time or group and no significant group by time interaction for the MCCB Neurocognition Composite. However, when examining individual domains, we found a significant time main effect for the Reasoning and Problem Solving domain with all groups improving. Additionally, there was a significant group x time interaction for the Social Cognition domain with the Brain Fitness group slightly improving, COGPACK not changing, and Sporcle getting worse. Means and standard deviations for each group’s cognitive performance pre- and post-training are shown in Table 2.
Table 2.
Change in primary outcome measure among groups
| MCCB | COGPACK | Brain Fitness | Sporcle | Group x Time | Time | |||
|---|---|---|---|---|---|---|---|---|
| (n = 35) | (n = 29) | (n = 19) | ||||||
| Pre | Post | Pre | Post | Pre | Post | |||
|
| ||||||||
| NC Comp | 41.57 | 41.92 | 42.52 | 42.02 | 41.18 | 41.94 | F = .58 | F = .18 |
| (8.34) | (8.47) | (7.64) | (7.96) | (8.68) | (8.72) | p = .56 | p = .67 | |
| SoP | 40.45 | 40.09 | 40.28 | 40.83 | 36.85 | 39.22 | F = .76 | F = .93 |
| (12.94) | (13.20) | (11.89) | (12.48) | (13.47) | (13.56) | p = .47 | p = .34 | |
| Attention | 39.02 | 38.64 | 39.08 | 39.93 | 40.15 | 36.17 | F = 2.08 | F = 1.59 |
| (12.25) | (12.55) | (11.28) | (11.94) | (12.76) | (12.88) | p = .13 | p = .21 | |
| WM | 39.65 | 39.72 | 39.33 | 39.14 | 36.80 | 39.32 | F = .70 | F = .71 |
| (11.51) | (11.84) | (10.60) | (11.33) | (11.99) | (12.12) | p = .50 | p = .40 | |
| Verbal | 40.85 | 40.66 | 42.23 | 39.59 | 40.60 | 39.68 | F = .70 | F = 1.70 |
| (9.23) | (9.62) | (8.49) | (9.34) | (9.59) | (9.77) | p = .50 | p = .19 | |
| Visual | 43.27 | 45.41 | 46.13 | 43.53 | 45.00 | 47.43 | F = 2.02 | F = .30 |
| (11.54) | (12.02) | (10.60) | (11.67) | (12.02) | (12.21) | p = .14 | p = .58 | |
| RPS | 46.20 | 46.64 | 48.08 | 50.18 | 47.70 | 50.11 | F = .60 | F = 3.88 |
| (9.64) | (9.95) | (8.88) | (9.52) | (10.03) | (10.16) | p = .55 | p = .05 | |
| SC | 34.60 | 34.06 | 35.72 | 37.10 | 36.15 | 31.79 | F = 5.11 | F = 2.88 |
| (9.70) | (9.92) | (8.92) | (9.40) | (10.10) | (10.16) | p = .008 | p = .09 | |
NC Comp = Neurocognition Composite; SoP = Speed of Processing; WM = Working Memory; Verbal = Verbal Learning; Visual = Visual Learning; RPS = Reasoning and Problem Solving; SC = Social Cognition; Pre = Baseline; Post = 12 weeks.
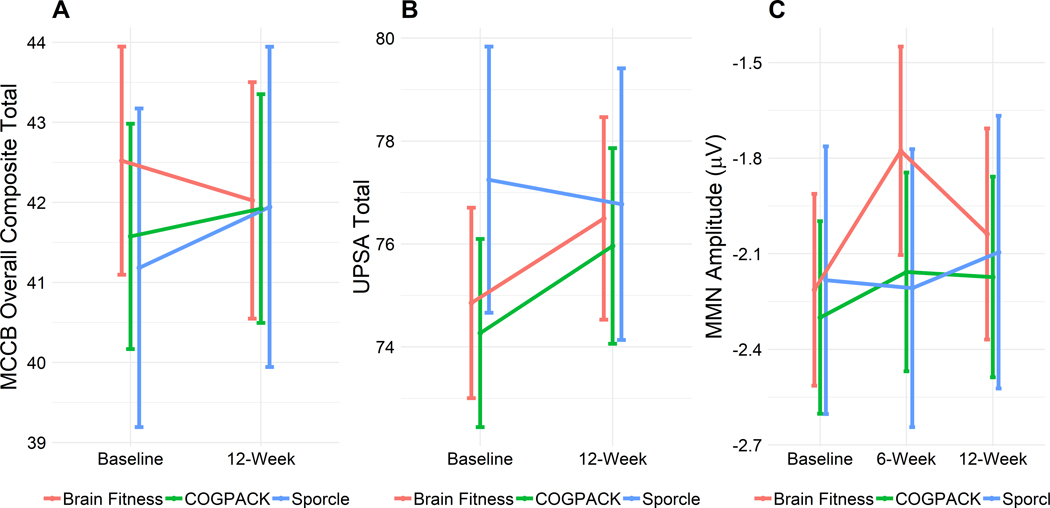
The analyses conducted on the UPSA total score did not yield significant time or group main effects or a significant group x time interaction. Furthermore, there were no significant main effects or interaction for the MMN amplitude (see descriptive data in Table 3). Figure 2 depicts the trajectory of MCCB, UPSA, and MMN over time in each group.
Table 3.
Change in secondary outcome measures among groups
| COGPACK | Brain Fitness | Sporcle | Group x Time | Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 35) | (n = 29) | (n = 19) | |||||||||
| Pre | Post | Pre | Post | Pre | Post | ||||||
|
| |||||||||||
| UPSA | 74.27 | 75.96 | 74.85 | 76.50 | 77.25 | 76.77 | F = .57 | F = 1.21 | |||
| (10.82) | (11.25) | (9.95) | (10.59) | (11.27) | (11.51) | p = .57 | p = .27 | ||||
| Pre | Mid | Post | Pre | Mid | Post | Pre | Mid | Post | |||
|
| |||||||||||
| MMN | −2.30 | −2.16 | −2.17 | −2.21 | −1.78 | −2.04 | −2.18 | −2.21 | −2.09 | F = .23 | F = .43 |
| (1.78) | (1.83) | (1.86) | (1.61) | (1.76) | (1.77) | (1.83) | (1.90) | (1.87) | p = .92 | p = .65 | |
Pre = Baseline; Mid = 6 weeks; Post =12 weeks.
Figure 2.
Mean performance on MCCB Neurocognition Composite (A), UPSA total score (B) and MMN amplitude (C) over time in each treatment group. The vertical lines represent the standard errors.
We examined correlations to identify baseline variables that were significantly associated with outcome at 12 weeks. SANS correlated negatively with MCCB (r = −.38, p < .001) and UPSA (r = −.43, p < .001). Age also correlated negatively with UPSA (r = −.28, p = .01). MMN and MCCB at baseline correlated with MMN (r = .61, p < .001) and MCCB (r = .89, p < .001) at 12 weeks, respectively. In the COGPACK group, two additional variables correlated positively with MCCB: education (r = .40, p = .02) and number of sessions completed (r = .46, p = .006). There were no significant correlations between IMI-SR (measured at 6 weeks) and any of the outcome measures. When symptom severity, age, education, number of sessions completed, and baseline cognitive performance were included as covariates in the analyses, the results did not change.
3.4. Within-group associations between changes in MMN and changes in MCCB
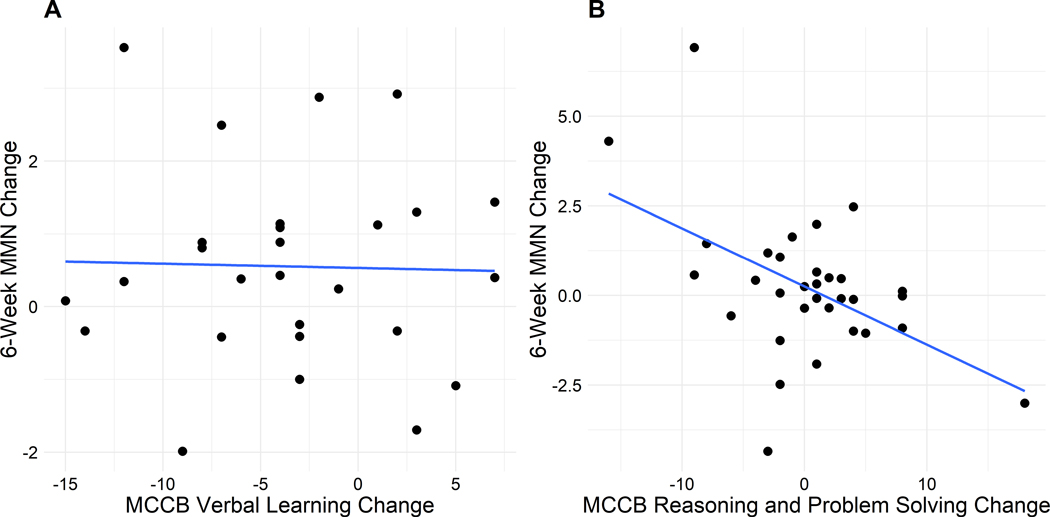
Although baseline MMN correlated with baseline MCCB (r = −.22, p = .03), there were no significant correlations between baseline MMN and changes in MCCB or between changes in MMN and changes in MCCB. Examining those relationships in each group separately, we found that in the Brain Fitness group, improvement in MMN (i.e., a larger amplitude or more negative value) from baseline to 6 weeks was correlated with improvement in MCCB Verbal Learning (r = −.38, p = .04). In the COGPACK group, improvement in MMN from baseline to 6 weeks was correlated with improvement in MCCB Reasoning and Problem Solving (r = −.50, p = .004). However, as shown in the scatterplots (Figure 3), the significant association in the Brain Fitness group was driven by one outlier, and is no longer large or significant using a Spearman’s correlation (rho = −.05, p = .78).
Figure 3.
Within-group correlations between 6-week MMN changes and 12-week MCCB changes in the Brain Fitness (A) and COGPACK (B) groups.
4. Discussion
This study aimed to address an important debate in the CT literature by comparing the effects of a “bottom-up” (Brain Fitness) versus “top-down” (COGPACK) CT approach in individuals with persistent schizophrenia, who had been ill, on average, for 30 years (mean age of 51 years). Our study had several strong features, including a reasonably large sample, blinded assessors, an active control condition, a reliable measure of neural plasticity, a broad neurocognitive battery, and a measure of functional capacity. While both CT approaches were successful at improving performance on the computerized training exercises, neither resulted in transfer effects to untrained cognitive tasks (MCCB), or secondary measures of neuroplasticity (MMN) and functional capacity (UPSA) in this sample.
The current results are consistent with those from a number of studies in persistent schizophrenia in which patients showed improvement on the training tasks with no extension to broader neurocognitive or functional outcome measures (Dickinson et al., 2010; Keefe et al., 2012; Murthy et al., 2012). A recent report on the benefits of adding social cognitive training to CT (Lindenmayer et al., 2018) did not find an improvement in the MCCB Neurocognition Composite in the CT alone group relative to the CT plus social cognitive training group. These studies suggest that CT is not effective for all patients, perhaps particularly for older, persistently ill, functionally disabled patients who may have different plasticity capacities or less dopamine system responsivity that can drive learning.
Our trial yielded negative findings despite reasonably good adherence to the interventions and large samples for this type of study. It is possible that the older age of the sample might have attenuated the effectiveness of CT, as younger age has been a predictor of positive response to CT in other studies (Lindenmayer et al., 2017; Vita et al., 2013). Furthermore, about 60% of the sample was on one or more antipsychotics plus other psychotropic medications with known anticholinergic effects. Given that anticholinergic burden has been associated with a lowered response to “neuroplasticity-based” auditory training (Vinogradov et al., 2009), it is likely that patients’ medication regimen played a role in their poor response to CT. It is also possible that, while we used what is considered an ideal dose of COGPACK, we used what might be a less than optimal dose of Brain Fitness, which is sometimes administered 4 or 5 times a week, rather than 3 times a week (Adcock et al., 2009; Fisher et al., 2009).
An innovative aspect of our study was the use of MMN to elucidate the neural changes that occur early in the course of training and might set the stage for enduring cognitive benefits. Although MMN malleability was seen after one hour of exposure to auditory “bottom-up” training in a previous study (Perez et al., 2017), we did not replicate this finding following a 30-hour course of treatment, as neither CT approach improved MMN in our sample. Interestingly, we observed a small decrease in the MMN amplitude from baseline to 6 weeks in the Brain Fitness group, but it did not reach statistical significance. Our results support the notion that MMN follows a dynamic pattern of change rather than a single static shift of amplitude in response to CT and does not look the same when measured at different time points throughout the training (Kantrowitz et al., 2018). Similar to Biagianti et al.’s findings, reduced MMN was associated with greater cognitive impairment (worse MCCB) at baseline in patients but we did not find that patients with a larger MMN benefited more from treatment. Yet, within-group analyses showed that an enhanced MMN response halfway through training (at 6 weeks) was significantly associated with improved reasoning/problem solving at the end of training (at 12 weeks) in the COGPACK group. This relationship suggests that patients who exhibit a larger neuroplastic potential may have an increased ability to benefit from top-down CT and supports the continued use and refinement of MMN as a target engagement biomarker for identifying those individuals most likely to respond to training.
Future studies should examine EEG measures of neuroplasticity as early indicators of treatment responsiveness in the context of stronger, dose-adequate CT interventions, possibly combining elements from both approaches with social cognitive training (Lindenmayer et al., 2018). As recommended (Wykes et al., 2011), adding a psychosocial rehabilitation skills training component to CT is likely to be necessary to induce changes in functional capacity and real-life functioning, perhaps especially in patients with long-standing illness who have been disabled for decades. Additionally, CT trials in schizophrenia should attempt to investigate factors that can influence treatment outcome (e.g., medication regimen, age, illness chronicity), an important step to guide treatment providers in choosing the type of CT that best suits each patient.
Supplementary Material
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S, 2009. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr. Bull. 35 (6), 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, 1984. The scale for the assessment of negative symptoms (SANS). The University of Iowa, Iowa City, IA. [Google Scholar]
- Bender S, Dittmann-Balcar A, Prehn G, Thienel R, Peters S, Gastpar M, 2004. [Subjective experience of a computer-assisted cognitive training by patients with schizophrenia]. Der Nervenarzt 75 (1), 44–50. [DOI] [PubMed] [Google Scholar]
- Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S, 2016. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology 30 (8), 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Roach BJ, Fisher M, Loewy R, Ford JM, Vinogradov S, Mathalon DH, 2017. Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia. Neuropsychiatr. Electrophysiol. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor J, Brunelin J, d’Amato T, Costes N, Suaud-Chagny MF, Saoud M, Poulet E, 2011. How can cognitive remediation therapy modulate brain activations in schizophrenia? An fMRI study. Psychiatr. Res. 192 (3), 160–166. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD, 2008. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol. Psychiatry 63 (5), 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Mogami T, Medalia A, 2010. Intrinsic motivation inventory: an adapted measure for schizophrenia research. Schizophr. Bull. 36 (5), 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbera S, Wexler BE, Poltorak A, Thime WR, Kurtz MM, 2017. Cognitive remediation for adults with schizophrenia: Does age matter? Psychiatry Res. 247, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Brown EG, Fisher M, Herman AB, Dowling AF, Hinkley LB, Subramaniam K, Nagarajan SS, Vinogradov S, 2016. Auditory Cortical Plasticity Drives Training-Induced Cognitive Changes in Schizophrenia. Schizophr. Bull. 42 (1), 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Findlay AM, Adcock RA, Vertinski M, Fisher M, Genevsky A, Aldebot S, Subramaniam K, Luks TL, Simpson GV, Nagarajan SS, Vinogradov S, 2010. Timing is everything: neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int. J. Psychophysiol. 75 (2), 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Tenhula W, Morris S, Brown C, Peer J, Spencer K, Li L, Gold JM, Bellack AS, 2010. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am. J. Psychiatry 167 (2), 170–180. [DOI] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS, 2010. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch. Gen. Psychiatry 67 (7), 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1997. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition, Biometrics Research Department. New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S, 2009. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatry 166 (7), 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Mellon SH, Wolkowitz O, Vinogradov S, 2016. Neuroscience-informed Auditory Training in Schizophrenia: A Final Report of the Effects on Cognition and Serum Brain-Derived Neurotrophic Factor. Schizophr. Res. Cogn. 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Sewell DR, Cooley EL, Leavitt N, 1993. Assessing levels of adaptive functioning: the Role Functioning Scale. Community Ment. Health J. 29 (2), 119–131. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J, 2000. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr. Bull. 26, 119–136. [DOI] [PubMed] [Google Scholar]
- Haut KM, Lim KO, MacDonald A 3rd, 2010. Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology 35 (9), 1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, Green MF, 2013. Relationship between auditory processing and affective prosody in schizophrenia. Schizophr. Res. 143 (2–3), 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Swerdlow NR, Dunn W, Vinogradov S, 2018. Auditory System Target Engagement During Plasticity-Based Interventions in Schizophrenia: A Focus on Modulation of N-Methyl-D-Aspartate-Type Glutamate Receptor Function. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (7), 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Vinogradov S, Medalia A, Buckley PF, Caroff SN, D’Souza DC, Harvey PD, Graham KA, Hamer RM, Marder SM, Miller DD, Olson SJ, Patel JK, Velligan D, Walker TM, Haim AJ, Stroup TS, 2012. Feasibility and pilot efficacy results from the multisite Cognitive Remediation in the Schizophrenia Trials Network (CRSTN) randomized controlled trial. J. Clin. Psychiatry 73 (7), 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RSE, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR, 2008. The MATRICS Consensus Cognitive Battery: Part 2. co-norming and standardization. Am. J. Psychiatry 165, 214–220. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A, 2014. Cognitive training in mental disorders: update and future directions. Am. J. Psychiatry 171 (5), 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Braff DL, 2005. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch. Gen. Psychiatry 62 (2), 127–136. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Khan A, McGurk SR, Kulsa MKC, Ljuri I, Ozog V, Fregenti S, Capodilupo G, Buccellato K, Thanju A, Goldring A, Parak M, Parker B, 2018. Does social cognition training augment response to computer-assisted cognitive remediation for schizophrenia? Schizophr. Res. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, McGurk SR, Khan A, Kaushik S, Thanju A, Hoffman L, Valdez G, Wance D, Herrmann E, 2013. Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation. Schizophr. Bull. 39 (3), 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP, Ozog VA, Khan A, Ljuri I, Fregenti S, McGurk SR, 2017. Predictors of response to cognitive remediation in service recipients with severe mental illness. Psychiatr. Rehabil. J. 40 (1), 61–69. [DOI] [PubMed] [Google Scholar]
- Loewy R, Fisher M, Schlosser DA, Biagianti B, Stuart B, Mathalon DH, Vinogradov S, 2016. Intensive Auditory Cognitive Training Improves Verbal Memory in Adolescents and Young Adults at Clinical High Risk for Psychosis. Schizophr. Bull. 42 Suppl 1, S118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT, 2007. A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry 164 (12), 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Choi J, 2009. Cognitive remediation in schizophrenia. Neuropsychol. Rev. 19 (3), 353–364. [DOI] [PubMed] [Google Scholar]
- Murthy NV, Mahncke H, Wexler BE, Maruff P, Inamdar A, Zucchetto M, Lund J, Shabbir S, Shergill S, Keshavan M, Kapur S, Laruelle M, Alexander R, 2012. Computerized cognitive remediation training for schizophrenia: an open label, multi-site, multinational methodology study. Schizophr. Res. 139 (1–3), 87–91. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Gaillard AW, Mantysalo S, 1978. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 42 (4), 313–329. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch D, Cohen J, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton R, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger D, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery: Part 1. test selection, reliability, and validity. Am. J. Psychiatry 165, 203–213. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV, 2001. UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr. Bull. 27 (2), 235–245. [DOI] [PubMed] [Google Scholar]
- Penades R, Pujol N, Catalan R, Massana G, Rametti G, Garcia-Rizo C, Bargallo N, Gasto C, Bernardo M, Junque C, 2013. Brain effects of cognitive remediation therapy in schizophrenia: a structural and functional neuroimaging study. Biol. Psychiatry 73 (10), 1015–1023. [DOI] [PubMed] [Google Scholar]
- Perez VB, Tarasenko M, Miyakoshi M, Pianka ST, Makeig SD, Braff DL, Swerdlow NR, Light GA, 2017. Mismatch Negativity is a Sensitive and Predictive Biomarker of Perceptual Learning During Auditory Cognitive Training in Schizophrenia. Neuropsychopharmacology 42 (11), 2206–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA, 2011. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol. Psychiatry 69 (5), 465–471. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ, 2006. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry 59 (10), 929–939. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Garrett C, Chung C, Fisher M, Nagarajan S, Vinogradov S, 2014. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage 99, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko M, Perez VB, Pianka ST, Vinogradov S, Braff DL, Swerdlow NR, Light GA, 2016. Measuring the capacity for auditory system plasticity: An examination of performance gains during initial exposure to auditory-targeted cognitive training in schizophrenia. Schizophr. Res. 172 (1–3), 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, 1998. Training and quality assurance with the Structured Clinical Interview for DSM-IV. Psychiatr. Res. 79, 163–173. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A, 1993. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. Int. J. Methods Psychiatr. Res. 3, 227–243. [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E, 2012. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology 37 (1), 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG, 2009. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am. J. Psychiatry 166 (9), 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, De Peri L, Barlati S, Cacciani P, Deste G, Poli R, Agrimi E, Cesana BM, Sacchetti E, 2011. Effectiveness of different modalities of cognitive remediation on symptomatological, neuropsychological, and functional outcome domains in schizophrenia: a prospective study in a real-world setting. Schizophr. Res. 133 (1–3), 223–231. [DOI] [PubMed] [Google Scholar]
- Vita A, Deste G, De Peri L, Barlati S, Poli R, Cesana BM, Sacchetti E, 2013. Predictors of cognitive and functional improvement and normalization after cognitive remediation in patients with schizophrenia. Schizophr. Res. 150 (1), 51–57. [DOI] [PubMed] [Google Scholar]
- Wykes T, Brammer M, Mellers J, Bray P, Reeder C, Williams C, Corner J, 2002. Effects on the brain of a psychological treatment: cognitive remediation therapy: functional magnetic resonance imaging in schizophrenia. Br. J. Psychiatry 181, 144–152. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry 168 (5), 472–485. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Sugar C, Horan WP, Kern R, Green MF, 2010. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol. Psychiatry 67 (10), 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.