Abstract
The BRAF inhibitor (BRAFi) vemurafenib improves survival of of patients with melanoma with BRAFV600E mutations. However, effects of sustained BRAFi on BRAFi-resistant melanomas with dual mutations in BRAF and NRAS are not well characterized. Jandova and Wondrak (2021) report that vemurafenib selectively enhances expression of genes involved in the epithelial-to-mesenchymal transition in BRAFV600E/NRASQ61K melanoma cells, paradoxically promoting tumor growth and metastasis in mice. This preclinical study provides compelling reasons to be cautious in the use of BRAFi in patients with NRAS-driven melanoma.
Introduction
Melanoma is one of the most aggressive cutaneous malignancies. It is typically driven by somatic mutations in oncogenes including BRAF and/or NRAS, leading to uncontrolled proliferation due to the activation of the MEK/ERK pathway (Adam et al., 2020). While more than 50% of advanced (unresectable or metastatic) melanomas harbor driver mutations in the BRAF gene (BRAFV600) as the most common mutation, NRAS mutations (including NRASQ61K) are the second most common oncogenic alterations in melanoma (Echevarria-Vargas and Villanueva, 2017). Significant therapeutic progress has been achieved in past few years targeting BRAF-driven melanomas. BRAF inhibitors (BRAFis) such as vemurafenib and dabrafenib have been approved for the treatment of BRAF-mutation-bearing melanomas, after demonstration of significant improvement over cytotoxic chemotherapy. The approved combination of the BRAF inhibitor dabrafenib with the MEK inhibitor trametinib demonstrated improved progression-free survival as compared to monotherapy (Robert et al., 2015). Despite advances in targeting BRAF-driven melanomas, fewer treatment options exist for NRAS-driven melanomas which are aggressive tumors with shorter patient survivals as compared to non-NRAS-mutant melanomas (Yin et al., 2019). Unfortunately, patients with NRAS mutations lack targetable BRAF mutations and feature a high degree of intrinsic and acquired resistance to MEK inhibition.
In previous studies, NRAS mutations (Q61R, Q61K) have been shown to cause resistance to BRAFi in melanomas with BRAFV600E mutations. This represents the unique co-occurrence of BRAF and NRAS mutations that is facilitated and selected by BRAFi therapy (Johnson et al., 2015). Extensive research has been performed to determine the molecular mechanisms underlying acquired NRAS-driven BRAFi-resistance. The most common mechanism is believed to be reactivation of the MAPK pathway that is stimulated by NRAS. However, there is a clear knowledge gap regarding the effects of BRAFi treatment on BRAFi resistant tumors displaying dual BRAF- and NRAS- mutations. Valid research questions remain to be addressed: i) should BRAFi treatment be continued in patients harboring dual mutations of BRAFV600E/NRASQ61K? and ii) what would the BRAFi-treatment response be in these patients with melanoma? Therefore, this is a very interesting and clinically relevant study in the context of BRAFi-treatment efficacy, treatment failure and acquired resistance in patients with melanoma.
In their new article in the Journal of Investigative Dermatology, (Jandova and Wondrak, 2021) analyzed the effects of an FDA-approved BRAFi (vemurafenib) on BRAFi-sensitive A375-BRAFV600E/NRASQ61 and an accepted genetic model of BRAFi resistance, A375-BRAFV600E/NRASQ61K melanoma cells, employing in vitro as well as in vivo approaches. This study provides novel information and suggests that BRAFi treatment selectively targets BRAFV600E/NRASQ61K melanoma cells upregulating epithelial-to-mesenchymal transition (EMT) gene expression, and paradoxically promoting invasiveness and metastasis. In this study, the authors conducted in vitro experiments using BRAFi-sensitive A375-BRAFV600E/NRASQ61 versus BRAFi-resistant A375- BRAFV600E/NRASQ61K melanoma cells followed by an in vivo validation in a SCID mouse bioluminescent melanoma metastasis model. They determined that BRAFi treatment enhanced lung tumor burdens caused by mutant A375-BRAFV600E/NRASQ61K cells, while inhibiting the metastatic potential of A375-BRAFV600E/NRASQ61. Herein we discuss the implications of these findings in the context of current research regarding the NRAS-driven BRAFi resistance in melanoma.
EMT and EMT to metastasis pathways in response to NRAS-driven BRAFi resistance in melanoma
EMT is a process by which malignant melanocytic cells lose their epithelial characteristics and acquire a mesenchymal phenotype leading to an increase in the migration, invasiveness and metastatic potential (Pearlman et al., 2017). The results obtained by Jandova and Wondrak (2021) regarding the increased expression of genes associated with ‘EMT’ and ‘EMT to metastasis’ pathways in BRAFi-resistant NRASQ61K melanoma cells are consistent with prior research showing increased metastatic potential of NRAS-driven melanomas (Eskandarpour et al., 2009). An important aspect of this study is that the authors performed a comprehensive high throughput NanoString™-based transcriptomic profiling and identified the differential upregulation of EMT- and metastasis-related gene expression networks. They identified modulation of ten genes encoding EMT- and metastasis-related transcription factors (TWIST1), extracellular matrix components (CLDN4, COL6A2, FN1, ITGA1, ITGA7, LAMA5), and components of inflammatory and growth factor signaling cascades (AREG, CXCL8, PDGFRB).
The authors further validated the differential expression of EMT-related genes as a function of NRASQ61K-genotype and BRAFi-treatment employing a more specific EMT-focused RT2-Profiler™ qPCR array. They observed that BRAFi paradoxically enhanced EMT-related gene expression as well as transcription factors ZEB1, ZEB2 in double mutant BRAFV600E/NRASQ61K melanoma cells in comparison to the BRAFV600E/NRASQ61melanoma cells that harbored only BRAF mutations. A review of the current literature shows that the EMT has a well-established role in melanoma progression and that EMT-related proteins are considered to be valid therapeutic targets for melanoma metastasis (reviewed in (Pedri et al., 2021)). Thus, the findings demonstrating that BRAFi treatment selectively targets BRAFV600E/NRASQ61K melanoma cells by promoting EMT-related gene expression (Figure 1) are relevant towards the development of future treatment strategies to overcome drug resistance in patients with melanoma.
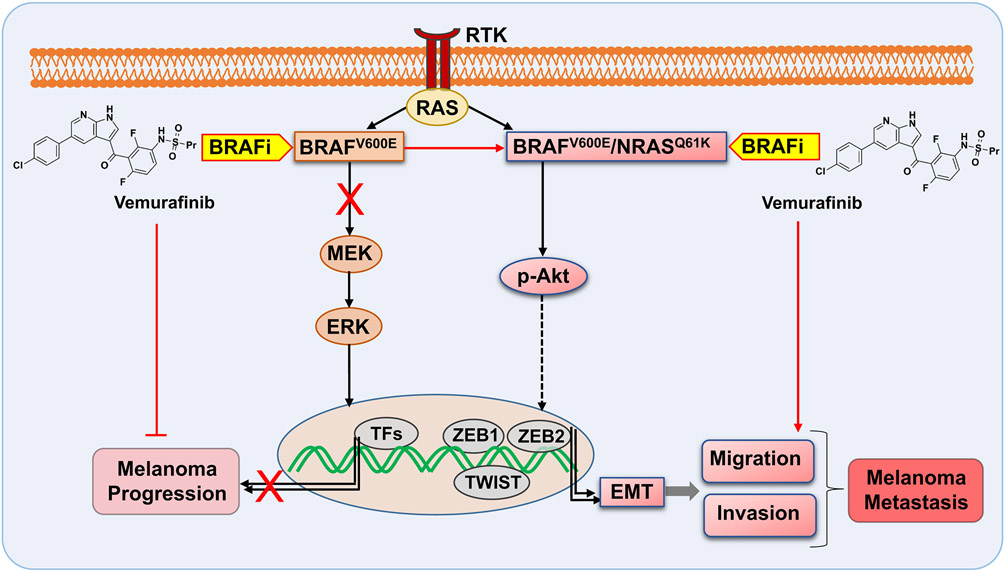
Figure 1. Opposing effects of the BRAF inhibitor vemurafinib on melanoma cells harboring BRAFV600E and BRAFV600E/NRASQ61K.
Vemurafenib treatment selectively targets BRAFV600E/NRASQ61K melanoma cells upregulating expression of epithelial-to-mesenchymal transition (EMT) genes, paradoxically promoting invasiveness and metastasis.
RTK: Receptor Tyrosine Kinase; TFs: transcription Factors; BRAFi: BRAF-inhibitor; ERK, extracellular signal–regulated kinase; MEK, MAPK/extracellular signal–regulated kinase kinase; p-Akt, phosphorylated protein kinase B.
Enhanced migratory and invasive potential of BRAFV600E/NRASQ61K melanoma cells in response to BRAFi-treatment
As melanoma progresses and penetrates the basal layer of the epidermis, tumor cells gain access to blood and lymph vessels leading to an increase in metastatic potential. This is often accompanied by phenotypic changes in melanoma cells with enhanced cell motility and migration as well as invasiveness. Together, migration and invasion potential of tumor cells are important parameters to assess metastatic potential. Using in vitro experiments, Jandova and Wondrak (2021) first determined the effect of vemurafinib on cellular viability, proliferation, and colony formation followed by migration and invasion potential, employing isogenic melanoma cell lines harboring BRAF-mutations and BRAF/NRAS double mutations. The authors demonstrated that vemurafenib-treatment affected cellular proliferation in opposing ways as a function of genotype. However, the viability of melanoma cells remained unaffected by BRAFi irrespective of genotype. The cellular proliferation and colony formation data showed that BRAFi-treatment inhibited BRAFi-sensitive A375-BRAFV600E/NRASQ61 melanoma cells. Interestingly, A375 melanoma cells with BRAFV600E/NRASQ61K displayed the opposite phenotype showing BRAFi-treatment caused an increase in proliferation, considered as a genotype-specific effect. Most importantly, Jandova and Wondrak (2021) observed a significant increase in migration and invasiveness of A375-BRAFV600E/NRASQ61K melanoma cells following BRAFi-treatment. As a possible mechanism for these genotype-specific effects of BRAFi-treatment, the authors observed loss of BRAFi-induced blockade of p-ERK1/2 and an increase in AKT-phosphorylation (p-AKT; Ser473) in BRAFV600E/NRASQ61K melanoma cells compared to BRAFi-treated BRAFV600E/NRASQ61 cells. However, a thorough evaluation and cause-and-effect validation of these mechanisms will require additional investigation.
Increased tumor growth and lung metastasis with BRAFi-treatment in BRAFV600E/NRASQ61K melanomas
Another important aspect of this study is that the authors used an animal model to validate their in vitro findings. They determined the effects of BRAFi-treatment in a bioluminescent SCID melanoma xenograft mouse model and compared tumor growth and metastatic potential of BRAFV600E/NRASQ61K and BRAFV600E/NRASQ61 melanoma cells. Corresponding to the in vitro phenotypic changes, the authors found significant enhancements of tumor growth and metastasis as a response to BRAFi-treatment of BRAFV600E/NRASQ61K-recipient mice. Immunohistochemical (IHC) analysis of tumor tissues demonstrated enhanced proliferation marker PCNA-positive cells only in BRAFV600E/NRASQ61K-recipent mice in response to BRAFi-treatment when compared to BRAFV600E/NRASQ61-recipent mice. It is important to note that one contradictory result was observed in IHC staining of tumor tissue sections regarding the expression of MMP9, an indicator of invasiveness in melanoma. In vitro data using RT-qPCR and immunoblot analysis on BRAFi-induced MMP9 upregulation in A375-BRAFV600E/NRASQ61K cells contrasted with the in vivo situation showing no increase in MMP9 expression in IHC staining of tumors from A375-BRAFV600E/NRASQ61K-recipient mice after BRAFi-treatment. In a recent study, MMP9 has been shown as a candidate marker of responses to BRAFi in melanoma patients harboring BRAFV600E mutation (Salemi et al., 2018). However, studies correlating MMP9 expression with NRAS mutations in melanoma are lacking.
Innovation and Limitations
This study is innovative in several respects. For the first time, the effects of BRAFi-treatment have been determined using a stringent genetic model with dual BRAF/NRAS-mutated BRAFi-resistant melanomas, employing a genetic model of NRASQ61K driven BRAFi-resistance in isogenic BRAFV600E-driven melanoma cell lines. Further, Nanostring nCounter™ profiling of isogenic malignant melanoma cells was used to determine gene expression changes in BRAFV600E/NRASQ61K cells relative to BRAFV600E/NRASQ61 cells. The genes involved in melanoma EMT have been observed to be responsive to BRAFi-treatment as a function of NRAS-genotype. Moreover to our knowledge, this study is the first to show invasiveness and lung metastasis imposed by BRAFV600E/NRASQ61K cells (while inactivating the BRAFV600E/NRASQ61-genotype), a preclinical finding that has the potential to inform clinical decisions in the future.
As in any research study, there are limitations that have been acknowledged by the authors. First, this study is focused on only one NRAS mutation (NRASQ61K) in combination with BRAFV600E. Further detailed investigations are required to show if these results are representative of other NRAS mutations that also confer BRAFi-resistance in malignant melanoma. Similarly, the findings are based on one specific BRAF inhibitor (vemurafenib) and the effects observed in this study should be validated with other clinically relevant BRAFi therapeutics. In addition, the findings in this study should be expanded to BRAFi and MEKi combination therapies as this is the current standard of care for melanoma in the first-line setting. Moreover, in this study, in vivo findings are restricted to one melanoma xenograft model. Future clinical validations of the findings of this study are needed using patient derived xenograft models and clinical BRAFi resistance patient tumors.
Conclusion
Successes of currently used targeted therapies for melanoma are limited owing to the occurrence of drug resistance and paradoxical oncogenesis. Therefore, understanding the mechanisms of resistance and effects of currently approved drugs on resistant tumors fills a knowledge gap and promotes development of new strategies to overcome drug resistance during melanoma management. The paper by Jandova and Wondrak (2021) provides substantial evidence regarding the opposing effects of the BRAFi vemurafinib on BRAFi-sensitive (BRAFV600E/NRASQ61) and BRAFi-resistant (BRAFV600E/NRASQ61K) isogenic melanoma cells, in vitro as well as in vivo. Although this is a preclinical study, the findings could help identify novel avenues of melanoma management for patients with melanoma with NRAS-driven BRAFi resistant tumors who are receiving BRAFi-treatment. In light of the observation by Jandova and Wondrak (2021) that the BRAFi vemurafinib increases proliferation, tumor growth and metastasis of BRAFi-resistant (BRAFV600E/NRASQ61K) melanoma cells (Figure 1), it is important that clinical decisions in the treatment of BRAF/NRAS double mutated patients with melanoma with vemurafinib, which could lead to enhanced invasiveness and metastasis, should be made deliberately. Because current research is increasingly moving towards personalized medicine that is based on specific markers and mutations in individual patients for effective cancer management, the work published by Jandova and Wondrak (2021) could be a valuable contribution related to treatment of melanoma, a highly heterogeneous malignancy.
Clinical Implications.
The BRAF inhibitor vemurafenib selectively targets BRAFV600E/NRASQ61K melanoma cells (with mutations in both BRAF and NRAS) to enhance the epithelial-to-mesenchymal transition (EMT), thereby enhancing invasion and metastasis.
Vemurafenib treatment enhances lung tumor burdens caused by vemurafenib-resistant BRAFV600E/NRASQ61K melanoma cells in mice.
Clinical decisions regarding targeted therapy in patients with NRAS-driven BRAFi-resistant tumors should be made deliberately.
ACKNOWLEDGMENTS
We acknowledge funding support from the National Institutes of Health (Bethesda, MD) (P30 CA014520) and the Department of Veterans Affairs (VA Merit Review Awards from BLR&D and CSR&D and a Research Career Scientist Award from BLR&D).
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Adam C, Fusi L, Weiss N, Goller SG, Meder K, Frings VG, et al. Efficient Suppression of NRAS-Driven Melanoma by Co-Inhibition of ERK1/2 and ERK5 MAPK Pathways. J Invest Dermatol 2020;140(12):2455–65 e10. [DOI] [PubMed] [Google Scholar]
- Echevarria-Vargas IM, Villanueva J. Combating Nras Mutant Melanoma: From Bench to Bedside. Melanoma Manag 2017;4(4):183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandarpour M, Huang F, Reeves KA, Clark E, Hansson J. Oncogenic NRAS has multiple effects on the malignant phenotype of human melanoma cells cultured in vitro. Int J Cancer 2009;124(1):16–26. [DOI] [PubMed] [Google Scholar]
- Jandova J, Wondrak GT. Vemurafenib drives EMT gene expression in BRAFi-resistant BRAF(V600E)/NRAS(Q61K) melanoma enhancing tumor growth and metastasis in a bioluminescent murine model. J Invest Dermatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DB, Menzies AM, Zimmer L, Eroglu Z, Ye F, Zhao S, et al. Acquired BRAF inhibitor resistance: A multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer 2015;51(18):2792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Potential therapeutic targets of epithelial-mesenchymal transition in melanoma. Cancer Lett 2017;391:125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedri D, Karras P, Landeloos E, Marine JC, Rambow F. Epithelial-to-mesenchymal-like transition events in melanoma. FEBS J 2021. [DOI] [PubMed] [Google Scholar]
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372(1):30–9. [DOI] [PubMed] [Google Scholar]
- Salemi R, Falzone L, Madonna G, Polesel J, Cina D, Mallardo D, et al. MMP-9 as a Candidate Marker of Response to BRAF Inhibitors in Melanoma Patients With BRAF(V600E) Mutation Detected in Circulating-Free DNA. Front Pharmacol 2018;9:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Zhu B, Zhang T, Liu T, Chen S, Liu Y, et al. Pharmacological Targeting of STK19 Inhibits Oncogenic NRAS-Driven Melanomagenesis. Cell 2019;176(5):1113–27 e16. [DOI] [PubMed] [Google Scholar]