Abstract
From 1991 through 1998, 1,266 cases of shellfish-related illnesses were attributed to Norwalk-like viruses. Seventy-eight percent of these illnesses occurred following consumption of oysters harvested from the Gulf Coast during the months of November through January. This study investigated the ability of eastern oysters (Crassostrea virginica) to accumulate indicator microorganisms (i.e., fecal coliforms, Escherichia coli, Clostridium perfringens, and F+ coliphage) from estuarine water. One-week trials over a 1-year period were used to determine if these indicator organisms could provide insight into the seasonal occurrence of these gastrointestinal illnesses. The results demonstrate that oysters preferentially accumulated F+ coliphage, an enteric viral surrogate, to their greatest levels from late November through January, with a concentration factor of up to 99-fold. However, similar increases in accumulation of the other indicator microorganisms were not observed. These findings suggest that the seasonal occurrence of shellfish-related illnesses by enteric viruses is, in part, the result of seasonal physiological changes undergone by the oysters that affect their ability to accumulate viral particles from estuarine waters.
Molluscan shellfish are vectors of bacterial and viral pathogens, including Salmonella typhi, Vibrio parahaemolyticus, V. vulnificus, hepatitis A virus, and Norwalk-like virus (NLV) (22, 23). The consumption of raw or partially cooked shellfish resulted in more than 2,100 illnesses in the United States from 1991 to 1998. The majority of these shellfish-associated illnesses (1,266 cases) were attributed to enteric viruses, particularly NLV (14, 26). Unlike illnesses caused by naturally occurring Vibrio spp. in shellfish, illnesses from enteric viruses in shellfish originate from the bodily wastes (including feces and vomit) from ill individuals. Seventy-eight percent of the reported illnesses due to NLV reported in the 1990s are associated with oysters harvested from the Gulf Coast during the months of November to January (Table 1) (14).
TABLE 1.
Gastrointestinal illnesses associated with NLV and the Eastern oysters (C. virginica) by month (1991 to 1997)a
| Mo of harvest | No. of outbreaks | No. of cases |
|---|---|---|
| November | 3 | 218 |
| December | 5 | 692 |
| January | 3 | 77 |
| February–April | 3 | 43 |
| May–October | 0 | 0 |
| Total | 14 | 1,030 |
Oysters are filter-feeding animals that can filter several liters of seawater daily (13). If pathogenic microorganisms are present in the water, oysters may accumulate the pathogens to levels considerably greater than those in the overlying water (20, 21). The bioaccumulation and elimination kinetics of enteric bacteria and viruses by bivalve mollusks vary with the species of shellfish (7), type of microorganism (4), environmental conditions, and season (4, 8).
The objective of this study was to identify factors that contribute to the temporal occurrence of enteric viruses in oysters from the Gulf Coast. Identification of these contributing factors was assessed by investigating the seasonal bioaccumulation of the traditional (fecal coliforms, Escherichia coli) and alternative sanitary indicator microorganisms (Clostridium perfringens and F+ coliphage) from estuarine water by oysters.
MATERIALS AND METHODS
Shellfish contamination.
Eastern oysters (Crassostrea virginica) were harvested from reefs in the Mississippi Sound near Dauphin Island, Ala. Oysters were depurated in a flow-through, natural-seawater, UV-disinfected depuration system for a minimum of 7 days prior to each bioaccumulation trial to provide the shellfish an opportunity to purge any background levels of indicator organisms. This length of time has been determined previously to effectively reduce fecal coliform densities by >99.9% under similar conditions (4).
Following depuration, 30 oysters were placed as a monolayer into a circular, fiberglass tank (80-cm diameter; 40-liter working volume). Seawater pumped from Little Dauphin Island Bay was continuously metered (2 liters/min) into this circular tank with a proportioning pump (Barnant Co., Barrington, Ill.). Wastewater collected from the City of Mobile (Ala.) Conception Street and the City of Bayou La Batre (Ala.) wastewater treatment facilities was metered into the circular contamination tank with a proportioning pump (model 7511-50; Ismatec Co.). The final dilution of seawater to sewage was approx 700:1. Wastewater served as the primary source of the indicator microorganisms present in the contamination system. Seawater and wastewater were continuously and thoroughly mixed in the contamination tank with a submersible pump (model 1; Little Giant, Inc., Oklahoma City, Okla.).
At the onset of each contamination trial, 30 additional oysters were collected from the depuration system and analyzed to determine the background levels of indicator microorganisms (see description below). These oysters served as the time zero control samples.
Sample collection.
Water samples from the contamination tank water were collected in sterile 500-ml polypropylene bottles on days 2, 6, and 7 for indicator organism density determinations. Samples were held under refrigeration until analysis, which was performed within 3 h of collection. Temperature, salinity, and dissolved oxygen (DO) of the contamination water were determined prior to the collection of each water sample by using a combination conductivity salinometer–DO meter (model 85; Yellow Springs Instruments, Yellow Springs, Ohio). Following 7 days of wastewater exposure, the oysters were collected and placed into a new, clean polyethylene bag for transport to the adjacent shucking room.
Water analysis.
Densities of the indicator organisms in the contamination water were determined using membrane filtration procedures (HC filters; Millipore Corp., Bedford, Mass.). Fecal coliform and E. coli densities were determined by using the mTEC procedure (Difco Laboratories, Detroit, Mich.) (12, 24); mCP agar was used to determine C. perfringens densities (3), and male-specific coliphage densities (F+ coliphage) were determined by using a modified double-agar-overlay procedure that incorporates E. coli strain (HS [pFamp] R) as the bacterial host strain (10).
Shellfish analysis.
Shellfish samples comprising of 24 to 30 oysters were randomly subdivided into three subsamples of 8 to 10 animals each. Each subsample was scrubbed, shucked, and independently analyzed in accordance with the recommended procedures for shellfish analysis (2). Fecal coliform and E. coli densities were determined by using the conventional five-tube multiple dilution most-probable-number (MPN) procedure. Lauryl tryptose broth (Difco) was chosen as the presumptive growth media, while presumptively positive tubes were confirmed for fecal coliforms and E. coli in EC media (Difco) with MUG (50 μg/ml; Biosynth International, Naperville, Ill.) (25). C. perfringens densities were determined by using a five-tube multiple dilution procedure incorporating iron milk media (1). F+ coliphage densities were determined by using a modified double-agar-overlay method described by Cabelli (6). Again, the E. coli strain HS (pFamp) R was utilized as the host strain.
Data analysis.
Densities of each indicator organism in the oysters were expressed as the geometric mean/100 g calculated from the three subsamples. Indicator densities, water temperature, salinity, and DO for the contamination water were calculated as the geometric mean of determinations made on days 2, 6, and 7. Accumulation factors were calculated as the geometric mean of the indicator density of each microorganism in the shellfish divided by the geometric mean density of the particular indicator found in the contamination water.
Statistical analyses of shellfish bioaccumulation of indicator microorganisms were conducted using SigmaStat (version 2.0; SPSS, Inc., Chicago, Ill.). Analysis of variance and Pearson regression analyses were performed to determine the relationships of indicator bioaccumulation and environmental parameters.
RESULTS
Study conditions.
Bioaccumulation of indicator organisms from estuarine water by the oysters was investigated for 12 consecutive months (October 1997 through September 1998) and consisted of 23 7-day trials. Trials were conducted to regulate contaminant exposure levels while allowing the shellfish to be exposed to estuarine conditions reflective of the area from which they were harvested. Temperature, salinity, and DO of the water varied considerably reflecting short-term local meteorological events and seasonal changes. The mean water temperature of the 23 trials was 22.5 ± 5.9°C with a range of 14 to 30°C (December to July, respectively) (Fig. 1). The mean dissolved oxygen concentrations and salinities were 6.9 ± 1.5 ppm and 19.3 ± 7.4 ppt, respectively. Dissolved oxygen concentrations were significantly related to water temperatures (r = 0.82; P < 0.001). The mean densities (± the standard error) of fecal coliforms, F+ coliphage, and C. perfringens in the water for the 23 trials were 1,300 (±150), 212 (±37), and 19 (±2.8) per 100 ml, respectively.
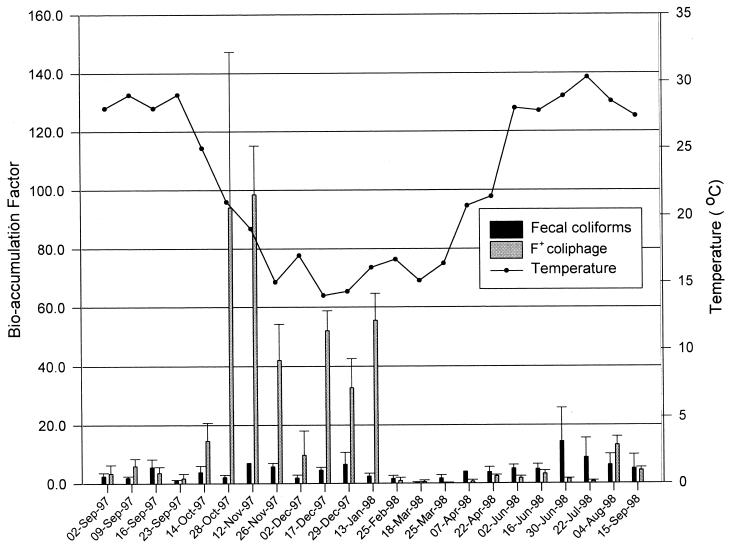
FIG. 1.
Geometric mean bioaccumulation of fecal coliforms and F+ coliphage by C. virginica in Gulf Coast estuarine water assessed with respect to season and temperature.
Indicator organism accumulation.
Oysters accumulated F+ coliphage to densities averaging 19 times greater than the levels in the contaminated estuarine water; however, accumulation varied, from <1- to 99-fold. From late October through January, the accumulation was significantly (P < 0.001) greater than during any other time of the year (Table 2). This period of increased bioaccumulation activity we identified as a period of hyperaccumulation, defined as a period when the mean accumulation factor of a particular organism is greater than one standard deviation (SD) of the mean for the entire data set. During the period of hyperaccumulation, the mean accumulation factor for F+ coliphage was 49.9. In contrast, the mean accumulation factor for F+ coliphage during the months of February through early October was 2.9. Regression analysis on the bioaccumulation of F+ coliphage throughout all 23 trials demonstrated that the F+ coliphage bioaccumulation factors were inversely related to temperature (r = −0.401; P < 0.001).
TABLE 2.
Bioaccumulation of indicator organisms by the eastern oyster (C. virginica)
| Indicator organism(s) | February–early October (n = 15)
|
Late October–January (n = 8)
|
||
|---|---|---|---|---|
| BAFa | SE | BAFa | SE | |
| Fecal coliforms | 4.4 | 0.5 | 4.2 | 0.5 |
| E. coli | 4.1 | 0.5 | 3.3 | 0.5 |
| C. perfringens | 58.1 | 7.8 | 62.0 | 12.2 |
| F+ coliphage | 2.9 | 0.5 | 49.9b | 7.4 |
BAF, bioaccumulation factor. Value is the mean ratio of the indicator organism in shellfish compared to water.
Significant increase (P < 0.001) as determined by the Mann-Whitney rank sum test.
In contrast to F+ coliphage, neither fecal coliforms, E. coli, nor C. perfringens exhibited a period of hyperaccumulation during any period of the year. The mean accumulation factors ± the SE for fecal coliforms, E. coli, or C. perfringens were 4.4 ± 0.5, 3.8 ± 0.5, and 59.5 ± 6.6, respectively (Table 2). A significant correlation between fecal coliforms and E. coli accumulation by Gulf Coast oysters was found throughout all seasons and trials (r = 0.845; P <0.001). However, the accumulation factors of fecal coliforms and E. coli (data not shown) were unrelated to those of F+ coliphage or C. perfringens (P > 0.05). In addition, the bioaccumulation factors of F+ coliphage were unrelated to those of C. perfringens (r = 0.081; P = 0.47). Background levels of fecal coliforms and F+ coliphage in the shellfish were always below the sensitivity of their respective assays (<20/100 g for fecal coliforms; <6/100 g for F+ coliphage) prior to the initiation of each bioaccumulation trial. C. perfringens organisms were generally below the level of detection (<20/100 g); however, when C. perfringens was detected, the levels were subtracted from those at the conclusion of bioaccumulation to account for the elevated levels at the onset.
DISCUSSION
Our investigation of bioaccumulation of several groups of traditional and alternative sanitary indicator organisms by Eastern oysters from the Gulf Coast attempted to identify a connective link with the seasonal occurrence of shellfish-related illnesses from oysters from the Gulf Coast. Conditions were selected to reflect conditions experienced by oysters in a nearby reef but to allow control of the type and level of contaminant exposure. In comparison, previous studies have investigated the bioaccumulation of microorganisms by shellfish in ways which are inherently different from the way shellfish are usually exposed to pollutants, including (i) utilization of laboratory strains of microorganisms, (ii) use of synthetic seawater, and (iii) batch or short-term exposure (24 h) of contaminants. In addition, these earlier studies paid little attention to the physiological state (e.g., dormancy, spawning condition) of the shellfish prior to these investigations.
Fecal coliforms are a group of gram-negative bacteria generally associated with the waste of warm-blooded animals and are used as indicators of sanitary quality of shellfish-growing waters. This study found that eastern oysters in the estuarine environment of the Gulf Coast accumulated fecal coliforms to levels that averaged 4.4 times (SD = 4.0) greater than levels in the water to which they were exposed.
Shellfish harvest areas are classified, in part, by the densities of fecal coliforms present in their surface waters. Estuarine waters approved for unrestricted shellfish harvest have a mean fecal coliform MPN of ≤14/100 ml, with 10% of the samples not to exceed an MPN of 43/100 ml in a five-tube decimal dilution test (28). Assuming oysters are harvested from a growing area where the mean fecal coliform level is 14/100 ml and the uppermost accumulation factor for fecal coliforms by oysters is 8.4 (mean + the SD), a prediction of fecal coliform density can be made by multiplying the concentration factor of fecal coliforms by oysters and the mean maximum level of fecal coliforms in the water: fecal coliform density would not exceed 118/100 g of oysters. In addition, assuming all of the fecal coliforms in a harvest area were E. coli, and the accumulation factor of E. coli by oysters is 7.9 (mean + the SD), the predicted densities of E. coli in the oysters would generally not exceed 111/100 g. This scenario assumes that the level of contamination exposure is constant, that the indicator densities in bottom waters reflect those of the surface water, and that the period of shellfish exposure is lengthy. These conditions, however, are not generally maintained in shellfish harvest areas, and therefore these densities would likely be the upper levels encountered in approved growing areas. Conversely, assuming the minimum accumulation of fecal coliforms by oysters is 0.4 (mean − the SD), while maintaining fecal coliform levels in the water at 14/100 ml, the predicted density of fecal coliforms in shellfish could be 6/100 g or less. On the basis of these calculations, therefore, it can be assumed that oysters harvested from an approved growing area could be expected to have fecal coliform densities of <6 to 118/100 g.
C. perfringens, a spore-forming anaerobic bacteria, is more resistant to disinfection (4, 15) and environmental conditions than fecal coliforms and E. coli (5). However, unlike fecal coliforms, there are no established standards for using C. perfringens densities to assess the sanitary quality of shellfish or shellfish-growing waters. This is attributed to the inability to distinguish the age of the contamination due to the extreme stability of this organism in the environment. The present study demonstrates that C. perfringens bioaccumulation was not significantly influenced by the environmental conditions (temperature, salinity, or DO) or seasonal changes. However, since this organism is highly accumulated (up to 245-fold), it may serve as a sentinel to determine if an oyster harvest area had been impacted by waste. Unfortunately, the extreme range of accumulation (5- to 245-fold) makes its utility in determining the magnitude of such an impact speculative.
F+ coliphage are a heterogeneous group of bacteriophage that are more resistant to chlorine, UV, and ozone than fecal coliforms (4, 16, 17) but which, like the Norwalk virus, are resistant to chlorine (18). The survival of F+ coliphage in seawater is similar to the enteric viruses hepatitis A virus, poliovirus, and rotavirus (9).
We found that the accumulation of F+ coliphage by Gulf Coast oysters was highly variable, with accumulation factors ranging from <1 to 99. Hyperaccumulation of F+ coliphage was not related to environmental conditions (e.g., temperature, salinity, and DO). However, it appeared that the period of hyperaccumulation began as water temperature declined in the fall, and ended when water temperature began to rise in early spring. This is significant since the period of hyperaccumulation of these coliphage corresponds with the months when oysters harvested from the Gulf Coast result in the majority of illnesses due to NLVs (Table 1).
This study complements previous studies that suggest there are at least three factors responsible for the occurrence and timing of shellfish-associated viral illnesses. First, the enteric viral pathogen must be present in the population. The prevalence of enteroviruses in wastewater and sewage fluctuates seasonally, with the greatest levels occurring in the winter and the lowest occurring in the summer (27). Unfortunately, little is known specifically about the carrier rate of NLVs in the population or its seasonal occurrence. Second, the viral pathogen(s) must survive in the estuarine environment for a period long enough to impact a shellfish-growing area. Viral survival in estuarine water is modulated by temperature and sunlight exposure (5, 9, 19). The occurrence of shellfish-related illnesses corresponds generally to periods when water temperature and sunlight intensity are at or near their lowest levels. We suggest a third factor influencing the timing and occurrence of shellfish-related outbreaks: the ability of shellfish to selectively accumulate viruses, as demonstrated by the accumulation of F+ coliphage. This aspect of selective accumulation can be attributed to the mechanism by which shellfish feed. The accumulation of viruses by shellfish during feeding is due, in part, to the ionic bonding of viral particles to the mucopolysaccharide moiety of shellfish mucus (11). The level of mucus production, in turn, corresponds generally to the glycogen content of the connective tissue and gonadal development (13). Further research is needed to determine if a connection exists between the concentration of glycogen, which is highest in oysters from late November through March, and the timing of illnesses. These findings, however, demonstrate clearly that the incidence of shellfish-related illness is a dynamic relationship between the level of fecal pollution and the ability of the shellfish to accumulate and retain enteric pathogens.
ACKNOWLEDGMENTS
We acknowledge the technical support of C. A. Collier and T. K. Previto of the U.S. Food and Drug Administration's Gulf Coast Seafood Laboratory, Dauphin Island, Ala. We also thank G. Hoskin, D. W. Cook, and R. M. McPhearson for their constructive comments during the preparation of the manuscript.
REFERENCES
- 1.Abeyta C. Comparison of iron milk and official AOAC methods for enumeration of Clostridium perfringens from fresh seafood. J Assoc Off Anal Chem. 1983;66:1175–1177. [PubMed] [Google Scholar]
- 2.American Public Health Association. Recommended procedures for the examination of seawater and shellfish. 4th ed. Washington, D.C.: American Public Health Association; 1970. [Google Scholar]
- 3.Bisson J W, Cabelli V J. Membrane filter enumeration of Clostridium perfringens. Appl Environ Microbiol. 1979;37:55–66. doi: 10.1128/aem.37.1.55-66.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkhardt W, III, Rippey S R, Watkins W D. Depuration rates of northern quahogs Mercenaria mercenaria and eastern oysters Crassostrea virginica in ozone- and ultraviolet light-disinfected seawater systems. J Shellfish Res. 1992;11:105–109. [Google Scholar]
- 5.Burkhardt, W., III, K. R. Calci, W. D. Watkins, S. R. Rippey, and S. J. Chirtel. Inactivation of indicator organisms in estuarine waters. Water Res., in press.
- 6.Cabelli V J. Microbial indicator levels in shellfish, water, and sediments from the upper Narragansett Bay conditional shellfish-growing area. 1988. Report to the Narragansett Bay Project. Narragansett Bay Project, Providence, R.I. [Google Scholar]
- 7.Cabelli V J, Heffernan W P. Elimination of bacteria by the soft shell clam Mya arenaria. J Fish Res Bd Canada. 1970;27:1579–1587. [Google Scholar]
- 8.Cabelli V J, Heffernan W P. Seasonal factor relevant to coliform levels in the northern quahaug. Proc Nat Shellfisheries Assoc. 1971;61:95–101. [Google Scholar]
- 9.Chung H, Sobsey M D. Comparative survival of indicator viruses and enteric viruses in seawater and sediment. Water Sci Technol. 1993;27:425–428. [Google Scholar]
- 10.DeBartolomeis J, Cabelli V J. Evaluation of an Escherichia coli of F male-specific bacteriophages. Appl Environ Microbiol. 1991;57:1301–1305. doi: 10.1128/aem.57.5.1301-1305.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiGirolamo R, Liston J, Matches J. Ionic bonding, the mechanism of viral uptake by shellfish mucus. Appl Environ Microbiol. 1977;33:19–25. doi: 10.1128/aem.33.1.19-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufour A P, Strickland E R, Cabelli V J. Membrane filter method for enumerating Escherichia coli. Appl Environ Microbiol. 1981;41:1152–1158. doi: 10.1128/aem.41.5.1152-1158.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galtstoff P S. Fisheries bulletin of the U.S. Fish and Wildlife Service. Vol. 64. Washington, D.C.: U.S. Government Printing Office; 1964. The American oyster, Crassostrea virginica, Gmelin. [Google Scholar]
- 14.Glatzer M B. Shellfish-borne disease outbreaks in the U.S., 1992–1998. 1998. Internal technical report. U.S. Food and Drug Administration, Southeast Regional Office, Atlanta, Ga. [Google Scholar]
- 15.Havelaar A H. Bacteriophages as model organisms in water treatment. Microbiol Sci. 1987;4:362–364. [PubMed] [Google Scholar]
- 16.Havelaar A H, Nieuwstad T J, Meulemans C C E, van Olphen M. F-specific RNA bacteriophages as model viruses in UV disinfection of wastewater. Water Sci Technol. 1991;24:347–352. [Google Scholar]
- 17.Havelaar A H, Nieuwstad T J. Bacteriophages and fecal bacteria as indicators of chlorination efficiency of biologically treated wastewater. J Water Pollut Cont Fed. 1985;57:1084–1088. [Google Scholar]
- 18.Keswick B H, Satterwhite T K, Johnson P C, DuPont H L, Secor S L, Bitsura J, Gary G W, Hoff J C. Inactivation of Norwalk virus in drinking water by chlorine. Appl Environ Microbiol. 1985;50:261–264. doi: 10.1128/aem.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo S, Gilbert J, Getrick F. Stability of human enteroviruses in estuarine and marine waters. Appl Environ Microbiol. 1976;32:245–249. doi: 10.1128/aem.32.2.245-249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metcalf T G, Mullin B, Eckerson D, Moulton E, Larkin E P. Bioaccumulation and depuration of enteroviruses by the soft-shelled clam, Mya arenaria. Appl Environ Microbiol. 1979;38:275–282. doi: 10.1128/aem.38.2.275-282.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rippey S R. Infectious diseases associated with molluscan shellfish consumption. Clin Microbiol Rev. 1994;7:419–425. doi: 10.1128/cmr.7.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards G P. Microbial purification of shellfish: a review of depuration and relaying. J Food Prot. 1988;51:218–251. doi: 10.4315/0362-028X-51.3.218. [DOI] [PubMed] [Google Scholar]
- 23.Richards G P. Shellfish-associated enteric virus illness in the United States, 1934–1984. Estuaries. 1987;10:84–85. doi: 10.4315/0362-028X-48.9.815. [DOI] [PubMed] [Google Scholar]
- 24.Rippey S R, Adams W N, Watkins W D. Enumeration of fecal coliforms and E. coli in marine and estuarine waters; an alternative to the APHA-MPN approach. Water Pollut Cont Fed. 1987;59:795–798. [Google Scholar]
- 25.Rippey S R, Chandler L A, Watkins W D. Fluorometric method for enumeration of Escherichia coli in molluscan shellfish. J Food Prot. 1987;50:685–690. doi: 10.4315/0362-028X-50.8.685. [DOI] [PubMed] [Google Scholar]
- 26.Shieh, Y.-S. C., S. S. Monroe, R. L. Fankhauser, G. W. Langlois, W. Burkhardt III, and R. S. Baric. Detection of Norwalk like virus in shellfish implicated in illness. J. Infect. Dis., in press. [DOI] [PubMed]
- 27.Simkova A, Cervenka J. Coliphages as ecological indicators of enteroviruses in various water systems. Bull W H O. 1981;59:611–618. [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. National shellfish sanitation program manual of operations, 1997 revision. Washington, D.C.: Department of Health and Human Services-Public Health Service-Food and Drug Administration; 1997. [Google Scholar]