Abstract
Phosphodiesterase 5 inhibition (PDE5i) activates cGMP-dependent protein kinase (PKG) and ameliorates heart failure; however, its impact on cardiac mitochondrial regulation has not been fully determined. Here, we investigated the role of the mitochondrial regulator peroxisome proliferator-activated receptor γ co-activator-1α (PGC1α) in the PDE5i-conferred cardioprotection, utilizing PGC1α null mice. In PGC1α+/+ hearts exposed to 7 weeks of pressure overload by transverse aortic constriction, chronic treatment with the PDE5 inhibitor sildenafil improved cardiac function and remodeling, with improved mitochondrial respiration and upregulation of PGC1α mRNA in the myocardium. By contrast, PDE5i-elicited benefits were abrogated in PGC1α−/− hearts. In cultured cardiomyocytes, PKG overexpression induced PGC1α, while inhibition of the transcription factor CREB abrogated the PGC1α induction. Together, these results suggest that the PKG–PGC1α axis plays a pivotal role in the therapeutic efficacy of PDE5i in heart failure.
Keywords: cyclic guanosine monophosphate, heart failure, mitochondria, PGC1α
Cyclic guanosine 3’,5’-monophosphate (cGMP) and its primary effector protein kinase G (PKG) mediate physiological actions of nitric oxide (NO) and natriuretic peptides (NPs), playing central roles in the maintenance of cardiovascular system [1,2]. Enhancing cGMP-PKG signaling has emerged as a potent therapeutic strategy to treat heart failure [1,3-6]. Phosphodiesterase type 5 (PDE5) inhibitors inhibit cGMP hydrolysis, and have shown cardioprotective effects in experimental models of heart failure [1,7]. Consistently, a single-center study of heart failure patients with reduced systolic function (HFrEF) revealed that one year of treatment with the PDE5 inhibitor sildenafil improved cardiac function, geometry, and clinical status [4]. On the other hand, a multi-center clinical trial of heart failure patients with preserved systolic function (HFpEF) failed to show benefits [8], which might be due to the heterogeneous pathology of HFpEF and to the complexity of cGMP-PKG regulation [9-11]. These highlight the need for better understanding of molecular mechanisms underlying the benefits of cGMP-PKG in heart failure. A recent study demonstrated cGMP-PKG-mediated autophagy is a significant contribution [12]; however, other mechanisms might also work.
The last decade of research provided convincing evidence that mitochondrial dysfunction could be a significant contributor to the pathogenesis of heart failure [13,14]. Cardiac mitochondria generate most of the ATP required to meet the demand of the high-energy-consuming organ, and also regulate intracellular redox balance and intracellular Ca2+. Although a small defect of mitochondrial function could lead to a vicious feed-forward cycle resulting in cell death, enough cardiomyocytes are thought to remain viable to potentially rescue function in failing hearts [13]. Peroxisome proliferator-activated receptor γ co-activator-1α (PGC1α) is a transcriptional coactivator that critically regulates mitochondrial biology and ancillary programs relevant to mitochondrial biology, including ATP production, ROS detoxification, biogenesis, and angiogenesis [15]. While human data remain a matter of debate, PGC1α down-regulation has been well-documented in experimental heart failure and is suggested to contribute to its pathophysiology [16,17]. Mice genetically lacking PGC1α (PGC1α−/− show poor contractile reserve and accelerated transition to failure during pressure overload by transverse aortic constriction (TAC) [18]. Interestingly, prior studies have shown that cGMP-PKG activation induces PGC1α in non-cardiac contexts. For example, an NO donor or PDE5 inhibition induces PGC1α mRNA and mitochondrial biogenesis in adipose tissues [19]. Moreover, ANP induces PGC1α in skeletal muscle, conferring metabolic adaptations to physiological exercise [20]. However, similar regulation in the heart remains underexplored. In the current study, we tested a hypothesis that cGMP/PKG-PGC1α axis plays a critical role in reversing the metabolic and contractile dysfunction in the treatment of heart failure with PDE5 inhibition.
Materials and methods
Animals
PGC1α−/− mice were previously described [18]; controls were littermate wild-type (PGC1α+/+) mice. Male mice were used for all experiments. The mice were allowed ad libitum access to water and normal chow diet. The mice were housed in cages with 12-h light-dark cycle in a temperature-controlled laboratory. Experimental procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins Medical Institutions and performed in accordance with the institutional guidelines and carried out in compliance with the ARRIVE guidelines.
TAC and Sildenafil treatment
Transverse aortic constriction (TAC) or sham surgery was performed on 3- to 4-month-old mice as described [21]. Briefly, animals were anesthetized with isoflurane (1–1.5%) and 100 mg kg−1 etomidate, intubated, and mechanically ventilated. The transverse aorta was constricted with a 27-gauge needle using 7-0 prolene suture. After ensuring the lack of excessive bleeding, the chest was closed, and the animal was allowed to recover from anesthesia. Animals were euthanized at 1 week or 3 weeks after TAC. Total heart weights were measured and normalized to tibial length (TL), and the left ventricular tissues were harvested. Snap-frozen heart samples were stored at −80 °C until analysis. Sildenafil (200 mg kg−1 day−1) was given orally as described [22].
Echocardiography
Transthoracic echocardiography (Acuson Sequoia C256, 13MHz transducer; Siemens) was performed in conscious mice. M-mode LV end-systolic and end-diastolic dimensions were measured and LVFS (%) was calculated as described previously [21,22]. These assessments were performed, on the day of TAC/sham surgery and at 1 and 3 weeks after TAC/sham surgery, by investigators blinded to the genotype and heart condition.
Hemodynamics
In vivo LV function was assessed by PV analysis in anesthetized mice as described previously [21,22]. The LV apex was exposed through an incision between the seventh and eighth ribs. 1.4-French PV catheter (SPR-839; Millar Instruments, Houston, Texas) was inserted from the LV apex and advanced into the LV lumen to lie along the longitudinal axis. The absolute volume was calibrated, and PV data were assessed at the steady state and during preload reduction phase. Data were analyzed using the LabChart application (AD Instruments, Dunedin, New Zealand).
Mitochondrial function
Mitochondrial respiratory function was assessed in cultured cardiac myocytes and left ventricular fibers after saponin permeabilization, using a Clark-type oxygen probe (World Precision Instruments) in a sealed respiration system as described [23]. Buffers contained (mM): for myocytes 65 KCl, 20 HEPES, 1 MgCl2, 0.5 KH2PO4, 0.1 EGTA-Tris, and 1 mg mL−1 BSA; pH 7.1 at 37 °C; for fibers 125 KCl, 20 HEPES, 3 Mg(CH3COO)2, 5 KH2PO4, 0.4 EGTA, 0.3 DTT and 2 mg mL−1 BSA; pH 7.1 at 25 °C. Additions were: 20 μM palmitoyl-L-carnitine (PC) and 5 mM malate for assessing PC respiration; 10 mM pyruvate and 5 mM malate for pyruvate respiration; 5 MM glutamate and 2 MM malate for glutamate respiration. Following measurement of basal respiration, maximal (State 3) respiration was determined by exposure to 1 mM ADP, and ATP was determined by sequential collection of 30μl of respiration buffer. Post-oligomycin respiration was evaluated after the addition of oligomycin (1 μg mL−1) to inhibit ATP synthase. Respiration rates were expressed as nanomoles O2/min/1 × 107 cells, or per milligram dry weight of fibers. Respiratory control ratio was calculated as maximal respiration divided by basal respiration.
Histology
Heart samples were fixed with 10% formalin and embedded in paraffin. Samples were sliced into 4–5-mm short-axis slices. The size of cardiomyocytes was analyzed as previously described [9].
RNA analysis
Total RNA was extracted from mouse LV heart samples using Trizol (Invitrogen, Waltham, MA, USA) [9]. The mRNA was reverse transcribed into cDNA using a High Capacity RNA-to-cDNA Kit (Applied Biosystems, Life Technologies, Rockville, Maryland). TaqMan primers and probes for PGC1α (Ppargc1a) and Sod2 were purchased from Applied Biosystems. The SYBR Green primers for Nppb are as described [9].
Cardiac myocyte culture and viral transfection
Primary cultures of neonatal rat ventricular myocytes were prepared [21]. Adenoviral expression vectors for PKGIα and PGC1α shRNA were prepared as described [24]. 10 MOI of overexpressing or shRNA adenovirus was employed, resulting in threefold protein increase (PKGIα) or 70% mRNA knockdown (PGC1α). PGC1α shRNA was a generous gift from Dr. Montminy (Salk Institute) [25].
GSH/GSSG assay
Reduced and oxidized glutathione (GSH/GSSG) were determined using a commercially available kit (OXIS Health Products) according to the manufacturer’s protocol.
Statistics
Each experiment was powered to detect putative effect size according to previous studies. Sample sizes were decided to provide 80% power to detect a change with putative SD difference when using a two-sided alpha value of 0.05. Results are reported as mean ± SD. Two groups were compared by unpaired Student’s t test. More than two groups were compared by 2- or 1-way ANOVA, followed by Tukey-Kramer post-hoc test comparison between groups. P < 0.05 was considered to denote statistical significance.
Results
Sildenafil fails to improve cardiac function in mice lacking PGC1α
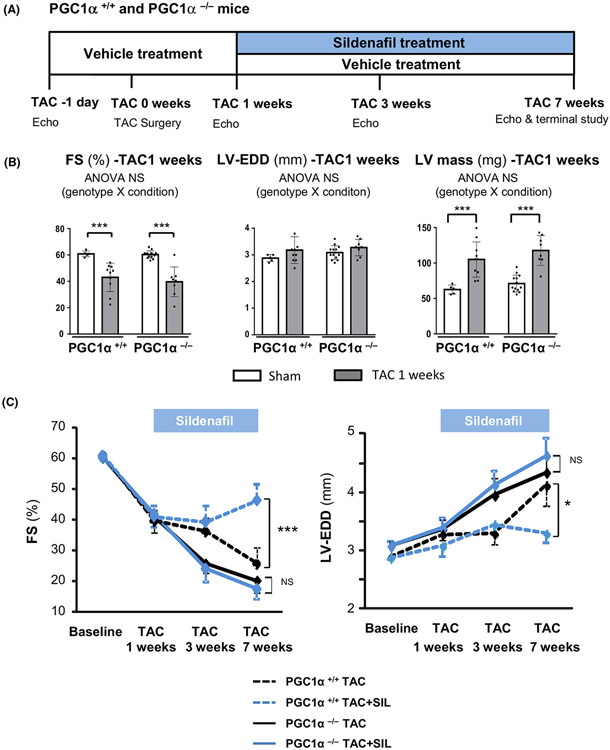
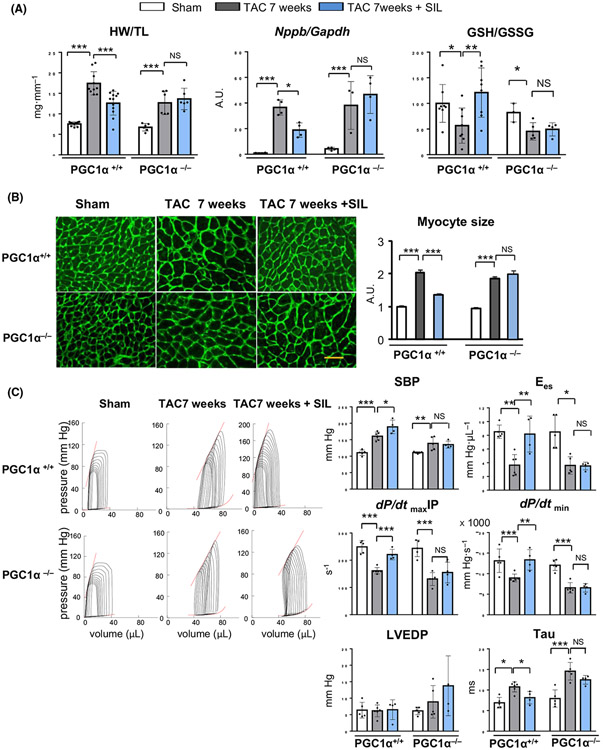
To determine the role for cGMP-PGC1α axis in the cardio-protection by PDE5 inhibition against heart failure, we employed PGC1α null (PGC1α−/−) mice and tested the effects of sildenafil in heart failure induced by pressure overload (TAC) (Fig. 1A). PGC1α−/− hearts did not present baseline cardiac abnormality or exacerbated early hypertrophic response by 1 week as assessed by echocardiographic fractional shortening (FS), left ventricular (LV) size (LV-EDD), and LV mass (Fig. 1B), but revealed accelerated transition to failure by 3 week TAC (Fig. 1C), consistent with the previous report by Arany et al [18]. Echocardiographic assessment revealed that chronic sildenafil treatment (200 mg kg−1 day−1 in rodent chow), initiated 1 week after TAC surgery, prevented development of heart failure in controls (PGC1α+/+), but failed to do so in PGC1α−/− TAC hearts (Fig. 1C). Histological assessment at 7 weeks after TAC revealed that sildenafil treatment blunted the increase in heart weight (Fig. 2A) and myocyte size (Fig. 2B) in PGC1α+/+ TAC hearts, which was associated with reduced levels of myocardial BNP mRNA (Fig. 2A) and oxidative stress (GSH/GSSG ratio) (Fig. 2A). By contrast, none of such beneficial effects were observed in PGC1α−/− 7 week-TAC hearts.
Fig. 1.
Study protocol and echocardiography. (A) A schematic diagram of the study protocol. (B) Fractional shortening (FS), left ventricular (LV) end-diastolic dimension (LV-EDD) and LV mass from echocardiograms of PGC1α+/+ and PGC1α−/− hearts after 1 week exposure to pressure overload (TAC) before sildenafil treatment (n = 14–15 per group). Groups were compared by 2-way ANOVA followed by Tukey-Kramer post-hoc test. (C) Serial echocardiographic assessments of FS and LV-EDD during sildenafil treatment (n = 7–9 per group). Sildenafil treatment (blue) significantly improved FS and ameliorated LV-EDD enlargement in PGC1α+/+ hearts (dotted lines), but not in PGC1α−/− hearts (solid lines). Groups were compared by 1-way ANOVA followed by Tukey-Kramer post-hoc test. *P < 0.05, ***P < 0.001.
Fig. 2.
Sildenafil fails to ameliorate cardiac dysfunction in mice lacking PGC1α. (A) Heart weight normalized by tibia length (n = 8–12 per group), myocardial BNP (Nppb) expression (n = 8 per group) and oxidative stress levels (GSH/GSSG ratio) (n = 4–8 per group). Groups were compared by 2-way ANOVA followed by Tukey-Kramer post-hoc test. (B) Histological analysis of cardiac myocyte size using wheat germ agglutinin (WGA) staining. Scale bar indicates 20 μm. Quantification results are shown in right bar graphs (n = 5 hearts per group, > 500 cells per heart). Groups were compared by 2-way ANOVA followed by Tukey-Kramer post-hoc test. (C) Representative pressure-volume (PV) loops during preload reduction. Shallow upper left relations (Ees) and rightward shift of loops by TAC were improved by sildenafil treatment in PGC1α+/+ hearts, but not in PGC1α−/− hearts. Contractile (dP/dtmaxIP and Ees) and relaxation (dP/dtmin and Tau) parameters from PV loop analysis were shown (n = 4–6). Groups were compared by 2-way ANOVA followed by Tukey-Kramer post-hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
In vivo pressure-volume analyses revealed that sildenafil improved both systolic and diastolic measures (dP/dtmax normalized to instantaneous pressure: dP/dtmaxIP; end-systolic elastance: Ees; dP/dtmin, and Tau) (Fig. 2C) in PGC1α+/+ TAC hearts, whereas none of these parameters was affected in hearts without PGC1α. Systolic blood pressure (SBP) defined by LV peak systolic pressure was not decreased by sildenafil treatment in TAC hearts of both genotypes (Fig. 2C).
Sildenafil improves cardiac mitochondrial function in a PGC1α-dependent manner
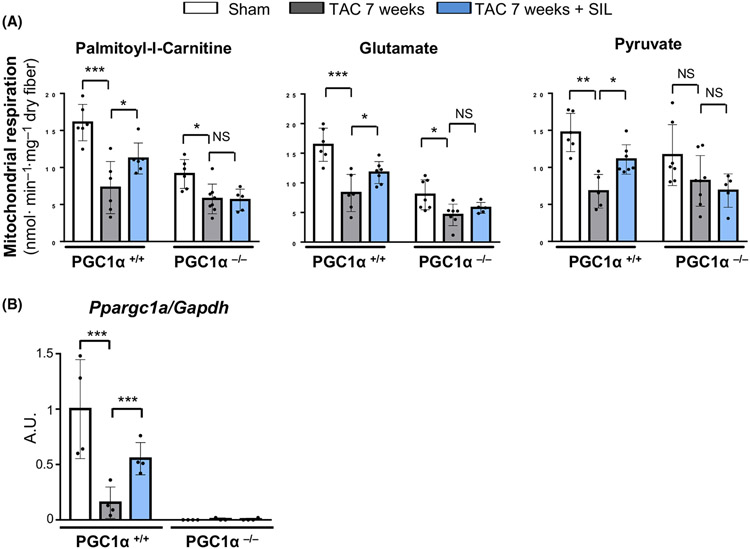
To determine the impact of sildenafil treatment on mitochondrial energy metabolism, we isolated left ventricular muscle fibers and assessed mitochondrial respiratory function using three substrates including palmitoyl-L-carnitine, glutamate, and pyruvate. Respiration was markedly reduced in PGC1α+/+ 7 week-TAC hearts with all the three substrates tested and was significantly improved by chronic sildenafil treatment (Fig. 3A). Importantly, such improvement with sildenafil treatment was accompanied by the recovery of myocardial PGC1α mRNA levels, whereas PGC1α was otherwise markedly down-regulated (Fig. 3B). By contrast, sildenafil treatment failed to improve state 3 respiration with any of the three substrates in PGC1α−/− 7 week-TAC hearts (Fig. 3A). These results indicate an essential role for PGC1α to the mitochondrial functional recovery by sildenafil treatment that critically contributes to the improvement of cardiac performance and ventricular remodeling.
Fig. 3.
Sildenafil regulation of mitochondrial function in PGC1α+/+ and PGC1α−/− TAC hearts. (A) Mitochondrial respiration in cardiac muscle fibers using palmitoyl-L-carnitine, glutamate or pyruvate as substrate in PGC1α+/+ and PGC1α−/− TAC hearts after sildenafil treatment (SIL) (n = 4–5 per group). Groups were compared by 2-way ANOVA followed by Tukey-Kramer post-hoc test. (B) Myocardial PGC1α expression in TAC hearts after sildenafil or vehicle treatment (n = 7–8 per group). Groups were compared by 2-way ANOVA followed by Tukey-Kramer post-hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
cGMP-PKG induces cardiac myocyte PGC1α, improving cellular mitochondrial function
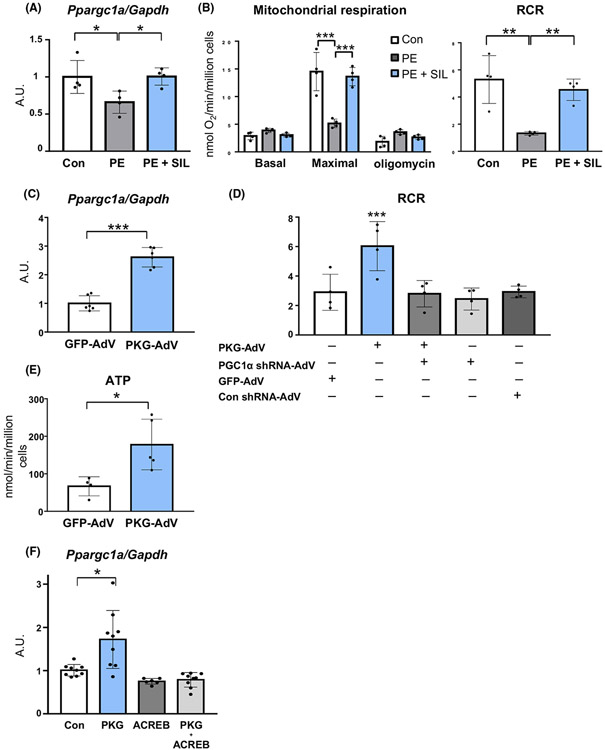
We then utilized cultured rat neonatal cardiac myocytes (RNCMs) and examined whether cGMP-PKG directly induced PGC1α and improved mitochondrial function in cardiac myocytes. We first assessed the effect of sildenafil in RNCMs exposed to prolonged phenylephrine (PE, 20 μM for 96 h), a well-known condition to down-regulate PGC1α [26]. Sildenafil (10 μM) potently inhibited PGC1α repression (Fig. 4A) and respiratory dysfunction assessed by maximal oxygen consumption and respiratory control ratio (RCR) (Fig. 4B), indicating the regulation of PGC1α by sildenafil at the level of the cardiac myocyte. Next, we examined the effect of PKGIα, a kinase that is activated by cGMP. Forced expression of PKGIα in RNCMs induced PGC1α (Fig. 4C), enhancing mitochondrial respiratory control ratio (Fig. 4D) and ATP synthesis (Fig. 4E). Importantly, PKGIα-induced enhancement of mitochondrial function did not occur when PGC1α was genetically silenced (~ 70% knock-down) using adenoviral-shRNA (Fig. 4D). These support the important role for cGMP-PKG-PGC1α axis in mitochondrial functional regulation in cardiac myocytes.
Fig. 4.
cGMP-PKG regulation of mitochondrial respiratory function via PGC1α in rat neonatal cardiac myocytes. (A) PGC1α expression and (B) mitochondrial respiratory function (glutamate/malate substrate) with or without sildenafil (SIL) in RNCMs exposed to prolonged phenylephrine (PE) (n = 4 per group). Groups were compared by 1- or 2-way ANOVA followed by Tukey-Kramer post-hoc test. (C) PGC1α (Ppargc1a) mRNA up-regulation by adenoviral PKGI α induction (n = 6 per group). Groups were compared by unpaired Student’s t test. (D) PKGIα regulation of mitochondrial respiratory function (RCR, respiratory control ratio with glutamate/malate substrate with or without PGC1α silencing) (n = 4 per group). Groups were compared by 1-way ANOVA followed by Tukey-Kramer post-hoc test. (E) ATP generation during state 3 respiration by PKGIα induction (n = 4-5 per group). Groups were compared by unpaired Student’s t test. (F) PGC1α expression by PKGIα in HEK cells with or without ACREB (CREB inhibitor) (n = 6–9 per group). Groups were compared by 1-way ANOVA followed by Tukey-Kramer post-hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
Cyclic-nucleotide regulatory element binding protein (CREB) activation is required for PKG-induction of PGC1α
The regulation of PGC1α gene is complex, which involves several transcription factors, including activating transcription factor 2 (ATF2), myocyte enhance factor 2 (MEF2), and forkhead box class O (FoxO1) and cAMP-responsive element-binding protein (CREB). Given that PGC1α transcription is highly reactive to CREB activation [27], we tested the role for CREB in PKG-induction of PGC1α. Because of the low transfection efficacy of plasmids into cardiomyocytes, we instead utilized HEK cells. PGC1α induction elicited by PKGIα over-expression was completely abrogated by co-expression with A-CREB (a dominant-negative CREB containing a serine-to-alanine mutation at S133) (Fig. 4F), indicating an essential role for CREB activation in this regulation.
Discussion
The present study demonstrated an essential role for PGC1α in the therapeutic benefits of PKG activation elicited by the PDE5 inhibitor sildenafil in a rodent model of heart failure. Sildenafil replenished myocardial PGC1α expression, maintained mitochondrial ATP generating capacity, and reduced oxidative stress, leading to the amelioration of maladaptive remodeling in later stages of heart failure during pressure overload, whereas neither metabolic nor physiological benefits of sildenafil treatment were observed in hearts lacking PGC1α. Our data provide evidence for the cardio-protection conferred by cGMP-PKG-PGC1α axis, and this might also underlie the therapeutic benefits from other cGMP-activating interventions such as PDE9 inhibition, neprilysin inhibition, or sGC stimulation [3,5,28].
PKG activation by PDE5 inhibition has been shown to target several pathways related to cardiac pathophysiology, including Gq-coupled signaling, calcium influx via transient receptor potential canonical (TRPC) and proteasome protein degradation [2,29]. Our results indicate that the maintenance of mitochondrial function via PGC1α replenishment is a key to the anti-failure remodeling from PKG activation by sildenafil in later stages of pressure overload-induced heart failure. Importantly, experimental studies have revealed excessive induction of PGC1α is harmful rather than beneficial to the heart. Transgenic mice with high-level cardiac overexpression of PGC1α develop spontaneous dilative cardiomyopathy [26], and even moderate overexpressors respond poorly to pressure overload [30]. Therefore, PGC1α levels need to be maintained within certain levels for proper mitochondrial function; sildenafil works as a mild inducer of PGC1α so that mitochondrial homeostasis might be appropriately maintained.
Our results suggest that CREB activation might be a significant contributor to the induction of PGC1α by cGMP-PKG. Our prior studies demonstrated that sildenafil treatment activates cGMP-PKG but not cAMP-PKA pathway in the hearts [21], and that sildenafil’s cardio-protective effect was blocked by PKG inhibition [31]. Accordingly, the sildenfil’s impact on PGC1α in the current study might be reasonably attributed to the activation of PKG. However, we did not directly rule out the potential involvement of cAMP-PKA pathway, which is a limitation of the study.
The present data showed the critical role of CREB activation in PGC1α upregulation, consistently with previous studies using various cell types including vascular smooth muscle cells, neuronal cells, and BHK cells [27,32,33]. Waton et al. also reported that CREB phosphorylation/activation correlates with PGC1α expression and is linked to adaptive cardiac hypertrophy to exercise [34]. The precise regulatory mechanisms in vivo, however, could be more complex, involving multiple factors to fine-tune its expression levels, given that the PGC1α promoter contains binding sites for MEF2, FoxO1, ATF2 as well as CREB and these can be modulated by several signaling pathways depending on cell-type and pathophysiological context [34]. In adipocytes, for example, ANP-activated PKG phosphorylates p38, which in turn phosphorylates ATF2 to enhance the transcription of PGC1α [35]. The precise regulatory mechanism underlying the transcriptional induction of PGC1α by PKG in cardiac myocytes in vivo warrants further investigation.
Besides transcriptional regulation, PGC1α can be activated post-translationally and this has been suggested to be involved in the benefits by PDE5 inhibition in diabetic hearts [14]. In type-II diabetic cardiomyopathy in ob/ob mice, tadalafil improves cardiac mitochondrial glutamate respiration, associated with increased Sirt1 activity (de-acetylation) and AMPK phosphorylation (phosphorylation) in the myocardium [36]. Although the metabolic remodeling in diabetic hearts is distinct from that of pressure-overloaded hearts: the former favoring fatty acid utilization without PGC1α repression and the latter glucose utilization with PGC1α repression, similar post-translational mechanisms of PGC1α activation might also contribute to the reverse remodeling process in our study. In turn, Li et al. reported the role for de-acetylation of PGC1α via Sirt3-mediated regulation by sildenafil in cardio-protection in a myocardial infarction model [37], which might be also involved in our TAC-induced heart failure model. The present study determined the mRNA levels of PGC1α and their functional impacts in the context of cGMP-PKG regulation, but did not assess the protein levels or function. This is the limitation of the study; however, it is likely that PGC1α mRNA expression levels might serve as substitutes for its protein levels as the strong correlation between PGC1α mRNA and protein expression levels has been demonstrated in rodent hearts [38].
Clinical application of PDE5 inhibition in heart failure has been demonstrated to be beneficial in a clinical study of heart failure patients with reduced ejection fraction (HFrEF) [4]. On the other hand, a large clinical trial of patients with heart failure with preserved cardiac ejection fraction (HFpEF) revealed no benefits from 6 months of sildenafil treatment [8]. With regard to the latter negative results, we found that the efficacy of sildenafil critically depends on estrogen levels in female animal models [9]. This may have contributed to the negative results in the HFpEF study in which older individuals were examined, nearly half of whom were women. However, there are other several potential explanations, including low levels of myocardial cGMP and PKG activity in this disorder, unknown levels of myocardial PDE5 (the phosphodiesterase is up-regulated in human HFrEF) [10] and modest or minimal structural and functional heart disease in many of the subjects enrolled in this study.
In conclusion, we have demonstrated that PGC1α regulation by cGMP-PKG plays a key role in the physiological benefits of PDE5 inhibition in the treatment of heart failure. The significance of PKG-PGC1α axis to maintaining mitochondrial function might be shared with other cGMP augmenting therapies in heart failure, including PDE9 inhibition, neprylisin inhibition, and sGC activation. The present study provides another line of mechanistic basis for the utility of PKG activating agents for treating heart diseases by revealing its role in metabolic signaling.
Acknowledgments
We thank Dr. Mark J. Ranek for critical reading of the manuscript. This work was supported by NIH grant HL-093432 and American Heart Association Grant-in-Aid 11GRNT7700071 (to ET), NIH grant HL-131831 (to RMB), and NIH grant HL-089297, Muscular Dystrophy Association Grant 186454, Foundation Leducq, and Abraham and Virginia Weiss Endowment (to DAK). The respective funding sources were not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- ANP
natriuretic peptide A
- ATP
adenosine triphosphate
- BNP
natriuretic peptide B
- cGMP
cyclic guanosine monophosphate
- CREB
cAMP-responsive element binding protein
- HFpEF
heart failure with preserved systolic function
- HFrEF
heart failure with reduced systolic function
- NO
nitric oxide
- PDE5
phosphodiesterase 5
- PDE5i
phosphodiesterase 5 inhibition
- PGC1α
peroxisome proliferator-activated receptor γ co-activator-1α
- PKG
cGMP-dependent protein kinase
- PV loop
pressure-volume loop
- TAC
transverse aortic constriction
Data accessibility
The data that support the findings of this study are available from the corresponding author (etakimo1@jhmi.edu) upon reasonable request.
References
- 1.Rainer PP and Kass DA (2016) Old dog, new tricks: novel cardiac targets and stress regulation by protein kinase G. Cardiovasc Res 111, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takimoto E (2012) Cyclic GMP-dependent signaling in cardiac myocytes. Circ J 76, 1819–1825. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E et al. (2015) Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 314, 2251–2262. [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Vicenzi M, Arena R and Guazzi MD (2011) PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail 4, 8–17. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K et al. (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371, 993–1004. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G et al. (2020) Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 382, 1883–1893. [DOI] [PubMed] [Google Scholar]
- 7.Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC Jr, Roessig L, Stasch JP, Solomon SD, Paulus WJ et al. (2013) The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc 2, e000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL et al. (2013) Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309, 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki H, Nagayama T, Blanton RM, Seo K, Zhang M, Zhu G, Lee DI, Bedja D, Hsu S, Tsukamoto O et al. (2014) PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest 124, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma K and Kass DA (2014) Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 115, 79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura T, Ranek MJ, Lee DI, Shalkey Hahn V, Kim C, Eaton P and Kass DA (2015) Prevention of PKG1α oxidation augments cardioprotection in the stressed heart. J Clin Invest 125, 2468–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranek MJ, Kokkonen-Simon KM, Chen A, Dunkerly-Eyring BL, Vera MP, Oeing CU, Patel CH, Nakamura T, Zhu G, Bedja D et al. (2019) PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 566, 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayeva M, Gheorghiade M and Ardehali H (2013) Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol 61, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW 2nd, Kitsis RN, Otsu K, Ping P, Rizzuto R et al. (2016) Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circ Res 118, 1960–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patten IS and Arany Z (2012) PGC-1 coactivators in the cardiovascular system. Trends Endocrinol Metab 23, 90–97. [DOI] [PubMed] [Google Scholar]
- 16.Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, Stevens RD, Wenner B, Ilkayeva O, Loebe M et al. (2014) Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet 7, 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schilling J and Kelly DP (2011) The PGC-1 cascade as a therapeutic target for heart failure. J Mol Cell Cardiol 51, 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A and Spiegelman BM (2006) Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA 103, 10086–10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S et al. (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299, 896–899. [DOI] [PubMed] [Google Scholar]
- 20.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I et al. (2012) Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest 122, 4675–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y and Kass DA (2005) Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 11, 214–222. [DOI] [PubMed] [Google Scholar]
- 22.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, Bedja D, Gabrielson KL, Blanton R, Siderovski DP et al. (2009) Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest 119, 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED et al. (2008) The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol 295, H185–H196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiche JD, Schlutsmeyer SM, Bloch DB, de la Monte SM, Roberts JD Jr, Filippov G, Janssens SP, Rosenzweig A and Bloch KD (1998) Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem 273, 34263–34271. [DOI] [PubMed] [Google Scholar]
- 25.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J and Montminy M (2004) PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med 10, 530–534. [DOI] [PubMed] [Google Scholar]
- 26.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM and Kelly DP (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannessen M, Delghandi MP and Moens U (2004) What turns CREB on? Cell Signal 16, 1211–1227. [DOI] [PubMed] [Google Scholar]
- 28.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, Danner T, Zhang M, Rainer PP et al. (2015) Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 519, 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranek MJ, Terpstra EJ, Li J, Kass DA and Wang X (2013) Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins. Circulation 128, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karamanlidis G, Garcia-Menendez L, Kolwicz SC Jr, Lee CF and Tian R (2014) Promoting PGC-1alpha-driven mitochondrial biogenesis is detrimental in pressure-overloaded mouse hearts. Am J Physiol Heart Circ Physiol 307, H1307–H1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, Koenke T, O’Rourke B, Champion HC, Crow MT et al. (2008) Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal 20, 2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai-Kusuhara A, Nakamura M, Mukuno H, Kanamori A, Negi A and Seigel GM (2007) cAMP-responsive element binding protein mediates a cGMP/protein kinase G-dependent anti-apoptotic signal induced by nitric oxide in retinal neuro-glial progenitor cells. Exp Eye Res 84, 152–162. [DOI] [PubMed] [Google Scholar]
- 33.Xu F, Lv C, Deng Y, Liu Y, Gong Q, Shi J and Gao J (2020) Icariside II, a PDE5 inhibitor, suppresses oxygen-glucose deprivation/reperfusion-induced primary hippocampal neuronal death through activating the PKG/CREB/BDNF/TrkB signaling pathway. Front Pharmacol 11, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson PA, Reusch JE, McCune SA, Leinwand LA, Luckey SW, Konhilas JP, Brown DA, Chicco AJ, Sparagna GC, Long CS et al. (2007) Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J Physiol Heart Circ Physiol 293, H246–H259. [DOI] [PubMed] [Google Scholar]
- 35.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R and Collins S (2012) Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122, 1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koka S, Aluri HS, Xi L, Lesnefsky EJ and Kukreja RC (2014) Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1alpha signaling. Am J Physiol Heart Circ Physiol 306, H1558–H1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Yuan Y, Li S, Zeng C, Yu W, Shen M, Zhang R, Li C, Zhang Y and Wang H (2016) PDE5 inhibitors protect against post-infarction heart failure. Front Biosci 21, 1194–1210. [DOI] [PubMed] [Google Scholar]
- 38.Thu VT, Kim HK, le Long T, Nyamaa B, Song IS, Thuy TT, Huy NQ, Marquez J, Kim SH, Kim N et al. (2016) NecroX-5 protects mitochondrial oxidative phosphorylation capacity and preserves PGC1α expression levels during hypoxia/reoxygenation injury. Korean J Physiol Pharmacol 20, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (etakimo1@jhmi.edu) upon reasonable request.