Abstract
Background
To compare the prognosis of first-line systemic chemotherapy of AS (Albumin-bound paclitaxel and S-1) versus SOX (S-1 and oxaliplatin) regimen in Chinese gastric cancer patients with peritoneal metastasis.
Methods
This was a real-world study of gastric cancer patients with peritoneal metastasis who have been treated with AS or SOX regimen as first-line chemotherapy. Patients were matched by the method of propensity score matching (PSM). The primary and secondary endpoints were overall survival (OS) and progress-free survival (PFS).
Results
A total of 108 gastric cancer patients with peritoneal metastasis were enrolled after PSM analysis. There was no significant difference between AS and SOX regimen based on gender, age, ascites, treatment cycles, gastric cancer resection, received checkpoint inhibitors, and HER-2 expression after PSM analysis. The median OS (14.13 vs. 11.17 months, p = 0.0356) and median PFS (10.30 vs. 6.70 months, p = 0.0003) of patients who received AS regimen were longer than those treated by SOX regimen as first-line systemic chemotherapy. In sub-group analysis, the median OS and median PFS were longer for patients in AS regimen than SOX regimen in Lauren diffuse type. The occurrence of toxicity between the two groups was shown no significant difference.
Conclusions
The results verified that AS regimen was more effective than SOX chemotherapy in gastric cancer patients with peritoneal metastasis, especially in Lauren diffuse type.
Keywords: Gastric cancer, Peritoneal metastasis, First-line, Lauren type, Preoperative chemotherapy
Introduction
Gastric cancer is reported as the fifth most common cancer in humans and the third leading cause of cancer-related death in the world [1]. The standard first-line systemic chemotherapy for advanced gastric cancer is the combination of fluoropyrimidine and platinum, with trastuzumab used in patients with HER-2 positive [2–4]. Currently, the most commonly used first-line chemotherapy for gastric cancer patients is S-1 (tegafur, gimeracil, oteracil) or capecitabine combined with cisplatin or oxaliplatin [2, 5, 6], especially the combination of S-1 with oxaliplatin (SOX) [7]. Although these chemotherapy treatments have survival benefits, the median survival time for patients with advanced gastric cancer was only about one-year time, and might worsen in those patients with peritoneal metastasis.
As we knew, one of the major causes of the poor prognosis in gastric cancer patients was peritoneal metastasis, accounting for 20 to 40% of all deaths. The incidence of peritoneal metastasis in gastric cancer patients was reported at about 40% [8–10]. The median overall survival (OS) of patients in gastric cancer with peritoneal metastasis was once reported as about 3 to 4 months [11]. Despite the progress of cancer treatment, there was still no standard and effective systemic treatment strategy for these patients with one of the main reasons being the peritoneal-plasma barrier which resisted drug diffusion. Therefore, it is necessary to explore a novel treatment method with high penetrability to lengthen the survival period of gastric cancer patients with peritoneal metastasis.
Albumin-bound paclitaxel (ABX), also known as nab-paclitaxel, has a better metastatic effect on tumor tissue and a higher inhibitory effect than solvent-based paclitaxel. ABX plus ramucirumab which served as second-line chemotherapy showed a slightly longer PFS compared to paclitaxel plus ramucirumab in advanced gastric cancer patients with peritoneal metastasis (5.8 vs 3.5 months, HR 0.66; 95% CI 0.40–1.10, p = 0.109) [12]. The weekly ABX regimen showed longer OS than the paclitaxel regimen (9.9 vs. 8.7 months) of peritoneal metastasis in gastric cancer patients in ABSOLUTE trial [13]. A phase II clinical trial of S-1 combined with ABX (AS regimen) in untreated patients with metastatic gastric cancer showed the median OS was approximately 14 months [14]. However, until now there was still no efficacy comparison between AS regimen and the standard therapy SOX regimen in gastric cancer patients with peritoneal metastasis. Thus, the objective of this study was to compare the prognosis of patients who received first-line treatment of AS or SOX regimen in gastric cancer patients with peritoneal metastasis.
Methods
Study design and patients
This was a retrospective study of gastric cancer patients with peritoneal metastasis between January 2016 and July 2021 conducted at Zhongshan Hospital of Fudan University. This study was approved by the Ethics Committee of Zhongshan Hospital of Fudan University. The committee removed the individual consent requirement due to the real-world study collecting data from medical records retrospectively. All data were anonymous before data processing.
Inclusion criteria: (1) gastric adenocarcinoma confirmed by histology; (2) all patients were diagnosed with peritoneal metastasis; (3) first-line AS or SOX chemotherapy was used; (4) no synchronous or metachronous cancer; (5) Eastern Cooperative Oncology Group performance status 0 or 1; 6) Trastuzumab was allowed to be used if HER-2 positive.
Diagnosis standards of peritoneal metastasis: (1) CT/MRI/PET-CT scan: omental cake, irregular nodules and thickening of the peritoneum, multiple cord shadows in fat space (peritoneum, omentum, mesentery, and intestinal wall), the density of peritoneal adipose tissue increased, the intestinal wall thickened; (2) clinical signs: board-like rigidity of the abdomen which cannot be attributed to other reason except peritoneal dissemination; (3) laparoscopy examination or laparotomy confirmed by the pathological diagnosis of peritoneal metastasis.
Data collection
Gender, age, chemotherapy regimen, pathological information, dates of diagnosis and follow-up, dates of initiation and termination of chemotherapy, and dates of progress and death were collected. Adverse events were assessed through the National Cancer Institute-Common Toxicity Criteria version 5.0. All patients underwent routine physical, hematological, and imaging examinations.
Chemotherapy regimen
All patients received first-line systemic chemotherapy under the guidelines of the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO). The AS regimen included 3-week cycles of ABX (125 mg/m2 on day 1, day 8 of each cycle, intravenously) plus S-1. The SOX regimen included 3-week cycles of oxaliplatin (130 mg/m2 intravenously on day 1 of each cycle) combined with S-1. The oral doses of S-1 were the same in both groups, with 40 mg (BSA < 1.25 m2), 50 mg (1.25 ≤ BSA < 1.50 m2), and 60 mg (BSA ≥ 1.50 m2) bid on day 1 to 14. Patients were allowed to be maintained with a single drug until tumor progression after the combined AS or SOX treatment. Trastuzumab was allowed to be used in gastric cancer patients with peritoneal metastasis with HER-2 positive. There were no restrictions on second or posterior treatment. The number of cycles was determined by researchers. Treatment continued until unacceptable toxicity, disease progression, patients’ refusal, or the decision by physicians.
Endpoints
The primary and secondary endpoints were OS and PFS, respectively. OS was defined as the period from the initiation date of treatment to the final follow-up or death for any reason. PFS was defined as the period from the initiation date of treatment to the progression date. The progression was determined as the appearance of new lesions in some cases, the appearance or increase of ascites, or the worsening of clinical findings. The assessment of progression was based on RECIST version 1.1. Adverse event (AEs) were graded according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
Age was divided into two groups at 65. Categorical data were displayed in numbers and percentages and further examined by the chi-square test. OS and PFS were estimated by the Kaplan–Meier method. Propensity score matching (PSM) was carried out through a logistic regression model and nearest neighbor matching algorithm with a ratio of 1:1. PSM accounted for factors of age, gender, ascites, and Lauren type. A difference of less than 10% of the absolute value was considered balanced. Estimates of treatment benefits were calculated as hazard ratios (HR) with 95% confidence intervals (CI). Comparisons in categorical data were evaluated by Fisher’s exact test or chi-square test. SPSS 26.0 (IBM, Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software, La Jolla, CA) were executed for analysis. p-value < 0.05 was considered statistically significant.
Results
Characteristics of the patients
From January 2016 to July 2021, a total of 143 patients met all the inclusion criteria, including 62 cases of AS regimen and 81 cases of SOX regimen. There were 54 cases in each group after PSM analysis. The second or posterior treatment was no restriction. The flowchart of the patients’ enrollment was displayed in Fig. 1. Comprehensive data collection and monitoring were conducted for all patients. The ultimate date of follow-up was July 31, 2021. The baseline features of patients before and after PSM were presented in Table 1. The two groups were all well balanced concerning age, gender, ascites, and Lauren tissue type after PSM analysis. Total of 113 patients died (79.6%; 38 and 75 in the AS and SOX groups, respectively) by the final follow-up day. The median cycles of chemotherapy were both 6 in two groups and the cycle numbers of less than 3 and more than 3 in AS and SOX group was balanced by PSM analysis. To further minimize the effect of other variables, we analyzed the surgery and immune checkpoint inhibitor implement in these patients which were all no significant difference after PSM. Five patients in AS and five patients in SOX group underwent palliative surgery. There were 8 patients in AS group and 5 patients in SOX group treated with a checkpoint inhibitor, including 6 patients treated with PD1 inhibitor and 2 patients treated with MET inhibitor in AS regimen, while 5 patients used PD1 inhibitor and 1 patient used MET inhibitor in SOX regimen after PSM analysis. The common used PD1 inhibitors were nivolumab, pembrolizumab, and toripalimab. There were 5 patients with HER2 positive expression in both groups after PSM, while 4 of them were taken with trastuzumab during first-line treatment in each group. Thus, the two groups were also well balanced in terms of the number of chemotherapy cycles, palliative surgery, HER2 expression, and immune checkpoint inhibitors application.
Fig. 1.
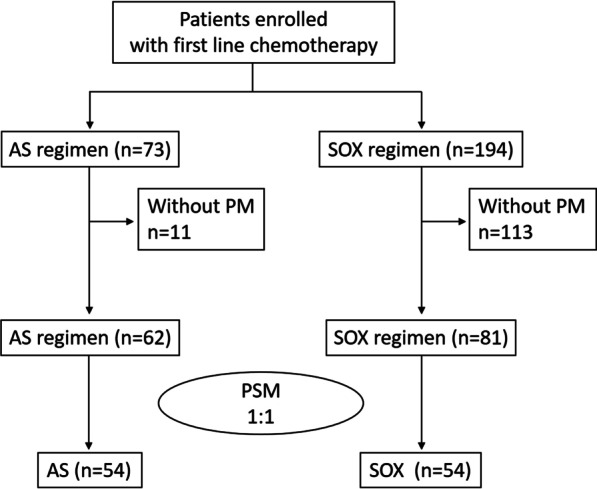
The flowchart of the study. AS, Albumin-bound paclitaxel combined with S-1 regimen, SOX, S-1 combined with oxaliplatin regimen; PM, peritoneal metastases; PSM, propensity score matching
Table 1.
Comparison of characteristics before and after propensity score matching (PSM)
| Variable | Before PSM | p-value | After PSM | p-value | ||
|---|---|---|---|---|---|---|
| AS (%) | SOX (%) | AS (%) | SOX (%) | |||
| Gender | 0.0098 | 1.0000 | ||||
| Male | 29 (46.8) | 56 (69.1) | 29 (53.7) | 29 (53.7) | ||
| Female | 33 (53.2) | 25 (30.9) | 25 (46.8) | 25 (46.8) | ||
| Age | 0.3440 | 1.0000 | ||||
| ≤ 65 | 48 (77.4) | 56 (69.1) | 43 (79.6) | 43 (79.6) | ||
| > 65 | 14 (22.6) | 25 (30.9) | 11 (20.4) | 11 (20.4) | ||
| Ascites | 0.2177 | 1.0000 | ||||
| Without | 26 (41.9) | 25 (30.9) | 21 (38.9) | 20 (37.0) | ||
| With | 36 (58.1) | 56 (69.1) | 33 (61.1%) | 34 (63.0) | ||
| Lauren type | 0.0006 | 0.0172 | ||||
| Intestinal | 4 (6.5) | 26 (32.1) | 0.3169a | 4 (7.4) | 15 (27.8) | 0.5255b |
| Diffuse | 42 (67.7) | 35 (43.2) | 34 (63.0) | 24 (44.4) | ||
| Mixed | 16 (25.8) | 20 (24.7) | 16 (29.6) | 15 (27.8) | ||
| Cycles | 0.0290 | 0.2318 | ||||
| ≤ 3 | 9 (14.5) | 25 (30.9) | 8 (14.8) | 14 (25.9) | ||
| > 3 | 53 (85.5) | 56 (69.1) | 46 (85.2) | 40 (74.1) | ||
| Surgery | 0.6602 | 1.0000 | ||||
| Yes | 5 (8.1) | 5 (6.2) | 5 (9.3) | 5 (9.3) | ||
| No | 57 (91.9) | 76 (93.8) | 49 (90.7) | 49 (90.7) | ||
| Inhibitor | 0.4099 | 0.3750 | ||||
| Yes | 8 (12.9) | 7 (8.6) | 8 (14.8) | 5 (9.3) | ||
| No | 54 (87.1) | 74 (91.4) | 46 (85.2) | 49 (90.7) | ||
| HER2 | 0.7631 | 1.0000 | ||||
| Negative | 56 (90.3) | 75 (92.6) | 49 (90.7) | 49 (90.7) | ||
| Positive | 6 (9.7) | 6 (7.4) | 5 (9.3) | 5 (9.3) | ||
| Trastuzumabc | 1.0000 | 1.0000 | ||||
| No | 1 (16.7) | 1 (16.7) | 1 (20) | 1 (20) | ||
| Yes | 5 (83.3) | 5 (83.3) | 4 (80) | 4 (80) | ||
| Total | 62 (100) | 81 (100) | 54 (100) | 54 (100) | ||
ap-value between diffuse and mixed lauren type before PSM
bp-value between diffuse and mixed lauren type after PSM
ctrastuzumab in HER2 positive patients; Surgery, palliative surgery during or after first line chemotherapy; Inhibitor, checkpoint inhibitor
Follow-up
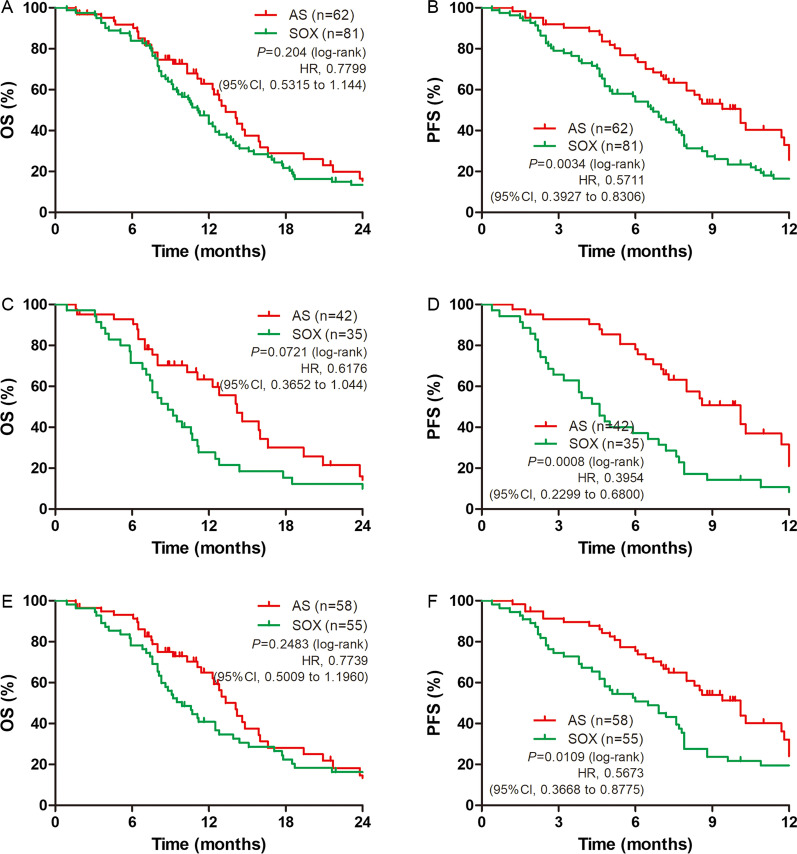
The median age of the patients was 58 (range from 19 to 78) years. The median follow-up period was 30.11 months. The median number of chemotherapy cycles was 6 (range from 1 to 9). Before PSM, there was no significant difference between the median OS of patients receiving AS and SOX (HR = 0.7799, 95% CI: 0.5315–1.144) (Fig. 2A; Table 2). However, we discovered a significant difference in median PFS (HR = 0.5711, 95% CI: 0.3927–0.8306) between patients receiving AS and SOX treatment (Fig. 2B). In subgroup analysis, we discovered that there was still no significant difference in diffuse only type and Lauren non-intestinal (diffuse and mixed) types between patients receiving AS and SOX of median OS (Fig. 2C and E). However, a significant difference in median PFS between these sub-groups was discovered (Fig. 2D and F).
Fig. 2.
Kaplan–Meier survival curves for overall survival (OS) and progression-free survival (PFS) before propensity score matching (PSM). OS (A) and PFS (B) analyses for the AS (n = 62) and SOX (n = 81) regimens. OS (C) and PFS (D) analyses of gastric cancer patients with Lauren non-intestinal type (diffuse and mixed type). OS (E) and PFS (F) analyses of gastric cancer patients with Lauren diffuse type
Table 2.
Progress free survival time and overall survival time of patients before and after PSM
| Variable | N | OS (months) | PFS (months) | ||
|---|---|---|---|---|---|
| Median | 95%CI | Median | 95%CI | ||
| Before PSM | |||||
| AS | 62 | 13.27 | 11.35–15.19 | 10.07 | 8.40–12.09 |
| SOX | 81 | 11.17 | 9.61–12.72 | 6.70 | 5.51–7.89 |
| AS (non-intestinal) | 58 | 13.27 | 11.46–15.09 | 10.07 | 8.12–12.02 |
| SOX (non-intestinal) | 55 | 9.90 | 7.71–12.09 | 6.53 | 4.31–8.76 |
| AS (diffuse) | 42 | 14.23 | 11.70–16.77 | 10.07 | 7.81–12.33 |
| SOX (diffuse) | 35 | 8.83 | 6.67–11.00 | 4.60 | 3.54–5.66 |
| After PSM | |||||
| AS | 54 | 14.13 | 11.96–16.31 | 10.30 | 7.06–13.54 |
| SOX | 54 | 11.17 | 9.09–13.24 | 6.70 | 6.08–7.33 |
| AS (non-intestinal) | 50 | 14.23 | 12.14–16.33 | 10.30 | 7.53–13.07 |
| SOX (non-intestinal) | 39 | 9.90 | 7.30–12.50 | 6.87 | 5.26–8.48 |
| AS (diffuse) | 34 | 15.93 | 13.58–18.28 | 10.30 | 7.27–13.33 |
| SOX (diffuse) | 24 | 8.83 | 6.51–11.15 | 4.60 | 2.25–6.95 |
OS, overall survival time; PFS, progress free survival time; CI, confidence intervals; non-intestinal, including diffuse lauren type and mixed lauren type; PSM, propensity score matching; N, number
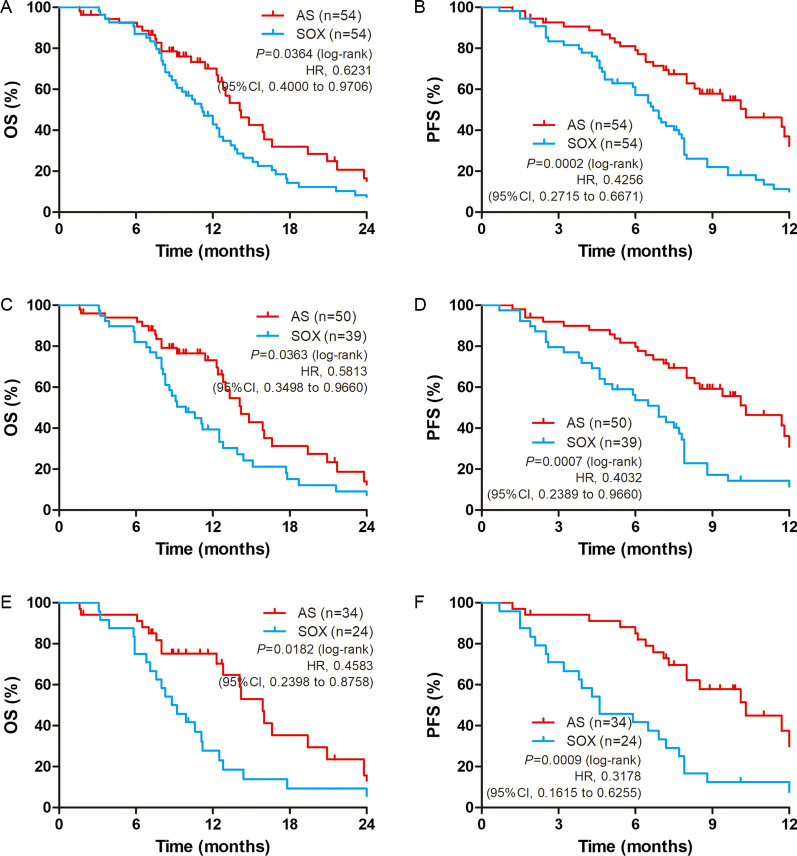
After PSM analysis, the median OS was 14.13 and 11.17 months (p = 0.0364; HR = 0.6231, 95% CI 0.4000–0.9706) between the AS and SOX groups, while median PFS was 10.30 and 6.70 months (p = 0.0002; HR = 0.4256, 95% CI 0.2715–0.6671) respectively (Fig. 3A and B, Table 2). Significant differences were observed in Lauren non-intestinal subgroup (diffuse and mixed type) analysis with median OS (AS 14.23 vs. SOX 9.90 months, p = 0.0363; HR = 0.5813, 95% CI 0.3498–0.9660) and median PFS was 10.30 and 6.87 months (p = 0.0007; HR = 0.4032, 95% CI 0.2389–0.9660) (Fig. 3C and D). Further in-deep analysis in Lauren mixed type subgroup, we found the median OS (AS 15.93 vs. SOX 8.83 months, p = 0.0182; HR = 0.4583, 95% CI: 0.2398–0.8758) and median PFS was 10.30 and 4.60 months (p = 0.0009; HR = 0.3178, 95% CI 0.1615–0.0.6225) with significant value (Fig. 3E and Fig. 3F).
Fig. 3.
Kaplan–Meier survival curves for OS and PFS after PSM. OS (A) and PFS (B) analyses for the AS (n = 54) and SOX (n = 54) regimens. OS (C) and PFS (D) analyses of gastric cancer patients with Lauren non-intestinal type (diffuse and mixed type). OS (E) and PFS (F) analyses of gastric cancer patients with Lauren diffuse type
Adverse events
Adverse events of each group were shown in Table 3. The myelosuppression, including leukocytopenia, anemia, and thrombocytopenia was the most frequent hematological adverse event in these two groups. Diarrhea, hepatic dysfunction, vomiting, peripheral neurotoxicity, hand-foot syndrome, and alopecia were also found in each group, but all without significant difference (p > 0.05).
Table 3.
Incidence of adverse events
| Event | AS group (n = 62) | SOX group (n = 81) | p-Value | ||
|---|---|---|---|---|---|
| Grade1/2 | Grade3/4 | Grade1/2 | Grade3/4 | ||
| Leukocytopenia | 3 (4.84%) | 3 (4.84%) | 2 (2.47%) | N/A | 0.0800 |
| Anemia | 2 (3.23%) | N/A | 1 (1.23%) | N/A | 0.4207 |
| Thrombocytopenia | 1 (1.61%) | 1 (1.61%) | 5 (6.17%) | N/A | 0.4000 |
| Vomiting | 2 (3.23%) | N/A | 3 (3.70%) | N/A | 0.8816 |
| Diarrhea | 2 (3.23%) | N/A | 2 (2.47%) | N/A | 0.7915 |
| Hepatic dysfunction | 1 (1.61%) | N/A | 1 (1.23%) | N/A | 0.8507 |
| Peripheral neurotoxicity | 3 (4.84%) | N/A | 2 (2.47%) | N/A | 0.5611 |
| Hand-foot syndrome | 3 (4.84%) | N/A | 2 (2.47%) | N/A | 0.5611 |
| Alopecia | 3 (4.84%) | N/A | 2 (2.47%) | N/A | 0.5611 |
Discussion
Despite the advances in systemic chemotherapy, the prognosis of gastric cancer patients with peritoneal metastasis remains very poor, even worse than those with other metastatic sites [15]. It was indicated that the peritoneal metastasis rate of gastric cancer patients was about 14% at initial diagnosis, accounting for 20 to 40% of death for gastric cancer which was regarded as the most frequent death cause [11]. To date, peritoneal metastasis is one of the most frequent types of metastasis and recurrence in human gastric cancer. The OS of patients with gastric cancer peritoneal metastasis was about 3–10 months [9, 16, 17]. The first-line systemic strategy for gastric cancer patients with peritoneal metastasis is also the combination of platinum and 5-FU-based regimens [18]. The phase III (SPIRITS) trial proved that cisplatin plus S-1 (CS regimen) was significantly better than S-1 alone for advanced gastric cancer, which was established as the standard first-line therapy in Japan in 2008 [2]. The median OS was longer in patients assigned to CS regimen (13.0 months) than S-1 alone (11.0 months; HR 0.77, 95%CI 0.61–0.98; p = 0.04). The median OS of the other commonly used oxaliplatin plus capecitabine (XELOX regimen) was reported as 11.1 months in a phase II study [19]. The median OS of first-line CS and SOX regimen was 13.1 and 14.1 months in the phase III study of patients with advanced gastric cancer respectively [20], which is similar to the SPIRITS study. In addition, the median OS of paclitaxel with S-1 in advanced gastric cancer patients was displayed as 14.0 months in one randomized phase II study [21]. Here, one thing that needs to emphasize is that the patients enrolled in these trials including advanced gastric cancer patients mostly without peritoneal metastasis. Thus, the median OS of gastric cancer patients with peritoneal metastasis might be worse than that.
Patients with peritoneal disseminated gastric cancer have a low response rate to systemic chemotherapy, mainly due to the existence of the barrier between peritoneal and blood that separates the abdominal cavity from intravenous chemotherapy. Recently, with the emergence of new chemotherapeutic drugs, such as docetaxel and ABX, they have shown good clinical efficacy and controllable toxicity.
It is well known that docetaxel and ABX are very effective for peritoneal metastasis because it has effective transferability to tumor tissues and high antitumor effects for peritoneal metastasis compared with paclitaxel [22–24]. The median OS was 20.0 months with docetaxel and S-1 (DS regimen) and 15.8 months with CS regimen in the phase II HERBIS-3 study [25]. However, the phase III START trial further showed that the DS regimen only improved OS as 12.5 months compared with S-1 alone as 10.8 months (HR of 0.837, 95%CI 0.711–0.985) [26]. In the START trial, the gastric patients with only non-measurable lesions such as peritoneal metastasis showed a better OS of DS regimen than the S-1 alone group, with 17.9 months vs. 12.0 months respectively [26]. A phase I/II study of docetaxel, cisplatin, and S-1 (DCS regimen) enrolled advanced gastric cancer patients with peritoneal metastasis, which showed high anti-tumor efficacy with an OS of 15.5 months but more frequencies grade of 3 or 4 toxicity [16]. The HERBIS-3 study reported that the OS of DS regimen was superior to CS regimen in gastric cancer patients with positive peritoneal lavage cytology, with the 2-year OS rates being 70.0% versus 16.7% (HR 0.153, 95% CI 0.037–0.632) [25]. Thus, the docetaxel-based three agents regimen could improve OS of gastric cancer patients with peritoneal metastasis but more toxicity which limits its use in the clinic.
Compared with traditional paclitaxel, ABX has shown significant vasopermeability and tissue penetrability [27]. ABX has many better characteristics than solvent paclitaxel, such as higher plasma clearance and enhanced intratumor delivery, which was encouraged to be used in gastric cancer with peritoneal dissemination [28, 29]. In comparison with traditional paclitaxel, ABX treatment increases the proportion of activated paclitaxel in plasma reported by Gardner et al. [30]. ABX plus ramucirumab was then used in patients with peritoneal metastasis of unresectable advanced or recurrent gastric cancer who have relapsed after first-line therapy [31]. The ABSOLUTE trial showed the weekly ABX regimen had longer OS than the paclitaxel regimen (9.9 vs. 8.7 months) of peritoneal metastasis in gastric cancer patients [13, 32]. Recently, the combination of intraperitoneal paclitaxel and systemic chemotherapy in advanced gastric cancer patients with peritoneal metastasis could enhance the OS to 20.0 months [33]. ABX following intravenous administration was thought to be infiltrated into the peritoneal tumor to the same degree as intraperitoneal injection [34]. Therefore, ABX was a proper systemic agent recommended for the peritoneal metastasis of gastric cancer patients. Our data was manifested that the patients of gastric cancer with peritoneal metastasis who received AS regimen reached a superior median OS (14.13 vs. 11.17 months, p = 0.0364) and PFS (10.30 vs. 6.7 months, p = 0.0363) than SOX regimen. With the increasing evidence of immune checkpoint inhibitors in gastric cancer, the data of nivolumab plus chemotherapy showed a longer OS and PFS in ATTRACTION-4 and CheckMate 649 trail [35, 36]. However, immune checkpoint inhibitors were rarely used as a first-line treatment in the study. We think combined immunotherapy can prolong the survival time of patients with peritoneal metastasis of gastric cancer, but further research is needed.
Lauren’s classification is the most extensively used classification system of gastric cancer [37–39]. The clinical trial has proved that gastric cancer patients with the diffuse type got a worse prognosis than those with intestinal-type [38]. The report once showed that 46.3% of gastric patients were intestinal type, 32.6% were diffuse type, and 21.1% were mixed type [40]. The OS of gastric cancer patients with diffuse and mixed type was significantly less than those with intestinal-type. Some researchers suggested combining mixed and diffuse gastric cancer into the same category of diffuse-type because the prognosis and survival pattern of the diffuse and mixed survival curves seem to be similar [40]. Our results displayed that about 21.0% of gastric patients with peritoneal metastasis were intestinal type, while 53.8% were diffuse type, and 25.2% were mixed type. Thus, our data indicated that the Lauren diffuse type was the primary type of gastric cancer with peritoneal metastasis. After PSM, the median OS is 14.23 months in AS regimen compared to 9.90 months in the SOX regimen of Lauren diffuse and mixed type (p = 0.0363). Further to analyze gastric patients of Lauren diffuse, the OS is 15.93 months in AS regimen compared to 8.83 months in SOX regimen (p = 0.0182). The median OS was prolonged from 9.90 months (SOX regimen) to 14.23 months (AS regimen) of diffuse and mixed type in patients with gastric cancer peritoneal metastasis. Meanwhile, the median OS was prolonged by 7.10 months by AS regimen than SOX regimen in Lauren diffuse mixed-type gastric patients (15.93 vs. 8.83 months). Thus, we concluded that patients with gastric cancer with peritoneal metastasis could benefit from AS treatment especially those patients with Lauren diffuse mixed type.
Although there was no difference between our statistics of adverse events in patients treated with AS and SOX, the relatively high incidence of bone marrow suppression was also worthy of attention. We found that leukocytopenia was more common in patients with AS regimen, while the incidence of thrombocytopenia in patients with SOX regimen was higher, which was in line with what we have observed in the clinic. Most AEs were no more than grade 2. There were 3 cases of leukocytopenia and 1 cases of thrombocytopenia with grade ≥ 3 AEs in AS group, while no case with grade ≥ 3 AEs was found in SOX group. The study also had limitations. The main limitation of this study was that it was a nonrandomized retrospective study and the number of cases that we followed up was not enough.
Conclusions
In this first-line systemic chemotherapy study in Chinese patients of gastric cancer with peritoneal metastasis, we first demonstrated the benefits of AS regimen compared with the SOX regimen. Meanwhile, our data highlighted the evidence that gastric cancer patients of Lauren diffuse-type could get extremely survival time by AS regimen as a first-line strategy. In conclusion, the study indicated that AS regimen was an effective and well-tolerated therapy for the first-line treatment of gastric cancer with peritoneal metastasis, especially in Lauren diffuse type.
Acknowledgements
We thank the patients involved in this study.
Abbreviations
- AS
Albumin-bound paclitaxel combined with S-1 regimen
- SOX
S-1 combined with oxaliplatin regimen
- ABX
Albumin-bound paclitaxel
- PSM
Propensity score matching
- OS
Overall survival
- PFS
Progression-free survival
Author contributions
Each author participated sufficiently in the work to take responsibility for appropriate portions of the content. LZ and JZ participated in the research design, coded the patient database, analyzed the data, and wrote the manuscript. YW, WL, SY, QL, YY, and TL contributed to the conception and study design. Y C designed the project, supervised the study, and edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
The study was supported by grants from the National Natural Science Foundation of China (grants no. 81902624).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhongshan Hospital of Fudan University. Written consent from study patients was not obtained because the database maintained by the Department of Medical Oncology of Zhongshan Hospital, Fudan University consists of deidentified secondary data for research purposes, and the Institutional Ethics Board of Zhongshan Hospital, Fudan University issued a formal written informed waiver for the need for consent. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lingyun Zhang, Jiayu Zhang contributed equally
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 3.Hamada C, Yamada Y, Azuma M, Nishikawa K, Gotoh M, Bando H, Sugimoto N, Nishina T, Amagai K, Chin K, et al. Meta-analysis supporting noninferiority of oxaliplatin plus S-1 to cisplatin plus S-1 in first-line treatment of advanced gastric cancer (G-SOX study): indirect comparison with S-1 alone. Int J Clin Oncol. 2016;21(4):668–675. doi: 10.1007/s10147-015-0938-9. [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 5.Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol Off J Eur Soc Med Oncol. 2009;20(4):666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka K, Tsushima T, Mizusawa J, Hironaka S, Tsubosa Y, Kii T, Shibuya Y, Chin K, Katayama H, Kato K, et al. A randomized controlled Phase III trial comparing 2-weekly docetaxel combined with cisplatin plus fluorouracil (2-weekly DCF) with cisplatin plus fluorouracil (CF) in patients with metastatic or recurrent esophageal cancer: rationale, design and methods of Japan clinical oncology group study JCOG1314 (MIRACLE study) Jpn J Clin Oncol. 2015;45(5):494–498. doi: 10.1093/jjco/hyv012. [DOI] [PubMed] [Google Scholar]
- 7.Zhong DT, Wu RP, Wang XL, Huang XB, Lin MX, Lan YQ, Chen Q. Combination chemotherapy with S-1 and oxaliplatin (SOX) as first-line treatment in elderly patients with advanced gastric cancer. Pathol Oncol Res POR. 2015;21(4):867–873. doi: 10.1007/s12253-015-9903-1. [DOI] [PubMed] [Google Scholar]
- 8.Tahara M, Ohtsu A, Boku N, Nagashima F, Muto M, Sano Y, Yoshida M, Mera K, Hironaka S, Tajiri H, et al. Sequential methotrexate and 5-fluorouracil therapy for gastric cancer patients with peritoneal dissemination: a retrospective study. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2001;4(4):212–218. doi: 10.1007/s10120-001-8012-x. [DOI] [PubMed] [Google Scholar]
- 9.Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–628. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 10.Maeda H, Kobayashi M, Sakamoto J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J Gastroenterol. 2015;21(39):10936–10947. doi: 10.3748/wjg.v21.i39.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Endou Y, Sasaki T. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N Am. 2003;12(3):635–648. doi: 10.1016/S1055-3207(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa M, Iwasa S, Nagashima K, Aoki M, Imazeki H, Hirano H, Shoji H, Honma Y, Okita N, Takashima A, et al. Retrospective comparison of nab-paclitaxel plus ramucirumab and paclitaxel plus ramucirumab as second-line treatment for advanced gastric cancer focusing on peritoneal metastasis. Invest New Drugs. 2020;38(2):533–540. doi: 10.1007/s10637-019-00822-3. [DOI] [PubMed] [Google Scholar]
- 13.Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(4):277–287. doi: 10.1016/S2468-1253(16)30219-9. [DOI] [PubMed] [Google Scholar]
- 14.Bando H, Shimodaira H, Fujitani K, Takashima A, Yamaguchi K, Nakayama N, Takahashi T, Oki E, Azuma M, Nishina T, et al. A phase II study of nab-paclitaxel in combination with ramucirumab in patients with previously treated advanced gastric cancer. Eur J Cancer. 2018;91:86–91. doi: 10.1016/j.ejca.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Chen JQ, Liu JL, Tian L. Issues on peritoneal metastasis of gastric cancer: an update. World J Surg Oncol. 2019;17(1):215. doi: 10.1186/s12957-019-1761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohnuma H, Sato Y, Hirakawa M, Kikuchi S, Miyanishi K, Sagawa T, Takahashi Y, Nobuoka T, Okamoto K, Miyamoto H, et al. Docetaxel, cisplatin and S-1 (DCS) combination chemotherapy for gastric cancer patients with peritoneal metastasis: a retrospective study. Cancer Chemother Pharmacol. 2018;81(3):539–548. doi: 10.1007/s00280-018-3523-x. [DOI] [PubMed] [Google Scholar]
- 17.Chan DY, Syn NL, Yap R, Phua JN, Soh TI, Chee CE, Nga ME, Shabbir A, So JB, Yong WP. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are we ready? J Gastrointest Surg Off J Soc Surg Aliment Tract. 2017;21(3):425–433. doi: 10.1007/s11605-016-3336-3. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer A: Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2021, 24(1):1–21 [DOI] [PMC free article] [PubMed]
- 19.Luo HY, Xu RH, Wang F, Qiu MZ, Li YH, Li FH, Zhou ZW, Chen XQ. Phase II trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy. 2010;56(2):94–100. doi: 10.1159/000305256. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(1):141–148. doi: 10.1093/annonc/mdu472. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Wang ML, Zhou LY, Lu XY, Yang JF, Yu HG. Randomized phase II study comparing paclitaxel with S-1 vs. S-1 as first-line treatment in patients with advanced gastric cancer. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mexico. 2013;15(10):836–842. doi: 10.1007/s12094-013-1012-6. [DOI] [PubMed] [Google Scholar]
- 22.Naitoh H, Kawaguch A, Yamamoto H, Mekata E, Tan T, Morii H, Chiba M. Measurement of docetaxel concentration in blood and ascites after drip infusion into each vessel and intraperitoneal cavity of gastric cancer. Gan To kagaku Ryoho Cancer Chemother. 2004;31(12):2031–2034. [PubMed] [Google Scholar]
- 23.Kinoshita J, Fushida S, Tsukada T, Oyama K, Watanabe T, Shoji M, Okamoto K, Nakanuma S, Sakai S, Makino I, et al. Comparative study of the antitumor activity of Nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol Rep. 2014;32(1):89–96. doi: 10.3892/or.2014.3210. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki Y, Nishina T, Yasui H, Goto M, Muro K, Tsuji A, Koizumi W, Toh Y, Hara T, Miyata Y. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105(7):812–817. doi: 10.1111/cas.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurokawa Y, Matsuyama J, Nishikawa K, Takeno A, Kimura Y, Fujitani K, Kawabata R, Makari Y, Terazawa T, Kawakami H, et al. Docetaxel plus S-1 versus cisplatin plus S-1 in unresectable gastric cancer without measurable lesions: a randomized phase II trial (HERBIS-3) Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2021;24(2):428–434. doi: 10.1007/s10120-020-01112-1. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START) J Cancer Res Clin Oncol. 2014;140(2):319–328. doi: 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7(8):1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 28.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12(4):1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 29.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, Beals B, Figg WD, Hawkins M, Desai N. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11(11):4136–4143. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 30.Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, Hawkins MJ, Sparreboom A, Figg WD. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(13):4200–4205. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirata K, Hamamoto Y, Ando M, Imamura CK, Yoshimura K, Yamazaki K, Hironaka S, Muro K. Weekly paclitaxel plus ramucirumab versus weekly nab-paclitaxel plus ramucirumab for unresectable advanced or recurrent gastric cancer with peritoneal dissemination refractory to first-line therapy-the P-SELECT trial (WJOG10617G)-a randomised phase II trial by the West Japan Oncology Group. BMC Cancer. 2020;20(1):548. doi: 10.1186/s12885-020-07047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takashima A, Shitara K, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, et al. Peritoneal metastasis as a predictive factor for nab-paclitaxel in patients with pretreated advanced gastric cancer: an exploratory analysis of the phase III ABSOLUTE trial. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2019;22(1):155–163. doi: 10.1007/s10120-018-0838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DW, Jee YS, Kim CH, Kim JJ, Park S, Choi SI, Park JM, Kim JH. group P-Gs: multicenter retrospective analysis of intraperitoneal paclitaxel and systemic chemotherapy for advanced gastric cancer with peritoneal metastasis. J Gastric Cancer. 2020;20(1):50–59. doi: 10.5230/jgc.2020.20.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura M, Ojima T, Katsuda M, Hayata K, Kitadani J, Nakamori M, Yamaue H. Phase 1 study of combined chemotherapy of nab-paclitaxel, S-1, and oxaliplatin for gastric cancer with peritoneal metastasis (NSOX Study) Oncology. 2021;99(1):57–61. doi: 10.1159/000509396. [DOI] [PubMed] [Google Scholar]
- 35.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–247. doi: 10.1016/S1470-2045(21)00692-6. [DOI] [PubMed] [Google Scholar]
- 37.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 38.Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, Xu RH. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med. 2013;11(1):58. doi: 10.1186/1479-5876-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014;20(19):5679–5684. doi: 10.3748/wjg.v20.i19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH. Clinicopathological variation of lauren classification in gastric cancer. Pathol Oncol Res POR. 2016;22(1):197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.