Abstract
Oxytocin (OXT) is a nine amino acid neuropeptide hormone that has become one of the most intensively studied molecules in the past few decades. The vast majority of OXT is synthesized in the periventricular nucleus and supraoptic nucleus of the hypothalamus, and a few are synthesized in some peripheral organs (such as the uterus, ovaries, adrenal glands, thymus, pancreas, etc.) OXT modulates a series of physiological processes, including lactation, parturition, as well as some social behaviors. In addition, more and more attention has recently been focused on the analgesic effects of oxytocin. It has been reported that OXT can relieve tension and pain without other adverse effects. However, the critical role and detailed mechanism of OXT in analgesia remain unclear. This review aims to summarize the mechanism of OXT in analgesia and some ideas about the mechanism.
Keywords: Oxytocin, receptor, analgesia, nervous system, spinal cord, peripheral nervous system
1. INTRODUCTION
Pain, as defined by the International Association for the Study of Pain (IASP), defines pain as an unpleasant subjective and emotional experience associated with tissue damage or potential tissue damage. Suffering causes tremendous pressure to human society. However, the current clinical anti-injury treatment effect is not ideal. Therefore, finding new therapeutic strategies for pain becomes a hot and important topic in this area.
Oxytocin (OXT) is a uterine contraction drug extracted or chemically synthesized from the posterior pituitary gland of animals. In 1906, Dell discovered a kind of neurohypophysis extract, which has a significant contraction effect on the uterus of a pregnant cat [1]. A few years later, Ott and Scott reported another neurohypophysis extract that could promote lactation [2]. Subsequently, related studies have shown that the physiological effects of these two neurohypophysis extracts belong to the same substance, namely OXT [3]. OXT is a peptide composed of nine amino acids (Gly-Leu-Pro-Cys-Asn-Gln-Ile-Tyr-Cys) with a molecular weight of 1.0kd. Because of its role in breastfeeding and childbirth, OXT was originally considered a hormone. However, subsequent studies have shown that OXT is also a neurotransmitter and regulates the nervous system [4]. In addition, researchers found that OXT has a high degree of sequence homology with another neuropeptide substance, arginine vasopressin (AVP) (only two amino acids are different), so OXT can interact with the arginine vasopressin receptor (AVPR) combined with neuromodulation [5].
Recent studies have shown that OXT has great potential in regulating pain and is an ideal analgesic target molecule without obvious side effects and addiction. This article reviews the clinical and basic research progress of OXT treatment of pain, which is helps understand the mechanism of oxytocin in regulating pain.
2. BASIC PROPERTIES AND PHYSIOLOGICAL FUNCTIONS OF OXT
OXT is mainly synthesized and secreted by neurons in the paraventricular nucleus (PVN) and supraoptic nucleus (SON), and widely distributed in the central nervous system (such as midbrain aqueduct gray matter, dorsal raphe nucleus, spinal cord, etc.) and surrounding tissues (such as uterus, placenta, breast, etc.). It is mainly involved in the regulation of fertility-related functions such as reproduction and breastfeeding, as well as the regulation of cognition, social behavior, and addiction. The first is fluid regulation, which is transported by OXT from the nerve fibers of the hypothalamic-pituitary axis to the posterior pituitary, where it is released into the bloodstream and delivered to target organs [6]. Another is neuromodulation, OXT secreted by nerve fibers from the different parts of the nervous system, paraventricular nucleus of the nerve endings OXT directly projected to other areas of the central nervous system.
In the central nervous system of mammals, there are two forms of OXT-releasing cells: Magnocellular OXT (Magn-OXT) neurons and Parvocellular OXT (Parv-OXT) neurons. The diameter of Magn-OXT neurons is 20-30 μm, and they are distributed in the paraventricular nucleus, supraoptic nucleus and middle accessory nucleus of the inferior colliculus. Magn-OXT neurons release OXT into the blood through the neurohypophysis gland [7]. Then OXT acts on target organs, such as the uterus and breast, through the systemic blood circulation, thereby promoting women's delivery and lactation. Moreover, Magn-OXT neurons have extensive projections in forebrain regions, such as the nucleus accumbens [8] and the central amygdala [9]. The OXT released from the ends of their axons can precisely regulate the activity of the relevant areas. For example, Magn-OXT neurons in the central amygdala can attenuate emotional responses such as fear [9]. In addition, Magn-OXT neurons may also exert analgesic effects by targeting C-type fibers in the dorsal root ganglion (DRG) [10]. Different from Magn-OXT neurons, Parv-OXT neurons are smaller in diameter, approximately 10-20 μm, distributed in the paraventricular nucleus of the PVN. Parv-OXT neurons project to the brainstem and spinal cord [11]. Based on the projection of Parv-OXT neurons in the brain stem and spinal cord, it has been demonstrated that OXT produced by axon terminals of Parv-OXT neurons could affect cardiovascular functions, respiratory movement, and food intake [12]. Recently, it has been found that a group of new Parv-OXT neurons can regulate the activity of Magn-OXT neurons, either by rapid direct neuronal projections that is releasing OXT from axons into spinal cord neurons and inhibiting their activity, or by slow indirect modulation of Magn-OT neurons to release OXT into the peripheral nervous system, acting as analgesics in an inflammatory pain model [13]. There are approximately 30 kinds of Parv-OXT neurons that can regulate Magn-OXT neurons and spinal cord deep neurons, respectively, through collateral projections. It has been reported that triggering the release of OXT from this group of OXT neurons can effectively inhibit the sense of injury. Personnel found that Parv-OXT primarily regulated blood pressure, heart rhythm, gut response, and pain sensation [14].
3. STRUCTURE AND FUNCTION OF OXT RECEPTOR
The OXT system consists of two functional units: OXT and OXT receptor (OXTR). OXT only works when combined with specific OXTR in the brain, so the effect of OXT depends on the quantity and quality of OXTR [15]. OXTR belongs to the family of G Protein-Coupled Receptors (GPCRs) of rhodopsin (A) and is coupled to phospholipase Cβ (PLCβ) via Gq/11 protein. The OXTR gene is located on chromosome 3p25 and contains three introns and four exons, which play an essential role in the structural coding and activity of the OXTR [16]. Moreover, the OXTR gene has dozens of single nucleotide polymorphisms (SNP); these SNPs directly affect the expression of the OXTR and then affect OXT [17]. Generally, OXT receptors are widely distributed in the central nervous system, such as the cortex, basal ganglia, brain stem, and spinal cord [18]. Specifically, OXT receptors are highly expressed in key brain areas that control mental, emotional, and social behaviors, such as the autonomic nervous system, frontal cortex, olfactory system, basal ganglia, limbic system, thalamus, hypothalamus, brainstem, and spinal cord [19]. Besides, OXT receptors are also present in surrounding tissues such as the kidney, uterus, heart, and thymus. In addition, OXT has different functions in different organizations [20]. Specifically, when OXT binds to OXTR, the phospholipase Cβ-phosphatidylinositol diphosphate inositol triphosphate (PLCβ-PIP2-IP3) pathway is activated by the Gq/11 protein, resulting in the release of Ca2+ from the endoplasmic reticulum to form Ca2+Calmodulin complex, further activates nitric oxide synthase (NOS) and triggering the nitric oxide-guanylate cyclase-cyclic guanosine monophosphate (NO-GC-cGMP) pathway [21]. Furthermore, OXT receptors may also increase prostaglandin E2 (PGE2) synthesis through Gq/11 protein and promote F-actin polymerization to increase smooth muscle excitability [22].
Since OXT and AVP have similar structures, they share some receptors. Therefore, OXT can also act on arginine vasopressin receptor 1a (AVPR1a). However, the distribution of the OXT receptor and AVP receptor in the body is different [23]. Even if the two receptors exist in the same nucleus at the same time, there are differences in most of their distribution between subnuclei [24]. For example, in the rat hippocampus, AVPR is mainly distributed in the dentate gyrus, while OXT receptors are only expressed in the CA1 area.
Above all, OXTR is an important decisive factor in OXT signal transduction, and the expression of OXTR is affected by many factors such as genetic variation and physiological hormone fluctuation [25].
4. THE ANALGESIC EFFECT OF OXT
The definition of pain in modern medicine refers to a rather unpleasant emotional feeling, a complex physical and psychological activity, and one of the most common clinical symptoms. It contains pain caused by noxious stimuli that are sensitive to the body, and the pain response of the human body can be used as a warning to the body, leading to a series of defensive and protective reactions. Various injury stimuli are transmitted into the spinal cord through sensory nerve fibers and finally reach the sensory area of the cerebral cortex, causing pain, affecting the patient's quality of life, and even causing unbearable torture to the body. Inflammatory pain is mainly caused by inflammatory factors (also known as pain-causing factors) released after tissue injury and is the main pathological basis of pain. Pain-causing factors include pro-inflammatory factors, prostaglandins (PG), bradykinin (BRK), etc, which are mainly released by macrophages, mast cells, and lymphocytes. PGE2 is one of the most important pain-causing factors among these transmitters [26].
Chronic pain is one of the most common health problems. Exploring different analgesic strategies and related mechanisms has always been a hot topic. OXT is a self-secreted neurotransmitter. In addition to its well-known functions, such as strengthening uterine contractions during childbirth and promoting lactation, the role of OXT in analgesia has also received increasing attention [27]. OXT can upregulate the activity of 5'-adenosine monophosphate (AMP) activated protein kinase (AMPK) mediated by G protein [28]. After combining with OXTR, OXT can activate protein kinase C (PKC), phospholipase C (PLC), and other systems and trigger a series of biological events by increasing the concentration of endogenous Ca2+ [29].
Meanwhile, OXT can also increase glutamic pyruvic transaminase (GPT) and PLC [30]. In recent years, the analgesic effect of OXT at the central system level, especially the analgesic effect at and above the spinal cord level, has been widely confirmed [31]. However, many studies have shown that the analgesic mechanism of OXT at the spinal cord level is different from that at the spinal cord level. At the spinal cord level, the analgesic effect of OXT is related to endogenous opioid peptides, while above the spinal cord, the analgesic effect of OXT is not related to endogenous opioid peptides. The underlying mechanisms of different levels of OXT analgesia are discussed below.
4.1. The Mechanism of Analgesic Effect of OXT at Spinal Cord Level
As early as 30 years ago, researchers discovered that OXT-containing nerve fibers project from the paraventricular nucleus of the hypothalamus to the superficial layer of the spinal dorsal horn [32]. The dorsal horn of the spinal cord is an important part of signal transmission and regulation of peripheral injury. PVN-OXT neurons project axons to the spinal dorsal horn and establish axon-dendritic synaptic connections with neurons in layers I and II of the spinal dorsal horn. This neural pathway has been proved to be involved in the regulation of the transmission of pain information in traumatic stimulation [33]. Besides, another study found that OXT receptors were highly expressed in pain-related areas of the spinal dorsal horn [34]. The results of immunohistochemistry showed that there are a large number of OXT binding sites in the superficial layer of the spinal dorsal horn. On the contrary, the superficial layer of the spinal dorsal horn plays a key regulatory role in the transmission of pain information from the periphery to the center [35], which was most likely to be the target of the projection of PVN neurons in the spinal cord. It was suggested that OXT might be involved in the regulation of pain at the spinal cord level. However, the projection of OXT fibers is limited to the gelatinous substance, marginal division, and the mediolateral column of the central gray matter of the spinal cord [36]. However, studies have shown that the projection position of the hypothalamic spinal cord OXT neurons matches the distribution of the OXT binding sites in the spinal cord [37], which also indicates that OXT produced by the hypothalamus can be transported to the spinal cord level through the hypothalamo-spinal projection to play an analgesic role.
At the same time, OXT may play an indirect analgesic effect by regulating the intermediate neurons. It has been observed that continuous epidural anesthesia with OXT exhibits a better impact on relieving incision pain after an operation. In the inflammatory pain model, the intrathecal injection of OXT upregulated the pain threshold in a dose-dependent and mechanical manner. Intrathecal injection of ferulic acid can weaken the anti-noxious effect of μ-opioid receptor antagonist and its application κ-opioid receptor antagonist. The analgesic effect was achieved [38]. Condes-Lara observed significant analgesic effects by injecting OXT into the dural sac of carrageenan-induced inflammatory pain model rats, which lasted for more than 5 hours [39].
Studies have shown that intrathecal injection of OXT can also effectively relieve patients' low back pain, and this effect can last for several hours [40]. A clinical trial reported that intravenous injection of high-concentration OXT (300 μg) in patients with refractory cancer pain who is not sensitive to opioid analgesics has a significant analgesic effect, and lasts for 70 minutes [41]. All these pieces of evidence indicate that the endogenous opioid peptide system is involved in the pain regulation of OXT, and the μ opioid receptor (MOR) and κ opioid receptor (KOR) in the spinal cord are involved in the analgesic effect of OXT. Also, OXT had an analgesic effect on the inflammatory pain model in rats [42]. Administration of exogenous OXT can alleviate the pain-induced by inflammation at the level of the spinal cord [36, 43].
On the other hand, many studies have suggested that the analgesic effect of OXT is mediated by the γ-aminobutyric acid (GABA) receptor [44]. The analgesic effect induced by the stimulus on PVN or local administration of OXT in the superficial dorsal horn of the spinal cord can be reversed by bicuculline (GABAA receptor antagonist). Therefore, it is speculated that OXT may activate GABA intermediate neurons in the superficial dorsal horn of the spinal cord, and the released GABA targets the GABAA receptors on the pain transmission neurons in the spinal cord, making them hyperpolarized, thus reducing the transmission of pain information to the center. Breton explored the mechanism of analgesia mediated by OXT at the spinal cord level by using patch-clamp and immunohistochemical techniques [45]. The results showed that OXT stimulated PVN to release activated intermediate glutamatergic neurons in the superficial layer of the spinal dorsal horn, leading to glutamate release and activation of inhibitory GABA interneurons, and finally inhibiting the expression of pain-related neurons. C fiber is the incoming pain, which plays the role of pain modulation. However, this inhibition can be reversed by selective OXT antagonists [46].
In short, the analgesic mechanism of oxytocin at the spinal cord level may be through the direct projection of oxytocin neurons to wide dynamic range (WDR) neurons, or direct activation of inhibitory GABA interneurons or activation of glutamatergic neurons to promote glutamine Acid release, activate downstream inhibitory GABA interneurons and regulate the endogenous opioid peptide system.
4.2. The Mechanism of Analgesic Effect of OXT at the Level Above the Spinal Cord
At the level above the spinal cord, both the increase of endogenous OXT and the administration of exogenous OXT can show significant analgesic effects. In the brain, OXT is mainly produced and secreted by SON and PVN. Studies have found that direct stimulation of supraventricular or paraventricular nuclear energy can increase endogenous OXT and play an analgesic effect. After stimulating SON and PVN, the animal's pain threshold was significantly increased, and after destroying SON and PVN, the animal's pain threshold was significantly reduced [37].
Recently, it has also been reported that stimulus on PVN can increase the release of OXT at the spinal level [13]. Similarly, the electrical stimulus on PVN can increase the concentration of OXT in the cerebrospinal fluid (CSF) and exhibit an analgesic effect [37]. These results suggest that OXT may be involved in the regulation of pain in the brain. In the cerebral cortex, the hypothalamic OXT neurons have a wide range of projections in the cortex [47], such as the prefrontal cortex (PFC), the cingulate cortex (ACC), the insular cortex (IC), the orbital frontal cortex (OFC) [9], where a large number of OXT receptors exist [48]. ACC and IC, in particular, are activated during acute pain in rodents and humans [49]. However, no study focused on the relationship between the changes of cortex and pain after the activation of OXT, and only a few studies cared about imaging of OXT and pain. Some researchers used functional neuroimaging to explore the effect of OXT on neural pain circuits and found that when the subjects watched others suffered, they would suffered enormously enhance the neural activity of some cortex (insular lobe and sensorimotor area). However, after administration of OXT via the nose, the neural activity of the cortex (especially the left insular lobe) of the subjects decreased significantly compared with that before, indicating that OXT could reduce pain empathy by inhibiting the neural activity of the cortex [50]. But, at present, there is no study exploring the direct analgesic effect of OXT in rodents and the human cortex. Periaqueductal gray matter (PAG) of the midbrain is an important structure of the endogenous pain modulation system. It is closely related to the hypothalamus and the rostral ventromedial reticular structure (RVM). It regulates the primary afferent activity of the dorsal horn of the spinal cord through a downward inhibitory pathway [51]. It was found that PAG was a region of extensive expression of the OXT receptor [47]. Some researchers have found that injecting OXT into PAG can increase the pain threshold, and this effect can be removed by giving OXT receptor antagonists [52]. After treatment with the OXT receptor antagonist, the concentrations of endogenous opioid peptides (including leucine enkephalin, methionine enkephalin, and β-endorphin) in PAG perfusion decreased significantly. Also, treatment of naloxone, an opioid receptor antagonist, effectively attenuated OXT induced analgesia, and the concentration of endogenous opioid peptide increased after administration of exogenous OXT [53]. In the same way, RVM, which contains OXT and OXT receptor, is also used as the target area for studying the analgesic mechanism of OXT [54]. Injection of OXT into PAG or RVM can reduce pain [55]. A large number of OXT terminals from PVN were also found in the brain area of the rostral ventrolateral medulla (RVLM) “PAGRVM-spinal cord” nociceptive signal pathway, and OXT could activate the neurons involved in the analgesic effect in RVM [56]. Recent studies have also found that in neuropathologic pain models, microinjection of OXT into the ventrolateral orbital cortex (VLOC) can alleviate pain, which may be mediated by the opioid receptor of PAG [57]. According to the abovementioned studies, it can be concluded that OXT, as a non-opioid analgesic, plays a key role in pain regulation and will be one of the potential analgesic drugs in the future.
The forebrain, including the amygdala, plays an essential role in emotional and cognitive responses to pain. The amygdala is a key structure for regulating pain and related emotions. Hypothalamus OXT neurons were projected to the amygdala, in which there some interneurons specifically expressing OXT receptors in the lateral of the central nucleus (CeL) part [58]. Stimulating the release of OXT by these neurons could reduce fear behavior [9]. Recent evidence indicates that the mesolimbic dopamine system, a circuit implicated in the rewarding properties of drugs of abuse, is also critical for the rewarding effects of social stimuli. The key element of this circuit is the dopamine neuron, which projects from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). For a long time, OXT has been recognized for its important role in a variety of social behaviors [59]. It participates in mediating social rewards through its behavior in VTA and NAc. Inhibition of OXT receptors in male mice [60] NAc or male hamsters [61] or mice [62] VTA can significantly weaken attachment behaviors and the rewarding characteristics of certain social interactions. In recent years, studies have found that OXT is also an important regulator of emotion and motivation and plays a critical regulatory role in various neuropsychiatric diseases. For example, OXT related pathways may be involved in regulating the occurrence and development of depression [63]. As we all know, the central amygdala (CeA) is involved in the regulation of pain. Therefore, OXT may participate in analgesia by projecting to the end of CeA. Some researchers have confirmed that after OXT is injected into the central amygdala of rats, the thermal pain threshold and mechanical pain threshold are significantly increased, and this effect can be antagonized by OXT receptors [64]. In addition, in arthritis pain models, OXT receptors in the central amygdala mediate analgesia by inhibiting pain-related destructive emotions [65]. Therefore, we speculate that OXT may play an analgesic role by regulating pain-related emotional responses through the amygdala. It was found that OXT receptors and OXT fibers were distributed in the NAc of rats. After the administration of OXT in the NAcc of rats, the hind paw heat threshold and mechanical pain threshold increased significantly [66], indicating that the administration of OXT in the NAcc has a good anti-injury effect. The application of naloxone in NAcc weakened the analgesic effect of OXT, suggesting that the endogenous opioid system may be involved in the analgesic effect of OXT.
OXT may regulate pain sensitivity in many ways. For example, receiving direct projection from hypothalamic OXT neurons, stimulating inhibitory GABA interneurons in the spinal cord, directly inhibiting afferent neurons that receive pain information in the periphery, or acting on the cerebral cortex, PAG, amygdala, NAcc, and other central areas (Fig. 1). At present, although the analgesic effect of OXT at the central level has been widely reported, the specific mechanism of OXT action is still unclear, especially the mechanism of its upper central and downstream signaling pathways at the level of the spinal cord. For example, advanced areas of the brain that regulate pain, such as the prefrontal lobe and the cingulate gyrus, require further research.
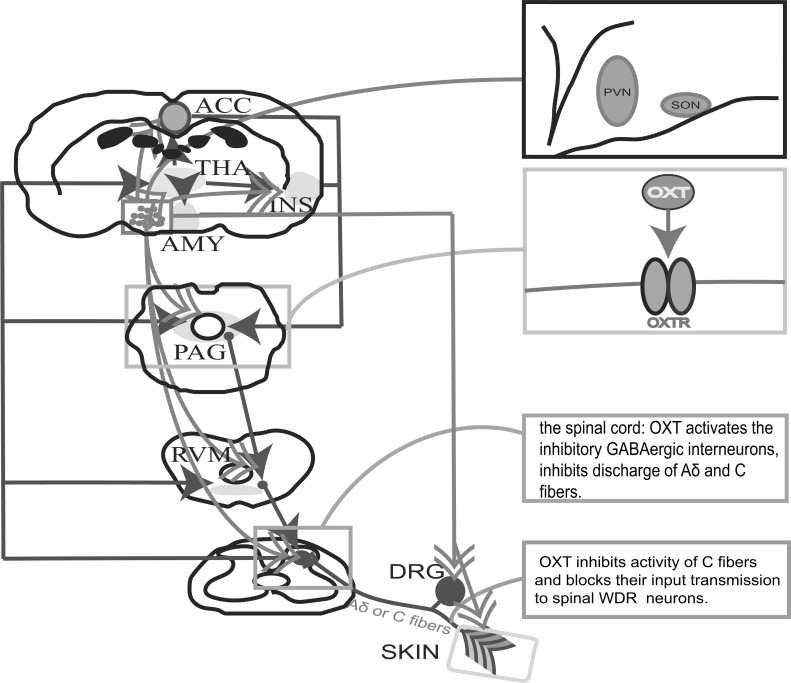
Fig. (1).
The analgesic mechanisms of oxytocin. A: The conduction pathways of pain and the regulatory sites of OXT. Ascending pain pathways (Red line): peripheral stimulation or inflammation activates Aδ and C pain afferent fibers, Aδ and C pain afferent fibers transmit nociceptive sensation to the spinal dorsal horn, which further transmits nociceptive sensation through the ascending spinothalamic tract, and the thalamus then projects to many brain regions such as ACC, insular (INS) and amygdala (AMY). Descending pain pathways (Blue line): Cortex, ACC, INS and AMY regulate the nociceptive signals to the PAG, PAG regulate the signal of RVM, RVM regulate the signals to the spinal dorsal horn. The regulatory sites of OXT are Cortex, AMY, PAG, RVM, NAc, spinal cord, DRG neurons and primary afferent fibers. B: SON and PVN neurons secrete OXT. C: in PAG, NAc and the spinal cord, OXT (Green line) relieves pain by regulating the opioid system. D: in the spinal cord, OXT can activate inhibitory GABAergic intermediate neurons and glutamatergic neurons that release glutamate, and then excite downstream inhibitory GABAergic neurons, thus inhibiting the discharges of Aδ and C fibers. OXT also inhibited the excitability of neurons in the substantia gelatinosa. E: OXT inhibits the activity of C fiber and blocks its afferent transmission to spinal WDR (wide dynamic range) neurons.
5. THE MECHANISM OF ANALGESIC EFFECT OF OXT AT THE PERIPHERAL NERVOUS SYSTEM
The mechanism by which OXT regulates pain at the level of the central nervous system has attracted widespread attention, but the mechanism of peripheral analgesia is poorly understood. Recent studies have shown that subcutaneous injection of OXT can relieve pain, but the exact mechanism is still unclear. Some studies have shown that painful stimulation can cause the activation of the entire OXT system, which leads to the release of a large amount of OXT into the blood, thereby producing analgesic effects. Some researchers have detected the immune signal of the OXT receptor at the end of the noxiousness of the epidermis and found that subcutaneous injection of OXT can activate the OXT receptor on the epidermis [67]. The peripheral OXT in regulating nociceptive sensation is the peripheral nociceptive terminal axons in the skin [68].
Furthermore, it was verified in the formalin pain model that OXT played an analgesic effect by activating the OXT receptor on the epidermis to inhibit the discharge of C fibers and block the nociceptive sensory signals transmitted to wide WDR neurons in the spinal dorsal horn. What’s more, OXT and its receptors are also expressed in DRG and peripheral skin [68], suggesting that OXT may be involved in the process of pain modulation [35]. It has been shown that OXT can rapidly decrease the excitatory ATP-activated current on the membrane of DRG neurons, and this inhibitory effect is triggered by activating the concentrations of PKA and Ca2+. Exogenous OXT can also reduce the excitability of isolated rat DRG neurons by increasing membrane outward current and membrane hyperpolarization, which is mediated by Ca2+/nNOS/NO/KATP pathway [13].
OXT receptors exist in the injury-specific terminals of the skin, and OXT effectively suppresses nociceptive sensory signals of WDR neurons from the peripheral to the dorsal horn of the spinal cord. Therefore, it is likely to reveal a new potential role of OXT in pain regulation by inhibiting the antinociceptive effect of peripheral nociceptive terminal axons in the skin. But at present, compared with the study of OXT regulating DRG neurons to reduce pain perception and pain perception, the research on the mechanism of OXT-mediated analgesia in the skin is still minimal. These new findings give rise to many forward-looking problems, such as the source of OXT acting on the skin, the signal transduction mechanism of OXT receptors in axonal terminals, and so on. In terms of analgesic mechanism, it is generally believed that non-narcotic analgesics (such as aspirin) mainly act on peripheral receptors, while narcotic analgesics (such as morphine) mainly act on CNS. Opioids or opioid analgesics are commonly used by clinicians in the treatment of physical pain, but these drugs can cause severe addiction and dependence. Aspirin is a widely used peripheral analgesia, but the gastrointestinal side effects are more pronounced. Studies have found that neuropeptides (such as galanin and calcitonin gene-related peptide) participate in the analgesic effect on rats at the central level, and oxytocin is also a tiny molecular peptide and a neuropeptide naturally secreted by mammals, which can avoid the addiction of opioids and the gastrointestinal reaction of aspirin. Therefore, it is of great clinical significance to further study the peripheral analgesic effect and mechanism of oxytocin [69].
6. THE ANALGESIC EFFECT OF OXT IN DIFFERENT GENDERS
It is worth noting that according to reports, the analgesic effect of OXT is different between men and women. Intrathecal injection of OXT in male rats has obvious analgesic effects. The same dose of OXT still has no analgesic effect on female rats. Injecting 0.125 nmol OXT into the dural sac of female rats has no obvious analgesic effect, but when the OXT concentration is increased to 1.25 nmol, it produced an obvious analgesic effect [70]. Therefore, it is believed that the analgesic effect of OXT at the level of the spinal cord of female rats is not small. Nasal administration of OXT can increase the perception of noxious heat stimuli in women with chronic neck and shoulder pain, but not in men. Men are more sensitive to pain in the cervical spine and deltoid muscle, while women are more sensitive to pain near the tibial muscle. The interaction between OXT and endogenous sex hormones may affect the sensitivity of nociceptive stimuli [39]. Therefore, the influence of gender should be considered when applying OXT analgesia.
CONCLUSION AND PERSPECTIVE
As people pay more and more attention to the impact of various pains on the quality of life of patients, many new analgesic substances have attracted researchers. As a polypeptide, oxytocin is widely used in some countries for labor induction and delivery [71], prevention of postpartum bleeding [72], and breastfeeding support in some countries [74]. Importantly, more and more studies support that oxytocin exerts analgesic effects through various central and peripheral mechanisms (Table 1), which indicates that oxytocin may be an effective substance for the treatment of pain or side pain. Compared with the analgesic effect of the peripheral nervous system, the analgesic mechanism of OXT in the central nervous system has been described more frequently and in detail. However, recent studies have shown that OXT can effectively regulate peripheral pain, providing new ideas for pain treatment. Whether in the central nervous system or the peripheral nervous system, OXT mainly acts by binding to OXT receptors. Therefore, whether the receptor exists, how it works, and the exact mechanism of OXT's analgesic effect remains to be studied in depth.
Table 1.
Overview of the analgesic mechanism of OXT.
| OXT Regulatory Premises | Existence of Hydroxylamine Energy Fibers | Expression of OXT Receptors | The Analgesic Mechanism of OXT | References |
|---|---|---|---|---|
| CeA | √ | √ | Reduces anxiety and depression to do indirect analgesia | [64, 76, 77] |
| NAc | √ | √ | Regulates the opioid system | [40, 78] |
| PAG | √ | √ | - | [57, 79, 80] |
| DRG | × | √ | Activate the cAMP-PKA pathway to increase intracellular Ca2+ and decrease the DRG neuron current mediated by ATP The intracellular calcium signals of primary sensory neurons in rats activated by OXT have dose and PKC dependent mechanisms Increases intracellular calcium in normal KCl concentration in neurons by inhibiting membrane depolarization Activates the neuronal membrane hyperpolarization by Ca2+/nNOS/NO/K-ATP pathway |
[46, 81-83] [84] [85, 86] [81, 82, 87] |
| Skin | × | √ | Activates TRPV1 channel to promote Ca2+ influx Skin Inhibits discharge of C fibers and blocks the input transmission to spinal WDR neurons |
[21, 88, 89] [35, 67] |
| Spinal cord | √ | √ | Associates with extracellular signal-related protein kinase ERK1/2 Blocking Aδ and C fibers by activating the inhibitory GABAergic interneurons Promotes glutamate release, stimulate a large quantity of inhibitory GABA interneurons, block Aδ and C fiber Regulates the opioid system Inhibits the excitability of the membrane of gelatinous cells |
[90-92] [44, 93] [94-96] [43, 93] [97, 98] |
In recent years, studies have found that oxytocin can be transported to the hippocampus, amygdala, hypothalamus, nucleus accumbens, and spinal dorsal horn to exert its neurotransmitter function. Since the amygdala and the dorsal horn of the spinal cord play an important role in regulating mood and pain, oxytocin receptors are also expressed in these parts, suggesting that oxytocin and its receptors may play an important role in pain information transmission, social behavior, and emotional processes affect. On the other hand, social exclusion can increase the unpleasant emotions caused by harmful heat stimuli. As we all know, oxytocin can stimulate satisfaction, calmness, security, and other emotions. These results indicate that oxytocin can reduce pain by inhibiting the part of the brain that controls fear and anxiety, and behavioral regulation. At the same time, some studies have shown that oxytocin will become a new method for the treatment of autism. Therefore, the analgesic effect supported by social behavior is closely related to the extensive effects of oxytocin to a certain extent. As a self-produced neurotransmitter, oxytocin is natural, safe, and reliable. Previous studies have fully proved that pain stimulation can increase the content of oxytocin in the central nervous system, and transmit oxytocin to different areas of the brain and spinal cord through a variety of neural pathways, participate in the modulation of pain information and play a central analgesic effect [75]. This is the automatic analgesic mechanism of individual mammals. However, it is difficult and risky to inject oxytocin into CNS for analgesia.
In conclusion, a series of scientific issues related to the analgesic effect of OXT need to be further explored, such as the role of OXT in DRG. Suppose we can further clarify the analgesic mechanism of OXT in the peripheral nervous system. In that case, it will contribute to the clinical treatment of refractory pain (including neuropathic pain and inflammatory pain) and open up a new direction for the opening of clinical drugs.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AC-cAMP-PKA
Adenylate Cyclase-cyclic Adenosine Monophosphate-Protein Kinase A
- ACC
Anterior Cingulate Cortex
- AMP
Adenosine 5‘-monophosphate
- AMPK
Adenosine 5‘-monophosphate (AMP)-activated protein kinase
- AMY
Amygdala
- AVP
Arginine Vasopressin
- AVPR
Arginine Vasopressin Receptor
- AVPR1a
Arginine Vasopressin Receptor 1a
- BRK
Bradykinin
- CeA
Central Amygdaloid Nucleus
- CeL
Lateral of the Central Amygdaloid Nucleus
- CSF
Cerebrospinal Fluid
- DRG
Dorsal Root Ganglion
- GABA
γ-aminobutyric acid
- GPCRS
G Protein-Coupled Receptors
- GPT
Glutamic Pyruvic Transaminase
- IASP
The International Association for the Study of Pain
- IC
Insular Cortex
- INS
Insula
- KOR
κ opioid receptor
- Magn-OXT
Magnocellular OXT
- MOR
μ opioid receptor
- NAc
Nucleus Accumbens
- NO-GC-cGMP
Nitric Oxide-guanylate Cyclase-cyclic Guanosine Monophosphate
- NOS
Nitric Oxide Synthetase
- OFC
Orbital Frontal Cortex
- OXT
Oxytocin
- OXTR
Oxytocin Receptor
- PAG
Periaqueductal Gray
- Parv-OXT
Parvocellular OXT
- PFC
Prefrontal Cortex
- PG
Prostaglandin
- PGE2
Prostaglandin E2
- PKC
Protein Kinase C
- PLC
Phospholipase C
- PLCβ
Phospholipase Cβ
- PLCβ-PIP2-IP3
Phospholipase Cβ-phosphatidylinositol Diphosphate Inositol Triphosphate
- PVN
Paraventricular Nucleus
- RVLM
Rostral Ventrolateral Medulla
- RVM
Rostral Ventromedial Reticular
- SNP
Single Nucleotide Polymorphisms
- SON
Supraoptic Nucleus
- VLOC
Ventrolateral Orbital Cortex
- WDR
Wide Dynamic Range
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The dissertation is partially supported by the National Natural Science Foundation of China (82030032, 32070960, 81961128005, 81871108, 81829002, 81771150), Top-Notch Young Talents Program of China of 2014, and Academic Frontier Youth Team of Huazhong University of Science and Technology to Dr. Ling-Qiang Zhu, National Key Research and Development Program of China (Grant No. 2019YFE0121200).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dale H.H. On some physiological actions of ergot. J. Physiol. 1906;34(3):163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ott I., Scott J.C. The action of glandular extracts upon the contractions of the uterus. J. Exp. Med. 1909;11(2):326–330. doi: 10.1084/jem.11.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David J.C., Vareed C. A Preliminary Note on the Action of Vasopressin and Oxytocin. Ind. Med. Gaz. 1929;64(2):73–76. [PMC free article] [PubMed] [Google Scholar]
- 4.García-Boll E., Martínez-Lorenzana G., Condés-Lara M., González-Hernández A. Oxytocin inhibits the rat medullary dorsal horn Sp5c/C1 nociceptive transmission through OT but not V1A receptors. Neuropharmacology. 2018;129:109–117. doi: 10.1016/j.neuropharm.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Poisbeau P., Grinevich V., Charlet A. Oxytocin signaling in pain: cellular, circuit, system, and behavioral levels. Curr. Top. Behav. Neurosci. 2018;35:193–211. doi: 10.1007/7854_2017_14. [DOI] [PubMed] [Google Scholar]
- 6.Gruber C.W. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Exp. Physiol. 2014;99(1):55–61. doi: 10.1113/expphysiol.2013.072561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbach J.P., Luckman S.M., Murphy D., Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 2001;81(3):1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 8.Ross H.E., Cole C.D., Smith Y., Neumann I.D., Landgraf R., Murphy A.Z., Young L.J. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knobloch H.S., Charlet A., Hoffmann L.C., Eliava M., Khrulev S., Cetin A.H., Osten P., Schwarz M.K., Seeburg P.H., Stoop R., Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Juif P.E., Poisbeau P. Neurohormonal effects of oxytocin and vasopressin receptor agonists on spinal pain processing in male rats. Pain. 2013;154(8):1449–1456. doi: 10.1016/j.pain.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Swanson L.W., Sawchenko P.E. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 12.Garrott K., Dyavanapalli J., Cauley E., Dwyer M.K., Kuzmiak-Glancy S., Wang X., Mendelowitz D., Kay M.W. Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure. Cardiovasc. Res. 2017;113(11):1318–1328. doi: 10.1093/cvr/cvx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliava M., Melchior M., Knobloch-Bollmann H.S., Wahis J., da Silva Gouveia M., Tang Y., Ciobanu A.C., Triana Del Rio R., Roth L.C., Althammer F., Chavant V., Goumon Y., Gruber T., Petit-Demoulière N., Busnelli M., Chini B., Tan L.L., Mitre M., Froemke R.C., Chao M.V., Giese G., Sprengel R., Kuner R., Poisbeau P., Seeburg P.H., Stoop R., Charlet A., Grinevich V. A New population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89(6):1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Althammer F., Grinevich V. Diversity of oxytocin neurons: Beyond magno- and parvocellular cell types? J. Neuroendocrinol. 2017 doi: 10.1111/jne.12549. [online a head of print]. [DOI] [PubMed] [Google Scholar]
- 15.Feng C., Lori A., Waldman I.D., Binder E.B., Haroon E., Rilling J.K. A common oxytocin receptor gene (OXTR) polymorphism modulates intranasal oxytocin effects on the neural response to social cooperation in humans. Genes Brain Behav. 2015;14(7):516–525. doi: 10.1111/gbb.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamasue H. Function and structure in social brain regions can link oxytocin-receptor genes with autistic social behavior. Brain Dev. 2013;35(2):111–118. doi: 10.1016/j.braindev.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Chen F.S., Kumsta R., Dvorak F., Domes G., Yim O.S., Ebstein R.P., Heinrichs M. Genetic modulation of oxytocin sensitivity: A pharmacogenetic approach. Transl. Psychiatry. 2015;5(10):e664. doi: 10.1038/tp.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bethlehem R.A.I., Lombardo M.V., Lai M.C., Auyeung B., Crockford S.K., Deakin J., Soubramanian S., Sule A., Kundu P., Voon V., Baron-Cohen S. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Transl. Psychiatry. 2017;7(4):e1099. doi: 10.1038/tp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasanbuyan N., Yoshida M., Takayanagi Y., Inutsuka A., Nishimori K., Yamanaka A., Onaka T. Oxytocin-oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology. 2018;159(2):763–775. doi: 10.1210/en.2017-00606. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X.B., Lutz S., Steffens F., Korth M., Wieland T. Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and nonpregnant rat uterine myocytes: Implications for myometrial contractility. Mol. Endocrinol. 2007;21(3):740–752. doi: 10.1210/me.2006-0220. [DOI] [PubMed] [Google Scholar]
- 21.Gimpl G., Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y.T., Hsu K.S. Oxytocin receptor signaling in the hippocampus: Role in regulating neuronal excitability, network oscillatory activity, synaptic plasticity and social memory. Prog. Neurobiol. 2018;171:1–14. doi: 10.1016/j.pneurobio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 23.de Wied D., Diamant M., Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front. Neuroendocrinol. 1993;14(4):251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- 24.Veinante P., Freund-Mercier M.J. Histo autoradiographic detection of oxytocin- and vasopressin-binding sites in the amygdala of the rat. Adv. Exp. Med. Biol. 1995;395:347–348. [PubMed] [Google Scholar]
- 25.Macdonald K., Macdonald T.M. The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiatry. 2010;18(1):1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- 26.Sauer R.S., Rittner H.L., Roewer N., Sohajda T., Shityakov S., Brack A., Broscheit J.A. A Novel Approach for the Control of Inflammatory Pain: Prostaglandin E2 Complexation by Randomly Methylated β-Cyclodextrins. Anesth. Analg. 2017;124(2):675–685. doi: 10.1213/ANE.0000000000001674. [DOI] [PubMed] [Google Scholar]
- 27.Gao L., Yu L.C. Involvement of opioid receptors in the oxytocin-induced antinociception in the central nervous system of rats. Regul. Pept. 2004;120(1-3):53–58. doi: 10.1016/j.regpep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Ohmichi M., Koike K., Nohara A., Kanda Y., Sakamoto Y., Zhang Z.X., Hirota K., Miyake A. Oxytocin stimulates mitogen-activated protein kinase activity in cultured human puerperal uterine myometrial cells. Endocrinology. 1995;136(5):2082–2087. doi: 10.1210/endo.136.5.7536662. [DOI] [PubMed] [Google Scholar]
- 29.Petersen R.C., Lopez O., Armstrong M.J., Getchius T.S.D., Ganguli M., Gloss D., Gronseth G.S., Marson D., Pringsheim T., Day G.S., Sager M., Stevens J., Rae-Grant A. Practice guideline update summary: Mild cognitive impairment: Report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology. 2018;90(3):126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku C.Y., Qian A., Wen Y., Anwer K., Sanborn B.M. Oxytocin stimulates myometrial guanosine triphosphatase and phospholipase-C activities via coupling to G alpha q/11. Endocrinology. 1995;136(4):1509–1515. doi: 10.1210/endo.136.4.7895660. [DOI] [PubMed] [Google Scholar]
- 31.Rojas-Piloni G., Mejía-Rodríguez R., Martínez-Lorenzana G., Condés-Lara M. Oxytocin, but not vassopressin, modulates nociceptive responses in dorsal horn neurons. Neurosci. Lett. 2010;476(1):32–35. doi: 10.1016/j.neulet.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 32.Cechetto D.F., Saper C.B. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J. Comp. Neurol. 1988;272(4):579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 33.Miranda-Cardenas Y., Rojas-Piloni G., Martínez-Lorenzana G., Rodríguez-Jiménez J., López-Hidalgo M., Freund-Mercier M.J., Condés-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122(1-2):182–189. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Wrobel L., Schorscher-Petcu A., Dupré A., Yoshida M., Nishimori K., Tribollet E. Distribution and identity of neurons expressing the oxytocin receptor in the mouse spinal cord. Neurosci. Lett. 2011;495(1):49–54. doi: 10.1016/j.neulet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Moreno-López Y., Martínez-Lorenzana G., Condés-Lara M., Rojas-Piloni G. Identification of oxytocin receptor in the dorsal horn and nociceptive dorsal root ganglion neurons. Neuropeptides. 2013;47(2):117–123. doi: 10.1016/j.npep.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Goodin B.R., Ness T.J., Robbins M.T. Oxytocin - a multifunctional analgesic for chronic deep tissue pain. Curr. Pharm. Des. 2015;21(7):906–913. doi: 10.2174/1381612820666141027111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez-Lorenzana G., Espinosa-López L., Carranza M., Aramburo C., Paz-Tres C., Rojas-Piloni G., Condés-Lara M. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain. 2008;140(2):265–273. doi: 10.1016/j.pain.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Van Wimersma Greidanus T.B., Kroodsma J.M., Pot M.L., Stevens M., Maigret C. Neurohypophyseal hormones and excessive grooming behaviour. Eur. J. Pharmacol. 1990;187(1):1–8. doi: 10.1016/0014-2999(90)90334-3. [DOI] [PubMed] [Google Scholar]
- 39.Tracy L.M., Labuschagne I., Georgiou-Karistianis N., Gibson S.J., Giummarra M.J. Sex-specific effects of intranasal oxytocin on thermal pain perception: A randomised, double-blind, placebo-controlled cross-over study. Psychoneuroendocrinology. 2017;83:101–110. doi: 10.1016/j.psyneuen.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Wang J.W., Lundeberg T., Yu L.C. Antinociceptive role of oxytocin in the nucleus raphe magnus of rats, an involvement of mu-opioid receptor. Regul. Pept. 2003;115(3):153–159. doi: 10.1016/S0167-0115(03)00152-6. [DOI] [PubMed] [Google Scholar]
- 41.Ostrowski N.L. Oxytocin receptor mRNA expression in rat brain: Implications for behavioral integration and reproductive success. Psychoneuroendocrinology. 1998;23(8):989–1004. doi: 10.1016/S0306-4530(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 42.Ando M., Hayashi Y., Hitomi S., Shibuta I., Furukawa A., Oto T., Inada T., Matsui T., Fukaya C., Noma N., Okubo M., Yonehara Y., Kaneko T., Iwata K., Shinoda M. Oxytocin-dependent regulation of TRPs expression in trigeminal ganglion neurons attenuates orofacial neuropathic pain following infraorbital nerve injury in rats. Int. J. Mol. Sci. 2020;21(23):E9173. doi: 10.3390/ijms21239173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu S.Q., Lundeberg T., Yu L.C. Involvement of oxytocin in spinal antinociception in rats with inflammation. Brain Res. 2003;983(1-2):13–22. doi: 10.1016/S0006-8993(03)03019-1. [DOI] [PubMed] [Google Scholar]
- 44.Rojas-Piloni G., López-Hidalgo M., Martínez-Lorenzana G., Rodríguez-Jiménez J., Condés-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137(1):69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 45.Breton J.D., Veinante P., Uhl-Bronner S., Vergnano A.M., Freund-Mercier M.J., Schlichter R., Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in lamina I-II which amplify GABAergic inhibition. Mol. Pain. 2008;4:19. doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan Z. J., Wei J. B., Li Z. W., Shao M., Hu Q. S., Peng B. W. Modulation of GABA-activated currents by oxytocin in rat dorsal root ganglion neurons. Sheng li xue bao : Acta physiologica Sinica, 2000;52(5):381–384. [PubMed] [Google Scholar]
- 47.Campbell P., Ophir A.G., Phelps S.M. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J. Comp. Neurol. 2009;516(4):321–333. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- 48.Vargas-Martínez F., Uvnäs-Moberg K., Petersson M., Olausson H.A., Jiménez-Estrada I. Neuropeptides as neuroprotective agents: Oxytocin a forefront developmental player in the mammalian brain. Prog. Neurobiol. 2014;123:37–78. doi: 10.1016/j.pneurobio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Orenius T.I., Raij T.T., Nuortimo A., Näätänen P., Lipsanen J., Karlsson H. The interaction of emotion and pain in the insula and secondary somatosensory cortex. Neuroscience. 2017;349:185–194. doi: 10.1016/j.neuroscience.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 50.Bos P.A., Montoya E.R., Hermans E.J., Keysers C., van Honk J. Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. Neuroimage. 2015;113:217–224. doi: 10.1016/j.neuroimage.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wager T.D., Atlas L.Y. The neuroscience of placebo effects: Connecting context, learning and health. Nat. Rev. Neurosci. 2015;16(7):403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J., Li P., Liang J.Y., Pan Y.J., Yan X.Q., Yan F.L., Hao F., Zhang X.Y., Zhang J., Qiu P.Y., Wang D.X. Oxytocin in the periaqueductal grey regulates nociception in the rat. Regul. Pept. 2011;169(1-3):39–42. doi: 10.1016/j.regpep.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Yang J., Liang J.Y., Li P., Pan Y.J., Qiu P.Y., Zhang J., Hao F., Wang D.X. Oxytocin in the periaqueductal gray participates in pain modulation in the rat by influencing endogenous opiate peptides. Peptides. 2011;32(6):1255–1261. doi: 10.1016/j.peptides.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Figueira R.J., Peabody M.F., Lonstein J.S. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav. Neurosci. 2008;122(3):618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- 55.Yang J., Liang J.Y., Zhang X.Y., Qiu P.Y., Pan Y.J., Li P., Zhang J., Hao F., Wang D.X., Yan F.L. Oxytocin, but not arginine vasopressin is involving in the antinociceptive role of hypothalamic supraoptic nucleus. Peptides. 2011;32(5):1042–1046. doi: 10.1016/j.peptides.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Lee S.K., Ryu P.D., Lee S.Y. Differential distributions of neuropeptides in hypothalamic paraventricular nucleus neurons projecting to the rostral ventrolateral medulla in the rat. Neurosci. Lett. 2013;556:160–165. doi: 10.1016/j.neulet.2013.09.070. [DOI] [PubMed] [Google Scholar]
- 57.Taati M., Tamaddonfard E. Ventrolateral orbital cortex oxytocin attenuates neuropathic pain through periaqueductal gray opioid receptor. Pharmacol. Rep. 2018;70(3):577–583. doi: 10.1016/j.pharep.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Huber D., Veinante P., Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 59.Caldwell H.K., Albers H.E. Oxytocin, vasopressin, and the motivational forces that drive social behaviors. Curr. Top. Behav. Neurosci. 2016;27:51–103. doi: 10.1007/7854_2015_390. [DOI] [PubMed] [Google Scholar]
- 60.Dölen G., Darvishzadeh A., Huang K.W., Malenka R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song Z., Borland J.M., Larkin T.E., O’Malley M., Albers H.E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 2016;74:164–172. doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hung L.W., Neuner S., Polepalli J.S., Beier K.T., Wright M., Walsh J.J., Lewis E.M., Luo L., Deisseroth K., Dölen G., Malenka R.C. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357(6358):1406–1411. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janeček M., Dabrowska J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res. 2019;375(1):143–172. doi: 10.1007/s00441-018-2889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han Y., Yu L.C. Involvement of oxytocin and its receptor in nociceptive modulation in the central nucleus of amygdala of rats. Neurosci. Lett. 2009;454(1):101–104. doi: 10.1016/j.neulet.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 65.Cragg B., Ji G., Neugebauer V. Differential contributions of vasopressin V1A and oxytocin receptors in the amygdala to pain-related behaviors in rats. Mol. Pain. 2016;12:12. doi: 10.1177/1744806916676491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu X.L., Yu L.C. Involvement of opioid receptors in oxytocin-induced antinociception in the nucleus accumbens of rats. Am. Pain Soc. 2007;8(1):85–90. doi: 10.1016/j.jpain.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 67.González-Hernández A., Manzano-García A., Martínez-Lorenzana G., Tello-García I.A., Carranza M., Arámburo C., Condés-Lara M. Peripheral oxytocin receptors inhibit the nociceptive input signal to spinal dorsal horn wide-dynamic-range neurons. Pain. 2017;158(11):2117–2128. doi: 10.1097/j.pain.0000000000001024. [DOI] [PubMed] [Google Scholar]
- 68.Grinevich V., Charlet A. Oxytocin: Pain relief in skin. Pain. 2017;158(11):2061–2063. doi: 10.1097/j.pain.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 69.Shi J., Fu L.B., Yu L.C. Involvement of protein kinase C in the galanin-induced antinociception in the brain of rats. Neurosci. Lett. 2011;497(1):60–63. doi: 10.1016/j.neulet.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 70.Chow L.H., Chen Y.H., Wu W.C., Chang E.P., Huang E.Y. Sex difference in oxytocin-induced anti-hyperalgesia at the spinal level in rats with intraplantar carrageenan-induced inflammation. PLoS One. 2016;11(9):e0162218. doi: 10.1371/journal.pone.0162218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei S.Q., Luo Z.C., Qi H.P., Xu H., Fraser W.D. High-dose vs low-dose oxytocin for labor augmentation: A systematic review. Am. J. Obstet. Gynecol. 2010;203(4):296–304. doi: 10.1016/j.ajog.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Sentilhes L., Merlot B., Madar H., Sztark F., Brun S., Deneux-Tharaux C. Postpartum haemorrhage: Prevention and treatment. Expert Rev. Hematol. 2016;9(11):1043–1061. doi: 10.1080/17474086.2016.1245135. [DOI] [PubMed] [Google Scholar]
- 73.Ruis H., Rolland R., Doesburg W., Broeders G., Corbey R. Oxytocin enhances onset of lactation among mothers delivering prematurely. Br. Med. J. (Clin. Res. Ed.) 1981;283(6287):340–342. doi: 10.1136/bmj.283.6287.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fewtrell M.S., Loh K.L., Blake A., Ridout D.A., Hawdon J. Randomised, double blind trial of oxytocin nasal spray in mothers expressing breast milk for preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2006;91(3):F169–F174. doi: 10.1136/adc.2005.081265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.González-Hernández A., Rojas-Piloni G., Condés-Lara M. Oxytocin and analgesia: Future trends. Trends Pharmacol. Sci. 2014;35(11):549–551. doi: 10.1016/j.tips.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Neugebauer V. Amygdala pain mechanisms. Handb. Exp. Pharmacol. 2015;227:261–284. doi: 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neumann I.D., Landgraf R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Li L., Wang X., Yu L.C. Involvement of opioid receptors in the CGRP-induced antinociception in the nucleus accumbens of rats. Brain Res. 2010;1353:53–59. doi: 10.1016/j.brainres.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 79.Li S.F., Zhang Y.Y., Li Y.Y., Wen S., Xiao Z. Antihyperalgesic effect of 5-HT7 receptor activation on the midbrain periaqueductal gray in a rat model of neuropathic pain. Pharmacol. Biochem. Behav. 2014;127:49–55. doi: 10.1016/j.pbb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Zhao M., Wang J.Y., Jia H., Tang J.S. Roles of different subtypes of opioid receptors in mediating the ventrolateral orbital cortex opioid-induced inhibition of mirror-neuropathic pain in the rat. Neuroscience. 2007;144(4):1486–1494. doi: 10.1016/j.neuroscience.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Molokanova E.A., Tamarova Z.A. Effect of oxytocin on rat dorsal root ganglia in vitro. Neurophysiology. 1989;21(3):420–422. [PubMed] [Google Scholar]
- 82.Tamarova Z.A. Effect of vasopressin and oxytocin on the dorsal root potentials of the isolated perfused spinal cord in rat pups. Neurophysiology. 1988;20(6):757–763. [PubMed] [Google Scholar]
- 83.Yang Q., Wu Z.Z., Li X., Li Z.W., Wei J.B., Hu Q.S. Modulation by oxytocin of ATP-activated currents in rat dorsal root ganglion neurons. Neuropharmacology. 2002;43(5):910–916. doi: 10.1016/S0028-3908(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 84.Ayar A., Ozcan M., Alcin E., Serhatlioglu I., Ozcan S., Kutlu S., Kelestimur H. Oxytocin activates calcium signaling in rat sensory neurons through a protein kinase C-dependent mechanism. J. Physiol. Biochem. 2014;70(1):43–48. doi: 10.1007/s13105-013-0278-z. [DOI] [PubMed] [Google Scholar]
- 85.Jo Y.H., Stoeckel M.E., Freund-Mercier M.J., Schlichter R. Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J. Neurosci. 1998;18(7):2377–2386. doi: 10.1523/JNEUROSCI.18-07-02377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hobo S., Hayashida K., Eisenach J.C. Oxytocin inhibits the membrane depolarization-induced increase in intracellular calcium in capsaicin sensitive sensory neurons: A peripheral mechanism of analgesic action. Anesth. Analg. 2012;114(2):442–449. doi: 10.1213/ANE.0b013e31823b1bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gong L., Gao F., Li J., Li J., Yu X., Ma X., Zheng W., Cui S., Liu K., Zhang M., Kunze W., Liu C.Y. Oxytocin-induced membrane hyperpolarization in pain-sensitive dorsal root ganglia neurons mediated by Ca(2+)/nNOS/NO/KATP pathway. Neuroscience. 2015;289:417–428. doi: 10.1016/j.neuroscience.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 88.Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 89.Nersesyan Y., Demirkhanyan L., Cabezas-Bratesco D., Oakes V., Kusuda R., Dawson T., Sun X., Cao C., Cohen A.M., Chelluboina B., Veeravalli K.K., Zimmermann K., Domene C., Brauchi S., Zakharian E. Oxytocin modulates nociception as an agonist of pain-sensing trpv1. Cell Rep. 2017;21(6):1681–1691. doi: 10.1016/j.celrep.2017.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poisbeau P., Patte-Mensah C., Keller A.F., Barrot M., Breton J.D., Luis-Delgado O.E., Freund-Mercier M.J., Mensah-Nyagan A.G., Schlichter R. Inflammatory pain upregulates spinal inhibition via endogenous neurosteroid production. J. Neurosci. 2005;25(50):11768–11776. doi: 10.1523/JNEUROSCI.3841-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blume A., Bosch O.J., Miklos S., Torner L., Wales L., Waldherr M., Neumann I.D. Oxytocin reduces anxiety via ERK1/2 activation: Local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci. 2008;27(8):1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 92.Juif P.E., Breton J.D., Rajalu M., Charlet A., Goumon Y., Poisbeau P. Long-lasting spinal oxytocin analgesia is ensured by the stimulation of allopregnanolone synthesis which potentiates GABA(A) receptor-mediated synaptic inhibition. J. Neurosci. 2013;33(42):16617–16626. doi: 10.1523/JNEUROSCI.3084-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Condés-Lara M., Rojas-Piloni G., Martínez-Lorenzana G., López-Hidalgo M., Rodríguez-Jiménez J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 2009;1247:38–49. doi: 10.1016/j.brainres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 94.Breton J.D., Poisbeau P., Darbon P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol. Pain. 2009;5:63. doi: 10.1186/1744-8069-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robinson D.A., Wei F., Wang G.D., Li P., Kim S.J., Vogt S.K., Muglia L.J., Zhuo M. Oxytocin mediates stress-induced analgesia in adult mice. J. Physiol. 2002;540(Pt 2):593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Condés-Lara M., González N.M., Martínez-Lorenzana G., Delgado O.L., Freund-Mercier M.J. Actions of oxytocin and interactions with glutamate on spontaneous and evoked dorsal spinal cord neuronal activities. Brain Res. 2003;976(1):75–81. doi: 10.1016/S0006-8993(03)02690-8. [DOI] [PubMed] [Google Scholar]
- 97.Jiang C.Y., Fujita T., Kumamoto E. Synaptic modulation and inward current produced by oxytocin in substantia gelatinosa neurons of adult rat spinal cord slices. J. Neurophysiol. 2014;111(5):991–1007. doi: 10.1152/jn.00609.2013. [DOI] [PubMed] [Google Scholar]
- 98.Jiang C.Y., Fujita T., Kumamoto E. Developmental change and sexual difference in synaptic modulation produced by oxytocin in rat substantia gelatinosa neurons. Biochem. Biophys. Rep. 2016;7:206–213. doi: 10.1016/j.bbrep.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]