Abstract
A growing number of copper(II) complexes have been identified as suitable candidates for biomedical applications. Here, we show that the biocompatibility and stability of copper(II) complexes can be tuned by directed ligand design and complex geometry. We demonstrate that azamacrocycle-based chelators that envelope copper(II) in a five-coordinate, distorted trigonal-bipyramidal structure are more chemically inert to redox-mediated structural changes than their six-coordinate, Jahn-Teller–distorted counterparts, as evidenced by electrochemical, crystallographic, electron paramagnetic resonance, and density functional theory studies. We further validated our hypothesis of enhanced inertness in vitro and in vivo by employing Cu-64 radiolabeling of bifunctional analogues appended to a prostate-specific membrane antigen targeting dipeptide. The corresponding Cu-64 complexes were tested for stability in vitro and in vivo, with the five-coordinate system demonstrating the greatest metabolic stability among the studied picolinate complex series.
Graphical Abstract

INTRODUCTION
The versatile coordination and redox chemistry of copper, as well as the importance of close control of the endogenous levels of this metal ion, has motivated broad interest from the medicinal inorganic, bioinorganic, and nuclear medicine communities. 1-4 Nuclear medicine applications have flourished because of the ease of production and potential application of copper radioisotopes for nuclear imaging applications, including the development of accessible nuclear production routes for Cu-64 (t1/2 = 12.7 h) as well as the Zn-62/Cu-62 generator.7 However, the lack of long-term clinical success of 64Cu-ATSM, a hypoxia tumor imaging agent, temporarily dampened research efforts to accelerate the advancement of other copper-isotope-based tracers.8 More recently, the availability of the therapeutic nuclide Cu-67 (t1/2 = 61.8 h) has renewed interest in radioactive copper isotopes: preclinical and clinical trials are ongoing to investigate applications of the theranostic Cu-64/Cu-67 isotope pair.9 Other recent medicinal inorganic chemistry applications of copper-based chelators and chelates detail sensing of aberrant, endogenous copper levels prevalent in metabolic disease such as Wilson’s or fatty liver disease,3,12 selective chelation of copper(II) in the CXCR4 receptor, or direct application of copper complexes as potential chemotherapeutics and immune modulators.14
One of the main challenges for the successful application of copper complexes in vivo for imaging or treatment of disease is the propensity of copper(II) to undergo rapid ligand dissociation reactions. The d9 configuration of copper(II) complexes results in pronounced Jahn–Teller distortion for six-coordinate systems and subsequent kinetic lability of axial ligands even in chelate systems.4,15-17 Furthermore, under physiological conditions, the reduction to copper(I) can also occur and results in considerable structural rearrangement, further contributing to structural destabilization and ligand dissociation.1,4,18 The subsequent premature release of the copper results in a lack of efficacy of the corresponding compound. A recent resurgence in the investigation of the antiproliferative activity of copper complexes due to their rich redox chemistry has resulted in the investigation of ternary, nonchelate copper(II) complexes, which may show efficacy in in vitro settings19,20 but will rapidly dissociate in vivo; thus, application of a polydentate chelator is necessary to prevent premature dissociation and release of the metal in biological environments. In addition to chelation, preorganization and enhanced structural rigidity are paramount for in vivo compatible, six-coordinate copper(II) complex species.4
Figure 1 collates some of the previously explored, six-coordinate chelators that have demonstrated high in vivo inertness of the corresponding radiochemical copper species. Table 1 summarizes the corresponding radiolabeling, thermodynamic, electrochemical, and acid stability properties. In spite of the importance of determining the thermodynamic stability constants as comparative benchmarks for characterization, these parameters remain poor predictors of in vivo inertness, as evidenced by the wide variation of established log KML values of select copper(II) complexes (Table 1). Similarly, kinetic inertness in hydrochloric acid (HCl) solutions is also not a reliable predictor of successful in vivo application, as exemplified by much of the promising literature data accumulated with 1,4,7-triazacyclononane (NOTA) and its derivatives.21,22 The six-coordinate, acetic acid functionalized triazamacrocyle exhibits low thermodynamic stability and fast rates of dissociation under strongly acidic conditions compared to structurally stabilized, cross-bridged chelators;1,23 however, [Cu(NOTA)]− derivatives remain among the most extensively and successfully used chelators for imaging applications with copper radioisotopes. As new biological imaging and therapy targets of disease continue emerge, the ability to select chelators with appropriate charge and chemical properties motivates the investigation of new ligand structures with appropriate chemical and biological properties.
Figure 1.

Coordination complex structures of select, previously reported, six-coordinate chelators for copper(II).
Table 1.
Summary of Previously Explored Ligand Designs and Their Corresponding Thermodynamic Stability Constant, Acid Stability, and Electrochemical Behaviora
| chelator | radiolabeling conditions required for >95% radiochemical yield | log KML | acid stability (conditions) | E1/2 (V) |
|---|---|---|---|---|
| DOTA1,5 | 5–90 °C, 30–60 min, pH 5–6 | 22.7 | <3 min (5 M HCl, 90 °C) | −0.74 (irrev) |
| TETA6 | 25 °C, 60 min, pH 5–7 | 21.9 | <4.5 min (5 M HCl, 90 °C) | −0.980 (irrev) |
| CB-TE2A6 | 95 °C, 60 min, pH 5–6 | 27.1 | 254 h (5 M HCl, 90 °C) | −0.88 (quasi-rev) |
| NOTA | 25 °C, 30–60 min, pH 5.5 | 21.6 | <3 min (5 M HCl, 90 °C) | − 0.7 (irrev) |
| Hbispa10 | 25 °C, <1 min, pH 5.5 | 18.9 | n/a | −0.6 (quasi-rev) |
| Diamsar11 | 25 °C, 5–30 min, pH 5.5 | n/a | 40 h (5 M HCl, 90 °C) | 0.198 (irrev) |
| NO3th13,24 | 25 °C, 15 min, pH 5.5 | 18.8 | ~55 h (5 M HCl, 95 °C) | −0.40 (quasi-rev) |
The ligand systems evaluated in this work are included for comparison.
Herein, we investigated a strategy to impart enhanced in vivo inertness to copper (II) chelate complexes that diverts from the construction of preorganized, rigid, cagelike chelators that may be limited in use by their slow complexation kinetics. We proposed that chelation with five donors to produce a trigonal-bipyramidal or square-pyramidal complex structure may be beneficial for stabilization of the corresponding copper complex in vivo. Specifically, we compared the consequences of complexation of copper(II) with five- or six-coordinate, azamacrocycle-based, picolinate-functionalized chelators. Five-coordinate copper(II) complexes form either a square-pyramidal or trigonal-bipyramidal structure, where angular distortion may be observed but variation in the copper-to-ligand bond lengths remains typically below 0.2 Å in structures attained by X-ray crystallography. Picolinate donors provide a good fit for copper(II) with respect to chemical hardness and can further accelerate complexation kinetics. Here, we synthesized three picolinate-functionalized copper chelates with variable coordination geometry and a number of donor atoms (Figure 2) and characterized them using X-ray crystallography, electron paramagnetic resonance (EPR), cyclic voltammetry (CV), and density functional theory (DFT) calculations. We also synthesized proof-of-concept conjugates with a small-molecule inhibitor of the prostate-specific membrane antigen (PSMA). Radiolabeling with Cu-64, followed by in vitro studies on kinetic inertness in plasma and in vivo evaluation of the corresponding complexes in a mouse xenograft tumor model using imaging, biodistribution, and urine metabolite analysis, was carried out to probe our hypothesis.
Figure 2.

Copper complexes of nompa, nompa-ac, and mpatcn ligands.
RESULTS AND DISCUSSION
Ligand Synthesis and Bioconjugation.
In analogy with Bartholomä’s and Tripier’s approaches,13,24 we sought to employ a versatile ligand structure that enables facile functionalization and modulation of the donor type and number. Recently, we have reported the application of the H3mpatcn chelator for the rapid and inert chelation of scandium(III).25 The seven-coordinate ligand environment would, however, likely destabilize the copper(II) complex; thus, we turned our attention to synthesis of the six- and five-coordinate analogues. Synthesis of all three model systems is easily achieved by alkylation of tacn with 1, followed by alkylation with tert-butylbromoacetate and then by subsequent isolation of protected ligand precursors 2–4 with varying donor sets. Subsequent deprotection in trifluoroacetic acid (TFA)/dichloromethane (DCM) produces the three individual chelators Hnompa, H2nompa-ac, and H3mpatcn by way of a shortened, albeit also lower yielding, route compared to that previously published, using tert-butyl protecting groups for all carboxylic acids to enable orthogonal conjugation chemistry (Scheme S1). Copper complexation was achieved using conventional aqueous complexation protocols with copper perchlorate salts. The thermodynamic stability constant (log KML = 21.07) of [Cu(nompa)]+ has already been determined by Tripier, Platas-Iglesias, and co-workers, producing a value very comparable to that of the [Cu(NOTA)]− complex (log KML = 21.9),26 confirming that five-coordinate complexation also leads to high thermodynamic complex stability comparable to that of [Cu(NOTA)]− and other in vivo validated Cu-64 complex architectures (Table 1). Application of tert-butyl protective groups also enabled the orthogonal protection of carboxylic acids for bioconjugation with orthogonally protected Glu-urea-Glu-hexylamine, a targeting vector with high affinity for the PSMA27,28 (Scheme S1) to produce nompa-DUPA, nompa-ac-DUPA, and mpatcn-DUPA (Figure 3).
Figure 3.
Structures of bifunctional, targeting-vector-conjugated chelator structures synthesized in this work.
As a reference compound, NODAGA-DUPA was also synthesized because 64Cu(NOTA) and 64Cu(NODAGA) derivatives have been extensively characterized in vivo in both preclinical and clinical settings and found to perform well with respect to in vivo stability.
Crystallographic Characterization of Copper(II) Complexes.
Single-crystal X-ray diffraction experiments revealed mononuclear five- and six-coordinate copper(II) ions in [Cu(nompa)]+ and [Cu(nompa-ac)], respectively. Views of the crystal structures are presented in Figure 4, and selected bond distances and angles are displayed in Tables S11 and S12. Both crystal structures feature extensive hydrogen-bonding networks between the copper(II) complexes and interstitial water molecules, and partial packing structures with select hydrogen-bonding distances are displayed in Figures S46 and S47.
Figure 4.
Views of the X-ray structures of (A) [Cu(nompa)]+, (B) [Cu(nompa-ac)], and (C) [Cu3(mpatcn)2] with 50% thermal ellipsoid probability. Hydrogen atoms on carbon atoms, along with the ClO4− counterion for [Cu(nompa)]+ and interstitial water molecules for [Cu3(mpatcn)2] are omitted for clarity.
The coordination environment of the copper(II) ion in [Cu(nompa)]+ is best described as distorted square-pyramidal, where the calculated index of trigonality (τ),29 0.43, indicates a geometry that is intermediate between square-pyramidal and trigonal-bipyramidal. The base of the square pyramid consists of mixed nitrogen and oxygen donors, the pyridyl nitrogen [N(1)] and carboxylate oxygen [O(1)] from the picolinate fragment along with tertiary and secondary amines from the triazacyclononane fragment [N(2) and N(4)], while the apical position is comprised of the remaining secondary amine [N(3)]. Unlike square-pyramidal or minimally distorted square-pyramidal structures30,31 typically found in other monosubstituted triazacyclononane chelates (τ = 0.15732 and 0.37433), the primary coordination sphere of copper in [Cu(nompa)]+ displays minimal elongation of the secondary amine in the apical position. The equatorial and apical bond distances fall within a narrow range [1.9007(19)–2.092(2) Å] and support a stabilized copper primary coordination sphere with N(4) positioned away from the fully occupied and formally antibonding dz2 orbital.
Determination of the X-ray structure of [Cu(nompa)]+ provides an opportunity to compare the structural preferences of copper(II) ions featuring monopicolinate pendant donors within different privileged azamacrocycles. Previously, Tripier and co-workers have reported the monopicolinate copper(II) complexes with cyclen, [Cu(do1pa)]+, cyclam, [Cu(te1pa)]+,34 and cross-bridged cyclam, [Cu(cb-te1pa)]+.35 While these complexes and [Cu(nompa)]+ all feature five-coordinate copper(II) ions, the coordination geometry and donor set vary significantly as a result of the macrocycle size and conformation. The smallest azamacrocycles, tacn, [Cu(nompa)]+, and cyclen, [Cu(do1pa)]+, feature N4O1 donor sets and copper(II) geometries that are intermediate between square-pyramidal and trigonal-bipyramidal (τ: [Cu(nompa)]+, 0.43; [Cu(do1pa)]+, 0.46) but contain donor arrangements that are unique to the parent macrocycle. For example, the apical donor of [Cu(nompa)]+ is a secondary amine, while the apical donor of [Cu(do1pa)]+ is the picolinate oxygen. Unlike the smaller azamacrocycles, cyclam, [Cu(te1pa)]+, and cross-bridged cyclam, [Cu(cb-te1pa)]+ favor N5 donor sets with the anionic picolinate oxygen engaging in intramolecular hydrogen bonding with the secondary amine located trans to the picolinate-appended macrocycle nitrogen. Despite the similarity of the donor sets, the ethylene cross-bridge perturbs the macrocycle conformation to favor a distorted trigonal-bipyramidal coordination mode in [Cu(cb-te1pa)]+ (τ = 0.58) rather than the square-pyramidal mode in [Cu(te1pa)]+ (τ = 0.14). These distinct structural preferences ultimately manifest in the electronic structure and observed spectroscopic properties, where [Cu(cb-te1pa)]+ has a dz2 ground state (trigonal-bipyramidal) instead of the dx2–y2 ground state (square-pyramidal).
The coordination environment of the copper(II) ion in [Cu(nompa-ac)] is best described as distorted octahedral, with the picolinate and acetate groups adopting a clockwise configuration. The equatorial plane consists of nitrogen and oxygen donors, a secondary and tertiary amine from the triazacyclononane framework [N(3) and N(4)], the pyridyl nitrogen [N(1)], and acetate [O(3)], while the apical donors consist of a tertiary amine from the triazacyclononane framework [N(3)] and carboxylate oxygen of the picolinate [O(1)]. The distorted octahedral geometry is readily captured by the significant deviation of the apical bond angle, O(1)─Cu(1)─N(2), from ideal [148.20(10) vs 180°]. The apical bond distances are significantly elongated [Cu(1)─N(2) 2.307(3) Å; Cu(1)─O(1) 2.349(3) Å] in comparison to the equatorial bond distances [1.958(3)–2.040(3) Å], which is consistent with the expected Jahn–Teller distortion.
In contrast to the mononuclear complexes crystallized with the nompa and nompa-ac ligands, single crystals obtained from an aqueous solution of mpatcn with excess CuCl2 led to a trinuclear complex, [Cu3(mpatcn)2]. This is similar to the trinuclear species detected during potentiometric and X-ray diffraction studies for a mixed heterocycle/carboxylate chelate based on triazacyclononane, Cu(no2th1tha).24,36 In our hands, we have not been able to obtain X-ray-quality single crystals of the mononuclear complex [Cu(mpatcn)]−; however, the desired mononuclear species is the dominant species in solution with stoichiometric or excess ligand. The formation of a 3:2 metal/ligand complex under radiochemical-labeling conditions is strongly disfavored because typical conditions involve a metal/ligand ratio of 1:100 or 1:1000. Despite limited relevance to solution speciation under labeling conditions, a brief description of the solid-state structure of [Cu3(mpatcn)2] is provided below. Structural models of the pertinent mononuclear complex, [Cu(mpatcn)]−, will be discussed in Computational Studies.
Unlike nompa and nompa-ac, coordinative saturation of the small copper(II) ion in the donor-rich constrained macrocycle, mpatcn, can be accomplished without coordination of all of the donor arms. The three crystallographically unique copper(II) ions adopt distorted square-pyramidal [Cu(1) and Cu(2); τ = 0.141 and 0.119, respectively] and axially distorted octahedral [Cu(3)] geometries. The bases of the square pyramid for Cu(1) and Cu(2) are comprised of two nitrogen donors from the triazacyclonane fragment [N(3), N(4) and N(7), N(8) respectively], a μ2-1,1-carboxylate donor [O(3) and O(11), respectively] and a terminal carboxylate donor [O(5) and O(9), respectively], while the elongated apical site is the remaining tertiary nitrogen [N(2) and N(6), respectively] appending the picolinate donor. The axially elongated octahedron of Cu(3) is comprised of an equatorial plane of nitrogen and oxygen donors from the dissociated picolinate arms from mpatcn bound to Cu(1) and Cu(2) [N(4), O(1) and N(8), O(7), respectively], while the elongated apical sites are μ2-1,1-carboxylates from mpatcn [O(5) and O(9)]. As noted, a similar trinuclear structural motif is observed for the triazacyclononane-based chelates no2th1tha and no3tha; however, the apical sites of these elongated octahedra are oxygen atoms of the perchlorate counterion rather than a bridged carboxylate.36
Computational Studies.
While an initial snapshot of the possible binding modes of nompa, nompa-ac, and mpatcn with copper(II) was provided by single-crystal X-ray diffraction experiments, a detailed investigation of the possible solution structures was pursued using DFT. Geometry optimizations in water using implicit solvation37 were explored at the M06-L38 level of theory (see Computational Studies for further information). Bonding metrics for the minimized structures of [Cu(nompa)]+ (dSPy-[Cu(nompa)]+; τ = 0.34; mean unsigned error (MUE) = 0.059 Å; root-mean-square deviation (RMSD) = 0.131 Å) and [Cu(nompa-ac)] (dO-[Cu(nompa-ac)]; MUE = 0.039 Å; RMSD = 0.217 Å) are in good agreement with those obtained from the single-crystal X-ray diffraction experiments (Table S14) and confirm the reliability of M06-L for our modeling studies.
It is well-known that macrocyclic copper complexes,39-42 including those derived from the triazacyclononane framework,43-45 can access multiple conformations in solution. Even in our simplest picolinate triazacyclononane framework, [Cu(nompa)]+, geometry optimizations provide two energy minima. These minima correspond to two picolinate conformers (Figure 5A): (i) a distorted square-pyramidal structure, dSPy-[Cu(nompa)]+ (τ = 0.34; 0 kcal mol−1) and (ii) a square-pyramidal structure, SPy-[Cu(nompa)]+ (τ = 0.07; +1.5 kcal mol−1). The two conformers arise from a slight canting of the picolinate unit in dSPy-[Cu(nompa)]+ and SPy-[Cu(nompa)]+, which is captured by the Cu(1)─N(2)─C(7)─C(6) torsion angles of −20.20° and +17.12°, respectively. The base of the square pyramid found in SPy-[Cu(nompa)]+ is comprised of two nitrogen donors of the triazacyclononane fragment [N(2) and N(3)] and both picolinate donors [N(4) and O(1)], where the remaining secondary amine [N(1)] occupies the apical site [elongated Cu─N(1)]. Notably, the picolinate arm in both isomers remains completely associated and, unlike related cyclam and cross-bridged cyclam derivatives (te1pa and cb-te1pa), does not engage in intramolecular hydrogen-bonding interactions with secondary amines of the azamacrocycle.34,35 Despite being sterically accessible, water coordination at SPy-[Cu(nompa)]+ to form [Cu(nompa)(OH2)]+ is unfavorable by ~+4.4 kcal mol−1 (Figure S51). Despite the conserved donor sets in the square-pyramidal (SPy) and distorted square-pyramidal (dSPy) isomers, one would expect to observe these readily spectroscopically (vide infra; Spectroscopic Studies).
Figure 5.
Two lowest-energy-calculated solution structures for (A) [Cu(nompa)]+, (B) [Cu(nompa-ac)], and (C) [Cu(mpatcn)]−. dSPy: distorted square-pyramidal. SPy: square-pyramidal. dO: distorted octahedral. Energies relative to the lowest-energy structures are given in parentheses (kcal mol−1). See the Supporting Information for the bond metrics and higher-energy structures.
The addition of a single pendant acetate arm leads to distinct conformational preferences in [Cu(nompa-ac)] (Figure 5B). Geometry optimizations of [Cu(nompa-ac)] identified two conformers that varied in their clockwise or counterclockwise arrangement of the acetate arm: (i) an axially distorted six-coordinate copper(II) ion, dO-[Cu(nompa-ac)], and (ii) a five-coordinate distorted square-pyramid copper(II) ion, dSPy-[Cu(nompa-ac)]. The preferred, clockwise arrangement, dO-[Cu(nompa-ac)] (0 kcal mol−1), adopts an axially distorted octahedral geometry that is in close agreement with the X-ray structure (vide supra). The disfavored, counterclockwise arrangement, dSPy-[Cu(nompa-ac)] (+3.3 kcal mol−1), adopts a five-coordinate distorted square-pyramidal geometry (τ = 0.43), where the picolinate oxygen [O(1)] is dissociated. Similar picolinate binding modes were also observed by Tripier and co-workers in their modeling studies of the mixed picolinate–picolyl triazacyclononane systems Cu(NO3PA)−, Cu(NO2PA1PY), and Cu(NO1PA2PY)+.43 The base of the square pyramid is comprised of three nitrogen donors [N(2), N(3), and N(4)] and a pendant acetate arm [O(3)], where the remaining secondary amine [N(1)] occupies the apical site [elongated Cu─N(1)].
The introduction of two pendant acetate arms presents several potential structures for [Cu(mpatcn)]−. Geometry optimization of [Cu(mpatcn)]− revealed two low-energy axially distorted six-coordinate structures that differed by ~1.1 kcal mol−1 (Figure 5C). In the lowest-energy structure, A-[Cu(mpatcn)]−, one of the pendant acetate arms is dissociated, while the coordinated picolinate and acetate donors adopt a clockwise binding mode. In the higher-energy structure, B-[Cu(mpatcn)]− (+1.1 kcal mol−1), both acetate arms are bound, while the picolinate is significantly canted and the picolinate oxygen [O(1)] is dissociated, similar to dSPy-[Cu(nompa-ac)]. Several other monomeric [Cu(mpatcn)]− structures could be optimized (e.g., picolinate dissociated, C-[Cu(mpatcn)]−, or two acetate arms dissociated, D-[Cu(mpatcn)]−) but were much higher in energy (≥+8 kcal mol−1; Figures S56 and S57).
In summary, our modeling studies have uncovered that additional conformers must be considered for copper(II) ions supported by the monopicolinate triazacyclononane ligands (nompa, nompa-ac, and mpatcn) in solution. Both [Cu(nompa)]+ and [Cu(mpatcn)]− have two minimum-energy conformers with relative energy differences of less than 2 kcal mol−1, which suggests that multiple species may be present at room temperature in solution. Furthermore, the primary coordination sphere of A-[Cu(mpatcn)]− is strikingly similar to that of [Cu(nompa-ac)] (Table S14) and suggests that the two complexes may display similar solution properties (e.g., EPR, UV–vis, and electrochemistry).
Spectroscopic Studies.
Room temperature electronic absorption spectra of [Cu(nompa)]+, [Cu(nompa-ac)], and [Cu(mpatcn)]− were recorded in deionized water at the natural pH of the redissolved copper(II) complexes (pH 5.0; Figures S24-S26). The complexes display two prominent features in the UV–vis region (Table 2): a strong and sharp absorption originating from the π–π* transition of the picolinate fragment (λmax = 270 nm; ε ~ 4 × 103 M−1 cm−1),34,46 along with a weaker and broad absorption band characteristic of the d–d transition of copper(II) ions (λmax = 650–667; ε = 74–128 M−1 cm−1). While all of the complexes display significant absorption into the NIR, we were only able to resolve the peak of a lower-energy d–d transition at 954 nm (65 M−1 cm−1) in the case of [Cu(mpatcn)]− because of the resolution of our spectrophotometer. These observations are consistent with elongated axial structures such as axially elongated octahedral and square-pyramidal geometries;43,47-49 however, the use of additional spectroscopic techniques are needed to resolve the solution structure of these complexes.
Table 2.
Summary of Copper Complex Properties with Respect to Structural and Spectroscopic Characterization and Electrochemical Stability
| [Cu] | geometrya | g z b | g x b | g y b | A z c | A x c | A y c | λmax(d–d) (nm) | ε (M−1 cm−1) | E1/2d (V)e |
|---|---|---|---|---|---|---|---|---|---|---|
| [Cu(nompa)]+ | SPyf | 2.265 | 2.069 | 2.069 | 164 | 12 | 12 | 667 | 128 | −0.555 |
| dSPyf | 2.237 | 2.049 | 2.049 | 155 | 14 | 14 | 667 | 128 | −0.555 | |
| [Cu(nompa-ac)] | dO | 2.271 | 2.056 | 2.057 | 170 | 11 | 11 | 665 | 79 | −0.680 |
| [Cu(mpatcn)]− | dO (A)g | 2.269 | 2.055 | 2.055 | 159 | 14 | 14 | 655 | 117 | −0.685 |
| dSPy (B)g | 2.233 | 2.047 | 2.047 | 168 | 20 | 20 | 655 | 117 | −0.685 |
dSPy: distorted square-pyramidal. Spy: square-pyramidal. dO: distorted octahedral.
Obtained from simulations performed with EasySpin.
×10−4 cm−1.
Epc + Epa/2; 100 mV s−1.
V vs Ag/AgCl.
1:1 ratio.
3:1 ratio (A/B).
EPR spectroscopy was performed at 77 K on degassed ~10 mM frozen solutions (50:50 H2O/ethylene glycol) of [Cu(nompa)]+, [Cu(nompa-ac)], and [Cu(mpatcn)]− (Figure 6 and Table 2). Although the number of species and effective coordination geometry observed in solution varied for all three complexes, axial spectra characteristic of tetragonal complexes with dx2–y2 ground states were observed for all samples (ge < gx ~ gy < gz). Furthermore, hyperfine coupling (A) to the copper nucleus () was clearly resolved for three of the four lines expected at low field, while superhyperfine coupling to ligand nitrogen atoms was not observed in any of the samples.
Figure 6.
Experimental (—) and simulated (- - -) X-band EPR spectra (~10 mM, 50:50 H2O/ethylene glycol, 77 K) of [Cu(nompa)]+ (black, bottom), [Cu(nompa-ac)] (blue, middle), and [Cu(mpatcn)]− (red, top) collected at 77 K.
The EPR spectrum of [Cu(nompa)]+ clearly indicated the presence of two paramagnetic species because two sets of gz values with distinct Az were observed (Figure 6). The presence of two unique copper(II) species was further corroborated from simulations of the experimental EPR spectrum using EasySpin,50 where excellent agreement between the experiment and simulation was only achieved by considering two copper(II) species in a ~1:1 molar ratio (Table 2). This was consistent with our crystallographic and computational modeling studies (vide supra), where the two lowest-energy conformers of [Cu(nompa)]+, dSPy-[Cu(nompa)]+, and SPy-[Cu(nompa)]+ differed by only 1.5 kcal mol−1. Therefore, we have assigned the two paramagnetic species as dSPy-[Cu(nompa)]+ and SPy-[Cu(nompa)]+.
In contrast, the EPR spectrum of [Cu(nompa-ac)] revealed a single paramagnetic species (gz = 2.271; gx and gy ~ 2.056). This is consistent with our expectations from our modeling studies, where the two minimum-energy structures, dO-[Cu(nompa-ac)]and dSPy-Cu(nompa-ac), differed by ~3 kcal mol−1. Both the X-ray, [Cu(nompa-ac)], and lowest-energy DFT, dO-[Cu(nompa-ac)], structures feature an N3O equatorial donor set that shares similarities with the square-pyramidal nompa isomer, SPy-[Cu(nompa)]+. Therefore, we have assigned the single paramagnetic species as dO-[Cu(nompa-ac)], the species identified by X-ray crystallography and the lowest-energy structure predicted by DFT.
Similar to [Cu(nompa)]+, the experimental and simulated EPR spectra of [Cu(mpatcn)]− supported two paramagnetic species in solution (~3:1 ratio). The observation of two paramagnetic species was consistent with our computational modeling studies (vide supra), where the two lowest-energy conformers of [Cu(mpatcn)]−, A-Cu(mpatcn)− and B-[Cu(mpatcn)]−, differed by only 1.1 kcal mol−1. The g values for the major paramagnetic species of [Cu(mpatcn)]− were nearly identical with that of [Cu(nompa-ac)]. As noted in our modeling studies, the primary coordination spheres of [Cu(nompa-ac)] and A-[Cu(mpatcn)]− were highly conserved. As such, we have assigned the major (gz = 2.269; gx and gy = 2.055) and minor (gz = 2.233; gx = 2.047 and gy = 2.047) paramagnetic species as A-[Cu(mpatcn)]− and B-[Cu(mpatcn)]−, respectively.
Electrochemical Studies.
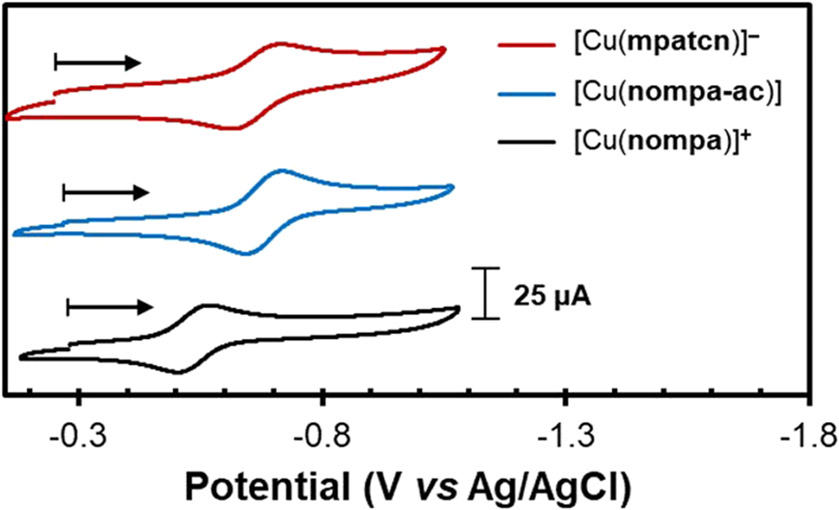
Facile reduction of copper(II) to copper(I) in aqueous solutions by typical bioreductants (−0.4 V vs NHE)4 can lower the in vivo stability due to copper dissociation through transchelation, especially when the corresponding copper(I) species is poorly stabilized by the coordination environment. 1,18 CV can provide critical insight into both the thermodynamics and kinetics of copper(II) redox events to help further understand behavior in vivo.1,18,24,51 CV of [Cu(nompa)]+, [Cu(nompa-ac)], and [Cu(mpatcn)]− was performed at pH 5.0 (natural pH of the redissolved compounds) using sodium perchlorate as the supporting electrolyte (Figure 7 and Table 2). The scan-rate-dependent CV curves of the copper(II/I) couple, Randles–Sevcik plots, and full scans are provided in Figures S28-39.
Figure 7.
Cyclic voltammograms of [Cu(nompa)]+ (black, bottom), [Cu(nompa-ac)] (blue, middle), and [Cu(mpatcn)]− (red, top) collected at 100 mV s−1. Conditions: 1 mM copper, 100 mM sodium perchlorate, 25 °C, and pH 5.
The three complexes featured quasi-reversible copper(II/I) redox potentials that spanned a ~130 mV range (E1/2 = −555 to −685 mV vs Ag/AgCl at 100 mV s−1), where [Cu(nompa)]+ displays the most positive and reversible copper(II/I) couple (E1/2 = −0.555 V; ΔEp = 64 mV; Ipc/Ipa = 1.01). The ligand geometry and donor type have a profound effect on the observed copper(II/I) couple.52-54 Related copper(II) monopicolinate complexes of cyclen (E1/2 = −0.729 V), cyclam (E1/2 = −0.804 V), and cross-bridged cyclam (E1/2 = −0.815 V) display much more negative copper(II/I) potentials (174–260 mV) than [Cu(nompa)]+ (i.e., triazacyclononane).34,35 Despite a more positive copper(II/I) couple, [Cu(nompa)]+ displays improved electrochemical reversibility and resistance to demetalation on the CV time scale (seconds). Without appropriate kinetic stabilization, copper(I) formed upon the reduction of copper(II) could demetallate and rapidly form copper(0) and copper(II) through disproportionation in water.55,56 Such a process would be readily apparent by the observation of an additional anodic event at ~+0.1 V versus Ag/AgCl, which corresponds to the oxidation of copper(0) to free copper(II).56,57 Copper demetalation on the CV time scale was reported for the five-coordinate copper(II) monopicolinate complexes with cyclen and cyclam frameworks, [Cu(do1pa)]+ and [Cu(te1pa)]+,34 but notably absent for [Cu(nompa)]+. This further highlights the importance of macrocycle size/conformation with the appended picolinate donor, where the tacn derivative, nompa, provides excellent kinetic stabilization of both copper(II) and copper(I) redox states (Figure S30).
The addition of pendant acetate arms in [Cu(nompa-ac)] and [Cu(mpatcn)]− shifted the copper(II/I) redox couple to more negative potentials (E1/2 = −0.680 V; E1/2 = −0.685 V) but also decreased the electrochemical reversibility (ΔEp = 74 and 103 mV). The similar CV behaviors for [Cu(nompa-ac)] and [Cu(mpatcn)]− were consistent with our earlier modeling and EPR studies (vide supra), which indicated that the largely conserved primary coordination sphere of A-[Cu(mpatcn)]−, the major species of [Cu(mpatcn)]− in solution, and [Cu(nompa-ac)] resulted in similar properties in solution. The decreased electrochemical reversibility led to significant copper demetalation, as evidenced by the strong anodic current at +0.1 V versus Ag/AgCl [copper(0/II)]. While suitable thermodynamic stabilization also needs to be considered, the improved electrochemical reversibility of [Cu(nompa)]+ compared to the more thermodynamically stabilized [Cu(nompa-ac)], [Cu(mpatcn)]−, cyclen, and cyclam derivatives underscores the importance of kinetics when considering the stability of copper(II) under physiologically relevant conditions.
Radiolabeling.
In order to competently assess the complex inertness and in vivo compatibility, we proceeded to radiochemical labeling with Cu-64 using PSMA-functionalized derivatives. First, optimized radiolabeling properties were assessed by determination of the optimal pH, radiolabeling temperature, reaction time, and apparent molar activity (Table 3). Each conjugate complexed Cu-64 at pH 5.5 and room temperature rapidly, with apparent molar activities ranging from 0.02 to 0.11 Ci μmol−1. The observed apparent molar activities remain 1–2 orders of magnitude below values measured for the NODAGA derivative, which produced an apparent molar activity of 2 Ci μmol−1 under the same conditions (Table 3 and Figures S40 and S41) possibly because of the diminished metal-ion selectivity or higher proton affinity of picolinate derivatives, which result in diminished radiochemical complexation rates with 64CuII. 64Cu(nompa)-DUPA, 64Cu(nompa-ac)-DUPA, 64Cu(mpatcn)-DUPA, and 64Cu(NODAGA)-DUPA complexes were purified using reverse-phase chromatography to remove any residual, uncomplexed 64CuII; the product complex was eluted with acetonitrile (MeCN) following a water wash to remove uncomplexed Cu-64. Following isolation in the product fraction, MeCN was removed, and the product was reconstituted and formulated in sterile phosphate-buffered saline (PBS) for stability, in vitro, and in vivo studies.
Table 3.
Summary of the Radiochemical-Labelling Conditions and Properties of the Investigated Picolinate and NOTA Conjugate Cu-64 Complexes
| complex | radiolabeling conditions at 23 °C |
radiochemical yield (%) |
apparent molar activity (Ci μmol−1) |
formulation stability after 12 h (PBS; %) |
|---|---|---|---|---|
| 64Cu(NODAGA)-DUPA | 10 min, pH 5.5 | 99 | 2 | 93.5 |
| 64Cu(nompa)-DUPA | 5 min, pH 5.5 | 99 | 0.02 | 88.7 |
| 64Cu(nompa-ac)-DUPA | 10 min, pH 5.5 | 92 | 0.06 | 88.5 |
| 64Cu(mpatcn)-DUPA | 10 min, pH 5.5 | 98 | 0.11 | 95.6 |
In Vitro Inertness and Binding Affinity to PSMA.
In addition to assessment of the stability in the PBS formulation, we also evaluated the copper-64 complex integrity using a 100× ethylenediaminetetraacetic acid (EDTA) challenge at pH 5.5. All complexes remained at least >88% intact when formulated in PBS, with stability differing only minimally (Table S6 and Figure S43); however, stark differences were observed in the excess EDTA challenge experiment, revealing a ranking of the complex stability under these conditions as follows from highest to lowest: 64Cu(NODAGA)-DUPA > 64Cu(mpatcn)-DUPA > 64Cu(nompa-ac)-DUPA > 64Cu(nompa)-DUPA (Table S5 and Figure S42). These results indicate significantly diminished kinetic inertness of the picolinate derivatives to transchelation with the EDTA chelator, especially when compared with the NOTA derivative.
We proceeded further with cell binding assays to determine the specificity of these copper-64-labeled compounds for cells expressing the PSMA prior to targeted in vivo imaging. Specifically, we investigated the selectivity of the radiolabeled compounds to bind PSMA-expressing cancer cells. For this purpose, we employed the transgenic PC-3 PiP/flu cell lines, a widely established and characterized model for the PSMA-expressing cell line and its corresponding background, nonexpressing control. Figure 8 shows the data obtained for all four compounds tested; each conjugate retains target specificity to PSMA, as demonstrated by the enhanced binding in the PSMA-expressing, PC-3 PiP cell line. Of note, the 64Cu(NODAGA)-DUPA conjugate exhibits considerably decreased nonspecific binding compared to all picolinate-based conjugates.
Figure 8.
Cell binding to PC-3 PiP and PC-3 flu cell lines for each copper-64 complex formed, indicating that target specificity is maintained for each conjugate.
In Vivo Studies.
Next, we assessed the ability of the copper-64-radiolabeled constructs to localize in a target of interest in vivo. We administered radiolabeled, purified, and formulated conjugates to mice bearing PSMA+ (PC-3 PiP) and PSMA− (PC-3 Flu) tumor xenografts on the right and left flanks, respectively.27,58 Mice were imaged at 90 min postinjection using positron emission tomography–computed tomography (Figure S44), followed by euthanasia at 120 min postinjection for biodistribution and urine metabolite analysis.
The animal studies reveal that 64Cu(nompa)-DUPA, 64Cu(nompa-ac)-DUPA, 64Cu(mpatcn)-DUPA, and 64Cu(nODAGA)-DUPA all exhibit greater uptake in the PSMA+ cancer xenografts than in the PSMA− tumors; however, all picolinate derivatives exhibit high hepatic uptake (ranging from 10.9 to 15.9% ID g−1; Figure 9; not statistically significant), which is typically indicative of copper complex degradation and concomitant copper-64 release. The difference in the lipophilicity of these compounds likely contributes to the observed trend in the hepatic uptake; indeed, log D7.4 measurements conducted using the shake-flask method show a measured log D7.4 of 64Cu(NODAGA)-DUPA of −2.21 ± 0.35, in comparison with −1.66 ± 0.26 for 64Cu(nompa)-DUPA and the siginificantly more lipophilic 64Cu(nompa-ac)-DUPA and 64Cu(mpatcn)-DUPA derivatives, measured at −1.19 ± 0.42 and −1.17 ± 0.47, respectively (Table S4).
Figure 9.
Comparative biodistribution 2 h postinjection (p.i.) of four copper-64 complexes analyzed in a PSMA± tumor model using the PC-3 PiP/ flu cell lines (n = 5–6). While all compounds exhibit target-specific tumor uptake, the off-target uptake in PSMA-nonexpressing tumor tissue as well as the liver is increased for picolinate derivatives. Statistical significance determined using the Holm–Sidak method, with α = 0.05.
This is further corroborated by significant copper-64 uptake in the nontarget tumor observed for all picolinate derivatives, while the 64Cu(NODAGA)-DUPA complex exhibits not only statistically significant lower liver uptake (3.8% ID g−1) but also a vastly improved target-to-nontarget tumor uptake ratio. On the basis of the biodistribution data, we can rank the estimated in vivo compound inertness based on the hepatic uptake and target specificity as follows: 64Cu(NODAGA)-DUPA > 64Cu(nompa)-DUPA > 64Cu(nompa-ac)-DUPA > 64Cu(mpatcn)-DUPA.
In addition to the biodistribution panel, renally cleared metabolites were also collected and analyzed using reverse-phase radio-high-performance liquid chromatography (radio-HPLC; Figure S45). The collected chromatograms showed that over 50% of 64Cu(nompa)-DUPA remained intact in the urine, while free copper-64 elutes with the solvent front (rt = 2.0–2.5 min). In contrast, all other compounds investigated, including 64Cu(NODAGA)-DUPA, remained less than 10% intact (Table S9 and Figure S45). For the picolinate derivatives, this reflects the observed trend in the hepatic uptake of copper-64 well. On the basis of the log D7.4 measurements conducted, we cautiously hypothesize that the enhanced lipophilicity and rich redox chemistry of pyridyl and picolyl derivatives predispose these conjugates to metabolic processing in the liver, while the hydrophilic 64Cu(NODAGA)-DUPA conjugate more effectively evades the liver by way of renal clearance. Nevertheless, the chemical stability trends predicted by our structural, spectroscopic, and electrochemical characterization studies prevail, with the distorted square-pyramidal Cu(nompa) chelate conjugate exhibiting an improved in vivo distribution and metabolic stability profile.
CONCLUSIONS
The stabilization of coordinatively labile metal ions in dynamic, biological environments remains an ongoing challenge in the field of medicinal inorganic chemistry. Controlling the speciation of copper(II) is particularly difficult because both redox and ligand dissociation-mediated processes can destabilize the corresponding coordination complexes. We have recently identified triazamacrocycle-based picolinates as a versatile chelator platform for rare earths.25 Here, we show that minor changes of the donor environment provided by triazamacrocycle-based picolinates produce copper(II) complexes that significantly differ in structural, spectroscopic, and electrochemical behaviors. Specifically, we have demonstrated that the five-coordinate, distorted square-pyramidal complex [Cu(nompa)]+ displays improved kinetic stability compared to the distorted octahedral [Cu(nompa-ac)] and [Cu(mpatcn)]− complexes in electrochemical experiments. Despite a ~100 mV shift of the copper(II/I) couple to more positive potentials compared to [Cu(nompa-ac)] and [Cu(mpatcn)]−, [Cu(nompa)]+ displays excellent electrochemical stability and highlights the importance of kinetics and stabilization of both copper(II) and copper(I) states to resist demetalation or transchelation under physiogically relevant conditions. We believe that this stabilization originates from the intermediate five-coordinate geometry found in [Cu(nompa)]+, which, unlike the axially distorted structures found in [Cu(nompa-ac)] and [Cu(mpatcn)]−, results in minimal elongation of the apical bond.
Nonfunctionalized copper complexes typically clear rapidly before extensive metabolic degradation can occur. To accurately assess the consequences of the structural changes to complex geometry in vivo, we synthesized proof-of-concept bifunctional versions of the three chelators and appended them to a peptide-based targeting vector DUPA with high affinity to the PSMA. The corresponding conjugates were radiolabeled with copper-64 and assessed for their in vitro binding affinity, biodistribution, and metabolic stability in tumor-bearing mice. Our biodistribution and metabolite analysis results affirmed trends predicted by our structural, spectroscopic, and electrochemical experiments of the nonfunctionalized complexes, with the 64Cu(nompa)-DUPA complex exhibiting the highest metabolic integrity in the urine compared with the 64Cu(nompa-ac)-DUPA and 64Cu(mpatcn)-DUPA complexes. The charge of the coordination complexes can play an integral role in the biodistribution behavior of radiopharmaceuticals. However, in this instance, the anionic, neutral, and cationic complexes did not indicate a distribution behavior that could have been traced to charge discrepancies.
Interestingly, the trends in the metabolic stability did not correlate with the results from EDTA challenge experiments conducted on the formed radiochemical complexes. It is important to note that while ex vivo ligand exchange experiments can forecast in vivo inertness in some instances, they are not reliably predictive. Overall, the picolinate derivatives studied herein exhibit enhanced, nonspecific binding to off-target tissues and hepatic uptake in comparison to the gold-standard conjugate 64Cu(NODAGA)-DUPA. On the basis of our results, we can conclude that copper-labeled nompa, nompa-ac, and mpatcn derivatives are not suitable for future applications aimed at the targeted delivery of copper(II) in vivo.
Our study shows that while careful evaluation of the coordination environment using the analytical toolkit of coordination chemistry can predict trends with respect to the biological behavior, a rigorous and critical in vivo assessment is required to ultimately qualify the suitability of novel coordination complex constructs for biomedical applications.
EXPERIMENTAL SECTION
General Methods.
All starting materials were purchased from Acros Organics, Alfa Aesar, Macrocyclics, Sigma-Aldrich, or TCI America and used without further purification. NMR spectra (1H and 13C) were collected on Bruker 700 MHz Advance III and 500 or 400 MHz instrument at 25 °C and processed using TopSpin 3.5pl7 (Figures S1-S23). Chemical shifts are reported as parts per million. Low-resolution electrospray ionization (ESI) mass spectrometry was carried out at the Stony Brook University Institute for Chemical Biology and Drug Discovery (ICB&DD) Mass Spectrometry Facility with an Agilent LC/MSD spectrometer. Preparative HPLC was carried out using a Shimadzu HPLC-20AR equipped with a Binary Gradient, pump, UV–vis detector, and manual injector on a Phenomenex Luna C18 column (250 mm × 21.2 mm, 100 Å, AXIA packed). Method A (preparative purification method): A = 0.1% TFA in water, B = 0.1% TFA in MeCN. Gradient: (A) 0–5 min, 95%; (B) 5–24 min, 5–95%. Benzyl tert-butyl 2-(methylsulfonyloxy)-glutarate and tert-butyl 6-(bromomethyl)-2-pyridinecarboxylate (1) were synthesized according to previously published procedures.25
Synthesis and Characterization of Compounds 11, 13, and 18.
Compound H3(mpatcn)-DUPA was prepared according to our previously published report.25 Full spectral assignments of 11, 13, and 18 are provided within the Supporting Information, as well as a detailed description of the synthesis and characterization of precursors and building blocks (Figures S1-S23).
(2S)-2-({[(1S)-1-Carboxy-3-{[5-(2-{4-[(6-carboxypyridin-2-yl)-methyl]-1,4,7-triazonan-1-yl}35 acetamido)pentyl]carbamoyl}-carbamoyl}propyl]carbamoyl}amino)pentanedioic Acid [H-(nompa)-DUPA; 11].
Compound 10 (0.0067 g, 0.007 mmol, 1 equiv) was dissolved in a solution of 2:1 TFA/DCM (1 mL). The reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo, and 11 was purified using reverse-phase chromatography (method A, product elutes at 35% B, rt = 9.79) and isolated as an off-white solid (0.0044 g, 0.006 mmol, 82%). 1H NMR (700 MHz, CD3OH): δ 8.15 (d, 1H, H-18), 8.07 (t, 1H), 7.65 (d, 1H), 4.31 (m, 2H), 3.78 (m, 2H), 3.57 (m, 2H), 2.88–3.39 (m, 16H), 2.44 (m, 2H, H-38), 2.33 (m, 2H), 2.18 (m, 2H), 1.90 (m, 2H), 1.38–1.71 (4H), 1.29 (m, 2H). 13C NMR (175 MHz, CD3OH): δ 175.0, 174.6, 174.5, 174.4, 173.6, 173.5, 166.2, 158.7, 147.3, 139.2, 126.5, 124.2, 59.1, 57.6, 56.1, 53.1, 52.2, 52.0, 49.7, 49.0, 43.1, 39.2, 38.7, 38.4, 31.8, 29.6, 28.5, 28.4, 27.3, 26.7, 23.7. Calcd monoisotopic mass for 11 (C33H50N8O13): 766.35. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 709.351531. Found: m/z 709.3514.
(2S)-2-({[(1S)-1-Carboxy-3-[(5-{2-[4-(carboxymethyl)-7-[(6-carboxypyridin-2-yl)methyl]-1,4,7-triazonan-1-yl]acetamido}pentyl)-carbamoyl]propyl]carbamoyl}amino)pentanedioic Acid [H2(nompa-ac)-DUPA; 13].
Compound 12 (0.0060 g, 0.006 mmol, 1 equiv) was dissolved in a solution of 2:1 TFA/DCM (1 mL). The reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo, and 13 was purified using reverse-phase chromatography (method A, product elutes at 40% B, rt = 9.85 min) and isolated as an off-white solid (0.0044 g, 0.006 mmol, 96%). 1H NMR (700 MHz, D2O): δ 8.16 (m, 1H), 8.12 (m, 1H), 7.80 (m, 1H), 4.17 (m, 1H), 4.07 (m, 1H), 3.87 (m, 2H), 3.77 (m, 2H), 3.45 (m, 2H), 3.29 (m, 3H), 3.22 (m, 2H), 3.19 (m, 2H), 2.88–3.13 (m, 9H), 3.22 (m, 2H), 3.19 (m, 2H), 2.41 (m, 2H), 2.25 (m, 2H), 2.06 (m, 2H), 1.89 (m, 2h), 1.65 (m, 4h), 1.23 (m, 2H). Calcd monoisotopic mass for 11 (C33H50N8O13): 766.35. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 767.3570. Found: m/z 767.3579.
(2R)-2-({[(1R)-3-[(5-{4-[4,7-Bis(carboxymethyl)-1,4,7-triazonan-1-yl]-4-carboxybutanamido}pentyl)carbamoyl]-1-carboxypropyl]-carbamoyl}amino)pentanedioic acid [H3(NODAGA)-DUPA; 18].
17 was dissolved in a solution of 2:1 TFA/DCM (1 mL). The reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo, and 18 was purified using reverse-phase chromatography (method A, product elutes at 25% B, rt = 6.34 min) and isolated as an off-white solid (0.0049 g, 0.006 mmol, 80%). 1H NMR (700 MHz, D2O): δ 4.16 (m, 1H), 4.07 (m, 1H), 3.84 (m, 4H), 3.52 (m, 1H), 2.95–3.26 (m, 16H), 2.40 (m, 2H), 2.33 (m, 2H), 2.24 (m, 2H), 2.05 (m, 3H), 1.94 (m, 1H), 1.84 (m, 2H), 1.37 (m, 4H), 1.21 (m, 2H). 13C NMR (175 MHz, D2O): 177.2, 175.4, 175.0, 174.9, 174.6, 176.2, 159.1, 63.2, 61.2, 55.4, 55.2, 54.3, 52.6, 52.5, 50.9, 50.8, 39.2, 32.7, 32.7, 30.0, 27.9, 27.8, 27.1, 26.2, 24.3, 23.3. Calcd monoisotopic mass for (C31H51N7O15): 761.34. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 762.3516. Found: m/z 762.3522.
Preparation of Nonradioactive Copper Complexes.
Caution! Perchlorate salts of metal complexes with organic ligands may present an explosion hazard and should be handled with care.
[Cu(nompa)]+.
Cu(ClO4)2·6H2O (0.0231 g, 0.062 mmol) was added to a solution of Hnompa (0.0165 g, 0.062 mmol) in water. The pH of the solution was adjusted to 5 with 0.1 M KOH. The solution was complexed for 1 h at room temperature with intermittent vortexing. The blue solution was loaded onto a C18 Sep-Pak cartridge, and washed with 1 mL of deionized water to remove unbound copper salts, and the product was eluted with 2 mL of 1:3 ethanol/water. Fractions containing the product were collected, and the solvent was removed in vacuo to afford a blue solid. The solid was resuspended in deionized water, and crystals were obtained by slow diffusion in THF. Calcd monoisotopic mass for (C13H17CuN4O2H): 325.05. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 326.07985. Found: m/z 326.0799. λmax (ε) = 270 (4520), 667 (128).
[Cu(nompa-ac)].
Cu(ClO4)2·6H2O (0.0259 g, 0.070 mmol) was added to a solution of H2nompa-ac (24.8 0.077) in water. The pH of the solution was adjusted to ~5 with 0.1 M KOH. The solution was complexed at 40 °C overnight. The solution was loaded onto a C18 Sep-Pak cartridge, washed with 1 mL of deionized water, and eluted with 2 mL of 1:3 ethanol/deionized water. The solvent was removed in vacuo to afford a blue solid. The solid was resuspended in deionized water, and crystals were obtained by slow diffusion in THF. Calcd monoisotopic mass for (C15H19CuN4O4H): 383.06. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 384.085329. Found: m/z 384.0852. λmax (ε) = 270 (4130), 665 (79).
Cu(mpatcn).
Cu(ClO4)2·6H2O (0.0202 g, 0.077 mmol) was added to a solution of H3mpatcn (0.0267 g, 0.070 mmol) in water. The pH of the solution was adjusted to ~5 with 0.1 M KOH. The solution was complexed at 40 °C for 18 h. The solution was loaded onto a C18 Sep-Pak cartridge, washed with 5 mL of deionized water, and eluted with 2 mL of MeCN. The solvent was removed in vacuo to afford a blue solid. The solid was resuspended in deionized water, and crystals were obtained by layering in methanol. Calcd monoisotopic mass for (C17H22CuN4O6): 441.08. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 442.0908. Found: m/z 442.0916. λmax (ε) = 270 (4780), 655 (74).
Cu(nompa-ac)-DUPA.
13 in deionized water (10 μL) and CuCl2 in deionized water (5 μL) were added to a 0.25 M solution of ammonium acetate at pH 5.5 (100 μL). The resulting solution was heated at 40 °C for 30 min. Complex formation was confirmed by mass spectrometry, followed by purification by C18 Sep-Pak. Calcd monoisotopic mass for (C33H48CuN8O13): 827.26. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 828.2710. Found: m/z 828.2721. Rt of a copper-radiolabeled product on HPLC [gradient: (A) H2O, 0.1% TFA; (B) CH3CN; 5–100% B gradient 20 min]: 6.21 min.
Cu(NODAGA)-DUPA.
Calcd monoisotopic mass for (C31H48CuN7O15): 822.26. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 823.2655. Found: m/z 823.2661. Rt of copper-labeled product on HPLC [gradient: (A) H2O, 0.1% TFA; (B) CH3CN; 5–100% B gradient 20 min]: 6.05 min.
Cu(nompa)-DUPA.
Calcd monoisotopic mass for (C31H46CuN8O11): 769.26. Found: 770.4 ([M + H]+). HR-MS (H2O, ESI+). Calcd for [M]+: m/z 770.265479. Found: m/z 770.2647. Rt of copper-labeled product on HPLC [gradient: (A) H2O, 0.1% TFA; (B) CH3CN; 5–100% B gradient 30 min]: 9.70 min.
Cu(mpatcn)-DUPA.
Calcd monoisotopic mass for (C36H51CuN8O15H): 899.28. HR-MS (H2O, ESI+). Calcd for [M + H]+: m/z 900.2921. Found: m/z 900.2975. Rt of copper-labeled product on HPLC [gradient: (A) H2O, 0.1% TFA; (B) CH3CN; 5–100% B gradient 20 min]: 5.95 min.
Determination of log D7.4.
The distribution coefficient at pH 7.4 (log D7.4) was determined by measuring the concentration of Cu(nompa)-DUPA, Cu(nomp-ac)-DUPA, Cu(NODAGA)-DUPA, or Cu(mpatcn)-DUPA concentration distribution in 1-octanol and PBS. A 50 μL sample of the copper complex was added to a vial containing 250 μL of PBS and 300 μL of 1-octanol. After vortexing, the vial was placed on a shaker for 1 h. The vial was centrifuged for 5 min, 100 μL of the aqueous layer was removed, and the concentration of the complex layer was measured by UV–vis HPLC. Assay was performed in triplicate for each complex.
X-ray Crystallography.
A sample was removed from the mother liquor, rinsed with acetone, and then transferred covered in paratone oil to a glass slide, where it was evaluated and mounted with the assistance of an optical microcope. X-ray reflection intensity data were collected on a Bruker D8 Quest with a Photon 100 CMOS detector employing graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at a temperature of 173(1) K. Rotation frames were integrated using SAINT,59 producing a listing of unaveraged F2 and σ(F2) values that were then passed to the SHELXT60 program package for further processing and structure solution. The intensity data were corrected for Lorentz and polarization effects and for absorption using SADABS.61 The structures were solved by direct methods (SHELXT).60 Refinement was by full-matrix least squares based on F2 using SHELXL.62 All reflections were used during refinements. Non-hydrogen atoms were refined anisotropically, and hydrogen atoms were refined using a riding model. For [Cu(nompa-ac)], the Flack parameter of 0.071(8) indicates a racemic twinning of this crystal. Refinement with a component of 7.1% results in a lower R value (R1 = 2.90 vs 2.93; wR2 = 6.63 vs 6.70). For [Cu3(mpatcn)2], hydrogen atoms were not assigned for O(15) (water molecule) because of issues with reliable placement from the difference map, which resulted in models with close contact between other hydrogen atoms of water molecules and higher R values. The (two) hydrogen atoms were still included in the formula. Crystallographic parameters are summarized in Table S10, bond distances and angles are summarized in Tables S11-S13, and thermal ellipsoid plots (50% probability) are shown in Figures S46-S48.
Computational Details.
All calculations were performed employing the Gaussian 09 package (revision D.01).63 Geometries were optimized at the M06-L level of theory38 with Grimme’s D3 dispersion correction,64 using the Stuttgart [8s7p6d2f1g∣6s5p3d2f1g] ECP10MWB contracted pseudopotential basis set on copper65 and the 6-31G(d′,p′) basis set on all other atoms.66,67 Solvation effects associated with water were accounted for by using the SMD continuum solvation model.37 An “ultrafine” grid was used for numerical integration, and self-consistent-field (SCF) convergence criteria of 10−6 were used for all calculations. Optimized geometries were confirmed as minima by frequency analysis (the absence of negative frequencies). The input geometries for [Cu(nompa)]+ and [Cu(nompa-ac)] were generated from the crystallographic data, while all others were constructed using Avogadro.68,69 A comparison of the bond distances and angles of the X-ray- and DFT-calculated structures is provided in Table S13. Tables of bond metrics and Cartesian coordinates are given in Table S15-S26), along with capped-stick images in Figures S49-S57.
UV–Vis.
Electronic absorbance spectra were collected at 298 K on an Agilent 8454 UV–vis spectrometer equipped with a diode-array detector. Sample solutions were prepared in deionized water and measured at pH 5.0 using concentrations of ~5 and ~0.5 mM for the copper(II) d–d and ligand π–π* transitions, respectively.
EPR Spectroscopy.
EPR spectra were collected on a Bruker EMX Premium-X spectrometer with a field strength of 9.65 GHz and a microwave power of 2.0 mW at 77 K using a liquid-nitrogen finger dewar (Wilmad, 50 mL Suprasil). Sample solutions were prepared as a 50:50 mixture of deionized water and ethylene glycol (~10 mM, pH 5.0). All samples were degassed with nitrogen and placed in 4-mm-o.d. quartz EPR tubes. Glasses were prepared by slowly lowering the sample into liquid nitrogen (~2 mm s−1). The experimental spectra were simulated using EasySpin.50
CV.
CV was performed using a CH730E Electrochemical Analyzer/Workstation, and the data were processed using CHI software version 12.04. Experiments were performed with deoxygenated aqueous solutions blanketed with an nitrogen atmosphere in a 10 mL glass cell using an Ag/AgCl (saturated KCl) reference electrode, glassy carbon working electrode, and platinum counter electrode. Prior to each scan, the working electrode was polished for 60 s in a figure-eight motion on a microcloth polishing pad with 0.05 μm alumina powder and rinsed with deionized water. Solutions were composed of ~1 mM analyte and 100 mM supporting electrolyte (NaClO4). All potentials are reported with respect to Ag/AgCl, and the potential stability was confirmed by including a water-soluble ferrocene derivative, ferrocenylmethanol [FeII(C5H4CH2OH)(C5H5)],70 as an internal redox standard.
64Cu Radiolabeling.
Radio-HPLC analysis was carried out using a Shimadzu HPLC-20AR chromatograph equipped with a binary gradient, pump, UV–vis detector, autoinjector, and Laura radio-detector on a HALO C18 column (2.7 μm, 4.6 × 150 mm, Advanced Materials Technology, USA). Method B: (A) 0.1% TFA in water and (B) 0.1% TFA in MeCN with a flow rate of 0.8 mL min−1 and UV detection at 254 and 280 nm.
The time-dependent complexation yield for each 64Cu2+/ligand ratio and the time-dependent plasma stability were determined by radio thin-layer chromatography (radio-TLC) using aluminum-backed normal-phase (silica) TLC plates as the stationary phase and 0.15 M NH4OAc/0.20 M EDTA pH 5 as the mobile phase. Under these conditions, free radiometals move with the solvent front (Rf = 0.8–1.0) and complexed copper-64 remains at Rf = 0–0.1. Radioactivity distribution on TLC plates was visualized and quantified using a LabLogic Dual Scan-RAM radio-TLC/HPLC scanner.
Radiochemical complexes for animal experiments were prepared according to the following general method: 64CuCl2 was obtained from the University of Wisconsin—Madison in a 0.1 M HCl solution. The stock solution was diluted in 0.05 M HCl to a volume of 100 μL. To a solution of ligand (20–100 μg, 0.025 μmol) in 100 μL of pH 5.5 ammonium acetate was added 3.4–3.6 mCi of 64CuCl2 in 0.05 M HCl. Complexation was carried out at room temperature or at 60 °C for 5–30 min. Complex formation was confirmed by radio-HPLC, and compounds were purified by C18 Sep-Pak to remove free copper-64. Eluted fractions were reduced in vacuo to remove excess ethanol and reformulated in PBS for in vivo experiments.
EDTA Challenge Experiment.
Following radiolabeling, conjugates in solution were added to a 10.1 mM EDTA solution at pH 5.5 The final ligand/EDTA molar ratio was 1:100. The resulting solutions were incubated at 37 °C for 1–12 h. A total of 5 μL (10% of the solution) was removed at each time point and spotted onto a TLC strip. Dose formulation solutions were evaluated for room temperature stability in Dulbecco’s phosphate-buffered saline (DPBS) at pH 7.4. Aliquots were removed at each time point and evaluated by radio-HPLC for intact complex and free copper.
The plasma stability (n = 3) was tested by incubation of 50 μL of a dose formulation solution in 200 μL of rat plasma at 37 °C. Aliquots were removed at each time point and evaluated by radio-HPLC for intact complex and free copper.
Cell-Binding Assay.
A total of 26.4–36.0 μCi of each copper-64 complex was added to 1 × 106 PSMA positive PC-3 PIP cells and PSMA negative PC-3 flu cells in 100 μL of 1% fetal bovine serum (FBS)/DPBS to assess the binding specificity. The resulting mixtures were incubated at 37 °C for 40 min. After incubation, the mixtures were centrifuged, and the supernatant was separated from the cell pellet. The resulting cells were washed twice with 100 μL of 1% FBS/DPBS. After a second washing, the resulting cell pellet was counted on a γ-counter. Counts per minute (CPM) values were decay-corrected, and the results were calculated as percent of bound activity/activity added to an unwashed cell sample standard.
Small Animal PET Imaging and Biodistribution.
All animal experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee at Stony Brook Medicine. Male NCr nude mice (12 weeks, Taconic Biosciences, Rensselaer, NY) were implanted subcutaneously on the forward right flank with PSMApos PC-3 PIP cells and on the forward left flank with PSMAneg PC-3 flu cells (0.7 × 105/mouse in 1:1 DPBS (pH 7.4)/Matrigel). When tumors reached 4–6 mm, the animals were anesthetized with isoflurane and injected with radiolabeled conjugates (103–272 μCi in DPBS, 100 μL), which were intravenously administered via a tail-vein catheter. Animals were imaged at 90 min postinjection using a Siemens Inveon PET/CT or SPECT Multimodality System, and images were reconstructed using ASIPro software. Upon completion of imaging at 2 h postinjection, animals were sacrificed by cervical dislocation and selected tissues collected to preweighed γ-counter tubes. The activity of preweighed tissues was counted in a γ-counter. CPM values were decay-corrected, and the results were calculated as percent ID per gram of wet tissue.
Metabolic Stability Analysis.
Urine was collected at 120 min postinjection by a bladder puncture and aspiration, followed by analysis by HPLC to determine intact complex and metabolites (method B). Following sample injection, an HPLC eluent was collected in 30 s fractions into γ-counter tubes. The activity of each fraction was counted in a γ-counter, and CPM values were used to plot the activity trace to improve the sensitivity of the experiment. The activity at the complex retention time was compared to the total activity of metabolites or unbound copper to determine the percent of intact complex in urine.
Supplementary Material
ACKNOWLEDGMENTS
E.B. acknowledges funding sources, specifically the NIH for a Pathway to Independence Award (NHLBI R00HL125728) and Stony Brook University. Nan Wang is warmly acknowledged for high-resolution mass spectrometry data acquisition. J.R acknowledges Brown University for financial support. J.R and A.B. thank Dr. Mehmed Z. Ertem (BNL) for helpful discussions related to the DFT modelling studies.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.0c02314.
Experimental procedures and additional figures and tables (PDF)
Accession Codes
CCDC 2020765–2020767 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Contributor Information
Brett A. Vaughn, Department of Chemistry, Stony Brook University, Stony Brook, New York 11794, United States
Alexander M. Brown, Department of Chemistry, Brown University, Providence, Rhode Island 02912, United States
Shin Hye Ahn, Department of Chemistry, Stony Brook University, Stony Brook, New York 11794, United States.
Jerome R. Robinson, Department of Chemistry, Brown University, Providence, Rhode Island 02912, United States.
Eszter Boros, Department of Chemistry, Stony Brook University, Stony Brook, New York 11794, United States.
REFERENCES
- (1).Woodin KS; Heroux KJ; Boswell CA; Wong EH; Weisman GR; Niu W; Tomellini SA; Anderson CJ; Zakharov LN; Rheingold AL Kinetic Inertness and Electrochemical Behavior of Copper(II) Tetraazamacrocyclic Complexes: Possible Implications for in Vivo Stability. Eur. J. Inorg. Chem 2005, 2005, 4829–4833. [Google Scholar]
- (2).Young MJ; Chin J Dinuclear Copper(II) Complex That Hydrolyzes RNA. J. Am. Chem. Soc 1995, 117, 10577–10578. [Google Scholar]
- (3).Zeng L; Miller EW; Pralle A; Isacoff EY; Chang CJ A selective turn-on fluorescent sensor for imaging copper in living cells. J. Am. Chem. Soc 2006, 128 (1), 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wadas TJ; Wong EH; Weisman GR; Anderson CJ Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev 2010, 110 (5), 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Liu S The role of coordination chemistry in the development of target-specific radiopharmaceuticals. Chem. Soc. Rev 2004, 33 (7), 445–461. [DOI] [PubMed] [Google Scholar]
- (6).Wong EH; Weisman GR; Hill DC; Reed DP; Rogers ME; Condon JS; Fagan MA; Calabrese JC; Lam KC; Guzei IA; Rheingold AL Synthesis and characterization of cross-bridged cyclams and pendant-armed derivatives and structural studies of their copper(II) complexes. J. Am. Chem. Soc 2000, 122 (43), 10561–10572. [Google Scholar]
- (7).Fujibayashi Y; Matsumoto K; Yonekura Y; Junji K; Yokoyama A A New Zinc-62/Copper-62 Generator as a Copper-62 Source for PET Radiopharmaceuticals. J. Nucl. Med 1989, 30 (11), 1838–1842. [PubMed] [Google Scholar]
- (8).Vāvere AL; Lewis JS Cu-ATSM: A radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007, 0, 4893–4902. [DOI] [PubMed] [Google Scholar]
- (9).Smith A; Alberto R; Bläuenstein P; Novak I; Maecke H; Schubiger PA Preclinical Evaluation of Cu-67 labeled Intact and Fragmented Anti-Colon Carcinoma Monoclonal Antibody Mab 35. Cancer Res. 1993, 53, 5727–5733. [PubMed] [Google Scholar]
- (10).Gillet R; Roux A; Brandel J; Huclier-Markai S; Camerel F; Jeannin O; Nonat AM; Charbonnière LJ A Bispidol Chelator with a Phosphonate Pendant Arm: Synthesis, Cu (II) Complexation, and 64Cu Labeling. Inorg. Chem 2017, 56 (19), 11738–11752. [DOI] [PubMed] [Google Scholar]
- (11).Di Bartolo NM; Sargeson AM; Donlevy TM; Smith SV Synthesis of a new cage ligand, SarAr, and its complexation with selected transition metal ions for potential use in radioimaging. Dalton Trans. 2001, No. 15, 2303–2309. [Google Scholar]
- (12).Su TA; Shihadih DS; Cao W; Detomasi TC; Heffern MC; Jia S; Stahl A; Chang CJ A modular ionophore platform for liver-directed copper supplementation in cells and animals. J. Am. Chem. Soc 2018, 140 (42), 13764–13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Le Fur M; Beyler M; Le Poul N; Lima LM; Le Mest Y; Delgado R; Platas-Iglesias C; Patinec V; Tripier R Improving the stability and inertness of Cu (II) and Cu (I) complexes with methylthiazolyl ligands by tuning the macrocyclic structure. Dalton Trans. 2016, 45 (17), 7406–7420. [DOI] [PubMed] [Google Scholar]
- (14).Burke BP; Miranda CS; Lee RE; Renard I; Nigam S; Clemente GS; D’Huys T; Ruest T; Domarkas J; Thompson JA; et al. 64Cu PET Imaging of the CXCR4 Chemokine Receptor Using a Cross-Bridged Cyclam Bis-Tetraazamacrocyclic Antagonist. J. Nucl. Med 2020, 61 (1), 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Silversides JD; Allan CC; Archibald SJ Copper (II) cyclam-based complexes for radiopharmaceutical applications: synthesis and structural analysis. Dalton Trans. 2007, No. 9, 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Halcrow MA Jahn-Teller distortions in transition metal compounds, and their importance in functional molecular and inorganic materials. Chem. Soc. Rev 2013, 42 (4), 1784–1795. [DOI] [PubMed] [Google Scholar]
- (17).Powell DH; Helm L; Merbach AE 17O nuclear magnetic resonance in aqueous solutions of Cu2+: The combined effect of Jahn-Teller inversion and solvent exchange on relaxation rates. J. Chem. Phys 1991, 95 (12), 9258–9265. [Google Scholar]
- (18).Heroux KJ; Woodin KS; Tranchemontagne DJ; Widger PCB; Southwick E; Wong EH; Weisman GR; Tomellini SA; Wadas TJ; Anderson CJ; Kassel S; Golen JA; Rheingold AL The long and short of it: the influence of N-carboxyethyl versusN-carboxymethyl pendant arms on in vitro and in vivo behavior of copper complexes of cross-bridged tetraamine macrocycles. Dalton Trans. 2007, No. 21, 2150–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ude Z; Kavanagh K; Twamley B; Pour M; Gathergood N; Kellett A; Marmion CJ A new class of prophylactic metallo-antibiotic possessing potent anti-cancer and anti-microbial properties. Dalton Trans. 2019, 48 (24), 8578–8593. [DOI] [PubMed] [Google Scholar]
- (20).Prosser KE; Chang SW; Saraci F; Le PH; Walsby CJ Anticancer copper pyridine benzimidazole complexes: ROS generation, biomolecule interactions, and cytotoxicity. J. Inorg. Biochem 2017, 167, 89–99. [DOI] [PubMed] [Google Scholar]
- (21).White JB; Hu LY; Boucher DL; Sutcliffe JL ImmunoPET Imaging of α v β 6 Expression Using an Engineered Anti-α v β 6 Cys-diabody Site-Specifically Radiolabeled with Cu-64: Considerations for Optimal Imaging with Antibody Fragments. Mol. Imaging Biol 2018, 20 (1), 103–113. [DOI] [PubMed] [Google Scholar]
- (22).Prasanphanich AF; Nanda PK; Rold TL; Ma L; Lewis MR; Garrison JC; Hoffman TJ; Sieckman GL; Figueroa SD; Smith CJ [64Cu-NOTA-8-Aoc-BBN (7–14) NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (30), 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bevilacqua A; Gelb RI; Hebard WB; Zompa LJ Equilibrium and thermodynamic study of the aqueous complexation of 1,4,7-triazacyclononane-N, N’, N”-triacetic acid with protons, alkaline-earth-metal cations, and copper(II). Inorg. Chem 1987, 26 (16), 2699–2706. [Google Scholar]
- (24).Guillou A; Lima LM; Esteban-Gómez D; Le Poul N; Bartholoma MD; Platas-Iglesias C; Delgado R; Patinec V. r.; Tripier R. l. Methylthiazolyl Tacn Ligands for Copper Complexation and Their Bifunctional Chelating Agent Derivatives for Bioconjugation and Copper-64 Radiolabeling: An Example with Bombesin. Inorg. Chem 2019, 58 (4), 2669–2685. [DOI] [PubMed] [Google Scholar]
- (25).Vaughn BA; Ahn SH; Aluicio-Sarduy E; Devaraj J; Olson AP; Engle J; Boros E Chelation with a twist: a bifunctional chelator to enable room temperature radiolabeling and targeted PET imaging with scandium-44. Chem. Sci 2020, 11 (2), 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Molnar E; Camus N; Patinec V. r.; Rolla GA; Botta M; Tircso G; Kálmán FK; Fodor T; Tripier R; Platas-Iglesias C Picolinate-containing macrocyclic Mn2+ complexes as potential MRI contrast agents. Inorg. Chem 2014, 53 (10), 5136–5149. [DOI] [PubMed] [Google Scholar]
- (27).Banerjee SR; Pullambhatla M; Foss CA; Nimmagadda S; Ferdani R; Anderson CJ; Mease RC; Pomper MG 64Cu-labeled inhibitors of prostate-specific membrane antigen for PET imaging of prostate cancer. J. Med. Chem 2014, 57 (6), 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hillier SM; Maresca KP; Lu G; Merkin RD; Marquis JC; Zimmerman CN; Eckelman WC; Joyal JL; Babich JW 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen for molecular imaging of prostate cancer. J. Nucl. Med 2013, 54 (8), 1369–1376. [DOI] [PubMed] [Google Scholar]
- (29).Addison AW; Rao TN; Reedijk J; van Rijn J; Verschoor GC Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc., Dalton Trans 1984, No. 7, 1349–1356. [Google Scholar]
- (30).Joshi T; Kubeil M; Nsubuga A; Singh G; Gasser G; Stephan H Harnessing the Coordination Chemistry of 1,4,7-Triazacyclononane for Biomimicry and Radiopharmaceutical Applications. ChemPlusChem 2018, 83 (7), 554–564. [DOI] [PubMed] [Google Scholar]
- (31).Joshi T; Graham B; Spiccia L Macrocyclic Metal Complexes for Metalloenzyme Mimicry and Sensor Development. Acc. Chem. Res 2015, 48 (8), 2366–2379. [DOI] [PubMed] [Google Scholar]
- (32).Qian J; Wang L-P; Tian J-L; Xie C-Z; Yan S-P A copper(II) complex of 1,4,7-triazacyclononane featuring acetate pendant: an efficient DNA cleavage agent. J. Coord. Chem 2012, 65 (1), 122–130. [Google Scholar]
- (33).Tetilla MA; Aragoni MC; Arca M; Caltagirone C; Bazzicalupi C; Bencini A; Garau A; Isaia F; Laguna A; Lippolis V; Meli V Colorimetric response to anions by a “robust” copper(ii) complex of a [9]aneN3 pendant arm derivative: CN- and I- selective sensing. Chem. Commun 2011, 47 (13), 3805–3807. [DOI] [PubMed] [Google Scholar]
- (34).Lima LMP; Esteban-Gómez D; Delgado R; Platas-Iglesias C; Tripier R Monopicolinate Cyclen and Cyclam Derivatives for Stable Copper(II) Complexation. Inorg. Chem 2012, 51 (12), 6916–6927. [DOI] [PubMed] [Google Scholar]
- (35).Lima LMP; Halime Z; Marion R; Camus N; Delgado R; Platas-Iglesias C; Tripier R Monopicolinate Cross-Bridged Cyclam Combining Very Fast Complexation with Very High Stability and Inertness of Its Copper(II) Complex. Inorg. Chem 2014, 53 (10), 5269–5279. [DOI] [PubMed] [Google Scholar]
- (36).Guillou A; Lima LMP; Esteban-Gómez D; Delgado R; Platas-Iglesias C; Patinec V; Tripier R endo- versus exo-Cyclic coordination in copper complexes with methylthiazolylcarboxylate tacn derivatives. Dalton Trans. 2019, 48 (24), 8740–8755. [DOI] [PubMed] [Google Scholar]
- (37).Marenich AV; Cramer CJ; Truhlar DG Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113 (18), 6378–6396. [DOI] [PubMed] [Google Scholar]
- (38).Zhao Y; Truhlar DG A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys 2006, 125 (19), 194101. [DOI] [PubMed] [Google Scholar]
- (39).Geraldes CFGC; Marques M; Paula M; de Castro B; Pereira E Study of Copper(II) Polyazamacrocyclic Complexes by Electronic Absorption Spectrophotometry and EPR Spectroscopy. Eur. J. Inorg. Chem 2000, 2000 (3), 559–565. [Google Scholar]
- (40).Meyer M; Dahaoui-Gindrey V; Lecomte C; Guilard R Conformations and coordination schemes of carboxylate and carbamoyl derivatives of the tetraazamacrocycles cyclen and cyclam, and the relation to their protonation states. Coord. Chem. Rev 1998, 178–180, 1313–1405. [Google Scholar]
- (41).Kotek J; Lubal P; Hermann P; Císařová I; Lukeš I; Godula T; Svobodová I; Táborský P; Havel J High Thermodynamic Stability and Extraordinary Kinetic Inertness of Copper(II) Complexes with 1,4,8,11-Tetraazacyclotetradecane-1,8-bis-(methylphosphonic acid): Example of a Rare Isomerism between Kinetically Inert Penta- and Hexacoordinated Copper(II) Complexes. Chem. - Eur. J 2003, 9 (1), 233–248. [DOI] [PubMed] [Google Scholar]
- (42).Hubin TJ; McCormick JM; Alcock NW; Clase HJ; Busch DH Crystallographic Characterization of Stepwise Changes in Ligand Conformations as Their Internal Topology Changes and Two Novel Cross-Bridged Tetraazamacrocyclic Copper(II) Complexes. Inorg. Chem 1999, 38 (20), 4435–4446. [DOI] [PubMed] [Google Scholar]
- (43).Guillou A; Lima LMP; Roger M; Esteban-Gómez D; Delgado R; Platas-Iglesias C; Patinec V; Tripier R 1,4,7-Triazacyclononane-Based Bifunctional Picolinate Ligands for Efficient Copper Complexation. Eur. J. Inorg. Chem 2017, 2017 (18), 2435–2443. [Google Scholar]
- (44).Wieghardt K; Bossek U; Chaudhuri P; Herrmann W; Menke BC; Weiss J 1,4,7-Triazacyclononane-N, N’, N’’-triacetate (TCTA), a new hexadentate ligand for divalent and trivalent metal ions. Crystal structures of [CrIII(TCTA)], [FeIII(TCTA)], and Na[CuII(TCTA)].bul.2NaBr.bul.8H2O. Inorg. Chem 1982, 21 (12), 4308–4314. [Google Scholar]
- (45).Roger M; Lima LMP; Frindel M; Platas-Iglesias C; Gestin J-F; Delgado R; Patinec V; Tripier R Monopicolinate-dipicolyl Derivative of Triazacyclononane for Stable Complexation of Cu2+ and 64Cu2+. Inorg. Chem 2013, 52 (9), 5246–5259. [DOI] [PubMed] [Google Scholar]
- (46).Platas-Iglesias C; Mato-Iglesias M; Djanashvili K; Muller RN; Elst LV; Peters JA; de Blas A; Rodríguez-Blas T Lanthanide Chelates Containing Pyridine Units with Potential Application as Contrast Agents in Magnetic Resonance Imaging. Chem. - Eur. J 2004, 10, 3579–3590. [DOI] [PubMed] [Google Scholar]
- (47).Hathaway B; Billing D The electronic properties and stereochemistry of mono-nuclear complexes of the copper (II) ion. Coord. Chem. Rev 1970, 5 (2), 143–207. [Google Scholar]
- (48).Hathaway BJ Copper. Coord. Chem. Rev 1983, 52, 87–169. [Google Scholar]
- (49).Lima LMP; Delgado R; Drew MGB; Brandão P; Félix V Cyclam derivatives containing three acetate pendant arms: synthesis, acid-base, metal complexation and structural studies. Dalton Trans. 2008, No. 46, 6593–6608. [DOI] [PubMed] [Google Scholar]
- (50).Stoll S; Schweiger A EasySpin, a comprehensive software package for spectral simulation and analysis in EPR J. Magn. Reson 2006, 178 (1), 42–55. [DOI] [PubMed] [Google Scholar]
- (51).Pandya DN; Kim JY; Park JC; Lee H; Phapale PB; Kwak W; Choi TH; Cheon GJ; Yoon Y-R; Yoo J Revival of TE2A; a better chelate for Cu(II) ions than TETA? Chem. Commun 2010, 46 (20), 3517–3519. [DOI] [PubMed] [Google Scholar]
- (52).Ambundo EA; Deydier M-V; Grall AJ; Aguera-Vega N; Dressel LT; Cooper TH; Heeg MJ; Ochrymowycz LA; Rorabacher DB Influence of Coordination Geometry upon Copper(II/I) Redox Potentials. Physical Parameters for Twelve Copper Tripodal Ligand Complexes. Inorg. Chem 1999, 38 (19), 4233–4242. [Google Scholar]
- (53).Sando GN; Blakley RL; Hogenkamp HPC; Hoffmann PJ Studies of the Mechanism of Adenosylcobalamin-dependent Ribonucleotide Reduciton by the Use of Analogs of the Coenzyme. J. Biol. Chem 1975, 250 (22), 8774–8779. [PubMed] [Google Scholar]
- (54).Hatcher LQ; Karlin KD In Advances in Inorganic Chemistry; van Eldik R, Reedijk J, Eds.; Academic Press, 2006; Vol. 58, pp 131–184. [Google Scholar]
- (55).Randles JEB 142. The equilibrium between cuprous and cupric compounds in presence of metallic copper. J. Chem. Soc 1941, No. 0, 802–811. [Google Scholar]
- (56).Anderson JL; Shain I Cyclic voltammetric studies of the pH dependence of copper(II) reduction in acidic aqueous nitrate and perchlorate solutions. Anal. Chem 1976, 48 (9), 1274–1282. [Google Scholar]
- (57).García-Rodríguez DE; Mendoza-Huizar LH; Rios-Reyes CH; Alatorre-Ordaz MA Copper electrodeposition on glassy carbon and highly oriented pyrolytic graphite substrates from perchlorate solutions. Quim. Nova 2012, 35, 699–704. [Google Scholar]
- (58).Szabo Z; Mena E; Rowe SP; Plyku D; Nidal R; Eisenberger MA; Antonarakis ES; Fan H; Dannals RF; Chen Y; Mease RC; Vranesic M; Bhatnagar A; Sgouros G; Cho SY; Pomper MG Initial Evaluation of [18F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol. Imaging Biol 2015, 17 (4), 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).SAINT, version 8.37a; Bruker AXS Inc.: Madison, WI, 2012. [Google Scholar]
- (60).Sheldrick G SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv 2015, 71 (1), 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).SADABS, version 2014/5; Bruker AXS Inc.: Madison, WI, 2001. [Google Scholar]
- (62).Sheldrick G Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem 2015, 71 (1), 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Frisch MJTGW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Pe-Tersson GA; Nakatsuji H; Caricato M; Li X; Hratchian HP; Izmaylov AFBJ; Zheng G; Sonnenberg JL; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Montgomery JA Jr.; Peralta JE; Ogliaro F; Bearpark M; Heyd JJ; Brothers E; Kudin KN; Staroverov VN; Kobayashi R; Normand J; Raghavachari K; Rendell ABJC; Iyengar SS; Tomasi J; Cossi M; Rega N; Millam NJ; Klene M; Knox JE; Cross JB; Bakken V; Adamo C; Jaramillo J; Gomperts R; Stratmann RE; Yazyev O; Austin AJ; Cammi R; Pomelli C; Ochterski JW; Martin RL; Morokuma K; Zakrzewski VG; Voth GA; Salvador P; Dannenberg JJ; Dapprich S; Daniels AD; Farkas Ö; Foresman JB; Ortiz JV; Cioslowski J; Fox DJ Gaussian 09, revision D.01; Gaussian Inc.: Wallingford, CT, 2009. [Google Scholar]
- (64).Grimme S; Antony J; Ehrlich S; Krieg H A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys 2010, 132 (15), 154104. [DOI] [PubMed] [Google Scholar]
- (65).Dolg M; Wedig U; Stoll H; Preuss H Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys 1987, 86 (2), 866–872. [Google Scholar]
- (66).Petersson GA; Bennett A; Tensfeldt TG; Al-Laham MA; Shirley WA; Mantzaris J A complete basis set model chemistry.I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys 1988, 89 (4), 2193–2218. [Google Scholar]
- (67).Petersson GA; Al-Laham MA A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys 1991, 94 (9), 6081–6090. [Google Scholar]
- (68).Appleton TG; Bailey AJ; Bedgood DR; Hall JR Amino Acid Complexes of Palladium(II). 1. NMR Study of the Ractions of the Diaqua(ethylenediamine)palladium(II) Cation with Ammonia, Betaine, and the Amino Acids + NH3(CH2nCO2- (n = 1–3)1. Inorg. Chem 1994, 33, 217–226. [Google Scholar]
- (69).Hanwell MD; Curtis DE; Lonie DC; Vandermeersch T; Zurek E; Hutchison GR Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf 2012, 4 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Cannes C; Kanoufi F; Bard AJ Cyclic voltammetry and scanning electrochemical microscopy of ferrocenemethanol at monolayer and bilayer-modified gold electrodes. J. Electroanal Chem 2003, 547 (1), 83–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.