Abstract
This paper reports on the effects of both reducing and nonreducing transgalactooligosaccharides (TOS) comprising 2 to 8 residues on the growth of Bifidobacterium adolescentis DSM 20083 and on the production of a novel β-galactosidase (β-Gal II). In cells grown on TOS, in addition to the lactose-degrading β-Gal (β-Gal I), another β-Gal (β-Gal II) was detected and it showed activity towards TOS but not towards lactose. β-Gal II activity was at least 20-fold higher when cells were grown on TOS than when cells were grown on galactose, glucose, and lactose. Subsequently, the enzyme was purified from the cell extract of TOS-grown B. adolescentis by anion-exchange chromatography, adsorption chromatography, and size-exclusion chromatography. β-Gal II has apparent molecular masses of 350 and 89 kDa as judged by size-exclusion chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis, respectively, indicating that the enzyme is active in vivo as a tetramer. β-Gal II had an optimal activity at pH 6 and was not active below pH 5. Its optimum temperature was 35°C. The enzyme showed highest Vmax values towards galactooligosaccharides with a low degree of polymerization. This result is in agreement with the observation that during fermentation of TOS, the di- and trisaccharides were fermented first. β-Gal II was active towards β-galactosyl residues that were 1→4, 1→6, 1→3, and 1↔1 linked, signifying its role in the metabolism of galactooligosaccharides by B. adolescentis.
The human colonic flora has both beneficial and pathogenic potential with respect to host health. There is now much interest in the manipulation of the microbiotic composition of the colon in order to improve the potentially beneficial effects of the microorganisms (10). Probiotics are live microbial supplements which beneficially affect the host by improving its intestinal microbial balance (7, 9) and have been used for many years for this reason. As the viability of live bacteria in food products and during transit through the gastrointestinal tract may be variable, the concept of prebiotics has been developed. A prebiotic is considered to affect the host beneficially by selectively stimulating the growth and/or activity of one or a limited number of naturally present or introduced bacterial species in the colon. It has been claimed that this will also lead to an improvement in host health (11). Increasingly, probiotics and prebiotics are used in combination and are called synbiotics (4, 7, 32). Fructooligosaccharides are considered prebiotics (10), and oral dosages of transgalactooligosaccharides (TOS) appear to result in increased numbers of bifidobacteria in the human fecal flora (2, 12, 13). It is claimed that a high number of bifidobacteria is beneficial for the health of the host. This may prevent colonization by pathogens and may have positive effects on intestinal peristalsis, on the immune system, in cancer prevention, on cholesterol metabolism, and on carbohydrate metabolism in the colon (11, 17).
TOS are oligosaccharides produced by transgalactosylation of lactose using a β-galactosidase (β-Gal). The linkage between the galactose units and the components in the final product depend on the enzymes and the conditions used in the reaction. The production and characterization of these TOS have been described in various publications (19, 21, 25). Different linkages between galactose and the reducing glucose unit have been identified, namely, β-d-Galp-(1→2)-d-Glcp, β-d-Galp-(1→3)-d-Glcp, β-d-Galp-(1→4)-d-Glcp, and β-d-Galp-(1→2)-d-Glcp. Also, branched Glcp residues occur, whereas oligogalactose fragments contain mainly 1→4 or 1→6 linkages (26, 33). Recently, Fransen et al. (8) showed that nonreducing galactooligosaccharides were also formed during transgalactosylation of lactose.
Up to now no information has been available about the contribution of the various oligosaccharides in TOS to growth of Bifidobacterium species, and nothing is known about their effect on the synthesis and activities of enzymes involved in oligosaccharide metabolism. Only artificial substrates, such as para-nitrophenyl β-d-galactoside, have been used to determine β-Gal activities in bifidobacteria (1, 5, 6, 22, 23, 27, 28). However, the use of such substrates does not supply information about the number of enzymes involved and their specificities. Using classical culture methods and also molecular techniques, it was shown that Bifidobacterium adolescentis is a major bifidobacterial species in the adult intestinal microflora (16, 18). In this paper we focus on the fermentation of various oligosaccharides in TOS by B. adolescentis. In addition to a lactose-degrading β-Gal (β-Gal I), a novel β-Gal (β-Gal II) involved in the degradation of TOS was purified and characterized. The role of the enzyme in the metabolism of TOS by B. adolescentis is discussed.
MATERIALS AND METHODS
Substrates.
TOS were obtained by transgalactosylation of lactose with a β-Gal. The TOS mixture (Borculo Whey Products, Borculo, The Netherlands) was partially purified using charcoal chromatography in order to decrease the levels of mono- and disaccharides. The enriched oligosaccharide mixture contained 99% oligomers as well as some residual galactose, glucose, and lactose.
Enriched fractions containing [β-d-Galp-(1→6)]n-d-Glcp and [β-D-Galp-(1→4)]n-d-Glcp were obtained by fractionation of TOS from, respectively, Oligomate-50 (Yakult Pharmaceutical Co. Ltd.) and CUP-oligo (Nissin Sugar, Tokyo, Japan). Enriched fractions containing [β-d-Galp-(1→4)]n-d-Galp (n = 1 to 3) were obtained by incubation of extracted soy arabinogalactan with an endo-galactanase. After partial purification, their structure was confirmed using nuclear magnetic resonance spectroscopy. [β-d-Galp-(1→4)]n-d-Glcp (n = 2 to 3) and β-d-Galp-(1→1)-d-Glcp were purified and characterized as described by Fransen et al. (8). 3′ Fucosyllactose, lacto-N-fucopentaose I, lacto-N-fucopentaose II, and β-d-Galp(1→6)-d-Galp were obtained from Dextra Laboratories Ltd. (Reading, United Kingdom), β-d-Galp(1→3)-Araf was obtained from ICN Biomedicals Inc. (Aurora, Ohio). p-Nitrophenyl (NP)-glycosides were obtained from Sigma (St. Louis, Mo.) or from Koch and Light, Ltd. (Haverhill, United Kingdom). Lactulose was obtained from Solvay (Weesp, The Netherlands). Melibiose was obtained from Jansens Chimica (Beerse, Belgium). Other chemicals were of analytical grade and obtained from commercial sources.
Bacterial strain, culture conditions, and oligosaccharide fermentation.
B. adolescentis DSM 20083 was obtained from the Deutsche Sammlung von Mikroorganismen und Zelkulturen GmbH (Braunschweig, Germany). Cell extracts were prepared from B. adolescentis grown in M17 broth (Oxoid, Hampshire, England) for 48 h at 37°C in an anaerobic chamber with an atmosphere consisting of CO2 (10%), H2 (10%), and N2 (80%). The pH of the medium was adjusted to pH 6.5 with KOH prior to sterilization. Sugars (TOS, melibiose, lactose, galactose, and glucose) (0.5% [wt/vol] sugars in the M17 medium) were added from filter-sterilized stock solutions.
Analysis of TOS.
TOS from Borculo Whey Products, Yakult, and Nissin were fractionated by Bio-Gel P-2 gel size-exclusion chromatography (100 by 2.6 cm with a 200/400 mesh (Bio-Rad) and with the column thermostated at 60°C, using a Pharmacia Hiload system equipped with a Pharmacia P50 pump. A Shodex RI-72 detector was used to monitor the refractive index of the water used as the eluent (0.3 ml/min). The oligosaccharide compositions of various fractions were established using high-performance anion-exchange chromatography (HPAEC). For this purpose a Bio-LC system (Dionex, Sunnyvale, Calif.) that included a quaternary gradient pump, an eluent degas (He) module, and a 4- by 250-mm Carbopac PA100 column with matching guard column (pulsed amperometric detection mode) was used. Samples (20 μl) were injected into the system using a Spectra Physics (San Jose, Calif.) SP8800 autosampler, and chromatograms were recorded using a PC 1000 system. The sodium acetate gradient (1 ml/min) in 100 mM NaOH was for 0 to 30 min with a linear gradient of 0 to 200 mM. The effluent was monitored using a pulsed electrochemical detector detector containing a gold electrode with an Ag-AgCl reference electrode. The column was washed for 5 min with 1 M sodium acetate and equilibrated again for 15 min with 100 mM NaOH before the next run was performed.
Preparation of cell extracts.
B. adolescentis DSM 20083 was grown as described previously, and the cells were harvested by centrifugation (10,000 × g, 10 min, 4°C) upon reaching the stationary phase. Cells were washed once in 20 mM phosphate buffer (pH 6.5) and then resuspended in 10 ml of the same buffer. Cells were disrupted on ice by sonic treatment (15 min; duty cycle, 30%). Subsequently, the suspension was centrifuged at 10,000 × g for 10 min to remove nondisrupted cells and the resulting supernatant was centrifuged at 30,000 × g for 60 min to pellet cell debris. The supernatants (enzyme extracts) were filter sterilized and assayed for enzyme activity. The protein contents of the enzyme extracts were determined using the method of Bradford (3) with bovine serum albumin as a standard.
Enzyme assays.
β-Gal activity was measured by determining the hydrolysis of p-NP-β-d-galactopyranoside (PNPG) at 40°C after 60 min of incubation. The reaction mixture (125 μl) contained 75 μl of 50 mM phosphate buffer (pH 6), 25 μl of 0.1% PNPG solution, and 25 μl of cell extract. The increase in adsorbance (405 nm) was measured. A unit of enzyme activity was defined as 1 μmol of galactose liberated per min in 50 mM phosphate buffer (pH 6) at 40°C. The molar extinction coefficient under these assay conditions was 13,700 M−1 cm−1.
The hydrolytic activities of β-Gal on TOS and the different oligosaccharides and polysaccharides were calculated from the amount of galactose released as determined by HPAEC. The incubation was performed at 40°C for 1 h, and the reaction mixture consisted of 100 μl of 0.1% (wt/vol) substrate in 50 mM phosphate buffer (pH 6).
Production and purification of β-Gal II.
β-Gal II was purified from the crude enzyme extract from B. adolescentis grown on 0.5% (wt/vol) TOS. Unless stated otherwise, all procedures were carried out at room temperature. All buffers contained 0.01% (wt/vol) sodium azide to prevent microbial growth. Collected fractions were screened for protein content (A280 or the Sedmak method) and for β-Gal activity.
The purification steps of the enzyme extract involved Bio-Gel HTP hydroxyapatite (Bio-Rad Laboratories, Richmond, Calif.), Q-Sepharose, Mono Q HR5/5, and Sephacryl S200 HR16/60. The last three columns were from Pharmacia LKB Biotechnology, Uppsala, Sweden.
pH and temperature optimum of β-Gal II at the conditions used.
The optimum temperature of β-Gal II was determined by incubation of the β-Gal with 0.1% (wt/vol) TOS in 50 mM phosphate buffer (pH 7) at 20, 30, 35, 40, 45, 50, 60°C for 1 h. The optimum pH was determined by incubating the β-Gal with 0.1% (wt/vol) TOS in citrate-phosphate buffer in a pH range of 2.5 to 8.0 for 1 h at 40°C.
Kinetic parameters of β-Gal II.
Different substrates [PNPG, lactose, β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Glcp, β-d-Galp-(1→4)-β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Glcp, β-d-Galp-(1→4)-d-Galp, β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Galp, or β-d-Galp-(1→4)-β-d-Galp-(1→4)-β-d-Galp-(1→4)-D-Galp] with concentrations ranging from 0.1 to 30 mM in a 50 mM phosphate buffer (200-μl total volume, pH 6.0) were incubated with β-Gal II (0.025 μg of protein/200 μl) at 40°C for 1 h. The Km and catalytic constant values were calculated from the initial rates of the hydrolyses of oligosaccharides. Data analysis for calculation of kinetic parameters, using nonlinear regression, was performed with the Enzfitter program (Biosoft, Cambridge, United Kingdom).
Gel electrophoresis.
Native electrophoresis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) were carried out with a PhastSystem (Pharmacia LKB Biotechnology) according to the instructions of the supplier by using a 8 to 25% polyacrylamide gel or a 10 to 15% polyacrylamide gel (Pharmacia LKB Biotechnology).
Activity staining in acrylamide gels.
Different enzyme extracts each containing 2 μg of protein were loaded and electrophoresed on a nondenaturing PAGE system (Pharmacia LKB Biotechnology). β-Gal activity was detected by incubating the gel in a 4′ umbelliferyl β-Galactoside solution (1 mg/ml in 50 mM phosphate buffer, pH 7). Fluorescent bands were visualized under UV light and photographed after incubation for 5 and 60 min.
RESULTS
Composition of the TOS mixture.
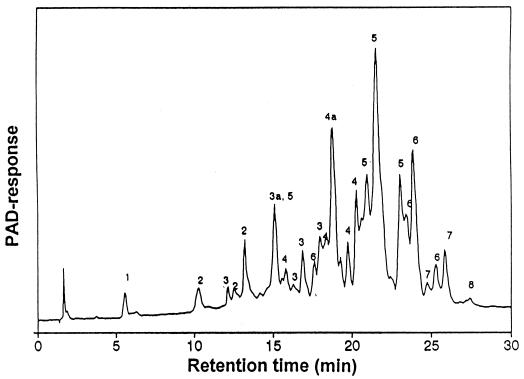
The TOS mixture was composed mainly of oligosaccharides (99%) and small amounts of residual galactose (0.1%), glucose (0.3%), and lactose (0.6%). In order to determine the degrees of polymerization of the oligosaccharides present, the oligosaccharide mixture was subjected to size-exclusion chromatography on a Bio-Gel P-2 column. Fractions corresponding to a given peak were pooled and subjected to HPAEC. All fractions were found to contain several components (data not shown) having the same degree of polymerization (8). In Fig. 1 the HPAEC elution profile of the complete TOS mixture is given and the degrees of polymerization of the various peaks are indicated.
FIG. 1.
HPAEC elution pattern of TOS. Numbers 1 to 8 indicate monomers, dimers, trimers, tetramers, pentamers, hexamers, heptamers, and octamers, respectively. Peaks 3a, 4a, and 5a have been identified as 4′ galactosyllactose, 4′ galactosyl galactosyl lactose, and 4′ galactosyl galactosyl galactosyl lactose, respectively. Note that peak areas do not supply information about the concentrations of the oligosaccharides, since the response factors of the pulsed electrochemical detector vary significantly with the various oligomers, with lowest responses occurring with oligosaccharides with a high DP. PAD, pulsed amperometric detection.
The TOS mixture contains 0.5, 2, 6, 17, 37, 27, 8.5, and 2% mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, and octamers, respectively, as determined using a refractive index. The structures of the purified oligosaccharides are described elsewhere (8).
Degradation of TOS by B. adolescentis.
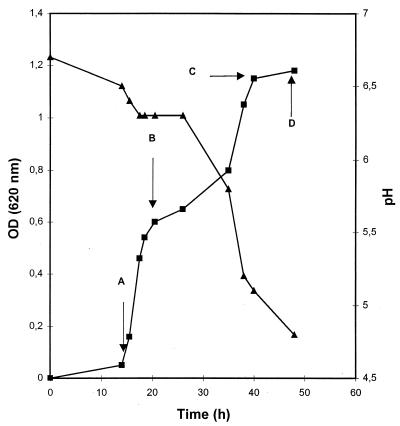
B. adolescentis grown in M17 containing glucose (0.5% [wt/vol]) (GM17) was transferred to fresh M17 medium (anaerobic, batch) containing 0.5% (wt/vol) TOS. The growth (optical density) and acidification (pH) of the culture were measured (Fig. 2), and residual TOS was analyzed using HPAEC (Fig. 3). After a lag phase in which no carbohydrates were fermented (Fig. 3A), exponential growth occurred. At the beginning of this exponential growth phase only monomeric material was fermented. At the end of the first exponential growth phase dimeric material and part of the trimeric material were fermented (Fig. 3B). Upon reaching an optical density of 0.6, a second lag phase occurred. In the second exponential growth phase oligomers with a DP of ≥3 were fermented (Fig. 3C), with the low-DP oligosaccharides being fermented first. Some residual TOS was observed after 50 h (Fig. 3D), which may have been due to inactivation of the enzyme at the low pH value reached at this point of fermentation (Fig. 2 and see below). When B. adolescentis was precultured on TOS, no biphasic growth could be observed and the stationary phase was reached within 30 h. These results suggest that cells have adapted to metabolize TOS more efficiently.
FIG. 2.
Growth of B. adolescentis in M17 containing 0.5% (wt/vol) TOS. Cells were precultured in M17 containing 0.5% (wt/vol) glucose. Arrows A to D indicate at which times samples were taken for HPAEC analysis of TOS in the supernatant (see Fig. 3). OD, optical density.
FIG. 3.
HPAEC elution pattern of TOS fermented by B. adolescentis. Samples were taken at 14 h (A), 22 h (B), 40 h (C), and 50 h (D) (see Fig. 2). m1, m2, m3, and m4 are components present in M17 broth. Numbers 1 to 8 indicate monomers, dimers, trimers, tetramers, pentamers, hexamers, heptamers, and octamers, respectively. Peaks 3a, 4a, and 5a have been identified as 4′ galactosyllactose, 4′ galactosyl galactosyl lactose, and 4′ galactosyl galactosyl galactosyl lactose, respectively. PAD, pulsed amperometric detection.
Glycosidase activities in B. adolescentis.
Subsequently, glycosidase activities in different cell extracts were determined with PNPG as a substrate. Specific β-Gal activity was highest in cells grown on TOS (Table 1). Surprisingly, other glycosidase activities were also higher in TOS-grown cells, including α-Gal, β-glucosidase, β-xylosidase, and α-l-arabinofuranosidase. Although high levels of glycosidases were present, no endo-glycanase activity could be detected.
TABLE 1.
Specific enzyme activities in cell extracts from B. adolescentis DSM 20083 grown in the presence of different substrates
| p-NP-glycoside | Sp act (mU/mg of protein) on growth substrate:
|
||||
|---|---|---|---|---|---|
| Galp | Glcp | Lactose | TOS | Melibiose | |
| p-NP-α-Galp | 530 | 995 | 745 | 2,430 | 2,220 |
| p-NP-β-Galp | 65 | 155 | 40 | 1,820 | 15 |
| p-NP-α-Glcp | 10 | 270 | 30 | 100 | 900 |
| p-NP-β-Glcp | 240 | 270 | 200 | 1,010 | 235 |
| p-NP-β-Xylp | 60 | 80 | 55 | 300 | 75 |
| p-NP-α-Araf | 5 | 10 | 5 | 50 | 10 |
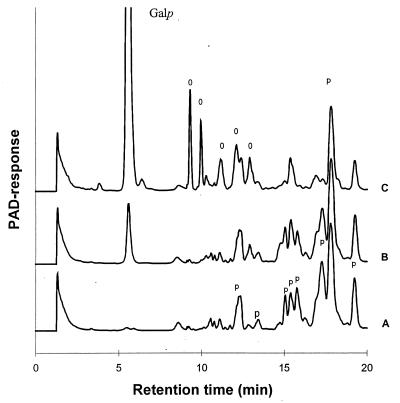
The activities of enzyme extracts from lactose- and TOS-grown cells towards TOS-pentamer were analyzed by determining the release of galactose residues (Fig. 4). The amount of galactose released by the enzyme extract from TOS-grown cells was approximately 20-fold higher than that with enzyme extract of lactose-grown cells. Comparable low amounts of galactose were also released with enzyme extracts from galactose- and glucose-grown cells (data not shown).
FIG. 4.
HPAEC elution patterns of galactopentasaccharides before (A) and after (B and C) degradation with the enzyme extracts of B. adolescentis grown on lactose (B) and on TOS (C). Galp, galactose; p, pentamers; O, oligosaccharides formed by degradation of the galactopentasaccharides; PAD, pulsed amperometric detection.
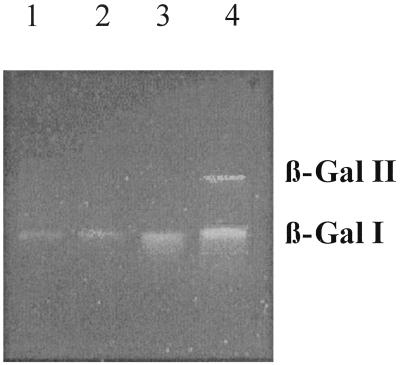
β-Gal activities of enzyme extracts from galactose-, glucose-, lactose-, and TOS-grown B. adolescentis were also assayed after PAGE under nondenaturating conditions using 4′ umbelliferyl β-galactoside as the substrate (Fig. 5). In extracts from galactose-, glucose-, and lactose-grown cells one β-Gal band (β-Gal I) was observed, whereas in extracts from TOS-grown cells, besides β-Gal I, the band of which exhibited an increased intensity, a second β-Gal (β-Gal II) was detected. This second activity band was only faintly visible after prolonged incubation with cell extracts from galactose-, glucose-, and lactose-grown cells (data not shown), indicating that this enzyme is indeed present at very low levels in cells grown on sugars other than TOS (see below).
FIG. 5.
β-Gal activity staining on a nondenaturing polyacrylamide gel. Crude enzyme preparations of B. adolescentis DSM 20083 grown on galactose (lane 1), glucose (lane 2), lactose (lane 3), and TOS (lane 4), with each preparation containing 2 μg of protein, were supplied to the gel. β-Gal activity was visualized under UV light after 5 min of incubation with a 4′ umbelliferyl β-galactoside solution.
Purification and characterization of β-Gal II.
B. adolescentis was grown on M17 and TOS, and after reaching stationary phase, cells were harvested and the enzyme extract was prepared. No β-Gal activity could be detected in the culture supernatant. The enzyme extract contained 2 g of protein, and a total β-galactosidase activity of 880 U towards PNPG was measured. Cell extract from B. adolescentis was fractionated using Q-Sepharose, Bio-Gel HTP hydroxyapatite, Mono Q HR5/5, and Sephacryl S200 HR16/60. The Q-Sepharose separation resulted in the separation of two different β-Gal's (β-Gal I and β-Gal II). The β-Gal II-containing fraction showed activity towards TOS and no activity towards lactose, while the β-Gal I-containing fraction was active towards lactose and not towards TOS. The TOS-active β-Gal II action was purified further on a Sephacryl S200 column and MonoQ column, and this resulted in an electrophoretically pure β-Gal II preparation (not shown). The molecular mass was approximately 350 kDa, as estimated using Superose 12. By sodium dodecyl sulfate-PAGE, a single protein band was found at 89 kDa, suggesting that the enzyme is active as a tetramer in vivo.
Substrate specificity and kinetic experiments with β-Gal II.
Subsequently, substrate specificity and physicochemical properties of β-Gal II were determined. Using PNPG, the enzyme had a specific activity of 5.5 U/mg and optimal activity at pH 6 and 35°C. β-Gal II was tested on a range of substrates. β-Gal II was active towards the nonreducing oligosaccharide β-d-Galp-(1↔1)-d-Glcp and to the other oligosaccharides present in the mixture, including TOS (with a DP of 8) (data not shown). Activity was also observed towards the galactooligosaccharides of other origins, such as β-d-Galp-(1→3)-Araf, β-d-Galp-(1→6)-d-Galp, [β-d-Galp-(1→6)]n-d-Glcp (n = 2 to 3), and [β-d-Galp-(1→4)]n-d-Galp (n = 1 to 3). The enzyme showed no detectable activity towards lactose, lactulose, 3′ fucosyllactose, lacto-N-fucopentaose I, or lacto-N-fucopentaose II. Incubation of β-Gal II in assays with increasing concentrations of β1→4-linked galactooligosaccharides resulted in hyperbolic plots of substrate versus reaction rate, indicating typical Michaelis-Menten saturation kinetics. Km and Vmax values for the enzyme towards different galactooligosaccharides and PNPG are given in Table 2. These data clearly show a general decrease in the Vmax and catalytic efficiency (expressed as Vmax/Km) of β-Gal II with an increase in the size of the oligosaccharides.
TABLE 2.
Kinetic parameters of β-Gal II from B. adolescentis
| Substrate | Km (mM) | Vmax (U/ml) | Vmax/Km |
|---|---|---|---|
| PNPG | 2.2 ± 0.2 | 5.0 ± 0.2 | 2.3 |
| β-d-Galp-(1→4)-d-Glcp | 0 | 0 | |
| β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Glcp | 2.2 ± 0.2 | 93.0 ± 3.5 | 42.2 |
| β-d-Galp-(1→4)-β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Glcp | 4.0 ± 0.4 | 68.1 ± 3.2 | 17.0 |
| β-d-Galp-(1→4)-d-Galp | 3.7 ± 0.3 | 95.3 ± 2.4 | 25.7 |
| β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Galp | 6.4 ± 0.2 | 48.6 ± 0.5 | 7.6 |
| β-d-Galp-(1→4)-β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Galp | 5.2 ± 0.7 | 17.2 ± 0.8 | 3.3 |
DISCUSSION
This paper reports on the isolation and characterization of a novel β-Gal (β-Gal II) from B. adolescentis, active towards TOS. B. adolescentis produces two different β-Gal's when it is cultured on TOS. The first one (β-Gal I), which was also detected in cells grown on glucose, galactose, and lactose, appeared to be active towards lactose but not towards TOS. The TOS-active β-Gal II was present at high levels only in TOS-grown cells, indicating that synthesis is induced by the substrate. β-Gal II was subsequently characterized further and was optimally active at pH 6 and 35°C, conditions mimicking those found in the colon. Remarkably, purified β-Gal II showed no activity at pH 5 and below, which may affect the action of the enzyme in environments such as the colon, where the pH may decrease below pH 6 due to microbial production of short-chain fatty acids. β-Gal II was active toward all the oligosaccharides present in the TOS mixture, including those with high DP. Kinetic characterization of β-Gal II revealed highest Vmax values towards oligosaccharides with low DP, which is in line with the sequential degradation observed during TOS fermentation. The β-Gal II was also active towards β-galactooligosaccharides derived from soy. However, β-Gal II showed no activity towards fucosylated galactooligosaccharides isolated from human milk. The nonreducing disaccharide β-d-Galp-(1↔1)-d-Glcp isolated by Fransen et al. (8) from the TOS mixture was completely hydrolyzed into galactose and glucose monomers, indicating that these novel types of nonreducing oligosaccharides can be degraded by B. adolescentis.
Since microbial sugar transport systems described so far can take up monomers, dimers, or trimers (20), it is speculated that β-Gal II, in contrast to lactose-hydrolyzing β-Gal I, is located extracellularly. It is conceivable that the enzyme is cell wall or membrane attached, since no activity was found in the culture supernatant. With TOS, extracellularly released galactose residues from TOS by β-Gal II would be accumulated via a galactose transport system and subsequently metabolized. The final remaining lactose may be taken up via a lactose transport system (14, 20) and split intracellularly by β-Gal I, and the galactose and glucose residues may then metabolized. Several other B. adolescentis enzymes active towards large extracellular substrates were not found in the supernatant, which indicates that they are cell wall or membrane bound (30, 31). The recently characterized (15) and cloned (29) α-Gal showing activity towards α-galactooligosaccharides was shown to possess a signal sequence, which indicates that the enzyme is translocated to and active on the outside of the cell. Also, two arabinoxylan arabinofuranohydrolases from B. adolescentis were supposed to be membrane or cell wall associated and to degrade an extracellular substrate, as they are active towards arabinose-containing xylooligosaccharides which contain up to 10 sugar units (30, 31). Whether the binding of these glycosidases to the cell wall provides a competitive advantage, e.g., in releasing substrates at the cell surface, remains to be elucidated.
The utilization of the trisaccharides 4′ galactosyllactose and 6′ galactosyllactose by intestinal bacteria has been studied previously (24), and it has been shown that these substrates are fermented not only by bifidobacteria but also by Lactobacillus, Bacteroides, and Clostridium species. It is conceivable that these bacteria produce β-Gal's active towards these trisaccharides. So far the utilization of galactooligosaccharides with a higher degree of polymerization has not been reported. Our work shows that B. adolescentis can degrade also oligosaccharides with a DP of >3 under these conditions. Other tested bacteria such as Bifidobacterium infantis and Lactobacillus acidophilus could utilize only the TOS with a DP of ≤3 present in the mixture (data not shown). Metabolism of high concentrations of TOS by B. adolescentis is linked to the production of β-Gal II, which is active towards these oligosaccharides. This enzyme might allow B. adolescentis to utilize the oligosaccharides more efficiently than other microorganisms. Therefore, this TOS mixture, containing mainly higher-molecular-weight material, might be an interesting prebiotic substrate, as it is metabolized by B. adolescentis, one of the predominant human fecal bacteria (16).
Strikingly, during growth of B. adolescentis on TOS a large number of glycosidases are produced, including two arabinofuranohydrolases which are involved in the degradation of arabinoxylooligosaccharides (30, 31). This may offer an additional competitive advantage, since it allows the organism to scavenge the environment for a range of substrates and use the degradation products for growth. No endo-glycanase activity could be detected in the cell extract (data not shown), suggesting that B. adolescentis adopted a strategy aimed at utilizing polysaccharide degradation products generated by other microorganisms instead of taking part in the initial depolymerization stage of polysaccharides.
The obtained results provide new insights into the oligosaccharide metabolism of bifidobacteria. Furthermore, the induction of β-Gal II during growth on TOS indicates that optimal performance of a synbiotic product containing B. adolescentis (probiotic) and TOS (prebiotic) can be obtained only by preculturing the microorganism on this substrate.
ACKNOWLEDGMENTS
This work was supported by the Netherlands Ministry of Agriculture, Nature Management and Fishery, The Dutch Dairy Foundation on Nutrition and Health, AVEBE, Nutreco (all in The Netherlands), and ORAFTI (Belgium).
REFERENCES
- 1.Blanchette D R L, Savoie L, Ward P, Chevalier P. Alpha- and beta-galactosidase properties of Bifidobacterium infantis. Milchwissenschaft. 1992;47:18–21. [Google Scholar]
- 2.Bouhnik Y, Flourie B, D'Agay L, Abensour, Pochart P, Gramet G, Durand M, Rambaud J C. Administration of transgalactooligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J Nutr. 1997;127:444–448. doi: 10.1093/jn/127.3.444. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Crittenden R G, Tannock G W. Prebiotics. In: Tannock G W, editor. Probiotics: a critical review. Wymondham, United Kingdom: Horizon Scientific Press; 1999. pp. 141–156. [Google Scholar]
- 5.Desjardins M L, Roy D, Goulet J. Growth of bifidobacteria and their enzyme profiles. J Dairy Sci. 1990;73:299–307. [Google Scholar]
- 6.Dumortier V, Brassart C, Bouquelet S. Purification and properties of a beta-d-galactosidase from Bifidobacterium bifidum exhibiting a transgalactosylation reaction. Biotechnol Appl Biochem. 1994;19:341–354. [Google Scholar]
- 7.Fooks L J, Fuller R, Gibson G R. Prebiotics, probiotics and human gut microbiology. Int Dairy J. 1999;9:53–61. [Google Scholar]
- 8.Fransen C T M, Van Laere K M J, van Wijk A A C, Brüll L P B, Dignum M, Thomas-Oates J E T, Haverkamp J, Schols H A, Voragen A G J, Kamerling J P, Vliegenthart J F G. α-d-Glcp-(1↔1)-β-d-Galp-containing oligosaccharides, a new class of oligosaccharides produced by β-galactosidase from lactose. Carbohydr Res. 1998;314:101–114. doi: 10.1016/s0008-6215(98)00285-7. [DOI] [PubMed] [Google Scholar]
- 9.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 10.Gibson G R. Dietary modulation of the human gut microflora using prebiotics. Br J Nutr. 1998;80:S209–S212. [PubMed] [Google Scholar]
- 11.Gibson G R, Roberfroid M B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 12.Ito M, Deguchi Y, Miyamori A, Matsuomote K, Kikuchi H, Matsumoto K, Yajima T, Kan T. Effect of administration of galactooligosaccharides on the human faecal microflora, stool weight and abdominal sensation. Microb Ecol Health Dis. 1990;3:285–292. [Google Scholar]
- 13.Ito M, Deguchi Y, Matsumoto K, Kimura M, Onodera N, Yajima T. Influence of galactooligosaccharides on the human fecal microflora. J Nutr Sci Vitaminol. 1993;39:635–640. doi: 10.3177/jnsv.39.635. [DOI] [PubMed] [Google Scholar]
- 14.Krzewinski F, Brassart C, Gavini F, Bouquelet S. Characterization of the lactose transport system in the strain Bifidobacterium bifidum DSM 20082. Curr Microbiol. 1996;32:301–307. doi: 10.1007/s002849900054. [DOI] [PubMed] [Google Scholar]
- 15.Leder S, Hartmeier W, Marx S P. α-Galactosidase of Bifidobacterium adolescentis DSM 20083. Curr Microbiol. 1999;38:101–106. doi: 10.1007/s002849900411. [DOI] [PubMed] [Google Scholar]
- 16.Matsuki T, Watanabe K, Tanaka R, Fukuda M, Oyaizu H. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl Environ Microbiol. 1999;65:4506–4512. doi: 10.1128/aem.65.10.4506-4512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsuoka T. Bifidobacteria and their role in human health. J Ind Microbiol. 1990;6:263–268. [Google Scholar]
- 18.Mutai M, Tanaka R. Ecology of Bifidobacterium in the human intestinal flora. Bifidobacteria Microflora. 1987;6:33–41. [Google Scholar]
- 19.Onishi N, Tanaka T. Purification and characterization of galactooligosaccharide-producing beta-galactosidase from Sirobasidium magnum. Lett Appl Microbiol. 1997;24:82–86. [Google Scholar]
- 20.Poolman B. Energy transduction in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:125–148. doi: 10.1111/j.1574-6976.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 21.Prenosil J E, Stuker E, Bourne J R. Formation of oligosaccharides during enzymatic lactose hydrolysis. I. State of the art. Biotechnol Bioeng. 1987;30:1019–1025. doi: 10.1002/bit.260300904. [DOI] [PubMed] [Google Scholar]
- 22.Roy D, Berger J L, Reuter G. Characterization of dairy-related Bifidobacterium spp. based on their beta-galactosidase electrophoretic patterns. Int J Food Microbiol. 1994;23:55–70. doi: 10.1016/0168-1605(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 23.Roy D, Blanchette L, Savoie L, Ward P, Chevalier P. alpha- and beta-galactosidase properties of Bifidobacterium infantis. Milchwissenschaft. 1992;47:18–21. [Google Scholar]
- 24.Sako T, Matsumoto K, Tanaka R. Recent progress on research and applications of non-digestible galactooligosaccharides. Int Dairy J. 1999;9:69–80. [Google Scholar]
- 25.Smart J B. Transferase reactions of the beta-galactosidase from Streptococcus thermophilus. Appl Microbiol Biotechnol. 1991;34:495–501. [Google Scholar]
- 26.Smart J B. Transferase reactions of beta-galactosidases—new product opportunities. Bull Int Dairy Fed. 1993;289:16–22. [Google Scholar]
- 27.Smart J B, Pillidge C J, Garman J H. Growth of lactic acid bacteria and bifidobacteria on lactose and lactose-related mono-, di- and trisaccharides and correlation with distribution of beta-galactosidase and phospho-beta-galactosidase. J Dairy Res. 1993;60:557–568. [Google Scholar]
- 28.Tochikura T, Saka K, Fujiyoshi T, Tachiki T, Kumagai H. p-Nitrophenyl glycoside-hydrolysing activities in bifidobacteria and characterisation of β-d-galactosidase of Bifidobacterium longum 401. Agric Biol Chem. 1986;50:2279–2286. [Google Scholar]
- 29.van den Broek L A M, Ton J, Verdoes J C, Van Laere K M J, Voragen A G J, Beldman G. Synthesis of α-galactooligosaccharides by a cloned α-galactosidase from Bifidobacterium adolescentis. Biotechnol Lett. 1999;21:441–445. [Google Scholar]
- 30.Van Laere K M J, Voragen C H L, Kroef T, van den Broek L A M, Beldman G, Voragen A G J. Purification and mode of action of two different arabinoxylan arabinofuranohydrolases from Bifidobacterium adolescentis. Appl Microbiol Biotechnol. 1999;51:606–613. [Google Scholar]
- 31.Van Laere K M J, Beldman G, Voragen A G J. A new arabinofuranohydrolase from Bifidobacterium adolescentis able to remove arabinosyl residues from double-substituted xylose units in arabinoxylan. Appl Microbiol Biotechnol. 1997;47:231–235. doi: 10.1007/s002530050918. [DOI] [PubMed] [Google Scholar]
- 32.Walker W A, Duffy L C. Diet and bacterial colonization: role of probiotics and prebiotics. J Nutr Biochem. 1998;9:668–675. [Google Scholar]
- 33.Zarate S, Lopez-Leiva M H. Oligosaccharide formation during enzymatic lactose hydrolysis: a literature review. J Food Prot. 1990;53:262–268. doi: 10.4315/0362-028X-53.3.262. [DOI] [PubMed] [Google Scholar]