Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease (MND) that typically causes death within 3-5 years after diagnosis. Regardless of the substantial scientific knowledge accrued more than a century ago, truly effective therapeutic strategies remain distant. Various conventional drugs are being used but are having several adverse effects.
Objective/Aim
The current study aims to thoroughly review plant-derived compounds with well-defined ALS activities and their structure-activity relationships. Moreover, the review also focuses on complex genetics, clinical trials, and the use of natural products that might decrypt the future and novel therapeutics in ALS.
Methods
The collection of data for the compilation of this review work was searched in PubMed Scopus, Google Scholar, and Science Direct.
Results
Results showed that phytochemicals like-Ginkgolides, Protopanaxatriol, Genistein, epigallocatechingallate, resveratrol, cassoside, and others possess Amyotrophic lateral sclerosis (ALS) activity by various mechanisms.
Conclusion
These plant-derived compounds may be considered as supplements for conventional (ALS). Moreover, further preclinical and clinical studies are required to understand the structure-activity relationships, metabolism, absorption, and mechanisms of plant-derived natural agents.
Keywords: Plant-derived products, SOD1 mutations, CNS disorders, SAR, clinical trials, Therapeutic effects
1. INTRODUCTION
Amyotrophic lateral sclerosis (ALS), also termed as Lou Gehrig’s disease, is an idiopathic, fatal cumulative neurodegenerative disease initiated by motor neurons dysfunction in the spinal cord and brain within weeks or months, which progresses into paralysis and finally death [1, 2]. There is no treatment available to cure this destructive disease. The majority of the deaths in ALS patients occur due to respiratory failure within 3 to 5 years from the onset of various signs and symptoms [3, 4]. The incidence of ALS in Western European countries is 2-3 in 100,000 individuals per year and has a prevalence of 4.6 per 100,000 [5-7]. ALS is more commonly found in men than women, affecting 1.2–1.5 men for every woman [8]. Evidence indicates that the incidence and prevalence are lesser in mixed ancestral origin populations than European people, with differences in age of onset in genetically heterogeneous populations [9]. Compared to Alzheimer’s disease, the maximum occurrence of the disease is between the age of 50 to 75 years and decreases after that [7]. However, chances of lower incidences among non-Caucasian populations or American Indians and Eskimos are still controversial, but most epidemiological studies accord with insignificant male/women predominance of 1.2-1.5/1 [10-12].
The etiology of ALS is highly multifactorial [1, 13]. It is associated with multiple cellular pathologies that are restricted to oxidative stress, loss of neurotrophic factors, glutamate-induced excite-toxicity, inflammation, insufficient protein quality control, accumulation and misfolding of proteins, and mitochondrial dysfunction [14, 15]. The clinical manifestations of sporadic amyotrophic lateral sclerosis (sALS) and familial amyotrophic lateral sclerosis (fALS) are almost very similar, and the median age of onset of sALS is around 60 years, and the age of onset for fALS is about ten years earlier than sALS. In juvenile ALS (JALS) families, mutations in ALS2 and SETX genes have been reported [16, 17].
Due to the secondary phenomena, deficiencies in a few of these pathways occur. To identify the primary pathophysiological processes underlying ALS, genetics would be the rational primary perspective. The ALS shows genetic predisposition, about 5-15% of patients diagnosed with ALS have a family history of the disease. A single defect in genetics is believed to lead ALS [18, 19]. While most people lack family background of ALS, in such cases, it is accepted that both genetic and environmental risk factors contribute to the development of disease [20]. Several genetic risk factors have been recognised that are involved in sporadic ALS. However, environmental risk factors exploration has been less successful. Many genetic and molecular pathways are most likely responsible for developing and progressing neurodegenerative changes in ALS.
Several pathological pathways have been suggested, yet no authenticated target for researchers while designing new molecules to impact the disease has been evidenced. To date, various molecules have failed in clinical trials so far while targeting the above-mentioned potential pathways. Thus, attempts carried in this field so far have not provided any success in new drug development [15]. Therefore, to successfully develop new medicine that will change the motor neuron degeneration process, several pathways need to be targeted due to the involvement of multiple pathways. Despite various preclinical and clinical studies, the accurate pathway of pathogenesis and progression of ALS is still not fully known. Thus, the development of successful and targeted therapy is challenging and is a major problem faced by scientists to treat ALS. Over the past two decades, the only FDA approved drug is riluzole, an anti-glutamatergic agent that acts by blocking glutamatergic neurotransmission in the CNS. However, riluzole’s efficacy is questionable, without any effects on disease symptoms and nominal therapeutic benefits of about 2-3 months of survival increase in ALS patients [21, 22]. After 22 years, another drug, edaravone, a free radical scavenging agent, was approved by FDA in May 2017, which was found to be effective in slowing ALS progression but its mechanistic pathway in ALS is not fully known yet [23, 24].
2. MATERIALS AND METHODS
2.1. Data Sources and Search Strategy
Databases like Scopus, Science Direct, Pubmed, Google Scholar, Web of Science were used to collect literature for the compilation of the present review by searching the terms including plant-derived bioactive compounds against (excitatory amino acid toxicity, neuroinflammation, calcium cytotoxicity and oxidative stress) in amyotrophic lateral sclerosis, traditional herbal medicines and.
3. PLANT-DERIVED NATURAL COMPOUNDS FOR THE TREATMENT OF AMYOTROPHIC LATERAL SCLEROSIS
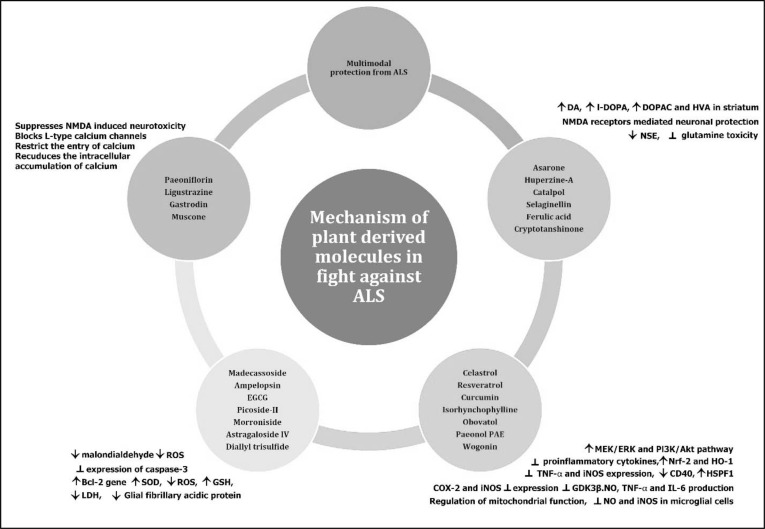
Regardless of the fact that drug design and discovery have a high reliance on synthetic chemistry, the contribution of natural products cannot be ignored [25-30]. WHO's list of essential drugs consists of 252, of which 11% are of plant origin [31]. So, there is an absolute chance of finding a natural molecule having desired ALS activity. The phytochemicals, including flavonoids, alkaloids, terpenes, and saponins from plant sources may instill positive change, which researchers are looking for, as they possess unique chemical diversity. Some of these cannot be synthesized by currently known methods [30, 32]. As a result, these natural compounds as novel drug molecules for ALS treatment remain untapped. Different scientific reports have focused on the validation of the phytoconstituents isolated from various medicinal plants. Scientific investigations claiming various phytochemicals as ameliorative agents in ALS are limited. However, some key findings have demonstrated flavonoids, alkaloids, terpenes, and saponins isolated from multiple medicinal plants exhibit ALS activity. In this review, we have discussed the potential of various plant origin phytochemicals for the treatment of ALS. This review will try to understand the mechanism of action of selected molecules (Fig. 1), and in vivo and in vitro activities of these Phytoconstituents will also be covered.
Fig. (1).
Plant derived natural compounds for the treatment Amyotrophic Lateral Sclerosis.
3.1. Phytochemicals Acting against Oxidative Stress
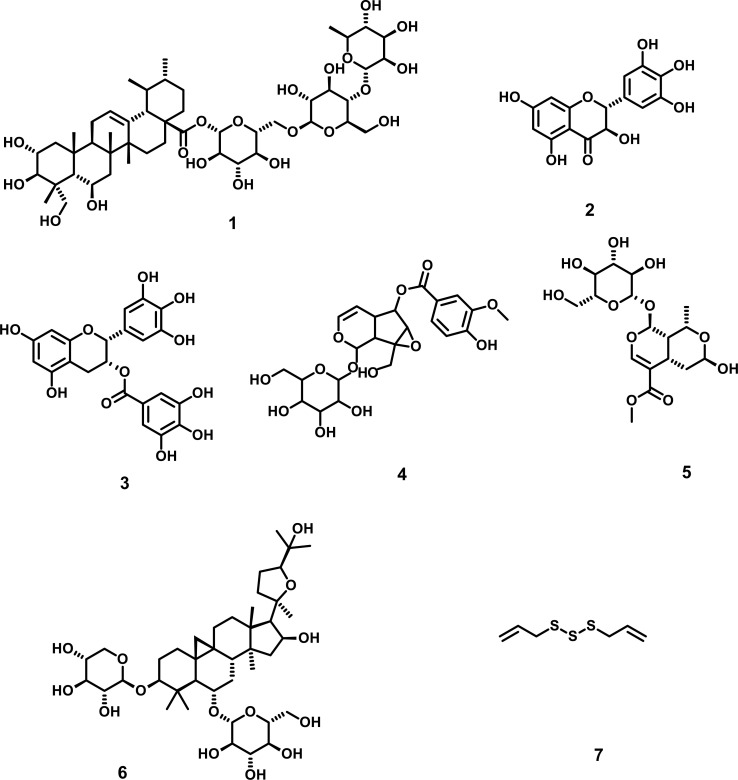
Oxidative stress imparts a major role in the process of neurodegeneration and is one of the most common pathways of all neurodegenerative diseases [33-36]. The death of neurons occurs mainly due to increases in the reactive oxygen species (ROS) generation and malfunctioning of the anti-oxidative system [37]. Herbal medicines impart a prospective role in oxidative stress regulation by improving the antioxidant activity of various enzymatic and non-enzymatic systems, decreasing the levels of (ROS) and maintaining the expression and regulation of various genes involved in ALS [38, 39]. Madecassoside, isolated from Centella asiatica is a triterpenoid saponin. It has been reported that in ALS involving transgenic SOD1-G93A mice model, madecassoside safeguards the motor neurons from degeneration and increases the survival time of mice. In another study, it was revealed that madecassoside reduces malondialdehyde levels and enhances the activity of SOD in the brain. In ALS mouse model, madecassoside protects the neurons from apoptosis due to free radicals by increasing the antioxidant activity. It has also been reported that madecassoside improves the LPS mediated neurotoxicity in rats by upregulating the Nrf2-HO pathway [40-44]. Ampelopsin, isolated from Ampelopsis grossedentata, belongs to the flavonoid class and exhibits prominent antioxidant activity. It has been reported that ampelopsin showed neuroprotective effects against H2O2-induced apoptosis in PC 12 cells by suppressing the ROS generation, upregulating the expression of HO-1 protein and hampering the expression of caspase-3. Moreover, in PC-12 cells, 1/2 (ERK1/2) and Akt-dependent signalling pathways play a role in the HO-1 protein upregulation. The studies suggested that ampelopsin could be a strong candidate in the ministration of various neurodegenerative diseases, including ALS [45-48]. Epigallocatechin gallate (EGCG), isolated from green tea, is its main constituent and is a water-soluble polyphenolic compound. It was reported to have strong antioxidant activity, besides acting as a radical scavenger mediating antioxidant activity in various neurodegenerative diseases, including ALS. The antioxidant activity of (EGCG) against ALS was further evaluated in transgenic SOD1 mice, where it slows down the beginning of symptoms and prolongs the lifespan. Moreover, upregulation of Bcl-2 gene, which is an anti-apoptotic gene, was also detected with (EGCG), suggesting the antioxidant activity of (EGCG) in ALS is associated with the upregulation of Bcl-2 gene [49-56]. Picroside-II, isolated from Picrorhi zarhizoma is a type of iridoid glycoside that is widely found in Tibet as well as in India. It was reported that in PC-12 cells, picroside-II strengths nerve growth factor (NGF) mediated neurite outgrowth besides acting synergistically against oxidative stress. Due to their synergistic effect, they are used to manage various nervous disorders, including ALS. Moreover, the neuroprotective activity against oxidative stress of picroside-II was also evaluated in various models, including in vitro model of glutamate-treated PC12 cells and in vivo model of AlCl3-induced male mice. Picroside -II also enhances the SOD levels in the brain of mice which results in suppression of ROS generation depicting picroside-II protects the brain from a neuronal injury that occurs due to oxidative stress [57-60]. Morroniside, isolated from Cornus officinalis is a type of iridoid glycoside reported to have a strong neuroprotective activity against oxidative stress. It was also reported that in SH-SY5Y cells, when exposed to H2O2-mediated cytotoxicity, Morroniside elevates the levels of cellular GSH and reduces the levels of lactate dehydrogenase (LDH), besides maintaining the Matrix metalloproteinases (MMP) and cell stability. Moreover, it suppresses the intracellular activity of SOD and ROS generation. In addition, Upregulation of Bcl- 2 genes was also reported, which confirms the anti-apoptotic and anti-oxidative activity of this compound [61-65]. Astragaloside IV, a saponin isolated from Radix astragali, is generally used for ALS treatment in China and is reported to have a strong antioxidant activity in various In-vitro and in-vivo studies. Astragaloside IV also showed a protective role against H2O2 mediated oxidative stress in PC-12 cells. Moreover, it also improves the viability of PC-12 cells, activation of HO-1, suppresses the intracellular production of ROS as well as apoptotic cell death [66-70]. Diallyl trisulfide (DATS), an active monomer of allicin isolated from bulbs of Liliaceae allium, was reported to exhibit diverse pharmacological activity owing to its capability to pass through the (BBB). It was reported that (DATS) acts as an inducer of phase II enzymes resulting in the amelioration of oxidative stress besides safeguards the activity of various antioxidant enzymes, thus imparting an important role in ALS. Diallyl trisulfide acts via multiple pathways in ALS, including activating the heme oxygenase-1 (HO-1), downregulating the expression of glial fibrillary acidic protein, activating the antioxidant activity of various enzymes [71-76].The various plant-derived phytochemicals (Fig. 2), along with their diverse mechanistic insights against oxidative stress, are shown in Table 1.
Fig. (2).
Phytochemicals against oxidative stress in ALS (1) Madecassoside (2) Ampelopsin (3) Epigallocatechin gallate (EGCG) (4) Picroside-II (5) Morroniside (6) Astragaloside IV (7) Diallyl trisulfide (DATS).
Table 1.
Phytochemicals along with their diverse mechanistic insights against oxidative stress in ALS.
|
Phytochemical
Constituent |
Study Type
Cellular/Animal/ Clinical |
Study Description | Mechanism | Refs. |
|---|---|---|---|---|
| Madecassoside (1) | In-vivo (Transgenic mice SOD1-G93A) | Two dissimilar doses of 61.1 ± 11.0 and 185.6 ±18.7mg/kg/day, markedly increased the time of survival in mice by 11 and 9 days, respectively |
Increase the mice survival time and reduces the malondialdehyde levels, and enhances the SOD activity in the brain | [40] |
| Ampelopsin (2) |
In-vitro (PC12 cells) |
Ampelopsin Neuroprotective effect against H2O2-induced cell death in PC12 cells is well observed. |
Suppressing the production of ROS, upregulating the expression of HO-1 protein and hampering the expression of caspase-3 | [45, 47] |
| Epigallocatechin gallate (EGCG) (3) | In-vivo Transgenic mice SOD1-G93A | EGCG given in doses of 1.5, 2.9, 5.8 µg/g body weight after 60 days of age suggest that it significantly delays the disease onset by 1.4weeks and prolongs the survival time by 1.8 weeks. |
upregulation of Bcl-2 gene |
[49] |
| Picroside-II (4) |
In-vitro PC12 cells In-vivo mice AlCl3-induced toxicity |
Neuroprotective action of picroside-II has been observed in glutamate-treated PC12 cells and improved SOD activity in the brain of mice |
Enhances the SOD levels in the brain of mice, suppression of ROS generation | [58] |
| Morroniside (5) |
In-vitro SH-SY5Y cells |
Morroniside exhibits both anti-oxidative and anti-apoptotic properties against oxidative stress-induced cell damage |
Elevates the levels of cellular GSH and reduce the levels of (LDH), Upregulation of Bcl- 2 genes | [62] |
| Astragaloside IV (6) |
In-vitro PC12 cells |
It enhances neuronal cellular viability in vitro, decreases intracellular production of ROS induced by H2O2 and improves cell survival. |
Activation of HO-1 suppresses the intracellular production of ROS |
[66] |
| Diallyl trisulfide (DATS) (7) |
In-vivo Transgenic mice SOD1-G93A |
A dose of 80 mg/kg/day significantly improves life span by 1week |
Activating the heme oxygenase-1 (HO-1) Downregulating the expression of glial fibrillary acidic protein |
[72] |
3.2. Phytochemicals Acting against Neuroinflammation
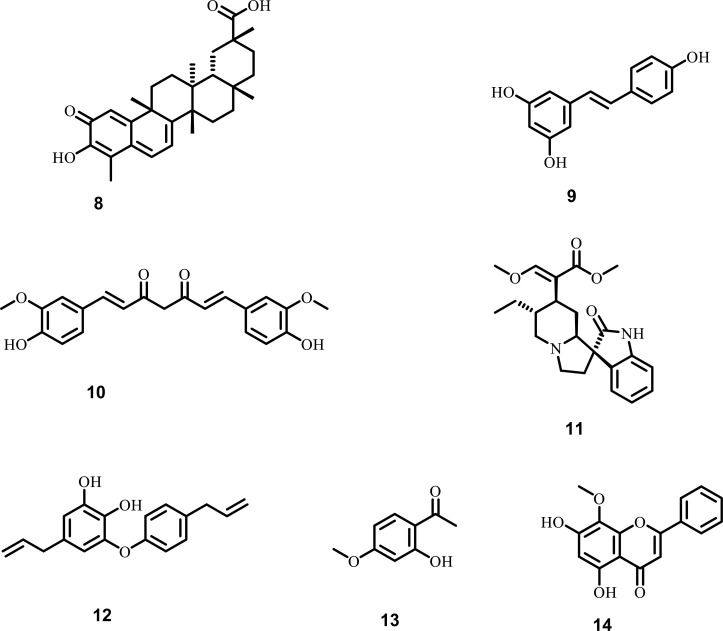
A strong correlation exists between inflammation and various CNS disorders, particularly ALS. Microglia cells in the CNS impart an essential role in ALS pathogenesis due to their primary role in the release of various pro-inflammatory factors, including (TNF-α), (iNOS), (COX-2). So, one of the targets for ALS involves decreasing the activation of microglia cells, which in turn, inhibits neuroinflammation [77-80]. Celastrol, isolated from Tripterygium wilfordii, is a triterpenoid pigment that inhibits cancer cell proliferation and inflammation-related various auto-immune diseases. In transgenic mice, SOD1-G93A model of ALS, Celastrol suppresses (TNF-α) and (iNOS) expression, decreased the expression of CD40 and glial fibrillary acidic protein in the lumbar spinal cord section of mice, resulting in delayed onset of disease and improvement in the motor function. Moreover, it was observed that celastrol at the molecular level inhibits LPS mediated activation of mitogen-activated protein kinase/ERK1/2 signaling pathway and (NF-kB), which plays a vital role in the damage to cells and stress. So celastrol suppresses the activation of microglia cells that further decreases the generation of pro-inflammatory cytokines [81-86]. Resveratrol, mainly isolated from Veratrum nigrum and Rhizoma polygoni, is a type of flavonoid (polyhydroxy) diphenyl ethylene, and has intense antioxidant activity due to various hydroxyl groups. Studies also revealed that resveratrol inhibits the release of pro-inflammatory cytokines instigated by LPS in mouse N-9 microglial and rat cortical microglia cells, besides inhibiting the degradation of IkBα and iNOS N-9 microglial cells expression, disclosing the role of resveratrol in the amelioration of various neurodegenerative disease including ALS [87-94]. Curcumin, isolated from Curcuma longa, is a polyphenolic monomer known for its neuroprotective and anti-inflammatory activity. In LPS stimulated microglia cells, curcumin suppresses the release of nitric oxide and iNOS expression. Moreover, curcumin also upregulates the expression of (Nrf-2) and (HO-1), exhibiting strong neuroprotective activity during inflammatory stress. The neuroprotective role of curcumin in ALS has also been reported due to the downregulation of NF-kB signaling pathway, which suppresses the pro-inflammatory cytokines, including IL-6, IL-1, and TNF-α [39, 95-100]. Isorhynchophylline (IRN), isolated from Uncaria rhynchophylla, has been reported to exhibit strong neuroprotective activity due to its ability to inhibit cytokine release like IL-6, IL-1, and TNF-α in LPS stimulated microglial cells. Moreover, the synthesis of inflammatory mediators and expression of mRNA and iNOS has also been reduced by IRN, which impart an essential role in various neurodegenerative disease, including ALS [101-103]. Obovatol, isolated from Magnolia officinalis leaves, is a type of neolignan. The neuroprotective activity of obovatol has been examined in various models of neuroinflammation mediated by LPS. It has also been reported that obovatol suppresses the release of NO and iNOS in microglial cells by inhibiting the signaling pathways of mitogen-activated protein kinase and NF-kB, besides one of the primary molecular targets of obovatol in microglia is Peroxiredoxin 2 (Prx2), which played an essential role in the various signalling pathways of neuroinflammation [104-106]. Paeonol, isolated from the bark of Paeonia suffruticosa, acts as a neuroprotective agent by inhibiting inflammation mediated by microglia as well as oxidative stress. In LPS induced inflammation in cortical neurons, paeonol downregulates the expression of COX-2 and iNOS, which results in reduced production of ROS and NO. Moreover, the phosphorylation of ERK induced by LPS was also suppressed by paeonol, which results in an increase in cell viability [107-109]. Wogonin, isolated from the Scutellaria root, acts as a neuroprotective agent by inhibiting the NO, TNF-α, and IL-6 production. Furthermore, wogonin also shows neuroprotective activity in LPS induced microglia injury by suppressing the various mediators of inflammation [110-112]. The various plant-derived phytochemicals (Fig. 3), along with their diverse mechanistic insights against neuroinflammation, are shown in Table 2.
Fig. (3).
Phytochemicals against neuroinflammation in ALS (8) Celastrol (9) Resveratrol (10) Curcumin (11) Isorhynchophylline (IRN) (12) Obovatol (13) Paeonol (14) Wogonin.
Table 2.
Phytochemicals along with their diverse mechanistic insights against neuroinflammation in ALS.
| Phytochemical constituent |
Study type
Cellular/Animal/clinical |
Description of Study | Mechanism | Refs. |
|---|---|---|---|---|
| Celastrol (8) | G93A SOD1 transgenic mouse model of ALS | Transgenic mice were transfected with NSC34 cells and then treated with hydrogen peroxide and celastrol at different doses | Activation in MEK/ERK and PI3K/Akt pathway | [82, 113, 114] |
| Transgenic mouse model of ALS | Celastrol was administered to the mice at 30 days of age, and reduction in body weight, improvement in motor function along with delayed onset of ALS was achieved. | Suppresses the TNF-α and iNOS expression Downregulated the expression of CD40 | ||
| SH-SY5Y neuronal cell model | There was Increased induction of Heat shock proteins (HSPs) after Co-application of celastrol and arimoclomol | Activation of HSPF1. | ||
| Resveratrol (9) | Rat cortical neuron cell model | Cell survival increased up to 75 % on the application of RSV with protection against neurodegeneration | Inhibits the release of pro-inflammatory cytokines | [88, 91, 93, 115-118] |
| VSC 4.1 hybrid cell line | Mutant SOD1 expression was induced in the cell line, and on administration of RSV, the cell survival was enhanced with respect to dose, and at highest dose of RSV, cell survival was fully restored. | |||
| Transgenic SOD mice model | Intraperitoneal administration of RSV led to a significant reduction in motor neuron death along with increased survival rates | |||
| Curcumin (10) | Motor neuron Cell model | Cell line transfected with mutant Q331K and wild TDP43 was treated with curcumin that led to altered membrane permeability of neurons. | Upregulates the expression of (Nrf-2) and (HO-1) | [39, 99, 119] |
| Double-blind therapeutic trial for 42 patients | Patients were divided into Group A & B. group A received a placebo for three months followed by curcumin for other three months, while Group B received curcumin for six months | |||
| Isorhynchophylline (IRN) (11) | BM-hMSCs model | Regulation of the intracellular pluripotency mechanisms was examined. | Regulation of mitochondrial function, NMDA subunit, FGFβ levels, BDNF, OXTR, ATP, BM-MSC proliferation and differentiation. | [101, 102, 120, 121] |
| Mouse N9 microglial cells | Inhibitory tendencies of RIN and IRN against cytokines and NO were a point of focus | Inhibits the pro-inflammatory cytokines release in LPS stimulated microglial cells | ||
| Obovatol (12) | Microglia BV-2 cell line | LPS induced stimulation was carried out in the cell line to mark changes with respect to NO, cytokines, along with activation of signalling cascades. | Suppresses the release of NO and iNOS in microglial cells | [104, 122, 123] |
| Paeonol PAE (13) | N9 microglia cell model | Role of PAE in the production of pro-inflammatory markers in LPS stimulated microglia cells and proteins formed in immune signalling cascade were observed | Downregulates the COX-2 and iNOS expression. Involvement of TLR4 signalling pathway to reduce the expression of TRAF6, MAPK molecules, etc. | [107, 109] |
| Wogonin (14) | SH-SY5Y cells | Aβ changes were observed in the cell line with treatment by wogonin. | GSK3β inhibition via the mediation of mTOR signalling pathway | [110, 124, 125] |
| Microglia cell | Lps stimulated microglial cells were subjected to treatment to monitor changes with regard to TNF, NO and IL-6. | Inhibiting the NO, TNF-α, and IL-6production. |
3.3. Phytochemicals Acting against Calcium Cytotoxicity
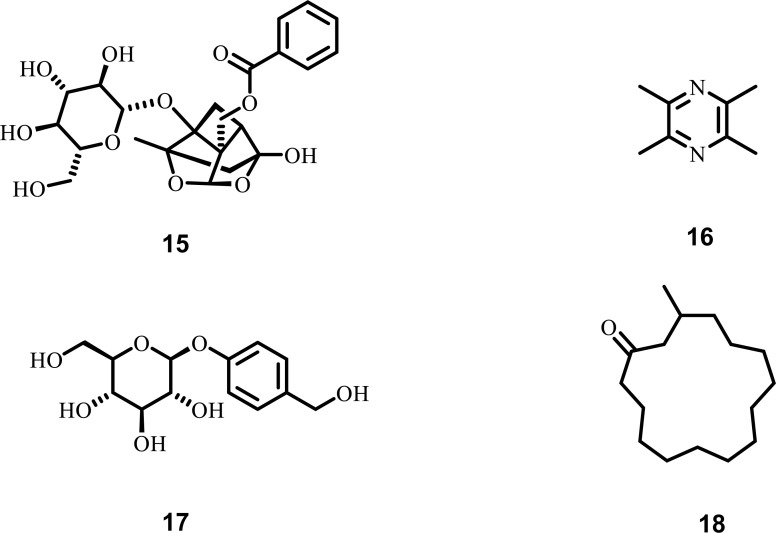
One of the prime factors that are involved in ALS is calcium toxicity. When the calcium channels are opened up, a massive influx of calcium via NMDA receptors piles up in the nuclear cell membrane. This results in nerve cell damage and even death of cells. Nowadays, the focus shifts to herbal medicines to find a phytochemical that can be beneficial in treating ALS [126-128]. Paeoniflorin, isolated from Paeoniae radix, has an essential role as a neuroprotective agent in ALS management by inhibiting the influx of calcium in cytoplasm in PC12 cell-injury models. Moreover, it also inhibits the extra intracellular level of calcium which is generated due to glutamate and suppresses the apoptosis in PC-12 cells. Further, in PC-12 cells, Paeoniflorin shows its neuroprotective effect by suppressing NMDA induced neurotoxicity [129-134]. Ligustrazine, isolated from Rhizoma chuanxiong, is known for its neuroprotective activity by blocking calcium channels. It has been reported that in SH-SY5Y cells, ligustrazine blocks L-type calcium channels, which impart a vital role in neurotoxicity development in ALS [135, 136]. Gastrodin, isolated from Gastrodia elata, can cross the (BBB) and exert its effect on CNS. In SH-SY5Y cells, Gastrodin was reported to limit calcium entry via acting on voltage-gated calcium channels, inhibiting the degeneration of neurons due to calcium toxicity [137-139]. Muscone, obtained from natural muskies, is its principal active component. In PC12 cells stimulated with glutamate, muscone administration exhibits its neuroprotective activity by reducing the intracellular accumulation of calcium [140]. The various plant-derived phytochemicals (Fig. 4), along with their diverse mechanistic insights against calcium cytotoxicity, are shown in Table 3.
Fig. (4).
Phytochemicals against calcium cytotoxicity in ALS (15) Paeoniflorin (16) Ligustrazine (17) Gastrodin (18) Muscone.
Table 3.
Phytochemicals along with their diverse mechanistic insights against calcium cytotoxicity in ALS.
| Phytochemical Constituent |
Study Type
Cellular/Animal/clinical |
Study Description | Mechanism | Refs. |
|---|---|---|---|---|
| Paeoniflorin (15) |
In-vitro PC12 cells |
Paeoniflorin reverses the amplified intracellular levels of calcium level caused by excitatory glutamate and reduced PC12 cell death in a dose-dependent manner |
Suppressing NMDA induced neurotoxicity | [129] |
| Ligustrazine (16) |
In-vitro SH-SY5Y cells |
Whole-cell patch-clamp technique demonstrated that in the nervous system, there is inhibitory action on the calcium channel due to ligustrazine |
Blocks L-type calcium channels |
[135] |
| Gastrodin (17) |
In-vitro SH-SY5Y cells |
The free calcium accumulation can be suppressed by Gastrodin, inhibits the enhanced glutamate to protect neurons |
Restricted the entry of calcium by acting on voltage-gated calcium channels | [139] |
| Muscone (18) |
In-vitro PC12 cells |
It suppresses the calcium overload-induced by glutamate and prevents neuronal cell death |
Reducing the intracellular accumulation of calcium | [140] |
3.4. Phytochemical Acting against Excitatory Amino Acid Toxicity
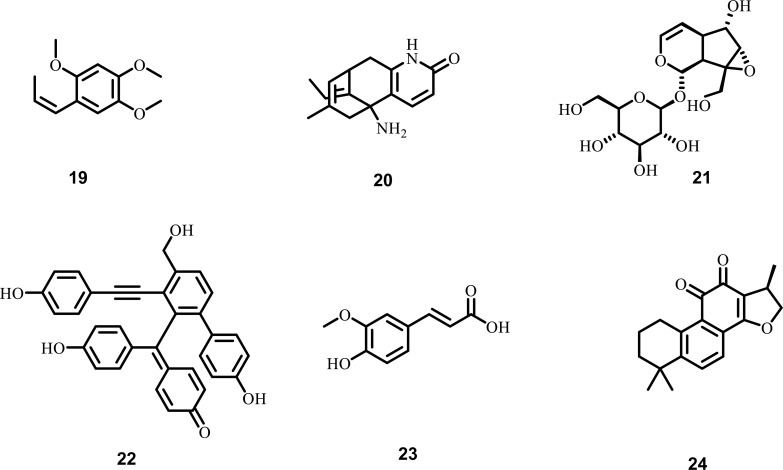
The primary excitatory neurotransmitter in the CNS is glutamate. To maintain the optimum level of glutamate, various metabolic enzymes, as well as transporters, are available, failure in the function of which leads to excessive accumulation of glutamate in the CNS resulting in various nervous disorders, including ALS [141-143]. Different phytochemicals are involved in maintaining the optimum level of glutamate in CNS, which include: β-Asarone, isolated from Acorus tatarinowii, acts as a neuroprotective agent due to its ability to cross the (BBB). It was reported that in ALS, β-Asarone suppresses (NMDA) or produces glutamate-induced excitotoxicity. Moreover, in PC-12 cells, β-Asarone increases the survival rate of cells, reduces the leakage of LDH, apoptosis ratio, and intracellular accumulation of calcium [144-147]. Huperzine-A, isolated from Huperzia serrata, is a novel alkaloid commonly used in the treatment of Alzheimer’s disease due to its ability to block glutamate-mediated neurotransmission. (Hup A) also inhibits glutamate toxicity by blocking NMDA receptors. In patients with ALS, (Hup A) acts as a neuroprotective agent by preventing damage to motor neurons [56, 148-150]. Catalpol, isolated from Rehmannia glutinosa, acts as a neuroprotective agent in various neurological disease, including ALS, by suppressing glutamate excitotoxicity. Moreover, it also increases the viability of cells, protects the neurons from various damages mediated via NMDA receptors [151-153]. Selaginellin, isolated from Saussurea pulvinata, exhibits neuroprotective activity in PC-12 cells by suppressing glutamate toxicity. It also decreases the ROS generation and expression of klotho gene [154-156]. Ferulic acid, a phenolic acid monomer mainly present in Chinese herbs, including angelica and Szechwan, crosses the BBB with ease. It shows its neuroprotective activity by preventing damage to neurons due to glutamate excitotoxicity and apoptosis in cortical neurons. Furthermore, it also protects the In-vitro PC-12 cells from hypoxia, free radicals, and excitatory amino acids [157-160]. Cryptotanshinone, isolated from Salvia miltiorrhiza, suppresses glutamate toxicity by activating phosphoinositide 3-kinase signalling pathway and inhibiting the downregulation of Bcl-2, an anti-apoptotic protein. The PI3K/Akt pathway plays an important role in controlling the pathogenesis of ALS [161, 162]. The various plant-derived phytochemicals (Fig. 5), along with their diverse mechanistic insights against excitatory amino acid toxicity, are shown in Table 4.
Fig. (5).
Phytochemicals against excitatory amino acid toxicity in ALS (19) β-Asarone (20) Huperzine-A (21) Catalpol (22) Selaginellin (23) Ferulic acid (24) Cryptotanshinone.
Table 4.
Phytochemicals with diverse mechanistic insights against excitatory amino acid toxicity in ALS.
|
Phytochemical
Constituent |
Study type
Cellular/Animal/Clinical |
Description of Study | Mechanism | Refs. |
|---|---|---|---|---|
| β-Asarone (19) |
In-vitro Cultured rat cortical cells |
Anti-excitotoxicity effect of isolated α & β asarone as compared to commercially available asarone. | Suppresses (NMDA) or glutamate-induced excitotoxicity | [44, 144, 145, 163] |
|
In-vivo PD rat model |
6-OHDA was used to induce Parkinson’s disease in rats that were divided into different groups like untreated, l-dopa, β-asarone and co-administered l-dopa and β-asarone. | Downregulation of NSE and improved levels of DA, l-DOPA, DOPAC and HVA in striatum. | ||
| Huperzine-A (20) |
In-vitro NSC34 and rat spinal cord organotypic culture |
Inducers like staurosporine, hydrogen peroxide, CCCP, THA etc. were used in a cell line, and the effects of huperazine A were noted | Inhibits glutamate toxicity by blocking NMDA receptors | [149] |
| Catalpol (21) |
In-vitro PC12 cell lines |
Effect of catalpol was observed against Cell injury induced by glutamate | Protects the neurons from various damages mediated via NMDA receptors | [44, 120, 151, 152] |
| Selaginellin (22) |
In-vitro PC12 cells |
Glutamate induced excitotoxicity in PC12 cells was exposed with selaginellin administration | Decreases the ROS generation and expression of klotho gene | [155, 164] |
| Ferulic acid (23) |
In-vitro PC12 cells |
The protective effects against hypoxia and excitotoxicity were monitored. | Preventing the damage to neurons due to glutamate excitotoxicity and apoptosis in cortical neurons. | [44, 157, 165] |
|
In-vivo Male Sprague Dawley rat |
Protective effects of ferulic acid against hypoxia-induced cerebral injury was the focus of the study | TLR and MyD88 pathways inactivation | ||
| Cryptotanshinone (24) |
In-vitro Rat cortical neurons |
Glutamate was used to entice neurotoxicity in a cell line. | Activating phosphoinositide 3-kinase pathway and inhibiting the downregulating Bcl-2, an anti-apoptotic protein | [120, 161, 162] |
CONCLUSION
There is currently only one drug available in the market approved by FDA in the treatment of ALS. However, various attempts have been carried out to develop an efficient therapeutic agent against ALS. Majority of the drugs passed the preclinical animal studies, but the results are not promising in human clinical trials. Herbal medicines, on the other hand, act as an alternative and complementary medicinal approach for ALS treatment. The phytochemicals, including flavonoids, alkaloids, terpenes, and saponins from plant sources may instill positive change, which researchers are looking for, as they possess unique chemical diversity. Some of these cannot be synthesized by currently known methods. As a result, these natural compounds as novel drug molecules for ALS treatment remain untapped. Different scientific reports have focused on the validation of the Phytoconstituents isolated from various medicinal plants. The phytochemicals isolated from herbal medicines act via multiple pathways, including an antioxidant, anti-inflammatory and an anti-apoptotic agent in ALS. The requirement of natural products to be used in the treatment of ALS has increased because of their safety and efficacy compared to conventional drugs as an alternative treatment measure. The review explains that natural products could be used as a new approach in relieving the intensity of various ALS symptoms. In addition, the review mentions that natural antioxidant compounds with multi targets, multi links, or multi pathways that can be used in the modern pharmacology of ALS. However, all these data underline the importance of testing the tolerability and efficacy of natural products to ameliorate the symptoms or disease progression in ALS in the context of controlled clinical trials.
ACKNOWLEDGEMENTS
The Authors acknowledge the university of Kashmir for providing the necessary facilities to carry out this work.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat. Rev. Neurosci. 2006;7(9):710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V., Islam A., Hassan M.I., Ahmad F. Therapeutic progress in amyotrophic lateral sclerosis-beginning to learning. Eur. J. Med. Chem. 2016;121:903–917. doi: 10.1016/j.ejmech.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Gil J., Funalot B., Verschueren A., Danel-Brunaud V., Camu W., Vandenberghe N., Desnuelle C., Guy N., Camdessanche J.P., Cintas P., Carluer L., Pittion S., Nicolas G., Corcia P., Fleury M.C., Maugras C., Besson G., Le Masson G., Couratier P. Causes of death amongst French patients with amyotrophic lateral sclerosis: A prospective study. Eur. J. Neurol. 2008;15(11):1245–1251. doi: 10.1111/j.1468-1331.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 4.Spataro R., Lo Re M., Piccoli T., Piccoli F., La Bella V. Causes and place of death in Italian patients with amyotrophic lateral sclerosis. Acta Neurol. Scand. 2010;122(3):217–223. doi: 10.1111/j.1600-0404.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- 5.Robberecht W., Philips T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013;14(4):248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 6.Pradas J., Puig T., Rojas-García R., Viguera M.L., Gich I., Logroscino G. Amyotrophic lateral sclerosis in Catalonia: A population based study. Amyotroph. Lateral Scler. Frontotemporal Degener. 2013;14(4):278–283. doi: 10.3109/21678421.2012.749915. [DOI] [PubMed] [Google Scholar]
- 7.Joensen P. Incidence of amyotrophic lateral sclerosis in the Faroe Islands. Acta Neurol. Scand. 2012;126(1):62–66. doi: 10.1111/j.1600-0404.2011.01611.x. [DOI] [PubMed] [Google Scholar]
- 8.Chiò A., Logroscino G., Traynor B.J., Collins J., Simeone J.C., Goldstein L.A., White L.A. Global epidemiology of amyotrophic lateral sclerosis: A systematic review of the published literature. Neuroepidemiology. 2013;41(2):118–130. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wittie M., Nelson L.M., Usher S., Ward K., Benatar M. Utility of capture-recapture methodology to assess completeness of amyotrophic lateral sclerosis case ascertainment. Neuroepidemiology. 2013;40(2):133–141. doi: 10.1159/000342156. [DOI] [PubMed] [Google Scholar]
- 10.Zufiría M., Gil-Bea F.J., Fernández-Torrón R., Poza J.J., Muñoz-Blanco J.L., Rojas-García R., Riancho J., López de Munain A. ALS: A bucket of genes, environment, metabolism and unknown ingredients. Prog. Neurobiol. 2016;142:104–129. doi: 10.1016/j.pneurobio.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Logroscino G., Traynor B.J., Hardiman O., Chiò A., Mitchell D., Swingler R.J., Millul A., Benn E., Beghi E. Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry. 2010;81(4):385–390. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaldivar T., Gutierrez J., Lara G., Carbonara M., Logroscino G., Hardiman O. Reduced frequency of ALS in an ethnically mixed population: A population-based mortality study. Neurology. 2009;72(19):1640–1645. doi: 10.1212/WNL.0b013e3181a55f7b. [DOI] [PubMed] [Google Scholar]
- 13.Ferraiuolo L., Kirby J., Grierson A.J., Sendtner M., Shaw P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011;7(11):616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 14.Shaw P.J. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J. Neurol. Neurosurg. Psychiatry. 2005;76(8):1046–1057. doi: 10.1136/jnnp.2004.048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunkel P., Chai C.L., Sperlágh B., Huleatt P.B., Mátyus P. Clinical utility of neuroprotective agents in neurodegenerative diseases: Current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin. Investig. Drugs. 2012;21(9):1267–1308. doi: 10.1517/13543784.2012.703178. [DOI] [PubMed] [Google Scholar]
- 16.Contestabile A. Amyotrophic lateral sclerosis: From research to therapeutic attempts and therapeutic perspectives. Curr. Med. Chem. 2011;18(36):5655–5665. doi: 10.2174/092986711798347289. [DOI] [PubMed] [Google Scholar]
- 17.Al-Saif A., Al-Mohanna F., Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann. Neurol. 2011;70(6):913–919. doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- 18.Swinnen B., Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014;10(11):661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- 19.Byrne S., Walsh C., Lynch C., Bede P., Elamin M., Kenna K., McLaughlin R., Hardiman O. Rate of familial amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2011;82(6):623–627. doi: 10.1136/jnnp.2010.224501. [DOI] [PubMed] [Google Scholar]
- 20.Al-Chalabi A., Hardiman O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013;9(11):617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- 21.Nagoshi N., Nakashima H., Fehlings M.G. Riluzole as a neuroprotective drug for spinal cord injury: From bench to bedside. Molecules. 2015;20(5):7775–7789. doi: 10.3390/molecules20057775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glicksman M.A. The preclinical discovery of amyotrophic lateral sclerosis drugs. Expert Opin. Drug Discov. 2011;6(11):1127–1138. doi: 10.1517/17460441.2011.628654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz M.P. Edaravone (Radicava): a novel neuroprotective agent for the treatment of amyotrophic lateral sclerosis. P&T. 2018;43(1):25–28. [PMC free article] [PubMed] [Google Scholar]
- 24.Abe K., Aoki M., Tsuji S., Itoyama Y., Sobue G., Togo M., Hamada C., Tanaka M., Akimoto M., Nakamura K. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505–512. doi: 10.1016/S1474-4422(17)30115-1. [DOI] [PubMed] [Google Scholar]
- 25.Mir R.H., Sawhney G., Verma R., Ahmad B., Kumar P., Ranjana S., Bhagat A., Madishetti S., Ahmed Z., Jachak S.M. Oreganum Vulgare: In-vitro assessment of cytotoxicity, Molecular docking studies, Antioxidant, and evaluation of anti-inflammatory activity in LPS stimulated RAW 264.7 cells. Medicinal Chemistry: Shariqah, United Arab Emirates, 2020. [DOI] [PubMed]
- 26.Hassan Mir R., Godavari G., Siddiqui N.A., Ahmad B., Mothana R.A., Ullah R., Almarfadi O.M., Jachak S.M., Masoodi M.H. Design, synthesis, molecular modelling, and biological evaluation of oleanolic acid-arylidene derivatives as potential anti-inflammatory agents. Drug Des. Devel. Ther. 2021;15:385–397. doi: 10.2147/DDDT.S291784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohi-Ud-Din R., Mir R.H., Mir P.A., Farooq S., Raza S.N., Raja W.Y., Masoodi M.H., Singh I.P., Bhat Z.A. Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus berberis linn: A comprehensive review. Comb. Chem. High Throughput Screen. 2021;24(5):624–644. doi: 10.2174/1386207323999201102141206. [DOI] [PubMed] [Google Scholar]
- 28.Mir R.H., Bhat M.F., Sawhney G., Kumar P., Andrabi N.I., Shaikh M., Mohi-Ud-Din R., Masoodi M.H. Prunella vulgaris L: Critical pharmacological, expository traditional uses and extensive phytochemistry: A review. Curr. Drug Discov. Technol. 2021 doi: 10.2174/1570163818666210203181542. [DOI] [PubMed] [Google Scholar]
- 29.Hassan R., Masoodi M.H. Saussurea lappa: A comprehensive review on its pharmacological activity and phytochemistry. Curr. Tradit. Med. 2020;6(1):13–23. doi: 10.2174/2215083805666190626144909. [DOI] [Google Scholar]
- 30.Mir R.H., Masoodi M.H. Anti-inflammatory plant polyphenolics and cellular action mechanisms. Curr. Bioact. Compd. 2020;16(6):809–817. doi: 10.2174/1573407215666190419205317. [DOI] [Google Scholar]
- 31.Rates S.M.K. Plants as source of drugs. Toxicon. 2001;39(5):603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 32.Mir R.H., Shah A.J., Mohi-Ud-Din R., Pottoo F.H., Dar M.A., Jachak S.M., Masoodi M.H. Natural anti-inflammatory compounds as drug candidates in Alzheimer’s disease. Curr. Med. Chem. 2020;28(23):4799–4825. doi: 10.2174/0929867327666200730213215. [DOI] [PubMed] [Google Scholar]
- 33.Van Raamsdonk J.M., Vega I.E., Brundin P. Oxidative stress in neurodegenerative disease: Causation or association? Oncotarget. 2017;8(7):10777–10778. doi: 10.18632/oncotarget.14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules. 2019;24(8):1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rekatsina M., Paladini A., Piroli A., Zis P., Pergolizzi J.V., Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020;37(1):113–139. doi: 10.1007/s12325-019-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukui K. Reactive oxygen species induce neurite degeneration before induction of cell death. J. Clin. Biochem. Nutr. 2016;59(3):155–159. doi: 10.3164/jcbn.16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forni C., Facchiano F., Bartoli M., Pieretti S., Facchiano A., D’Arcangelo D., Norelli S., Valle G., Nisini R., Beninati S. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019;7:8748253. doi: 10.1155/2019/8748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chico L., Ienco E.C., Bisordi C., Lo Gerfo A., Petrozzi L., Petrucci A., Mancuso M., Siciliano G. Amyotrophic Lateral Sclerosis and oxidative stress: A double-blind therapeutic trial after curcumin supplementation. CNS Neurol. Disord. Drug Targets. 2018;17(10):767–779. doi: 10.2174/1871527317666180720162029. [DOI] [PubMed] [Google Scholar]
- 40.Bai J-R., Liu Y-J., Song Y. The mechanism of interfere effects of madecassoside (MC) on neurodegeneration in mice. Zhongguo Laonianxue Zazhi. 2008;28:2297–2300. [Google Scholar]
- 41.Kobayashi Y., Liu Y., Tobinaga S., Tsunematsu T., Nakamura M. In journal of pharmacological sciences.Japanese pharmacological soc editorial off, kantohya bldg gokomachi, 2007, 103, 136.
- 42.Liu Y. Therapeutic potential of madecassoside in transgenic mice of amyotrophic lateral sclerosis. Chinese Traditional Herbal Drugs. 1994:5. [Google Scholar]
- 43.Liu S., Li G., Tang H., Pan R., Wang H., Jin F., Yan X., Xing Y., Chen G., Fu Y., Dong J. Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci. Lett. 2019;709:134386. doi: 10.1016/j.neulet.2019.134386. [DOI] [PubMed] [Google Scholar]
- 44.Kumar V., Preeti G., Md Imtaiyaz H. Mechanism and implications of traditional Chinese medicine in amyotrophic lateral sclerosis therapy. J. Proteins Proteom. 2019;10(2):131–147. [Google Scholar]
- 45.Kambara T., Zhou Y., Oda M., Tamura Y., Miyakoshi M., Mizutani K., Ikeda T., Tanaka O., Chou W. 120th Annual Meeting of Pharmaceutical Society of Japan; Gifu. 2000. [Google Scholar]
- 46.Murakami T., Miyakoshi M., Araho D., Mizutani K., Kambara T., Ikeda T., Chou W-H., Inukai M., Takenaka A., Igarashi K. Hepatoprotective activity of tocha, the stems and leaves of Ampelopsis grossedentata, and ampelopsin. Biofactors. 2004;21(1-4):175–178. doi: 10.1002/biof.552210136. [DOI] [PubMed] [Google Scholar]
- 47.Kou X., Shen K., An Y., Qi S., Dai W.X., Yin Z. Ampelopsin inhibits H2O2-induced apoptosis by ERK and Akt signaling pathways and up-regulation of heme oxygenase-1. Phytother. Res. 2012;26(7):988–994. doi: 10.1002/ptr.3671. [DOI] [PubMed] [Google Scholar]
- 48.Kim T.Y., Leem E., Lee J.M., Kim S.R. Control of reactive oxygen species for the prevention of parkinson’s disease: The possible application of flavonoids. Antioxidants. 2020;9(7):583. doi: 10.3390/antiox9070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandel S., Weinreb O., Amit T., Youdim M.B. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: Implications for neurodegenerative diseases. J. Neurochem. 2004;88(6):1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 50.Koh S-H., Lee S.M., Kim H.Y., Lee K-Y., Lee Y.J., Kim H-T., Kim J., Kim M-H., Hwang M.S., Song C., Yang K.W., Lee K.W., Kim S.H., Kim O.H. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci. Lett. 2006;395(2):103–107. doi: 10.1016/j.neulet.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 51.Xu Z., Chen S., Li X., Luo G., Li L., Le W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem. Res. 2006;31(10):1263–1269. doi: 10.1007/s11064-006-9166-z. [DOI] [PubMed] [Google Scholar]
- 52.Hockenbery D.M., Oltvai Z.N., Yin X-M., Milliman C.L., Korsmeyer S.J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75(2):241–251. doi: 10.1016/0092-8674(93)80066-N. [DOI] [PubMed] [Google Scholar]
- 53.Nabavi S.F., Daglia M., D’Antona G., Sobarzo-Sánchez E., Talas Z.S., Nabavi S.M. Natural compounds used as therapies targeting to amyotrophic lateral sclerosis. Curr. Pharm. Biotechnol. 2015;16(3):211–218. doi: 10.2174/1389201016666150118132224. [DOI] [PubMed] [Google Scholar]
- 54.Srinivasan E., Rajasekaran R. Probing the inhibitory activity of epigallocatechin-gallate on toxic aggregates of mutant (L84F) SOD1 protein through geometry based sampling and steered molecular dynamics. J. Mol. Graph. Model. 2017;74:288–295. doi: 10.1016/j.jmgm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Maher P. The potential of flavonoids for the treatment of neurodegenerative diseases. Int. J. Mol. Sci. 2019;20(12):3056. doi: 10.3390/ijms20123056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solanki I., Parihar P., Parihar M.S. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem. Int. 2016;95:100–108. doi: 10.1016/j.neuint.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Li P., Matsunaga K., Ohizumi Y. Nerve growth factor-potentiating compounds from picrorhizae rhizoma. Biol. Pharm. Bull. 2000;23(7):890–892. doi: 10.1248/bpb.23.890. [DOI] [PubMed] [Google Scholar]
- 58.Cao Y., Liu J.W., Yu Y.J., Zheng P.Y., Zhang X.D., Li T., Guo M.C. Synergistic protective effect of picroside II and NGF on PC12 cells against oxidative stress induced by H2O2. Pharmacol. Rep. 2007;59(5):573–579. [PubMed] [Google Scholar]
- 59.Li T., Liu J-W., Zhang X-D., Guo M-C., Ji G. The neuroprotective effect of picroside II from hu-huang-lian against oxidative stress. Am. J. Chin. Med. 2007;35(4):681–691. doi: 10.1142/S0192415X0700517X. [DOI] [PubMed] [Google Scholar]
- 60.Guo N., Jin C., Shen L., Wu F., Lin X., Feng Y. Chemical components, pharmacological actions, and clinical applications of Rhizoma Picrorhizae. Phytother. Res. 2020;34(5):1071–1082. doi: 10.1002/ptr.6591. [DOI] [PubMed] [Google Scholar]
- 61.Wang W., Xu J., Li L., Wang P., Ji X., Ai H., Zhang L., Li L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 2010;83(5):196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Wang W., Huang W., Li L., Ai H., Sun F., Liu C., An Y. Morroniside prevents peroxide-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells. Cell. Mol. Neurobiol. 2008;28(2):293–305. doi: 10.1007/s10571-007-9168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W., Sun F., An Y., Ai H., Zhang L., Huang W., Li L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharmacol. 2009;613(1-3):19–23. doi: 10.1016/j.ejphar.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J-X., Wang R., Xi J., Shen L., Zhu A-Y., Qi Q., Wang Q-Y., Zhang L-J., Wang F-C., Lü H-Z., Hu J.G. Morroniside protects SK-N-SH human neuroblastoma cells against H2O2-induced damage. Int. J. Mol. Med. 2017;39(3):603–612. doi: 10.3892/ijmm.2017.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin Y., Lin B., Lin D. Effects of morroniside on the viability of random skin flaps in rats. J. Invest. Surg. 2020;33(2):182–188. doi: 10.1080/08941939.2018.1479007. [DOI] [PubMed] [Google Scholar]
- 66. Wang, Shi-bo.; Jing-fu, Qiu.; Qun-hua, B.; Jia-Jia, Li.; Jin-yu, He.; Yan-jun G.; and Chao Y. A study on protection of astragaioside IV about oxidative stress on PC12 cells induced by H2O2. Chinese Pharmacol. Bull., 2011;11 [Google Scholar]
- 67.Rong J., Cheung C.Y-H., Lau A.S-Y., Shen J., Tam P.K-H., Cheng Y-C. Induction of heme oxygenase-1 by traditional Chinese medicine formulation ISF-1 and its ingredients as a cytoprotective mechanism against oxidative stress. Int. J. Mol. Med. 2008;21(4):405–411. doi: 10.3892/ijmm.21.4.405. [DOI] [PubMed] [Google Scholar]
- 68.Yu J., Guo M., Li Y., Zhang H., Chai Z., Wang Q., Yan Y., Yu J., Liu C., Zhang G-X. Astragaloside IV protects neurons from microglia-mediated cell damage through promoting microglia polarization. Folia Neuropathol. 2019;57(2):170–181. doi: 10.5114/fn.2019.86299. [DOI] [PubMed] [Google Scholar]
- 69.Shahzad M., Shabbir A., Wojcikowski K., Wohlmuth H., Gobe G.C. The antioxidant effects of Radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets. 2016;17(12):1331–1340. doi: 10.2174/1389450116666150907104742. [DOI] [PubMed] [Google Scholar]
- 70.Zhu J., Shen L., Lin X., Hong Y., Feng Y. Clinical research on traditional chinese medicine compounds and their preparations for amyotrophic lateral sclerosis. Biomed. Pharmacother. 2017;96:854–864. doi: 10.1016/j.biopha.2017.09.135. [DOI] [PubMed] [Google Scholar]
- 71.Sun M-M., Bu H., Li B., Yu J-X., Guo Y-S., Li C-Y. Neuroprotective potential of phase II enzyme inducer diallyl trisulfide. Neurol. Res. 2009;31(1):23–27. doi: 10.1179/174313208X332959. [DOI] [PubMed] [Google Scholar]
- 72.Guo Y., Zhang K., Wang Q., Li Z., Yin Y., Xu Q., Duan W., Li C. Neuroprotective effects of diallyl trisulfide in SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. Brain Res. 2011;1374:110–115. doi: 10.1016/j.brainres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Calò L.A., Fusaro M., Davis P.A. HO-1 attenuates hypertension-induced inflammation/oxidative stress: support from Bartter’s/Gitelman’s patients. Am. J. Hypertens. 2010;23(9):936–936. doi: 10.1038/ajh.2010.130. [DOI] [PubMed] [Google Scholar]
- 74.Liu C., Leng B., Li Y., Jiang H., Duan W., Guo Y., Li C., Hong K. Diallyl trisulfide protects motor neurons from the neurotoxic protein TDP-43 via activating lysosomal degradation and the antioxidant response. Neurochem. Res. 2018;43(12):2304–2312. doi: 10.1007/s11064-018-2651-3. [DOI] [PubMed] [Google Scholar]
- 75.Silva-Islas C.A., Chánez-Cárdenas M.E., Barrera-Oviedo D., Ortiz-Plata A., Pedraza-Chaverri J., Maldonado P.D. Diallyl trisulfide protects rat brain tissue against the damage induced by ischemia-reperfusion through the Nrf2 pathway. Antioxidants. 2019;8(9):410. doi: 10.3390/antiox8090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jain K.K. In The Handbook of Neuroprotection. Springer; 2019. pp. 609–641. [DOI] [Google Scholar]
- 77.Weydt P., Möller T. Neuroinflammation in the pathogenesis of amyotrophic lateral sclerosis. Neuroreport. 2005;16(6):527–531. doi: 10.1097/00001756-200504250-00001. [DOI] [PubMed] [Google Scholar]
- 78.Weydt P., Weiss M.D., Möller T., Carter G.T. Neuro-inflammation as a therapeutic target in amyotrophic lateral sclerosis. Curr. Opin. Investig. Drugs. 2002;3(12):1720–1724. [PubMed] [Google Scholar]
- 79.Subedi L., Lee S.E., Madiha S., Gaire B.P., Jin M., Yumnam S., Kim S.Y. Phytochemicals against TNFα-mediated neuroinflammatory diseases. Int. J. Mol. Sci. 2020;21(3):764. doi: 10.3390/ijms21030764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ong W.Y., Farooqui T., Ho C.F.Y., Ng Y.K., Farooqui A.A. Use of Phytochemicals against neuroinflammation. Neuroprotective Effects of Phytochemicals in Neurological Disorders; 2017. p. 648. [Google Scholar]
- 81.Morita T. Celastrol: A new therapeutic potential of traditional Chinese medicine. Am. J. Hypertens. 2010;23(8):821–821. doi: 10.1038/ajh.2010.87. [DOI] [PubMed] [Google Scholar]
- 82.Kiaei M., Kipiani K., Petri S., Chen J., Calingasan N.Y., Beal M.F. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener. Dis. 2005;2(5):246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 83.Jung H.W., Chung Y.S., Kim Y.S., Park Y-K. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp. Mol. Med. 2007;39(6):715–721. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- 84.Venkatesha S.H., Dudics S., Astry B., Moudgil K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016;74(6):ftw059. doi: 10.1093/femspd/ftw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu Y., Fotinos A., Mao L.L., Atassi N., Zhou E.W., Ahmad S., Guan Y., Berry J.D., Cudkowicz M.E., Wang X. Neuroprotective agents target molecular mechanisms of disease in ALS. Drug Discov. Today. 2015;20(1):65–75. doi: 10.1016/j.drudis.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 86.Zhang R., Zhu Y., Dong X., Liu B., Zhang N., Wang X., Liu L., Xu C., Huang S., Chen L. Celastrol attenuates cadmium-induced neuronal apoptosis via inhibiting Ca2+ -CaMKII-dependent Akt/mTOR pathway. J. Cell. Physiol. 2017;232(8):2145–2157. doi: 10.1002/jcp.25703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burns J., Yokota T., Ashihara H., Lean M.E., Crozier A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 88.Bi X.L., Yang J.Y., Dong Y.X., Wang J.M., Cui Y.H., Ikeshima T., Zhao Y.Q., Wu C.F. Resveratrol inhibits nitric oxide and TNF-α production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2005;5(1):185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Meng X-L., Yang J-Y., Chen G-L., Wang L-H., Zhang L-J., Wang S., Li J., Wu C-F. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem. Biol. Interact. 2008;174(1):51–59. doi: 10.1016/j.cbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 90.Contestabile A. Oxidative stress in neurodegeneration: mechanisms and therapeutic perspectives. Curr. Top. Med. Chem. 2001;1(6):553–568. doi: 10.2174/1568026013394723. [DOI] [PubMed] [Google Scholar]
- 91.Mancuso R., del Valle J., Modol L., Martinez A., Granado-Serrano A.B., Ramirez-Núñez O., Pallás M., Portero-Otin M., Osta R., Navarro X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics. 2014;11(2):419–432. doi: 10.1007/s13311-013-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yun Y.C., Jeong S.G., Kim S.H., Cho G.W. Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J. Tissue Eng. Regen. Med. 2019;13(1):110–115. doi: 10.1002/term.2776. [DOI] [PubMed] [Google Scholar]
- 93.ALS Untangled no. 49. Resveratrol. Amyotroph. Lateral Scler. Frontotemporal Degener. 2019;20(7-8):619–624. doi: 10.1080/21678421.2019.1593596. [DOI] [PubMed] [Google Scholar]
- 94.Laudati G., Mascolo L., Guida N., Sirabella R., Pizzorusso V., Bruzzaniti S., Serani A., Di Renzo G., Canzoniero L.M.T., Formisano L. Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical neurons overexpressing SOD1-G93A to Thimerosal toxicity through SIRT1/DREAM/PDYN pathway. Neurotoxicology. 2019;71:6–15. doi: 10.1016/j.neuro.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Kaiyan Y., Jianlan G., Dongmei Y., Qin S. Effect of curcumin on iNOS expression in LPS-activated microglia cells and anti-oxidation. 2010.
- 96.Yin W., Shi X., Zhang X., Yu L. Curcumins upregulate expression of HO-1 via inducing Nrf-2 in SH-SY5Y cells. Chinese Pharmacol. Bulletin, 2003.
- 97.Sikora E., Scapagnini G., Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun. Ageing. 2010;7(1):1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bedlack R., Group A. ALSUntangled 44. Curcumin. Amyotroph. Lateral Scler. Frontotemporal Degener. 2018;19(7-8):623–629. doi: 10.1080/21678421.2018.1440738. [DOI] [PubMed] [Google Scholar]
- 99.Chico L., Ienco E.C., Bisordi C., Gerfo A.L., Schirinzi E., Siciliano G. Curcumin as an ROS scavenger in amyotrophic lateral sclerosis. React. Oxygen Species. 2016;2(5):339–354. [Google Scholar]
- 100.Adami R., Bottai D. Curcumin and neurological diseases. Nutr. Neurosci. 2020:1–21. doi: 10.1080/1028415X.2020.1760531. [DOI] [PubMed] [Google Scholar]
- 101.Yuan D., Ma B., Yang J.Y., Xie Y.Y., Wang L., Zhang L.J., Kano Y., Wu C.F. Anti-inflammatory effects of rhynchophylline and isorhynchophylline in mouse N9 microglial cells and the molecular mechanism. Int. Immunopharmacol. 2009;9(13-14):1549–1554. doi: 10.1016/j.intimp.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 102.Song Y., Liu J., Shi F., Lan Z., Li L., Ma S. Inhibitory effect of isorhynchophylline on lipopolysaccharide stimulated release of inflammatory mediators in primary rat astrocytes. Pharmacol Clin Res. 2011;19(2):311–314. [Google Scholar]
- 103.Rahman M.A., Rahman M.R., Zaman T., Uddin M.S., Islam R., Abdel-Daim M.M., Rhim H. Emerging potential of naturally occurring autophagy Modulators against neurodegeneration. Curr. Pharm. Des. 2020;26(7):772–779. doi: 10.2174/1381612826666200107142541. [DOI] [PubMed] [Google Scholar]
- 104.Ock J., Han H.S., Hong S.H., Lee S.Y., Han Y.M., Kwon B.M., Suk K. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br. J. Pharmacol. 2010;159(8):1646–1662. doi: 10.1111/j.1476-5381.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J., Su G., Gao J., Tian Y., Liu X., Zhang Z. Effects of peroxiredoxin 2 in neurological disorders: A review of its molecular mechanisms. Neurochem. Res. 2020;45(4):720–730. doi: 10.1007/s11064-020-02971-x. [DOI] [PubMed] [Google Scholar]
- 106.Rehman M.U., Wali A.F., Ahmad A., Shakeel S., Rasool S., Ali R., Rashid S.M., Madkhali H., Ganaie M.A., Khan R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019;17(3):247–267. doi: 10.2174/1570159X16666180911124605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tseng Y-T., Hsu Y-Y., Shih Y-T., Lo Y-C. Paeonol attenuates microglia-mediated inflammation and oxidative stress-induced neurotoxicity in rat primary microglia and cortical neurons. Shock. 2012;37(3):312–318. doi: 10.1097/SHK.0b013e31823fe939. [DOI] [PubMed] [Google Scholar]
- 108.Wang X., Zhu G., Yang S., Wang X., Cheng H., Wang F., Li X., Li Q. Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via downregulation of ERK activation and inhibition of apoptosis. Planta Med. 2011;77(15):1695–1701. doi: 10.1055/s-0030-1271033. [DOI] [PubMed] [Google Scholar]
- 109.He L.X., Tong X., Zeng J., Tu Y., Wu S., Li M., Deng H., Zhu M., Li X., Nie H., Yang L., Huang F. Paeonol suppresses neuroinflammatory responses in LPS-activated microglia cells. Inflammation. 2016;39(6):1904–1917. doi: 10.1007/s10753-016-0426-z. [DOI] [PubMed] [Google Scholar]
- 110.Lee H., Kim Y.O., Kim H., Kim S.Y., Noh H.S., Kang S.S., Cho G.J., Choi W.S., Suk K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003;17(13):1943–1944. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- 111.Piao H., Cui H., Piao R., Yingjun L. Effects of wogonin on LPS-induced production of proinflammatory cytokines. J. Xi’an Jiaotong University. Medical Sciences; 1981. p. 2. [Google Scholar]
- 112.Zhang J., He Y., Jiang X., Jiang H., Shen J. Nature brings new avenues to the therapy of central nervous system diseases-An overview of possible treatments derived from natural products. Sci. China Life Sci. 2019;62(10):1332–1367. doi: 10.1007/s11427-019-9587-y. [DOI] [PubMed] [Google Scholar]
- 113.Li Y., Liu S., Zhang H., Zhou F., Liu Y., Lu Q., Yang L. Antioxidant effects of celastrol against hydrogen peroxide-induced oxidative stress in the cell model of amyotrophic lateral sclerosis. Sheng Li Xue Bao. 2017;69(6):751. [PubMed] [Google Scholar]
- 114.Deane C.A., Brown I.R. Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol. Cell Stress Chaperones. 2016;21(5):837–848. doi: 10.1007/s12192-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim D., Nguyen M.D., Dobbin M.M., Fischer A., Sananbenesi F., Rodgers J.T., Delalle I., Baur J.A., Sui G., Armour S.M., Puigserver P., Sinclair D.A., Tsai L.H. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J., Zhang Y., Tang L., Zhang N., Fan D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 2011;503(3):250–255. doi: 10.1016/j.neulet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 117.Markert C.D., Kim E., Gifondorwa D.J., Childers M.K., Milligan C.E. A single-dose resveratrol treatment in a mouse model of amyotrophic lateral sclerosis. J. Med. Food. 2010;13(5):1081–1085. doi: 10.1089/jmf.2009.0243. [DOI] [PubMed] [Google Scholar]
- 118.Mancuso R., Del Valle J., Morell M., Pallás M., Osta R., Navarro X. Lack of synergistic effect of resveratrol and sigma-1 receptor agonist (PRE-084) in SOD1G93A ALS mice: Overlapping effects or limited therapeutic opportunity? Orphanet J. Rare Dis. 2014;9(1):78. doi: 10.1186/1750-1172-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dong H., Xu L., Wu L., Wang X., Duan W., Li H., Li C. Curcumin abolishes mutant TDP-43 induced excitability in a motoneuron-like cellular model of ALS. Neuroscience. 2014;272:141–153. doi: 10.1016/j.neuroscience.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X., Hong Y.L., Xu D.S., Feng Y., Zhao L.J., Ruan K.F., Yang X.J. A review of experimental research on herbal compounds in amyotrophic lateral sclerosis. Phytother. Res. 2014;28(1):9–21. doi: 10.1002/ptr.4960. [DOI] [PubMed] [Google Scholar]
- 121.Kaneko Y., Coats A.B., Tuazon J.P., Jo M., Borlongan C.V. Rhynchophylline promotes stem cell autonomous metabolic homeostasis. Cytotherapy. 2020;22(2):106–113. doi: 10.1016/j.jcyt.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y-H., Zeng K-W. Natural products as a crucial source of anti-inflammatory drugs: Recent trends and advancements. Trad. Med. Res. 2019;4(5):257–268. [Google Scholar]
- 123.Lee S-H., Suk K. Identification of glia phenotype modulators based on select glial function regulatory signaling pathways. Expert Opin. Drug Discov. 2018;13(7):627–641. doi: 10.1080/17460441.2018.1465925. [DOI] [PubMed] [Google Scholar]
- 124.Zhu Y., Wang J. Wogonin increases β-amyloid clearance and inhibits tau phosphorylation via inhibition of mammalian target of rapamycin: Potential drug to treat Alzheimer’s disease. Neurol. Sci. 2015;36(7):1181–1188. doi: 10.1007/s10072-015-2070-z. [DOI] [PubMed] [Google Scholar]
- 125.Yousuf M., Khan P., Shamsi A., Shahbaaz M., Hasan G.M., Haque Q.M.R., Christoffels A., Islam A., Hassan M.I. Inhibiting CDK6 activity by quercetin is an attractive strategy for cancer therapy. ACS Omega. 2020;5(42):27480–27491. doi: 10.1021/acsomega.0c03975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.von Lewinski F., Keller B.U. Ca2+, mitochondria and selective motoneuron vulnerability: Implications for ALS. Trends Neurosci. 2005;28(9):494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Appel S.H., Beers D., Siklos L., Engelhardt J.I., Mosier D.R. Calcium: The darth vader of ALS. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2001;2(1) Suppl. 1:S47–S54. [PubMed] [Google Scholar]
- 128.Prell T., Lautenschläger J., Grosskreutz J. Calcium-dependent protein folding in amyotrophic lateral sclerosis. Cell Calcium. 2013;54(2):132–143. doi: 10.1016/j.ceca.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 129.Chen D-M., Xiao L., Cai X., Zeng R., Zhu X-Z. Involvement of multitargets in paeoniflorin-induced preconditioning. J. Pharmacol. Exp. Ther. 2006;319(1):165–180. doi: 10.1124/jpet.106.104380. [DOI] [PubMed] [Google Scholar]
- 130.Mao Q.Q., Zhong X.M., Li Z.Y., Huang Z. Paeoniflorin protects against NMDA-induced neurotoxicity in PC12 cells via Ca2+ antagonism. Phytother. Res. 2011;25(5):681–685. doi: 10.1002/ptr.3321. [DOI] [PubMed] [Google Scholar]
- 131.Yang J., He L.N., He S.B. Effect of paeoniflorin on calcium overloading injury in cultured PC12 cells. Zhongguo Xin Yao Zazhi. 2001;6:413–416. [PubMed] [Google Scholar]
- 132.Mao Q-Q., Zhong X-M., Feng C-R., Pan A-J., Li Z-Y., Huang Z. Protective effects of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via antioxidant mechanisms and Ca(2+) antagonism. Cell. Mol. Neurobiol. 2010;30(7):1059–1066. doi: 10.1007/s10571-010-9537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.da S Hage-Melim, L.I.; Ferreira, J.V.; de Oliveira, N.K.; Correia, L.C.; Almeida, M.R.; Poiani, J.G.; Taft, C.A.; de Paula da Silva, C.H. The impact of natural compounds on the treatment of neurodegenerative diseases. Curr. Org. Chem. 2019;23(3):335–360. [Google Scholar]
- 134.Zhang L-C. Paeoniflorin reduces the spinal cord injury in rats through TLR4 inflammatory pathway and Nrf2 oxidative stress pathway: The experimental study. Hainan Yixueyuan Xuebao. 2017;23(8):26–30. [Google Scholar]
- 135.Yifeng D., Zhaolin S., Yang L., Zhongyan H., Shuli S. Effects of ligustrazine on L-type calcium current in SH-SY5Y human neuroblastoma. Chinese J. Neuroimmunol. Neurol. 2004;11(1):43–45. [Google Scholar]
- 136.Masoomzadeh S., Aminroaia P., Darchin Tabrizi F., Rashvand S., Rostamizadeh K. Lipid based nanoparticles for treatment of CNS diseases. Nanomed. Res. J. 2020;5(2):101–113. [Google Scholar]
- 137.Li Y-M., Chen F-P., Liu G-Q. Studies on inhibitive effect of gastrodin on PC12 cell damage induced by glutamate and H~ 2O~ 2. Zhongguo Yaoke Daxue Xuebao. 2003;34(5):456–460. [Google Scholar]
- 138.Xu X., Lu Y., Bie X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007;73(7):650–654. doi: 10.1055/s-2007-981523. [DOI] [PubMed] [Google Scholar]
- 139.Du F., Wang X., Shang B., Fang J., Xi Y., Li A., Diao Y. Gastrodin ameliorates spinal cord injury via antioxidant and anti-inflammatory effects. Acta Biochim. Pol. 2016;63(3):589–593. doi: 10.18388/abp.2016_1272. [DOI] [PubMed] [Google Scholar]
- 140.Sun R., Zhang Z., Huang W., Lv L., Yin J. Protective effects and machanism of muskone on pheochromocytoma cell injure induced by glutamate. Zhongguo Zhongyao Zazhi. 2009;34(13):1701–1704. [PubMed] [Google Scholar]
- 141.Van Damme P., Dewil M., Robberecht W., Van Den Bosch L. Excitotoxicity and amyotrophic lateral sclerosis. Neurodegener. Dis. 2005;2(3-4):147–159. doi: 10.1159/000089620. [DOI] [PubMed] [Google Scholar]
- 142.Rosenblum L.T. Trotti, D. Glial Amino Acid Transporters. Springer; 2017. pp. 117–136. [DOI] [Google Scholar]
- 143.Malik A.R., Willnow T.E. Excitatory amino acid transporters in physiology and disorders of the central nervous system. Int. J. Mol. Sci. 2019;20(22):5671. doi: 10.3390/ijms20225671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho J., Kim Y.H., Kong J-Y., Yang C.H., Park C.G. Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci. 2002;71(5):591–599. doi: 10.1016/S0024-3205(02)01729-0. [DOI] [PubMed] [Google Scholar]
- 145.Chen Y., Fang Y., Liang Y., Wang Q., He Y. Protective effects of β-asarone on PC12 cells damage induced by glutamate. Zhongguo Zhongyiyao Xinxi Zazhi. 2007;14:22–23. [Google Scholar]
- 146.Zheng M., Fan D. Different distribution of NMDA receptor subunits in cortex contributes to selective vulnerability of motor neurons in amyotrophic lateral sclerosis. Beijing Da Xue Xue Bao. 2011;43(2):228–233. [PubMed] [Google Scholar]
- 147.Deolankar S.C., Modi P.K., Subbannayya Y., Pervaje R., Prasad T.S.K. Molecular targets from traditional medicines for neuroprotection in human neurodegenerative diseases. OMICS. 2020;24(7):394–403. doi: 10.1089/omi.2020.0033. [DOI] [PubMed] [Google Scholar]
- 148.Gordon R.K., Nigam S.V., Weitz J.A., Dave J.R., Doctor B.P., Ved H.S. The NMDA receptor ion channel: A site for binding of Huperzine A. J. Appl. Toxicol. 2001;21(S1) Suppl. 1:S47–S51. doi: 10.1002/jat.805. [DOI] [PubMed] [Google Scholar]
- 149.Hemendinger R.A., Armstrong E.J., III, Persinski R., Todd J., Mougeot J-L., Volvovitz F., Rosenfeld J. Huperzine a provides neuroprotection against several cell death inducers using In-vitro model systems of motor neuron cell death. Neurotox. Res. 2008;13(1):49–61. doi: 10.1007/BF03033367. [DOI] [PubMed] [Google Scholar]
- 150.Kumar S.S. Application of phytochemicals for the treatment of neurodegenerative diseases.Drug Invention Today, 2018;10(3) [Google Scholar]
- 151.Jiang B., Liu J.H., Bao Y.M., An L.J. Catalpol inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Toxicon. 2004;43(1):53–59. doi: 10.1016/j.toxicon.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 152.Wang J., Kang B., Hu Y. XIA, Z.-q. Catalpol attenuates PC12 cells injury induced by L-glutamate. Zhongguo Yaolixue Tongbao. 2008;24:1258–1259. [Google Scholar]
- 153.Zheng X-w., Yang W-t., Chen S., Xu Q-q., Shan C-s., Zheng G-q., Ruan J-c. Neuroprotection of catalpol for experimental acute focal ischemic stroke: Preclinical evidence and possible mechanisms of antioxidation, anti-inflammation, and antiapoptosis. Oxid. Med. Cell. Longev. 2017;2017:5058609. doi: 10.1155/2017/5058609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu R., Liu J-f., Xu K-p. ZOU, H.; SONG, L.-y.; DANG, R.-L.; ZOU, Z.-x.; LI, G.; TAN, G.-s. Chemical constituents in Selaginella tamariscina. Cent. South Pharm. 2011;9(8):564–566. [Google Scholar]
- 155.Wang C-J., Hu C-P., Xu K-P., Yuan Q., Li F-S., Zou H., Tan G-S., Li Y-J. Protective effect of selaginellin on glutamate-induced cytotoxicity and apoptosis in differentiated PC12 cells. Naunyn Schmiedebergs Arch. Pharmacol. 2010;381(1):73–81. doi: 10.1007/s00210-009-0470-4. [DOI] [PubMed] [Google Scholar]
- 156.Pérez-Hernández J., Zaldívar-Machorro V.J., Villanueva-Porras D., Vega-Ávila E., Chavarría A. A potential alternative against neurodegenerative diseases. Phytodrugs. Oxid. Med. Cell. Longev. 2016;2016:8378613. doi: 10.1155/2016/8378613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yi D., Ning W., Quan Z. Neuroprotective effects of ferulic acid against glutamate-induced neurotoxicity in PC12 cells. Pharmacol. Clin. Chinese Materia Medica; 2008. p. 6. [Google Scholar]
- 158.Jin Y., Yan E.Z., Fan Y., Guo X.L., Zhao Y.J., Zong Z.H., Liu Z. Neuroprotection by sodium ferulate against glutamate-induced apoptosis is mediated by ERK and PI3 kinase pathways. Acta Pharmacol. Sin. 2007;28(12):1881–1890. doi: 10.1111/j.1745-7254.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 159.Luo L., Sun Y. Neuroprotective effect of ferulic acid In-vitro. Zhong Yao Cai. 2011;34(11):1750–1753. [PubMed] [Google Scholar]
- 160.Holeček V., Rokyta R. Possible etiology and treatment of amyotrophic lateral sclerosis. Neuroendocrinol. Lett. 2018;38(8):528–531. [PubMed] [Google Scholar]
- 161.Zhang F., Zheng W., Pi R., Mei Z., Bao Y., Gao J., Tang W., Chen S., Liu P. Cryptotanshinone protects primary rat cortical neurons from glutamate-induced neurotoxicity via the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. Exp. Brain Res. 2009;193(1):109–118. doi: 10.1007/s00221-008-1600-9. [DOI] [PubMed] [Google Scholar]
- 162.Kanekura K., Hashimoto Y., Kita Y., Sasabe J., Aiso S., Nishimoto I., Matsuoka M.A. Rac1/phosphatidylinositol 3-kinase/Akt3 anti-apoptotic pathway, triggered by AlsinLF, the product of the ALS2 gene, antagonizes Cu/Zn-superoxide dismutase (SOD1) mutant-induced motoneuronal cell death. J. Biol. Chem. 2005;280(6):4532–4543. doi: 10.1074/jbc.M410508200. [DOI] [PubMed] [Google Scholar]
- 163.Fang X. Impaired tissue barriers as potential therapeutic targets for Parkinson’s disease and amyotrophic lateral sclerosis. Metab. Brain Dis. 2018;33(4):1031–1043. doi: 10.1007/s11011-018-0239-x. [DOI] [PubMed] [Google Scholar]
- 164.Chandran G. Insights on the neuromodulatory propensity of Selaginella (Sanjeevani) and its potential pharmacological applications. CNS Neurol. Disord. Drug Targets. 2014;13(1):82–95. doi: 10.2174/18715273113126660188. [DOI] [PubMed] [Google Scholar]
- 165.Ren Z., Zhang R., Li Y., Li Y., Yang Z., Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017;40(5):1444–1456. doi: 10.3892/ijmm.2017.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]