Abstract
Objectives:
COVID-19 abruptly halted in-person clinical care and research requiring a shift to virtual assessment and treatment. This unexpected transition of a two-arm randomized controlled trial (RCT) examining interdisciplinary graded exposure treatment (GET Living) compared to multidisciplinary pain management (MPM) for youth with chronic pain provided an opportunity to implement the first remotely delivered exposure treatment and remotely delivered biomechanical assessment for pediatric chronic pain. Here we describe these new approaches and provide lessons learned to inform future efforts in digital healthcare.
Methods:
A total of 68 youth (M=14.2 years; 80.9% female) were enrolled in the RCT (n=31 in-person, n=5 hybrid, n=32 virtual, n = 9 withdrew). Of those withdrawn, n = 3 withdrew due to COVID-19 related reasons. Some RCT elements required slight modification (e.g., e-consent, actigraphy deployment, recruitment and screening) while others were significantly altered (e.g., session format and lab-based biomechanical assessment). Data from Exit Interviews were also examined to assess perspectives on the virtual format transition.
Results:
Results show increased enrollment rate when virtual care was an option (70.7%) compared to in-person (44.3%). Equivalent rates of completion for daily assessment (in-person, 72.8%; virtual, 73.3) were also observed, and participants described enhanced experience when able to complete exercises and exposures in their home environment during session (vs. a rehabilitation gym) allowing for genuine in-vivo exposures (e.g., household chores, riding bicycles).
Discussion:
Overall, our data demonstrate acceptability, feasibility, and equivalent patient engagement to virtual treatment. Novel methods implemented in this RCT can inform trial design and measures of clinical endpoints for future digital health interventions.
Keywords: graded-exposure treatment, virtual care, pediatric chronic pain
Introduction
The SARS Coronavirus-2 (COVID-19) pandemic forced significant changes in the delivery of health care and clinical research. Following Shelter-In-Place orders in early March 2020, many medical and mental health clinics abruptly halted in-person treatment and research participation, requiring rapid adaptation of assessment and care via technology. Fortunately, the efficacy of internet-based interventions for pediatric chronic pain populations has been an area of active study, with data suggesting small to moderate reductions in pain, functional disability, and distress[1]. Further, remotely delivered physical therapy has also been found to be beneficial at reducing pain when compared to no care/wait list, and outcomes have been comparable to in-person care as usual [2]. At the beginning of the COVID-19 pandemic, recommendations were put forth by the academic pediatric pain community urging researchers and clinicians to consider the risks and burdens on youth with chronic pain during a pandemic, highlighting implications of delivering care remotely for this vulnerable population, as well as the necessity of continuing research and treatment delivery during COVID-19. [3–5]. These recommendations also included practical resources related to best practices for use of technology, navigating technical issues, and capitalizing on enhanced opportunities for experiential learning during treatment sessions, as well as consideration of the stressful nature of the context of the pandemic [3]. Several research specific recommendations included assessment of the implementation of clinical research via digital technologies, examination of factors assessing uptake and engagement in virtual care, satisfaction with treatment delivery, and preferences for virtually delivered interventions [3].
In January 2019, our group began a two-arm randomized controlled trial (RCT) at the outpatient Pediatric Pain Management Clinic (PPMC) at Stanford Children’s Health (SCH) examining graded exposure treatment (GET) for youth with chronic pain compared to multidisciplinary pain management (MPM)[6]. GET Living is an outpatient interdisciplinary graded in-vivo exposure-based treatment focused on reducing of pain-related fear and increasing function via values-based goal setting and activity exposures. GET Living is jointly delivered by a pain psychologist and physical therapist. MPM consists of separately delivered standard outpatient cognitive behavioral therapy and physical therapy (PT) for pain management. The PPMC at Stanford has utilized telehealth services for medical and psychology follow-up appointments for over two years, with roughly 10% of visits conducted via telehealth prior to March 2020[7] . One week prior to Shelter-in-Place orders for California, the PPMC shifted all patient visits, including new patient interdisciplinary evaluations, and medical and psychology follow-up appointments, to telehealth. Given that recruitment for the trial was conducted following new patient evaluations at the PPMC, we were able to maintain our recruitment efforts with the same methods following the transition to virtual care within the clinic. Simultaneously, our clinical-research team also adapted the RCT protocol to telehealth, with priority placed on transitioning youth already in active in-person treatment. We reinitiated clinical care with families via Zoom three days before statewide Shelter-in-Place orders went into place on March 19, 2020. Assessment procedures were adapted within the first few weeks of the shutdown, resulting in the development of a virtual protocol to continue collecting secure and accurate data during the trial.
As a result of our rapid pivot in assessment and treatment, we are uniquely positioned to describe the first remotely delivered exposure-based pain management treatment and remotely delivered biomechanical assessment for pediatric chronic pain. We aim to present these new approaches, provide lessons learned throughout the process of converting to a virtual care format, and discuss Implications for future research, trial design, and utilization of these virtual methods in patient care.
Methods
Study Overview
The GET Living trial protocol is published[6], registered (NCT03699007), and funded by the National Institutes of Health (R21AR072921). The study was a two-arm, randomized, controlled design to test GET Living for youth with chronic musculoskeletal pain and elevated pain-related fear, compared to a MPM control arm. For both GET Living and MPM treatment arms, intervention consisted of 12 sessions total, each lasting one-hour, with twice weekly appointments for approximately 6 weeks at the PPMC, where there was access to a rehabilitation gym space with a variety of equipment to use[6]. GET Living sessions were jointly delivered by a psychologist and physical therapist, with youth and caregiver present, and treatment targets including pain-related fear and avoidance in youth via activity exposures to improve functioning. Examples of exposure activities chosen by youth included riding a bike, walking the dog, and cleaning their room. MPM consisted of six sessions of pain psychology and six sessions of impairment-based physical therapy, delivered independently of each other. Generally, with two appointments each week, MPM patients were scheduled for one session of PT and Pain Psychology every week. Of the six pain psychology sessions, two sessions (session 1 and 6) are delivered to the caregiver and youth, while sessions 2–5 are delivered one-on-one to the youth.
Primary outcomes were pain-related fear and avoidance (Fear of Pain Questionnaire [FOPQ-C][8] and Photographs of Daily Activities [PHODA-Youth][9]). Secondary outcome was disability (Functional Disability Inventory [FDI][10]). Additional measures included the Pain Catastrophizing Scale- child (PCS-C)[11] and parent (PCS-P)[12], Chronic Pain Acceptance Questionnaire for Adolescents (CPAQ-A)[13]; Parent Pain Acceptance Questionnaire (PPAQ)[14], and Adult Responses to Child’s Symptoms (ARCS) [15]. Exploratory outcomes included adolescent biomechanics assessment via motion analysis, daily physical activity and sleep (Actigraphy[16]), and a weekly healthcare costs[17]. All procedures were devised for in-person recruitment, assessment, and treatment.
This RCT was enhanced with a sequential replicated and randomized single-case experimental design (SCED) methodology. In SCED, a subject is observed repeatedly at different levels of at least one independent variable (e.g., baseline, treatment), acting as their own control, with primary and secondary outcomes assessed daily via daily diaries, allowing for the collection of statistically rigorous data at the level of the individual patient.
Procedures
Youth were eligible to participate if they 1) had musculoskeletal pain (e.g., localized [back, limb] or diffuse, not due to an acute trauma (active sprain or fracture); 2) were between 8 and 18 years old; 3) had moderate to high pain-related fear (Fear of Pain Questionnaire- FOPQ-C] ≥ 35); 4) had moderate to high functional disability (Functional Disability Inventory ≥ 13) and 5) were proficient in the English language. Potentially eligible youth were referred to GET Living by a team of interdisciplinary providers (physician, psychologist, physical therapist) following initial or follow-up visits at the PPMC at SCH or through the Kaiser Santa Clara Chronic Pain Clinic [6]. Once referred, eligibility was confirmed by the research team via a brief introductory phone call and by completing measures (FOPQ-C[8] and FDI[10]). Once deemed eligible, youth were randomized to treatment arm and scheduled for an initial baseline study visit, which was conducted at the Motion and Sports Performance Lab (MASPL) at SCH. During this baseline visit, consent and assent procedures were completed. Youth also completed a biomechanical assessment consisting of a 6-minute walk test [18, 19], a jogging task, single-and double leg squats[20], and a drop vertical jump task[21–23], with data collected from 3-D motion capture and force plates[24]. Both the youth and caregiver completed a battery of self-report measures assessing primary, secondary, and additional outcomes via REDCap during this visit. An Actigraph device was initialized by the research team and administered to enrolled youth at the baseline session.
To create a multiple baseline methodology with SCED, patients were randomized to a baseline period ranging from 10 to 21 days which began immediately following the baseline visit. During the baseline phase, Actigraphy data and daily self-report measures (i.e., Daily Diaries) for youth and caregiver were collected. Following the completion of treatment, patients were scheduled for a discharge visit during which the biomechanical assessment and self-reported test measures were repeated. All youth and caregivers also completed an exit interview. This interview was a semi-structured free response format with a list of questions for both the youth and the caregiver in order to obtain feedback on the intervention. Youth and caregiver also complete the battery of self-report measures via REDCap at 3-and 6-month follow-up. See Figure 1 for study design flow chart.
Figure 1. Study Design.

Eligibility Screening: Once a potential adolescent (age 8–18) was referred to the GET Living trial, the coordinator confirms eligibility, youth were randomized and baseline assessment was scheduled. Baseline: At the baseline assessment the adolescent and parent began daily diaries, the Actigraph watch was activated, and biomechanical assessment completed. At the baseline visit the adolescent and parent received a treatment start date and a schedule for all sessions . Active Treatment: Adolescent and one caregiver/parent present for 12 sessions of treatment scheduled over the course of 6–8 weeks, accounting for holidays/vacations. Discharge assessment occurred at the conclusion of treatment. Actigraph was returned to the research team and daily diaries end. Follow-up: Adolescents and parents were contacted via phone initially with REDCap surveys then sent with one battery of questionnaires and daily diaries for a 7-day period at 3-month and 6-month follow-up. Note. DD=daily diary; SR=self-report; W=week.
Remote Procedures.
We modified assessment and treatment procedures to an entirely remote format, allowing for continuation the clinical trial uninterrupted. Below we outline the changes made to recruitment and eligibility, data collection, and intervention. Virtual treatment sessions in both arms were designed to mimic the in-person experience as much as possible.
Remote Recruitment and Eligibility.
As pain clinic services were fully converted to telehealth in March 2020, we maintained access to our referral base. Remote recruitment workflow remained predominately identical to in-person procedures. All patients identified as potentially eligible were flagged by the research team who notified the clinical team treating the patient via secure messaging. If the clinical team deemed it an appropriate referral, participation in the trial was presented as a treatment option to the family at the end of the telehealth clinic evaluation. Research coordinators also joined the end of the telehealth clinic visit whenever possible to mimic the usual in-person introduction to research. If the family was interested in enrolling, a PT screening was conducted by the physical therapist over the phone at a later day to assess for safety concerns specific to remote delivery of PT, such as fall risk and identifying a capable and willing adult available in the home during PT sessions. An inventory of home supplies available to the family (both exercise equipment and technology such as computer, tablet, and/or printer) was also taken. Once the family completed the PT screening and was deemed appropriate for remote participation, enrollment and scheduling proceeded per the adapted virtual procedures. In terms of eligibility criteria for the virtual format, distance from the clinic (i.e., >100 miles) was no longer considered exclusionary so that youth across the state could participate virtually once eligibility was confirmed.
Remote Data Collection.
Baseline visits maintained the same structure as the in-person protocol. Starting with the consent process, an email describing the baseline procedures was sent to the family prior to the Zoom call, along with all links to the electronic eConsent/eAssent forms and other materials that were needed for the call. We utilized a pre-existing, secure REDCap eConsent/eAssent framework that had originally been IRB approved for the trial [25]. During the remote baseline visit, a research coordinator reviewed the consent/assent forms with the youth and caregiver, then the forms were electronically signed through their own device. Additionally, a physical therapy telehealth treatment agreement form was reviewed and signed in the same electronic consent form. This IRB-approved form was created so that youth can be informed of and agree to the risks and benefits of PT telehealth. The specific components detailed in this form included the following:
Telemedicine/telehealth description
Outline of risks, benefits, and limitations of telehealth
Consent to being recorded and storage of data through video conferencing
Emergency & client safety procedures
The research coordinator initialized the Actigraph during the virtual baseline visit based on information provided by youth on the call (current weight, height, and handedness). Then the watch was shipped to youth via USPS priority mail (generally delivered within 1–2 days). The MASPL research team developed a shortened biomechanical assessment taking into consideration ability to perform safely, accuracy of the data collected, and feasibility of guiding youth through the exercises remotely. Three tasks were selected: 6 Minute Walk Test, and double-leg squat, and single-leg squat task.
The 6-minute walk test was selected as it is a standard assessment readily used by clinicians to examine functional performance for a variety of pediatric patients[18, 19, 26]. This task was also selected based on ease of description and data collection, facilitated by the iWalkAssess app (University of Toronto, Toronto ON, Canada) which families downloaded and activated for remote test administration. During the 6-minute walk test, the MASPL team instructed the family to measure an 8m long walking track with a tape measure and instructed the caregiver on how to operate the app to accurately collect the data. The double-leg and single-leg squat tasks were adopted into the remote assessment because of the ease of description, collection, and translation when collected from two-dimensional data[24]. The examination of squatting has previously been reported as a tool to assess physical function[20, 24]. In order to collect these data efficiently over Zoom, subjects were asked to position and stand away from their camera so that the entire body (head to toe) was in view and the video was recorded in the Zoom application (Figure 2). Youth stood so that the camera had a sagittal plane view of all squats performed. For the single leg squat task, youth were asked to face the direction so that the current stance leg was facing the camera. To control for length scale in the videos, youth were told to hold up an object with known dimensions prior to starting their squats (e.g., standard 8.5 × 11’ paper). For both tasks, the youth were instructed to perform a maximum of five squats to whatever depth they felt comfortable with, consistent with in-person assessment. The video data was then exported to MaxTraq 2.0 (Innovation Systems, Columbiaville, MI) for data processing (Figure 3).
Figure 2.

Example of patient engaging in the double-leg squat task over Zoom
Figure 3.
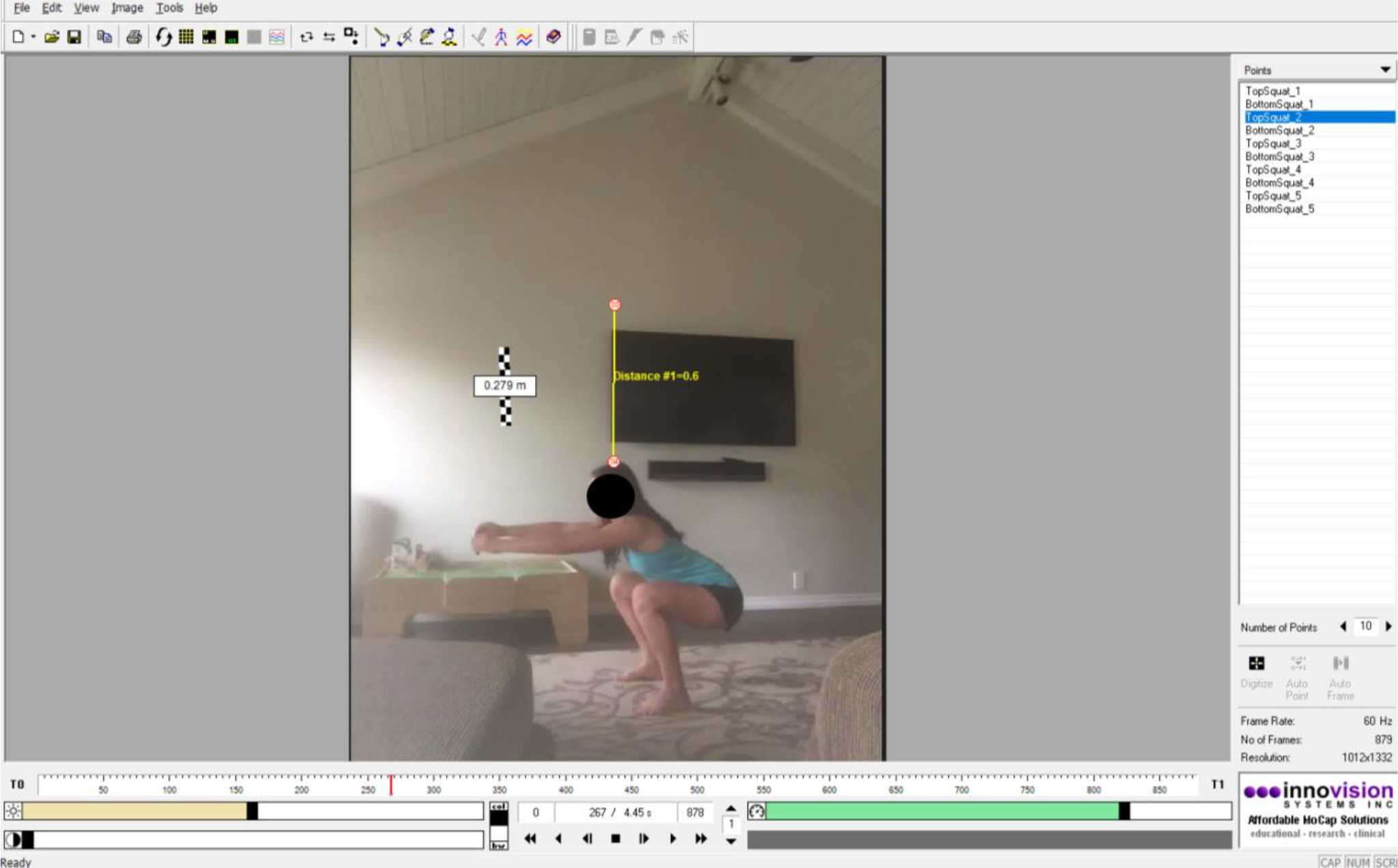
Video of double-leg squat task in the MaxTraq 2.0 software. *Consent was obtained to use video and images in presentations and publications.
At discharge, Zoom and REDCap links were sent to families and the virtual biomechanical assessment was repeated. Families also received a prepaid USPS shipping label for easy mailbox drop off to return the Actigraph device to the study team. Lastly, the exit interview was administered and audio recorded via the secure Zoom platform.
Prior to transition to virtual assessment and treatment delivery, the majority of PROs, including batteries assessed at baseline, discharge, and follow-up, and daily assessments were already being delivered online via REDcap, which facilitated for a straightforward transition during the virtual format. There were only two PROs typically presented on paper to the youth and caregiver during weekly treatment sessions: a weekly healthcare cost survey completed by caregivers when they arrived at in-person treatment sessions each week, and a treatment expectancies survey which was typically presented to youth and caregivers on paper at the end of their first session. Both were modified to be deployed with a REDcap email survey sent at the time of their remote session, with the clinicians verbally prompting the family to complete the survey while in the session. The COVID-19 Exposure and Family Impact Survey (CEFIS[27]) was added as an additional measure to assess the impact of the pandemic on families, and was completed by a caregiver within the baseline battery.
Remote Intervention.
The intervention format and content remained the same in virtual delivery, with sessions were conducted via Zoom videoconferencing. Unique to the GET Living intervention is the focus on activity exposures. As a result of the virtual format, many exposures were able to be truly in-vivo as youth were completing exposures in their home (versus a rehabilitation gym), allowing for guided practice of activities within their daily environment (e.g., completing chores, riding bike, walking a dog, Figure 4). In the MPM arm, the psychology and physical therapy (PT) sessions also transitioned to remote delivery. For PT, traditional assessments and in-person hands-on physical examination, such as manual muscle testing and goniometric measurements, cannot be conducted virtually. Instead, completing standard functional tasks such as squatting, single-limb balance, heel and toe walking, and transfers (sit to stand or floor to stand) are employed to study strength, balance, coordination, posture, and endurance.
Figure 4.

Patient engaging in an activity exposure during a virtual treatment session
During in person treatment, we provide the youth with a treatment binder containing hardcopies of all worksheets prior to the first session. For remote treatment, all session worksheets were emailed to families prior to treatment. Additionally, Zoom session links became a core component of treatment materials to distribute to youths. A unique meeting link for each of the twelve sessions is created by the research team and distributed to the clinician team (via shared Outlook calendars) and families (via email). Consistent with in- person treatment, all sessions are recorded to assess treatment fidelity (Figure 5).
Figure 5.

Pain psychologist and physical therapist co-leading a virtual exposure treatment session
Acceptability and COVID Impact Measures
Exit Interview.
We continued to conduct the post-treatment exit interview during the discharge visit. No questions were intentionally modified according to rationale to maintain consistency across the trial, as well as to keep the focus on the content of the interventions. However, most families openly discussed the virtual aspects of their experience in response to free response questions. Questions included: “What did you like best about the treatment?”, “What would you change?”, “What will help you to stay motivated to continue to work on the things you learned in treatment?”, and “What advice would you give to a new patient just starting the treatment program?”.
COVID-19 Exposure and Family Impact Survey (CEFIS)[27].
The CEFIS assessed the impact of COVID-19 in the community such as ‘stay at home’ orders, school closures, and health and financial impacts. There are 3 parts of the survey, assessing both exposure and impact. Part 1 of the survey consists of 25 items rated yes/no (no = 0; yes = 1) measuring the family’s “exposure” to COVID-19 related events. Items are summed to create an Exposure score, ranging from 0–25, with higher scores indicating greater exposure. Part 2 assesses the impact of COVID-19 via eight items on a four-point Likert scale. The eight items are summed to create an impact score, ranging from 0–40, with higher scores indicating greater negative impact. Impact is also assessed via 2 items that use a 0–10 Likert scale where parents rate the overall distress they and their child has experienced due to COVID-19, with 0 = No Distress and 10 = Extreme Distress, with scores ranging from 0–20. Part 3 of the survey is one open-ended question that asks, “Please tell us about other effects of COVID-19 on your child/ren and your family, both negative and/or positive.” Initial validation data for the CEFIS subscales demonstrated good-excellent reliability (Exposure, α = .80; Impact, α = .92)[28]. Internal consistencies in the current sample were α = .62 for the Exposure scale and α = .81 for Impact scale.
Results
Participants
A total of 68 youth (M=14.2 years; 80.9% female) were enrolled in the RCT (n=31 in-person, n=5 hybrid, n=32 virtual, n = 9 withdrew). Participant demographics are provided in Table 1. Of note, hybrid participants were those who were actively receiving the intervention but were then transitioned to virtual care during the beginning of the pandemic. Median distance from clinic for in-person treatment was 43.81 miles, while it was 72.41 for hybrid/virtual. As of March 2021, in-person enrollment resumed, although the PPMC reopened with heavily reduced capacity which limited the number of patients able to receive in-person treatment. Moreover, due to continued interest from families living in distant, under resourced areas, it was decided to maintain virtual enrollment as an option to families.
Table 1.
Patient demographics and treatment characteristics (n=68)
| Variable | Range | Mean (SD) | Frequency % (n) |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 8–18 | 14.18 (2.6) | |
| Female | 80.9 (55) | ||
| Ethnicity | |||
| Caucasian | 76.4 (52) | ||
| Hispanic or Latino | 22.1(15) | ||
| Other | 1.5 (1) | ||
| Parent Marital Status | |||
| Married | 77.6 (52) | ||
| Single | 3.0 (2) | ||
| Divorced/Separated | 16.4 (11) | ||
| Treatment Characteristics | |||
| Duration of baseline (days) | 7–24 | 14.66 (4.3) | |
| Number of treatment sessions | 6–12 | 11.8 (0.5) | |
| Number of treatment days | 43–143 | 74.6 |
Outcomes and Lessons Learned from Remote Transition
Below we outline the lessons learned and insights from changes made to recruitment and eligibility, data collection, and intervention delivery. Table 2 summarizes lessons learned from the transition.
Table 2.
Summary of key modifications and insights derived from the transition of an interdisciplinary behavioral health clinical trial to virtual delivery during COVID-19.
| Process | Specific modification | Lessons Learned |
|---|---|---|
| Recruitment and Eligibility | Outpatient services were transitioned to telemedicine visits, allowing for maintenance of recruitment pool | •Outpatient clinics should be ready and willing to deploy telehealth to allow for ongoing patient visits |
| Research coordinator recruits virtually through outpatient telehealth Zoom conference visit | •Adjust virtual workflow to be similar to the pre-existing workflow to allow for easier transition to remote recruitment, and prioritize “face-to-face” interaction with patient families for recruitment as usual | |
| Participants living far are now eligible for virtual treatment | •Any patient within-state is eligible to participate in virtual care, and may recruit from other clinics •Patients traveling to temporarily stay out-of-state are unable to enroll due to physical therapy licensing restrictions across state lines |
|
| Additional screening step added to assess factors relating to participation in virtual care | •To ensure all things be considered to screen for safety of home environment, patient access to equipment and materials, and internet connectivity •To ensure that an adult is present in the location where the patient is being seen and contact information is verified in case of an emergency |
|
| Consent | Research coordinator conducts the consent process virtually via Zoom, utilizing REDCap eConsent/eAssent framework | •The REDCap eConsent/eAssent framework is a simple and secure way to consent virtually •Research coordinators can review the consent document while participants follow along to electronically sign the corresponding sections using a smartphone |
| A telehealth liability form is included for participants to be informed of and agree to receiving remote care | •This form should be IRB-approved and integrated into the e-consent process to sign along with the main consent | |
| Data Collection | Wearable device (Actigraph) is initialized by the research team during remote baseline, then is mailed to the participant | •Shipping costs should be considered when mailing wearable devices to participants •Additional costs may incur in cases where devices break or are lost (from participant use, or while in transit) and requires the research team to re-initialize and ship new devices |
| Adapt biomechanical assessment protocol to collect data virtually | •Select certain tests that are safe and feasible to utilize videoconferencing capabilities for accurate assessment •Conducting remote assessments require a consistent protocol to account for differences in environment, technological familiarity, and ease of instruction with participants at home |
|
| Self-report outcomes adjusted to be entirely delivered online via REDCap | •Without in-person opportunities for oversight on survey completion and sending surveys all via email/text, closer tracking of survey adherence may be beneficial | |
| Include a measure to assess the impact of COVID-19 on families and outcomes | •The COVID-19 Exposure and Family Impact Survey (CEFIS) was included in the parent baseline survey battery; researchers may consider collecting this measure at multiple points throughout a trial | |
| Intervention | Intervention transitioned to take place via Zoom videoconferencing | •Despite variability in reliable internet connection as well as equipment and materials, providers were flexible and adapted to work with each participant and their family •Without access to hands-on examinations, physical therapy needs were assessed functionally. Physical therapy is different to deliver with limited access to equipment and space; therapists must ensure a safe exercise environment as well as other unforeseen issues when treating remotely •Certain activities and exercises may be enhanced as participants can complete these in a natural home environment |
Remote Recruitment and Eligibility.
The rapid transition to remote delivery of all services at the PPMC allowed for the maintenance of the study recruitment pool. Our research team worked closely with clinic providers to carefully consider the best virtual recruitment procedure taking into consideration prior workflow, what aspects of in-person strategies could be deployed for successful recruitment, and potential patient factors such as fatigue and attention in the virtual context that would hinder same-day recruitment. Research coordinators joining the Zoom call at the end of each initial evaluation visit helped to establish a comparable level of personal contact between the research team and youth and their families that is typically present during an in-person evaluation.
In terms of recruitment rates, prior to the pandemic 29.8% of potentially eligible patients were referred to the trial (n = 61 referred), with 44.3% meeting eligibility criteria and enrolled (n = 27 enrolled). During the pandemic, 31.5% of potentially eligible patients were referred (n = 59 referred), with 70.7% meeting eligibility criteria and enrolled (n = 42 enrolled). Further, chi-squared analyses demonstrated increased rates of enrollment in patients referred to GET Living during virtual treatment delivery compared to in-person treatment delivery, X2(1, 119) = 8.89, p = .003. These numbers might reflect increased accessibility due to virtual participation. Of note, our team was able to add two treatment providers (one physical therapist and one psychology provider) in September 2020, allowing us to increase approximately two additional treatment slots per week, which contributed to an overall increase in enrollment. See Table 3 for data regarding number of patients screened, excluded, and enrolled, as well as reasons for withdrawal across treatment contexts.
Table 3.
In-person vs virtual recruitment, enrollment, and trial participation
| Total N | % | n withdrawn | Reasons for Withdrawal | |
|---|---|---|---|---|
| In-Person (Jan 2018-March 2020) | ||||
| Potentially Eligible | 205 | |||
| Referred | 61 | 29.8 | ||
| Enrolled | 27 | 44.3 | ||
| Withdrew | 4 | 14.8 | 1 | Psychiatric acuity (possible psychosis) |
| 1 | Family decision to withdraw | |||
| 1 | Psychiatric acuity (child highly anxious, family decided to withdraw) | |||
| 1 | Psychiatric acuity (treatment team recommended withdraw) | |||
|
| ||||
| Virtual/Dual (March 2020-July 2021) | ||||
| Potentially Eligibile | 184 | |||
| Referred | 58 | 31.5 | ||
| Enrolled | 41 | 70.7 | ||
| Virtual | 32 | |||
| In-Person | 8 | |||
| Hybrid | 1 | |||
| Withdrew | 5 | 12.2 | 2 | Initially in-person; withdrew because did not want virtual treatment |
| 1 | Virtually enrolled; family moved out of state during pandemic | |||
| 1 | High medical/psychiatric acuity (treatment team recommended withdraw) | |||
| 1 | Family was unable to commit to treatment schedule due to increased extracurricular demands | |||
Note: Adaptation to virtual format occurred in March 2020. Items highlighted in blue indicate COVID-19 related reasons for withdrawal. % referred = eligible patients (screened, not excluded) referred for trial; % enrolled = patients referred and enrolled; % withdrawn = youth withdrawn out of total enrolled. Two enrolled youth for in-person treatment immediately withdrew during the initial transition to the virtual format, as both did not want virtual treatment. Four in-person enrolled youth decided to continue with virtual treatment during the initial transition (these youth were considered “hybrid” because they received both in-person and virtual components). The “dual” phase occurred March-July 2021; both in-person and virtual options were available and individual youth remained in the chosen format. One youth, however, in the dual phase received a combination of formats in order to work with the family’s availability.
Regarding dropout rates, during the initial pivot to virtual care the research team reached out to all youth already in active treatment to discuss the option to continue treatment via remote means. Two youth decided not to continue with virtual treatment (due to wanting in-person treatment, or from overwhelming stress of the pandemic) and withdrew immediately. Four youth continued with virtual treatment (“hybrid participants”). Dropout rates from the overall in-person phase was 14.8%, dropout rates during the virtual format was 12.2% (see Table 3). One unexpected consequence of statewide Shelter-in-Place orders was that referred patients moved out-of-state temporarily to stay with other family members. While there was a national waiver of state licensing restrictions for psychologists, it was unclear for physical therapy providers, meaning that we were unable to deliver the intervention to them.
Remote Data Collection.
The REDCap e-consent process was quick to deploy as the eConsent framework was already an option available on the platform. Overall, families found it easy to use and understand once given a thorough explanation of the process. To support the ease of use, the research team displayed the consent and assent forms to the families via screenshare on Zoom while leading the consent process, as typically done when consenting in-person. Simultaneously the youth and caregiver opened the REDCap e-consent form on their personal smartphone device to complete the electronic signatures.
While shipping the Actigraph to families was a successful way to sustain the collection of daily activity and sleep data, several lessons were learned. First, one watch arrived damaged to a participant family, thus we started shipping in well-padded envelopes. Second, we needed to account for additional costs incurred by events like devices not being returned or devices requiring recalibration. Previously, when youth were regularly visiting the clinic twice weekly for the intervention, the clinician was able to re-initialize the device or address any additional issues without delay and return it to the individual within the same session. In the virtual format, the additional time it takes to ship the device between participant family and research team can be several days, which resulted in data loss. Particularly, in unforeseen circumstances such as a dead battery, broken strap, or a misplaced watch, there was no way to anticipate earlier shipping for replacements. Actigraph issues arose frequently for both formats; among in-person youth, five needed replacements during treatment, and eight among virtual youth needed replacements. Especially working with pediatric populations, watches are often broken and misplaced, making this an important consideration when utilizing this form of data collection particularly in remote procedures.
The remote biomechanical assessment required creativity and flexibility from the research team and participants. Youth had to set up for assessments precisely (e.g., download and use apps, measure exact walk length), requiring specific and clear verbal instructions from the research team. We found it important to clearly describe the equipment necessary (e.g., tape measure, paper, phone) to set up for each task to participants prior to the assessment video call. However, a few participant families did not own a tape measure, and thus the 6-minute-walk test needed to be omitted as accurate data assessment would not have been possible. The other tasks were still feasible without requiring the additional tool. Researchers implementing this type of remote data collection should also consider variables such as camera placement (i.e., camera should be completely stable throughout the test to ensure that the scale utilized does not change during one session), utilizing scale references for accurate measurement, and reliable internet connection, which are necessary to maintain a stable data collection frame rate and minimize any potential data loss.
To ensure adherence to PRO’s, closer tracking efforts and oversight from the research team have been required. Without in-person opportunities during treatment sessions to verify surveys are completed, remote research required consistent reminders to encourage youth and caregivers to complete the surveys on their own. With constant tracking and reminders, patients who fully completed in-person treatment (n = 24) completed on average 72.78% of their daily surveys. Participants who have completed virtual treatment (both hybrid and fully virtual, n = 35) completed 73.3% of their daily surveys.
Remote Intervention.
Overall, the virtual intervention was successful. There was variability in the ability to deliver simultaneous child and parent sessions when there was a lack of multiple devices (e.g., for sessions where caregivers needed to be one-on-one with psychology while the youth was with physical therapist) and/or poor (or no) Wi-Fi connectivity. Additional research team support was also critical to successfully implement remote clinical intervention. For example, in the early stages of virtual delivery, youth often forgot to join virtual sessions without the physical prompt of commuting to the clinic, and rescheduling missed sessions would impact the length of treatment, taking up additional treatment slots, and subsequently the ability to enroll new patients. Thus, we implemented text and email reminders for each family on the day of treatment sessions, which improved attendance to virtual sessions. Regardless of treatment arm, interventions were designed to be completed across 6 weeks, or roughly a duration of 42 days. Missed and rescheduled appointments during virtual delivery prolonged the duration of active treatment. To demonstrate the quantity of missed and rescheduled sessions, youth who completed in-person treatment spent on average 43.1 days in active treatment, whereas those in virtual treatment spent an average of 47.2 days in active treatment (or approximately a week longer, equaling two rescheduled appointments). Researchers should consider factors such as these that impact and challenge adherence with virtual participation when conducting remote research and interventions. Solutions to automate as much of the process as possible to reduce the amount of burden falling on the research team in virtual study design are a valuable resource.
During the intervention, the team experienced a multitude of external stimuli present in the home environments of participants (e.g., noise from family members, clutter in homes) that produced distractions for youth. Additionally, we needed to modify treatment based on unforeseen events such as variability in the weather (e.g., summer temperatures exceeding 100 degrees F) given that not all families live in a climate-controlled home. For instance, physical activities in both treatment conditions had to be modified occasionally due to heat levels and lack of air conditioning within the home. Also, completion of in-person exposures occasionally had to flex around availability of space when other family members were also working and doing school remote. When possible and appropriate, family members were engaged in exposure sessions (e.g., kicking a ball back and forth, joining for a bike ride, providing materials for the completion of chores).
Specifically, for telehealth physical therapy there was a limited access to cardiovascular and strength training equipment. For instance, we adapted household items to serve as weights (e.g., canned goods, gallon of milk), incorporated body-weight training, and the use of towels to assist with stretching. There was also more reliance on verbal instructions, cues, and feedback on what a youth ‘should’ be feeling since the therapist is not physically able to make necessary corrections to form.
Exit Interview.
The exit interview provided a qualitative examination of the family experience to better understand the feasibility and acceptability of the virtual assessment and intervention procedures. The exit interview was originally structured to primarily focus on the content of the in-person intervention, however several themes derived from the exit interviews from youth within the virtual treatment format that addressed aspects of the remote participation experience. These themes are summarized below:
Benefits of pain psychology.
During in-person delivery, youth and caregivers often noted that their favorite part of treatment was physical therapy. Contrarily, in virtual delivery youth and caregivers have generally preferred the psychology portions of their treatment, expressing that they felt physical therapy was limited and not as helpful. Those families who preferred the psychology portion shared that learning about and better understanding their chronic pain (pain science education) was the most beneficial aspect of the treatment. In the context of the pandemic, those receiving the virtual intervention during the pandemic stated that they enjoyed pain psychology because it gave them the opportunity to speak to someone outside of their family during Shelter-In-Place orders.
Motivation to get moving.
Youth enrolled in GET Living during the COVID-19 pandemic (both virtual and in-person) reported that the intervention was helpful to motivate them “to get up and move”, which was an important overall goal of the interventions. Families specifically mentioned the treatment sessions encouraged adolescents to socialize, increased motivation, added structure throughout their weeks, and supported increased confidence carrying out daily activities from the convenience and familiarity of their homes. Youth and parents shared that this motivation was especially valuable when there were even more barriers to movement and social engagement within the context of COVID-19.
Pros and cons of virtual format.
During the in-person delivery format, families often shared that the one thing they would change about the treatment would be the long commute. Some youth mentioned that the long drive would often cause them additional pain from sitting in the car for an extended period of time. In the virtual format, families mentioned that due to living far from the clinic they would not have been able to have the opportunity participate if it were still in-person. Although, while most caregivers acknowledged that the virtual intervention increased accessibility, some still expressed that they would have preferred the opportunity to receive in-person treatment. A few families even expressed that, regardless of distance, the long drive might have been worth it due to the perspective that receiving in-person physical therapy would have improved their overall experience. Youth also often expressed the burden of the sheer number of various online surveys they needed to complete as well as Zoom fatigue, given that all aspects of the virtual trial were either through online surveys or Zoom.
COVID-19 Impact.
To understand the context of the remote transition for our trial, we also examined the CEFIS. Parents who completed the CEFIS (n = 51) had a mean score of 7.84 (SD = 2.75; range 1–16) on the Exposure scale, with a mean on the 2-item distress scale of 5.74 (SD = 2.34; range 1.00–10.0). While there are no published clinical interpretations of these subscales, our results were similar to those published in the measure validation paper (Exposure, M = 8.71; Distress, M = 5.95)[28]. Further, with regard to the Impact subscale, Kazak et al., (2021) suggest that a score of ≤2.5 indicated a positive valence, while a score of >2.5 indicating negative impact. The mean of the 10-item Impact Score for our sample was 2.31 (SD = 0.75; range 0.00–3.40). There was no significant association between the Exposure and Impact scales (r = 0.152, p = 0.29) or Exposure and Distress scales (r =0.25, p = 0.07, however there was a significant association between the Impact and Distress scale (r = 0.57, p < 0.001). See Supplemental Table 1 for frequencies for the Exposure scale items. For the open-ended question that asks parents to expand on the impact of COVID-19, several topics were raised including negative impacts to social life with friends and family, negative impact on mental health, difficulties with school, and disruptions to routines. Responses are summarized in Supplemental Table 2.
Discussion
With a rapid pivot to virtual assessment and treatment, we provided remotely delivered RCT examining multi-disciplinary pain management treatment and graded exposure treatment , as well as a remotely delivered biomechanical assessment for pediatric chronic pain. Here we provide proof-of-concept data regarding the feasibility and acceptability of these new approaches. Families in our trial reported several benefits of virtual treatment, including reduced burden of a lengthy commute and expanded access to care. Telehealth also provided a unique opportunity to conduct behavioral exposures and home-based exercises in the natural context of the patients own home, which was more reflective of the youths’ day-to-day lives. Qualitative data from exit interviews provided insight into a potential preference for cognitive-behavioral pain psychology via telehealth compared to physical therapy, which may be due to the limitations imposed on PT in the virtual space. However, despite limitations of virtual PT and the lack of in-person direction and access to equipment, youth still expressed benefit in the form of motivation to move, an important goal for pediatric pain. Best telehealth strategies for specific interventions such as physical therapy are still unclear, and growing implications for pediatric pain researchers and therapists include understanding family and child factors, such as age group, level of risk/need, and personal preferences, as well as logistical factors, such as access to technology and equipment[30].Quantitative data suggest an enhanced referral and enrollment rate compared to in-person treatment delivery, likely due to increased accessibility for those living at distance from the clinic, as well as a reduction in burden to commute to the clinic for several weeks of treatment. Additionally, we found equivalent rates of completion for daily assessment in virtual care compared to in-person. Overall, our data demonstrate promising indications of the acceptability, feasibility, and patient engagement to virtual pediatric pain treatment.
The COVID-19 pandemic presented additional challenges to our participants. The results from the CEFIS revealed that certain consequences of the pandemic were widely experienced by youth and their families, such as schools closing and missing an important family event. Open-ended responses from the CEFIS provided insight into the areas of daily life most impacted by the pandemic, with commonly endorsed themes including negative impacts to social life with friends and family, negative impact on mental health, difficulties with school, and disruptions to routines. Unfortunately, pediatric pain patients likely experienced disproportionately greater impacts from the pandemic because of the drastic changes to factors impacting functioning, which many of our participants expressed[31]. According to qualitative exit interviews, our participants did benefit from having access to support from providers through virtual treatment during stay-at-home orders. Beyond the impact of the COVID-19 pandemic, virtual treatment for vulnerable pediatric pain patients may be a valuable resource to support adherence and self-management when functioning is impacted by a variety of circumstances, such as medical trauma and adjustment to serious illness [5].
Remote data collection for the clinical trial was demonstrated to be feasible. We administered complex digital biomechanical assessments through the use of innovative technologies, such as wearable devices, advanced video conferencing capabilities, and analytical programs for video data processing. This effort is consistent with the recent focus on the development of technologies and methodologies to remotely collect a variety of physiological and behavioral data for clinical trials, previously only possible through in-person visits[32]. These mobile health tools offer the ability to monitor physiological and behavioral data in naturalistic settings and can be applied in clinical research to facilitate the improvement of treatment outcomes for pediatric pain patients. For instance, video analysis software such as OpenPose[33] utilizes deep learning algorithms to estimate joint positions from video collected from mobile phones. We hope to further implement this proof-of-concept method of remote biomechanical assessments within the context of digitally delivered interdisciplinary pain rehabilitation to promote adaptive outcomes.
Our team returned to in-person data collection and treatment delivery in March 2021, with the option for virtual care still available to patients and families. Although, there has been more variation in this trial than originally intended due to the unexpected pandemic (ie. format of intervention, pandemic related stressors, assessments and data collection), one unique aspect of our study design is the use of a single-case experimental phase design (SCED) with multiple measures. The SCED methodology will allow for examination of outcomes within each type of treatment delivery (i.e., in-person and virtual) pertaining to individual responses for when and what changes occurred during the course of treatment. This data will supplement outcomes obtained from the larger investigation of group level differences regarding treatment effects.
Limitations
Several limitations to the current study exist. First, optimization of evaluating the transition from in-person to virtual was not possible given the rapid change of intervention delivery. Ideally, additional iterative development processes could have been possible based on the initial feedback regarding acceptability and feasibility of virtual delivery we received from patients and their families. Additionally, had this transition to virtual care been planned, it may have been possible to trial a true hybrid model, such as offering patients in-person PT sessions and virtual pain psychology. Further examination of preferences, as well as barriers, for virtual versus in-person care could help shape continued virtual treatment delivery beyond the COVID-19 pandemic. Another limitation was variability in access to treatment materials across virtual patients. While this is mostly relevant for the PT sessions, there was also significant variability in patients who printed the pain psychology materials that were electronically sent to families and those who did not print any materials. A benefit to in-person care is that the clinician provides patients with all necessary worksheets and materials, allowing the patient to write and reflect directly on those materials, as well as have the option to reference them later if preferred. It is possible that the saliency of these materials was less for patients who passively viewed them via Zoom compared to those who printed and engaged with hard copies of materials.
Future Directions
While there have been pre-pandemic efforts to encourage the virtual delivery of care for pain management, the pandemic created a push for health care systems to develop strategies to integrate remote care into practice. Digital delivery of pain management treatments offers promise for expanding access to treatment beyond the context of a global pandemic. Prior to COVID-19, considerable research was being conducted to develop digital solutions and assess the feasibility and effectiveness of interventions for youth with chronic pain delivered via the Internet and smartphone/mobile technologies[34–38]. Feasibility and effectiveness of these remotely delivered health interventions for youth with chronic pain has been demonstrated via clinical trials, with effectiveness demonstrated in real-world settings as well[39]. Yet, there are still many future directions to be explored within the context of digital behavioral healthcare delivery. These include advancing the current evidence base, examining the efficacy of translating empirically supported in-person interventions into the digital sphere, and reimagining the delivery of behavioral health interventions by pulling inspiration from advancements in digital health technology. Future trials should also examine patient characteristics and factors predicting when and for whom to best utilize virtual interventions in order to alleviate disparities and prioritize resources, while still providing patient-centered care. Likewise, research will be needed to determine which treatments can be more feasibly and effectively delivered virtually compared to other therapies. For instance, through the transition of physical therapy to virtual delivery during the pandemic, we have gained insight via acceptability and exit interview data supporting the value of prioritizing in-person physical therapy for pain patients possibly combined with virtual pain psychology, in order to reduce the burden. Furthermore, in order to successfully evaluate the efficacy of virtual care, continued research efforts are necessary to develop and determine precise ways to measure patient outcomes through remote means. These measurements have the potential to also guide the integrated use of remote monitoring devices and mobile applications to supplement tailored and precise interventions for pediatric pain care.
In summary, the pivot from in-person RCT examining graded exposure treatment delivered by an interdisciplinary team to virtual data collection and treatment delivery was found to be promising. Moreover, we provided proof-of-concept methods for remote biomechanical assessment and in-vivo exposures in youth with chronic pain. While this paper highlights a transition propelled by the global pandemic, the recommendations for transition to and implementation of remotely delivered pain management apply broadly to digital and remotely delivered pain management interventions.
Supplementary Material
Acknowledgements:
We want to thank the additional clinicians involved in delivering GET Living (Hannah Toyama, Meaghan O’Day) and additional research coordinators in supporting the study implementation (Marissa Heirich, Gillian Rush).
Disclosures: This investigation was supported by NIAMS/R21 AR072921 awarded to LES. There are no conflicts of interest to report.
Disclosure of funding: This clinical trial is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases
References
- 1.Fisher E, Law E, Dudeney J, Eccleston C and Palermo TM. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2019;4:CD011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamse C, Dekker-Van Weering MG, van Etten-Jamaludin FS and Stuiver MM. The effectiveness of exercise-based telemedicine on pain, physical activity and quality of life in the treatment of chronic pain: A systematic review. Journal of telemedicine and telecare 2018;24:511–526. [DOI] [PubMed] [Google Scholar]
- 3.Eccleston C, Blyth FM, Dear BF, Fisher EA, Keefe FJ, Lynch ME, Palermo TM, Reid MC and de C Williams AC. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain 2020;161:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padala PR, Jendro AM and Padala KP. Conducting clinical research during the COVID-19 Pandemic: Investigator and participant perspectives. JMIR Public Health and Surveillance 2020;6:e18887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiles-Shields C, Plevinsky JM, Psihogios AM and Holmbeck GN. Considerations and future directions for conducting clinical research with pediatric populations during the COVID-19 pandemic. Journal of Pediatric Psychology 2020;45:720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons LE, Harrison LE, O’Brien SF, Heirich MS, Loecher N, Boothroyd DB, Vlaeyen JW, Wicksell RK, Schofield D and Hood KK. Graded exposure treatment for adolescents with chronic pain (GET Living): Protocol for a randomized controlled trial enhanced with single case experimental design. Contemporary clinical trials communications 2019;16:100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krane E Telemedicine implementation during COVID-19: Experience and recommendations [Web Page]. Available at https://csahq.org/news/blog/detail/csa-online-first/2020/04/16/telemedicine-implementation-during-covid-19-experience-and-recommendations. 2020.
- 8.Simons LE, Sieberg CB, Carpino E, Logan D and Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. The Journal of Pain 2011;12:677–686. [DOI] [PubMed] [Google Scholar]
- 9.Simons LE, Pielech M, McAvoy S, Conroy C, Hogan M, Verbunt JA and Goossens ME. Photographs of Daily Activities–Youth English: validating a targeted assessment of worry and anticipated pain. Pain 2017;158:912–921. [DOI] [PubMed] [Google Scholar]
- 10.Walker LS and Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of pediatric psychology 1991;16:39–58. [DOI] [PubMed] [Google Scholar]
- 11.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L and Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain 2003;104:639–646. [DOI] [PubMed] [Google Scholar]
- 12.Goubert L, Eccleston C, Vervoort T, Jordan A and Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain 2006;123:254–263. [DOI] [PubMed] [Google Scholar]
- 13.Gauntlett-Gilbert J, Alamire B and Duggan GB. Pain Acceptance in Adolescents: Development of a Short Form of the CPAQ-A. J Pediatr Psychol 2019;44:453–462. [DOI] [PubMed] [Google Scholar]
- 14.Smith AM, Sieberg CB, Odell S, Randall E and Simons LE. Living Life With My Child’s Pain: The Parent Pain Acceptance Questionnaire (PPAQ). Clin J Pain 2015;31:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LS, Levy RL and Whitehead WE. Validation of a measure of protective parent responses to children’s pain. Clin J Pain 2006;22:712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson AC and Palermo TM. Physical activity and function in adolescents with chronic pain: a controlled study using actigraphy. The Journal of Pain 2012;13:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goossens ME, Rutten-van Mölken MP, Vlaeyen JW and van der Linden SM. The cost diary: a method to measure direct and indirect costs in cost-effectiveness research. Journal of clinical epidemiology 2000;53:688–695. [DOI] [PubMed] [Google Scholar]
- 18.Lelieveld OT, Takken T, van der Net J and van Weert E. Validity of the 6-minute walking test in juvenile idiopathic arthritis. Arthritis and rheumatism 2005;53:304–307. [DOI] [PubMed] [Google Scholar]
- 19.Nixon PA, Joswiak ML and Fricker FJ. A six-minute walk test for assessing exercise tolerance in severely ill children. The Journal of pediatrics 1996;129:362–366. [DOI] [PubMed] [Google Scholar]
- 20.Myer GD, Kushner AM, Brent JL, Schoenfeld BJ, Hugentobler J, Lloyd RS, Vermeil A, Chu DA, Harbin J and McGill SM. The back squat: A proposed assessment of functional deficits and technical factors that limit performance. Strength and conditioning journal 2014;36:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourdier P, Birat A, Rochette E, Doré E, Courteix D, Dutheil F, Pereira B, Ratel S, Merlin E and Duche P. Muscle function and architecture in children with juvenile idiopathic arthritis. Acta Paediatrica 2020. [DOI] [PubMed] [Google Scholar]
- 22.Ford KR, Myer GD, Melson PG, Darnell SC, Brunner HI and Hewett TE. Land-jump performance in patients with juvenile idiopathic arthritis (JIA): a comparison to matched controls. International journal of rheumatology 2009;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sil S, Thomas S, DiCesare C, Strotman D, Ting TV, Myer G and Kashikar-Zuck S. Preliminary evidence of altered biomechanics in adolescents with juvenile fibromyalgia. Arthritis care & research 2015;67:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schurr SA, Marshall AN, Resch JE and Saliba SA. Two-dimensional video analysis is comparable to 3D motion capture in lower extremity movement assessment. International journal of sports physical therapy 2017;12:163. [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Turner SP, Sholle ET, Brown SW, Blau VL, Brouwer JP, Lewis AN, Cole CL, Nanus DM and Shah MA. Evaluation of a REDCap-based Workflow for Supporting Federal Guidance for Electronic Informed Consent. AMIA Summits on Translational Science Proceedings 2019;2019:163. [PMC free article] [PubMed] [Google Scholar]
- 26.Gulmans V, Van Veldhoven N, De Meer K and Helders P. The six-minute walking test in children with cystic fibrosis: reliability and validity. Pediatric pulmonology 1996;22:85–89. [DOI] [PubMed] [Google Scholar]
- 27.Kazak A, Canter K, Phan-Vo T, McDonnell G, Hildenbrand A, Alderfer M and Deatrick J COVID-19 Exposure and Family Impact Survey (CEFIS). 2020.
- 28.Kazak AE, Alderfer M, Enlow PT, Lewis AM, Vega G, Barakat L, Kassam-Adams N, Pai A, Canter KS, Hildenbrand AK, McDonnell GA, Price J, Schultz C, Sood E and Phan TL. COVID-19 Exposure and Family Impact Scales: Factor Structure and Initial Psychometrics. J Pediatr Psychol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calouro C, Kwong MW and Gutierrez M. An analysis of state telehealth laws and regulations for occupational therapy and physical therapy. International Journal of Telerehabilitation 2014;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camden C and Silva M. Pediatric teleheath: opportunities created by the COVID-19 and suggestions to sustain its use to support families of children with disabilities. Physical & Occupational Therapy In Pediatrics 2021;41:1–17. [DOI] [PubMed] [Google Scholar]
- 31.Plevinsky JM, Young MA, Carmody JK, Durkin LK, Gamwell KL, Klages KL, Ghosh S and Hommel KA. The Impact of COVID-19 on Pediatric Adherence and Self-Management. Journal of pediatric psychology 2020;45:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izmailova ES, Ellis R and Benko C. Remote monitoring in clinical trials during the covid-19 pandemic. Clinical and Translational Science 2020;13:838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rammohan S, Kidzinski Ł and Delp S. MobileClinic: An end-to-end software architecture for analyzing human movement on a mobile device. 2019.
- 34.Palermo TM, Law EF, Fales J, Bromberg MH, Jessen-Fiddick T and Tai G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. Pain 2016;157:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson PA, Harrison LE, Heathcote LC, Rush G, Shear D, Lalloo C, Hood K, Wicksell RK, Stinson J and Simons LE. mHealth for pediatric chronic pain: state of the art and future directions. Expert Review of Neurotherapeutics 2020;20:1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stinson JN, Jibb LA, Nguyen C, Nathan PC, Maloney AM, Dupuis LL, Gerstle JT, Alman B, Hopyan S and Strahlendorf C. Development and testing of a multidimensional iPhone pain assessment application for adolescents with cancer. Journal of medical Internet research 2013;15:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinson JN, Lalloo C, Harris L, Isaac L, Campbell F, Brown S, Ruskin D, Gordon A, Galonski M and Pink LR. iCanCope with Pain™: User-centred design of a web-and mobile-based self-management program for youth with chronic pain based on identified health care needs. Pain Research and Management 2014;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zetterqvist V, Gentili C, Rickardsson J, Sörensen I and Wicksell RK. Internet-Delivered Acceptance and Commitment Therapy for Adolescents with Chronic Pain and Their Parents: A Nonrandomized Pilot Trial. Journal of Pediatric Psychology 2020;45:990–1004. [DOI] [PubMed] [Google Scholar]
- 39.Palermo TM, de la Vega R, Murray C, Law E and Zhou C. A digital health psychological intervention (WebMAP Mobile) for children and adolescents with chronic pain: results of a hybrid effectiveness-implementation stepped-wedge cluster randomized trial. Pain 2020;161:2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


