Abstract
General anesthesia has been successfully used in clinics for over 170 years, but its mechanisms of effect remain unclear. Behaviorally, general anesthesia is similar to sleep as it produces a reversible transition between wakefulness and the state of being unaware of one’s surroundings. A discussion regarding the common circuits of sleep and general anesthesia has been ongoing as an increasing number of sleep-arousal regulatory nuclei are reported to participate in the consciousness shift occurring during general anesthesia. Recently, with progress in research technology, both positive and negative evidence for overlapping neural circuits between sleep and general anesthesia has emerged. This article provides a review of the latest evidence on the neural substrates for sleep and general anesthesia regulation by comparing the roles of pivotal nuclei in sleep and anesthesia.
Keywords: Sleep, anesthesia, neural circuits, wakefulness, unconsciousness, sleep-arousal regulatory nuclei
1. INTRODUCTION
Modern anesthesia serves millions of surgical patients worldwide every year. However, the underlying mechanisms of general anesthesia remain understudied and unclear. The most marked feature of general anesthesia is the reversible loss of consciousness. In fact, there are only two approaches that can reversibly change the consciousness of creatures in nature. One is natural sleep, and the other is general anesthesia. These two processes have many similarities in terms of behavior and electroencephalogram (EEG) patterns. An increase in slow-delta (0.1-4 Hz) oscillations during sleep onset was observed in the anesthetic stage induced by propofol, sevoflurane, and dexmedetomidine. However, how sequential loss and recovery of consciousness occur following the administration of anesthesia remains unclear.
Arousal was previously considered a passive procedure after adequate sleep (i.e., when the sleep pressure was wiped away). As for emergence from anesthesia, this has also been considered a passive process opposite to anesthesia induction (i.e., when the concentration of general anesthesia is decreased). However, passive recovery after anesthesia is insufficient to elucidate the mechanism of neurological disorders occurring after anesthesia, such as agitation, delirium, and cognitive dysfunction. In the past decades, the “flip-flop” theory of sleep mechanisms has supported the mechanism of active arousal, suggesting that some nuclei that promote sleep are activated during sleep induction, whereas nuclei that actively promote wakefulness are activated when arousal begins. Sleep-promoting neurons and wake- promoting neurons are mutually antagonistic (i.e., these neurons inhibit each other) and, therefore, build up a sleep-arousal circuit. Since the emergence of the “flip-flop” theory in sleep studies, an increasing number of sleep-arousal-mediating nuclei have been validated in regulating general anesthesia. Moreover, there has been growing interest in investigating whether general anesthesia-induced consciousness transitions share neural circuits with natural sleep. Answering this question could not only provide new insights into the mechanisms of consciousness shifts but could also help discover novel neural targets for developing sleep-like anesthetics.
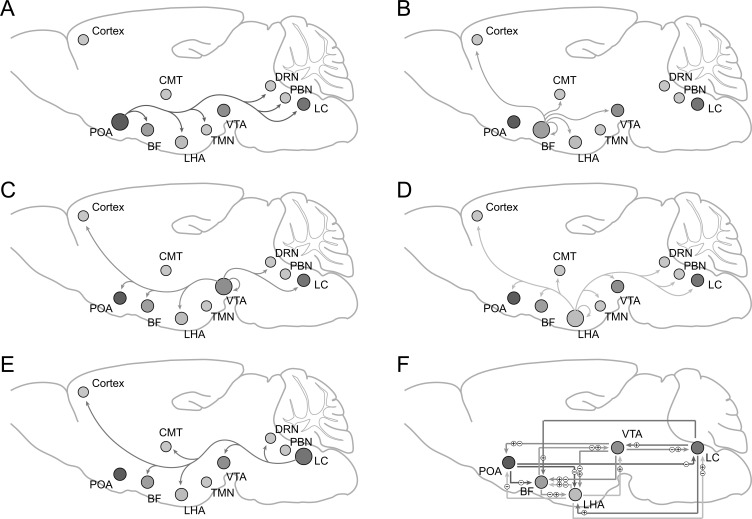
Among those nuclei which mediate sleep or anesthesia procedures, the preoptic area (POA) is the key structure of the sleep-promoting circuit and is widely studied for its common mechanism of both sleep and general anesthesia. Besides, basal forebrain (BF), lateral hypothalamic area (LHA), ventral tegmental area(VTA), and locus coeruleus (LC) are recognized as the sub-cortical arousal-mediating centers, which have been confirmed for their regulatory role in the sleep-arousal and anesthesia-arousal transitions, respectively. Recently, several brain areas have been newly found to be modulatory in consciousness transitions caused by sleep or anesthesia, such as the parabrachial nucleus (PBN), dorsal raphe nucleus (DRN), tuberomammillary nucleus (TMN), central medial thalamus (CMT), and certain cortical areas. Here, we review the latest evidence of these nuclei and their circuits in regulating the mechanism of sleep and general anesthesia (Fig. 1).
Fig. (1).
Long projections of brain areas implicated in the regulation of sleep / anesthesia-arousal transitions. A-E: Sleep / anesthesia-arousal related efferents of POA, BF, LHA, VTA, LC. F: Reciprocal connections among POA, BF, LHA, VTA, LC. POA: preoptic area; BF: basal forebrain; LHA: lateral hypothalamus; VTA: ventral tegmental area; LC: locus coeruleus. PBN: parabrachial nucleus; DRN: dorsal raphe nucleus; TMN: tuberomammillary nucleus; CMT: central medial thalamus ㊉ excitation; ㊀ inhibition.
2. PREOPTIC AREA
POA has long been considered a significant hub for sleep generation. POA is located in the anterior hypothalamus and spatially divided into the lateral preoptic area (LPO), the ventrolateral preoptic area (VLPO, core), the ventromedial preoptic area (VMPO), and the median preoptic area (MnPO) [1]. The neuronal subtype of VLPO has been well studied and mainly includes GABAergic and galaninergic neurons [2]. Recent single-cell RNA-sequencing techniques have revealed that POA has a rather high heterogeneity beyond traditional neuronal classifications. By analyzing approximately 1 million POA cells, 43 clusters within inhibitory neurons and 23 subpopulations within excitatory neurons are reported [3]. These clusters are characterized by distinct neuromodulatory signatures and spatial distributions in the POA. In addition to neuronal heterogeneity, the POA also diversely projects inhibitory innervations to the arousal-promoting nuclei, including the noradrenergic LC, the dopaminergic ventral tegmental area (VTA), the histaminergic TMN, acetylcholine in the pontine, the serotonergic dorsal and median raphe nuclei, the perifornical LHA, BF, and the PBN [4].
POA has been studied as a ‘sleep center’ for a long time. Early research on the role of the POA reported that broad activation of the POA by electrical stimulation resulted in the rapid onset of sleep [5]. Of note, sleep-active neurons have particularly high densities in the VLPO and MnPO. Selective VLPO lesions drastically induced insomnia in both clinical and animal experiments [4]. Severe lesions of the central VLPO caused a substantial decrease in delta power and a 50% decrease in both the non-rapid-eye-movement (NREM) sleep time and the rapid-eye-movement (REM) sleep [6]. GABAergic neurons in the VLPO are mostly NREM- and REM-active and are considered key triggers for promoting NREM and turning off wake-promoting neurons during NREM. GABAergic neurons in the MnPO are activated just prior to the onset of sleep, and the discharges of which are increased during sleep, suggesting a role in sleep initiation [7]. Chemogenetic stimulation of MnPO GABAergic neurons modulated sleep-wake architecture by increasing the NREM sleep time and decreasing the REM duration, whereas the wake time was not altered [8]. Galaninergic neurons, a small proportion of GABAergic neurons expressing galanin, also promote sleep. Galanin gene knockouts led to missing sleep rebound following sleep deprivation by medication and physical methods [9]. Chemogenetic activation of galaninergic neurons substantially increases NREM sleep [10]. Unexpectedly, recent studies have reported that optogenetic activation of galaninergic neurons at 8 Hz promotes wakefulness, while low-frequency (1–4 Hz) promotes sleep, indicating that the sleep regulatory effect of galaninergic neurons depends on the frequency of stimulation [10].
POA also plays an important role in general anesthesia. Isoflurane directly activates VLPO neurons in vitro, and propofol anesthesia or pentobarbital at a sedative dose significantly increases the expression of c-Fos in VLPO neurons [11]. Local administration of GABA receptor agonists in VLPO shortens the propofol-induced LORR and prolongs the RORR [12]. It has been observed that selective knockout of the vesicular GABA transporter gene (vgat) in the POA slowed the transition to sedation immediately after dexmedetomidine administration, but the mice were sedated 30 min after injection; this indicates that POA GABAergic neurons were necessary for the rapid induction of dexmedetomidine sedation but not sedative maintenance [13].
Given its pivotal role in sleep, POA has always been considered a typical target to study the overlapping mechanisms of sleep and anesthesia. Zhang et al. [13] used TetTagging with DREADD pharmacogenetics (TetTag-DREADD) to specifically label the POA neurons activated during dexmedetomidine sedation or deep sleep. Reactivation of genetically tagged POA neurons (especially the LPO neurons) following dexmedetomidine-induced sedation rendered the mice entering an NREM-sleep-like state, suggesting that sleep shares similar mechanisms and circuitry for the preoptic area with dexmedetomidine-induced sedation [13]. Mice without galaninergic neurons due to a gene-editing virus in the VLPO showed impairment of sleep homeostasis during sleep recovery after sleep deprivation and a decrease in the delta wave power of EEG; this was incapable of inducing delta waves following dexmedetomidine administration, indicating that sleep and dexmedetomidine require the involvement of POA [14]. Furthermore, activation of POA tachykinin 1 (Tac-1) neurons promoted and stabilized wake from NREM and REM sleep and showed reduced sensitivity to isoflurane anesthesia and an easier emergence from isoflurane and sevoflurane anesthesia [15]. Although this result was contrary to the previous finding that POA Tac1 neurons promoted NREM sleep [16], it provided more evidence for POA neurons mediating both sleep and anesthesia.
The hypothesis of joint POA circuits for sleep and general anesthesia has been challenged in the past few years. Eikermann et al. [17] reported in 2011 that the VLPO neuronal ablation-induced sleep loss sensitized animals to isoflurane anesthesia. Although the cumulative sleep loss was strongly related to the high sensitivity to anesthesia in this study, the number of remaining post-lesion VLPO neurons was not significantly correlated with the latency to loss of the righting reflex or to the onset time of slow-wave activity, potentially suggesting that sleep debt induced by VLPO neuronal loss (but not VLPO neurons themselves) are required for isoflurane anesthesia [17]. Recently, Vanini et al. [8] found that chemogenetic activation of MnPO GABAergic neurons increased total time spent in NREM sleep, and chemogenetic activation of VLPO glutamatergic neurons produced a substantial increase in wakefulness duration and a reduction in time spent in both NREM and REM sleep. However, neither activation of MnPOGABAergic neurons nor VLPO glutamatergic neurons altered the anesthetic state transitions induced by isoflurane, strongly contradicting the “sharing hypothesis.” The discrepancy among previous reports on the common target of POA for sleep and anesthesia could be due to experimental techniques as well as the heterogeneity of the POA cells.
3. BASAL FOREBRAIN
BF participates in the regulation of multiple brain functions, such as sleep-wake regulation, attention, learning, and sensory processing. Three main neuronal subtypes (glutamatergic, GABAergic, and cholinergic neurons, in descending order of quantity) spatially intermingled within the BF, which consists of the vertical and horizontal limbs of the diagonal band (VDB and HDB), medial septum (MS), the substantia innominate (SI), and the magnocellular preoptic nucleus (MCPO) [18, 19]. Extensive studies have been performed to delineate the role of connections within these neuronal subtypes and their long projections in the regulation of sleep and wakefulness [4].
During sleep-wake cycles, the activities of cholinergic and glutamatergic neurons are increased during the wake and REM sleep stages, as indicated by firing rates and calcium signals [20, 21]. Various neuronal subtypes of GABAergic neurons display distinct neuronal activities. Although parvalbumin (PV)-positive GABAergic neurons show similar patterns to cholinergic and glutamatergic neurons during wakefulness and REM activity, a subpopulation of somatostatin (SST)-positive GABAergic neurons shows increased activity during NREM stages [21].
Optogenetic stimulation of cholinergic neurons in the BF can induce cortical activation and rapid transitions from NREM sleep to wakefulness or REM sleep [21-23]. Activation of BF glutamatergic neurons also potently induces a transition from NREM sleep to wakefulness even at a higher efficiency [21]. However, chemogenetic manipulation of these neurons yields only slight alterations in the total time spent in each state, thereby highlighting the contribution of these neurons to cortical desynchronization and state consolidation (rather than a simple wake-promoting effect) [24, 25]. Overall activation of BF GABAergic neurons causes a prolonged duration of wakefulness and a shortened time duration of NREM and REM sleep [25], but different subtypes show wide discrepancies. Selective activation of PV-expressing GABAergic neurons causes rapid but brief arousals and high-frequency cortical oscillations [21, 26], which have been suggested to mediate arousal responses to sensory stimuli [27]. In contrast, SST-expressing GABAergic neurons, which have been shown to contain at least two heterogeneous subtypes, may exert a moderate inhibitory effect on wakefulness [18, 21, 28].
The regulatory effect of BF on sleep and wakefulness largely depends on the reciprocal connectivity within the cholinergic, glutamatergic, and GABAergic neurons and their long-range projections [19, 29, 30]. Electrophysiological studies have suggested that BF glutamatergic neurons excite local cholinergic and PV-expressing GABAergic neurons and that cholinergic neurons also excite nearby PV-expressing GABAergic neurons, thus forming a hierarchical excitatory microcircuit; this may partially mediate the strong wake-promoting effect of glutamatergic neurons [21]. Lesions within local BF cholinergic neurons barely impede glutamatergic neurons from robustly promoting wakefulness, indicating the involvement of long-range projections [31]. In addition, an opto-dialysis study showed that infusion of cholinergic antagonists within the BF could prevent the wake-promoting effect of activation of BF cholinergic neurons [32], indicating the involvement of local non-cholinergic neurons in the cholinergic regulation of sleep and wakefulness. Therefore, excitation of neighboring PV-expressing GABAergic neurons might mediate the wake-promoting effects of cholinergic neurons [32, 33]. In contrast, local inhibition of the other three types of wake-promoting neurons in the BF endows SST-expressing neurons with NREM-promoting potency [21].
In addition to local connections, BF glutamatergic neurons are known to have dense projections to several distant brain regions associated with sleep-wake control, including VTA, the central amygdala (CeA), LHA, and the lateral habenula (LHb) [19, 29]. Activation of projections from cholinergic neurons to several cortical regions produces desynchronized cortical states [34, 35], but optical stimulation of cholinergic fibers in the thalamic reticular nucleus (TRN) yields the opposite effect of promoting and stabilizing NREM sleep [36], suggesting that long-range cholinergic projections to different brain regions might play distinct functional roles. PV-expressing GABAergic neurons directly innervate neocortical interneurons to regulate fast cortical rhythms in terms of gamma frequency [26, 37-39], but whole-brain mapping of BF PV-expressing GABAergic neurons reveals extensive subcortical projections, including to the striatum, pallidum, LHA, and LHb [19, 29]; this may mediate the regulation of behavioral states and cortical oscillations. Distant areas innervated by SST-expressing neurons, including LHA, LHb, and VTA, may also be potential targets for sleep-promoting effects [29]. BF is involved in the regulation of sleep homeostasis [40]. Increased extracellular adenosine in the BF mediates accumulated sleep pressure following prolonged wakefulness [41], and activation of BF glutamatergic and cholinergic neurons contributes to an increase in adenosine [20].
Isoflurane anesthesia significantly decreases the acetylcholine efflux originating from the BF in the cortical areas [42], whereas BF cholinergic neurons increase their discharge rate during cortical activation [43]. Cholinergic activation of the cortex through muscarinic mechanisms disrupts slow rhythms in anesthetized animals [44], suggesting the involvement of BF cholinergic systems in the regulation of general anesthesia. Recent studies with calcium imaging have further confirmed the inhibition of BF neuronal activity during general anesthesia [45, 46]. In addition, non-specific lesions of the BF and immunological or electrolytic depletion of BF cholinergic neurons both potentiate anesthetic effects and prolong emergence from general anesthesia [45, 47, 48]. Additionally, genetic lesions within BF cholinergic neurons increase anesthetic potency, while chemogenetic or optogenetic activation of genetically defined BF cholinergic neurons reduces sensitivity to anesthetics, verifying the causal role of BF cholinergic neurons in the regulation of general anesthesia [46]. In addition, microinjection of orexin, histamine, or norepinephrine into the BF induces cortical activation, facilitates emergence from anesthesia, and elicits behavioral arousal [49-54]. Activation of orexinergic terminals from LH exerts a similar effect of facilitating recovery from general anesthesia [55], suggesting that BF is a mediator of the neuropeptidergic modulation of general anesthesia.
Potentiation of GABAA receptors underlies the mechanisms of most general anesthetics [56, 57]. Electrophysiological studies have found that propofol could decrease the excitability of both BF cholinergic and glutamatergic neurons by influencing the GABAA receptor without affecting glutamate transmission [58, 59], and that inhibition of BF through the infusion of the GABAA receptor agonist modulates anesthetic sensitivity, frontal cortex delta activity, and emergence from general anesthesia [45, 60]. However, it is intriguing that isoflurane significantly increases glutamate efflux and decreases GABA efflux in the BF [42], suggesting the possibility of a bidirectional mechanism of general anesthesia.
4. LATERAL HYPOTHALAMIC AREA
The hypothalamus is one of the most phylogenetically conserved areas in the vertebrate brain, reflecting its key role in maintaining physiological and behavioral homeostasis. The LHA, as the center for coordinating the sleep-wake state, eating, energy balance, and motivational behaviors, displays significant neuronal heterogeneity [61]. Single-cell sequencing identified neurons in the LHA into 15 subtypes of glutamatergic neurons and 15 subtypes of GABAergic neurons, both of which express known or novel cell markers [62].
A group of glutamatergic neurons expressing orexin (hypocretin, Hcrt) is strictly located in the perifornical area of the LHA and dorsomedial hypothalamus (DMH) but is projected to most brain regions. Orexinergic neurons exert a strong arousal-promoting effect through orexinergic signaling. The orexinergic system contains two peptides, orexin-A (OA) and orexin B (OB), and two receptors, orexin receptor 1 (OxR1) and orexin receptor 2 (OxR2).
The Orx1 receptor prefers to bind to OA, but Orx2 binds to OA and OB with similar high affinities [63]. The concentration of orexin in the CSF increases during wakefulness and decreases during sleep [64]. Accordingly, orexinergic neurons fire most rapidly during active wakefulness, become less active during wakefulness, and become silent during NREM and REM sleep. In addition, loss of orexin function could cause narcolepsy, with symptoms of excessive daytime sleepiness. Intracerebroventricular injection of orexin-A or an orexin receptor agonist could strongly promote wakefulness and reduce REM sleep time for hours. The arousal-promoting effect of the orexin system is likely mediated by the orexinergic projections to the BF, VTA, and LC [65]. In addition, optogenetic activation of orexin neurons could rouse mice from sleep, whereas inhibition of orexin neurons by optogenetics or chemogenetics promoted sleep [66].
Orexinergic neurons in the LHA also play a crucial role in anesthesia-arousal regulation. Previous studies have found that microinjection of orexin-A into the cerebral ventricles, BF, dorsal raphe nucleus, or VTA elicits arousal response on EEG and facilitates emergence from isoflurane-, sevoflurane-, or propofol-induced anesthesia [53, 54, 67, 68]. Notably, the findings indicate that orexinergic systems selectively affect anesthetic emergence without causing an associated change in induction, suggesting a distinguishable mechanism of emergence from that of induction and highlighting the emergence-promoting effect of orexin [69]. Specifically, chemogenetic activation of genetically defined orexinergic neurons shortens the emergency latency from general anesthesia [70], and optogenetic activation of orexinergic neurons or their projections to the BF, VTA, LC, or periventricular thalamus significantly reduces anesthetic depth [68, 71, 72].
Melanin-concentrating hormone (MCH) is synthesized and expressed by a large population of cells that are sparsely distributed throughout the LHA. In contrast to orexinergic neurons, MCH neurons are active during sleep, especially during REM sleep. MCH neurons project to brain areas that promote arousal and REM sleep, including the LC, DRN, laterodorsal, and pedunculopontine tegmental nuclei (LDT/PPT) as well as the sublaterodorsal tegmental nucleus (SLD). Intracerebroventricular or local administration of MCH into the DRN or BF induces both REM and NREM sleep. Optogenetic studies have confirmed the regulatory role of MCH neurons in sleep architecture. Selective activation of MCH neurons was found to enhance REM sleep. Ablation of MCH neurons increased wakefulness time and decreased NREM sleep duration with no effect on REM sleep. MCH neuronal activity, indicated by c-Fos expression, was not changed by inhaled anesthetics [69]; few results have been reported concerning the regulatory role of MCH neurons under general anesthesia. However, this should be investigated in future studies.
In addition to orexinergic and MCH neurons, GABAergic neurons in the LHA also participate in the regulation of sleep and wakefulness. With the help of circuit mapping in brain slices, orexinergic neurons were found to be inhibited by local GABAergic neurons via a microcircuit input; GABAergic neurons also send large putatively inhibitory projections to sleep-promoting and arousal-promoting nuclei, including the VLPO, TMN, ventral periaqueductal gray, and LC. Optogenetic activation of LHA GABAergic neurons during NREM sleep, but not during REM sleep, rapidly promoted the transition to wakefulness [73]; chemogenetic activation of LHA GABAergic neurons also profoundly promoted wakefulness and inhibited sleep [74]. Additionally, activation of GABAergic terminals in the VLPO or the TRN potently promoted wakefulness. Because of the dominant role of TRN in the thalamocortical network, the feedforward inhibiting effects on TRN GABAergic neurons position LHA GABAergic neurons as cardinal regulators in sleep-wake state transitions. Indeed, activation of the GABAergic circuit from the LHA to the TRN led to significant EEG alternations during anesthesia accompanied by limb movements. In addition, the application of propofol causes membrane hyperpolarization of LHA GABAergic neurons, indicating that LHA GABAergic neurons are involved in propofol-induced anesthesia. On the other hand, numerous LHA neurons synthesize the excitatory neurotransmitter glutamate, and optogenetic activation of these LHA glutamatergic neurons or their projections to the LHb during the maintenance of anesthesia could reduce the anesthetic depth and promote the emergence of anesthesia.
In general, intermingled various neuronal types with diverse molecular signatures, including various neurotransmitters and neuropeptides, make the LHA a central player in the regulation of multiple fundamental physiological behaviors, leading to its complex roles in arousal and consciousness. Further investigations with elaborate novel techniques are necessary to delineate the role of neurochemically distinct neuronal phenotypes of LHA in sleep-wake cycles and general anesthesia.
5. VENTRAL TEGMENTAL AREA
The VTA consists of five sub-regions with intermingled neuronal populations, including dopaminergic, glutamatergic, and GABAergic neurons [75]. Dopaminergic neurons can be further divided into neuronal subtypes with different peptides, proteins, or receptors, and numerous studies have shown that different functions of this area are mediated by diverse subpopulations of dopaminergic neurons involved in distinct neural circuits. VTA GABAergic and glutamatergic neurons also participate in the regulation of multiple functions independent of dopaminergic neurons [75, 76]. Dopaminergic neurons have been found to co-release GABA or glutamate [75].
Recent methodological advances have facilitated the mapping of afferent and efferent brain regions in different VTA neuronal populations. VTA dopaminergic neurons extensively project to forebrain areas through mesocortical and mesolimbic pathways and form reciprocal connections with the LH, POA, BF, the dorsal raphe nucleus (DRN), the LC, and the LDT/PPT [77]. Although VTA GABAergic neurons can function through dense local inhibitory connections, both GABAergic and glutamatergic neurons in the VTA also send long projections widely to forebrain and brainstem targets [78].
Apart from their predominant role in regulating reward and aversion, VTA dopaminergic neurons have been extensively reported in the modulation of sleep and wakefulness. Both multichannel recording and fiber photometry studies have shown that VTA dopaminergic neurons display elevated activities during REM sleep and wakefulness when compared to those during NREM sleep [79, 80], and studies have reported that these neurons switch to a burst firing pattern during REM sleep, further implying their involvement in the memory consolidation function of REM sleep [81]. Optogenetic activation of VTA dopaminergic neurons was sufficient to initiate and maintain wakefulness, even under high sleep pressure. Chemogenetic inhibition of these neurons could increase sleep, impede the wake-promoting effects of salient stimuli, and promote nesting behaviors, revealing a causal role of VTA dopaminergic neurons in the regulation of arousal and sleep-related behaviors [79]. However, manipulation of VTA dopaminergic projections to different downstream regions yielded inconsistent results in terms of arousal modulation, and it is believed that the local effects of dopaminergic neurons are mainly mediated by nucleus accumbens (NAc) projections to the medial prefrontal cortex (mPFC), CeA, and dorsal-lateral striatum [79]. Additionally, studies have shown that darkness-induced wakefulness is mediated by the inhibition of GABAergic inputs to VTA dopaminergic neurons from the superior colliculus (SC) [82], further implying the role of VTA in the regulation of circadian rhythm.
Similar to dopaminergic neurons, activation of glutamatergic neurons, which are wake- and REM-active, could produce wake sates through projections to the NAc and LHA; lesioning these areas impairs the consolidation of wakefulness [76]. In contrast, although VTA GABAergic neurons also increase their activities during wake and REM sleep, they produce a long-lasting NREM-resembling sedation state when activated [76]. The contradiction between the physiological activities and regulative effects of GABAergic neurons suggests that they may not directly promote natural NREM sleep but may limit wakefulness due to local microcircuits via arousal-promoting dopaminergic or glutamatergic neurons or long projections to the LHA [76, 83].
Systematical application of a dopamine transporter inhibitor, such as methylphenidate or a dopamine D1 receptor agonist, facilitated reanimation from general anesthesia, indicating the regulatory potency of the dopaminergic system on anesthetic-induced unconsciousness. The findings that electrical stimulation of the VTA, but not the adjacent substantia nigra, reliably induced a robust behavioral arousal response in rats during isoflurane and propofol anesthesia further highlights the role of VTA dopaminergic neurons [84]. Indeed, selective optogenetic activation of VTA dopaminergic neurons produced profound behavioral arousal responses, which could be blocked by dopamine D1 receptor antagonists during stable isoflurane-induced anesthetic states, confirming the critical role of VTA dopaminergic neurons in promoting emergence from general anesthesia [85]. Although the activity of VTA dopaminergic neurons and dopamine release in the downstream NAc region were significantly inhibited by sevoflurane anesthesia, prior findings have shown that dexmedetomidine could activate dopaminergic neurons in the VTA and increase dopamine concentrations in the related forebrain projection areas, including the NAc and mPFC. This discrepancy in activity alternations could partially explain the distinct arousability of dexmedetomidine and other anesthetics. In contrast, VTA GABAergic neurons could facilitate isoflurane anesthesia and postpone emergence through projections to the LHA [86].
Overall, VTA dopaminergic neurons exhibit general arousal-promoting effects in both natural sleep and general anesthesia, and GABAergic neurons restrict wakefulness and facilitate anesthesia. The changes in the physiological activity of VTA dopaminergic, GABAergic, and glutamatergic neurons during sleep-wake cycles suggest that excitatory and inhibitory hemostasis of the microcircuit within one nucleus could play a role in sculpting the function of this nucleus and even in regulating different vigilant states. Of note, VTA dopaminergic neurons have multiple firing patterns during consciousness states induced by natural sleep or anesthetics, which likely suggests diverse functional states through which VTA dopaminergic neurons could regulate consciousness. Moreover, because VTA dopaminergic neurons modulate physiological arousal through disparate projections, further research is still needed to verify how these neural circuits differentially regulate arousal following general anesthesia.
6. LOCUS COERULEUS
Sleep and general anesthesia are always mentioned together because of an important shared behavioral property (that is, reduced responsiveness to the external environment), yet the rapid reversibility of the quiescent state makes sleep easily distinguishable from anesthetic states [87]. Rapid arousability from sleep endows individuals with the ability to perceive threats and avoid dangers, thus possessing indispensable evolutionary significance. Within sleep-wake regulation neural networks, the LC, a noradrenergic (NA) center that sends broad projections to multiple brain areas, plays a pivotal role in regulating the transitions of vigilance states as well as a wide array of arousal-associated behaviors, such as attention, cognition, and orientation [88, 89].
Several seminal electrode recording studies have shown that LC-NA neurons display fluctuating firing activity across different arousal states and are most active during wakefulness, inactive during slow-wave sleep, and almost silent during paradoxical sleep [90, 91]. It is notable that awakening following alerting situations, wakefulness, and sleep interruptions are accompanied by phasic firing impulses of LC-NA neurons [91-93], indicating their role in the processing of salient external stimuli. Recent studies recording LC activities with silicon probes have further demonstrated that LC clusters fired short phasic bursts (responding to the onset of auditory stimulations), and the phasic responses were more robust during NREM sleep than during quiet or active wakefulness [94].
Previous lesioning and pharmacological studies have indicated that LC-NA neurons function to maintain activated brain states [95]. With advances in optogenetics, Carter et al. [96] found that inhibition of LC-NA neurons decreased the duration of wakefulness and increased the activity of the slow-wave oscillation and that stimulation of the LC-NA neurons caused immediate transitions from sleep to waking; these results suggested that these neurons were required for both maintaining wakefulness and promoting immediate transitions to wakefulness [96]. Long-term stimulation with different protocols yielded variant results that both tonic and phasic stimulation for 1 h caused an increase in the wakefulness, though only phasic stimulation prolonged the duration of wakefulness over a period of five hours [96]. In addition, the baseline tonic activity of LC-NA neurons during NREM sleep correlates with the probability of sound-evoked awakenings, and these neurons reliably respond to auditory stimuli by turning to fire phasic bursts, further revealing that lower LC-NA neuronal activities during sleep support reduced responsiveness to external stimuli [94]. In addition to adrenaline, several other neurotransmitters, including galanin, neuropeptide Y, and dopamine, are co-released by LC-NA neurons [88]. By specifically knocking down dopamine beta-hydroxylase, an enzyme for NE synthesis, Yamaguchi et al. further confirmed that NA was indispensable for the arousal regulation of LC stimulation [97].
For general anesthesia, propofol, etomidate, midazolam, thiopental, and isoflurane, which facilitate GABA signaling, significantly reduce noradrenergic activities [98-100]. However, chemicals acting as NMDA antagonists, including ketamine, nitrous oxide, and xenon, increase noradrenaline release in several brain regions [100], indicating a complicated engagement of LC noradrenergic systems in regulating general anesthesia. Adrenergic deficiency could cause hypersensitivity to the induction of volatile anesthesia and significantly retard the emergence from sevoflurane, isoflurane, or halothane anesthesia [101]. By modulating genetically defined LC-NA neurons via designer receptors under isoflurane anesthesia, Vazey et al. found that LC activation was sufficient to reduce anesthetic depth indicated by EEG measurements, including burst-suppression ratios or delta frequency power, and accelerate the recovery of consciousness; these effects could be prevented by adrenergic β or α1 receptor antagonists [102]. Similar to volatile anesthetics, the LC-NA system has been tested to regulate general anesthesia induced by propofol and etomidate [98]. Although LC noradrenergic projections to the midline thalamus exerted similar emergence-promoting effects [103, 104], the intracerebral injection of NA or the activation of noradrenergic terminals in the TRN delayed recovery from the propofol anesthesia [105], indicating diverse effects mediated by different neural circuits.
In addition to the abovementioned general anesthetics, dexmedetomidine (as an agonist of inhibitory metabotropic adrenergic α2 receptors) could induce loss of consciousness and a deeply sedative yet arousable state resembling NREM sleep. Selective knockdown of LC adrenergic α2 receptors abolished dexmedetomidine-induced LORR, but did not eliminate a sedative effect, indicative of disparate mechanisms with respect to these properties. Further investigation confirmed that the sedative state induced by dexmedetomidine was similar to recovery sleep, and neurons in the preoptic hypothalamus regulating NREM sleep played a role in this dexmedetomidine-induced sedation [13]; this implies a concurrent mechanism of sleep and dexmedetomidine-mediated anesthesia. In addition, the neuronal activities of VTA dopaminergic neurons and dopaminergic concentrations in the mPFC and NAc were increased after intraperitoneal injection of dexmedetomidine, suggesting that these easily arousing properties might be attributed to the activation of midbrain dopaminergic systems [106].
Generally, LC-NA neurons promote wakefulness from both sleep and general anesthesia. Notably, as a main regulatory target of dexmedetomidine anesthesia, LC-NA neurons could be an underlying knot for decoding inconsistent arousability within NREM sleep, dexmedetomidine-induced sedation, and other general anesthetics-induced unconsciousness states. The elaborate balance between sleep- and arousal-regulating neural circuits probably endows the NREM sleep and sedative states induced by dexmedetomidine with arousable properties. However, the overall breakdown of the adrenergic system and nearly all other neural systems by general anesthetics produces a profound unarousable unconscious state.
7. OTHER CIRCUITS INVOLVED IN THE AROUSAL OF ANESTHESIA
7.1. Parabrachial Nucleus
PBN, located in the brainstem, contains glutamatergic and GABAergic neurons. PBN GABAergic neurons are necessary not only for the quantity of normal sleep but also for the quality of sleep (the maintenance of slow-wave sleep requires PBN neuronal activation) [107]. PBN is also a predominant arousal-active nucleus. Genetically knocking out the vesicular glutamate transporter-2 (Vglut2) in PBN neurons increases NREM sleep during the dark period, indicating opposite effects of PBN glutamatergic and GABAergic neurons in sleep and arousal. Recent studies have validated the involvement of PBN in the consciousness transition induced by general anesthesia. Specifically, a group of PBN neurons is activated during emergence from isoflurane anesthesia. Electrical stimulation in the PBN substantially induces arousal during sleep under continuous isoflurane general anesthesia [108]. Chemogenetic activation of PBN glutamatergic neurons reduced the animals’ sensitivity to sevoflurane and decreased emergence time from sevoflurane inhalation. Optogenetic activation of PBN glutamatergic neurons during sevoflurane anesthesia maintenance produced the arousal-like changes observed in the EEG. The emergence facilitation of PBN could be mediated through its neural projections to the prefrontal cortex, the BF, the LH, the thalamus, and the supramammillary nucleus [109].
7.2. Dorsal Raphe Nucleus
The serotonin (5-HT) system participates in a broad range of neurophysiological regulations, including the regulation of sleep and wakefulness. 5-HT neurons in the DRN send extensive projections to the midbrain and forebrain and receive innervation from the cerebral cortex, limbic systems, BF, and hypothalamus. Optogenetic stimulation of 5-HT neurons in the DRN in a phasic pattern prolonged wakefulness and decreased NREM sleep while increasing NREM sleep when stimulated in a tonic pattern; this confirms the role of the DRN 5-HT system in stabilizing sleep homeostasis and the regulating of the sleep/wake cycle [110]. Electrolytic lesions of the DRN decreased the halothane MAC. Calcium activity of DRN 5-HT neurons imaged by fiber photometry decreased during isoflurane anesthesia and gradually recovered when isoflurane was discontinued. Using optogenetic and chemogenetic approaches, activation of DRN 5-HT neurons reduced the depth of anesthesia with respect to a decrease in the burst-suppression ratio during anesthesia maintenance and facilitated the emergence from isoflurane anesthesia. The emergence-promoting function of DRN 5-HT neurons could be achieved by 5-HT 1A or 2C receptors in the brain [111].
7.3. Tuberomammillary Nucleus
The central neural histamine system is originated from the TMN in the posterior hypothalamus [112]. Receiving the strong inhibition of VLPO GABAergic projections, the TMN histaminergic neurons are highly activated during active waking and less active during NREM and REM sleep [113]. Multiple studies have revealed that TMN histaminergic neurons have reciprocal interactions with GABAergic neurons of the preoptic area in regulating the sleep-wake cycle. On the other hand, histamine is involved in many anesthetics-induced loss of consciousness [114], such as isoflurane [115], dexmedetomidine, propofol [116], and pentobarbital [117], but not ketamine. Histamine levels were discovered to be markedly reduced in the brain during an anesthesia-induced loss of consciousness. The emergence facilitation effect of TMN histaminergic neurons is highly regulated by the inhibitory innervations from GABAergic neurons [117]. Bilaterally blocking the GABAA receptor in the TMN reduced the sensitivity of animals to dexmedetomidine anesthesia [114] and decreased the LORR time of propofol and pentobarbital [117].
7.4. Central Medial Thalamus
The integrity of thalamocortical connectivity is essential for the maintenance of arousal. Disruption of thalamocortical connectivity leads to unconsciousness. The CMT nucleus is a part of the middle thalamic complex that receives both subcortical and cortical innervations and also sends informative projections to cortices. Overexpression of the Kv1.2 protein in the CMT area facilitated sleep but decreased the pro-arousal effect of caffeine in a previous study [118]. Activating nicotinic acetylcholine receptors or blocking voltage-gated potassium channels in the CMT caused the arousal-like changes in the EEG and reversed sevoflurane-induced unconsciousness. The CaV3.1 isoform of T-type calcium channels is probably related to delta oscillation and burst-suppression patterns in EEG induced by isoflurane anesthesia [119]. Microinjections of norepinephrine into the CMT induced EEG signs of cortical arousal and accelerated the time to emergence from propofol anesthesia. These reports indicate a complex interaction between CMT neurons with various neurotransmitter systems. Baker et al. compared the local field potentials in the thalamus and cortex during the transition from awareness to unconsciousness induced by natural sleep and general anesthesia. They found that changes in local field potentials occurred first in the CMT (before changes could be detected in the neocortex for both propofol anesthesia and natural sleep), suggesting a potential initiatory role of the CMT in state transition [120].
7.5. Cortex
Disruption of cortical connective integrity is a feature of unconsciousness for general anesthesia [121, 122]. The neocortex exhibits higher activity during wakefulness and lower activity during slow-wave sleep [123, 124]. However, cerebral cortex also contains sleep-active neurons. The GABAergic interneurons are more active in slow-wave sleep than in wakefulness [125]. Particularly, as one of the limbic structures, the retrosplenial cortex was found to be REM-active and presented synchronized discharges with hippocampal theta waves during REM sleep. Neocortical neuronal activity was reported to decrease by 50% under the general anesthesia induced by volatile anesthetics and by 40% with propofol and etomidate [126]. Guo et al. [127] explored neurotransmission within the primary visual cortex during isoflurane anesthesia by using high-resolution approaches of in vivo two-photon imaging and genetically encoded neurotransmitter sensors. They found a general decrease in cortical GABA transmission during loss of consciousness, while glutamate transmission was mostly preserved in pyramidal cells but significantly reduced in inhibitory interneurons. Subtypes of cortical interneurons showed different changes in activity. After exposure to isoflurane, the activity of vasoactive intestinal peptide (VIP)-expressing neurons experienced a delayed inhibition preceded by a temporary increase and then showed a synchronized response pattern during the loss of consciousness. PV neuronal activity decreased slightly more than SOM interneurons, but they both were highly synchronized during the loss of consciousness. These findings depict a disrupted cortical excitatory-inhibitory network during the loss of consciousness induced by general anesthesia and emphasize the indispensable function of the inhibitory network in maintaining consciousness [127].
CONCLUSION
In this paper, we have reviewed several well-studied nuclei (from the brainstem to the neocortex) in modulating consciousness transitions caused by sleep and anesthesia, respectively. Among these, most neural circuits involved in sleep-arousal regulation also play a role in anesthesia-induced loss and recovery of consciousness. With modern advanced techniques, a few neuronal subtypes involved in natural sleep have been identified as overlapping in regulating general anesthetic effects. However, overlapping neurons labeled by specific markers are difficult to distinguish using traditional inhibitory or excitatory neuronal classifications. The current results may not completely indicate an overlapping neural circuitry mechanism between sleep and general anesthesia, though these findings raise a few points for discussion. First, a certain nucleus may regulate sleep or anesthesia, which does not necessarily mean that all neuronal types in this nucleus are involved in both sleep and anesthesia. Accumulating evidence supports the theory that sleep-promoting neurons are often spatially intermingled with wake-promoting neurons, making it difficult to target them selectively for circuit analysis. Second, although some nuclei have confirmed overlapping neurons in dual-regulating sleep and anesthesia, whether other sleep-arousal-regulating nuclei have such overlapping neurons needs to be further studied. Third, overlapping neurons are likely to distinguish themselves by having untraditional neuronal labels. Conventionally classified neuronal markers and responsible genetic tools may not be able to accurately anchor such neurons; therefore, more advanced regulative approaches are needed. Lastly, for most sleep-arousal nuclei, such as VTA and BF, there are multiple types of neurons that likely form the microcircuits participating in the regulation of unconscious and arousal transition. The configuration of intranuclear microcircuits of overlapping neurons requires further research.
In addition, with regard to the molecular targets of sleep and anesthesia, these two reversible unconscious procedures may not share an identical mechanism. Although the activation of GABAA receptors mediates both sleep and general anesthesia effects, the subsets of GABAA receptors activated by the endogenous GABA (primarily released from the POA GABAergic neurons) during sleep onset could be different from those driven by general anesthetics. Endogenous GABA normally binds to the area between the α and β subunits in the GABAA receptor [128]. In contrast, the anesthetic effect of propofol is related to α, β, and γ subunits of GABAA receptor and the inhalational anesthetic agents to the α1-subunit of the GABAA receptor [129]. The sensitivity of subsets of GABAA receptors to general anesthetics depends on the intrinsic sensitivity of receptor’s subunit as well as on their distribution in the related neural circuits [56]. It is also worth noting that high concentrations of general anesthetics can directly activate GABAA receptors without the involvement of GABA [56], indicating that there could be differences between anesthesia and natural sleep in the activation and functional regulation of GABAA receptors.
There are different stages in sleep, similar to anesthesia; sedation and surgical anesthesia are also different levels of unconsciousness. Large differences in EEG patterns are acquired in the NREM and REM sleep stages. Similarly, EEG features differ between light anesthesia for sedative procedures and deep anesthesia for surgical procedures. These findings suggest that diverse neural networks could be involved in the regulation of sleep and anesthesia, depending on the stage of unconsciousness. Studies have clarified that certain neurons regulate NREM sleep rather than REM sleep. Similarly, some neurons only participate in sedation, but not anesthesia. However, only a few studies have compared differences in neural circuits between light and deep anesthesia. Currently, only a few studies have investigated the mechanisms of these non-consciousness-related effects of general anesthetics; these studies have suggested that at least one group of central amygdala neurons is related to analgesia, but not unconsciousness. In contrast, a prior study found that sleep-accompanied immobility is mediated by the substantia nigra pars reticulata (SNr), which also regulates transitions in consciousness. These interesting studies impose more challenges onto the hypothesis that general anesthesia shares neural circuits with sleep. Therefore, it might not be sensible to conceptually conclude that anesthesia and sleep share the same neural circuits.
There are more things in heaven and earth than are dreamt of in your philosophy.
——William Shakespeare
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was funded by a grant from the National Natural Science Foundation of China, Grant Number: No. 82030038, awarded to Dr. Hailong Dong, and No. 82071554, awarded to Dr. Qianzi Yang.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Brown R.E., Basheer R., McKenna J.T., Strecker R.E., McCarley R.W. Control of sleep and wakefulness. Physiol. Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter A., van der Spek L., Hardy E., Bemelmans A.P., Rouach N., Rancillac A. Structural and functional connections between the median and the ventrolateral preoptic nucleus. Brain Struct. Funct. 2019;224(9):3045–3057. doi: 10.1007/s00429-019-01935-4. [DOI] [PubMed] [Google Scholar]
- 3.Moffitt J.R., Bambah-Mukku D., Eichhorn S.W., Vaughn E., Shekhar K., Perez J.D., Rubinstein N.D., Hao J., Regev A., Dulac C., Zhuang X. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science. 2018;362(6416):eaau5324. doi: 10.1126/science.aau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber F., Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538(7623):51–59. doi: 10.1038/nature19773. [DOI] [PubMed] [Google Scholar]
- 5.Sterman M.B., Clemente C.D. Forebrain inhibitory mechanisms: sleep patterns induced by basal forebrain stimulation in the behaving cat. Exp. Neurol. 1962;6:103–117. doi: 10.1016/0014-4886(62)90081-X. [DOI] [PubMed] [Google Scholar]
- 6.Lu J., Greco M.A., Shiromani P., Saper C.B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 2000;20(10):3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai K. Sleep-waking discharge profiles of median preoptic and surrounding neurons in mice. Neuroscience. 2011;182:144–161. doi: 10.1016/j.neuroscience.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Vanini G., Bassana M., Mast M., Mondino A., Cerda I., Phyle M., Chen V., Colmenero A.V., Hambrecht-Wiedbusch V.S., Mashour G.A. Activation of preoptic GABAergic or glutamatergic neurons modulates sleep-wake architecture, but not anesthetic state transitions. Curr. Biol. 2020;30(5):779–787.e4. doi: 10.1016/j.cub.2019.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichert S., Pavón Arocas O., Rihel J. The neuropeptide galanin is required for homeostatic rebound sleep following increased neuronal activity. Neuron. 2019;104(2):370–384.e5. doi: 10.1016/j.neuron.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Kroeger D., Absi G., Gagliardi C., Bandaru S.S., Madara J.C., Ferrari L.L., Arrigoni E., Münzberg H., Scammell T.E., Saper C.B., Vetrivelan R. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat. Commun. 2018;9(1):4129. doi: 10.1038/s41467-018-06590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J., Nelson L.E., Franks N., Maze M., Chamberlin N.L., Saper C.B. Role of endogenous sleep-wake and analgesic systems in anesthesia. J. Comp. Neurol. 2008;508(4):648–662. doi: 10.1002/cne.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan J., Luo Z., Zhang Y., Zhang Y., Wang Y., Cao S., Fu B., Yang H., Zhang L., Zhou W., Yu T. GABAergic ventrolateral pre optic nucleus neurons are involved in the mediation of the anesthetic hypnosis induced by propofol. Mol. Med. Rep. 2017;16(3):3179–3186. doi: 10.3892/mmr.2017.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z., Ferretti V., Güntan İ., Moro A., Steinberg E.A., Ye Z., Zecharia A.Y., Yu X., Vyssotski A.L., Brickley S.G., Yustos R., Pillidge Z.E., Harding E.C., Wisden W., Franks N.P. Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat. Neurosci. 2015;18(4):553–561. doi: 10.1038/nn.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y., Miracca G., Yu X., Harding E.C., Miao A., Yustos R., Vyssotski A.L., Franks N.P., Wisden W. Galanin neurons unite sleep homeostasis and α2-adrenergic sedation. Curr. Biol. 2019;29(19):3315–3322.e3. doi: 10.1016/j.cub.2019.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reitz S.L., Wasilczuk A.Z., Beh G.H., Proekt A., Kelz M.B. Activation of preoptic tachykinin 1 neurons promotes wakefulness over sleep and volatile anesthetic-induced unconsciousness. Curr. Biol. 2021;31(2):394–405.e4. doi: 10.1016/j.cub.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung S., Weber F., Zhong P., Tan C.L., Nguyen T.N., Beier K.T., Hörmann N., Chang W.C., Zhang Z., Do J.P., Yao S., Krashes M.J., Tasic B., Cetin A., Zeng H., Knight Z.A., Luo L., Dan Y. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature. 2017;545(7655):477–481. doi: 10.1038/nature22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eikermann M., Vetrivelan R., Grosse-Sundrup M., Henry M.E., Hoffmann U., Yokota S., Saper C.B., Chamberlin N.L. The ventrolateral preoptic nucleus is not required for isoflurane general anesthesia. Brain Res. 2011;1426:30–37. doi: 10.1016/j.brainres.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C., Thankachan S., McCarley R.W., Brown R.E. The menagerie of the basal forebrain: how many (neural) species are there, what do they look like, how do they behave and who talks to whom? Curr. Opin. Neurobiol. 2017;44:159–166. doi: 10.1016/j.conb.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agostinelli L.J., Geerling J.C., Scammell T.E. Basal forebrain subcortical projections. Brain Struct. Funct. 2019;224(3):1097–1117. doi: 10.1007/s00429-018-01820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng W., Wu Z., Song K., Zhang S., Li Y., Xu M. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science. 2020;369(6508):eabb0556. doi: 10.1126/science.abb0556. [DOI] [PubMed] [Google Scholar]
- 21.Xu M., Chung S., Zhang S., Zhong P., Ma C., Chang W.C., Weissbourd B., Sakai N., Luo L., Nishino S., Dan Y. Basal forebrain circuit for sleep-wake control. Nat. Neurosci. 2015;18(11):1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irmak S.O., de Lecea L. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37(12):1941–1951. doi: 10.5665/sleep.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Y., Shi Y.F., Xi W., Zhou R., Tan Z.B., Wang H., Li X.M., Chen Z., Feng G., Luo M., Huang Z.L., Duan S., Yu Y.Q. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr. Biol. 2014;24(6):693–698. doi: 10.1016/j.cub.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Chen L., Yin D., Wang T.X., Guo W., Dong H., Xu Q., Luo Y.J., Cherasse Y., Lazarus M., Qiu Z.L., Lu J., Qu W.M., Huang Z.L. Basal forebrain cholinergic neurons primarily contribute to inhibition of electroencephalogram delta activity, rather than inducing behavioral wakefulness in mice. Neuropsychopharmacology. 2016;41(8):2133–2146. doi: 10.1038/npp.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anaclet C., Pedersen N.P., Ferrari L.L., Venner A., Bass C.E., Arrigoni E., Fuller P.M. Basal forebrain control of wakefulness and cortical rhythms. Nat. Commun. 2015;6:8744. doi: 10.1038/ncomms9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim T., Thankachan S., McKenna J.T., McNally J.M., Yang C., Choi J.H., Chen L., Kocsis B., Deisseroth K., Strecker R.E., Basheer R., Brown R.E., McCarley R.W. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc. Natl. Acad. Sci. USA. 2015;112(11):3535–3540. doi: 10.1073/pnas.1413625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenna J.T., Thankachan S., Uygun D.S., Shukla C., McNally J.M., Schiffino F.L., Cordeira J., Katsuki F., Zant J.C., Gamble M.C., Deisseroth K., McCarley R.W., Brown R.E., Strecker R.E., Basheer R. Basal forebrain parvalbumin neurons mediate arousals from sleep induced by hypercarbia or auditory stimuli. Curr. Biol. 2020;30(12):2379–2385.e4. doi: 10.1016/j.cub.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anaclet C., De Luca R., Venner A., Malyshevskaya O., Lazarus M., Arrigoni E., Fuller P.M. Genetic activation, inactivation, and deletion reveal a limited and nuanced role for somatostatin-containing basal forebrain neurons in behavioral state control. J. Neurosci. 2018;38(22):5168–5181. doi: 10.1523/JNEUROSCI.2955-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do J.P., Xu M., Lee S.H., Chang W.C., Zhang S., Chung S., Yung T.J., Fan J.L., Miyamichi K., Luo L., Dan Y. Cell type-specific long-range connections of basal forebrain circuit. eLife. 2016;5:13214. doi: 10.7554/eLife.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaborszky L., Duque A. Local synaptic connections of basal forebrain neurons. Behav. Brain Res. 2000;115(2):143–158. doi: 10.1016/S0166-4328(00)00255-2. [DOI] [PubMed] [Google Scholar]
- 31.Lelkes Z., Abdurakhmanova S., Porkka-Heiskanen T. Cholinergic basal forebrain structures are not essential for mediation of the arousing action of glutamate. J. Sleep Res. 2018;27(4):e12605. doi: 10.1111/jsr.12605. [DOI] [PubMed] [Google Scholar]
- 32.Zant J.C., Kim T., Prokai L., Szarka S., McNally J., McKenna J.T., Shukla C., Yang C., Kalinchuk A.V., McCarley R.W., Brown R.E., Basheer R. Cholinergic neurons in the basal forebrain promote wakefulness by actions on neighboring non-cholinergic neurons: an opto-dialysis study. J. Neurosci. 2016;36(6):2057–2067. doi: 10.1523/JNEUROSCI.3318-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang C., McKenna J.T., Zant J.C., Winston S., Basheer R., Brown R.E. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J. Neurosci. 2014;34(8):2832–2844. doi: 10.1523/JNEUROSCI.3235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto L., Goard M.J., Estandian D., Xu M., Kwan A.C., Lee S.H., Harrison T.C., Feng G., Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 2013;16(12):1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eggermann E., Kremer Y., Crochet S., Petersen C.C.H. Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep. 2014;9(5):1654–1660. doi: 10.1016/j.celrep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Ni K.M., Hou X.J., Yang C.H., Dong P., Li Y., Zhang Y., Jiang P., Berg D.K., Duan S., Li X.M. Selectively driving cholinergic fibers optically in the thalamic reticular nucleus promotes sleep. eLife. 2016;5:5. doi: 10.7554/eLife.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freund T.F., Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc. Natl. Acad. Sci. USA. 1992;89(2):738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henny P., Jones B.E. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur. J. Neurosci. 2008;27(3):654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenna J.T., Yang C., Franciosi S., Winston S., Abarr K.K., Rigby M.S., Yanagawa Y., McCarley R.W., Brown R.E. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J. Comp. Neurol. 2013;521(6):1225–1250. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borbély A.A., Daan S., Wirz-Justice A., Deboer T. The two-process model of sleep regulation: a reappraisal. J. Sleep Res. 2016;25(2):131–143. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- 41.Porkka-Heiskanen T., Strecker R.E., McCarley R.W. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99(3):507–517. doi: 10.1016/S0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 42.Dong H.L., Fukuda S., Murata E., Higuchi T. Excitatory and inhibitory actions of isoflurane on the cholinergic ascending arousal system of the rat. Anesthesiology. 2006;104(1):122–133. doi: 10.1097/00000542-200601000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Détári L., Vanderwolf C.H. Activity of identified cortically projecting and other basal forebrain neurones during large slow waves and cortical activation in anaesthetized rats. Brain Res. 1987;437(1):1–8. doi: 10.1016/0006-8993(87)91521-6. [DOI] [PubMed] [Google Scholar]
- 44.Toth A., Hajnik T., Detari L. Cholinergic modulation of slow cortical rhythm in urethane-anesthetized rats. Brain Res. Bull. 2012;87(1):117–129. doi: 10.1016/j.brainresbull.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Liu C., Shi F., Fu B., Luo T., Zhang L., Zhang Y., Zhang Y., Yu S., Yu T. GABAA receptors in the basal forebrain mediates emergence from propofol anaesthesia in rats. Int. J. Neurosci. 2020:1–13. doi: 10.1080/00207454.2020.1840375. [DOI] [PubMed] [Google Scholar]
- 46.Luo T.Y., Cai S., Qin Z.X., Yang S.C., Shu Y., Liu C.X., Zhang Y., Zhang L., Zhou L., Yu T., Yu S.Y. Basal forebrain cholinergic activity modulates isoflurane and propofol anesthesia. Front. Neurosci. 2020;14:559077. doi: 10.3389/fnins.2020.559077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laalou F.Z., de Vasconcelos A.P., Oberling P., Jeltsch H., Cassel J.C., Pain L. Involvement of the basal cholinergic forebrain in the mediation of general (propofol) anesthesia. Anesthesiology. 2008;108(5):888–896. doi: 10.1097/ALN.0b013e31816d919b. [DOI] [PubMed] [Google Scholar]
- 48.Leung L.S., Ma J., Shen B., Nachim I., Luo T. Medial septal lesion enhances general anesthesia response. Exp. Neurol. 2013;247:419–428. doi: 10.1016/j.expneurol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Dong H.L., Fukuda S., Murata E., Zhu Z., Higuchi T. Orexins increase cortical acetylcholine release and electroencephalographic activation through orexin-1 receptor in the rat basal forebrain during isoflurane anesthesia. Anesthesiology. 2006;104(5):1023–1032. doi: 10.1097/00000542-200605000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Dong H., Niu J., Su B., Zhu Z., Lv Y., Li Y., Xiong L. Activation of orexin signal in basal forebrain facilitates the emergence from sevoflurane anesthesia in rat. Neuropeptides. 2009;43(3):179–185. doi: 10.1016/j.npep.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Luo T., Leung L.S. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology. 2009;111(4):725–733. doi: 10.1097/ALN.0b013e3181b061a0. [DOI] [PubMed] [Google Scholar]
- 52.Pillay S., Vizuete J.A., McCallum J.B., Hudetz A.G. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology. 2011;115(4):733–742. doi: 10.1097/ALN.0b013e31822c5ee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L.N., Li Z.J., Tong L., Guo C., Niu J.Y., Hou W.G., Dong H.L. Orexin-A facilitates emergence from propofol anesthesia in the rat. Anesth. Analg. 2012;115(4):789–796. doi: 10.1213/ANE.0b013e3182645ea3. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L.N., Yang C., Ouyang P.R., Zhang Z.C., Ran M.Z., Tong L., Dong H.L., Liu Y. Orexin-A facilitates emergence of the rat from isoflurane anesthesia via mediation of the basal forebrain. Neuropeptides. 2016;58:7–14. doi: 10.1016/j.npep.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Wang D., Guo Y., Li H., Li J., Ran M., Guo J., Yin L., Zhao S., Yang Q., Dong H. Selective optogenetic activation of orexinergic terminals in the basal forebrain and locus coeruleus promotes emergence from isoflurane anaesthesia in rats. Br. J. Anaesth. 2021;126(1):279–292. doi: 10.1016/j.bja.2020.09.037. [DOI] [PubMed] [Google Scholar]
- 56.Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008;9(5):370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 57.Kim J.J., Gharpure A., Teng J., Zhuang Y., Howard R.J., Zhu S., Noviello C.M., Walsh R.M., Jr, Lindahl E., Hibbs R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature. 2020;585(7824):303–308. doi: 10.1038/s41586-020-2654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L., Yang Z.L., Cheng J., Zhang P.P., Zhang L.S., Liu X.S., Wang L.C. Propofol decreases the excitability of cholinergic neurons in mouse basal forebrain via GABAA receptors. Acta Pharmacol. Sin. 2019;40(6):755–761. doi: 10.1038/s41401-018-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Chen L., Zhu D., Chen Y., Qin W., Cui J. Propofol downregulates the activity of glutamatergic neurons in the basal forebrain via affecting intrinsic membrane properties and postsynaptic GABAARs. Neuroreport. 2020;31(17):1242–1248. doi: 10.1097/WNR.0000000000001540. [DOI] [PubMed] [Google Scholar]
- 60.Ma J., Shen B., Stewart L.S., Herrick I.A., Leung L.S. The septohippocampal system participates in general anesthesia. J. Neurosci. 2002;22(2):RC200. doi: 10.1523/JNEUROSCI.22-02-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonnavion P., Mickelsen L.E., Fujita A., de Lecea L., Jackson A.C. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. 2016;594(22):6443–6462. doi: 10.1113/JP271946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mickelsen L.E., Bolisetty M., Chimileski B.R., Fujita A., Beltrami E.J., Costanzo J.T., Naparstek J.R., Robson P., Jackson A.C. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 2019;22(4):642–656. doi: 10.1038/s41593-019-0349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ammoun S., Holmqvist T., Shariatmadari R., Oonk H.B., Detheux M., Parmentier M., Akerman K.E., Kukkonen J.P. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Ther. 2003;305(2):507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- 64.Ono D., Yamanaka A. Hypothalamic regulation of the sleep/wake cycle. Neurosci. Res. 2017;118:74–81. doi: 10.1016/j.neures.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 65.Sasaki K., Suzuki M., Mieda M., Tsujino N., Roth B., Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6(5):e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adamantidis A.R., Zhang F., Aravanis A.M., Deisseroth K., de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang C., Zhang L., Hao H., Ran M., Li J., Dong H. Serotonergic neurons in the dorsal raphe nucleus mediate the arousal-promoting effect of orexin during isoflurane anesthesia in male rats. Neuropeptides. 2019;75:25–33. doi: 10.1016/j.npep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Li J., Li H., Wang D., Guo Y., Zhang X., Ran M., Yang C., Yang Q., Dong H. Orexin activated emergence from isoflurane anaesthesia involves excitation of ventral tegmental area dopaminergic neurones in rats. Br. J. Anaesth. 2019;123(4):497–505. doi: 10.1016/j.bja.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Kelz M.B., Sun Y., Chen J., Cheng Meng Q., Moore J.T., Veasey S.C., Dixon S., Thornton M., Funato H., Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc. Natl. Acad. Sci. USA. 2008;105(4):1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou W., Cheung K., Kyu S., Wang L., Guan Z., Kurien P.A., Bickler P.E., Jan L.Y. Activation of orexin system facilitates anesthesia emergence and pain control. Proc. Natl. Acad. Sci. USA. 2018;115(45):E10740–E10747. doi: 10.1073/pnas.1808622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao S., Wang S., Li H.M., Guo J., Li J.N., Wang D., Zhang X.X., Yin L., Li R., Li A., Li H.H., Fan Z., Yang Q.Z., Zhong H.X., Dong H.L. Activation of orexinergic neurons inhibits the anesthetic effect of desflurane on consciousness state via paraventricular thalamic nucleus in rats. Anesth. Analg. 2021;133(3):781–793. doi: 10.1213/ANE.0000000000005651. [DOI] [PubMed] [Google Scholar]
- 72.Ren S., Wang Y., Yue F., Cheng X., Dang R., Qiao Q., Sun X., Li X., Jiang Q., Yao J., Qin H., Wang G., Liao X., Gao D., Xia J., Zhang J., Hu B., Yan J., Wang Y., Xu M., Han Y., Tang X., Chen X., He C., Hu Z. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362(6413):429–434. doi: 10.1126/science.aat2512. [DOI] [PubMed] [Google Scholar]
- 73.Herrera C.G., Cadavieco M.C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 2016;19(2):290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Venner A., Anaclet C., Broadhurst R.Y., Saper C.B., Fuller P.M. A novel population of wake-promoting GABAergic neurons in the ventral lateral hypothalamus. Curr. Biol. 2016;26(16):2137–2143. doi: 10.1016/j.cub.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morales M., Margolis E.B. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017;18(2):73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 76.Yu X., Li W., Ma Y., Tossell K., Harris J.J., Harding E.C., Ba W., Miracca G., Wang D., Li L., Guo J., Chen M., Li Y., Yustos R., Vyssotski A.L., Burdakov D., Yang Q., Dong H., Franks N.P., Wisden W. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat. Neurosci. 2019;22(1):106–119. doi: 10.1038/s41593-018-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monti J.M., Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med. Rev. 2007;11(2):113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Taylor S.R., Badurek S., Dileone R.J., Nashmi R., Minichiello L., Picciotto M.R. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J. Comp. Neurol. 2014;522(14):3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eban-Rothschild A., Rothschild G., Giardino W.J., Jones J.R., de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016;19(10):1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fifel K., Meijer J.H., Deboer T. Circadian and homeostatic modulation of multi-unit activity in midbrain dopaminergic structures. Sci. Rep. 2018;8(1):7765. doi: 10.1038/s41598-018-25770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dahan L., Astier B., Vautrelle N., Urbain N., Kocsis B., Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32(6):1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z., Liu W.Y., Diao Y.P., Xu W., Zhong Y.H., Zhang J.Y., Lazarus M., Liu Y.Y., Qu W.M., Huang Z.L. Superior colliculus GABAergic neurons are essential for acute dark induction of wakefulness in mice. Curr. Biol. 2019;29(4):637–644.e3. doi: 10.1016/j.cub.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 83.Yu X., Ba W., Zhao G., Ma Y., Harding E.C., Yin L., Wang D., Li H., Zhang P., Shi Y., Yustos R., Vyssotski A.L., Dong H., Franks N.P., Wisden W. Dysfunction of ventral tegmental area GABA neurons causes mania-like behavior. Mol. Psychiatry. 2021;26(9):5213–5228. doi: 10.1038/s41380-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Solt K., Van Dort C.J., Chemali J.J., Taylor N.E., Kenny J.D., Brown E.N. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121(2):311–319. doi: 10.1097/ALN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor N.E., Van Dort C.J., Kenny J.D., Pei J., Guidera J.A., Vlasov K.Y., Lee J.T., Boyden E.S., Brown E.N., Solt K. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc. Natl. Acad. Sci. USA. 2016;113(45):12826–12831. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y.D., Luo Y.J., Xu W., Ge J., Cherasse Y., Wang Y.Q., Lazarus M., Qu W.M., Huang Z.L. Ventral pallidal GABAergic neurons control wakefulness associated with motivation through the ventral tegmental pathway. Mol. Psychiatry. 2021;26(7):2912–2928. doi: 10.1038/s41380-020-00906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anafi R.C., Kayser M.S., Raizen D.M. Exploring phylogeny to find the function of sleep. Nat. Rev. Neurosci. 2019;20(2):109–116. doi: 10.1038/s41583-018-0098-9. [DOI] [PubMed] [Google Scholar]
- 88.Poe G.R., Foote S., Eschenko O., Johansen J.P., Bouret S., Aston-Jones G., Harley C.W., Manahan-Vaughan D., Weinshenker D., Valentino R., Berridge C., Chandler D.J., Waterhouse B., Sara S.J. Locus coeruleus: a new look at the blue spot. Nat. Rev. Neurosci. 2020;21(11):644–659. doi: 10.1038/s41583-020-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sara S.J., Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76(1):130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 90.Chu N., Bloom F.E. Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science. 1973;179(4076):908–910. doi: 10.1126/science.179.4076.908. [DOI] [PubMed] [Google Scholar]
- 91.Aston-Jones G., Bloom F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rajkowski J., Kubiak P., Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res. Bull. 1994;35(5-6):607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 93.Foote S.L., Aston-Jones G., Bloom F.E. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. USA. 1980;77(5):3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayat H., Regev N., Matosevich N., Sales A., Paredes-Rodriguez E., Krom A.J., Bergman L., Li Y., Lavigne M., Kremer E.J., Yizhar O., Pickering A.E., Nir Y. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 2020;6(15):eaaz4232. doi: 10.1126/sciadv.aaz4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berridge C.W., Schmeichel B.E., España R.A. Noradrenergic modulation of wakefulness/arousal. Sleep Med. Rev. 2012;16(2):187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carter M.E., Yizhar O., Chikahisa S., Nguyen H., Adamantidis A., Nishino S., Deisseroth K., de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010;13(12):1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamaguchi H., Hopf F.W., Li S.B., de Lecea L. In vivo cell type-specific CRISPR knockdown of dopamine beta hydroxylase reduces locus coeruleus evoked wakefulness. Nat. Commun. 2018;9(1):5211. doi: 10.1038/s41467-018-07566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du W.J., Zhang R.W., Li J., Zhang B.B., Peng X.L., Cao S., Yuan J., Yuan C.D., Yu T., Du J.L. The locus coeruleus modulates intravenous general anesthesia of zebrafish via a cooperative mechanism. Cell Rep. 2018;24(12):3146–3155.e3. doi: 10.1016/j.celrep.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y., Yu T., Yuan J., Yu B.W. The ventrolateral preoptic nucleus is required for propofol-induced inhibition of locus coeruleus neuronal activity. Neurol. Sci. 2015;36(12):2177–2184. doi: 10.1007/s10072-015-2292-0. [DOI] [PubMed] [Google Scholar]
- 100.Kushikata T., Yoshida H., Kudo M., Kudo T., Kudo T., Hirota K. Role of coerulean noradrenergic neurones in general anaesthesia in rats. Br. J. Anaesth. 2011;107(6):924–929. doi: 10.1093/bja/aer303. [DOI] [PubMed] [Google Scholar]
- 101.Hu F.Y., Hanna G.M., Han W., Mardini F., Thomas S.A., Wyner A.J., Kelz M.B. Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine β-hydroxylase knockout mice. Anesthesiology. 2012;117(5):1006–1017. doi: 10.1097/ALN.0b013e3182700ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vazey E.M., Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. USA. 2014;111(10):3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ao Y., Yang B., Zhang C., Wu B., Zhang X., Xing D., Xu H. Locus coeruleus to paraventricular thalamus projections facilitate emergence from isoflurane anesthesia in mice. Front. Pharmacol. 2021;12:643172. doi: 10.3389/fphar.2021.643172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fu B., Yu T., Yuan J., Gong X., Zhang M. Noradrenergic transmission in the central medial thalamic nucleus modulates the electroencephalographic activity and emergence from propofol anesthesia in rats. J. Neurochem. 2017;140(6):862–873. doi: 10.1111/jnc.13939. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Fu B., Liu C., Yu S., Luo T., Zhang L., Zhou W., Yu T. Activation of noradrenergic terminals in the reticular thalamus delays arousal from propofol anesthesia in mice. FASEB J. 2019;33(6):7252–7260. doi: 10.1096/fj.201802164RR. [DOI] [PubMed] [Google Scholar]
- 106.Qiu G., Wu Y., Yang Z., Li L., Zhu X., Wang Y., Sun W., Dong H., Li Y., Hu J. Dexmedetomidine activation of dopamine neurons in the ventral tegmental area attenuates the depth of sedation in mice. Anesthesiology. 2020;133(2):377–392. doi: 10.1097/ALN.0000000000003347. [DOI] [PubMed] [Google Scholar]
- 107.Jacobs B.L., Fornal C.A. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2) Suppl.:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 108.Muindi F., Kenny J.D., Taylor N.E., Solt K., Wilson M.A., Brown E.N., Van Dort C.J. Electrical stimulation of the parabrachial nucleus induces reanimation from isoflurane general anesthesia. Behav. Brain Res. 2016;306:20–25. doi: 10.1016/j.bbr.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 109.Luo T., Yu S., Cai S., Zhang Y., Jiao Y., Yu T., Yu W. Parabrachial neurons promote behavior and electroencephalographic arousal from general anesthesia. Front. Mol. Neurosci. 2018;11:420. doi: 10.3389/fnmol.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oikonomou G., Altermatt M., Zhang R.W., Coughlin G.M., Montz C., Gradinaru V., Prober D.A. The serotonergic raphe promote sleep in zebrafish and mice. Neuron. 2019;103(4):686–701.e8. doi: 10.1016/j.neuron.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li A., Li R., Ouyang P., Li H., Wang S., Zhang X., Wang D., Ran M., Zhao G., Yang Q., Zhu Z., Dong H., Zhang H. Dorsal raphe serotonergic neurons promote arousal from isoflurane anesthesia. CNS Neurosci. Ther. 2021;27(8):941–950. doi: 10.1111/cns.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haas H., Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 2003;4(2):121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi K., Lin J.S., Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J. Neurosci. 2006;26(40):10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nelson L.E., Lu J., Guo T., Saper C.B., Franks N.P., Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 115.Luo T., Leung L.S. Involvement of tuberomamillary histaminergic neurons in isoflurane anesthesia. Anesthesiology. 2011;115(1):36–43. doi: 10.1097/ALN.0b013e3182207655. [DOI] [PubMed] [Google Scholar]
- 116.Xia C., Zhao Z., Yu L., Yan M. The role of the central histaminergic system in emergence from propofol anesthesia in rats model. Ann. Palliat. Med. 2021;10(6):6067–6078. doi: 10.21037/apm-20-2594. [DOI] [PubMed] [Google Scholar]
- 117.Nelson L.E., Guo T.Z., Lu J., Saper C.B., Franks N.P., Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat. Neurosci. 2002;5(10):979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 118.Cazzin C., Piccoli L., Massagrande M., Garbati N., Michielin F., Knaus H.G., Ring C.J., Morrison A.D., Merlo-Pich E., Rovo Z., Astori S., Lüthi A., Corti C., Corsi M. rKv1.2 overexpression in the central medial thalamic area decreases caffeine-induced arousal. Genes Brain Behav. 2011;10(8):817–827. doi: 10.1111/j.1601-183X.2011.00719.x. [DOI] [PubMed] [Google Scholar]
- 119.Timic Stamenic T., Feseha S., Valdez R., Zhao W., Klawitter J., Todorovic S.M. Alterations in oscillatory behavior of central medial thalamic neurons demonstrate a key role of CaV3.1 isoform of T-channels during isoflurane-induced anesthesia. Cereb. Cortex. 2019;29(11):4679–4696. doi: 10.1093/cercor/bhz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baker R., Gent T.C., Yang Q., Parker S., Vyssotski A.L., Wisden W., Brickley S.G., Franks N.P. Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J. Neurosci. 2014;34(40):13326–13335. doi: 10.1523/JNEUROSCI.1519-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferrarelli F., Massimini M., Sarasso S., Casali A., Riedner B.A., Angelini G., Tononi G., Pearce R.A. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc. Natl. Acad. Sci. USA. 2010;107(6):2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee U., Ku S., Noh G., Baek S., Choi B., Mashour G.A. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118(6):1264–1275. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cirelli C., Pompeiano M., Tononi G. Sleep deprivation and c-fos expression in the rat brain. J. Sleep Res. 1995;4(2):92–106. doi: 10.1111/j.1365-2869.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 124.Pompeiano M., Cirelli C., Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J. Sleep Res. 1994;3(2):80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 125.Gerashchenko D., Wisor J.P., Burns D., Reh R.K., Shiromani P.J., Sakurai T., de la Iglesia H.O., Kilduff T.S. Identification of a population of sleep-active cerebral cortex neurons. Proc. Natl. Acad. Sci. USA. 2008;105(29):10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andrada J., Livingston P., Lee B.J., Antognini J. Propofol and etomidate depress cortical, thalamic, and reticular formation neurons during anesthetic-induced unconsciousness. Anesth. Analg. 2012;114(3):661–669. doi: 10.1213/ANE.0b013e3182405228. [DOI] [PubMed] [Google Scholar]
- 127.Guo J., Ran M., Gao Z., Zhang X., Wang D., Li H., Zhao S., Sun W., Dong H., Hu J. Cell-type-specific imaging of neurotransmission reveals a disrupted excitatory-inhibitory cortical network in isoflurane anaesthesia. EBioMedicine. 2021;65:103272. doi: 10.1016/j.ebiom.2021.103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mody I., Pearce R.A. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27(9):569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 129.Brohan J., Goudra B.G. The role of GABA receptor agonists in anesthesia and sedation. CNS Drugs. 2017;31(10):845–856. doi: 10.1007/s40263-017-0463-7. [DOI] [PubMed] [Google Scholar]