Abstract
Early in the pandemic, New Jersey (NJ) long-term care facilities (LTCFs) witnessed severe COVID-19 illness. With limited surveillance to characterize the scope of infection, we estimated the prevalence of antibody to the SARS-CoV-2 nucleocapsid protein among residents and staff, to describe the epidemiology, and to measure antibody distribution by prior PCR/antigen status and symptomatology. 10 NJ LTCFs of 20 solicited with diverse geography and bed-capacities were visited between October 2020 and March 2021. A single serum was tested for total N-antibody (ELISA) by the state laboratory. Residents’ demographics and clinical history were transcribed from the patient record. For staff, this information was solicited directly from employees, supplemented by prior PCR/antigen results from facilities. 62% of 332 residents and 46% of 661 staff tested N-antibody positive. In a multivariable logistic regression in residents, odds ratios for older age and admission prior before March 1, 2020 were significant. Among the staff, odds ratios for older age, ethnic-racial group, nursing-related job, and COVID-19 symptoms were significantly associated with N-antibody positivity. In a sub-analysis in five better record-keeping LTCFs, 90% of residents and 85% of staff with positive PCR/antigen results were seropositive for N-antibody, yet 25% of residents and 22% of staff were N-antibody positive but PCR/antigen and symptoms negative. The high rate of clinically unsuspected infections likely contributed to the spread. These findings argue for robust surveillance, regular screening of asymptomatic individuals, and vaccinating both residents and staff to abate the pandemic. The data also provide guidance to prevent future outbreaks.
Keywords: SARS-Co-V-2, PCR, Antibody prevalence, Surveillance, Long-term care facility
Introduction
During the pandemic’s first wave, COVID-19 had a devastating impact on nursing homes and other long-term care facilities (LTCFs) in New Jersey, in other states, and in other high-income countries such as Canada, France, and the United Kingdom [1–7]. While LTCF staff experienced elevated morbidity and mortality, their residents—commonly elderly with multiple comorbid conditions—suffered from particularly high case fatality rates [8–10]. COVID-19 struck NJ early, and spread rapidly especially in LTCFs. The first case was diagnosed on March 4, 2020 in northern New Jersey, and by April 1st, more than 20% of NJ’s LTCFs had at least one COVID-19 case. As this first wave progressed from its epicenter in northern New Jersey through the entire state, very few LTCFs remained unscathed. By November 18, 2021, the total number of confirmed cases among LTCF residents exceeded 34,080 of whom 8006 died [11]. Cumulatively residents of LTCFs have accounted for an estimated 30% of COVID-19 deaths in New Jersey while comprising only 3% of the confirmed cases [11]. Among an estimated 55,000 staff, there have been 23,291 reported confirmed infections of whom 145 had died of COVID-19 as of November 18, 2021.
Complicating both the characterization of outbreaks and the infection control response, testing availability was limited early in the pandemic. In April 2020, real-time polymerase chain reaction (PCR) testing for SARS-CoV-2 was reserved for those LTCF residents and staff exhibiting symptoms of COVID-19, and it was not always possible to test all who were ill. In May 2020, as testing became more readily available, NJ required LTCFs to provide a baseline screening test for asymptomatic staff and residents [12]. In August 2020 regular weekly screening testing became required.
In addition to the State of New Jersey, the Centers for Medicare and Medicaid Services (CMS) also set requirements in the summer of 2020 for periodic testing of LTCF residents and staff [13]. Testing for detection of nucleocapsid antigen was implemented beginning in October, 2020 (personal communication, James Gonzalez, President, Broadway House for Continuing Care, Newark, NJ).
Given the difficulty characterizing the scope of infection in LTCFs early in the pandemic, we undertook this study (1) to estimate the prevalence of antibodies to the SARS-CoV-2 nucleocapsid protein among residents and staff of LTCFs in New Jersey, (2) to complement the serologic data with surveillance infection data available to the New Jersey Department of Health, and (3) to describe the epidemiology of COVID-19 in residents and staff. In addition, in those LTCFs with evidence of reliable reporting of antigen and PCR tests for SARS-CoV-2 as determined by matching facility records with those in New Jersey’s Communicable Diseases Reporting and Surveillance System (CDRSS), we sought to compare the distribution of SARS-CoV-2 antibody to PCR/antigen test reporting.
Methods
Sampling
The 356 NJ LTCFs were categorized into 3 constituent regions of New Jersey [north (130), central (119), and south (107)] as well as bed capacity [≤ 100 beds (164), 101–175 beds (77), and > 175 beds (115)]. We estimated that a total of 10 facilities would have provided approximately 500 residents and 1000 staff. It required solicitation from 20 LTCFs reflecting these geographic and bed capacity categories to acquire 10 participating LTCFs. Facilities were contacted by letter and by telephone to explain the details of the study. Once the facility manager indicated participation in writing, we disseminated an educational flyer for the residents and staff.
The study protocol and consent forms were approved by the Rutgers Institutional Review Board. Residents and staff were enrolled by written consent. Only residents who were able to consent for themselves were offered enrollment in the study. Within the facilities, we enrolled as many residents and staff as we could within an approximate 2 week period.
Procedures
Blood drawing on residents was done throughout the day to optimize their availability. Enrollment of staff was offered at various times of day in order to enroll both daytime and nighttime shift workers. Blood was drawn and transferred to the state’s public health laboratory (New Jersey Public Health and Environmental Laboratory, NJPHEL). Serological tests for nucleocapsid (N) antibodies (IgA, IgM, and IgG) were conducted using Bio-Rad Platelia SARS-CoV-2 Total Ab assay (authorized by the FDA under an EUA). Sensitivity and specificity of the test on serum were reported as 98.0% (95% CI 89.5, 99.6) and 99.3% (95% CI 98.3, 99.7) [14]. SARS-CoV-2 provokes antibody to the nucleoplastid (N) and spike (S) proteins. Antibody to N protein is produced only to natural infection, while antibody to S protein is made to both infection and to the vaccines administered during this study. N-antibody was chosen in this study to focus solely on infection.
Demographics and Infection Testing
Staff characteristics, such as demographics and employment type (nurse, social workers, doctor, physical or occupational therapist, administrator, maintenance and facilities) were obtained using a structured questionnaire and entered into a computerized database using REDCap (Research Electronic Data Capture) [15]. For residents, age, gender and ethnic-racial group were abstracted directly from charts available at the LTCF. For both residents and staff, ethnic-racial groups were characterized as non-Hispanic Black, non-Hispanic White, non-Hispanic Asian, Hispanic (of any race), and other or unknown. Staff were asked whether there had been a prior history of COVID-19 or symptoms of a COVID-19-like illness, and symptoms were measured against the Centers for Disease Control and Prevention (CDC) clinical case definition [16]. For residents and staff, PCR and antigen tests taken prior to the date of blood drawing were abstracted from LTCF records. Subjects were classified as ever positive on one of these viral tests if any PCR or antigen test taken prior to antibody testing was positive. When more than one test was positive, the date of the first PCR or antigen positive test was used.
Comparison of NJ DOH Surveillance Versus Study History of Infection
Per State of New Jersey Executive Directive No. 20-013 [12], no later than May 19, 2020, LTCFs were required by then Commissioner of Health Judith Persichilli to submit testing PCR and antigen results and dates to the CDRSS. To evaluate the study data of PCR and antigen results on staff and residents, we compared our study data with surveillance reporting for the facilities’ reporting to CDRSS. To estimate the completeness of reporting of PCR and antigen tests by residents or staff in each facility, we submitted the list of staff and residents participating in the study to the New Jersey State Department of Health (NJ DOH) list of SARS-CoV-2 positive individuals in the CDRSS. Without identifying individuals to our researchers, the NJ DOH reviewed the CDRSS for the number of these study participants positive by facility as of the date of our initial study visit. Facilities for whom the study team determined that PCR and antigen results were fully ascertained (for whom our positive reports accounted for at least 80% of CDRSS positives among these residents or staff) were considered “reliable” reporters. These residents and staff were then categorized by the presence of undiagnosed infection, defined as presence of the N-antibody in the absence of a history of (a) positive PCR/antigen test or (b) presumptive COVID-19 illness.
As a measure of the infection rate in residents at each facility, the percent of cumulative COVID-19 cases in CDRSS divided by the occupied beds was calculated. In a sensitivity analysis, the correlation between this ratio and the study’s N-antibody positivity for each facility was measured (Pearson) to gauge the accuracy of the N-antibody results.
Risk Factors Associated with Seropositivity
Bivariate and logistic-regression was used to estimate the odds ratio of N-antibody positivity by age groups, gender, and ethnic-racial groups, with facility serving as the matching variable. The matching by facility allowed pooling across the ten facilities to increase the sample power. We also conducted a sub-analysis of nurses and related staff defined as those who reported their position as registered nurse, licensed practical nurse, medical assistant, respiratory therapist, and phlebotomist. Analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Result
Ten sites agreed to participate. The distribution of facilities by region and bed occupancy is displayed in Table 1. Bed numbers for these LTCFs ranged from a minimum of 60 to a maximum of 574.
Table 1.
The 10 NJ LTCFs by region, bed occupancy, Staff, NJ CDRSS COVID-19 cases and deaths in residents and staff as of date of study visit to facility
| Facility | Region | Number of beds | Estimated staff | Cumulative total residents with COVID-19 | Cumulative total COVID-19 deaths in residents | Cumulative total staff with COVID-19a |
|---|---|---|---|---|---|---|
| 1 | North | < 100 | 128 | 10 | 1 | 26 |
| 2 | North | 100–175 | 174 | 44 | 11 | 33 |
| 3 | North | 100–175 | 214 | 100 | 28 | 24 |
| 4 | North | > 175 | 754 | 281 | 66 | 94 |
| 5 | North | > 175 | 284 | 82 | 15 | 37 |
| 6 | Central | < 100 | 142 | 45 | 14 | 24a |
| 7 | Central | 100–175 | 230 | 62 | 15 | 29 |
| 8 | Central | > 175 | 322 | 103 | 28 | 27 |
| 9 | South | < 100 | 108 | 29 | 2 | 21 |
| 10 | South | > 175 | 296 | 126 | 42 | 46 |
| Total | 2652 | 882 | 222 | 361 |
aOne death reported in a staff member
From October 20, 2020 through March 4, 2021, we obtained written consent and enrolled 361 residents and 730 staff at 10 LTCFs. The distribution of COVID-19 case and fatality counts by LTCF prior to visit by the study team is shown in Table 1. There was significant morbidity and mortality in residents and staff at all facilities regardless of bed count. Cumulative reported cases in residents in smaller facilities ranged from 10 to 45, and reported deaths from 1 to 14. In the largest facilities, cumulative reported cases varied from 82 to 281 with 15 to 66 reported deaths. Cases in staff across all facilities ranged from 21 to 94, and there was one COVID-19 death reported in a staff member [17].
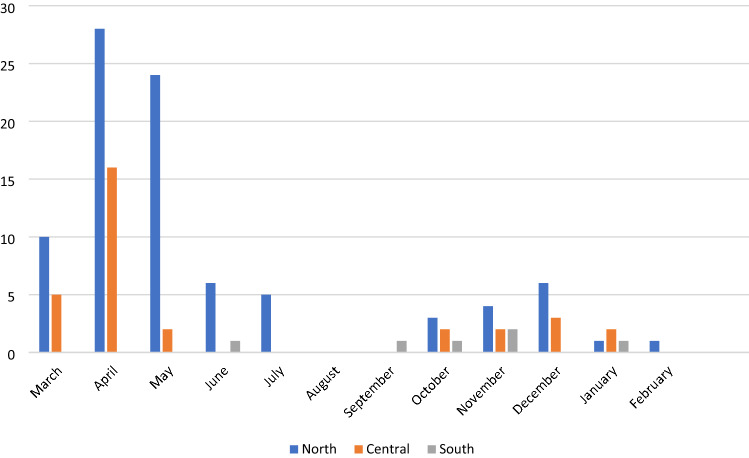
Staff case rates in the north and central regions of the state began rising sooner and peaked at a higher level than those in the south (Fig. 1). A similar trend was seen in the general population where southern case rates rose later and peaked later and lower than those in the Central and Northern New Jersey.
Fig. 1.
Staff PCR/antigen positive tests (first date) by month by facility and geography, n = 126
Serologic results were available for 337 residents: 208 (61.7%) were N-antibody positive, 124 (36.8%) were negative, and 5 (1.5%) had an equivocal result. Of the 332 residents with positive or negative N-antibody, gender was almost equally distributed: 162 residents were male and 170 were female (Table 2). The mean age of residents was 70.8 years (standard deviation 14.1). Ethnic-racial group was known for 95% of residents: 56.6% were non-Hispanic White, 19.1% were non-Hispanic African-American, 12.8% were non-Hispanic Asian and 11.6% were Hispanic.
Table 2.
Demographic characteristics of residents and staff
| Variable | Category | Residents n = 332a | Staff n = 661b | ||||
|---|---|---|---|---|---|---|---|
| Number | N-Ab percent | Chi square | Number | N-Ab percent | Chi square | ||
| Gender | Female | 170 | 65.3 | p = 0.3077 | 522 | 46.7 | p = 0.9968 |
| Male | 162 | 59.9 | 139 | 46.8 | |||
| Age in years, staff | < 25 | 24 | 20.8 | p = 0.0317 | |||
| 25–34 | 82 | 39.0 | |||||
| 35–44 | 112 | 43.8 | |||||
| 45–54 | 190 | 51.6 | |||||
| 55–64 | 171 | 51.5 | |||||
| ≥ 65 | 82 | 45.1 | |||||
| Median [IQR] | 51 | [41, 60] | |||||
| Age in years, residents | < 65 years | 114 | 46.5 | p < 0.0001 | |||
| 65–74 years | 84 | 66.7 | |||||
| 75–84 years | 67 | 71.6 | |||||
| ≥ 85 years | 67 | 76.1 | |||||
| Median [IQR] | 70 [61, 81] | ||||||
| Ethnicity/race | Non-Hispanic white | 179 | 69.8 | p = 0.0002 | 167 | 32.3 | p = 0.0002 |
| Non-hispanic black | 61 | 47.5 | 186 | 53.8 | |||
| Hispanic | 35 | 37.1 | 64 | 45.3 | |||
| Non-Hispanic Asian | 41 | 70.7 | 150 | 55.3 | |||
| Unknown/Other | 16 | 75.0 | 94 | 45.7 | |||
aFive residents had equivocal N-antibody test
bSix staff (0.90%) had an equivocal N-antibody test
Serologic results were available for 667 staff: 309 (46.3%) were N-antibody positive, 352 (52.8%) were negative, and 6 (0.9%) were equivocal. Of the 661 staff with positive or negative results, most staff (79.0%), were female. The mean age of staff was 49.3 years (standard deviation 13.0). Among the 86% of staff with known ethnic-racial group, 29.4% were non-Hispanic Whites, 32.8% were non-Hispanic African-American, 26.4% were non-Hispanic Asians, and 11.3% were Hispanics.
Of the 332 residents with positive or negative N-antibody, seropositivity in residents was higher in men (65.3% versus 59.9% in women) but not statistically significant. Incidence increased with age, from a low of 46.5% among those aged under 65 years to a high of 76.1% among those 85 years or older (Mantel–Haenszel Chi square < 0.0001, Tables 2 and 3, Residents). Non-Hispanic Whites and Asians had the highest seroprevalence (69.8% and 70.7% respectively) compared to non-Hispanic African-Americans and Hispanics (47.5% and 37.1% respectively) (Chi square p = 0.0002). Residents admitted to their LTCF before March 1, 2020, i.e., prior to the first wave, were more likely to be N-antibody positive (Chi square p = 0.0002). For the ten facilities, N-antibody positivity ranged from 19 to 84%, median 70%. The ratio of cumulative COVID-19 cases reported to CDRSS to the bed occupancy by facility ranged from 16 to 93%, median 60.5. The Pearson correlation between this ratio and N-antibody positivity by facility was 0.75 (95% CI 0.19, 0.93).
Table 3.
Bivariate and multivariate logistic regression of risk factors and N-antibody positivity: residents
| Factor | N-Ab positive, (percent) | Unadjusted OR | 95% CI | Adjusted OR | 95% CI | |
|---|---|---|---|---|---|---|
| Gender | M | 111 (65.3) | 1 | Ref | 1 | Ref |
| F | 97 (59.9) | 1.11 | 0.67, 1.83 | 0.96 | 0.548, 1.69 | |
| Age group (years) | Under 65 | 53 (46.5) | 1 | Ref | 1 | Ref |
| 65–74 | 56 (66.7) | 1.76 | 0.92, 3.39 | 1.88 | 0.92,3.849 | |
| 75–84 | 48 (71.6) | 2.04 | 0.99, 4.21 | 2.33 | 1.01, 5.39 | |
| 85 or older | 51 (76.1) | 5.16 | 2.13, 12.50 | 6.84 | 2.52, 18.58 | |
| Ethnic-racial group | Non-Hispanic white | 125 (69.8) | 1 | Ref | 1 | Ref |
| Non-Hispanic African-American | 29 (47.5) | 0.87 | 0.38, 2.02 | 1.15 | 0.45, 2.91 | |
| Hispanic | 13 (37.1) | 0.52 | 0.21, 1.31 | 0.76 | 0.27, 2.09 | |
| Non-Hispanic Asian | 29 (70.7) | 0.96 | 0.41, 2.27 | 0.71 | 0.28, 1.82 | |
| Admitted before 3/2020c | Yes | 146 (69.9) | 3.27 | 1.84, 5.79 | 3.52 | 1.90, 6.52 |
| No | 58 (49.2) | 1 | Ref |
Frequencies, percentages, unadjusted and adjusted odds ratios, and 95% confidence intervals
Odds ratios with confidence intervals excluding 1.0 are bolded
Model: N-antibody = gender, age group, ethnic-racial group, admitted to facility before 3/20/2020, n = 332a
aFive residents had an equivocal N-antibody test result
b16 residents with unknown race/ethnicity
cFive residents with unknown admission date
Of the 661 staff with positive or negative N-antibody results, the prevalence of N-antibody in men and women was equal. There was generally an increase in N-antibody positivity with age in staff, from a low of 20.8% in those less than 25 years to approximately 51.5% in the 45 to 54 and 55 to 64 year age groups (Mantel–Haenszel Chi square p = 0.0182, Tables 2 and 4, Staff). Staff who self-reported their ethnic-racial group as non-Hispanic African-American (53.8%) or non-Hispanic Asian (55.3%) were more likely than non-Hispanic Whites (32.3%) to be N-antibody positive (Chi square p = 0.001). Body mass index was not significantly associated with N-antibody positivity (p > 0.05). Nursing-related staff had a higher N-antibody positivity than non-nursing workers (OR = 1.68, 95% CI 1.10, 2.56, Chi square p = 0.0002). Staff who reported symptoms meeting the CDC case definition for COVID-19 were more than nine times more likely to be N-antibody positive (OR = 9.82, 95% CI 6.08, 15.87, Chi square p < 0.0001).
Table 4.
Bivariate and multivariate logistic regression of risk factors and N-antibody positivity: staff
| Factor | n (%) | Unadjusted OR | 95% CI | Adjusted OR | 95% CI | |
|---|---|---|---|---|---|---|
| Gender | Male | 65 (46.8) | 1 | Ref | 1 | Ref |
| Female | 244 (46.7) | 0.98 | 0.67, 1.44 | 0.79 | 0.48, 1.30 | |
| Age group (years) | Under 25 | 5 (20.8) | 1 | Ref | 1 | Ref |
| 25–34 | 32 (39.0) | 1.92 | 0.63, 5.84 | 1.41 | 0.42, 4.78 | |
| 35–44 | 49 (43.8) | 2.68 | 0.90, 7.95 | 2.24 | 0.67, 7.54 | |
| 45–54 | 98 (51.6) | 3.68 | 1.28, 10.60 | 3.65 | 1.14 11.66 | |
| 55–64 | 88 (51.5) | 3.54 | 1.23, 10.20 | 3.80 | 1.18, 12.30 | |
| ≥ 65 | 37 (45.1) | 2.72 | 0.89, 8.29 | 3.06 | 0.89, 10.54 | |
| Ethnic-racial group | Non-Hispanic White | 54 (32.3) | 1 | Ref | 1 | Ref |
| Non-Hispanic African-American | 100 (53.8) | 2.17 | 1.34, 3.52 | 3.59 | 1.99, 6.48 | |
| Hispanic | 29 (45.3) | 1.46 | 0.77, 2.75 | 2.16 | 1.04, 4.50 | |
| Non-Hispanic Asian | 83 (55.3) | 1.73 | 1.03, 2.91 | 2.29 | 1.24, 4.21 | |
| Nursing-related jobs | Other than nursing | 136 (40.1) | 1 | Ref | Ref | Ref |
| Nursing related | 173 (53.7) | 1.84 | 1.33, 2.54 | 1.68 | 1.10, 2.56 | |
| COVID-19 symptoms | Not a CDC case | 150 (33.2) | 1 | Ref | 1 | |
| CDC case | 159 (76.1) | 7.54 | 5.07, 11.23 | 9.82 | 6.08, 15.87 |
Frequencies, percentages, unadjusted and adjusted odds ratios, and 95% confidence intervals
Odds ratios with confidence intervals excluding 1.0 are bolded
Model: N-antibody = gender, age group, ethnic-racial group, nursing-related jobs, COVID-19 symptoms. n = 661a
a6 staff had an equivocal N-antibody test result
94 staff had unknown or other ethnic-racial group
In a multivariable logistic regression in residents including gender, age, racial ethnic group and admission date to the facility (Tables 3 and 4, Residents), the odds ratios were similar to those observed in the bivariate analysis. Older age and admission to the facility before March 1, 2020 remaining significant. In the staff, odds ratios for older age, Hispanic, non-Hispanic African-American, and Asian racial ethnic group, nursing-related job, and COVID-19 symptoms were significantly associated with N-antibody positivity. Restricting this multivariable analysis to nurses and nursing-related staff, the odds ratios for non-Hispanic African-Americans and Asians compared with non-Hispanic Whites remained significant OR = 4.64, 95% CI 1.96, 10.9, and OR = 2.96, 95% CI 1.16, 7.57, respectively.
There were 197 residents enrolled at sites with “reliable” reporting of PCR or antigen tests to NJ DOH; 101 (51.3%) had a positive PCR or antigen test recorded (Table 5). Of these 101, 91 (90.1%) were positive for N-antibody compared to 21 of 87 (24.1%) with a negative PCR/antigen test report (Chi square p < 0.001). There were 115 N-antibody positive residents (58.4%); 91 of these (79.1%) were PCR/antigen positive and 21 (24.7%) were PCR/antigen negative, and symptoms negative or missing information (Table 5).
Table 5.
Symptoms, PCR, and N-antibody in reliable reporting facilities
| COVID-19 symptoms or prior PCR/antigen positive result | Residents | Staff | ||||
|---|---|---|---|---|---|---|
| N-antibody | N-antibody | |||||
| Positive | Total | Percenta | Positive | Total | Percenta | |
| Negative both | 21 | 85 | 24.7 | 26 | 175 | 14.9 |
| Symptoms positive/PCR missing or negative | 0 | 2 | 0.0 | 26 | 50b | 52.0 |
| PCR positive/symptoms missing or negative | 85 | 95 | 89.5 | 21 | 29 | 72.4 |
| Both positive | 6 | 6 | 100.0 | 47 | 51 | 92.2 |
| Total | 112 | 188 | 59.6 | 120 | 305 | 39.3 |
Facilities for whom study team determined that PCR and antigen results were well-documented
aBoth residents and staff, p < 0.0001 Mantel–Haenszel Chi square for trend
bOf 50 staff, 44 were PCR negative (22 N-Ab positive), 6 lacked PCR result (4 N-Ab positive)
Nine residents (Three N-antibody positive) and Four staff (One N-antibody positive) were missing both symptoms and PCR information
There were 309 staff enrolled at sites with "reliable” reporting of PCR or antigen tests to NJ DOH; 80 (25.9%) had a positive PCR or antigen test recorded (Table 5). Of these 80, 68 (85.0%) were positive for N-antibody compared to 52 N-antibody positives (23.1%) among the 225 with a negative PCR/antigen test report (Chi square p < 0.001). Of the 44 staff who reported negative PCR/antigen history but who also reported symptoms meeting the CDC case definition of COVID-19, 22 (50%) were N-antibody positive. Overall, of the 121 N-antibody positive staff, 68 (56.2%) were PCR/antigen positive, 48 (39.7%) were PCR/antigen negative, and 26 (21.5%) were negative for PCR/antigen and symptoms (Table 5).
Discussion
This observational cross-sectional study found N-antibody in 62% of residents and 46% of staff in a sample from ten LTCFs in New Jersey during the fall and winter of 2020 to 2021. This prevalence is far higher than the 15% estimated in the general population by the Centers for Disease Control and Prevention using sera from commercial laboratories in September 2020 [18], it is also higher even than the 31% modeled using multiple data sources by the end of 2020 [7]. These findings are consistent with previous investigations into nursing home outbreaks where high antibody rates were found in residents and staff with symptoms or positive PCR tests [19, 20]. However, in the current study, there was also a sizable antibody seropositivity among residents (24.7%) and staff (14.9%) who lacked history of symptoms or positive PCR/antigen tests, even in facilities with reliable test reporting. Noted also by other investigators [21, 22] this finding suggests that asymptomatic or milder infections in either residents or staff were often not detected or diagnosed, especially in the early phase of the pandemic when testing was not widely available and routine screening testing was not performed [5]. The high rate of infection in both residents and staff underscores the ongoing need to screen for infection and vaccinate all who live or work in these sites.
The higher infection rate in nursing staff in this study was similar to findings in some but not all studies of SARS-CoV-2 in health care workers [23, 24]. In regard to race, there was a higher antibody positivity among staff but not residents who identified as African-American or Asian. Perhaps this is related to higher community exposure to the virus among staff in these groups [25, 26] This convergence of risk factors in nursing staff is of particular concern because of their interaction with residents, and again highlights the need for implementing infection control precautions including vaccination.
Limitations
The validity of PCR/antigen testing and symptomatology was dependent on the frequency of testing and documentation in the resident’s chart. Testing of residents and staff was done initially only on symptomatic individuals, so milder infection might not have been recognized. Staff with fewer symptoms or less severe illness may not have sought testing. Furthermore, testing was not available, especially earlier in the outbreak. It is also possible that staff who had positive PCR/antigen tests were undercounted because some staff were tested outside of the facility. Although our investigators reminded staff to report test results from outside providers, some tests were probably missed. Still, the finding of a trend in seropositivity from subjects with negative symptom history and PCR/antigen results, to those with symptoms but negative history of PCR/antigen, to those positive for both symptoms and PCR/antigen supports the validity of our findings.
With regard to the disagreement between staff’s history of PCR/antigen test and the reports in CDRSS, some matches were missed because of differences in name or other demographic identifiers.
We were able to enroll both small and large facilities LTCFs geographically distributed throughout NJ. However, we tested a relatively small sample of the total residents and staff in these LTCFs. The requirement that residents had to be able to consent for themselves, a minority in most of these sites, limited enrollment. Less mentally competent residents required more assistance from nursing staff, so they may have had a higher risk of infection. Their exclusion from our study may have resulted in an underestimate of the seroprevalence in residents. On the other hand, residents who consented may have actively interacted with other residents and staff socially, increasing their infection rate. Hundreds of infected residents died prior to the study period. Therefore, survivor bias most likely suppressed our seropositivity rate.
Enrollment was more successful for staff but still comprised less than half of eligible workers. As with the residents, our rate of enrollment limited our ability to generalize our findings. It is difficult to predict whether the residents and staff who agreed to be tested were more or less likely to have been infected than the non-participants.
This study used the NJ DOH SARS-Co-V-2 surveillance system to evaluate the study infection data. The validity of a high N-antibody seropositivity in residents and staff was supported by a similarly high cumulative prevalence of infection reported by these facilities to the NJ CDRSS. This is also the only study to document the movement of the infection of SARS-Co-V-2 across a cross-section of LTCFs in New Jersey or any populous state during the first wave of the pandemic.
Conclusions
In this survey of ten geographically diverse LTCFs across New Jersey, 62% of residents and 46% of the staff tested positive for antibody to SARS-CoV-2. The data suggested that there were large numbers of clinically asymptomatic or mild infections. Although undiagnosed, these infections likely contributed to spread of the virus in LTCFs. The study’s infection and seroprevalence data enhanced the picture of SARS-Co-V-2 surveillance in NJ, as the DOH’s CDRSS mutually evaluated and complemented the study findings. Regular screening of asymptomatic individuals, vaccinating both residents and staff, and the interweaving of surveillance and special surveys were integral to limiting the pandemic, and provide guidance for prompt and rapid action for future outbreaks.
Acknowledgements
The following individuals contributed to the acquisition of the data. Khyati Mehta, Aanal Patel, Nirja Chaudhari, Julia Gohler-Rubenstein. Jeffrey Li performed the N-antibody tests. Mr. Stephen Modica directed the references. The following individuals assisted in the coordination with the long-term care facilities: Ana Gomes, DO, James Gonzalez, Katherine Richardson, John Kasarda, Steven Isaac, Michael Lifschultz, Janet Merly-Liranzo, Jennifer Jones, Christina Lynn, Brian Corliss, and Jennifer Causer.
Author Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written by AED, PT, EL, MG, and SF. Subsequent versions were edited by Stephen Friedman, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the New Jersey Department of Health to the Rutgers New Jersey Medical School.
Data Availability
All data and materials as well as software application or custom code support comply with field standards.
Code Availability
Code for the code for analysis can be made availability at the discretion of the authors. The datasets generated during and/or analyzed during the current study are not publicly available due to their ongoing analysis and further study. They are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. All authors agreed with the study content and all gave explicit consent to Rutgers NJ Medical School where the work was carried out.
Consent to Participate
The authors state that they have approval from the Rutgers New Jersey Medical School Institutional Review Board. Consent to participate was signed by all study subjects.
Consent for Publication
Not applicable.
Ethics Approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McMichael TM, Currie DW, Clark S, et al. Epidemiology of covid-19 in a long-term care facility in king county Washington. New England Journal of Medicine. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu M, Maxwell CJ, Armstrong P, et al. COVID-19 in long-term care homes in Ontario and British Columbia. CMAJ. 2020;192(47):E1540–E1546. doi: 10.1503/cmaj.201860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmin J, Georges S, Franke F, Daniau C, Cochet A, Durand C, Noury U, Gomes do Espirito Santo M, Fonteneau L, Pariel S, Lafuente-Lafuente C, Danis K. Coronavirus disease 2019 in french residential care facilities: A nationwide study. Journal of the American Medical Directors Association. 2021;22(6):1142–1145. doi: 10.1016/j.jamda.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham NSN, Junghans C, Downes R, et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. Journal of Infection. 2020;81(3):411–419. doi: 10.1016/j.jinf.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New England Journal of Medicine. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagchi S, Mak J, Li Q, et al. Rates of COVID-19 among residents and staff members in nursing homes—United States, May 25-November 22, 2020. Morbidity and Mortality Weekly Report. 2021;70(2):52–55. doi: 10.15585/mmwr.mm7002e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen P, Yamana TK, Kandula S, Galanti M, Shaman J. Burden and characteristics of COVID-19 in the United States during 2020. Nature. 2021;598(7880):338–341. doi: 10.1038/s41586-021-03914-4. [DOI] [PubMed] [Google Scholar]
- 8.de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: A cross-sectional study. The Lancet Infectious Diseases. 2020;20(9):1034–1042. doi: 10.1016/s1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Internal Medicine. 2021;181(4):439–448. doi: 10.1001/jamainternmed.2020.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi SH, See I, Gouin KA, Zang S, Slifka KJ, Sauer AG, Kutty PK, Perz JF, Stone ND, Stuckey MJ, et al. Characteristics of COVID-19 in assisted living facilities - 39 states, October 2020. Morbidity and Mortality Weekly Report. 2020;69(46):1730–1735. doi: 10.15585/mmwr.mm6946a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New confirmed New Jersey State Department of Health COVID-19 Dashboard. State of New Jersey. Retrieved November 17, 2021, from https://www.nj.gov/health/cd/topics/covid2019_dashboard.shtml
- 12.Persichilli J. Covid-19 testing at licensed long-term care facilities. Signed by Commissioner Judith M. Persichilli, May 12, 2020. November 17, 2021, fromhttps://www.state.nj.us/health/legal/covid19/05-12-2020_LTC_COVID19testing.pdf
- 13.CMS. New COVID-19 testing and reporting requirements. Retrieved August 25, 2020, from https://www.cms.gov/files/document/covid-ppt-nh-all-call.pdf
- 14.FDA US. EUA authorized serology test performance. Retrieved November 17, 2021, from https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 15.https://projectredcap.org/software/
- 16.Pilishvili, T. (2020). Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States. Morbidity and Mortality Weekly Report,69(12), 343–346. https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2020-08-05/ [DOI] [PMC free article] [PubMed]
- 17.COVID-19 Nursing Home Data. Retrieved November 17, 2021, from https://data.cms.gov/covid-19/covid-19-nursing-home-data/data/november-7-2021
- 18.Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Internal Medicine. 2021;181(4):450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladhani SN, Jeffery-Smith A, Patel M, et al. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: Prospective cohort study. England EClinicalMedicine. 2020;28:100597. doi: 10.1016/j.eclinm.2020.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Candel FJ, Barreiro P, San Roman J, et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the Community of Madrid: The SeroSOS study. Age and Ageing. 2021;50(4):1038–1047. doi: 10.1093/ageing/afab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—king county, Washington, March 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stubblefield WB, Talbot HK, Feldstein LR, et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for patients with COVID-19-Nashvill Tennessee. Clinical Infectious Diseases. 2021;72(9):1645–1648. doi: 10.1093/cid/ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network—13 Academic Medical Centers, April-June 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69(35):1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sydney ER, Kishore P, Laniado I, Rucker LM, Bajaj K, Zinaman MJ. Antibody evidence of SARS-CoV-2 infection in healthcare workers in the Bronx. Infection Control & Hospital Epidemiology. 2020;41(11):1348–1349. doi: 10.1017/ice.2020.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaser M, Cailas MD, Canar J, Cooper B, Geraci P, Osiecki KM, Sambanis A. Analyzing COVID-19 Mortality Within the Chicagoland Area: Data Limitations and Solutions. Research Brief. 2020 doi: 10.25417/uic.13470324. [DOI] [Google Scholar]
- 26.Wrigley-Field E, Garcia S, Leider JP, Van Riper D. COVID-19 mortality at the neighborhood level: Racial and ethnic inequalities deepened in minnesota in 2020. Health Affairs (Millwood) 2021;40(10):1644–1653. doi: 10.1377/hlthaff.2021.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials as well as software application or custom code support comply with field standards.
Code for the code for analysis can be made availability at the discretion of the authors. The datasets generated during and/or analyzed during the current study are not publicly available due to their ongoing analysis and further study. They are available from the corresponding author on reasonable request.