Abstract
The elderly people are increasingly exposed to polymedication and therefore to the risks of drug–drug interactions (DDIs). However, there are few data available on the clinical consequences of these drug combinations. We investigated the impact of the various DDIs classified as severe in terms of emergency admissions in the elderly. A cross‐sectional study was conducted using information from the emergency department admissions of Bordeaux University Hospital between September 2016 and August 2017. Events of interest were frequency of concomitant uses of interacting drugs that are contraindicated or warned against and frequency of emergency admissions due to contraindicated or warned against concomitant uses of interacting drugs. Five thousand, eight hundred sixty (5860) admissions to the emergency department were analyzed. A total of 375 (6.4%) contraindicated or warned against concomitant uses were identified, including 163 contraindicated (43.5%) and 212 warned against (56.5%). Reason for admission appeared likely related to the underlying DDI in 58 cases. Within these, 36 admissions were assessed as probably due to a DDI (0.6% of hospitalizations) and 22 as certainly (0.4% of hospitalizations). Of these, there were 24 (45%) admissions related to a long QT syndrome (LQTS), nine (16%) related to a drug overdose, and eight (14%) related to a hemorrhage. An antidepressant was involved in 22 of the 24 cases of LQTS. Seven of the eight cases of hemorrhage involved the antithrombotic agents / non‐steroidal anti‐inflammatory drugs combination. Elderly patients admitted to emergency departments are particularly exposed to high‐risk potential DDIs. These drug combinations lead mainly to LQTS and involve certain antidepressants.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The prevalence of polypharmacy is high in elderly patients admitted to the emergency department, which theoretically exposes this population to many potential drug–drug‐interactions (DDIs).
WHAT QUESTION DID THIS STUDY ADDRESS?
What is the real clinical impact of potential DDIs classified as severe?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Reason for emergency admission appeared likely related to a potential DDI for 0.4–1% of elderly patients. Among the drugs involved, psychotropic drugs and in particular two antidepressants: citalopram and escitalopram, are the drugs which seem to cause the most hospitalizations. Particularly due to the risk of long QT syndrome.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
We strongly recommend systematizing electrocardiogram monitoring in all elderly patients admitted to the emergency department and treated with an antidepressant.
INTRODUCTION
Drug–drug interactions (DDIs) occur when the effects of one drug are modified in the presence of a concomitant drug. 1 Even though they may be prescribed on purpose, DDIs are mostly associated with a decrease in drug effectiveness or an increase in the risk of adverse drug reactions that both affect quality of care and are mostly predictable and avoidable. 2 , 3 DDIs are estimated to be responsible for about 1% of hospital admissions in the general population 4 , 5 and 2 to 5% of hospital admissions in elderly patients. 6 , 7 The reasons for this higher burden in the elderly are multiple and involve higher exposure to polymedication, changes in pharmacokinetics and/or pharmacodynamics, and a care trajectory involving a higher number of health professionals. 8 , 9 In this population, the prevalence of dispensing for drugs whose concomitant use is contraindicated was estimated to be 0.4%, and 2.3% for drugs whose concomitant use is discommended. 10
As not all concomitant use of drugs known to interact leads to situations of clinically meaningful DDIs and, potentially, to adverse reactions, prevalence/incidence studies of such at‐risk coprescribing or codispensing cannot preclude the concrete clinical impact of DDIs. Similarly, they cannot identify which of these situations of at‐risk coprescribing or codispensing are responsible for the most frequent adverse reactions resulting from DDIs. However, as shown in a recent meta‐analysis, very few studies have estimated the overall clinical impact of DDIs. 11 A French study based on patients admitted to a geriatric unit from the emergency unit showed that a quarter of patients exposed to a potential DDI had developed an adverse reaction. 12 A Canadian study focusing on elderly patients admitted to the hospital with specific drug toxicities found that many admissions occurred after administration of a drug known to cause DDIs. Among 1051 patients admitted to the hospital for digoxin toxicity, cases were ~ 13 times more likely to have received a prescription for clarithromycin in the week prior to hospital admission than controls without digoxin toxicity (odds ratio [OR], 13.6; 95% confidence interval [CI], 8.8–20.8). 13
In this context, we investigated the impact of the various DDIs classified as severe in terms of emergency admissions in the elderly patients. Our secondary objective was to determine the drugs most frequently encountered in this situation and the clinical consequences observed.
METHODS
Study design, settings, and participants
We performed a descriptive, cross‐sectional study using information from the emergency department admissions of Bordeaux University Hospital. The analysis considered data for all patients aged 75 years and over admitted to the emergency department between September 1, 2016, and August 31, 2017, and for whom at least one “usual treatment” questionnaire could have been completed. If a patient was admitted for different stays over the period, data for each emergency hospitalization was considered.
This information was obtained from the department electronic medical records, which include, in addition to all clinical data, a dedicated geriatric investigation for all patients aged 75 years and over. The latter registers all treatments the patient was taking before being admitted to the emergency department. From these patient medical records, we extracted information on demographic data (age and sex), drug uses informed at admission in the drug questionnaire, and reason for admission. The reason for admission was entered in a structured format according to the International Classification of Diseases 10th version. A medical review of each file of interest was carried out to determine the exact reason for admission.
Events of interest
The study investigated two events of interest. The first consisted in concomitant uses of interacting drugs that, because of this interaction and its serious potential health consequences, are contraindicated or warned against. These contraindicated or warned against concomitant uses of interacting drugs were searched in patient emergency department admissions data. The second consisted in the concrete clinical consequences of contraindicated or warned against concomitant uses of interacting drugs. These corresponded to emergency department admissions relating to health consequences of DDIs that occurred during contraindicated or warned against concomitant uses of interacting drugs.
Identification of contraindicated or warned against concomitant uses of interacting drugs (potential DDIs)
Identification of contraindicated or warned against concomitant uses of interacting drugs was performed using a three‐step method. These are hereafter referred to as contraindicated or warned against concomitant uses. First, we extracted the free text from the drug questionnaire. Second, we identified all drug international non‐proprietary names (INNs) mentioned in these free texts using an automated detection method developed and validated in a previous study. The tool has a positive predictive value (PPV) of 99.0% and a sensitivity of 96.0% for such INN identification from free text. 14 Third, within the drug INN data obtained from these processed free texts, we identified all contraindicated or warned against concomitant uses of interacting drugs using a second tool developed with the Synapse Medicine start‐up. 15 The tool analyses prescriptions to identify all drugs whose concomitant use is listed by the French National Agency for Medicines (ANSM) as contraindicated or not recommended because of clinically meaningful interactions. This official list (namely “Thesaurus des Interactions”) is made publicly available on the ANSM website. This second tool was also validated previously for the automatic identification of contraindicated or warned against concomitant uses of interacting drugs, and was shown to have a specificity of 100% and a PPV of 99.7% (Appendix S1 and Table S2).
Identification of emergency room admissions relating to contraindicated or warned against concomitant uses
For each patient for which a contraindicated or warned against concomitant use was retrospectively identified in admission data, medical charts were analyzed by two pharmacologists to determine whether the reason for admission corresponded to a health consequence of the underlying DDI. In the event of a disagreement, a third expert analyzed the files. This allowed all admissions of patients admitted with contraindicated or warned against concomitant uses to be classified into two categories: admissions likely related to the underlying DDI or admissions unlikely related to the underlying DDI. Among the admissions likely related to the underlying DDI, we differentiated those which were “probably” from “certainly” depending on the presence of information allowing the medical condition leading to the emergency department admission to be determined (e.g., information on cardiac rhythm disorder in admission diagnosis but no electrocardiogram [ECG] stored in the electronic hospital charts that would confirm the presence of long QT syndrome [LQTS]).
Finally, we examined the medical charts to determine whether the clinicians who managed the patients had identified the DDIs that were involved in admissions considered as certainly related to contraindicated or warned against concomitant uses.
Data analysis
Demographic characteristics of the population were described regarding the first admission for each patient and the number of drug distributions were described for all admissions. Quantitative variables were described in terms of median and interquartile range (IQR), and qualitative variables were described as prevalence and frequency. The most frequent therapeutic groups in the “usual treatments” questionnaires of the population were presented by Anatomical Therapeutical Chemical (ATC) classification therapeutic level three. 16 The prevalence of contraindicated or warned against concomitant uses over the study period was expressed as an absolute value and their overall frequency among emergency admissions was expressed as percentage with its 95% CI using a standard normal distribution. 17 The absolute value and the frequency of emergency department admissions relating to contraindicated or warned against concomitant uses were also measured. The drug pairs involved in emergency department admissions relating to contraindicated or warned against concomitant uses are presented according to the INN. The associated mechanisms and expected adverse events were detailed according to the information provided in the French ANSM Thesaurus des Interactions.
RESULTS
Characteristics of study subjects
Overall, 5860 admissions to the emergency department were considered, including 5022 elderly patients aged 75 and older (Figure 1). Patients’ median age was 85 (IQR: 81–90) years and 59.8% were women. The median number of different drugs they were taking before being admitted to the emergency department was five. 3 , 4 , 5 , 6 , 7 , 8 In total, 37,954 different drugs were identified among the patients’ geriatric investigation questionnaires. The most represented drugs were: non‐opioid analgesics and antipyretics (9.8%), antithrombotic agents (5.4%), beta‐blockers (5.0%) and drugs for peptic ulcer and gastro‐esophageal reflux disease (4.9%; see Table S3 for a detailed list).
FIGURE 1.
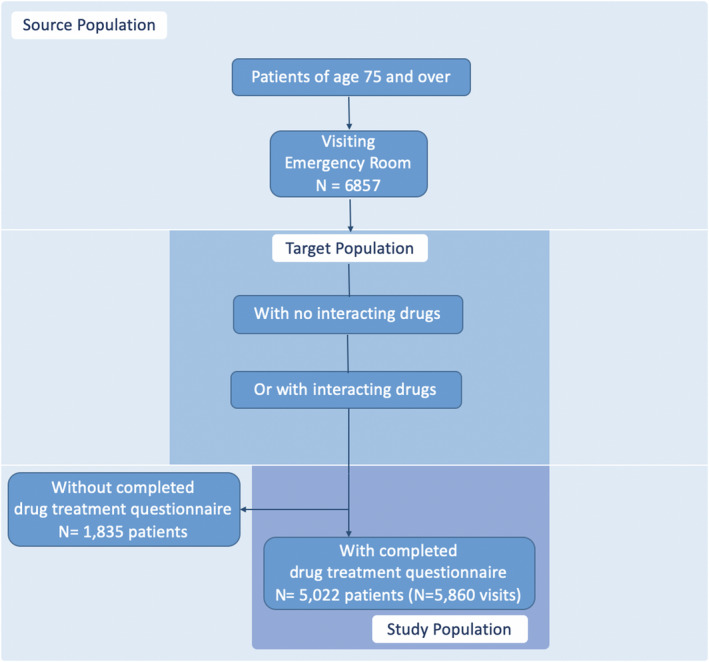
Flow chart of study population selection. If a patient presented several admissions, each admission drug questionnaire was included. “Created by the authors”
Main results
A total of 375 contraindicated or warned against concomitant uses were identified, including 163 contraindicated (43.5%) and 212 warned against (56.5%). The overall frequency was 6.4% (95% CI, 5.8%–7.0%), with 2.8% (2.4–3.2) contraindicated and 3.6% (3.1–4.1) warned against (Table 1). These 375 situations of potential DDIs included 42 different drug pairs corresponding to contraindicated concomitant uses and 93 drug pairs corresponding to warned against concomitant uses. The mechanisms involved in the associated potential DDIs were mostly pharmacodynamics (86.9% vs. 13.1% pharmacokinetics). The three most represented drug pairs in contraindicated concomitant use were: amiodarone/escitalopram, flecainide/bisoprolol, and hydroxyzine/amiodarone, which represented 17.2%, 13.5%, and 6.7% of identified contraindicated, respectively (see Table S4 for detailed list). The three most represented drug pairs in warned against concomitant use were: spironolactone/potassium, bisoprolol/rilmenidine, and fluindione/diclofenac, which represented 11.8%, 7.5%, and 7.1% of identified warned against, respectively (see Table S5 for a detailed list). The most frequent expected adverse events relating to these 375 situations of potential DDIs were LQTS (36.8%) and hemorrhage (16.3%; see Table S6 for a detailed list).
TABLE 1.
Prevalence, overall frequency, and mechanism of potential DDIs relating to contraindication or warned against concomitant uses identified among emergency admissions (n = 5860)
| n (%) | Overall frequency, % (95% CI) | Pharmacodynamic, n (%) | Pharmacokinetic, n (%) | |
|---|---|---|---|---|
| Overall | 375 | 6.4 (5.8, 7.0) | 326 (86.9) | 49 (13.1) |
| Contraindicated a | 163 (43.5) | 2.8 (2.4, 3.2) | 151 (92.6) | 12 (7.4) |
| Warned against b | 212 (56.5) | 3.6 (3.1, 4.1) | 175 (82.5) | 37 (17.5) |
Abbreviations: 95% CI, 95% confidence interval; DDIs, drug‐drug interactions.
Contraindicated concomitant uses.
Warned against concomitant uses.
Among patients presenting with contraindicated or warned against concomitant uses at emergency department admission, the reason for admission appeared likely related to the underlying potential DDI in 58 (16%) patients. After medical chart examination, 36 patients were assessed as probably due to a DDI and 22 as certainly (Table 2).
TABLE 2.
Prevalence and proportion of all emergency department admissions consecutive to DDIs relating to contraindicated or warned against concomitant uses, by level of certainty
| All DDIs, n = 375 | Contraindicated a , n = 163 | Warned against b , n = 212 | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Overall | 58 (16) | 26 (16) | 32 (15) |
| Probably consecutive to DDI | 36 (10) | 19 (12) | 17 (8) |
| Certainly consecutive to DDI | 22 (6) | 7 (4) | 15 (7) |
Abbreviation: DDIs, drug‐drug interactions.
Contraindicated concomitant uses.
Warned against concomitant uses.
Admissions probably consecutive to DDIs involved 25 different drug pairs (Table 3). Patients concerned were more likely to be women (75%) and their median age was 87 years (IQR: 81–91). The drug pairs amiodarone/citalopram or amiodarone/escitalopram accounted for 25% of them. An antidepressant was involved in 13 of the 19 drug pairs incriminated in contraindicated concomitant uses. Antiarrhythmic agents and beta blockers were involved in four. Among the incriminated drug pairs corresponding to warned against concomitant uses, seven out of 17 involved an antipsychotic.
TABLE 3.
Emergency admissions probably consecutive to contraindicated or warned against concomitant uses, n = 36
| Probable clinical effect | Drug A | Drug B | PK/PD | n (%) | Age, yearMedian (IQR) | Female ratio |
|---|---|---|---|---|---|---|
| ALL | 36 | 87 (81–91) | 0.75 | |||
| Contraindicated | 19 (53) | 89 (84–94) | 0.72 | |||
| LQTS a | Amiodarone | Escitalopram | PD | 6 (17) | ||
| Amiodarone | Citalopram | PD | 3 (8) | |||
| Amiodarone | Domperidone | PD | 1 (3) | |||
| Amisulpride | Hydroxyzine | PD | 1 (3) | |||
| Citalopram | Haloperidol | PD | 1 (3) | |||
| Citalopram | Escitalopram | PD | 1 (3) | |||
| Domperidone | Escitalopram | PD | 1 (3) | |||
| Haloperidol | Hydroxyzine | PD | 1 (3) | |||
| Heart failure | Bisoprolol | Flecainide | PD | 3 (8) | ||
| Flecainide | Nebivolol | PD | 1 (3) | |||
| Warned against | 17 (47) | 81 (78–88) | 0.78 | |||
| LQTS | Cyamemazine | Sotalol | PD | 2 (6) | ||
| Amiodarone | Haloperidol | PD | 1 (3) | |||
| Cyamemazine | Haloperidol | PD | 1 (3) | |||
| Drug overdose | Lithium b | Ramipril | PK | 2 (6) | ||
| Lamotrigine c | Valproic acid | PK | 1 (3) | |||
| Lamotrigine c | Valpromide | PK | 1 (3) | |||
| Psychotic disorders | Levodopa | Risperidone | PD | 1 (3) | ||
| Olanzapine | Piribedil | PD | 1 (3) | |||
| Cyamemazine | Levodopa | PD | 1 (3) | |||
| Hemorrhage | Diclofenac | Flurbiprofen | PD | 1 (3) | ||
| Apixaban | Ibuprofen | PD | 1 (3) | |||
| Hypotension | Nebivolol | Rilmenidine | PD | 1 (3) | ||
| Bisoprolol | Rilmenidine | PD | 1 (3) | |||
| Bradycardia | Diltiazem | Timolol | PD | 1 (3) | ||
| Hyperkalemia | Potassium | Spironolactone | PD | 1 (3) |
Abbreviations: IQR, interquartile range; LQTS, long QT syndrome; PD, pharmacodynamic; PK, pharmacokinetic.
Long QT syndrome.
Lithium overdose.
Lamotrigine overdose.
The most frequently represented adverse effect consecutive to DDI and having led to admission was rhythm disorder resulting from LQTS (n = 19; 53%).
Admissions certainly consecutive to DDIs involved 18 different drug pairs (Table 4). Patients concerned were more likely to be women (59%) and their median age was 84 years (IQR: 80–88). There were no pairs with more than two occurrences. An antidepressant was involved in four of the seven drug pairs incriminated in contraindicated concomitant uses and antiarrhythmic agents and beta blockers were involved in two. Among the incriminated drug pairs corresponding to warned against concomitant uses, five out of 15 involved combinations of antithrombotic agents and non‐steroidal anti‐inflammatory drugs (NSAIDs). The most frequent adverse effects certainly consecutive to DDI and having led to admission were hemorrhage (n = 6; 27%), rhythm disorder resulting from LQTS (n = 5; 23%) and lithium, and lamotrigine or alfuzosin overdose (n = 5; 23%). Only seven of these 22 (32%) adverse effects certainly consecutive to DDI had been identified by clinicians during patient management and none had been declared to the pharmacovigilance system.
TABLE 4.
Emergency admissions certainly consecutive to contraindication or warned against concomitant uses, n = 22
| Clinical effect | Drug A | Drug B | Pk/Pd | Identified by clinicians | n (%) | Age, yearMedian (IQR) | Sex ratio (F/M) |
|---|---|---|---|---|---|---|---|
| ALL | 22 | 84 (80–88) | 0.59 | ||||
| Contraindication | 7 (32) | 84 (79–87) | 0.43 | ||||
| LQTS a | Amiodarone | Escitalopram | PD | No | 2 (9) | ||
| Amisulpride | Hydroxyzine | PD | No | 1 (5) | |||
| Escitalopram | Hydroxychloroquine | PD | No | 1 (5) | |||
| Heart failure | Bisoprolol | Flecainide | PD | No | 2 (9) | ||
| Drug overdose | Alfuzosin b | Ketoconazole | PK | No | 1 (5) | ||
| Warned against | 15 (68) | 84 (81–89) | 0.67 | ||||
| Hemorrhage | Apixaban | Diclofenac | PD | Yes c | 2 (9) | ||
| Diclofenac | Enoxaparin | PD | No | 1 (5) | |||
| Fluconazole | Rivaroxaban | PK | No | 1 (5) | |||
| Dabigatran | Ketoprofen | PD | Yes | 1 (5) | |||
| Diclofenac | Fluindione | PD | No | 1 (5) | |||
| Drug overdose | Furosemide | Lithium d | PK | No | 2 (9) | ||
| Ketoprofen | Lithium d | PK | Yes | 1 (5) | |||
| Lamotrigine | Valproic acid e | PK | Yes | 1 (5) | |||
| Apixaban ineffectiveness | Apixaban | Phenobarbital | PK | No | 1 (5) | ||
| Hyponatremia | Oxcarbazepine | Sertraline | PD | No | 1 (5) | ||
| Hypotension | Silodosin | Urapidil | PD | Yes | 1 (5) | ||
| LQTS | Amiodarone | Domperidone | PD | Yes | 1 (5) | ||
| Psychotic disorders | Cyamemazine | Levodopa | PD | Yes | 1 (5) |
Abbreviations: DDI, drug‐drug interaction; IQR, interquartile range; LQTS, long QT syndrome; PD, pharmacodynamic; PK, pharmacokinetic.
Long QT syndrome.
Alfuzosin overdose.
Only one of these two DDI was identified by clinicians.
Lithium overdose.
Lamotrigine overdose.
Overall, there were 24 (45%) LQTS, nine (16%) drug overdoses (5 cases of lithium overdose) and eight (14%) cases of hemorrhage among the 58 admissions likely related to DDIs. An antidepressant was involved in 22 of the 24 cases of LQTS. Seven of the eight cases of hemorrhage involved the antithrombotic agents / NSAIDs combination. Lithium overdose was induced by ACE inhibitor, NSAID, or loop diuretic. Forty of the 58 admissions likely related to DDIs involved women (69%), including 18 among the 24 LQTS (75%).
DISCUSSION
Summary of findings
The clinical impact of DDIs and their avoidability are a major issue in geriatrics. Our study shows that elderly patients admitted to emergency departments are particularly exposed to high‐risk potential DDIs (6.4% exposed to a potential DDI, 2.8% to a contraindicated association and 3.6% to a warned against association). In comparison, our previous study based on out‐of‐hospital drug dispensings in the general French population showed an exposure to these potential DDIs of 1.6% (0.2% to contraindicated and 1.4% to warned against). 10 Although this is a different population, we observed a particularly high prevalence of potential DDIs in this study. This result is even more concerning as 16% of these DDIs were identified as likely responsible for emergency admissions (10% probably and 6% certainly). As these DDIs were contraindicated or not recommended, the resulting hospitalizations were theoretically avoidable. Considering that around 300,000 elderly patients are admitted to emergency departments yearly in France, the estimations we performed would extrapolate to 17,400 to 21,000 admitted elderly patients presenting with contraindicated or warned against concomitant uses, and to around 2800 to 3400 the number of avoidable hospitalizations due to high‐risk DDIs (16% of high‐risk potential DDIs). Additionally, our results show that most of these related admissions concerned only a few conditions that were consecutive to drug interactions involving a small number of drugs and drug classes. Prioritizing a communication and drug education campaign that would help diminish the burden of these accidents would thus appear quite feasible. Finally, we can estimate between 0.4 and 1% of the proportion of emergency hospitalizations in the elderly that can be explained by DDIs. Although the public health impact is not negligible, DDIs do not reflect all avoidable hospitalizations or the burden of iatrogenic events in this population. According to the literature, the proportion of hospital admissions due to adverse drug reactions has ranged from 6% to 12% of all admissions in the elderly. 18 In particular, iatrogenic events and not only DDIs, would be responsible for a significant proportion of avoidable re‐admissions in elderly patients. A French study showed that, in this population, 11.4% of the re‐admissions were drug‐related re‐admissions. 19
Emergency department admissions related to DDIs
A recent US study showed that among patients discharged from the emergency department, 1.6% presented with at least one contraindicated potential DDI and the majority of these DDIs involved QT prolongation. 20 Furthermore, LQTS is one of the most common reasons for drug withdrawal from the market. 21 It is a well‐known potential adverse event with anti‐arrhythmic drugs but also with other non‐cardiac drugs, such as antipsychotics and anti‐allergic drugs (list available on https://www.crediblemeds.org/). However, current knowledge on estimating the prevalence of LQTS induced by one or more drugs is very limited, yet the vast majority of acquired LQTS seem to be the result of adverse drug effects. 22 Our results highlight a particular risk of LQTS with antidepressants in elderly patients, and an antidepressant was involved in 22 of the 24 cases of LQTS. These results are consistent with a Swedish register‐based cohort study, which identified antidepressants as the drugs most at risk for LQTS and torsade de pointes in the elderly patients. 23 Citalopram / escitalopram was the most frequently incriminated combination. Our results also suggest that women may be at increased risk for this type of interaction (75% of cases), even though female sex is itself a known risk factor for LQTS. Most cases of LQTS were considered as possible by our experts. The reason for not rating them as certain was mostly the lack in the electronic medical chart of a digitalized version of the ECG performed in the emergency department. Altogether, the large percentage of LQTS in admissions related to DDIs in the elderly patients, and of antidepressants in the drug pairs involved in the incriminated DDIs in the elderly patients, plead for the systematic performing of an ECG in elderly patients treated with antidepressants and admitted to the emergency department, whatever the reason.
Hemorrhage was the main reason for admissions assessed as certainly resulting from a DDI. This is due partly to the fact that all were adequately documented in the medical charts (results of endoscopic explorations, hemoglobin assay, etc.) and probably partly to the large prevalence of use of the incriminated drugs in the elderly patients. Among the six cases of hemorrhage certainly due to DDIs, five concerned the NSAID / antithrombotic agent combination. In addition, two cases of hemorrhage were probably related to DDI and concerned this combination of drugs. Hemorrhage was the most frequent serious clinical consequence of DDIs in numerous studies. 2 , 24 , 25 However, even though the risk of gastrointestinal bleeding is well known by clinicians, especially in elderly patients, our study shows that the prevention of DDIs associated with this risk of this event should be enforced.
Aging causes deterioration in most organs, especially the kidneys and liver. 26 Several studies have shown that DDIs are often due to pharmacodynamic interactions in the elderly patients. 27 This was also the case in our study, although we found 19% of pharmacokinetic interactions of which 16% resulted in drug overdose. Lithium was the most affected drug (5 out of 9 cases). In each case, it was associated with a drug known to be able to impair kidney function. These results plead for systematic lithium overdose monitoring whenever an elderly patient on lithium is admitted for general, neurological, or cardiac disorders, or if their renal function is impaired.
Strengths and limitations
This study has several strengths. First, by investigating high‐risk potential DDIs and emergency admissions related to DDIs, it provides a more complete analysis of the impact of DDIs in clinical practice. The high prevalence of potential DDIs shows that action should be taken on the proper use of drugs in the elderly. Our results also suggest that a significant number of these potential DDIs lead to emergency admissions (16%). These results are consistent with a study conducted in Italy, which found a number of 9% for any type of potentials DDIs. 26 The estimate was slightly lower but this study was not focused on high‐risk potential DDIs. Second, we used an automated data analysis method coupled with pharmacological validation. Such methods could prove useful in practice for providing precise information to physicians about the combinations of drugs that may trigger severe interactions with clinical consequences. Such targeting would attenuate the risk of alert fatigue that clinicians often encounter with tools that assist prescription.
On the other hand, the study concerned only one emergency department, so the results cannot be generalized to the French population despite the large number of cases analyzed. Furthermore, the associations found in this French population might differ from those in other countries. However, the most frequently found DDIs likely responsible for emergency admissions, especially combinations of drugs causing LQTS or hemorrhage, are consistent with those reported elsewhere.
The main risk of bias in our study was a possible nonexhaustive collection of treatments taken by the patient. In particular concerning self‐medication, which may concern drugs with known risks of DDIs, such as NSAIDs for example. However, the existence of a dedicated geriatric investigation for patients admitted to the emergency department limits this risk, on the other hand, the risk of this bias is to underestimate the clinical impact of DDIs.
Finally, as we performed a cross‐sectional study, it would be necessary to complete our results with a longitudinal approach in order to quantitatively measure the impact of the DDIs that we have identified on the risk of hospitalization. This type of design will also allow us to use an active comparator to manage within‐person confounding. 28
CONCLUSIONS
Elderly patients admitted to emergency departments are particularly exposed to high‐risk potential DDIs. These drug combinations mainly lead to LQTS and less frequently to overdoses of psychotropic drugs and hemorrhages. We recommend systematizing ECG monitoring in all elderly patients admitted to the emergency department and treated with an antidepressant known to be at risk of LQTS, especially in case of treatment with citalopram or escitalopram. A stronger focus should be placed on preventing DDIs in emergency settings by targeting high‐risk potential DDIs and by explaining the expected clinical consequences.
CONFLICT OF INTEREST
L.L. and I.P. were employed by Synapse Medicine at the time this research was conducted or hold stock/stock options therein. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
L.L., I.P., C.G., and A.P. wrote the manuscript. L.L., I.P., P.D., G.E., F.S., C.G., and A.P. designed the research. L.L., I.P., and P.D. performed the research. L.L., F.S., C.G., and A.P. analyzed the data.
Supporting information
Appendix S1
Letinier L, Pujade I, Duthoit P, et al. Emergency department admissions induced by drug–drug interactions in the elderly: A cross‐sectional study. Clin Transl Sci. 2022;15:1472–1481. doi: 10.1111/cts.13262
Funding information
No funding was received for this work
REFERENCES
- 1. Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug‐drug interactions. Expert Opin Drug Saf. 2012;11:83‐94. [DOI] [PubMed] [Google Scholar]
- 2. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Obreli‐Neto PR, Nobili A, de Oliveira BA, et al. Adverse drug reactions caused by drug‐drug interactions in elderly outpatients: a prospective cohort study. Eur J Clin Pharmacol. 2012;68:1667‐1676. [DOI] [PubMed] [Google Scholar]
- 4. Bénard‐Laribière A, Miremont‐Salamé G, Pérault‐Pochat M‐C, Noize P, Haramburu F. The EMIR study group on behalf of the French network of pharmacovigilance centres. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundam Clin Pharmacol. 2015;29:106‐111. [DOI] [PubMed] [Google Scholar]
- 5. Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug–drug interactions: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2014;23:489‐497. [DOI] [PubMed] [Google Scholar]
- 6. Becker ML, Kallewaard M, Caspers PWJ, Visser LE, Leufkens HGM, Stricker BHC. Hospitalisations and emergency department visits due to drug‐drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641‐651. [DOI] [PubMed] [Google Scholar]
- 7. Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc J‐L, Lapeyre‐Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging. 2009;26:475‐482. [DOI] [PubMed] [Google Scholar]
- 8. Seymour RM, Routledge PA. Important drug‐drug interactions in the elderly. Drugs Aging. 1998;12:485‐494. [DOI] [PubMed] [Google Scholar]
- 9. Burato S, Leonardi L, Antonazzo IC, et al. Comparing the prevalence of polypharmacy and potential drug‐drug interactions in nursing homes and in the community dwelling elderly of Emilia Romagna region. Front Pharmacol. 2021;11:2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Létinier L, Cossin S, Mansiaux Y, et al. Risk of drug‐drug interactions in out‐hospital drug dispensings in France: results from the drug‐drug interaction prevalence study. Front Pharmacol. 2019;10:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng WY, Richardson LC, Li L, Day RO, Westbrook JI, Baysari MT. Drug‐drug interactions and their harmful effects in hospitalised patients: a systematic review and meta‐analysis. Eur J Clin Pharmacol. 2018;74:15‐27. [DOI] [PubMed] [Google Scholar]
- 12. Doucet J, Chassagne P, Trivalle C, et al. Drug‐drug interactions related to hospital admissions in older adults: a prospective study of 1000 patients. J Am Geriatr Soc. 1996;44:944‐948. [DOI] [PubMed] [Google Scholar]
- 13. Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug‐drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652‐1658. [DOI] [PubMed] [Google Scholar]
- 14. Cossin S, Lebrun L, Lobre G, et al. Romedi: an open data source about french drugs on the semantic web. MEDINFO 2019: Health and Wellbeing e‐Networks for All. Stud Health Technol Inform. 2019;264:79‐82. [DOI] [PubMed] [Google Scholar]
- 15. L Louis, G Clement, C Sebastien, et al. Patent FR1661257 – device and method for generating a database relating to drugs. https://patents.google.com/patent/FR3059118A1/fr.
- 16. WHOCC – ATC/DDD Index . ATC/DDD index 2022. https://www.whocc.no/atc_ddd_index/. Accessed February 15, 2022.
- 17. Confidence Intervals . http://www.stat.yale.edu/Courses/1997‐98/101/confint.htm. Accessed December 14, 2021.
- 18. Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Castelino RL, Bereznicki LR. Hospitalization in older patients due to adverse drug reactions – the need for a prediction tool. Clin Interv Aging. 2016;11:497‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwab C, Korb‐Savoldelli V, Escudie JB, et al. Iatrogenic risk factors associated with hospital readmission of elderly patients: a matched case‐control study using a clinical data warehouse. J Clin Pharm Ther. 2018;43:393‐400. [DOI] [PubMed] [Google Scholar]
- 20. Jawaro T, Bridgeman PJ, Mele J, Wei G. Descriptive study of drug‐drug interactions attributed to prescriptions written upon discharge from the emergency department. Am J Emerg Med. 2019;37:924‐927. [DOI] [PubMed] [Google Scholar]
- 21. Molokhia M, Pathak A, Lapeyre‐Mestre M, Caturla L, Montastruc JL, McKeigue P. Case ascertainment and estimated incidence of drug‐induced long‐QT syndrome: study in Southwest France. Br J Clin Pharmacol. 2008;66:386‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El‐Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and electrophysiology of torsade de pointes. Arrhythm Electrophysiol Rev. 2019;8:122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Danielsson B, Collin J, Nyman A, et al. Drug use and torsades de pointes cardiac arrhythmias in Sweden: a nationwide register‐based cohort study. BMJ Open. 2020;10:e034560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leone R, Magro L, Moretti U, et al. Identifying adverse drug reactions associated with drug‐drug interactions: data mining of a spontaneous reporting database in Italy. Drug Saf. 2010;33:667‐675. [DOI] [PubMed] [Google Scholar]
- 25. Marengoni A, Pasina L, Concoreggi C, et al. Understanding adverse drug reactions in older adults through drug‐drug interactions. Eur J Intern Med. 2014;25:843‐846. [DOI] [PubMed] [Google Scholar]
- 26. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67‐76. [DOI] [PubMed] [Google Scholar]
- 27. Hines LE, Murphy JE. Potentially harmful drug‐drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011;9:364‐377. [DOI] [PubMed] [Google Scholar]
- 28. Bykov K, Mittleman MA, Glynn RJ, Schneeweiss S, Gagne JJ. The case‐crossover design for drug‐drug interactions: considerations for implementation. Epidemiology. 2019;30:204‐211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


