CASE
A 48-year-old male with hypertension, uncontrolled type 2 diabetes mellitus, tobacco use, and opioid use disorder presented to a hospital in Baltimore, MD, in September with a right-foot plantar surface wound. He noticed the wound 3 weeks prior and attempted his own wound care and dressings while living in his car. The wound enlarged and developed increasingly purulent drainage, with visible maggots, and his foot became swollen. A review of systems was notable for malaise, fatigue, and anorexia.
Three months prior, he was hospitalized for a polymicrobial wound infection of multiple wounds on his right foot and bilateral toes. He was discharged on trimethoprim-sulfamethoxazole; however, he missed his outpatient magnetic resonance imaging (MRI) and vascular surgery follow-up appointments.
Upon arrival to the hospital, he was afebrile, and his heart rate was 110 beats/min; vital signs were otherwise within normal limits. Select laboratory results are as follows (with reference ranges in parentheses): white blood cell count of 15,200 cells/mm3 (4,500 to 11,000 cells/mm3); hemoglobin of 8.9 g/dL (13.9 to 16.3 g/dL); hematocrit of 25.7% (41.0 to 53.0%); sodium of 125 mmol/L (135 to 148 mmol/L); chloride of 87 mmol/L (96 to 109 mmol/L); blood urea nitrogen of 44 mg/dL (7 to 22 mg/dL); creatinine of 3.8 mg/dL (0.6 to 1.3 mg/dL); glucose of 651 mg/dL (71 to 99 mg/dL); hemoglobin A1C of 13.8% (<5.7%); lactate of 2.6 mmol/L (0.5 to 2.0 mmol/L); C-reactive protein of 30 mg/dL (<0.5 mg/dL); ferritin of 1,090 ng/mL (30 to 400 ng/mL); pH 7.36; and urine toxicology positive for fentanyl, codeine/morphine, and oxycodone/oxymorphone.
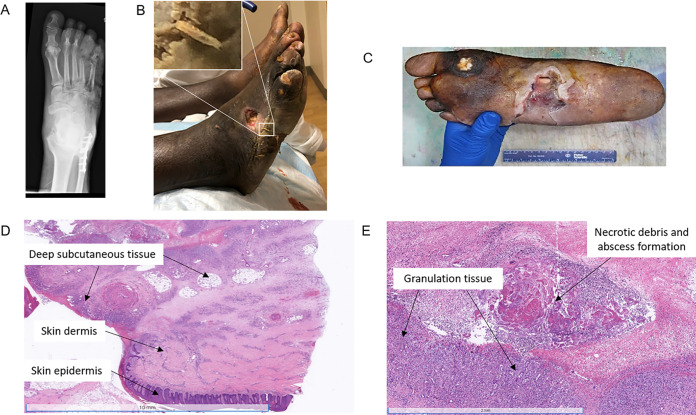
Physical examination revealed a large wound that appeared to pass through from the dorsal to the plantar surface of his right foot, with purulent drainage, visible bone, and multiple maggots (Fig. 1A). Foot X-ray showed acute osteomyelitis with extensive subcutaneous air and osseous erosion/destruction of multiple phalanges and metatarsals (Fig. 1B). He was empirically started on intravenous (IV) vancomycin and piperacillin-tazobactam, given IV fluids for dehydration, and treated with insulin for his hyperosmolar hyperglycemia. Given the extent of his infection and after discussion of plans and alternatives with the patient, his foot was amputated (Fig. 1C). Pathology showed deep soft tissue abscesses and tissue necrosis (Fig. 1D and E).
FIG 1.
(A) Clinical image of the patient’s foot demonstrating a purulent maggot-containing wound. (B) X ray of the affected foot showing acute osteomyelitis, subcutaneous air, and osseous erosion/destruction of multiple phalanges and metatarsals. (C) Gross image of the patient’s amputated foot demonstrating a 10.1- by 5.3-cm purulent ulcer exposing the underlying tendon. (D) Low-power histology image of the amputated foot showing inflammation involving the dermis and subcutaneous tissue. (E) High-power histology image showing abscess formation, necrosis, and granulation tissue within the deep soft tissue.
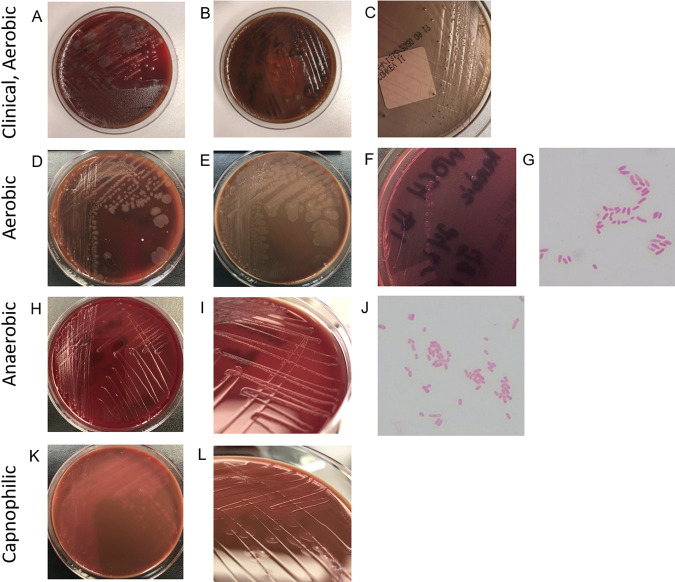
Due to concern for sepsis, on the day of admission, blood cultures (BD Bactec lytic anaerobic and BD Bactec Plus aerobic media) were obtained and grew a mixed population of small, beta-hemolytic colonies mixed with large, gray, mucoid colonies on blood agar after 24 h from the anaerobic bottle only (Fig. 2A). These larger colonies were subcultured onto blood agar (Fig. 2B) and MacConkey agar (Fig. 2C) plates. Colorless colonies were visible on MacConkey agar after 48 h, which developed a slight purple tinge over time (due to the uptake of crystal violet from the medium rather than lactose fermentation). The organism was confirmed to be Gram negative and oxidase positive.
FIG 2.
Original clinical culture plates and growth under aerobic, anaerobic, and capnophilic conditions. (A to C) Images of plates taken approximately 6 days after inoculation at 35°C under aerobic conditions. (A) Original 5% sheep blood agar plate. (B and C) Subculture of large spreading colonies on a 5% sheep blood agar plate (B) and MacConkey agar (C). (D to L) Plates and Gram stains representing aerotolerance testing at 48 h of the same isolate. (D) Five percent sheep blood agar, aerobic conditions. (E) Chocolate agar, aerobic conditions. (F) MacConkey agar, aerobic conditions. (G) Gram stain, aerobic conditions. (H) Brucella agar, anaerobic conditions. (I) Detail of colonies on Brucella agar under anaerobic conditions. (J) Gram stain, anaerobic conditions. (K) Chocolate agar, capnophilic (5% CO2) conditions. (L) Detail of colonies on chocolate agar under capnophilic conditions.
The organism was preliminarily identified as Wohlfahrtiimonas chitiniclastica by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Biotyper Compass version 4.1.100, research use only [RUO] database; Bruker, Germany) and subsequently confirmed by 16S rRNA gene PCR and sequencing. Blood cultures also grew Enterococcus faecalis and Streptococcus agalactiae, the beta-hemolytic colonies seen on the initial blood agar plate.
Susceptibility testing performed on the BD Phoenix M50 system revealed that this isolate of Wohlfahrtiimonas chitiniclastica was susceptible to amikacin, cefepime, ceftazidime, ciprofloxacin, gentamicin, meropenem, piperacillin-tazobactam, tobramycin, and trimethoprim-sulfamethoxazole, by applying Clinical and Laboratory Standards Institute non-Enterobacterales interpretations (1). Vancomycin was discontinued, and he continued on piperacillin-tazobactam. Subsequent blood cultures were negative, and he remained afebrile and hemodynamically stable. He was discharged on linezolid and ciprofloxacin for a total of 10 days of treatment and with home wound care, physical therapy, and occupational therapy. Three months after discharge, he was doing well, with no further infections or hospitalizations.
DISCUSSION
Wohlfahrtiimonas chitiniclastica is a facultatively anaerobic, nonmotile, non-spore-forming, non-lactose-fermenting Gram-negative rod that is positive for both catalase and oxidase; negative for urease, indole, and H2S (2, 3); and negative for the reduction of nitrate to nitrite. Its colonies are gray-white, flat, and smooth on chocholate agar and blood agar for which they also nonhemolytic, while they are generally colorless on MacConkey agar (3). It has strong chitinase activity (2), an enzyme that degrades chitin, a polysaccharide found in fungal cell walls and the exoskeletons of some animals. The strong chitinase activity likely reflects a symbiotic relationship with its host and may play a role in the metamorphosis of the fly (2).
W. chitiniclastica is transmitted to humans by obligate parasitic flies, including Wohlfahrtia magnifica (the first described vector), Lucilia sericata, Musca domestica, and Chrysomya megacephala (2). Larvae of these flies can be deposited in open wounds or on mucosal surfaces, leading to wound infections, cellulitis, osteomyelitis, and bacteremia (2). In addition to W. chitiniclastica, Ignatzschineria indica is another organism that expresses chitinase and is associated with myiasis. Both are nonmotile, non-spore-forming, Gram-negative rods that are both oxidase and catalase positive and can be differentiated using MALDI-TOF MS and 16S rRNA gene sequencing (4). Both organisms are included in the Bruker RUO database, with W. chitiniclastica recently added to the FDA-cleared Bruker Claim 6 database. Neither of the organisms is included in the Vitek MS RUO or FDA-cleared databases as of July 2021 (bioMérieux, Marcy-l’Etoile, France) (V. Girard, personal communication). Each microbiology laboratory must consult the technical specifications of the database that it uses to determine whether these organisms are present. Coinfection with W. chitiniclastica and I. indica causing bacteremia has been reported (4).
To date, approximately 23 cases of W. chitiniclastica have been published in the literature (4). Several risk factors have been identified among infected patients, including open wounds, poor personal and wound hygiene, unstable living conditions, older age, proximity to livestock, alcoholism, tobacco abuse, chronic cardiovascular/circulatory disease, and neurological disease (3, 4). Of note, many of these risk factors are shared with I. indica and are essentially the same risk factors as those for acquiring myiasis (3, 4). The typical presentation of W. chitiniclastica infection is a local wound infection, although sepsis is possible (3), as occurred in our patient. Additionally, the majority of reported cases have been polymicrobial in nature (3). After evaluating data from 2011 to date (March 2021), the Johns Hopkins Medical Microbiology Laboratory was able to identify 5 cases of W. chitiniclastica associated with polymicrobial wound infections, with the first case being identified in 2013.
The identification of W. chitiniclastica using traditional biochemical methods can be difficult and unreliable due to the lack of inclusion in databases, similarities to other Gram-negative rods, and the rarity of isolation. Misidentifications of W. chitiniclastica by API 20 NE (analytical profile index; bioMérieux, Germany) as Brevundimonas diminuta and Oligella urethralis; by the Vitek 2 system (bioMérieux) as Comamonas testosteroni, Acinetobacter lwoffii, and Rhizobium radiobacter; and by the MicroScan WalkAway system (Beckman Coulter) as Moraxella/Psychrobacter spp., Vibrio spp., Pasteurella/Actinobacillus spp., and Pseudomonas spp. have been reported (5). Newer techniques, including MALDI-TOF MS, PCR, and 16S rRNA gene sequencing, allow the reliable, reproducible identification of the organism (3).
W. chitiniclastica was initially reported as strictly aerobic (6), and accordingly, most reports of clinical infection also describe W. chitiniclastica as strictly aerobic (2–5). Analyses of closely related bacteria proposed as additional members of the Wohlfahrtiimonas species demonstrate facultatively anaerobic growth (7). Notably, W. chitiniclastica was isolated only from an anaerobic blood culture bottle in the case described here.
To address these discrepancies, we performed aerotolerance (growth in air or air plus 5% CO2) and anaerobic (5% H2, 95% N2) testing of the W. chitiniclastica isolate described here as well as a second isolate cryopreserved in the Johns Hopkins Medical Microbiology Laboratory. Isolates were subcultured onto sheep blood, chocolate, and MacConkey plates under aerobic conditions; chocolate plates under capnophilic (5% CO2) conditions; and chocolate and Brucella blood plates under anaerobic conditions (Fig. 2). Robust growth was documented on sheep blood and chocolate plates under aerobic conditions at 24 and 48 h. No growth was noted on MacConkey plates at 24 h, and only scant growth was apparent at 48 h. Modest growth was present on chocolate plates isolated under capnophilic conditions for both isolates at 24 and 48 h. Present but minimal growth was observed under anaerobic conditions, with slightly better growth on Brucella blood plates than on chocolate plates for both isolates at 24 and 48 h. Although we observed superior growth under aerobic conditions, these data demonstrate that these isolates of W. chitiniclastica are facultatively anaerobic and suggest that other isolates share the same metabolism. Gram staining was also performed at 48 h of growth under both aerobic and anaerobic conditions and demonstrated pleomorphic Gram-negative rods (Fig. 2). Staining under anaerobic conditions was more Gram variable and showed less robust uptake of the safranin counterstain, which correlates with its weaker growth under anaerobic conditions. As a facultatively anaerobic Gram-negative rod, it is also notable that W. chitiniclastica grows slowly on MacConkey agar regardless of the atmospheric culture conditions.
Similar to the current case, most reports reveal that W. chitiniclastica is susceptible to commonly prescribed agents with Gram-negative coverage, including β-lactams, fluoroquinolones, aminoglycosides, and trimethoprim-sulfamethoxazole (3).
In summary, W. chitiniclastica is a facultatively anaerobic Gram-negative rod that causes infections associated with myiasis. Increasing reports of W. chitiniclastica likely reflect the expansion of MALDI-TOF MS into clinical laboratories providing reliable identification of this previously unrecognized and misidentified organism.
SELF-ASSESSMENT QUESTIONS
-
1.
When presenting with Wohlfahrtiimonas chitiniclastica bacteremia, patients should undergo a thorough investigation for evidence of which of the following, if not already known at the time of microbiologic diagnosis?
-
a.
Myiasis
-
b.
Tattoos
-
c.
Implanted devices
-
d.
Prior leach therapy in a U.S. clinic or hospital
-
e.
i.v. use of recreational drugs
-
a.
-
2.
When performing biochemical tests on cultured growth, which one of the following characteristics would be expected for Wohlfahrtiimonas chitiniclastica?
-
a.
Urease positive
-
b.
Catalase negative
-
c.
Oxidase positive
-
d.
H2S production
-
e.
Indole positive
-
a.
-
3.
Which of the following methods can reliably identify Wohlfahrtiimonas chitiniclastica from cultured growth?
-
a.
API NE
-
b.
Rapid biochemical tests (e.g., oxidase and catalase, etc.)
-
c.
Vitek 2
-
d.
MALDI-TOF MS
-
e.
MicroScan Walkaway
-
a.
Footnotes
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.01081-21 in this issue.
Contributor Information
Patricia J. Simner, Email: psimner1@jhmi.edu.
Carey-Ann D. Burnham, Pattern Bioscience
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing; thirty-first informational supplement. M100-S30. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.Schröttner P, Rudolph WW, Damme U, Lotz C, Jacobs E, Gunzer F. 2017. Wohlfahrtiimonas chitiniclastica: current insights into an emerging human pathogen. Epidemiol Infect 145:1292–1303. 10.1017/S0950268816003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenwick AJ, Arora V, Ribes JA. 2019. Wohlfahrtiimonas chitiniclastica: two clinical cases and a review of the literature. Clin Microbiol Newsl 41:33–38. 10.1016/j.clinmicnews.2019.01.006. [DOI] [Google Scholar]
- 4.Snyder S, Singh P, Goldman J. 2020. Emerging pathogens: a case of Wohlfahrtiimonas chitiniclastica and Ignatzschineria indica bacteremia. IDCases 19:e00723. 10.1016/j.idcr.2020.e00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez JA, Alexander AJ, Balada-Llasat JM, Pancholi P. 2017. A case of Wohlfahrtiimonas chitiniclastica bacteremia in continental United States. JMM Case Rep 4:e005134. 10.1099/jmmcr.0.005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toth EM, Schumann P, Borsodi AK, Keki Z, Kovacs AL, Marialigeti K. 2008. Wohlfahrtiimonas chitiniclastica gen. nov., sp. nov., a new gammaproteobacterium isolated from Wohlfahrtia magnifica (Diptera: Sarcophagidae). Int J Syst Evol Microbiol 58:976–981. 10.1099/ijs.0.65324-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee JK, Lee YY, Park KH, Sim J, Choi Y, Lee S-J. 2014. Wohlfahrtiimonas larvae sp. nov., isolated from the larval gut of Hermetia illucens (Diptera: Stratiomyidae). Antonie Van Leeuwenhoek 105:15–21. 10.1007/s10482-013-0048-5. [DOI] [PubMed] [Google Scholar]