Abstract
Trazpiroben is a dopamine D2/D3 receptor antagonist under development for the treatment of gastroparesis. This phase I, open‐label, randomized, two‐way crossover study (NCT04121078) evaluated the effect of single‐dose intravenous rifampin, a potent inhibitor of the organic anion transporting polypeptides (OATPs) 1B1 and 1B3, on the pharmacokinetics and safety of trazpiroben in healthy adults. The utility of coproporphyrin (CP) I and CPIII as biomarkers of OATP inhibition was also assessed. Overall, 12 participants were enrolled and randomized (1:1) into one of two treatment sequences (AB and BA). Participants received either a single oral dose of trazpiroben 25 mg (treatment A) or a single oral dose of trazpiroben 25 mg immediately after a single 30‐min intravenous infusion of rifampin 600 mg (treatment B). After a washout period of at least 7 days, participants received the other treatment. Geometric mean area under the curve from time 0 extrapolated to infinity (AUC∞) and maximum serum concentration (C max) of plasma trazpiroben were higher in participants receiving treatment B than those receiving treatment A (AUC∞, 168.5 vs. 32.68 ng*h/ml; C max, 89.62 vs. 14.37 ng/ml); corresponding geometric mean ratios (90% confidence interval) showed 5.16 (4.25–6.25) and 6.24 (4.62–8.42)‐fold increases in these parameters, respectively. In this study, trazpiroben was confirmed as a substrate of OATP1B1/1B3, and therefore co‐administration of trazpiroben with moderate to strong inhibitors of OATP1B1/1B3 is not recommended. This is also the first assessment of the utility of CPI and CPIII as endogenous biomarkers of OATP1B1/1B3 inhibition after a single intravenous dose of rifampin.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Despite the disease burden of gastroparesis, treatment options remain limited. Affected patients are often treated for multiple comorbidities, along with receiving medication for symptomatic management of gastroparesis, making polypharmacy a common issue. Consequently, the potential for drug–drug interactions (DDIs) must be considered when developing new treatments for this disease.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study evaluated the effect of a single intravenous dose of rifampin, an organic anion transporting polypeptide (OATP) 1B1/1B3 inhibitor, on the single‐dose pharmacokinetics of oral trazpiroben in healthy adults and explored the use of coproporphyrin (CP) I and CPIII as endogenous biomarkers of OATP1B1/1B3 activity.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study confirms trazpiroben as an OATP1B1/1B3 substrate, and is the first exploring the utility of CPI and CPIII as endogenous biomarkers to assess clinical OATPIB1/1B3‐mediated DDIs after intravenous rifampin administration.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Co‐administration of trazpiroben with moderate to strong OATP1B1/1B3 inhibitors is not recommended. CPI and CPIII may be suitable biomarkers for assessing OATP1B1/1B3‐mediated DDIs after administering single‐dose intravenous rifampin.
INTRODUCTION
Gastroparesis is a chronic gastric motility disorder characterized by delayed emptying of the stomach without mechanical obstruction. 1 Cardinal symptoms include early satiety, postprandial fullness, nausea, vomiting, and bloating, and patients may additionally experience symptoms of gastroesophageal reflux, such as heartburn and regurgitation. 1 , 2 , 3 Gastroparesis is also associated with a significant healthcare burden 4 , 5 and reduction in a patient’s quality of life. 5
Treatment options for gastroparesis are limited, and there are no approved therapies for the treatment of chronic gastroparesis. 6 Available non‐pharmaceutical treatment options are varied and include surgery (e.g., venting gastrostomy, gastrojejunostomy, pyloroplasty, and gastrectomy), gastric electrical stimulation, and optimization of diet. Pharmaceutical interventions, such as prokinetics (e.g., metoclopramide and domperidone) are also available to help to control the signs and symptoms of the disease. 7 , 8 Metoclopramide, a centrally penetrating dopamine D2 receptor antagonist, is the only US Food and Drug Administration (FDA)‐approved medication available for patients with acute or recurrent diabetic gastroparesis in the United States. 9 However, owing to the risk of central nervous system side effects, metoclopramide carries a black box warning from the FDA, and its use is restricted to 12 weeks. 6 , 9 , 10 , 11 Domperidone, a dopamine D2/D3 receptor antagonist, acts peripherally and therefore does not elicit the central nervous system effects associated with long‐term use of metoclopramide. 12 Although domperidone has received approval for the short‐term treatment of nausea and vomiting in more than 20 countries, 13 it is not approved for the treatment of gastroparesis in the European Union 14 and is not a legally marketed human drug or approved for sale in the United States. 15 Risks associated with the use of domperidone include cardiovascular risks of QT prolongation, 16 ventricular arrhythmia, and sudden cardiac death, 17 which likely occur via the inhibition of the human ether‐à‐go‐go‐related gene (hERG) potassium channel. 18 , 19 , 20
Trazpiroben (previously referred to as TAK‐906 and ATC1906M) is a novel, peripherally restricted, and selective dopamine D2/D3 receptor antagonist being developed for the treatment of gastroparesis. Trazpiroben has a dopamine receptor antagonist profile similar to that of domperidone, but has lower affinity for the hERG potassium channel (half maximal inhibitory concentration of 15.6 μM vs. 0.1 μM, respectively), thereby reducing the risk of cardiovascular adverse events (AEs). 21 , 22 , 23 , 24 , 25
The absolute bioavailability of trazpiroben was ~20% after oral administration in rats and dogs. 26 , 27 Preclinically, plasma concentrations of trazpiroben generally increased in a dose‐proportional manner, with minor accumulation after repeated doses. 28 In vitro studies have demonstrated that trazpiroben is primarily metabolized via a non‐cytochrome P450 (CYP) pathway by multiple cytosolic nicotinamide adenine dinucleotide phosphate (NADPH)‐dependent reductases (56.7%) to a ketone product (M23; structure provided in reference 29 ), with the remaining pathways of metabolism occurring via CYP3A4 (25.8%) and CYP2C8 (11.8%). 29 Following oral administration of radiolabeled trazpiroben in fasted rats, ~94% of the dosed radioactivity was excreted in feces and 6% in urine after 24 h. In bile duct cannulated rats, ~86%, 5%, and 11% of the dosed radioactivity was excreted in bile, urine, and feces, respectively, suggesting biliary excretion could be one of the major excretion routes of trazpiroben (data on file). Evaluation of absolute bioavailability, and characterization of the absorption, distribution, metabolism, and excretion of trazpiroben are ongoing and not yet available in humans.
In vitro drug–drug interaction (DDI) studies have shown that trazpiroben does not inhibit several of the major CYP isoforms (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A) and does not induce CYP isoforms when administered at a clinically relevant dose (0.06 μM). 29 Additionally, in contrast to domperidone, 30 phase I DDI studies have demonstrated that trazpiroben is relatively insensitive to CYP3A4 inhibition and concluded that the cardiovascular concerns associated with domperidone use are unlikely to occur when trazpiroben is used in similar conditions. 31 The first‐in‐human study with trazpiroben, completed in the United States (AT‐01C study), showed that single and multiple doses of trazpiroben were well‐tolerated in healthy men and women. 22 Trazpiroben was rapidly absorbed (time to maximum concentration [T max] ~1.1 h) and eliminated, with a mean half‐life of 4 h and a mean apparent oral clearance of ~600–800 L/h across single‐dose groups of 25–300 mg. Minor accumulation occurred after administration of trazpiroben 50 and 100 mg, twice daily for 5 days (accumulation index of 1.9 and 1.4, respectively). Trazpiroben has also been evaluated in healthy Japanese individuals, and was found to be well‐tolerated in this population. 32
Trazpiroben has been evaluated in vitro as a substrate or inhibitor of clinically relevant transporters and demonstrated to be a substrate of p‐glycoprotein (P‐gp) and OATP1B1/1B3. 29 Trazpiroben is not a substrate of breast cancer resistance protein (BCRP), multidrug and toxin extrusion (MATE) 1, MATE2‐K, organic anion transporter (OAT)1, OAT3, or organic cation transporter (OCT) 2. 29 Furthermore, trazpiroben is not an inhibitor of BCRP, bile salt export pump, MATE1, MATE2‐K, OAT1, OAT3, OCT2, or OATP1B1/1B3 at a clinically relevant dose. 29 Importantly, these in vitro studies predicted that nearly all in vivo uptake of trazpiroben into hepatocytes occurs via transporters (predominantly OATP1B1) and that clinical evaluation was warranted to determine the potential for increased trazpiroben exposure after administration of a strong OATP1B1/1B3 inhibitor. 29
To assess the role of OATP1B1/1B3 on the uptake of specific compounds in a clinical setting, several inhibitors are recommended by the FDA. 33 , 34 Rifampin is known to have pleiotropic effects, including CYP3A4 induction 35 , 36 as well as OATP1B1/1B3 and P‐gp inhibition. 37 , 38 A single intravenous (i.v.) dose of rifampin allows for the inhibition of OATP1B1/1B3 without inhibiting P‐gp in the gut 37 and is often used to evaluate potential DDIs involving these transporters. 33 , 37 DDIs are particularly relevant when considering patients with gastroparesis. These individuals often receive symptomatic treatment for the disease, as well as for multiple comorbidities, meaning that polypharmacy is common in affected patients. 39 , 40 Therefore, understanding the potential impact of DDIs when assessing novel treatments for gastroparesis is essential.
Sensitive and specific biomarkers permitting the evaluation of OATP inhibition would be of clinical value in the management of patients receiving trazpiroben. Coproporphyrin (CP) I and III are byproducts of heme synthesis that are secreted from the body in bile or urine. 41 Prior clinical studies have demonstrated that inhibition of OATP1B1/1B3‐mediated transport leads to increased levels of CPI and CPIII in plasma and urine when compared with baseline readings, indicating that these byproducts are substrates of OATP1B1/1B3. 42 , 43 , 44 , 45 Similar observations have been reported from in vitro and preclinical analyses. 42 , 46 Accordingly, these findings suggest that CPI and CPIII may have clinical utility as endogenous biomarkers of OATP1B1/1B3 activity.
This study sought to evaluate the impact of i.v. rifampin, a potent OATP1B1/1B3 inhibitor, on the single‐dose pharmacokinetic (PK) and safety profile of trazpiroben, and explore the use of CPI and CPIII as endogenous biomarkers of OATP1B1/1B3 activity after a single‐dose i.v. rifampin administration.
METHODS
Study design
This was a phase I, open‐label, randomized, two‐way crossover study (NCT04121078; Figure 1). A randomized cross‐over design was selected to reduce the effect of interindividual variability on the study results, and to reduce the number of participants required to demonstrate DDI potential between trazpiroben and rifampin. The primary objective of this study was to evaluate the effect of single‐dose i.v. rifampin on the single‐dose PKs of orally administered trazpiroben, and the secondary objective was to evaluate the safety and tolerability of a single oral dose of trazpiroben in the presence and absence of rifampin. As an exploratory objective, the plasma concentrations of CPI and CPIII were evaluated as endogenous biomarkers of OATP1B1 and OATP1B3 activity. Details of how and when the primary, secondary, and exploratory objectives were assessed are detailed in the PK analysis, safety and tolerability assessment, and statistical methods sections of this article.
FIGURE 1.

Study design. aBefore dosing on day 1 of study period 1, participants were randomized in a ratio of 1:1 to one of two treatment sequences (AB and BA). Participants received each treatment on one occasion. bEach trazpiroben dosing was separated by at least 7 days from the time of study period 1 trazpiroben administration. cParticipants were confined from check‐in until after the 48‐h post‐trazpiroben‐dose blood draw during each study period. dTrazpiroben administration was defined as hour 0. PK, pharmacokinetic
Patients were enrolled by a single physician and assigned to interventions at a single clinical study site (Celerion). The study was conducted between October 15, 2019, and November 24, 2019. To assess the impact of OATP1B1/1B3 inhibition on plasma levels of trazpiroben, i.v. rifampin was used during the study. 37 , 38 The study contained two periods (period 1 and period 2) that each comprised 4 days (day −1, 1, 2, and 3). The study included a screening visit to determine eligibility (conducted between 28 and 2 days prior to dosing) and a follow‐up visit. Participants were randomized 1:1 into one of two treatment sequences (AB or BA; Figure 1) according to a computer‐generated schedule (developed by Celerion). The schedule comprised predetermined numbers linked to study treatment sequences, and participants were assigned to the next available consecutive number upon enrollment. Participants were required to fast for at least 10 h prior to trazpiroben dosing. Trazpiroben was administered orally, with trazpiroben as an active ingredient supplied in a gelatin capsule (1 × 25 mg). In study period 1, participants in sequence AB received a single oral dose of trazpiroben 25 mg (treatment A) and those in sequence BA received a single oral dose of trazpiroben 25 mg immediately after a single i.v. dose of rifampin 600 mg administered over 30 min (treatment B). Study period 1 was followed by a washout period of at least 7 days, comprising at least five terminal half‐lives (t 1/2) of trazpiroben. After the washout period, each group received the other treatment regimen (study period 2). Treatments were administered on day 1 of each study period, with PK sampling and safety monitoring conducted over days 2 and 3 until 48 h after dosing. To facilitate sample collection, completion of safety assessments, and to control for other external factors that could influence study results, participants were confined from check‐in (day −1) until after the 48‐h post‐trazpiroben‐dose blood draw during each study period. Follow‐up assessments occurred on day 14 (±2 days) after the last trazpiroben dose. All pertinent study documents were reviewed by Advarra Institutional Review Board (IRB) prior to study initiation. Study‐related documents were approved by the local IRB of the study site, and written informed consent was obtained from participants prior to them undergoing any study‐specific procedures. The study was conducted in compliance with the principles outlined in the Declaration of Helsinki and Good Clinical Practice regulations and guidelines.
Inclusion and exclusion criteria
Participants eligible for the study were healthy, continuous nonsmokers, 19–55 years of age, with a body mass index (BMI) greater than or equal to 18.0 and less than or equal to 30.0 kg/m2 at screening. Participants were excluded if they were unable to refrain from, or anticipated the use of, any medication, herbal remedies, or vitamin supplements within 14 days prior to initial dosing and throughout the study. Participants were also excluded if they were unable to refrain from, or anticipated the use of, any medication known to significantly affect the absorption, distribution, metabolism, or elimination of the study drugs (trazpiroben and rifampin), including any drug that affected the function of OATP1B1/1B3, within 28 days prior to the first dosing and throughout the study (for further details, see Table S1).
Pharmacokinetic analysis
Blood samples (4 ml) were collected predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36, and 48 h post trazpiroben dose during both study periods. Plasma concentrations of trazpiroben and its inactive metabolite, M23, were used to calculate PK parameters, such as area under the curve (AUC) from time 0 extrapolated to infinity (AUC∞), maximum serum concentration (C max), and T max, using a noncompartmental approach. For CPI and CPIII, PK measures included AUC from time 0 to 24 h (AUC0‐24) and C max. Plasma concentrations of trazpiroben and M23 were measured using a validated liquid chromatography/tandem mass spectrometry assay at Q2 Solutions. The analytical range of the assay for trazpiroben and M23 was 0.1–50.0 ng/ml; results below the limit of quantitation (BLOQ) were replaced with zero for the purposes of PK parameter calculations. Plasma concentrations of CPI and CPIII were measured using a validated liquid chromatography mass spectrometry assay at PPD Laboratories. The analytical range of the assay for CPI and CPIII was 50.0–10,000.0 pg/ml.
Safety and tolerability assessment
Safety was monitored throughout the study. Safety measures assessed included treatment‐emergent adverse events (TEAEs), physical and vital signs, and electrocardiogram (ECG) measures. Vital signs, and a 12‐lead safety ECG in triplicate, were recorded during the screening period (day −28 to −2), at predose, and at 1, 2, 4, 8, and 48 h post trazpiroben dose during both study periods. At follow‐up, participants received a full physical examination and underwent assessment of vital signs and a 12‐lead ECG.
Statistical methods
A sample size was selected to give an 80% probability that the 90% confidence intervals (CIs) would be within 65% and 135% of the point estimate of the geometric mean ratio (GMR) for C max and the AUC of trazpiroben with and without rifampin co‐administration. PK parameters were calculated using plasma trazpiroben, M23, and baseline‐adjusted CPI and CPIII using Phoenix WinNonlin version 7.0 using noncompartmental analysis. Actual sampling times were used in all computations. All evaluable concentration data were included in the calculation of PK parameters. The individual concentration–time plots (using actual sample times) and the mean and median concentration–time plots (using nominal sample times) were prepared. The PK parameters of CPI and CPIII were determined from the concentration–time profiles for all participants using a noncompartmental analysis method. To assess the potential effect of rifampin on trazpiroben and M23 PK parameters, an analysis of variance (ANOVA) was performed on the natural log‐transformed AUC∞ and C max, using a mixed effects model. The mixed effects model included sequence, treatment, and period as fixed effects and subject nested within sequence as a random effect. Each model included calculation of least‐squares means (LSMs) and the difference between treatment LSMs for AUC∞ and C max and their respective CIs. The GMRs (trazpiroben with rifampin relative to trazpiroben alone) and the associated 90% CIs were derived by exponentiation of the CIs obtained for the difference between treatment LSM (treatment B – treatment A) resulting from the analyses on the log‐transformed AUC∞ and C max. Frequency counts and percentages were reported for categorical data where appropriate. Continuous variables were summarized using sample size (n), mean, standard deviation (SD), minimum, median, and maximum values.
RESULTS
Participant disposition and baseline demographics
In total, 34 participants were assessed for eligibility, of which 12 were enrolled into the study and randomized. Of the randomized participants, 12 received the study drugs (treatment sequence AB: n = 6; treatment sequence BA: n = 6) and were included in the final analysis (Figure 2). Most of the participants were men (83%) and had a mean (SD) age and BMI of 37.3 (10.6) years and 26.0 (2.2) kg/m2, respectively (Table 1). There were no marked differences in any participant demographics or characteristics between study groups.
FIGURE 2.
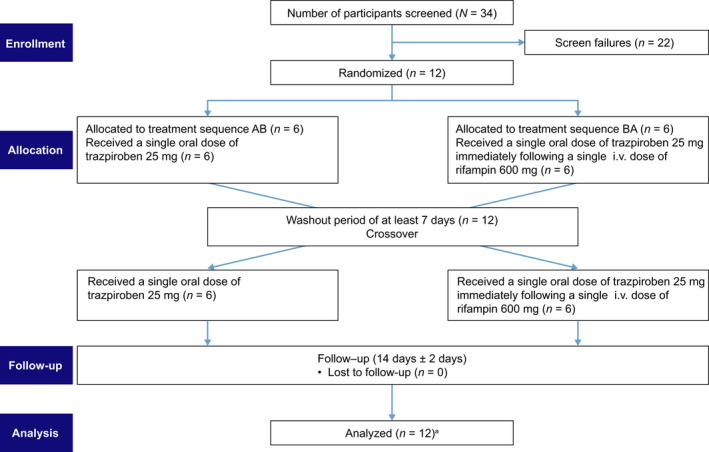
Consort Flow diagram. aAUC∞ of M23 (treatment A) could not be calculated for one subject as the terminal elimination phase could not be robustly characterized. AUC∞, area under the concentration–time curve from time 0 to infinity; i.v., intravenous
TABLE 1.
Baseline demographics
| Characteristic | Randomized treatment sequence a | Overall (n = 12) | |
|---|---|---|---|
| AB (n = 6) | BA (n = 6) | ||
| Sex, n (%) | |||
| Female | 1 (17) | 1 (17) | 2 (17) |
| Male | 5 (83) | 5 (83) | 10 (83) |
| Race, n (%) | |||
| Asian | 1 (17) | 0 (0) | 1 (8) |
| Black or African American | 1 (17) | 0 (0) | 1 (8) |
| Black or African American, White | 1 (17) | 0 (0) | 1 (8) |
| White | 3 (50) | 6 (100) | 9 (75) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 6 (100) | 6 (100) | 12 (100) |
| Age, years b | |||
| Mean (SD) | 36.7 (13.5) | 38.0 (8.1) | 37.3 (10.6) |
| Median (range) | 40.0 (21–52) | 39.5 (24–48) | 39.5 (21–52) |
| BMI, kg/m2 | |||
| Mean (SD) | 25.2 (2.4) | 26.8 (1.8) | 26.0 (2.2) |
| Median (range) | 24.9 (22.3–28.6) | 26.7 (24.8–29.1) | 25.7 (22.3–29.1) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Treatment sequence AB: Participants received a single oral dose of trazpiroben 25 mg. After a 7‐day washout period, participants received a single oral dose of trazpiroben 25 mg immediately after a single 30‐min intravenous infusion of rifampin 600 mg. Treatment sequence BA: Participants received a single oral dose of trazpiroben 25 mg immediately after a single 30‐min intravenous infusion of rifampin 600 mg. After a 7‐day washout period, participants received a single oral dose of trazpiroben 25 mg.
Age as recorded on the case report form.
Pharmacokinetic analysis
All participants had an evaluable PK profile. In total, 11 of 12 participants (92%) had detectable levels of plasma trazpiroben at 0.5 h after administration of trazpiroben alone, and all participants had detectable levels of trazpiroben at this time point when co‐administered with rifampin. At 12 h after treatment with trazpiroben alone, 11 of 12 participants (92%) had detectable levels of plasma trazpiroben, which dropped after 16 h to five of 12 participants (42%). After co‐administration of trazpiroben and rifampin, the majority of participants (10/12, 83%) had detectable levels of trazpiroben at 16 h post trazpiroben dose. The C max of plasma trazpiroben was higher in participants receiving trazpiroben in conjunction with rifampin than in those treated with trazpiroben alone (89.62 ng/ml vs. 14.37 ng/ml, respectively; Table 2).
TABLE 2.
Plasma trazpiroben pharmacokinetic parameters
| Parameter | Trazpiroben 25 mg (n = 12) | Trazpiroben 25 mg + rifampin 600 mg (n = 12) | Geometric mean ratio (90% CI) a | Intra‐participant CV% b |
|---|---|---|---|---|
| AUC∞, ng*h/ml, geometric mean c (mean CV%) | 32.68 (23.9) | 168.5 (49.9) | 5.16 (4.25–6.25) | 26.48 |
| C max, ng/ml, geometric mean c (geometric CV%) | 14.37 (38.7) | 89.62 (68.6) | 6.24 (4.62–8.42) | 42.31 |
| T max, h, median (min, max) | 1.037 (0.50, 2.00) | 1.002 (0.49, 2.00) | NR | NR |
| t 1/2z, h, mean (SD) | 4.229 ± 3.7897 | 2.028 ± 0.3449 | NR | NR |
| CL/F, L/h, geometric mean a (geometric CV%) | 765.1 (23.9) | 148.4 (49.9) | NR | NR |
| V z/F, L, geometric mean c (geometric CV%) | 3469 (70.7) | 428.1 (49.9) | NR | NR |
Abbreviations: ANOVA, analysis of variance; AUC∞, area under the concentration–time curve from time 0 to infinity; CI, confidence interval; C max, maximum observed concentration; CL/F, apparent clearance after extravascular administration; CV%, geometric coefficient of variation %; max, maximum; min, minimum; LSM, least‐squares means; MSE, mean square error; NR, not reported; SD, standard deviation; T max, time to first occurrence of C max; t 1/2z, terminal disposition phase half‐life; V z/F, apparent volume of distribution during the terminal disposition phase after extravascular administration.
Geometric mean ratio = (trazpiroben)/(trazpiroben + rifampin).
Intra‐participant CV% = 100 × square root (exp[MSE] − 1), where MSE = residual variance from ANOVA.
Geometric LSM were calculated by exponentiating the LSM from ANOVA.
The overall and peak plasma exposure of trazpiroben was higher after co‐administration of trazpiroben and rifampin versus trazpiroben alone, although median T max remained similar between study groups (Figure S1 and Table 2). Trazpiroben AUC∞ for participants treated with trazpiroben alone was 32.7 ng*h/ml versus 168.5 ng*h/ml after treatment with trazpiroben and rifampin, and a similar trend was observed for C max (Table 2). Although the mean terminal disposition phase half‐life (t 1/2z) of trazpiroben was numerically higher in participants treated with trazpiroben alone than in those receiving trazpiroben co‐administered with rifampin (Table 2), the median terminal profiles of plasma trazpiroben in participants receiving either trazpiroben or trazpiroben co‐administered with rifampin were visually similar (Figure 3). The differences in t 1/2z observed between participants treated with trazpiroben or trazpiroben and rifampin may have been due to interindividual variability. GMRs of AUC∞ and C max in participants treated with trazpiroben and rifampin were 5.16 (90% CI: 4.25–6.25)‐fold higher and 6.24 (90% CI: 4.62–8.42)‐fold higher, respectively, than those observed in participants treated with trazpiroben alone (Table 2).
FIGURE 3.
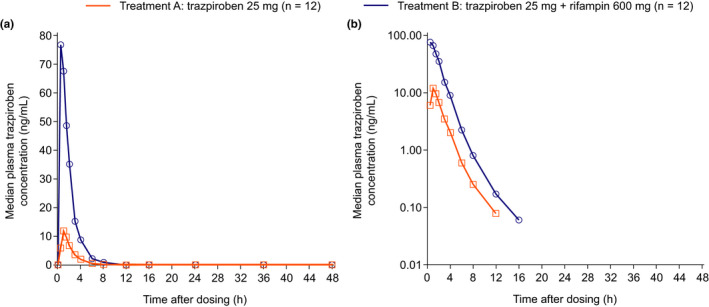
Median plasma concentration–time curves of trazpiroben on a linear scale (a) and semi‐log scale (b)
For M23, an inactive abundant metabolite of trazpiroben, the concentration–time curves, and PK parameters are presented in Figure S2 and Table S2, respectively, and the GMRs of M23 AUC∞ and C max are provided in Table S3. Generally consistent with the results observed for trazpiroben, plasma M23 AUC∞ and C max were higher in participants treated with trazpiroben and rifampin than in those treated with trazpiroben alone. The GMRs of plasma M23 AUC∞ (20.96, 90% CI: 15.62–28.13) and C max (24.48, 90% CI: 17.59–34.06) were approximately four to five‐fold higher than the equivalent GMRs observed for plasma trazpiroben AUC∞ (5.16, 90% CI: 4.25–6.25) and C max (6.24, 90% CI: 4.62–8.42), respectively.
Plasma CPI and CPIII concentrations were higher in participants receiving rifampin and trazpiroben compared with those receiving trazpiroben alone (peak arithmetic means of 3.15 vs. 0.64 nM and 0.50 vs. 0.12 nM, respectively; Figure S3), suggesting that OATP1B1/1B3‐dependent transport was successfully inhibited. Plasma CPI AUC0‐24 and C max increased 3.20‐ and 4.89‐fold, respectively, after co‐administration of trazpiroben and rifampin when compared with trazpiroben alone. Additionally, respective values for plasma CPIII AUC0‐24 and C max increased 2.86‐ and 4.31‐fold, respectively, after co‐administration of trazpiroben and rifampin versus trazpiroben alone.
Safety analysis
There were no deaths, serious AEs, or discontinuations due to an AE during this study. In total, 12 participants were included in the safety analysis, and 10 TEAEs were recorded, of which headache was the most frequently reported. All TEAEs were considered mild in severity. Of participants receiving trazpiroben alone, two reported a headache (17%), and other TEAEs included one report each of upper abdominal pain (8%), sensory disturbance (8%), sinus congestion (8%), and contact dermatitis (8%; Table 3). Of TEAEs reported for participants receiving trazpiroben and rifampin, single incidents of headache, cough, and nasal congestion were reported (Table 3). Overall, one headache event was considered to be related to both trazpiroben and rifampin, and the remaining nine AEs were considered unrelated to both study drugs. For all participants, serum chemistry, hematology, urinalysis, vital signs, and ECG results remained within normal limits throughout the study and changes from baseline were minimal. All out‐of‐range readings observed in these tests were deemed to be not clinically meaningful.
TABLE 3.
Overview of TEAEs reported by participants
| TEAE, n (%) | Trazpiroben 25 mg (n = 12) | Trazpiroben 25 mg + rifampin 600 mg (n = 12) | Overall (n = 12) |
|---|---|---|---|
| Number of participants with TEAEs | 6 (50) | 2 (17) | 6 (50) |
| Gastrointestinal disorders | 1 (8) | 0 (0) | 1 (8) |
| Abdominal pain, upper | 1 (8) | 0 (0) | 1 (8) |
| Nervous system disorders | 3 (25) | 1 (8) | 3 (25) |
| Headache | 2 (17) | 1 (8) | 2 (17) |
| Sensory disturbance | 1 (8) | 0 (0) | 1 (8) |
| Respiratory, thoracic, and mediastinal disorders | 1 (8) | 1 (8) | 2 (17) |
| Cough | 0 (0) | 1 (8) | 1 (8) |
| Nasal congestion | 0 (0) | 1 (8) | 1 (8) |
| Sinus congestion | 1 (8) | 0 (0) | 1 (8) |
| Sneezing | 1 (8) | 0 (0) | 1 (8) |
| Skin and subcutaneous tissue disorders | 1 (8) | 0 (0) | 1 (8) |
| Dermatitis contact | 1 (8) | 0 (0) | 1 (8) |
Note: Adverse events are classified according to the Medical Dictionary for Regulatory activities (MedDRA) version 22.1. If a participant had two or more clinical adverse events within a category, the participant is counted only once within the category.
Abbreviation: TEAE, treatment‐emergency adverse event.
DISCUSSION
The current study assessed the effect of i.v. rifampin, a potent inhibitor of OATP1B1/1B3, on the single‐dose PKs, safety, and tolerability of trazpiroben in healthy adult participants. The AUC∞ and C max of plasma trazpiroben were 5.16‐ and 6.24‐fold higher after co‐administration of rifampin with a single‐dose of trazpiroben than with trazpiroben alone, respectively, confirming trazpiroben as a sensitive (AUC increase of ≥5‐fold) 33 substrate of OATP1B1/1B3 in humans. On the basis of these data, it is recommended that combinations of trazpiroben with moderate to strong 33 OATP1B1/1B3 inhibitors should be avoided in ongoing clinical studies if co‐administration is expected to result in more than a twofold increase in plasma trazpiroben AUC. Co‐administration of trazpiroben with weak inhibitors of OATP1B1/1B3 (<2‐fold change in AUC) 33 is considered acceptable.
This study also investigated the plasma levels of M23, an inactive metabolite of trazpiroben, after administration of trazpiroben alone and in combination with rifampin. M23 was monitored in this analysis as it was deemed a borderline major (~11%) inactive metabolite of trazpiroben, based on early, incomplete exploratory data available at the time of this study. Generally, changes in M23 plasma levels and PK parameter values were similar to those seen for plasma trazpiroben. However, increases in plasma M23 AUC∞ and C max were approximately four to five‐fold higher than the corresponding measurements for plasma trazpiroben. As previously reported, 29 M23 is formed by non‐CYP cytosolic NADPH‐dependent reductases, and hypothesized to be predominantly formed extrahepatically. Co‐administration of rifampin with trazpiroben could block the hepatic uptake of M23 via OATP1B1/1B3, resulting in elevated M23 plasma concentrations (unpublished ongoing investigations). The impact of increased plasma M23 after OATP1B1/1B3 inhibition was assessed in preclinical models in vivo and no safety signals were identified (data on file). In addition, no clinically relevant AE relating to QT prolongation was reported during the current study.
In agreement with their potential role as endogenous biomarkers of OATP1B1/1B3 inhibition, 43 , 44 , 47 plasma CPI and CPIII levels increased after co‐administration of trazpiroben and i.v. rifampin when compared with trazpiroben alone. This indicates that OATP1B1/1B3‐dependent transport was successfully inhibited and further validated the experimental approach adapted from previous reports for oral rifampin administration. The PKs of rifampin after a single i.v. or oral dose have previously been shown to be comparable, and therefore it would be expected that plasma CPI/CPIII changes would also be similar. 48 Fold changes in plasma CPI/CPIII after i.v. administration of rifampin were comparable to those previously reported by studies utilizing oral rifampin. In the present study, fold changes in plasma CPI and CPIII AUC0‐24 and C max after co‐administration of trazpiroben and rifampin were 3.20‐ and 4.89‐fold (CPI) and 2.86‐ and 4.31‐fold (CPIII), respectively, when compared with trazpiroben alone. Shen et al. reported similar fold changes in AUC0‐24 and C max for CPI (3.30‐ and 5.60‐fold, respectively) and CPIII (4.75 and 2.57‐fold, respectively) after a single oral dose of rifampin 600 mg. 44 In the current study, basal levels of CPI were 0.64 nM, whereas CPIII were 0.12 nM, which are similar to those reported by Shen et al. (0.72 and 0.14 nM, respectively). 44 In addition, Kunze et al. also reported levels of plasma CPI and CPIII after a single oral dose of rifampin 600 mg, which were consistent with our findings. 47 The comparable fold changes in plasma CPI and CPIII observed after oral and i.v. rifampin support their use as endogenous biomarkers of OATP1B1/1B3 inhibition following i.v. administration of rifampin.
Trazpiroben was well‐tolerated during this study. No safety signals were observed when trazpiroben was administered as either a single agent or in combination with rifampin, and no clinically meaningful changes in ECG results or vital signs were reported. Additional studies have also been completed or are underway to further elucidate the safety profile of trazpiroben. A recent phase II study (NCT03268941) involving 51 patients with idiopathic or diabetic gastroparesis evaluated the safety, PKs, and pharmacodynamics of trazpiroben and did not identify any safety signals, 49 whereas an ongoing phase IIb clinical trial (NCT03544229) is investigating the efficacy and safety of trazpiroben in adult patients with symptomatic idiopathic or diabetic gastroparesis. Notably, these studies imposed restrictions on the use of certain medications (e.g., those that affect gastric motility or gastric pH and antiemetics) throughout the study period and therefore the potential for OATP‐mediated DDIs may have been limited.
The limitations of this study should be considered. The study was conducted in a small number of healthy volunteers who received the study treatment sequences over a shorter period than would typically be observed within a clinical setting. Based on this clinical DDI assessment, trazpiroben was confirmed as a sensitive substrate of OATP1B1/1B3, with a potential for DDIs when administered with inhibitors of these transporters. Owing to the increased exposure to trazpiroben observed in this study after administration with an OATP1B1/1B3 inhibitor compared with trazpiroben alone, co‐administration of trazpiroben with moderate to strong OATP1B1/1B3 inhibitors is not recommended.
CONCLUSION
Trazpiroben exposure was increased after co‐administration of an OATP1B1/1B3 inhibitor (single‐dose i.v. rifampin) in healthy adults compared with trazpiroben alone, confirming trazpiroben as a sensitive substrate of these transporters. Given the results of this study, co‐administration of trazpiroben with moderate to strong OATP1B1/1B3 inhibitors is not recommended. Both interventions were well‐tolerated by healthy participants. This study is the first to report on the utility of CPI and CPIII as endogenous biomarkers of OATP1B1/1B3‐mediated DDIs after administration of a single i.v. dose of rifampin.
CONFLICT OF INTEREST
J.K.M. was a salaried employee of Takeda Development Center Americas, Inc., and received stock or stock options at the time of the study, and is currently an employee of EMD Serono Research and Development Institute, Inc., Billerica, Massachusetts, USA. M.T., L.W., S.Y.H., and D.R. are employees of Takeda Development Center Americas, Inc., and receive stock or stock options. G.D. was a contracted employee of Takeda Development Center Americas, Inc., at the time of the study, and is currently an employee of Ironwood Pharmaceuticals, Inc. C.A. was an employee of Takeda Development Center Americas, Inc., and received stock or stock options at the time of the study, and is currently an employee of Ironwood Pharmaceuticals, Inc. M.N. is an employee of Takeda Pharmaceutical Company, Limited., Fujisawa, Kanagawa, Japan. C.C. was an employee of Takeda Development Center Americas, Inc., and received stock or stock options at the time of the study, and is currently an employee of Bayer Pharmaceuticals.
Study protocol: The full protocol for this study can be accessed at: https://clinicaltrials.gov/ProvidedDocs/78/NCT04121078/Prot_001.pdf.
ClinicalTrials.gov Identifier: NCT04121078.
AUTHOR CONTRIBUTIONS
C.A., C.C., G.D., S.H., J.K.M., M.N., D.R., M.T., and L.W. wrote the manuscript. J.K.M., G.D., L.W., C.A., M.N., and C.C. designed the research. J.K.M. performed the research. J.K.M., M.T., L.W., S.H., and D.R. analyzed the data.
Supporting information
Table S1 Excluded medications, supplements, and dietary products
Table S2 Plasma M23 pharmacokinetic parameters
Table S3 Plasma M23 pharmacokinetic parameters: summary of statistical comparisons
Figure S1 Mean (+ SD) plasma concentration–time curves of trazpiroben
Figure S2 Median plasma concentration–time curves of M23 on a linear scale (a) and semi‐log scale (b)
Figure S3 Mean plasma concentration–time curves of coproporphyrin (CP) I (a) and CPIII (b)
ACKNOWLEDGMENTS
Medical writing assistance was provided by Luke Humphreys, PhD, of Oxford PharmaGenesis, Oxford, UK, and was supported by Takeda Development Center Americas, Inc.
Mukker JK, Dukes G, Tolkoff M, et al. The pharmacokinetics of oral trazpiroben (TAK‐906) after organic anion transporting polypeptide 1B1/1B3 inhibition: A phase I, randomized study. Clin Transl Sci. 2022;15:1532‐1543. doi: 10.1111/cts.13274
Funding information
This study was sponsored by Takeda Development Center Americas, Ltd.
REFERENCES
- 1. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4:41. [DOI] [PubMed] [Google Scholar]
- 2. Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis cardinal symptom index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13:833‐844. [DOI] [PubMed] [Google Scholar]
- 3. Jehangir A, Parkman HP. Reflux symptoms in gastroparesis: correlation with gastroparesis symptoms, gastric emptying, and esophageal function testing. J Clin Gastroenterol. 2020;54:428‐438. [DOI] [PubMed] [Google Scholar]
- 4. Wadhwa V, Mehta D, Jobanputra Y, Lopez R, Thota PN, Sanaka MR. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;23:4428‐4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis: quality of life and health care utilization. J Clin Gastroenterol. 2018;52:20‐24. [DOI] [PubMed] [Google Scholar]
- 6. Myint AS, Rieders B, Tashkandi M, et al. Current and emerging therapeutic options for gastroparesis. Gastroenterol Hepatol. 2018;14:639‐645. [PMC free article] [PubMed] [Google Scholar]
- 7. Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of Gastroenterology . Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18‐37; quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Usai‐Satta P, Bellini M, Morelli O, Geri F, Lai M, Bassotti G. Gastroparesis: new insights into an old disease. World J Gastroenterol. 2020;26:2333‐2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ANI Pharmaceuticals, Inc . Reglan (metoclopromide) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017854s062lbl.pdf. Revised August 2017.
- 10. Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31:11‐19. [DOI] [PubMed] [Google Scholar]
- 11. Bateman DN, Rawlins MD, Simpson JM. Extrapyramidal reactions with metoclopramide. Br Med J. 1985;291:930‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barone JA. Domperidone: a peripherally acting dopamine2‐receptor antagonist. Ann Pharmacother. 1999;33:429‐440. [DOI] [PubMed] [Google Scholar]
- 13. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726‐733. [DOI] [PubMed] [Google Scholar]
- 14. European Medicines Agency . EMA/152501/2014 ‐ Domperidone‐containing medicinal products. 2014. https://www.ema.europa.eu/en/documents/referral/domperidone‐article‐31‐referral‐prac‐assessment‐report_en.pdf. Accessed June 22, 2021.
- 15. U.S. Food and Drug Administration . How to request domperidone for expanded access use. 2021. https://www.fda.gov/drugs/investigational‐new‐drug‐ind‐application/how‐request‐domperidone‐expanded‐access‐use#:~:text=Domperidone%20is%20not%20currently%20a,domperidone%2Dcontaining%20products%20is%20illegal. Accessed June 1, 2021.
- 16. Ortiz A, Cooper CJ, Alvarez A, Gomez Y, Sarosiek I, McCallum RW. Cardiovascular safety profile and clinical experience with high‐dose domperidone therapy for nausea and vomiting. Am J Med Sci. 2015;349:421‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leelakanok N, Holcombe A, Schweizer ML. Domperidone and risk of ventricular arrhythmia and cardiac death: a systematic review and meta‐analysis. Clin Drug Investig. 2016;36:97‐107. [DOI] [PubMed] [Google Scholar]
- 18. Giudicessi JR, Ackerman MJ, Camilleri M. Cardiovascular safety of prokinetic agents: a focus on drug‐induced arrhythmias. Neurogastroenterol Motil. 2018;30:e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hondeghem LM. Domperidone: limited benefits with significant risk for sudden cardiac death. J Cardiovasc Pharmacol. 2013;61:218‐225. [DOI] [PubMed] [Google Scholar]
- 20. Sanguinetti MC, Tristani‐Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463‐469. [DOI] [PubMed] [Google Scholar]
- 21. Kreckler LM, Osinski MA, Williams SM, Whiting R. Su1739 safety pharmacology evaluations of TAK‐906, a novel dopamine D2/D3 selective receptor antagonist for the management of gastroparesis. Gastroenterology. 2020;158:S‐627‐S‐628. doi: 10.1016/S0016-5085(1020)32267-32268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whiting RL, Darpo B, Chen C, et al. Safety, pharmacokinetics, and pharmacodynamics of trazpiroben (TAK‐906), a novel selective D2/D3 receptor antagonist: a phase 1 randomized, placebo‐controlled single‐ and multiple‐dose escalation study in healthy participants. Clin Pharmacol Drug Dev. 2021;10:927‐939. doi: 10.1002/cpdd.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jasper J, Whiting R. TAK‐906, a dopamine D2/D3 receptor antagonist with minimal brain penetration for gastrointestinal disorders. FASEB J. 2020;34:1. [Google Scholar]
- 24. Champion MC, Hartnett M, Yen M. Domperidone, a new dopamine antagonist. CMAJ. 1986;135:457‐461. [PMC free article] [PubMed] [Google Scholar]
- 25. Claassen S, Zunkler BJ. Comparison of the effects of metoclopramide and domperidone on HERG channels. Pharmacology. 2005;74:31‐36. [DOI] [PubMed] [Google Scholar]
- 26. Altos Therapeutics LLC . DATA ON FILE: evaluation of bioavailability of ATC‐1906 maleic salt in male beagle dog following intravenous and oral administration; 2014.
- 27. Altos Therapeutics LLC . DATA ON FILE: evaluation of bioavailability of 3 different formulations of ATC‐1906 in male Sprague‐Dawley rat following intravenous and oral administration; 2015.
- 28. Altos Therapeutics LLC . DATA ON FILE: 4‐week oral gavage toxicity, toxicokinetic, central nervous system, and respiratory safety pharmacology evaluation study with ATC‐1906 (maleate salt) in rats with a 2‐week recovery phase; 2014.
- 29. Nishihara M, Ramsden D, Balani SK. Evaluation of the drug‐drug interaction potential for trazpiroben (TAK‐906), a D2/D3 receptor antagonist for gastroparesis, towards cytochrome P450s and transporters. Xenobiotica. 2021;51:668‐679. [DOI] [PubMed] [Google Scholar]
- 30. Rossi M, Giorgi G. Domperidone and long QT syndrome. Curr Drug Saf. 2010;5:257‐262. [DOI] [PubMed] [Google Scholar]
- 31. Chen C, Zhang W, Bari M, Almansa C, Baratta M, Rosario M. Evaluation of the pharmacokinetics of trazpiroben (TAK‐906), a peripherally selective D2/D3 dopamine receptor antagonist, in the presence and absence of itraconazole, a potent CYP 3A4 inhibitor. Clin Pharmacol. 2021;13:145‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whiting, R. , Darpo, B. , Chen, C. et al. Ethnic similarity in pharmacokinetics and pharmacodynamics of trazpiroben (TAK‐906), a D2/D3 receptor antagonist: phase 1 single‐ and multiple‐ascending dose studies in healthy Japanese and US participants. Presented at the Annual Meeting of the American College of Clinical Pharmacology (ACCP‐20); 2020.
- 33. U.S. Food and Drug Administration . Drug development and drug interactions: table of substrates, inhibitors and inducers. 2020. https://www.fda.gov/drugs/drug‐interactions‐labeling/drug‐development‐and‐drug‐interactions‐table‐substrates‐inhibitors‐and‐inducers#table5‐2. Accessed April 28, 2021.
- 34. Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schuetz EG, Schuetz JD, Strom SC, et al. Regulation of human liver cytochromes P‐450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology. 1993;18:1254‐1262. [PubMed] [Google Scholar]
- 36. Li T, Chiang JY. Rifampicin induction of CYP3A4 requires pregnane X receptor cross talk with hepatocyte nuclear factor 4alpha and coactivators, and suppression of small heterodimer partner gene expression. Drug Metab Dispos. 2006;34:756‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reitman ML, Chu X, Cai X, et al. Rifampin's acute inhibitory and chronic inductive drug interactions: experimental and model‐based approaches to drug‐drug interaction trial design. Clin Pharmacol Ther. 2011;89:234‐242. [DOI] [PubMed] [Google Scholar]
- 38. Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584:57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee A, Kuo B. Metoclopramide in the treatment of diabetic gastroparesis. Expert Rev Endocrinol Metab. 2010;5:653‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Youssef AS, Parkman HP, Nagar S. Drug‐drug interactions in pharmacologic management of gastroparesis. Neurogastroenterol Motil. 2015;27:1528‐1541. [DOI] [PubMed] [Google Scholar]
- 41. Kaplowitz N, Javitt N, Kappas A. Coproporphyrin I and 3 excretion in bile and urine. J Clin Invest. 1972;51:2895‐2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bednarczyk D, Boiselle C. Organic anion transporting polypeptide (OATP)‐mediated transport of coproporphyrins I and III. Xenobiotica. 2016;46:457‐466. [DOI] [PubMed] [Google Scholar]
- 43. Barnett S, Ogungbenro K, Menochet K, Shen H, Humphreys WG, Galetin A. Comprehensive evaluation of the utility of 20 endogenous molecules as biomarkers of OATP1B inhibition compared with rosuvastatin and coproporphyrin I. J Pharmacol Exp Ther. 2019;368:125‐135. [DOI] [PubMed] [Google Scholar]
- 44. Shen H, Christopher L, Lai Y, et al. Further studies to support the use of coproporphyrin I and III as novel clinical biomarkers for evaluating the potential for organic anion transporting polypeptide 1B1 and OATP1B3 inhibition. Drug Metab Dispos. 2018;46:1075‐1082. [DOI] [PubMed] [Google Scholar]
- 45. Wolkoff AW, Wolpert E, Pascasio FN, Arias IM. Rotor's syndrome. A distinct inheritable pathophysiologic entity. Am J Med. 1976;60:173‐179. [DOI] [PubMed] [Google Scholar]
- 46. Shen H, Dai J, Liu T, et al. Coproporphyrins I and III as functional markers of OATP1B activity: in vitro and in vivo evaluation in preclinical species. J Pharmacol Exp Ther. 2016;357:382‐393. [DOI] [PubMed] [Google Scholar]
- 47. Kunze A, Ediage EN, Dillen L, Monshouwer M, Snoeys J. Clinical investigation of coproporphyrins as sensitive biomarkers to predict mild to strong OATP1B‐mediated drug‐drug interactions. Clin Pharmacokinet. 2018;57:1559‐1570. [DOI] [PubMed] [Google Scholar]
- 48. Loos U, Musch E, Jensen JC, Mikus G, Schwabe HK, Eichelbaum M. Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin Wochenschr. 1985;63:1205‐1211. [DOI] [PubMed] [Google Scholar]
- 49. Kuo B, Scimia C, Dukes G, et al. Randomised clinical trial: safety, pharmacokinetics and pharmacodynamics of trazpiroben (TAK‐906), a dopamine D2/D3 receptor antagonist, in patients with gastroparesis. Aliment Pharmacol Ther. 2021;54:267‐280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Excluded medications, supplements, and dietary products
Table S2 Plasma M23 pharmacokinetic parameters
Table S3 Plasma M23 pharmacokinetic parameters: summary of statistical comparisons
Figure S1 Mean (+ SD) plasma concentration–time curves of trazpiroben
Figure S2 Median plasma concentration–time curves of M23 on a linear scale (a) and semi‐log scale (b)
Figure S3 Mean plasma concentration–time curves of coproporphyrin (CP) I (a) and CPIII (b)


